Abstract
A model for the posttraumatic stress disorder (PTSD) as a disorder of memory is presented drawing both on psychological and neurobiological data. Evidence on intrusive memories and deficits in declarative memory function in PTSD-patients is reviewed in relation to three brain areas that are involved in memory functioning and the stress response: the hippocampus, amygdala, and the prefrontal cortex. Neurobiological studies have shown that the noradrenergic stress-system is involved in enhanced encoding of emotional memories, sensitization, and fear conditioning, by way of its effects on the amygdala. Chronic stress also affects the hippocampus, a brain area involved in declarative memories, suggesting that hippocampal dysfunction may partly account for the deficits in declarative memory in PTSD-patients. Deficits in the medial prefrontal cortex, a structure that normally inhibits the amygdala, may further enhance the effects of the amygdala, thereby increasing the frequency and intensity of the traumatic memories. Thus, by way of its influence on these brain structures, exposure to severe stress may simultaneously result in strong emotional reactions and in difficulties to recall the emotional event. This model is also relevant for understanding the distinction between declarative and non-declarative memory-functions in processing trauma-related information in PTSD. Implications of our model are reviewed.
Keywords: Memory, Posttraumatic stress disorder (PTSD), Hippocampus, Amygdala, Prefrontal cortex
1. Introduction
Although most often the experience of a trauma does not lead to long-term psychological problems, some individuals continue to experience trauma-related symptoms long after a trauma occurred, often qualifying for a diagnosis of posttraumatic stress disorder (PTSD), depression, or alcohol or substance abuse (Saigh and Bremner, 1999). Rates of PTSD following exposure to a traumatic event average around 25–30%, although certain traumatic events, such as rape, are associated with higher rates (Acierno et al., 1999). Studies of PTSD suggest a specific association between the extreme stress of a trauma and alterations in memory functioning (Pitman, 1989; American Psychiatric Association, 1994; Bremner et al., 1999a). Clinicians have pointed at these memory disturbances of PTSD-patients for decades. In a study from World War II about 5% of the soldiers who had been combatants in a campaign had no memory for events that had just occurred (Torrie, 1944). Follow-up studies of veterans of World War II showed that many people still suffered from episodes of ‘black-outs’ or loss of explicit memory (Archibald and Tuddenham, 1965). Also in civilians, for instance the survivors of the Boston’s Cocoanut Grove nightclub fire, memory related ‘posttraumatic complications’ have been identified (Adler, 1943).
Typically two types of memory disturbances have been identified in traumatized individuals; intrusive memories and impoverished memory functioning (APA, 1994). Intrusive memories are accompanied by high levels of arousal, involving a variety of sensoric modalities or behavioral responding, that may be experienced as reenactments of the original trauma (‘flashbacks’) (see for a comprehensive review; Van Oyen Witvliet, 1997). In general, these memories are triggered automatically by situations that reflect aspects of the traumatic event. The second category of memory disturbances in PTSD-patients is concerned with impoverished memory functioning due to diminished encoding or impaired retrieval abilities. PTSD-patients may report deficits in declarative memory (remembering events, facts or lists), fragmentation of memories (both autobiographical and trauma-related), and trauma-related amnesia (gaps in memory that can occur for minutes to days and are not due to ordinary forgetting; Table 1).
Table 1.
Major neural structures thought to underlie memory dysfunction in PTSD
| Brain structure | Memory function | Memory deficits in PTSD |
|---|---|---|
| Hippocampus | Declarative memory | Declarative memory ↓ |
| Integration in space and time | Fragmentation of memories | |
| Trauma-related amnesia | ||
| Amygdala | Fear conditioning | Conditioning↑ + sensitization↑ |
| Emotional memory | Enhanced traumatic memories | |
| Prefrontal cortex | Inhibition of irrelevant stimuli and responses | Failure to inhibit irrelevant cognitions |
| Working memory↓ | ||
| Working memory | Inhibition of emotions↓, intrusions↑ | |
| Inhibition of activation of the amygdala | Deficits in attention/concentration | |
| Sustained attention |
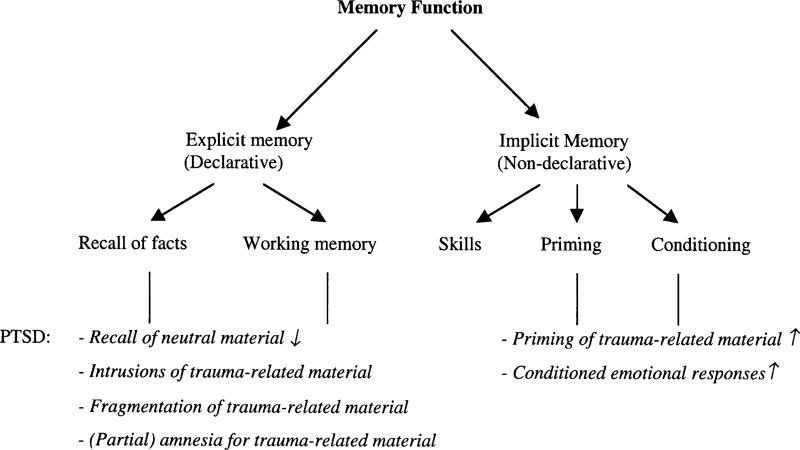
Memory functioning can be divided into declarative (explicit) and non-declarative (implicit) memory processes (Squire and Zola-Morgan, 1991; see also Fig. 1). This distinction is particularly useful for research in PTSD, since declarative and non-declarative memory processes may be affected in different ways (Kihlstrom, 1987; Brewin et al., 1996; McNally, 1998). Declarative memory refers to the ability to consciously remember and reproduce events and facts. Implicit or procedural memory refers to affective and behavioral knowledge of an event without conscious memory, including learning skills, priming, and conditioning. In patients with PTSD, implicit memory processes may automatically facilitate access to information about the traumatic event. Implicit memory may underlie fear conditioning and re-experiencing phenomena in PTSD. Explicit memory is related to declarative memories of the trauma that contain explicit information about the sensory features of the situation, the emotional and physiological reactions experienced, and the perceived meaning of the event.
Fig. 1.
Schematic diagram of the functional organization of memory based on Squire and Zola-Morgan (1991). Memory related disturbances in PTSD have been indicated in italics.
Memory alterations in PTSD represent a complex interrelationship between mind and brain. Accordingly, theories on PTSD and memory disturbances typically stem from a psychological (Foa et al., 1989; Brewin et al., 1996; McNally, 1998) or a neurobiological (Pitman, 1989; Sapolsky, 1996; Bremner et al., 1999a) perspective. Psychological theories have emphasized the social, psychological, and cognitive impact of life threatening experiences, many couched in terms of information processing analyses. These theories have offered valuable insights into the wide array of consequences that a traumatic experience may have. Psychological theories have one major limitation, however. Because experimental research on trauma-related memories is difficult to conduct for ethical considerations, psychological theories are merely based on post-hoc observations. On the basis of experiments with animals under stressful conditions neurobiological models of stress have been developed that are extremely valuable in understanding ‘how’ the stress-system works. A limitation of this approach is, however, the generalisability to humans. Furthermore, animal studies cannot inform us about the impact of trauma on verbal, declarative memory, and the meaning and consequences a trauma may have.
Taken into account the strengths and limitations of both psychological and neurobiological approaches, an interdisciplinary analysis may lead to a better understanding of the effects of trauma on memory functioning. The purpose of the present paper is to present a new model for PTSD and memory disturbances, drawing both on psychological and neurobiological data. We will first present psychological data on trauma-related intrusive memories, amnesia, and recovered memories, followed by neurobiological findings on stress-related memory alterations. Finally, we will present our model for memory disturbances as a core element of PTSD, incorporating data from psychological and neurobiological studies.
2. Neuropsychological studies of memory dysfunction in PTSD
2.1. Information processing models of PTSD
Following the footsteps of Kardiner (1941), who pointed out that patients with PTSD develop serious distortions in the way they process information, psychologists have increasingly applied experimental paradigms to elucidate information processing abnormalities in patients with PTSD (Foa et al., 1989; Brewin et al., 1996; McNally, 1998). Information processing theories focus on how trauma-related information is represented in the cognitive system, and how it is subsequently processed. Central to these theories is the concept of emotional memory networks that comprise stimulus (perceptual information), response (verbal, behavioral, and physiological efferents), and meaning units (declarative and semantic knowledge). When input (for example a noise) ‘matches’ representations in the emotional memory network (e.g. the noise of the ambulance), mutual activation automatically spreads among the units, activating verbal, behavioral and physiological responses, and declarative and semantic knowledge. This may result in reexperiencing symptoms, accompanied by physiological arousal, intrusive memories, and strong emotions, such as fear or anger.
Cognitive models share the assumption that a trauma provides highly salient information that does not fit into preexisting schema’s or models of the world (Foa et al., 1989; Brewin et al., 1996). Successful processing of the traumatic event (‘recovery’) is assumed to occur when trauma-related information is integrated into existing models, often by virtue of changing these models (Foa et al., 1989; Brewin et al., 1996). Because activation of the trauma-network may lead to excessive arousal, this is often avoided however, thereby impeding the integration of trauma-related information in preexisting models.
Although information processing theories generally assume only one level of processing, the dual representation model distinguishes between conscious and nonconscious processes (Brewin et al., 1996). In this model, conscious, verbally accessible memories contain information about the sensory features of the traumatic situation, the emotional and physiological reactions experienced, and the perceived meaning of the event. Nonconscious (automatic) processing of the trauma is assumed to result in situationally accessible knowledge that is accessed automatically when physical features or meaning (a picture, or smell) resemble those of the traumatic situation. Situationally accessible knowledge encompasses highly detailed, repetitive memories (flashbacks, or intrusions) that are difficult to edit and that are accompanied by physiological and emotional changes experienced during the trauma. It is assumed that through selective attention and memory processes trauma-related information is given priority over neutral material, further stimulating the processing of trauma-related information.
2.2. Automatic processing of trauma-related information
Experimental studies support the notion that automatic processing of trauma-related information is disturbed in patients with PTSD. Several studies have established that patients with PTSD compared to traumatized patients without the disorder exhibit an attentional bias that selectively favors the processing of trauma-related material. Vietnam veterans with PTSD exhibited an enhancement of implicit memory (i.e. recall following priming by words stems) for combat words, relative to neutral words in comparison subjects (Zeitlin and McNally, 1991; Amir et al., 1996). Furthermore, in emotional Stroop-tasks, where participants have to name the color of words that are presented to them, patients with PTSD take significantly longer to name the color of trauma-related words (i.e. ‘body-bag’) relative to several categories of control words. Delayed color-naming for trauma-related words was found in several samples of PTSD-patients, including Vietnam veterans (McNally et al., 1993), rape victims (Foa et al., 1991), and motor vehicle accident survivors (Bryant and Harvey, 1997). Since delays in color-naming are involuntary, this indicates that PTSD-patients have an automatic attentional bias to trauma words. PTSD-patients also demonstrated initial eye fixations on threat words more than controls, which further supports the automatic nature of processing trauma-related information (Bryant et al., 1995). Interestingly, Stroop-interference for trauma words appeared to be related to severity of PTSD-symptoms as measured by the Mississippi scale (McNally et al., 1990) and self-reported intrusive symptoms (Cassidy et al., 1992). All together, these studies support the notion that PTSD-patients exhibit automatic interference for trauma-related information, and exhibit more interference for trauma-related material than do trauma-exposed people without the diagnosis PTSD. It has been suggested that intrusions in PTSD may not be limited to trauma-related cognitions alone, however, but may instead reflect a more general pattern of disinhibition. On tasks of sustained attention, initial acquisition, and retroactive interference the performance of Persian Gulf War veterans with PTSD was typically characterized by a general response disinhibition and intrusions (Vasterling et al., 1998).
In line with information processing models, physiological hypersensitivity to traumatic material (or conditioning), has also been found in PTSD-patients, indicating that disturbances in automatic information processing are not limited to cognitive processes. Several studies have shown exaggerated startle response (such as eye blink) in response to a loud noise in patients with PTSD, presumably reflecting a sensitization of the fear/alarm response (Orr et al., 1995; Shalev et al., 1997; Grillon and Morgan, 1999). Moreover, exposure to internal or external cues that symbolize or resemble an aspect of the traumatic event induces strong sympathetic reactivity in PTSD-patients, even years after the trauma occurred (Blanchard and Buckley, 1999; Shin et al., 1999). Taken together, these studies indicate that automatic access to trauma-related information in PTSD-patients is disturbed, consistently resulting in strong physiological and emotional responses, and that the facilitated access is more strongly related to PTSD than to a history of exposure to trauma.
2.3. General memory impairment
PTSD is not only characterized by intrusive memories, but is also associated with general deficits in declarative memory, fragmentation of memories (both autobiographical and trauma-related), and trauma-related amnesia. Even though intrusions and amnesia may appear to be opposite phenomena, these may instead be interrelated processes in that the occupancy with intrusive memories may interfere with, and thus reduce, memory processing of other material. Several studies have established that patients with PTSD compared to traumatized subjects without a PTSD diagnosis have deficits in general declarative memory for information unrelated to the content of the trauma. Vietnam veterans with combat-related PTSD scored significantly lower (almost 50%) on the Wechsler Memory Scale (WMS)–Logical memory and the Selective Reminder Test compared to matched controls, without displaying any differences in IQ (Bremner et al., 1993a). In line with this, Vietnam veterans with PTSD have shown deficits in short-term memory as assessed with the Auditory Verbal Learning Test (AVLT) in comparison to veterans without PTSD (Uddo et al., 1993). Deficits in short-term and delayed declarative memory have also been found in adult survivors of childhood abuse (Bremner et al., 1995a), in rape victims with PTSD (Jenkins et al., 1998), and in child and adolescent patients with PTSD (Moradi et al., 1999). Veterans also manifested a significant decrement in retention of previously presented material following exposure to an intervening word list (Yehuda et al., 1995). In a study by Zeitlin and McNally (1991) PTSD-patients exhibited a general memory impairment for neutral information, whereas their memory for trauma-related information was enhanced, suggesting that intrusions of trauma-related material interfered with the encoding and retrieval of neutral information. Veterans with PTSD also exhibited more impairment in general cognitive functioning compared to veterans without this diagnosis (Barrett et al., 1996).
2.4. Trauma-related amnesia
Trauma-related amnesia is another memory failure that has been reported often by traumatized persons. In a survey about 25 studies a substantial proportion of individuals report having forgotten traumatic experiences for some period of time, especially from childhood (Scheflin and Brown, 1996). More than 30% of adults who had been sexually abused as children either failed to report or were amnesic for the event many years later (Williams, 1994). Although most of these studies suffer from methodological weaknesses related to the subjective nature of the reports, altogether these studies suggest that amnesia for a traumatic experience may occur at some point. The mechanism behind self-reported amnesia remains a controversial topic, however (Loftus et al., 1994; Freyd, 1996; Elzinga et al., 1998).
The distinction between conscious and nonconscious, implicit memory processes has played an important role in the understanding of trauma-related amnesia (Kihlstrom and Schacter, 1995; Elzinga et al., 2000). Based on this distinction, amnesia has been regarded as the absence of verbally accessible knowledge leaving only nonconscious, situationally accessible knowledge available (Brewin et al., 1996). Within this model amnesia may (partly) be seen as the result of a conscious strategy to avoid trauma-related information by disengaging attention from negative emotions associated with the traumatic event. Although clinical observations and subjective reports support the cognitive avoidance hypothesis, a study using the directed-forgetting paradigm in patients with PTSD as a result of childhood abuse was inconsistent with this idea (McNally et al., 1998). In this study, participants were presented trauma, positive and neutral words and were instructed to either remember or forget each word. Instead of exhibiting recall deficits for trauma-related words, the PTSD group exhibited recall deficits for the positive and neutral words they were supposed to remember (McNally et al., 1998). More generally, factors that are known to affect the depth of encoding and retrieval, including the degree to which information is elaborated, organized, and rehearsed, have been proposed to play a (additive) role in forgetting emotional information (Koutstaal and Schacter, 1997). As such, prohibitions against verbalizing and sharing information of traumatic experiences may undermine encoding, storage, and/or retrieval of traumatic memories (Freyd, 1996).
2.5. Recovered memories
There has been considerable controversy surrounding the validity of memories of childhood abuse that are recovered after they have been absent for a certain period (Bremner et al., 1996a; Pezdek and Banks, 1996). Reports of recovered memory have been criticized as representing possibly false memories of abuse suggested by over-zealous psychotherapists and by other influences in the media and popular culture (Lindsey and Read, 1994; Kihlstrom, 1995). Based on studies in normal subjects, consensus has been reached that under certain circumstances memories can be induced or altered by postevent interventions, including misinformation, suggestion, repeated imaging, and source confusion, especially in highly suggestible subjects (Ceci and Bruck, 1993; Zaragoza and Mitchell, 1996). In a study of Hyman and Billings (1998) approximately 25% of the students created false childhood memories after being encouraged to imagine the suggested childhood event. Interestingly, subjects with high scores on dissociative and imaginative capacities appeared to be more prone to create false memories.
Until now, only two studies have been conducted on false memories in patients with PTSD (Bremner et al., 2000; Clancy et al., 2000). In these studies, subjects were read lists of words, with each word in the list associated with a ‘critical lure’ not actually present in the list, but which is ‘falsely remembered’ in a substantial percentage of normal individuals. Women with abuse-related PTSD had a higher frequency of false recognitions of critical lures then women with abuse histories without PTSD, non-abused non-PTSD women, or men without abuse or PTSD (Bremner et al., 2000). PTSD women also showed a pattern of poorer memory for previously studied words, consistent with the findings of declarative memory deficits in PTSD. Clancy et al. (2000) found that women with recovered memories of childhood sexual abuse showed high levels of false recognition compared to other childhood sexual abuse victims and non-abused controls. As shown in the study of Bremner et al. (2000) memory deficits and proneness to the creation of false memories may co-occur. Thus, PTSD-patients may be more prone for the creation of false memories, not so much because they are more suggestible, but because of their general memory deficits, leaving more room for elaboration.
In sum, there seems to be no reason to doubt that under certain circumstances false memories can be created. On the other hand, the creation of false memories of rather common events, such as being lost in a shopping mall, is not a reasonable analogy for more aversive situations. False memories of experiences with the same emotional impact and severity as those of a traumatizing situation have never been created in a laboratory situation. In an attempt to suggest a false memory of a rectal enema Pezdek and Roe (1997) succeeded in none of the subjects. Because a study on false memories of traumatic events is inoperable for ethical reasons, the creation of false memories will remain an unproved possibility of which therapists should be aware off. Clearly, further studies are needed that directly assess the issue of memory functioning and suggestibility of patients with PTSD.
2.6. Dissociation and amnesia
There is evidence that dissociative phenomena may also play an important role in the memory impairments of PTSD-patients. Dissociation is defined as a disruption in the usually integrated functions of consciousness, memory, identity, or perception of the environment. PTSD-patients report more dissociative symptoms during a life-threatening situation compared to traumatized people without PTSD (Bremner et al., 1992; Marmar et al., 1994; Shalev et al., 1996; Bremner and Brett, 1997a). Moreover, in assessing dissociative symptoms with the use of the structured clinical interview for DSM-IV Dissociative Disorders (SCID-D) in Vietnam veterans, PTSD-patients had far more amnesia symptoms than non-PTSD patients (Bremner et al., 1993b). Dissociative reactions, such as derealisation or depersonalisation, may impede memory encoding, so that traumatic memories can not be stored properly. Moreover, difficulties with verbally accessing memories of the trauma can prevent integration of this information in preexisting networks. This may explain why people with high levels of dissociation at the time of trauma are more prone to develop PTSD. Although trauma-related amnesia may seem adaptive in chronically traumatic circumstances, empirical studies have shown that amnesia and related dissociative symptoms at the time of trauma are associated with a long-term increase in psychopathology (Bremner et al., 1992; Marmar et al., 1994; Shalev et al., 1996; Bremner and Brett, 1997a).
3. The neurobiology of memory functioning in PTSD
Findings from studies of the effects of stress on brain structures that mediate memory can add to the model for memory disturbances in PTSD. The hypothalamus–pituitary–adrenal (HPA)-axis (cortisol) and noradrenergic system are mutually inter-regulating systems that play an important role in the regulation of stress. Cortisol and (nor)adrenaline act on the hippocampus, amygdala, prefrontal cortex, and other brain areas to influence memory function. Adrenaline has a short-term effect to strengthen memory traces, while cortisol has a more long-term effect to inhibit the laying down of memory traces. Studies in animals show that stress can have reversible, acute effects on memory function and long-term, structural effects on the hippocampus.
3.1. The role of the noradrenergic system and the amygdala
The noradrenergic system is responsible for rapid responses to stress, including the fight or flight response. Chronic stress is associated with increased firing of noradrenaline neurons in the brainstem and potentiated release of noradrenaline in the brain with subsequent stressors (Bremner et al., 1996b). The noradrenergic system strengthens the formation of memory traces associated with emotional events. In several studies adrenaline is shown to enhance memory in dose-dependent way (Cahill and McGaugh, 1998). Furthermore, a medication that blocks the adrenaline beta receptor (propranolol) reduces the recall of an emotionally arousing story (but not a neutral story), suggesting that activation of the adrenergic receptors in the brain enhances the encoding of emotionally arousing stories (Cahill et al., 1994; Van Stegeren et al., 1998).
Adrenaline seems to enhance retention through its effects in limbic structures, including the amygdala complex. Several studies indicate that the memory enhancing effects of emotionally arousing events are mediated by the activation of β-adrenergic activity within the amygdala complex (Cahill and McGaugh, 1998). Lesions in this region block the memory enhancing effects of adrenaline. In patients with selective damage to the amygdala complex emotionally influenced memory is impaired whereas the memory for relatively unemotional material is normal (Cahill et al., 1995). Furthermore, in a PET-study high correlations were found between activity in the right amygdala complex while viewing an emotional film with the retention of those films, whereas no such correlation was found for neutral films (Cahill et al., 1996). Since the experience of a stressful event might be accompanied by adrenaline release, the noradrenergic system may play an important role in the enhanced encoding of trauma-related memories of PTSD-patients. Moreover, when retrieval of a traumatic event is accompanied by adrenaline release, this may further strengthen the traumatic memory trace. A positive feedback loop may then result in deeply engraved memories, which are clinically expressed as intrusive recollections and flashbacks that are difficult to erase.
Several studies support the role of noradrenaline in intrusive memories in PTSD. Infusion of lactate or yohimbine, substances that activate the noradrenergic system, induced intrusive memories and flashbacks in combat veterans with PTSD, besides other PTSD-symptoms including emotional numbing, and panic attacks (Southwick et al., 1993). In combat veterans with PTSD (Yehuda et al., 1992) and women with child abuse-related PTSD (Lemieux and Coe, 1995) 24-h urinary excretion of noradrenaline has been correlated with intrusive traumatic memories as measured by the IES (Impact of Event Scale). Furthermore, plasma noradrenaline was enhanced in veterans with PTSD after exposure to auditory stimuli reminiscent of combat (McFall et al., 1990; Blanchard et al., 1991). These findings indicate that adrenaline may be associated with the intrusive memories of PTSD-patients.
The amygdala also plays an important role in fear conditioning (Ledoux, 1996). Fear conditioning leads to increases in amygdala activity, as measured by functional magnetic resonance imagery (LaBar et al., 1998; Buchel et al., 1998). Electrical stimulation of the central nucleus of the amygdala has been shown to enhance the acoustic startle response (Davis, 1998). Furthermore, lesions to the central nucleus of the amygdala completely block fear potentiated startle (Hitchcock and Davis, 1986). Patients with unilateral temporal lobectomy (including amygdala resection) showed impaired acquisition of fear conditioning relative to controls, whereas their explicit memory showed no difference (LaBar et al., 1995; Bechara et al., 1995). Interestingly, functional imaging studies have shown that the amygdala is activated more strongly in the presence of subliminal presentation of fearful and angry faces than do freely seen ones, suggesting that the amygdala is specifically engaged in non-declarative, implicit emotional processing (Whalen et al., 1998). Given these findings, amygdala dysfunction may be responsible for the increased fear conditioning of PTSD-patients that is evident in the elevated startle response and the increase in physiological responding to exposure to traumatic reminders.
3.2. The prefrontal cortex
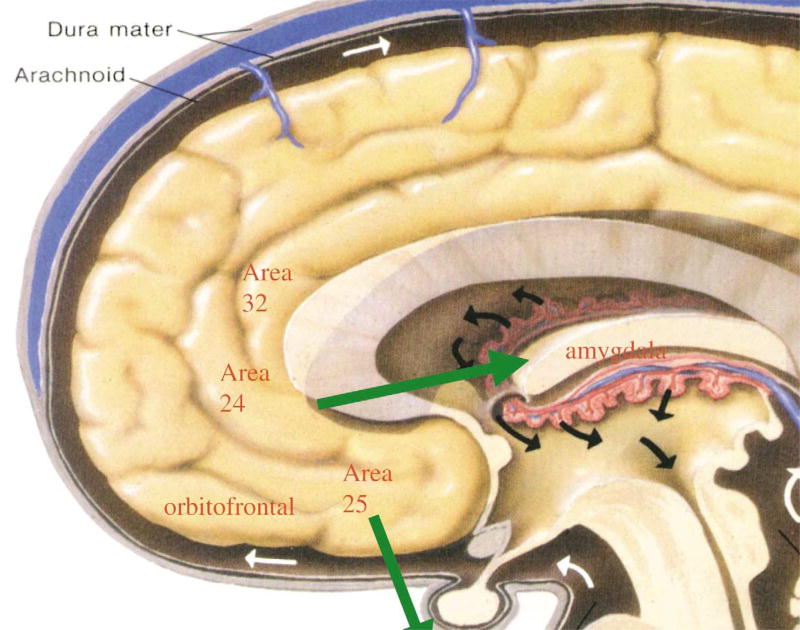
The amygdala has connections to the prefrontal cortex (PFC), terminating mainly in the medial and orbital areas of the PFC (Fig. 2). The PFC is critical for guiding behavior using working memory. The PFC allows the inhibition of inappropriate cognitive and emotional responses or distracting stimuli, thereby facilitating planning and the execution of effective organized behavior (Arnsten, 1998). The medial PFC may also be involved in the modulation of the emotional valence assigned to specific memories, through inhibition of responsivity of the amygdala. It has been shown that exposure to stress increases catecholamine release in the PFC (Thierry et al., 1976; Goldstein et al., 1996). Noradrenaline appears to have opposite actions at alpha-1 and alpha-2 receptors; noradrenaline can impair PFC function through its actions at post-synaptic alpa-1 adrenergic receptors, whereas actions at alpa-2 receptors may improve performance. Since noradrenaline has higher affinity for alpa-2 than for alpha-1 receptors, low levels of noradrenaline release may engage alpa-2 receptors, whereas higher concentrations may engage alpha-1 receptors, resulting in impaired PFC function, including impaired working memory and response inhibition (Arnsten, 1998). Besides the effects of noradrenaline, high levels of dopamine may also impair PFC function. These effects of catechola-mines may explain why persons have difficulties in sustaining attention, are easily distracted, and have inhibition problems during stress. In monkeys, there is evidence that noise stress impairs prefrontal cortical cognitive functioning (Arnsten and Goldman-Rakic, 1998). Thus, PFC dysfunction might account for working memory deficits of PTSD-patients (Bremner et al., 1993a, 1995a). Experiments using imaging techniques have observed enhanced activity in the anterior cingulate cortex during the Stroop task and selective attention tasks. Accordingly, PFC dysfunction may also play a role in the interference on Stroop-tasks (e.g. McNally et al., 1990, 1993) and lack of inhibition (Vasterling et al., 1998) of PTSD-patients.
Fig. 2.
Brain structures related to the prefrontal cortex. Areas of medial prefrontal cortex including anterior cingulate (Brodmann’s areas 32, or the ‘Stroop’ area, Brodmann’s area 24 (‘subgenual cortex’) and area 25 (subcallosal gyrus), as well as orbitofrontal cortex, have inhibitory projects to the amygdala, as well as outputs to activation of the peripheral stress response.
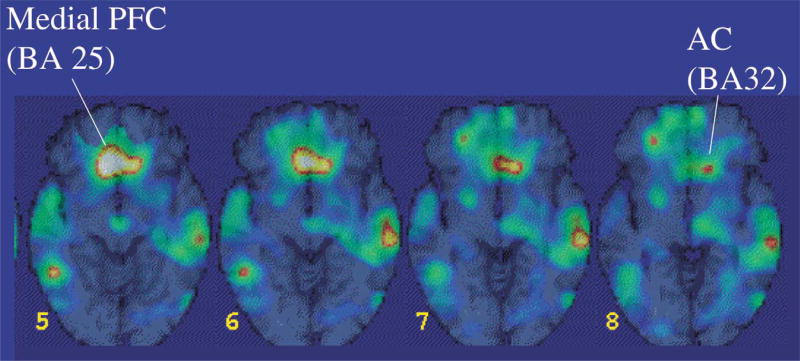
There are several indications that the PFC also has a function in the inhibition of emotions through its projections to the amygdala. Rats with PFC lesions were unable to extinguish fear responses after fear conditioning, whereas this fear response was easily extinguished when there was no damage to the PFC (Morgan et al., 1993). In humans, lesions to the PFC have also been associated with dysfunctions in emotion regulation and an inability to relate in social situations that require correct interpretation of the emotional expressions from others (Damasio et al., 1994). Neuroimaging studies are consistent with the idea that medial PFC dysfunction may be partially responsible for the failure to extinguish fear. Patients with PTSD as a result of childhood sexual abuse (Bremner et al., 1999b) and of combat exposure (Bremner et al., 1999c) showed a deactivation of the medial PFC when confronted with reminders of the trauma (Fig. 3). Decreased activation in the PFC could be related to the incapacity of PTSD-patients to inhibit intrusive memories. Medial PFC dysfunction together with amygdala activation might also offer an explanation for the finding that prior trauma sensitizes for developing PTSD in response to subsequent traumatic events.
Fig. 3.
Area of decreased blood flow with exposure to traumatic combat-related slides and sounds in patients with combat-related PTSD relative to veterans without PTSD. Yellow area shows decreased blood flow in medial prefrontal cortex with traumatic exposure.
3.3. The cortisol-system and the hippocampus
Prolonged exposure to stressful events is associated with a marked increase in the release of the stress-hormone cortisol from the adrenal. Cortisol or corticosterone, depending on the system, is part of the stress-regulating hypothalamic–pituitary–adrenal (HPA) axis. Cortisol release from the adrenal is regulated by the adrenocorticotropine releasing hormone (ACTH) from the pituary, which in turn is primarily regulated by corticotropin releasing factor (CRF) from the paraventricular nucleus of the hypothalamus. After an initial increase in cortisol, inhibitory feedback systems in the brain reduce further release of CRF and ACTH. Because of its glucocor-ticoid (GC) receptor sites, the hippocampus is the primary side of feedback for GC regulation keeping cortisol levels within physiological range. This makes the hippocampus particularly sensitive to stress (McEwen et al., 1992; Sapolsky, 1996). Moreover, unlike other brain structures, the dentate gyrus of the hippocampus formation undergoes continual structural remodeling in adulthood. This is another factor what makes the hippocampus particularly sensitive to environmental and experience-dependent changes (Gould and Tanapat, 1999).
The hippocampus is a brain structure involved in learning and memory, especially declarative (explicit) memory. Memories are initially stored in the hippocampus, and reorganized after several weeks, with storage in other brain areas, including the neocortex (Zola-Morgan and Squire, 1990). Another role of the hippocampus is to bring together memory elements from diverse neocortical areas at the time of retrieval of explicit memory. Interestingly, there is a biologically based distinction between declarative memory and non-declarative memory, which is mediated by structures outside the hippocampus. In patients with damage to the hippocampus, for instance the famous case of H.M., declarative memory is impaired, whereas procedural (implicit) memory is unaffected (Scoville and Milner, 1957).
Animal studies have provided ample support for the hypothesis that the hippocampus may be impaired due to high levels of GC release (Sapolsky, 1996; McEwen, 1999). Studies in a variety of animal species suggest that direct GC exposure results in a loss of neurons in the hippocampus (Uno et al., 1989; Sapolsky et al., 1990), in a decrease in dendritic branching (Woolley et al., 1990; Watanabe et al., 1992), in alterations in synaptic terminal structure (Magarinos et al., 1997), and in an inhibition of neuronal regeneration (Gould and Tanapat, 1999). Stress appears to affect long-term potentiation (LTP), which is used as a model for the molecular basis of new learning and memory, because it requires repeated and synchronous activation of two neurons (Diamond et al., 1995). Thus, reduced LTP due to high levels of stress may lead to a reduced ability to form new memory traces. Corticosterone has also been shown to have detrimental effects on a second model of memory, ‘primed burst’ (PB). In line with this, high levels of GC seen in stress have been associated with deficits in new learning. Rats that were exposed to 21 days of restraint stress for 6 h each day demonstrated impaired performance on the eight-arm radial maze (Luine et al., 1994).
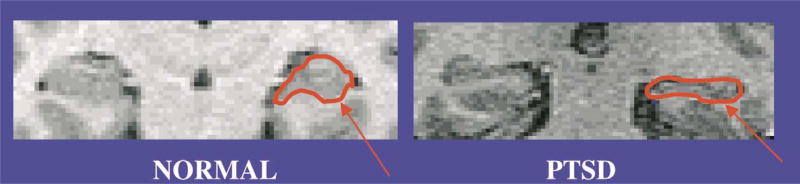
Studies in animals showing GC-mediated hippocampal toxicity and memory dysfunction with stress led to the hypothesis that severe stress, such as the experience of a trauma, may result in similar deficits in human subjects (Bremner, 1999). In clinical subjects, patients with elevated cortisol levels have been reported to show deficits in memory. Patients with Cushing’s Disease, involving excessive release of cortisol over long periods of time, have deficits of verbal declarative memory that are correlated with hippocampal volume reduction on magnetic resonance (MRI) (Starkman et al., 1992). Imaging studies in PTSD are consistent with the hypothesis that stress affects the hippocampus. Smaller hippocampal volumes have been observed in Vietnam veterans with PTSD (Bremner et al., 1995b; Gurvits et al., 1996), and in adults with PTSD as a result of a history of severe childhood abuse (see Fig. 4; Bremner et al., 1997b; Stein et al., 1997). In the later study hippocampal atrophy was correlated with level of dissociative symptomatology. In one study reduced hippocampal volume was associated with deficits in short-term memory (Bremner et al., 1995b). Thus, hippocampal atrophy may provide an explanation for the general memory deficits of PTSD patients. Inversely, cognitive deficits in PTSD have also been hypothesized to represent a risk factor for the development of PTSD (McNally and Shin, 1995).
Fig. 4.
Magnetic resonance imaging scan of a patient with PTSD and a normal control showing a visible reduction in volume of the hippocampus (arrow) in PTSD. Overall there was a 12% reduction of the left hippocampal volume in abuse-related PTSD relative to controls.
Relatively few studies have directly studied the effect of stress-related cortisol on memory processes in humans. Kirschbaum et al. (1996) found a significant negative relationship between stress-induced cortisol levels and performance on a memory task; i.e. subjects with higher cortisol response showed poorer memory performance. Several studies in normal subjects showed that administration of commonly used therapeutic doses of GCs resulted in declarative memory impairments (Kirschbaum et al., 1996; Newcomer et al., 1999). In one study significant effects of hydrocortisone were found on working memory, but not on paired-associate declarative memory function (Lupien et al., 1999). In line with the hypothesis that cortisol only impairs hippocampus-mediated memory, a differential effect was found between procedural and declarative memory: cortisol did impair declarative, but not procedural memory (Kirschbaum et al., 1996; Lupien et al., 1997; Newcomer et al., 1999). Moreover, the effects of cortisol seem to be reversible (Newcomer et al., 1999). Although more direct evidence is needed, cortisol secretion seems to be an important factor in the occurrence of amnesia for emotional events in PTSD-patients.
4. A psychoneurobiological model for memory disturbances in PTSD
Findings from neuropsychological and neurobiological studies suggest a working model for memory dysfunction as a core element of PTSD. While witnessing a traumatic event two stress reactions may occur. As a first rapid reaction, (nor)adrenaline is secreted. This may strengthen emotional memory traces, and enhance fear conditioning. Since the retrieval of traumatic memories is often accompanied by enhanced levels of adrenaline, this may further engrave the traumatic memory each time that it is retrieved. This may account for the sharpness of intrusive memories and conditioning reactions, such as startle, of PTSD-patients. Dysfunction of the medial PFC, a structure that normally inhibits the activation of the amygdala, may further enhance the effects of amygdala function, thereby increasing the emotional valence and the frequency of intrusive memories. Together with amygdala activation pre-frontal dysfunction may also play a role in stress sensitization, thereby accounting for the effects of prior trauma in developing PTSD after subsequent traumatisation. Dysfunction of the PFC may also account for deficits in working memory and inhibition of irrelevant stimuli in PTSD-patients.
Cortisol may also be released during stress. This may result in acute (but reversible) hippocampal dysfunction, and thus in verbal, declarative memory deficits, such as trauma-related amnesia, while leaving non-hippocampus mediated memory processes unaffected. Chronic cortisol secretion may eventually result in a permanent loss or atrophy of hippocampal cells. This may underlie the deficits in declarative memory that PTSD patients demonstrate. Since the hippocampus plays an important role in the integration of different aspects of a memory, especially locating it in time, place, and context (Squire and Zola-Morgan, 1991), we have hypothesized that dysfunction or atrophy of the hippocampus may also underlie distortions and fragmentations of trauma memories (see also Bremner et al., 1996a).
In sum, by way of its influence on amygdala, hippocampus, and PFC, severe stress may simultaneously result in strong emotional reactions and in an incapacity to deliberately recall the emotional event (Ledoux, 1996). This is in line with the information processing models that make a distinction between declarative and non-declarative functions in processing trauma-related information. In our model, non-declarative processes are mediated by the amygdala, that is found to be especially responsible to subliminal stimulation (LaBar et al., 1995; Bechara et al., 1995) whereas declarative memories are mediated by the hippocampus and PFC.
Although our model is supported by empirical evidence at several points, more studies are needed that directly assess our hypotheses. With regard to trauma-related amnesia, it should be tested in PTSD-patients whether encoding processes are specifically impaired under high levels of cortisol by assessing memory retrieval when cortisol levels are low. Until now, most studies have assessed memory encoding and retrieval under conditions of high cortisol levels in healthy subjects, preventing the possibility to distinguish the effects of cortisol on encoding and retrieval (Kirschbaum et al., 1996; Lupien et al., 1997; Newcomer et al., 1999). Also the relationship between amygdala function and PFC in PTSD-patients needs more investigation.
This model of PTSD as a disorder of memory may have several implications. Given the fact that most trauma survivors tend to recover after the initial trauma, many efforts have been put into predicting who is at risk for developing PTSD. According to our model, PTSD would especially occur in individuals with hippocampal dysfunction in response to trauma, resulting in fragmented memories or (partial) amnesia of the traumatic event. Hippocampal dysfunction may be due to high levels of cortisol secretion during or shortly after the traumatic event, or by enhanced sensitivity of cortisol receptors in the hippocampus, possibly due to prior trauma. High levels of adrenaline may further aggravate PTSD symptoms by increasing intrusive symptoms and conditioned responses. Hippocampus dysfunction as a predictor for the development of PTSD accords with information processing theories, posing that a lack of verbal representations of the trauma may inhibit its integration in preexisting networks, and thus recovery.
Although ultimately physiological parameters may underlie the development of PTSD, psychological factors (e.g. appraisal of the event, perceived control, social support) and personality characteristics (e.g. neuroticism, self-esteem) play a crucial role in the development of PTSD (Fauerbach et al., 2000). Those factors may directly influence the release of stress hormones during and after a traumatic event. Lack of self-esteem, for example, has been found to correlate with cortisol response to a stress task (Pruessner et al., 1999). In the past, efforts have been put into differentiating psychological factors specifically associated to adrenal versus GC activity. In animal models, adrenal activity has been related more to ‘defense’ reactions, whereas cortisol is more associated with ‘defeat’ (Henry and Stephens, 1977). If this would also apply to humans, a reaction of defeat in response to a traumatic event might yield larger cortisol-responses, and thus increased cortisol-related PTSD symptoms (e.g. memory impairments, and trauma-related amnesia) than defense responses. Until now, evidence in favor of such models is scarce, however, so that these ideas should be regarded merely as speculations. Also factors that influence hippocampal plasticity (e.g. age, gender) may be related to enhanced vulnerability for developing PTSD after exposure to a traumatic event. Thus, chronic abuse at a young age may yield higher rates of PTSD than abuse occurring at a later age, when hippocampal plasticity has decreased. Gender differences in hippocampal plasticity have also been found in rats, which potentially suggests that the hippocampus may be more vulnerable in women (Juraska, 1991).
It is assumed that this model on memory impairments in PTSD is generic to all types of traumatic events. Nevertheless, certain features of traumatic events have been associated with specific memory dysfunctions. It has been suggested, for example, that abuse by a parent or caregiver is more often accompanied by amnesia for the event than when the perpetrator is unknown to the victim (Freyd, 1996). Moreover, it could be hypothesized that events which are intrinsically more closely related to ‘defeat’ (e.g. loss of a loved one), might predominantly result in cortisol-related memory impairments, whereas events that are more closely related to ‘defense’ (e.g. war experiences), could result in intrusions and flashbacks.
It should be noted that if memory dysfunction in PTSD can (partly) be traced back to physiological processes, no intentional, psychological factors are necessarily needed to explain the occurrence of memory disturbances. This is particularly important with respect to traumatic amnesia, which has typically been described as the result of avoidance or repression. If amnesia is instead induced by hippocampal dysfunction, assigning intentions of deliberately forgetting may even be harmful to patients.
In line with the ideas on the development of PTSD, it is predicted that exposure therapy to recover and process trauma-related memories may only be useful under circumstances when cortisol and adrenaline levels are on low. Otherwise, exposure to trauma-related memory may further aggravate the fragmentation (or even amnesia) and intrusive-ness of traumatic memories. Under therapeutic conditions a facilitation of associations to related events may bring all of the aspects of the memory together so that eventually the memory may be verbally reconstructed. These memories are reconstructions, however, and it must be stressed that accordingly, these reconstructed memories are dependent on the emotions and needs of the context in which they are retrieved (Schacter et al., 1995).
Acknowledgments
This study was supported by a grant from the Foundation for Behavioral and Educational Sciences of the Netherlands Organization of Scientific Research (NWO).
References
- Acierno R, Kilpatrick DG, Resnick HS. Posttraumatic stress disorder in adults relative to criminal victimization: prevalence, risk factors and comorbidity. In: Saigh PA, Bremner JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn and Bacon; New York: 1999. pp. 44–68. [Google Scholar]
- Adler A. Neuropsychiatric complications in victims of Boston’s Cocoanut Grove disaster. J. Am. Med. Assoc. 1943;123:1098–1101. [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4. Washington DC: Author; 1994. [Google Scholar]
- Amir N, McNally RJ, Wiegartz PS. Implicit memory bias for threat in posttraumatic stress disorder. Cogn. Ther. Res. 1996;20:625–635. [Google Scholar]
- Archibald HC, Tuddenham RD. Persistent stress reaction after combat. Arch. Gen. Psychiatry. 1965;12:475–481. doi: 10.1001/archpsyc.1965.01720350043006. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine modulation of prefrontal cognitive function. Trends Cogn. Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Barrett DH, Green ML, Morris R, Giles WH, Croft JB. Cognitive functioning and posttraumatic stress disorder. Am. J. Psychiatry. 1996;153:1492–1494. doi: 10.1176/ajp.153.11.1492. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with Posttraumatic Stress Disorder. J. Nerv. Ment. Dis. 1991;179:371–373. doi: 10.1097/00005053-199106000-00012. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Buckley TC. Psychophysiological assessment of posttraumatic stress disorder. In: Saigh P, Bremner JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn and Bacon; New York: 1999. pp. 248–266. [Google Scholar]
- Bremner JD, Southwick S, Brett E, Fontana A, Rosenheck R, Charney DS. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. Am. J. Psychiatry. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, Southwick SM, Mason JW, Johnson DR, Innis RB, McCarthy G, Char-ney DS. Deficits in short-term memory in post-traumatic stress disorder. Am. J. Psychiatry. 1993a;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Steinberg M, Southwick SM, Johnson DR, Charney DS. Use of the structured clinical interview for DSM-IV dissociative disorders for systematic assessment of dissociative symptoms in posttraumatic stress disorder. Am. J. Psychiatry. 1993b;150:1011–1014. doi: 10.1176/ajp.150.7.1011. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Capelli S, Scott T, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res. 1995a;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in combat-related posttraumatic stress disorder. Am. J. Psychiatry. 1995b;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Charney DS, Southwick SM. Neural mechanisms in dissociative amnesia for childhood abuse: Relevance to the current controversy surrounding the ‘False Memory Syndrome’. Am. J. Psychiatry. 1996a;153:FS71–82. doi: 10.1176/ajp.153.7.71. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Brett E. Trauma-related dissociative states and long-term psychopathology in posttraumatic stress disorder. J. Traum. Stress. 1997a;10:37–49. doi: 10.1023/a:1024804312978. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Capelli S, Mazure CM, McCarthy G, Innis RB, Charney DS. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: A preliminary report. Biol. Psychiatry. 1997b;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. Does stress damage the brain? Biol. Psychiatry. 1999;45:797–805. doi: 10.1016/s0006-3223(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Charney DS. The neurobiology of posttraumatic stress disorder: An integration of animal and human research. In: Saigh P, Bremner JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn and Bacon; New York: 1999a. pp. 103–143. [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in woman with and without posttraumatic stress disorder. Am. J. Psychiary. 1999b;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry. 1999c;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krause K, Shobe K, Kihlstrom JF. False memories in women with self-reported childhood sexual abuse: an empirical study. Psychol. Sci. 2000;11:333–337. doi: 10.1111/1467-9280.00266. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Dalgleish T, Joseph S. A dual representation theory of posttraumatic stress disorder. Psychol. Rev. 1996;103:670–686. doi: 10.1037/0033-295x.103.4.670. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Attentional bias in post-traumatic stress disorder. J. Traum. Stress. 1997;10:635–644. doi: 10.1023/a:1024849920494. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG, Gordon E, Barry RJ. Eye movement and electrodermal responses to threat stimuli in post-traumatic stress disorder. Int. J. Psychophysiol. 1995;20:209–213. doi: 10.1016/0167-8760(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Alpha-adrenergic activation and memory for emotional events. Nature. 1994;371:702–703. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinksy R, Markowitsch H, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Hurer RJ, Fallon J, Alhire MI, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term free recall of emotional information. Proc. Natl. Acad. Sci. USA. 1996;23:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Cassidy KL, McNally RJ, Zeitlin SB. Cognitive processing of trauma cues in rape victims with post-traumatic stress disorder. Cogn. Ther. Res. 1992;16:283–295. [Google Scholar]
- Ceci SJ, Bruck M. Suggestibility of the child witness: A historical review and synthesis. Psychol. Bull. 1993;113:403–439. doi: 10.1037/0033-2909.113.3.403. [DOI] [PubMed] [Google Scholar]
- Clancy S, Schacter D, McNally R, Pitman RK. False recognition in women reporting recovered memories of sexual abuse. Psychol. Sci. 2000;11:26–31. doi: 10.1111/1467-9280.00210. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol. Psychiatry. 1998;15:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Branch BJ, Fleshner M, Rose GM. Effects of dehydroepiandosterone and stress on hippocampal electrophysiological plasticity. Ann. New York Acad. Sci. 1995;774:304–307. doi: 10.1111/j.1749-6632.1995.tb17393.x-i1. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Van Dyck R, van Spinhoven P. Three controversies about dissociative identity disorder. Clin. Psychol. Psychother. 1998;5:13–23. [Google Scholar]
- Elzinga BM, De Beurs E, Sergeant JA, Van Dyck R, Phaf RH. Dissociative style and directed forgetting. Cogn. Ther. Res. 2000;24:279–295. [Google Scholar]
- Fauerbach JA, Lawrence JW, Schmidt CW, Munster AM, Costa PT. Personality predictors of injury-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2000;188:510–517. doi: 10.1097/00005053-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualization of post-traumatic stress disorder. Behav. Ther. 1989;20:155–176. [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. Processing of threat-related information in rape victims. J. Abnormal Psychol. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Freyd JJ. Betrayal Trauma: The Logic of Forgetting Childhood Abuse. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J. Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and neurogenesis. Biol. Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J. Abnormal Psychol. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Gurvits TG, Shenton MR, Hokama H, Ohta H, Lasko NB, Gilberson MW, Orr SP, Kikinis R, Lolesz FA, McCarley RW, Pitman RK. Magnetic resonance imaging study of hippocampal volume in chronic combat-related post-traumatic stress disorder. Biol. Psychiatry. 1996;40:192–199. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. Stress, Health and the Social Environment; A Sociobiological Approach To Medicine. Springer; New York: 1977. [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav. Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hyman IE, Billings FJ. Individual differences and the creation of false childhood memories. Memory. 1998;6:1–20. doi: 10.1080/741941598. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Langlais PJ, Delis D, Cohen R. Learning and memory in rape victims with posttraumatic stress disorder. Am. J. Psychiatry. 1998;155:278–279. doi: 10.1176/ajp.155.2.278. [DOI] [PubMed] [Google Scholar]
- Juraska JM. Sex differences in cognitive regions of the brain. Psychoneuroendocrinology. 1991;16:105–119. doi: 10.1016/0306-4530(91)90073-3. [DOI] [PubMed] [Google Scholar]
- Kardiner A. The Traumatic Neuroses of War. Hoeber; New York, NY: 1941. [Google Scholar]
- Kihlstrom JF. The cognitive unconscious. Science. 1987;237:1445–1452. doi: 10.1126/science.3629249. [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF. The trauma-memory argument. Conscious. Cogn. 1995;4:63–67. doi: 10.1006/ccog.1995.1004. [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF, Schacter DL. Functional disorders of autobiographical memory. In: Baddeley AD, Wilson BA, et al., editors. Handbook of Memory Disorders. Wiley; Chichester, UK: 1995. pp. 337–364. [Google Scholar]
- Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Schacter DL. Intentional forgetting and voluntary thought suppression: two potential methods for coping with childhood trauma. In: Spiegel D, editor. Repressed Memories. American Psychiatric Press; Washington DC: 1997. pp. 79–122. [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 1995;15:6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Ledoux J. The Emotional Brain. Simon and Schuster; New York: 1996. [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosom. Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Lindsey DS, Read JD. Psychotherapy and memories of childhood sexual abuse: A cognitive perspective. Appl. Cogn. Psychol. 1994;8:281–338. [Google Scholar]
- Loftus EF, Garry M, Feldman J. Forgetting sexual trauma: What does it mean when 38% forget? J. Consult. Clin. Psychol. 1994;62:1177–1181. doi: 10.1037//0022-006x.62.6.1177. [DOI] [PubMed] [Google Scholar]
- Luine V, Villages M, Martinex C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gaudreau S, Tchiteya BM, Maken F, Sharma S, Nair NP, Hangu RL, McEwen BS. Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J. Clin. Endocrinol. Metab. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav. Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar CR, Weiss DS, Schlenger WE, Fairbank JA, Jordan BK, Kulka RA, Hough RL. Peritraumatic dissociation and posttraumatic stress in male Vietnam theater veterans. Am. J. Psychiatry. 1994;151:902–907. doi: 10.1176/ajp.151.6.902. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, Chao HM, Daniels D, Gannon MN, Gould E, Mendelson S, Sakai R, Spencer R, Woolley C. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol. Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC. Autonomic responses to stress in Vietnam combat veterans with posttraumatic stress disorder. Biol. Psychiatry. 1990;27:1165–1175. doi: 10.1016/0006-3223(90)90053-5. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Kaspi SP, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. J. Abnormal Psychol. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- McNally RJ, English GE, Lipke HJ. Assessment of intrusive cognition in PTSD: Use of a modified Stroop paradigm. J. Traum. Stress. 1993;6:33–41. [Google Scholar]
- McNally RJ, Shin LM. Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. Am. J. Psychiatry. 1995;152:936–938. doi: 10.1176/ajp.152.6.936. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Experimental approaches to cognitive abnormality in posttraumatic stress disorder. Clin. Psychol. Rev. 1998;18:971–982. doi: 10.1016/s0272-7358(98)00036-1. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Metzger LJ, Lasko NB, Clancy SA, Pitman RK. Directed forgetting of trauma cues in adult survivors of childhood abuse with and without posttraumatic stress disorder. J. Abnormal Psychol. 1998;107:596–601. doi: 10.1037//0021-843x.107.4.596. [DOI] [PubMed] [Google Scholar]
- Moradi AR, Doost HT, Taghavi MR, Yule W, Dalgleish T. Everyday memory deficits in children and adolescents with PTSD: performance on the Rivermead Behavioural Memory Test. J. Child Psychol. Psychiatry. 1999;40:357–361. [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AC. Decreased memory performance in healthy humans induced by stress level cortisol treatment. Arch. Gen. Psychiatry. 1999;56:527–533. doi: 10.1001/archpsyc.56.6.527. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiological responses to loud noises in Vietnam veterans with posttraumatic stress disorder. J. Abnorm. Psychol. 1995;104:75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- Pezdek K, Roe C. The suggestibility of children’s memory for being touched: planting, erasing, and changing memories. Law. Hum. Behav. 1997;21:95–106. doi: 10.1023/a:1024870127516. [DOI] [PubMed] [Google Scholar]
- Pezdek K, Banks WP, editors. The Recovered Memory/False Memory Debate. Academic Press; San Diego, CA: 1996. [Google Scholar]
- Pitman RK. Posttraumatic stress disorder, hormones and memory. Biol. Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Low self-esteem, induced failure and the adrenocortical stress response. Pers. Ind. Dif. 1999;27:477–489. [Google Scholar]
- Saigh PA, Bremner JD, editors. Posttraumatic Stress Disorder: A Comprehensive Text. Allyn and Bacon; New York: 1999. [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Coyle JT, Fischbach GD, Mesulam MM, Sullivan LE, editors. Memory Distortion: How Minds, Brains, And Societies Reconstruct the Past. Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- Scheflin AW, Brown D. Repressed memory or dissociative amnesia: what the science says. J. Psychiatry Law. 1996;24:143–188. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Canetti L, Schreiber S. Predictors of PTSD in injured trauma survivors: A prospective study. Am. J. Psychiatry. 1996;153:219–225. doi: 10.1176/ajp.153.2.219. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Orr SP, Bonne O, et al. Auditory startle responses in help-seeking trauma survivors. Psychiatry Res. 1997;69:1–7. doi: 10.1016/s0165-1781(96)03001-6. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional bloodflow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am. J. Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Starkman MN, Gebarksi SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s Syndrome. Biol. Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinksy J. Selective activation of the mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Torrie A. Psychosomatic casualties in the Middle East. Lancet. 1944;29:139–143. [Google Scholar]
- Uddo M, Vasterling JT, Brailey K, Sutker PB. Memory and attention in posttraumatic stress disorder. J. Psychopathol. Behav. Assessment. 1993;15:43–52. [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oyen Witvliet C. Traumatic intrusive imagery as an emotional memory phenomenon: a review of research and explanatory information processing theories. Clin. Psychol. Rev. 1997;17:509–536. doi: 10.1016/s0272-7358(97)00025-1. [DOI] [PubMed] [Google Scholar]
- Van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, Gooren LJG. Memory for emotional events: differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology. 1998;138:305–310. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, Sutker PB. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expression modulate amygdala activity without explicit knowledge. J. Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM. Recall of childhood trauma: a prospective study of child sexual abuse. J. Consult. Clin. Psychol. 1994;62:1167–1176. doi: 10.1037//0022-006x.62.6.1167. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Giller EL, Xiaowan MA, Mason JW. Urinary catecholamines excretion and severity of PTSD symptoms in Vietnam Veterans. J. Nerv. Ment. Dis. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefe RS, Harvey PD, Levengood RA, Gerber DK, Geni J, Siever LJ. Learning and memory in combat veterans with posttraumatic stress disorder. Am. J. Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- Zaragoza MS, Mitchell KJ. Repeated exposure to suggestion and the creation of false memories. Psychol. Sci. 1996;7:294–300. [Google Scholar]
- Zeitlin SB, McNally RJ. Implicit and explicit memory bias for threat in post-traumatic stress disorder. Behav. Res. Ther. 1991;29:451–457. doi: 10.1016/0005-7967(91)90129-q. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan SM, Squire LR. The primate hippocampal formation: evidence for a time limited role in memory storage. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]