Abstract
We present a CMOS-based high-density microelectrode array (HD-MEA) system that enables high-density mapping of brain slices in-vitro with multiple readout modalities. The 4.48×2.43 mm2 array consists of 59,760 micro-electrodes at 13.5 µm pitch (5487 electrodes/mm2). The overall system features 2048 action-potential, 32 local-field-potential and 32 current recording channels, 32 impedance-measurement and 28 neurotransmitter-detection channels and 16 voltage/current stimulation channels. The system enables real-time and label-free monitoring of position, size, morphology and electrical activity of brain slices.
Keywords: CMOS, High-density microelectrode arrays, Electrophysiology, Action potential, Impedance measurement, Brain slices
Introduction
Microelectrode arrays (MEAs) are well-established platforms for in-vitro extracellular investigation of electrogenic cells and tissues [1]. They are widely used for characterization of cell physiological responses of dissociated brain cells, acute or organotypic brain slices, and retinae [2]. Recent CMOS-based high-density MEA systems (HD-MEAs) comprise of thousands of electrodes at pitches down to a few µm [3][4], which is crucial to obtain detailed insights into cellular and sub-cellular phenomena. HD-MEAs feature mostly voltage recording and/or stimulation units [4] and they are limited either in their noise performance or spatial resolution [5]. The system presented in [6] features a combination of impedance, extracellular voltage recording, and optical detection functions, however, with only a very few number of channels and low spatial resolution. Moreover, it does not include stimulation capabilities. In this paper, we present a high-density MEA system that allows for performing six measurement modalities in parallel on any arbitrary set or subset of electrodes.
Design
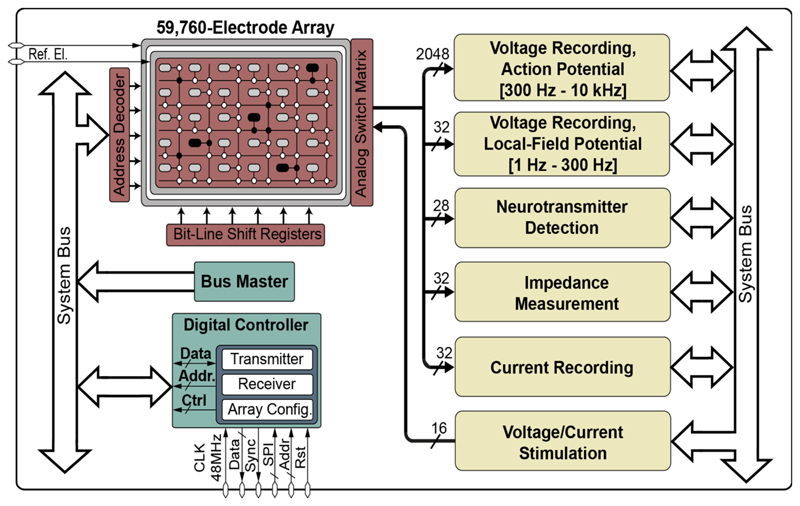
The electrode array routing scheme has been designed and implemented with a large number of wires and switches that can be configured in a flexible manner, so that any electrode in the array can be connected to any sensing/stimulation modality or channel (Figure 1).
Figure 1.
High-density microelectrode array (HD-MEA) system including an array of 59,760 electrodes and multiple functional measurement and stimulation blocks.
To simultaneously record electrophysiological activity of large cell networks, 2048 action-potential readout (300 Hz to 6 kHz) and 32 local-field-potential (1 Hz to 300 Hz) channels were integrated in the MEA system. Each electrophysiology recording channel consists of four fully differential amplification and filtering stages and a 10-bit analog/digital converter (ADC) to sample the channels at 20 kSamples/s. To sense the impedance magnitude and phase over a frequency range from 1 Hz to 1 MHz, 32 lock-in amplifiers were integrated on-chip. 28 neurotransmitter detection channels were included that can be used to perform, for example, cyclic voltammetry.
Experimental
Fabrication
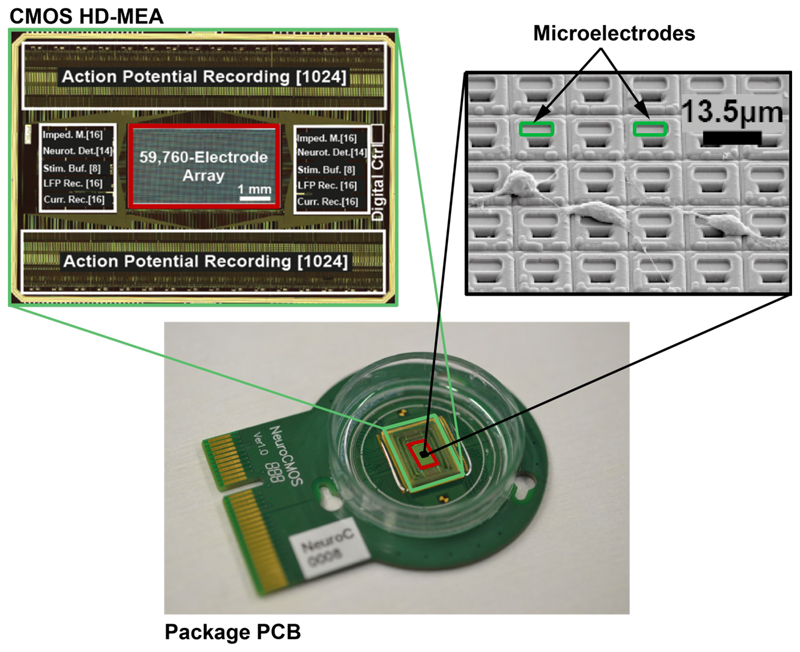
The HD-MEA chip was fabricated in 0.18-µm CMOS technology (6M1P) and has a size of 12.0×8.9 mm2 [7]. The platinum microelectrodes were post-processed at wafer level by means of ion-beam deposition and etching. A multilayer SiO2/Si3N4 passivation stack was deposited by plasma-enhanced chemical vapor deposition (PECVD), to protect the CMOS circuits against the saline solution that was used as cell culture medium. On the other hand, the passivation helps to prevent the release of toxic aluminum and copper from the CMOS metal layers into the cell culture. Openings in the passivation, defining the electrode areas and wire bonding contacts, were fabricated in a reactive-ion etching (RIE) step. Figure 2 shows the bio-compatible chip packaging used for the cell culturing, a micrograph of the chip, and an SEM image of the postprocessed array surface with rat cortical neurons.
Figure 2.
Bio-compatible chip packaging and PCB (bottom), chip micrograph (close-up top-left), and SEM image (close-up top-right) of the post-processed array surface with rat cortical neurons. The HD-MEA chip was fabricated in 0.18-µm CMOS technology.
Measurement setup
To characterize different cell physiological responses of the brain slice, the HD-MEA PCB was connected to a custom-designed chip support board, which provides interfacing circuitry to communicate with the HD-MEA chip. A serial peripheral interface (SPI) was used for sending commands to configure the electrode array and the on-chip circuitry. Data from the chip was acquired using a National Instruments PXIe-6544 DAQ card, which provided a high-speed interface to a desktop PC. A custom-designed LabVIEW (NI, Texas, United States) program was running on the PC to visualize experiments and perform recordings.
Recordings
The setup was kept at room temperature during electrophysiology and impedance recording session, while the acute tissue on the HD-MEA chip was continuously superfused with carbogen-bubbled Ca-free artificial cerebrospinal fluid (ACSF) at 36°C to maintain cell viability. By using the 32 parallel impedance measurement units on the chip, a full array scanning of the 59,760 electrodes at full spatial resolution will require ~1868 electrode configurations. Here, the impedance measurements were performed at 10 kHz over 120 electrode configurations to acquire the impedance values from 3200 uniformly distributed electrodes. As there are 2048 electrophysiology-recording channels that can be simultaneously used, a full electrophysiology activity scan of the entire array was done with a sparse electrode configuration (254 electrodes/mm2).
Results
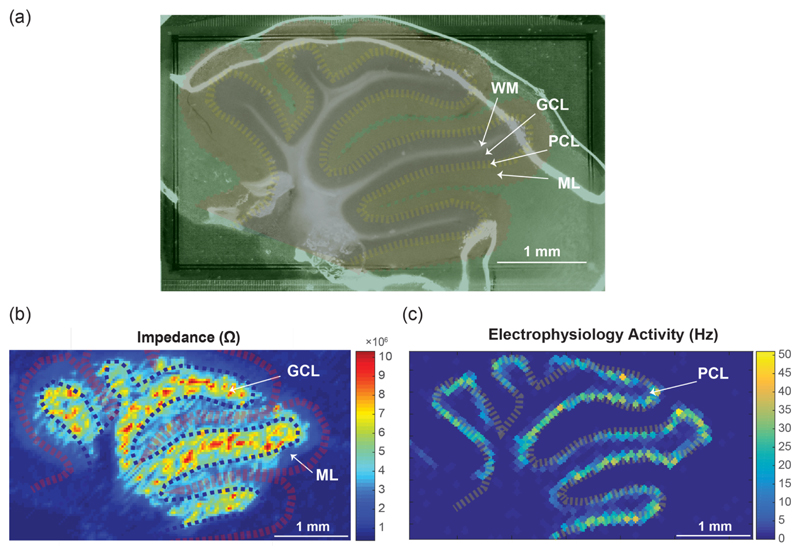
To perform multi-functional mapping of different tissues and cell layers, an acute cerebellar slice of a wildtype mouse was placed on the array (Figure 3.a). Four main cell layers could be differentiated: 1) white matter (WM) that consists of low-density fibers and axons but does not contain electrogenic cells, 2) the granular cell layer (GCL) featuring a high density of granular cells, 3) the Purkinje cell layer (PCL), which contains Purkinje neurons with high electrophysiological activity, and 4) the molecular layer (ML), which contains the flattened dendritic trees of Purkinje cells.
Figure 3.
Impedance imaging and measurement of the electrophysiological activity of a cerebellar mouse brain slice placed on the high-density array (a) Microscopic image of the cerebellar slice. The white regions show the white matter (WM), granular cells (GCL) are marked in violet, Purkinje neurons (PCL) are marked in yellow, and the molecular layers (ML) are marked in red. (b) Impedance magnitude “image” obtained by using 32 impedance measurement channels. To cover the entire array, impedance measurements were repeated with 120 electrode configurations using 3840 sparsely configured electrodes. (c) Extracellular electrophysiological activity: action potential rate (Hz) recorded by using the 2048 AP recording channels.
Impedance “images” (Figure 3.b) of the brain slice were acquired by using the 32 on-chip impedance measurement channels. Different cell layers could be distinguished in these impedance images. For instance, the granular cell layer with high cell density exhibited higher impedance magnitudes than the WM or the ML layers [8][9]. We then recorded the electrophysiological activity of the slice (Figure 3.c) for 20 seconds. The corresponding figure clearly shows the high spontaneous electrical activity of the Purkinje neurons.
Conclusions
In this paper, we presented a CMOS chip that can perform impedance measurements along with electrophysiology recordings on 59,760 electrodes at 13.5 µm spatial resolution. The circuitry was fabricated in a standard 0.18 µm CMOS process. All circuits needed to perform impedance and electrophysiology experiments were integrated on the chip. We demonstrated the system’s capability to investigate and monitor position, size, morphology and electrical activity of brain slices. The possibility to perform high-resolution impedance measurement in combination with electrophysiology recording makes our system a versatile tool for in-depth studies of tissue morphology as well as cellular behavior and dynamics.
Acknowledgements
All use of animals and all experimental protocols were approved by the Basel Stadt veterinary office according to Swiss federal laws on animal welfare. The authors thank A. Martel, ETH Zurich for the electrode processing and Evi Bieler, University of Basel, for providing the SEM image.
This work was supported by the European Community through the European Research Council Advanced Grants 267351‘NeuroCMOS’ (FP7) and 694829 ‘neuroXscales’ (Horizon 2020). A. Shadmani received individual support through FP7-MTN “EngCaBra” (Contract 264417). The funders had no role in study, design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- [1].Obien MEJ, et al. Revealing neuronal function through microelectrode array recordings. Front Neurosci. 2015 doi: 10.3389/fnins.2014.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morefield SI, et al. Drug evaluations using neuronal networks cultured on microelectrode arrays. Biosens Bioelectron. 2000 doi: 10.1016/s0956-5663(00)00095-6. [DOI] [PubMed] [Google Scholar]
- [3].Eversmann B, et al. A 128 × 128 CMOS Biosensor Array for Extracellular Recording of Neural Activity. IEEE J Solid-State Circuits. 2003 [Google Scholar]
- [4].Ballini M, et al. A 1024-channel CMOS microelectrode array with 26,400 electrodes for recording and stimulation of electrogenic cells in vitro. IEEE J Solid-State Circuits. 2014 doi: 10.1109/JSSC.2014.2359219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bertotti G, et al. A CMOS-based sensor array for in-vitro neural tissue interfacing with 4225 recording sites and 1024 stimulation sites. IEEE BioCAS. 2014 [Google Scholar]
- [6].Chi T, et al. A Multi-Modality CMOS Sensor Array for Cell-Based Assay and Drug Screening. IEEE TBioCAS. 2015 doi: 10.1109/TBCAS.2015.2504984. [DOI] [PubMed] [Google Scholar]
- [7].Viswam, et al. Multi-Functional Microelectrode Array System Featuring 59,760 Electrodes, 2048 Electrophysiology Channels, Impedance and Neurotransmitter Measurement Units. IEEE ISSCC. 2016 doi: 10.1109/ISSCC.2016.7418073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yedlin M, Kwan H, Murphy JT, Nguyen-Huu H, Wong YC. Electrical conductivity in cat cerebellar cortex. Exp Neurol. 1974;43(3):555–569. doi: 10.1016/0014-4886(74)90195-2. [DOI] [PubMed] [Google Scholar]
- [9].Okada YC, Huang JC, Rice ME, Tranchina D, Nicholson C. Origin of the apparent tissue conductivity in the molecular and granular layers of the in vitro turtle cerebellum and the interpretation of current source-density analysis. J Neurophysiol. 1994;72(2):742–53. doi: 10.1152/jn.1994.72.2.742. [DOI] [PubMed] [Google Scholar]