Abstract
Somatic genetic mutations in meningiomas are associated with histologic subtypes, anatomical location, and grade. Concomitant hyperostosis occurs with some meningiomas and the pathogenesis is not well understood. Cranial hyperostosis and meningiomas are common in patients with Proteus syndrome, which is caused by a somatic activating mutation in AKT1 c.49G>A. This same mutation has also been found in 6–9% of sporadic non-syndromic meningiomas. Sixty-one patients with Proteus syndrome meeting clinical diagnostic criteria were evaluated at the NIH from 1997 – 2014. Of these 61, 52 had a somatic activating mutation (c.49G>A, p.Glu17Lys) in AKT1 confirmed from affected tissue samples. Photographs, physical examination and/or autopsy, X-rays, CT and/or MRI scan of the head were reviewed in 29/52 patients. Of the 29 patients, the most common intracranial tumor was meningioma, all co-localizing with cranial hyperostosis and diagnosed at younger ages than typical for isolated, nonsyndromic meningiomas. These patients had progressive cranial overgrowth that consisted primarily of diploic space expansion, and was characterized by unilateral, parasagittal, and frontal bone involvement. We hypothesize that sporadic meningothelial and transitional subtype meningiomas are a forme fruste or microform of Proteus syndrome, and activation of the AKT/PI3K pathway drives hyperostosis in both non-syndromic and Proteus-related meningiomas.
Keywords: AKT1 mutations, cranial hyperostosis, hemimegalencephaly, meningiomas, Proteus syndrome
INTRODUCTION
Meningiomas are the one of the most common types of primary intracranial tumor in adults in the general population [Pham et al., 2011]. Cranial hyperostoses have been reported to be associated with sporadic meningiomas; however, the underlying mechanism is unclear [Yilmaz et al., 2010]. While multiple genetic mutations have been identified in sporadic nonsyndromic meningiomas [Riemenschneider et al., 2006], the potential causative role of specific genetic mutations with the co-occurrence of cranial hyperostosis and meningiomas has not been well described.
Proteus Syndrome (PS), which is a highly variable disorder with asymmetric and disproportionate overgrowth of the body, connective tissue nevi, epidermal nevi, dysregulated adipose tissue, and vascular malformations is caused by a somatic activating mutation in AKT1 [Lindhurst et al., 2012]. Hyperostoses or bony overgrowths of the cranium, maxilla, mandible, or auditory meatus occur frequently in patients with PS [Jamis-Dow et al., 2004; Turner et al., 2004]. While the hyperostoses are described as asymmetrical and patchy, the pattern of distribution of the cranial involvement has not been characterized in detail. Meningiomas, astrocytomas and characteristic brain malformations have also been reported in patients with PS [Gilbert-Barness et al., 2000]. Among patients confirmed to be affected with PS, central nervous system (CNS) malformations occurred in 40% and included neuronal formation and migration defects; astrocytomas, and meningiomas were also reported in this study [Turner et al., 2004].
The purposes of the present study were to review the clinical findings of 29 patients with PS having cranial overgrowth with or without CNS involvement, evaluate the pattern and relationship of the brain findings and intracranial tumors, including meningiomas to the overlying cranial hyperostosis, and discuss the potential pathogenetic mechanisms for these findings.
MATERIALS AND METHODS
Sixty-one patients with PS were evaluated at the NIH from 1997 through 2014. They were enrolled under the NHGRI IRB-approved protocol, 94-HG-0132, Phenotype and Etiology of Proteus Syndrome and Related Overgrowth Disorders. All patients met clinical diagnostic criteria [Biesecker et al., 1999], and 52 of 61 patients also had molecular confirmation of a somatic AKT1 mutation [Lindhurst et al., 2012]. The medical records of these 52 patients including photographs, autopsy reports, skull radiographs, cerebral computed tomography, and magnetic resonance imaging were reviewed. Of these 61 patients with PS, 29 had confirmed cranial hyperostosis: 20/29 had cranial CT or MRI scans, which were reviewed by neuroradiologists, and 9/29 had only cranial X-rays or autopsy findings. For the patients for whom this was available, cross-sectional imaging (CT or MRI) of the head was reviewed for the presence of intracranial findings. We then examined for patterns in the findings that could be observed in multiple patients.
RESULTS
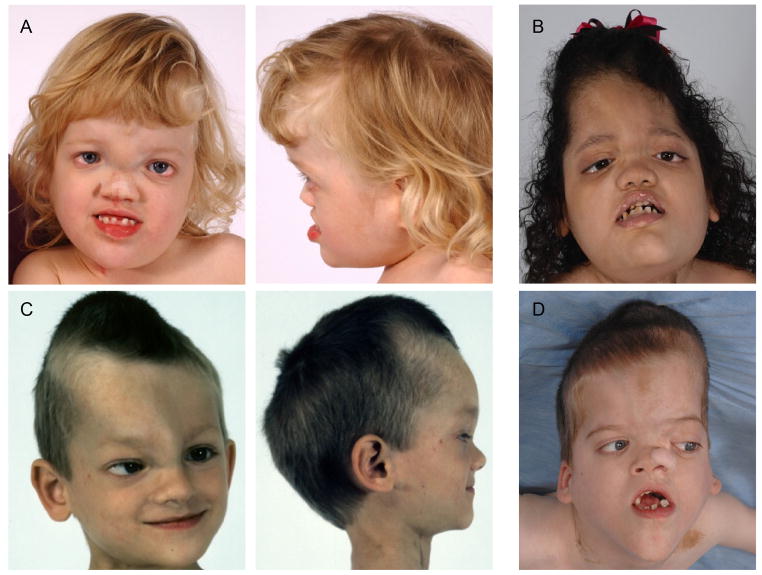
Twenty-nine of 61 patients (48%) had evidence of cranial hyperostosis, and of those the age range was two to 66 years with a median age of 16 years. There were 32 of 61 patients with no evidence of cranial hyperostosis on examination or X-rays, and of those the age range was two to 37 years with a median age of 14.5 years. There were no other appreciable differences between those with and those without cranial hyperostosis. All 61 patients met clinical diagnostic criteria for PS [Biesecker et al., 1999]. Figure 1 shows some of the patients with cranial overgrowth and its typical patterning. Frontal (N=18) or frontoparietal (N=5) hyperostosis was present in 23/29 patients, and 6/29 (20%) also had occipital hyperostosis. There were other less frequently involved areas of the cranium, including the petrous portion of the temporal bone, sphenoid, occipital bone, and mandibular ramus. Involvement was unilateral in 21/26 (80%). All were parasagittal. Progressive cranial hyperostosis associated with brain compression was reported in two patients, but otherwise hyperostoses typically extended extracranially. The cranial overgrowth was described as being radiologically similar in appearance to fibrous dysplasia with widening of the diploic compartment of the bone [Figure 2].
Figure 1.
Typical patterning of cranial hyperostosis in four individuals with Proteus syndrome. The pattern is predominantly unilateral, parasagittal, and involves the frontal and/or parietal bones. (A) 4 year old girl; (B) 2 year 5 month old girl; (C) 3 year 5 month old boy; (D) 6 year 10 month old boy.
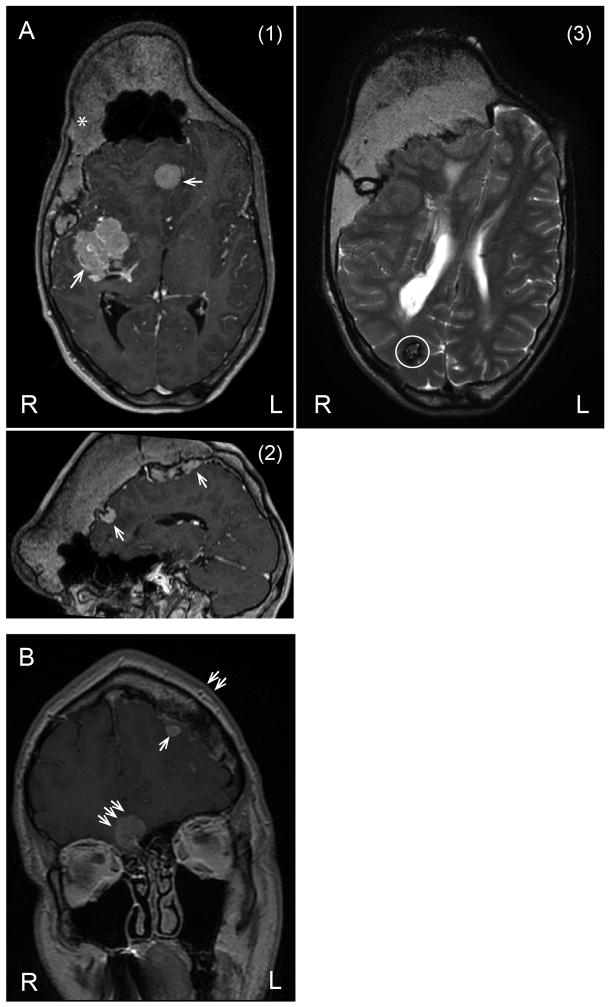
Figure 2.
33 year old with PS, (A) (1) Axial and (2) sagittal views on the MRI scan shows hyperostosis crossing the midline, predominantly involving the right side, meningiomas (thin arrows), and (3) another axial view shows one cavernoma or capillary telangiectasia (circled). 34 year old with PS, (B) MRI scan shows left frontal bone hyperostosis (double arrows) with adjacent meningioma (thin arrow); there is second meningioma (triple arrows) without hyperostosis adjacent to the sphenoid bone.
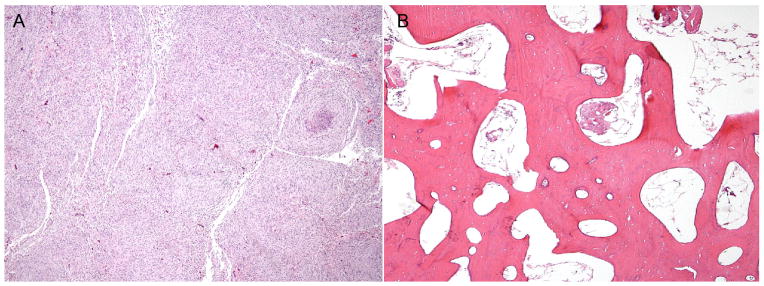
Meningiomas were identified in four patients. The ages of these patients were 9, 19, 24, and 34 years; two were males, and two were females. We found that two of the patients had more than one meningioma. Figure 2 shows meningiomas in two of the patients. We observed several interesting patterns regarding the location of the meningiomas within the cranial compartment. Most were located near the frontal hyperostosis (4/5), originating near the anterior falx (in 1/5) and dura of the skull base (2/5). Genetic testing was performed on the meningioma and cranial bone in one patient, who had the typical c.49G>A AKT1 mutation in the meningioma and the overlying cranial overgrowth (data not shown). The meningiomas were of the meningothelial type, and were located at the base of the skull, around the lesser and greater wings of the sphenoid. The overgrown frontoparietal bone along the left side of the sagittal suture showed thickened bony trabeculae and a lamellar bone appearance similar to an osteoma. The cranial overgrowth is radiographically similar to that of monostotic cranial fibrous dysplasia. On review of the CT scans of the cranium, the affected, overgrown regions have the appearance of fibroosseus tissue and on calculation of the Hounsfield unit (HU), measurements were in the range of ~+110 to +115, which was less than cancellous bone (+400), and dense bone (+1,000 to +3,000) and more than soft tissue [+40 to +80]. The marrow space was enlarged, and on histopathologic examination, the marrow of the skull was abnormal, being aplastic with <5% immature myeloid cells and replaced by fibrous-fatty tissue [Figure 3]. Two other intracranial neoplasms were identified: two patients had a hamartoma and one patient had a probable cavernous hemangioma.
Figure 3.
Histopathology from the autopsy of one of the patients with PS. The top panel (A) demonstrates a meningothelial type meningioma, which was located at the base of the skull, and adjacent to the lesser wing and greater wings of the sphenoid bone. The lower panel (B) shows the overgrown frontoparietal bone along the left side of the sagittal suture and adjacent to the meningioma shows thickened bony trabeculae, lamellar bone appearance similar to an osteoma, and abnormal marrow.
Thirteen of 20 patients having CT and/or MRI scans of the head were found to have structural brain abnormalities, including, hemimegalencephaly (HMEG), polymicrogyria, heterotopias, porencephaly, septum pellucidum dysplasia, colpocephaly, enlarged ventricles or white matter abnormalities, which included white matter cysts and decreased white matter. Brain anomalies were preferentially located ipsilateral to the cranial hyperostosis in 10/13 (77%) patients.
DISCUSSION
Meningiomas are the most common primary intracranial tumor in adults with annual incidence of 4.5 per 100,000 individuals, occurring more frequently in females [Riemenschneider et al., 2006]. Childhood meningiomas account for ~3–4%, and are more common in males [Bhat et al., 2014]. Meningiomas are also one of the more common benign brain tumors in patients with PS [Turner et al., 2004]. However, in patients with PS, the age of diagnosis of a meningioma is typically younger (<30 years) [Turner et al., 2004] than in the general population, where it is most commonly diagnosed between 40 to 70 years of age [Simon et al., 2007]. Multiple meningiomas are common in patients with PS, whereas solitary meningiomas are more common in the general population.
Intracranial meningiomas are dural-based with different locations and histopathological types. Histologically, the fibrous and transitional meningioma subtypes had features similar to the fibroblasts found in the deeper layers of the arachnoid, close to the subarachnoid space. In contrast, the meningothelial subtype resembled the arachnoid cap cells of the outer layers. Therefore, the differential leptomeningeal embryogenesis could result in the predominance of one cell type (i.e., arachnoid cap cells and/or trabecular cells) in certain locations [Lee et al., 2006].
While approximately 40–60% of sporadic meningiomas have NF2 mutations, there are a subset of meningiomas that have been found to have mutations in AKT1 (c.49G>A, p.Glu17Lys), [Briastianos et al., 2012; Clark et al., 2013; Sahm et al., 2013], which is the same somatic mutation that is found in patients with Proteus syndrome [Lindhurst et al., 2012]. El-Habr et al. [2014] found that 9% of 108 patients with sporadic meningioma had a somatic AKT1 mutation, similar to other reports [Briastianos et al., 2012; Clark et al., 2013]. Amongst 391 primary brain tumors and 1,046 tumors of the coverings of the central and peripheral nervous system, AKT1 (p.Glu17Lys) was exclusively seen in meningiomas and occurred in 65 of 958 of these tumors (61 of 705 WHO grade I meningiomas) (p<0.0001, Fisher’s exact test) [Brastianos et al., 2012]. Clark et al. [2013] found that in 38 meningiomas with the AKT1 (c.49G>A, p.Glu17Lys) mutation, 66% (N=25) also had a co-occurring TRAF7 mutation. There is evidence that certain gene mutations are associated with particular characteristics such as location and grade (aggressiveness/ severity, risk of recurrence, invasiveness, and stroke risk) of meningiomas. Isolated sporadic meningiomas with AKT1 mutations tend to be of meningothelial and transitional histopathological subtypes (compared to fibroblastic or atypical subtype with NF2 mutations), more commonly are located at the medial skull base, are usually benign with chromosomal stability (low malignant potential), and are classified as WHO grade I [Sahm et al., 2013]. The meningiomas in Proteus syndrome share these characteristics.
Hyperostoses are reported in 4.5 – 13% of isolated, sporadic meningiomas; however, hyperostosis involving the cranial vault is rare [Yilmaz et al., 2010; Pieper et al., 1999; Akutsu et al., 2004; Baek et al., 2008; Talachi et al., 2011]. In one study, hyperostosis was identified in 6% of patients with meningioma. However, only 1.7% of cases had hyperostosis of the vault, and of these, the frontoparietal region of the skull was predominantly affected, more extensive on the outer table, and unilateral in distribution with 99% of the associated meningiomas being the meningothelial type [Talachi et al., 2011]. The characteristics of the sporadic meningiomas with hyperostosis in that study correspond to those seen in the patients with PS presented herein.
Previously suggested potential causative mechanisms for the hyperostosis that accompanies some meningiomas have included a reactive process of the bone to the meningioma or meningeal tumor cell invasion into bone; however, the mechanisms are unclear [Yilmaz et al., 2010; Pieper et al., 1999; Akutsu et al., 2004; Baek et al., 2008; Talachi et al., 2011]. We propose two additional models for the presence of cranial hyperostosis with meningioma. One is that the hyperostosis is a cell autonomous event with a somatic mutation in the involved cranium and in the meningioma that represents a microform or forme fruste of Proteus syndrome [Wee et al., 2014]. This model of a discrete mutation of AKT1 in dura and cranium, derived from the same cells of the membranous neurocranium, is plausible in light of Proteus syndrome, which consists of multi-systemic, but mosaic abnormalities, including meningiomas, being caused by somatic mutations in multiple cell types. Proteus syndrome represents an example of a simple, single gene mosaic overgrowth disorder in a spectrum of genomic perturbation with meningioma and cranial hyperostosis - both manifestations of Proteus syndrome representing a limited, localized somatic mutation of the same gene, namely AKT1 [Wee et al., 2014]. Two, that the meningioma causes release of agents/ factors that lead to changes in the cells of the cranial bone causing hyperostosis, i.e., there is an autocrine signaling from the meningioma. There is evidence showing that there is increased expression of other growth factors, their receptors, and respective kinases in meningiomas, such as epidermal growth factor receptor, which is widely expressed on meningioma cells and tumors. Further, meningioma cells are capable of autocrine expression of the ligands of the epidermal growth factor receptor, such as TGR-alpha and EGF, associated with aggressive growth [Riemenschneider et al., 2006]. In this model, the meningioma with AKT1 mutations would potentially lead to increased expression of downstream growth factors in the AKT/PI3K signaling pathway [El-Habr et al., 2014; Johnston et al., 2010]. Both models have implications for potential therapeutic interventions.
We hypothesize that somatic AKT1 mutations in tumors and surrounding tissue cause the hyperostosis observed with a subset of non-syndromic meningiomas. This would have interesting etiologic implications for hyperostosis with meningiomas. These results further demonstrate the utility of studying rare disorders as they can identify pathogenetic mechanisms that may be relevant for common disease.
Summary
Cranial overgrowth in patients with PS consists primarily of expansion of the marrow or diploe with an appearance similar to monostotic cranial fibrous dysplasia. We found that the frontal bone is most commonly involved in the cranial overgrowth in patients with PS. Associated structural brain abnormalities in patients with PS are typically neuronal migration and organization defects that co-localize with the cranial overgrowth. Meningiomas are the most common intracranial tumor in PS, and are associated with a characteristic pattern of cranial hyperostosis, which is found in some sporadic, isolated meningiomas. We hypothesize that somatic AKT1 mutations also cause the hyperostosis observed with these non-syndromic meningiomas. Understanding the genetic and molecular mechanisms by which meningiomas and their associated findings develop by studying rare diseases, like Proteus syndrome, may provide insight into the natural history of these tumors and help direct management and potential therapeutic approaches. Indeed, we hypothesize that sporadic meningothelial and transitional subtype meningiomas are a forme fruste or microform of Proteus syndrome.
Acknowledgments
The authors are grateful to the patients and their families who participated in this research study. Thank you to Dr. Meggie Doucet, MD, Department of Pathology and Laboratory Medicine, Tulane University School of Medicine, New Orleans, LA for her preparation and review of the histology findings shown in Figure 3. Thank you to Dr. Nicholas Patronas, MD in the Department of Radiology at the NIH Clinical Center, who provided review of the manuscript. Thank you to Dr. Michael T. Collins, MD, Chief of the Skeletal Clinical Studies Section for his review and characterization of the cranial overgrowth findings in several of the CT and MRI images. EHB has no financial or personal conflicts of interest. MJL declares receipt of royalties from Genentech. LGB is an uncompensated advisor to the Illumina Corporation, receives royalties from Genentech and Amgen and honoraria from Wiley-Blackwell for editing. KK-N, JCS, MJL and LGB are supported by intramural funding of the National Human Genome Research Institute. The ideas and opinions expressed in this paper are those of the authors only and do not necessarily represent any position or policy of the National Institutes of Health or any other institution organization with which any of the authors are affiliated.
References
- Akutsu H, Sugita K, Sonobe M, Matsumura A. Parasagittal meningioma en plaque with extracranial extension presenting diffuse massive hyperostosis of the skull. Surg Neurol. 2004;61:165–69. doi: 10.1016/s0090-3019(03)00521-4. [DOI] [PubMed] [Google Scholar]
- Baek JU, Cho YD, Yoo JC. An osteolytic meningioma en plaque of the sphenoid ridge. J Korean Neurosurg Soc. 2008;43:34–36. doi: 10.3340/jkns.2008.43.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AR, Wani MA, Kirmani AR, Ramzan AU. Histological-subtypes and anatomical location correlated in meningeal brain tumors (meningiomas) J Neurosci Rural Pract. 2014;5:244–259. doi: 10.4103/0976-3147.133568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker LG, Happle R, Mulliken JB, Weksberg R, Graham JM, Viljoen DL, Cohen MM. Proteus syndrome: diagnostic criteria, differential diagnosis, and patient evaluation. Am J Med Genet. 1999;84:389–395. doi: 10.1002/(sici)1096-8628(19990611)84:5<389::aid-ajmg1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2012;45:285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avsar T, Li J, Murray PB, Henegariu O, Yilmaz B, Grady C, Tanrikulu B, Bakircioglu M, Kaymakcalan H, Caglayan AO, Sencar L, Ceyhun E, Atki AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilguvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kilic T, Lifton RP, Noonan JP, Yasuno K, Gunel M. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339:1077–1080. doi: 10.1126/science.1233009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Habr EA, Levidou G, Trigka E-A, Sakalidou J, Piperi C, Chatziandreou I, Spyropoulou A, Soldatos R, Tomara G, Kalliopi Petraki K, Samaras V, Zisakis A, Varsos V, Vrettakos G, Boviatrsis E, Patsouris E, Saetta AA, Korkolopoulou P. Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development. Virchows Arch. 2014;465:473–485. doi: 10.1007/s00428-014-1641-3. [DOI] [PubMed] [Google Scholar]
- Gilbert-Barness E, Cohen MM, Jr, Opitz JM. Multiple meningiomas, craniofacial hyperostosis and retinal abnormalities in Proteus syndrome. Am J Med Genet. 2000;93:234–240. doi: 10.1002/1096-8628(20000731)93:3<234::aid-ajmg15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Goyal N, Kakkar A, Sarkar C, Agrawal D. Does bony hyperostosis in intracranial meningioma signify tumor invasion? A radiopathologic study. Acta Neurochir (Wien) 2011;153:53–61. [Google Scholar]
- Jamis-Dow CA, Turner J, Biesecker LG, Choyke PL. Radiologic Manifestations of Proteus Syndrome. RadioGraphics. 2004;24:1051–1068. doi: 10.1148/rg.244035726. [DOI] [PubMed] [Google Scholar]
- Johnson MD, O’Connell MJ, Pilcher W, Reeder JE. Fibroblast growth factor receptor-3 expression in meningiomas with stimulation of proliferation by phosphoinositide 3 kinase-Akt pathway. J Neurosurg. 2010;112:934–939. doi: 10.3171/2009.7.JNS09726. [DOI] [PubMed] [Google Scholar]
- Lee JH, Sade B, Choi E, Golubic M, Prayson R. Meningothelioma as the predominant histological subtype of midline skull base and spinal meningioma. J Neurosurg. 2006;105:60–64. doi: 10.3171/jns.2006.105.1.60. [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, Blumhorst C, Brockmann K, Calder P, Cherman N, Deardorff MA, Everman DB, Golas G, Greenstein RM, Kato BM, Keppler-Noreuil KM, Kuznetsov SA, Miyamoto RT, Newman K, Ng D, O’Brien K, Rothenberg S, Schwartzentruber DJ, Singhal V, Tirabosco R, Upton J, Wientroub S, Zackai EH, Hoag K, Whitewood-Neal T, Robey PG, Schwartzberg PL, Darling TN, Tosi LL, Mullikin JC, Biesecker LG. Mosaic activating mutation in AKT1 associated with the Proteus syndrome. New Engl J Med. 2012;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham MH, Zada G, Mosich GM, Chen TC, Giannotta SL, Wang K, Mack WJ. Molecular genetics of meningiomas: a systematic review of the current literature and potential basis for future treatment paradigms. Neurosurg Focus. 2011;30:E7. doi: 10.3171/2011.2.FOCUS1117. [DOI] [PubMed] [Google Scholar]
- Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis Associated with Meningioma of the Cranial Base: Secondary Changes or Tumor Invasion. Neurosurgery. 1999;44:742–746. doi: 10.1097/00006123-199904000-00028. [DOI] [PubMed] [Google Scholar]
- Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- Sahm F, Bissel J, Koelsche C, Schweizer L, Capper D, Reuss D, Böhmer K, Lass U, Göck T, Kalis K, Meyer J, Habel A, Brehmer S, Mittelbronn M, Jones DT, Schittenhelm J, Urbschat S, Ketter R, Heim S, Mawrin C, Hainfellner JA, Berghoff AS, Preusser M, Becker A, Herold-Mende C, Unterberg A, Hartmann C, Kickingereder P, Collins VP, Pfister SM, von Deimling A. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol. 2013;126:757–762. doi: 10.1007/s00401-013-1187-5. [DOI] [PubMed] [Google Scholar]
- Simon M, Boström JP, Hartmann C. Molecular genetics of meningiomas: from basic research to potential clinical applications. Neurosurgery. 2007;60:787–798. doi: 10.1227/01.NEU.0000255421.78431.AE. [DOI] [PubMed] [Google Scholar]
- Talachi A, Corsini F, Gerosa M. Hyperostosing meningiomas of the cranial vault with and without tumor mass. Acta Neurochir. 2011;153:53–61. doi: 10.1007/s00701-010-0838-8. [DOI] [PubMed] [Google Scholar]
- Turner JT, Cohen MM, Jr, Biesecker LG. Reassessment of the Proteus Syndrome Literature: Application of Diagnostic Criteria to Published Cases. Am J Med Genet. 2004;130A:111–122. doi: 10.1002/ajmg.a.30327. [DOI] [PubMed] [Google Scholar]
- Wee JS, Mortimer PS, Lindhurst MJ, Chong H, Biesecker LG, Holden CA. A limited form of Proteus syndrome with bilateral plantar cerebriform collagenomas and varicose veins secondary to a mosaic AKT1 mutation. JAMA Dermatol. 2014;150:990–993. doi: 10.1001/jamadermatol.2013.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Musluman AM, Cavusoglu H, Colak I, Tanik C, Aydin Y. Meningioma causing hyperostosis of the cranial convexity in a child. J Clin Neurosci. 2010;17:926–929. doi: 10.1016/j.jocn.2009.11.008. [DOI] [PubMed] [Google Scholar]