Abstract
Influenza virus is a significant pathogen in humans and animals with the ability to cause extensive morbidity and mortality. Exuberant immune responses induced following infection have been described as a “cytokine storm”, associated with excessive levels of proinflammatory cytokines and widespread tissue damage. Recent studies have painted a more complex picture of cytokine networks and their contributions to clinical outcomes. While many cytokines clearly inflict immunopathology, others have non-pathological delimited roles in sending alarm signals, facilitating viral clearance, and promoting tissue repair, such as the IL-33 – amphiregulin axis, which plays a key role in resolving some types of lung damage. Recent literature suggests that type 2 cytokines, traditionally thought of as not involved in anti-influenza immunity, may play an important regulatory role. Here we discuss the diverse roles played by cytokines after influenza infection and highlight new, serene features of the cytokine storm, while highlighting the specific functions of relevant cytokines that perform unique immune functions and may have applications for influenza therapy.
Introduction
Influenza virus causes acute respiratory infection and significant rates of hospitalization and mortality [1]. After infection, influenza virus is internalized into upper and lower respiratory epithelial cells via endocytosis. Viral RNAs can be recognized by the infected cell as pathogen-associated molecular patterns (PAMPs) by numerous pathogen recognition receptors (PRRs), which in turn can promote downstream cellular and humoral responses, including the “cytokine storm” [2].
The term "cytokine storm” to describe an immune response to influenza infection was first used in late 2003 in reference to influenza-associated encephalopathy [3] [4]. Thus far, the influenza-induced cytokine storm has been linked to uncontrolled proinflammatory responses, which induce significant immunopathology and severe disease outcomes [5] [6] [7] [8]. As we better understand the varied roles of individual cytokines, the concept of the cytokine storm has become more complicated. Beyond the direct effects of these cytokines on different cell types, their cross-regulatory functions within the cytokine network can have important effects on the outcome of an infection.
One useful framework for considering the role of cytokines is to divide them by those that are directly induced by virus infection (primary cytokines) and those that are induced downstream by other cytokines or features of the immune response (secondary cytokines). Influenza virus infection in epithelial cells, endothelial cells, and alveolar macrophages leads to the primary wave of cytokine production, especially type I interferons (IFNs), which upregulate the expression of numerous interferon-stimulated genes (ISGs) [9]. Though originally not a primary focus of influenza virus biology, endothelial cells expressing the Sphingosine-1-phosphate (S1P1) receptor have been demonstrated to be key orchestrators of the cytokine storm [10]. Following the type I IFN release, higher expression of ISGs initiates downstream anti-viral responses and subsequent inflammatory cytokine production by innate immune cells, like dendritic cells (DCs), macrophages, neutrophils, and monocytes. In the adaptive phase of the immune response, different subsets of T cells and group 2 innate lymphoid cells (ILC2s) are activated and regulated to secrete the secondary cytokines that promote viral clearance, tissue homeostasis, and lung repair.
Here, we summarize some representative anti-influenza cytokines and introduce several new members into the storm, including IL-33 and amphiregulin, whose functions are relatively recently appreciated in influenza virus infection. In this review, we focus on the secretion, regulation, and functions of the cytokines in influenza immune responses to provide potential therapeutic targets for clinical treatments.
Cytokines directly induced by viral infection (Primary cytokines)
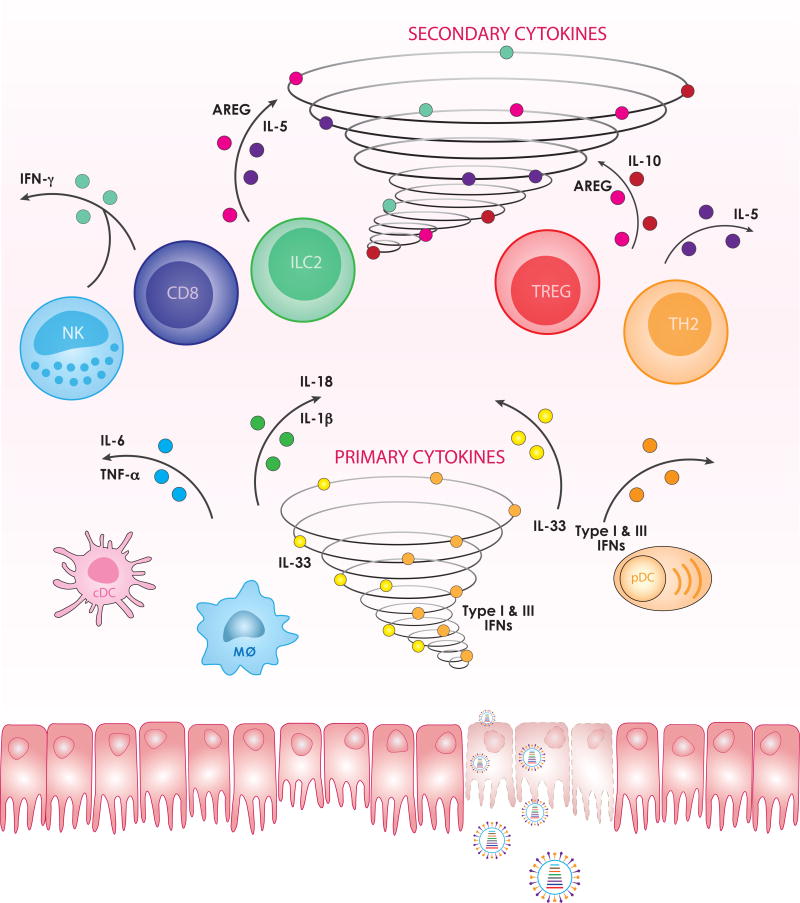
As soon as viral RNA is sensed by the innate immune system, it initiates a rapid anti-viral signaling cascade, leading to the production of various cytokines by the infected epithelial cell and professional innate immune cells. The number of identified cytokines produced by infected cells is greater than 15, without considering chemokines. Here we focus our discussion on several representative cytokines that play key roles, including type I and III interferons, IL-1β, IL-18, TNF-α, IL-6 and the “alarmin” IL-33 (Figure 1).
Figure 1. Spatial and temporal segregation of cytokine production after influenza infection.
After internalization into epithelial cells, influenza virus can be detected by innate immune sensors and trigger downstream immune responses, including tremendous cytokine production, sometimes called the “cytokine storm”. Cytokines directly induced in a virally infected cell versus those downstream from other cytokine signaling can be segregated as primary cytokines and secondary cytokines, respectively. In the primary cytokine wave, virus-infected lung epithelial, endothelial, and other immune cells produce type I and III IFNs, IL-1β, IL-18, TNF-α, IL-6, IL-33 and other cytokines, mainly to limit viral replication and spreading and to initiate downstream immune responses (bottom panel). Following their recruitment and activation by primary cytokines, CD8 T cells, NK cells, ILC2s, Tregs, and Th2 cells can secrete the secondary cytokines IFN-γ, IL-10, amphiregulin, and IL-5 to eliminate virus and virally infected cells, dampen inflammation, and restore lung function (top panel).
1. Type I and III interferons
The interferons (IFNs) are a family of well-studied cytokines that play a critical role in innate immunity by inducing the activation of an “antiviral state” in infected and neighboring cells. There are three types of interferons (type I, II and III), defined by their receptor specificity. Type I interferons (IFNα/β) were discovered first and named for their ability to interfere with viral replication [11]. Type I interferons can be produced by diverse cell types; however, macrophages, pneumocytes, dendritic cells (DCs) and inflammatory monocytes are the main immune cell producers in acute influenza infection [12] [13]. IFNα/β can bind to the type I IFN heterodimeric transmembrane receptor complex, IFNα receptor (IFNAR), which is composed of subunits IFNAR1 and IFNAR2, and initiate a signaling cascade through the Janus kinase - signal transducer and activator of transcription (JAK-STAT) pathway, leading to the transcription of ISGs [14]. The type III interferon family consists of IFNλ1 (IL-29), IFNλ2 (IL-28A), IFNλ3 (IL-28B) and IFNλ4, which can be expressed by different cell types and have similar functions as type I interferons through the JAK-STAT pathway [15]. The receptor for IFNλ, composed of IFNLR1 (also known as IL-28RA) and the shared IL-10R2 chain, is primarily expressed on epithelial cells. As a result, type III interferons have important, but restricted, anti-viral activities. The type II IFN category contains a single member, IFNγ, that has functions distinct from type I and type III IFNs and will be discussed in the section of “secondary cytokines”.
The key function of type I IFN signaling is to induce ISG-encoded proteins, which have potent antiviral activities to inhibit viral replication directly within the infected epithelial cell [16] [17]. The importance of type I IFNs can be assessed by studying infections in mice deficient in the IFNα Receptor (Ifnar−/− mice) [18]. Consistent with the functions of type I IFNs, the expression levels of numerous ISGs induced by H3N2 influenza virus infection were dramatically dampened in Ifnar−/− mouse lungs when compared to wildtype lungs [19]. Immortalized murine lung epithelial type I cells (LET1s) from Ifnar−/− mice also showed reduced expression of antiviral genes and increased permissiveness for viral protein production after in vitro H1N1 infection compared to wildtype epithelial cells, which suggests the capability to produce type I IFNs in epithelial cells is critical for local restriction of influenza virus replication [20]. Beyond their effects on epithelial cells, type I IFNs can enhance the lytic activity of memory CD8 T cells by boosting granzyme B production and facilitating viral clearance [21]. Mice that lack IFNβ alone also exhibit decreased survival and delayed viral clearance [22]. In contrast, Ifnar−/− and Stat1−/− mice have distinct inflammatory responses to influenza infection, with Stat1−/− showing unimpaired viral clearance by cytotoxic T lymphocytes but decreased IL-15 production and a bias towards type 2-biased immune responses [23]. This is likely due to the fact that multiple cytokines can signal through Stat1, especially type II and III IFNs, which may distinguish the downstream immune responses between infected Ifnar−/− and Stat1−/− mice.
Despite of the signaling and functional similarities between type I and III IFNs, also called the IFNλs, the receptor complex for the IFNλs (IFNLR) is heterodimeric, including one shared subunit of IL-10R2, and one specific subunit of IFNLR1 (IL-28RA), which is preferentially expressed on epithelial cells [24]. Type III IFNs can be highly induced by influenza virus infection, even in Ifnar−/− animals, and produced by both epithelial cells and myeloid-lineage cells, such as DCs [25]. IFNλs have been found to be critical in protecting mice against influenza infections. Mice deficient in type III IFN signaling (Il28rα−/−) exhibited enhanced susceptibility and delayed viral clearance during H1N1 influenza virus infection, and the outcomes were even more significant in Ifnar−/− and Il28rα−/− double knockout mice, which suggests the independent contribution of both type I and III IFNs to protection [26] [27]. Therefore, as the first line of defense, type I and III IFNs coordinately protect hosts against influenza infections by promoting viral clearance and antiviral immune responses.
2. Interleukin-1β (IL-1β) and IL-18
In addition to the Toll-like receptors 3, 7, 8, and RIG-I, which all recognize some form of viral RNA, influenza virus can also be detected by the NLRP3 inflammasome [28] [29], which is assembled by NLRP3, the adaptor ASC and pro-caspase 1. The upstream activator of NLRP3 appears to be the sensor DAI (DNA-dependent activator of IFN regulatory factors) or Zbp1 (Z-DNA binding protein 1), which recognizes viral RNA or the viral RNP complex [30] [31]. The activation of NLRP3 leads to the cleavage of pro-IL-1β and pro-IL-18 into their active forms for secretion, IL-1β and IL-18, by DCs and macrophages. Previous studies using IL-1R-deficient mice indicated that IL-1R signaling is critical for promoting survival, priming influenza-specific cytotoxic CD8 T cell responses, and generating IgA responses [32] [33] [34]. Interestingly, the administration of an NLRP3 inhibitor (blocking IL-1β and IL-18 maturation) during influenza infection suggested that IL-1β can promote recovery when present early in infection but is associated with a damaging inflammatory response leading to severe pathogenesis and mortality when present at late stages of infection [35]. Thus, the early induction of IL-1β by virus is protective to infected hosts by promoting CD8 T cell activity and antibody response, but has negative consequences if sustained throughout the response.
The maturation of IL-18 is also modulated by NLRP3 inflammasome activation. The functions of IL-18 are similar to IL-1β in post-influenza responses with IL-18 improving the outcome of influenza infection through enhancing cytokine production by virus-specific CD8 T cells and augmenting cytotoxicity of natural killer cells [36]. Il18−/− mice show reduced cytokine production by CD8 T cells and impaired ability for viral clearance [37]. This topic remains controversial, however, as another study shows that deficiency in IL-18 can enhance viral clearance in mice [38]. Furthermore, receptor interacting protein kinase 2 (RIPK2)-mediated mitophagy provides protection against virally triggered immunopathology by negatively regulating the activation of NLRP3 and the production of IL-18 suggesting that overproduction of IL-18 can have deleterious consequences [39]. In human studies in vitro, IL-18 synergizes with type I IFNs to induce IFN-γ production in T cells, which can promote viral clearance through numerous mechanisms [40]. In addition to these pro-inflammatory roles, IL-18R signaling can also negatively regulate IFN-α expression by pDCs in the presence of influenza virus, which may form a potential negative regulatory loop for type I IFN production [41]. Thus, IL-18 appears to play a complex role in the development of the cytokine storm, with functions that both promote viral clearance and immune pathology, and negatively regulate other inflammatory cascades.
3. Tumor Necrosis Factor alpha (TNF-α)
TNF-α can be produced by different cell types after influenza infection, including TNF/iNOS-producing DCs (tipDCs) [42], lung epithelial cells [43], and helper T cells and cytotoxic T lymphocytes [44]. TNF-α is considered to be the prototypical proinflammatory cytokine at the “center of the influenza cytokine storm”, escalating the severity of disease in humans with highly pathogenic and pathological influenza infections[45] [46] [47]. Supporting this claim, TNF receptor 1 (TNFR1)-deficient mice exhibit significantly reduced morbidity but no difference in viral replication during highly pathogenic H5N1 influenza infection [48]. In addition, mice lacking both TNF-R1/TNF-R2 and IL-1 receptor exhibited decreased morbidity and delayed mortality with reduced airway inflammation but similar viral clearance after lethal H5N1 challenge [49]. These data indicate that TNF-α may contribute to the symptoms of severe disease after infection, but a more limited role in reducing viral replication, representing the quintessential features of the “cytokine storm”. Anti-TNF treatment can reduce the severity of weight loss and illness after A/X31 H3N2 virus challenge, indicating it may be a promising therapeutic target [50].
4. IL-6
IL-6 has been primarily considered a proinflammatory cytokine and a marker for inflammation in inflammatory arthritis and inflammatory bowel diseases [51] [52] [53] [54]. Clinical studies also implicate IL-6 as correlated with the disease severity in influenza-infected patients [55] [56] [57]. However, like IL-18, the role of IL-6 in severe influenza infection is still somewhat ambiguous. By using IL-6 or IL-6 receptor deficient mice (Il6−/− or Il6r−/−), it has been demonstrated that IL-6 is essential for protecting the host from H1N1-associated mortality by preventing virus-induced neutrophil cell death. The insufficiency of neutrophils in Il6−/− mice appears to impair viral clearance and is associated with severe lung damage [58]. Consistent with this, another study indicated that IL-6 is required to control the extent of influenza-induced lung inflammation and enhance viral clearance and survival [59]. Furthermore, IL-6 is crucial in secondary infections to recall virus-specific memory CD4 T cells, but not CD8 T cells, by limiting the activity of virus-specific regulatory T cells (Tregs), which in turn favors virus clearance and host survival [60]. Therefore, despite the association of IL-6 with poor clinical outcomes, animal studies indicate it may be promoting important protective responses that improve disease resolution.
5. IL-33
As a member of the IL-1 family, IL-33 plays critical roles in innate and adaptive immunity, promoting tissue repair and maintaining tissue homeostasis [61] [62] [63] [64] [65] [66]. IL-33 was discovered by its binding to IL-1RL1 (ST2 receptor), which belongs to the IL-1 receptor family and is structurally similar to the receptors for IL-1 and IL-18. IL-33 drives type 2 immune responses via MyD88 - NF-κB signaling [67]. IL-33 is a chromatin – associated nuclear cytokine, which associates with chromatin via protein-protein interactions in vivo [68]. Due to the lack of a signal sequence, the release of IL-33 occurs primarily through cell death via infectious insults or allergen exposure as an “alarmin” by numerous cell types, including epithelial cells, endothelial cells, macrophages, and fibroblasts [69] [70]. Influenza virus infection has the ability to induce IL-33 production by lung epithelial cells in vivo and in vitro [71]. In addition, IL-33 can promote lung tissue repair and homeostasis by inducing ILC2s and Tregs to produce amphiregulin (AREG) after infection (discussed in detail below) [72] [73]. Although the necessity of IL-33 in promoting type 2 responses following influenza has been well-established, the induction and regulation of IL-33 in lung infections still remain to be investigated.
Cytokines induced by immune responses (Secondary cytokines)
The “primary cytokine storm” derived from the direct infection of epithelial, endothelial and innate immune cells resident in the lung helps restrain the virus from spreading and replicating, recruits and activates effector cells that can perform more extensive elimination of the virus and virally-infected cells, and various cell types that can contribute to the restoration of the lung tissue. These cells secrete a second wave of cytokines, which continue the process of viral clearance, dampen inflammation, and attempt to restore lung function. In this section, we summarize the functions of IFN-γ, IL-10, amphiregulin, and IL-5 (Figure 1).
1. Type II interferon (IFN-γ)
IFN-γ, the only type II IFN, is produced throughout influenza infection. IFN-γ binds to the type II IFN receptors (IFNGR1 and IFNGR2) to signal through the classic JAK-STAT pathway and induce the formation of STAT1-STAT1 homodimers to promote ISGs [74]. IFN-γ is a potent antiviral cytokine with numerous functions, including promoting the activation of DCs, enhancing the cytotoxicity by other immune cells, and inducing antibodies by B cells [75]. IFN-γ is mainly produced by T cells and natural killer (NK) cells after influenza infection. IL-18, produced in the “primary cytokine storm”, drives the differentiation of highly activated antigen-specific CD8 and NK cells producing IFN-γ contributing to viral control and other immune regulatory activities [36] [37]. The importance of IFN-γ during influenza infection appears to depend in part on the specifics of the infection model, with some reports finding no difference in viral clearance and disease survival in IFN-γ deficient mice [76] [77], while in other systems IFN-γ-deficiency is associated with loss of protection [78] [79] [80].
As innate immune cells, NK cells produce IFN-γ at an early stage of infection (days 3–5 after infection) to control viral replication. Endogenous IL-12 contributes to the early NK cell IFN-γ production, but not to IFN-γ from T cells at day 7. Additionally, exogenous IFN-γ treatment at the early stage of A/PR8 H1N1 infection can protect mice through NK cell activation and proliferation [81]. Thus, IFN-γ, produced rapidly after infection by NK cells, is protective for virus-infected hosts.
Neutralization of IFN-γ by using monoclonal antibodies in influenza-infected mice indicates that IFN-γ is important for generating virus-induced humoral responses (immunoglobulin (Ig) G2a/c and IgG3) and affects local cellular responses [78].
Later in the response, T cells become the major source of IFN-γ. T cells are activated in the local draining lymph node by migratory CD103+ and CD11b+ DCs [82] [83] carrying viral antigens [84]. Once activated, T cells differentiate into antigen-specific effector T cells. The influenza-specific effector CD8 T cells can function by various antigen-dependent routes to limit infection, such as producing cytokines (including IFN-γ and TNF-α) and mediating infected-cell killing (perforin/granzyme-mediated cytolysis, FasL/Fas-mediated apoptosis and TRAIL/TRAIL-DR-mediated apoptosis) [85].
Some of the activities of IFN-γ have been associated with inflammation and lung injury [86] [87] but, for the most part, the activities of IFN-γ are protective. One study that transferred in vitro differentiated Tc1 (CD8 T cells primarily producing IFN-γ) effector CD8 T cells from wild-type animals into naïve wildtype recipients followed by infection with H1N1 virus 1 day after cell transfer demonstrates that production of IFN-γ by Tc1 cells protect recipients against influenza infection [88]. Furthermore, IFN-γ produced by memory CD4 T cells during secondary influenza challenge is required to mediate the protection of immuno-deficient hosts [89] [90] [91].
IFN-γ is critical for the migration antigen-specific CTLs to the lungs and helps maintain CTL homeostasis in the spleen after infection [76]. After infection with an H3N2 virus, wild-type mice have significantly higher numbers of epitope-specific CD8 T cells in the bronchoalveolar lavage (BAL) than in IFN-γ−/− mice, while knockout mice have more antigen-specific CD8 T cells in the spleen, which suggests the importance of IFN-γ for CD8 T cell trafficking to the site of infection. In addition, the results of a transfer of H1N1-primed CD8 T cells to wild-type, IFN-γ−/− or IFNGR1−/− mice followed by H3N2 challenge show that extrinsic IFN-γ produced by host cells is required for CD8 T cell recruitment and homeostasis.
In sum, IFN-γ secreted by T cells and NK cells has multiple functions following influenza infection, including promoting viral clearance, boosting cellular and humoral immune responses, and improving host disease outcomes.
2. IL-10
IL-10 is the proto-typical anti-inflammatory cytokine that negatively regulates innate and adaptive immunity during bacterial and viral infections [92] [93] [94]. In influenza infection, IL-10 is highly abundant, especially during the adaptive immune response [95]. Effector CD4 and CD8 T cells expressing high levels of the transcription factors T-bet and IFN-γ are the main producers of IL-10. Neutralization of CD4 and CD8 T cells significantly decreases the IL-10 and IFN-γ levels in H1N1-infected lungs [96]. Consistent with its immunosuppressive functions, animals deficient in IL-10 (Il10−/−) or given an antibody to block IL-10 receptor signaling produce higher levels of numerous proinflammatory cytokines, such as IL-17A, IL-17F, IFN-γ, and TNF-α [95] [96] [97]. However, the role of IL-10 in influenza infection is extremely complex. Blocking IL-10 receptor with an antibody at days 3, 4 and 6 after sublethal A/PR8 H1N1 infection of BALB/c mice leads to lethal pulmonary inflammation and cell infiltration, no difference in viral clearance, and increased expression of proinflammatory cytokines [96]. Interestingly, other studies demonstrated that IL-10 has minimal effects on a low-dose of A/PR8 H1N1 influenza infection, but Il10−/− mice are more resistant to influenza than wildtype mice challenged with a lethal dose of virus; in one set of studies, IL10−/− animals displayed similar viral clearance yet had better lung function [95] [97]. Previous results suggested that IL-10 had a detrimental role in suppressing the protective CD4 T cell-dependent influenza-specific Th17- [95] and antibody-mediated responses [97]. In addition, early-phase administration (Day 0–3 post-infection) of IL-10 can attenuate the level of viral neuraminidase-activated TGF-β and promote type I responses resulting in severe pulmonary inflammation and death, while late-phase modulation (Day 4–7 post-infection) can aid in recovery and outcome of the infection [98]. In human influenza infection, increased levels of IL-10 are correlated with severe lung inflammation and fatality [99] [100]. However, high levels of IL-10 may indicate the host’s attempt to regulate the inflammatory damage caused by other members of the cytokine storm. In this view, IL-10 itself may not be causative of poor outcome, but an indicator that inflammation is unrestrained. Taken together, IL-10 appears to play a time-dependent regulatory role, and may serve as an indicator of a detrimental response. Additionally, its effects seem highly sensitive to the precise conditions of the model (virus strain, dose).
3. Amphiregulin (AREG)
Recent studies identified an important role for amphiregulin after influenza infection. AREG belongs to the epidermal growth factor (EGF) family and binds EGFR to promote EGFR dimerization and trigger diverse intracellular signals [101] [102]. AREG is constitutively expressed in numerous cell types to promote cell proliferation, tissue homeostasis, [101] [103] and plays a key role in lung repair after influenza virus infection [72] [73]. During infection, AREG is primarily produced by ILC2s [72], Tregs, and T helper cells (Th2 cells) [73] at the stage when adaptive immunity dominates. These cell types, which also express the IL-33R (ST2), can be regulated by IL-33 production in the “primary cytokine storm” and express AREG to improve lung tissue homeostasis. Specific blockade of AREG worsens tissue repair and disease outcomes. Granulocyte macrophage colony-stimulating factor (GM-CSF)-induced AREG production reduces susceptibility of post-influenza staphylococcal pneumonia co-infection [104]. Additionally, progesterone (P4) can protect female mice against influenza infection by upregulating AREG production in respiratory epithelial cells to improve wound healing [105]. Based on the current studies on AREG in the context of influenza infection, AREG contributes to lung recovery and restores lung function, and may have great potential as a therapeutic.
4. IL-5
As a classic type 2 cytokine, IL-5, is mainly produced by Th2 cells and ILC2s in allergies and helminth infections to promote eosinophil maturation and infiltration [106] [107]. IL-5-induced eosinophilia has been correlated with the severity of asthma and some clinical trials of anti-IL-5 therapy for asthma have shown a reduction in disease outcomes [108]. However, during adult influenza infection, a type 2 anti-inflammatory immune response is mounted in parallel to the conventionally measured type 1 response; the magnitude of this type 2 response also appears to be somewhat lower than the type 1 profile. In addition, it remains unclear how hosts with biased type 2 immunity, such as infants or asthma patients, respond to influenza infection. Thus, the studies of type 2 immunity during the influenza response may facilitate the thorough understanding of certain infection scenarios. It has been suggested that IL-33-producing NKT cells regulate ILC2s to produce IL-5, leading to eosinophil recruitment in influenza infection [109]. Subsequently, IL-5, together with IL-13, is correlated with influenza-induced airway hyper-responsiveness (AHR) development via the IL-33 dependent innate pathway, instead of viral clearance [110]. Furthermore, adoptive transfer of virus-specific Th2 CD4+ T cell clones fails to promote recovery from influenza infection, but causes delayed viral clearance and pulmonary eosinophilia [111] [112]. As for the functions of IL-5-induced eosinophils, one study has shown that mice with high eosinophilia display robust viral clearance and preserved airway epithelial integrity after acute allergic asthma [113]. Therefore, for the most part IL-5 has not correlated with strong protection and viral clearance but rather with the alteration of the lung cellular environment, particularly with respect to eosinophils.
The potential functions of eosinophils are only recently receiving significant focus in the context of influenza infection. One study demonstrated that H1N1 virus infection can induce the degranulation and facilitate the activation and proliferation of CD8 T cells in vitro. In this work, adoptive transfer of eosinophils from infected animals could protect the recipients against influenza challenge by enhancing antigen-specific cellular responses [114]. The combination of these immune regulatory and enhancing functions with the known effects of eosinophils on lung function requires further studies to determine the relative contributions of each activity and the regulatory role of IL-5 in these processes.
Conclusions and future perspectives
As we develop a comprehensive understanding of the multifaceted roles of innate and adaptive immune effectors during influenza infection, the traditional cytokine storm” should be expanded to encompass the diversity of cytokines promoting pathogenic and protective immunity, including inducing local inflammation, eliminating infected cells, modulating cellular and molecular immune responses, and promoting tissue repair and homeostasis. Beyond the well-established functions of traditional antiviral cytokines like the IFNs, recent studies have implicated new cytokine subsets as being relevant to influenza infection. Most of the literature has focused on the strong type 1 immune response induced during infection, but type 2 cytokines have now been demonstrated to play important roles following infection, including IL-33, IL-5, and AREG. However, their functions remain, in many cases, ambiguous or controversial, with significant work needed to define their place in the immune network.
Immunomodulatory drugs, which blunt the inflammatory cytokine storm during influenza infection, have been suggested as clinical therapeutics, including S1P1 receptor agonists, COX inhibitors, anti-TNF therapy, peroxisome proliferator-activated receptors (PPARs) agonists, and antioxidants [7] [115] [116]. S1P1 signaling lowers the level of early inflammatory cytokines and chemokines, and suppresses the infiltration of leukocytes during post-influenza responses, and treating infected mice with S1P1 receptor agonists protected animals better than treating with oseltamivir [10] [117]. To date, several S1P1 receptor agonists are being tested in clinical trials for multiple sclerosis and ulcerative colitis, which may provide insight into the potential of these drugs for acute respiratory infections [118]. The effects of these drugs on the “serene” components of the cytokine storm also need to be assessed.
Much of the work referenced in this review, and most influenza immunology research, still utilizes healthy adult mice, whereas disease manifests disproportionately in immuno-compromised patients, the obese, and in pregnant women, infants, and the elderly [119]. Defining the potentially unique cytokine networks in these populations is an important goal if these insights are to be translated therapeutically.
Acknowledgments
This work was supported by HHSN272201400006C (St. Jude Center of Excellence for Influenza Research and Surveillance), R01 AI121832, and ALSAC. We apologize to any investigators whose relevant work was not included due to space limitations.
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-Associated Hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Dash P, Thomas PG. Host Detection and the Stealthy Phenotype in Influenza Virus Infection. In: Oldstone MBA, Compans RW, editors. Influenza Pathog. Control - Vol. II. Springer International Publishing; 2014. pp. 121–147. [DOI] [PubMed] [Google Scholar]
- 3.Yokota S. Influenza-associated encephalopathy--pathophysiology and disease mechanisms. Nihon Rinsho Jpn J Clin Med. 2003;61:1953–1958. [PubMed] [Google Scholar]
- 4.Clark IA. The advent of the cytokine storm. Immunol Cell Biol. 2007;85:271–273. doi: 10.1038/sj.icb.7100062. [DOI] [PubMed] [Google Scholar]
- 5.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Zhou Y, Yang Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teijaro JR. The Role of Cytokine Responses During Influenza Virus Pathogenesis and Potential Therapeutic Options. In: Oldstone MBA, Compans RW, editors. Influenza Pathog. Control - Vol. II. Springer International Publishing; 2014. pp. 3–22. [DOI] [PubMed] [Google Scholar]
- 8.D’Elia RV, Harrison K, Oyston PC, et al. Targeting the “Cytokine Storm” for Therapeutic Benefit. Clin Vaccine Immunol. 2013;20:319–327. doi: 10.1128/CVI.00636-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato H, Sato S, Yoneyama M, et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial Cells Are Central Orchestrators of Cytokine Amplification during Influenza Virus Infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaacs A, Lindenmann J. Virus interferenceI. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 12.McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wack A, Terczyńska-Dyla E, Hartmann R. Guarding the frontiers: the biology of type III interferons. Nat Immunol. 2015;16:802–809. doi: 10.1038/ni.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadler AJ, Williams BRG. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller U, Steinhoff U, Reis LF, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 19.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberger CM, Podyminogin RL, Askovich PS, et al. Characterization of innate responses to influenza virus infection in a novel lung type I epithelial cell model. J Gen Virol. 2014;95:350–362. doi: 10.1099/vir.0.058438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohlmeier JE, Cookenham T, Roberts AD, et al. Type I Interferons Regulate Cytolytic Activity of Memory CD8+ T Cells in the Lung Airways during Respiratory Virus Challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koerner I, Kochs G, Kalinke U, et al. Protective Role of Beta Interferon in Host Defense against Influenza A Virus. J Virol. 2007;81:2025–2030. doi: 10.1128/JVI.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durbin JE, Fernandez-Sesma A, Lee C-K, et al. Type I IFN Modulates Innate and Specific Antiviral Immunity. J Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 24.Lazear HM, Nice TJ, Diamond MS. Interferon-λ: Immune Functions at Barrier Surfaces and Beyond. Immunity. 2015;43:15–28. doi: 10.1016/j.immuni.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jewell NA, Cline T, Mertz SE, et al. Lambda Interferon Is the Predominant Interferon Induced by Influenza A Virus Infection In Vivo. J Virol. 2010;84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mordstein M, Kochs G, Dumoutier L, et al. Interferon-λ Contributes to Innate Immunity of Mice against Influenza A Virus but Not against Hepatotropic Viruses. PLOS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mordstein M, Neugebauer E, Ditt V, et al. Lambda Interferon Renders Epithelial Cells of the Respiratory and Gastrointestinal Tracts Resistant to Viral Infections. J Virol. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas PG, Dash P, Aldridge JR, Jr, et al. The Intracellular Sensor NLRP3 Mediates Key Innate and Healing Responses to Influenza A Virus via the Regulation of Caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen IC, Scull MA, Moore CB, et al. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thapa RJ, Ingram JP, Ragan KB, et al. DAI Senses Influenza A Virus Genomic RNA and Activates RIPK3-Dependent Cell Death. Cell Host Microbe. 2016;20:674–681. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuriakose T, Man SM, Malireddi RKS, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1:aag2045–aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 Is Responsible for Acute Lung Immunopathology but Increases Survival of Respiratory Influenza Virus Infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat Immunol. 2013;14:246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichinohe T, Lee HK, Ogura Y, et al. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate MD, Ong JDH, Dowling JK, et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci Rep. 2016;6:27912. doi: 10.1038/srep27912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Mori I, Hossain MJ, et al. Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol. 2004;85:423–428. doi: 10.1099/vir.0.19596-0. [DOI] [PubMed] [Google Scholar]
- 37.Denton AE, Doherty PC, Turner SJ, La Gruta NL. IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol. 2007;37:368–375. doi: 10.1002/eji.200636766. [DOI] [PubMed] [Google Scholar]
- 38.Van Der Sluijs KF, Van Elden LJR, Arens R, et al. Enhanced viral clearance in interleukin-18 gene-deficient mice after pulmonary infection with influenza A virus. Immunology. 2005;114:112–120. doi: 10.1111/j.1365-2567.2004.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupfer C, Thomas PG, Anand PK, et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A Virus-Induced IFN-α/β and IL-18 Synergistically Enhance IFN-γ Gene Expression in Human T Cells. J Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- 41.Chao Y, Kaliaperumal N, Chretien A-S, et al. Human plasmacytoid dendritic cells regulate IFN-α production through activation-induced splicing of IL-18Rα. J Leukoc Biol. 2014;96:1037–1046. doi: 10.1189/jlb.2A0813-465RR. [DOI] [PubMed] [Google Scholar]
- 42.Aldridge JR, Moseley CE, Boltz DA, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veckman V, Österlund P, Fagerlund R, et al. TNF-α and IFN-α enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2006;345:96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 44.Hufford MM, Kim TS, Sun J, Braciale TJ. The Effector T Cell Response to Influenza Infection. In: Oldstone MBA, Compans RW, editors. Influenza Pathog. Control - Vol. II. Springer International Publishing; 2014. pp. 423–455. [Google Scholar]
- 45.Peiris JSM, Cheung CY, Leung CYH, Nicholls JM. Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol. 2009;30:574–584. doi: 10.1016/j.it.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheung C, Poon L, Lau A, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? The Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 47.Chan M, Cheung C, Chui W, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szretter KJ, Gangappa S, Lu X, et al. Role of Host Cytokine Responses in the Pathogenesis of Avian H5N1 Influenza Viruses in Mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrone LA, Szretter KJ, Katz JM, et al. Mice lacking both TNF and IL-1 receptors exhibit reduced lung inflammation and delay in onset of death following infection with a highly virulent H5N1 virus. J Infect Dis. 2010;202:1161–1170. doi: 10.1086/656365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussell T, Pennycook A, Openshaw PJM. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur J Immunol. 2001;31:2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. doi: 10.1002/1521-4141(200109)31:9<2566::AID-IMMU2566>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 52.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–195. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 54.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012;33:571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Oshansky CM, Gartland AJ, Wong S-S, et al. Mucosal Immune Responses Predict Clinical Outcomes during Influenza Infection Independently of Age and Viral Load. Am J Respir Crit Care Med. 2013;189:449–462. doi: 10.1164/rccm.201309-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaiser L, Fritz RS, Straus SE, et al. Symptom pathogenesis during acute influenza: Interleukin-6 and Other cytokine responses. J Med Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 57.Hagau N, Slavcovici A, Gonganau DN, et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care. 2010;14:R203. doi: 10.1186/cc9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dienz O, Rud JG, Eaton SM, et al. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258–266. doi: 10.1038/mi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lauder SN, Jones E, Smart K, et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur J Immunol. 2013;43:2613–2625. doi: 10.1002/eji.201243018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longhi MP, Wright K, Lauder SN, et al. Interleukin-6 Is Crucial for Recall of Influenza-Specific Memory CD4+ T Cells. PLOS Pathog. 2008;4:e1000006. doi: 10.1371/journal.ppat.1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liew FY, Girard J-P, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 63.Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol. 2016;17:122–131. doi: 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- 64.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cayrol C, Girard J-P. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Peine M, Marek RM, Löhning M. IL-33 in T Cell Differentiation, Function, and Immune Homeostasis. Trends Immunol. 2016;37:321–333. doi: 10.1016/j.it.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz J, Owyang A, Oldham E, et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Carriere V, Roussel L, Ortega N, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kearley J, Silver JS, Sanden C, et al. Cigarette Smoke Silences Innate Lymphoid Cell Function and Facilitates an Exacerbated Type I Interleukin-33-Dependent Response to Infection. Immunity. 2015;42:566–579. doi: 10.1016/j.immuni.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Bonilla WV, Fröhlich A, Senn K, et al. The Alarmin Interleukin-33 Drives Protective Antiviral CD8+ T Cell Responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 71.Le Goffic R, Arshad MI, Rauch M, et al. Infection with Influenza Virus Induces IL-33 in Murine Lungs. Am J Respir Cell Mol Biol. 2011;45:1125–1132. doi: 10.1165/rcmb.2010-0516OC. [DOI] [PubMed] [Google Scholar]
- 72.Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arpaia N, Green JA, Moltedo B, et al. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 75.Billiau A, Matthys P. Interferon-γ: A historical perspective. Cytokine Growth Factor Rev. 2009;20:97–113. doi: 10.1016/j.cytogfr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 76.Turner SJ, Olivas E, Gutierrez A, et al. Disregulated Influenza A Virus-Specific CD8+ T Cell Homeostasis in the Absence of IFN-γ Signaling. J Immunol. 2007;178:7616–7622. doi: 10.4049/jimmunol.178.12.7616. [DOI] [PubMed] [Google Scholar]
- 77.Graham MB, Dalton DK, Giltinan D, et al. Response to influenza infection in mice with a targeted disruption in the interferon gamma gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karupiah G, Chen J-H, Mahalingam S, et al. Rapid Interferon γ–dependent Clearance of Influenza A Virus and Protection from Consolidating Pneumonitis in Nitric Oxide Synthase 2–deficient Mice. J Exp Med. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bot A, Bot S, Bona CA. Protective Role of Gamma Interferon during the Recall Response to Influenza Virus. J Virol. 1998;72:6637–6645. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss ID, Wald O, Wald H, et al. IFN-γ Treatment at Early Stages of Influenza Virus Infection Protects Mice from Death in a NK Cell-Dependent Manner. J Interferon Cytokine Res. 2010;30:439–449. doi: 10.1089/jir.2009.0084. [DOI] [PubMed] [Google Scholar]
- 82.Kim TS, Braciale TJ. Respiratory Dendritic Cell Subsets Differ in Their Capacity to Support the Induction of Virus-Specific Cytotoxic CD8 + T Cell Responses. PLOS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.GeurtsvanKessel CH, Willart MAM, Rijt LS van, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heer AK, Harris NL, Kopf M, Marsland BJ. CD4+ and CD8+ T Cells Exhibit Differential Requirements for CCR7-Mediated Antigen Transport during Influenza Infection. J Immunol. 2008;181:6984–6994. doi: 10.4049/jimmunol.181.10.6984. [DOI] [PubMed] [Google Scholar]
- 85.Duan S, Thomas PG. Balancing Immune Protection and Immune Pathology by CD8+ T-Cell Responses to Influenza Infection. Immunol Mem. 2016;25 doi: 10.3389/fimmu.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramana CV, DeBerge MP, Kumar A, et al. Inflammatory impact of IFN-γ in CD8+ T cell-mediated lung injury is mediated by both Stat1-dependent and - independent pathways. Am J Physiol - Lung Cell Mol Physiol. 2015;308:L650–L657. doi: 10.1152/ajplung.00360.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiley JA, Cerwenka A, Harkema JR, et al. Production of Interferon-γ by Influenza Hemagglutinin-Specific CD8 Effector T Cells Influences the Development of Pulmonary Immunopathology. Am J Pathol. 2001;158:119–130. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamada H, Garcia-Hernandez M de la L, Reome JB, et al. Tc17, a Unique Subset of CD8 T Cells That Can Protect against Lethal Influenza Challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teijaro JR, Verhoeven D, Page CA, et al. Memory CD4 T Cells Direct Protective Responses to Influenza Virus in the Lungs through Helper-Independent Mechanisms. J Virol. 2010;84:9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Teijaro JR, Turner D, Pham Q, et al. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McKinstry KK, Strutt TM, Kuang Y, et al. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J Clin Invest. 2012;122:2847–2856. doi: 10.1172/JCI63689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 93.Ouyang W, Rutz S, Crellin NK, et al. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 94.Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 95.McKinstry KK, Strutt TM, Buck A, et al. IL-10 Deficiency Unleashes an Influenza-Specific Th17 Response and Enhances Survival against High-Dose Challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun K, Torres L, Metzger DW. A Detrimental Effect of Interleukin-10 on Protective Pulmonary Humoral Immunity during Primary Influenza A Virus Infection. J Virol. 2010;84:5007–5014. doi: 10.1128/JVI.02408-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dutta A, Huang C-T, Chen T-C, et al. IL-10 inhibits neuraminidase-activated TGF-β and facilitates Th1 phenotype during early phase of infection. Nat Commun. 2015;6:6374. doi: 10.1038/ncomms7374. [DOI] [PubMed] [Google Scholar]
- 99.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al HS, et al. Immunologic Changes during Pandemic (H1N1) 2009, China - Volume 17, Number 6—June 2011 - Emerging Infectious Disease journal - CDC. doi: 10.3201/eid1706.100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berasain C, Avila MA. Amphiregulin. Semin Cell Dev Biol. 2014;28:31–41. doi: 10.1016/j.semcdb.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Shoyab M, Plowman GD, McDonald VL, et al. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- 103.Zaiss DMW, Gause WC, Osborne LC, Artis D. Emerging Functions of Amphiregulin in Orchestrating Immunity, Inflammation, and Tissue Repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Subramaniam R, Barnes PF, Fletcher K, et al. Protecting Against Post-influenza Bacterial Pneumonia by Increasing Phagocyte Recruitment and ROS Production. J Infect Dis jit. 2013:830. doi: 10.1093/infdis/jit830. [DOI] [PubMed] [Google Scholar]
- 105.Hall OJ, Limjunyawong N, Vermillion MS, et al. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLOS Pathog. 2016;12:e1005840. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–1309. doi: 10.1093/intimm/dxp102. [DOI] [PubMed] [Google Scholar]
- 107.Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mukherjee M, Sehmi R, Nair P. Anti-IL5 therapy for asthma and beyond. World Allergy Organ J. 2014;7:32. doi: 10.1186/1939-4551-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gorski SA, Hahn YS, Braciale TJ. Group 2 Innate Lymphoid Cell Production of IL-5 Is Regulated by NKT Cells during Influenza Virus Infection. PLOS Pathog. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chang Y-J, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Graham MB, Braciale VL, Braciale TJ. Influenza virus-specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 113.Samarasinghe AE, Woolard SN, Boyd KL, et al. The immune profile associated with acute allergic asthma accelerates clearance of influenza virus. Immunol Cell Biol. 2014;92:449–459. doi: 10.1038/icb.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Samarasinghe AE, Melo RCN, Duan S, et al. Eosinophils Promote Antiviral Immunity in Mice Infected with Influenza A Virus. J Immunol. 2017:1600787. doi: 10.4049/jimmunol.1600787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Q, Zhou Y, Yang Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. 2016;13:3–10. doi: 10.1038/cmi.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tisoncik JR, Korth MJ, Simmons CP, et al. Into the Eye of the Cytokine Storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oldstone MBA, Teijaro JR, Walsh KB, Rosen H. Dissecting influenza virus pathogenesis uncovers a novel chemical approach to combat the infection. Virology. 2013;435:92–101. doi: 10.1016/j.virol.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez-Cabrera PJ, Brown S, Studer SM, Rosen H. S1P signaling: new therapies and opportunities. F1000Prime Rep. 2014 doi: 10.12703/P6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chang DH, Bednarczyk RA, Becker ER, et al. Trends in U.S. hospitalizations and inpatient deaths from pneumonia and influenza, 1996–2011. Vaccine. 2016;34:486–494. doi: 10.1016/j.vaccine.2015.12.003. [DOI] [PubMed] [Google Scholar]