Abstract
Exposure to chlorpyrifos (CPF) during the late preweanling period in rats inhibits the endocannabinoid metabolizing enzymes fatty acid hydrolase (FAAH) and monoacylglycerol lipase (MAGL), resulting in accumulation of their respective substrates anandamide (AEA) and 2-arachidonylglycerol (2-AG). This occurs at 1.0 mg/kg, but at a lower dosage (0.5 mg/kg) only FAAH and AEA are affected with no measurable inhibition of either cholinesterase (ChE) or MAGL. The endocannabinoid system plays a vital role in nervous system development and may be an important developmental target for CPF. The endocannabinoid system plays an important role in the regulation of anxiety and, at higher dosages, developmental exposure to CPF alters anxiety-like behavior. However, it is not clear whether exposure to low dosages of CPF that do not inhibit ChE will cause any persistent effects on anxiety-like behavior. To determine if this occurs, 10-day old rat pups were exposed daily for 7 days to either corn oil or 0.5, 0.75, or 1.0 mg/kg CPF by oral gavage. At 12 h following the last CPF administration, 1.0 mg/kg resulted in significant inhibition of FAAH, MAGL, and ChE, whereas 0.5 and 0.75 mg/kg resulted in significant inhibition of only FAAH. AEA levels were significantly elevated in all three treatment groups as were palmitoylethanolamide and oleoylethanolamide, which are also substrates for FAAH. 2-AG levels were significantly elevated by 0.75 and 1.0 mg/kg but not 0.5 mg/kg. On day 25, the latency to emerge from a dark container into a highly illuminated novel open field was measured as an indicator of anxiety. All three CPF treatment groups spent significantly less time in the dark container prior to emerging as compared to the control group, suggesting a decreased level of anxiety. This demonstrates that repeated preweanling exposure to dosages of CPF that do not inhibit brain ChE can induce a decline in the level of anxiety that is detectable during the early postweanling period.
1. Introduction
Exposure to the organophosphorus insecticides (OP) is suspected to exert a negative impact on the developing nervous system in children. Despite the voluntary restrictions that have eliminated the use of many of these compounds from residential use in the United States, they are still used heavily in agriculture. Thus, the potential for childhood exposure to OP insecticides in agricultural communities still exists (Koch et al., 2002; Arcury et al., 2007). Developmental exposure to OP insecticides has been suggested to have lasting negative impacts, including decreased motor skills, decreased cognitive abilities, increased signs of attention deficit disorder and attention deficit/hyperactivity disorder (ADHD), and altered brain morphology (Ruckart et al., 2004; Marks et al., 2010; Engel et al., 2011; Bouchard et al., 2011; Rauh et al., 2006; 2011; 2012). Of the OP insecticides frequently linked to these issues, chlorpyrifos (CPF) is one of the most frequently applied (Grube et al., 2011).
The canonical toxicological target for CPF is brain acetylcholinesterase (ChE), the inhibition of which results in the accumulation of acetylcholine and subsequent hyperactivity in the cholinergic system. However, multiple developmental studies have reported that CPF induces neurochemical and behavioral aberrations at dosage levels that do not produce characteristic signs of cholinergic hyperactivity and induce only minimal amounts of ChE inhibition (Levin et al., 2002; Slotkin et al., 2006, 2007; Timofeeva et al., 2008a,b). Such observations have led to the hypothesis that some of the developmental toxic effects of CPF occurs through non-cholinergic mechanisms.
Previous studies have reported the disruption of endocannabinoid metabolism following acute in vivo exposure of adult mice and rats to high dosages of OP compounds including CPF (Quistad et al., 2001, 2002, 2006; Nallapaneni et al., 2008; Nomura et al., 2008; Nomura and Casida, 2011; Lui et al., 2013; 2015). Work performed in our laboratory has expanded this work by investigating the effect of repeated low level CPF exposure on endocannabinoid metabolism in developing rats. Developmental CPF exposure resulted in significant inhibition of the brain endocannabinoid metabolizing enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) and significant elevation of their respective substrates the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (Carr et al., 2011; 2013). However, the levels of CPF used in these studies also resulted in significant inhibition of ChE activity even at the lowest dosage used (1.0 mg/kg). A subsequent study utilized a lower dosage (0.5 mg/kg) of CPF that yielded no measurable inhibition of ChE or MAGL, but induced significant inhibition of FAAH (Carr et al., 2014). This dosage resulted in a significant elevation of brain AEA levels but no change in 2-AG levels.
Although these data demonstrate that developmental CPF exposure results in a biochemical disruption of the endocannabinoid system, it is not clear if this altered signaling results in any functional effects in the whole animal. Appropriate functioning of the endocannabinoid system is necessary for the control of the responses that are induced by environmental stimuli (Wotjak, 2005; Lutz, 2009). Furthermore, the modulation of endocannabinoid signaling can alter normal responses (Naidu et al., 2007; Zanettini et al., 2011). In addition, altering endocannabinoid signaling during development can induce persistent changes in anxiety (O’Shea et al., 2006). It has also been demonstrated that developmental CPF induces changes in behaviors associated with anxiety (Aldridge et al., 2005; Ricceri et al., 2003; 2006; Venerosi et al., 2006; 2008; 2010; 2015; De Felice et al., 2014), but these effects were observed at dosages that inhibit ChE.
One test of anxiety is the emergence test, which measures an animal’s response to a novel environment (Archer, 1973). This study was designed to determine the response of preadolescent rats exposed developmentally to CPF to a novel aversive environment by measuring the time required for a rat to emerge from a dark area (safe) into a well-lit area (novel aversive environment). In an attempt to obtain a dose response, two dosages previously demonstrated to induce biochemical effects (0.5 and 1.0 mg/kg) and a third new middle dosage (0.75 mg/kg) were utilized in the behavioral test. To match previous data, the activities of ChE, MAGL, and FAAH and the levels of the endocannabinoids AEA and 2-AG were determined in the forebrain at 12 hrs following the last administration of CPF. In addition, the levels of the non-cannabinoid N-acylethanolamides palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) in the brain were also determined. OEA and PEA are substrates for FAAH and genetic deletion or inhibition of FAAH activity also increases their levels (Cravatt et al., 1996; Fegley et al., 2005). However, it is not clear whether the inhibition of FAAH resulting from low level exposure to CPF will result in changes in their levels.
2. Materials and Methods
2.1. Chemicals
Chlorpyrifos (> 99% purity) was a generous gift from DowElanco Chemical Company (Indianapolis, IN). All other chemicals were purchased from Cayman Chemicals (Ann Arbor, MI) or Sigma Chemical Co. (St. Louis, MO).
2.2 Animal Treatment
Animals were housed in a temperature controlled (22 ± 2°C)
Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility with a 12-h dark-light cycle and with lights on between 0700 and 1900. LabDiet rodent chow and tap water were freely available during the experimentation. All procedures were approved by the Mississippi State University Institutional Animal Care and Use Committee. Adult male and female Sprague Dawley rats (CD IGS; Harlan Laboratories, Indianapolis, IN) were bred and following parturition, male and female rat pups within the same litter were assigned to different treatment groups. There were always representative control animals of the same sex present in each litter to match the CPF treated animals. For the behavioral portion of this project, rats from 15 litters were used. The day of birth was considered as postnatal day 0 (PND 0).
Chlorpyrifos was administered at a volume of 0.5 ml/kg body weight by oral gavage (per os) every day from PND10 through PND16, a time frame corresponding to the postnatal age in humans in which significant brain maturation occurs (Andersen, 2003; Counotte et al., 2011; Tau and Peterson, 2010). Oral gavage was performed using a 25 µl tuberculin syringe equipped with a 1-inch 24-gauge straight intubation needle (Popper and Sons, Inc., New Hyde Park, NY) to deliver the solution to the back of the throat. Daily body weights were recorded and weight gain was calculated as the difference between the body weights on PND11-16 and the original body weight at initiation of treatment on PND10.
The treatment groups selected for study were control (corn oil), low CPF (0.5 mg/kg), medium CPF (0.75 mg/kg), and high CPF (1 mg/kg). These dosages were designed to span the range between no inhibition of brain ChE and low inhibition of brain ChE (Carr et al., 2013; 2014). These dosages are below the oral repeated No Observed Effect Level (NOEL) for signs of toxicity (4.5 mg/kg) for postnatal rats but do span the oral repeated NOEL for inhibition of brain ChE activity (0.75 mg/kg) as reported by Zheng et al. (2000). However, it is unclear how these dosages compare to environmental exposure levels in children.
2.3 Behavioral Testing
On PND25, each rat was subjected to an emergence test similar to that described by Smith et al. (1998) and Lalonde and Strazielle (2008; 2009; 2010) with some modifications. The behavioral arena was a clear plexiglass open-field (28 × 28 × 46 cm). A black plexiglass barrier was present between the experimenter and the open-field to prevent the rat from observing the experimenter. The open-field contained a small stainless steel cup (7.5 × 10.25 cm) that attached to the floor of one corner with the open end facing into the box. The room was illuminated by fluorescent lights resulting in a light intensity of 300 lux. Each test session was filmed and recorded using Limelight Video Tracking Software (Actimetrics, Wilmette, IL). The rats were naive to the testing room and the testing apparatus. On the day of testing, each rat was removed from its cage and carried to the testing room. Having been handled daily during treatment, the rats do not require physical restraint. The rat was then placed into the cup facing the enclosed end of the cup. The rat remained in the arena until it emerged (all four paws) into the open-field or until a 300 sec time limit had elapsed. After each test, the open field and the cup were cleaned with 70% ethanol and water and dried with tissue.
2.4 Tissue Collection
An additional cohort of non-behavioral rats were used to determine the activities of ChE, FAAH, and MAGL, the levels of the endocannabinoids AEA and 2-AG, and the levels of the non-cannabinoid N-acylethanolamides palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) in the forebrain of each treatment group. Rat pups were treated as described above with either corn oil or 0.5, 0.75 or 1.0 mg/kg CPF and then sacrificed at 12 h after the last administration of CPF on PND16, which is the time for peak inhibition of ChE and FAAH as determined by our previous time course study (Carr et al., 2013). Brains were rapidly removed and dissected to obtain the forebrain (excluding the medulla and cerebellum). The forebrain was divided into right and left hemispheres and frozen on a stainless steel plate on top of dry ice, and maintained at −80°C until assay. The forebrain was the region used to determine the level of enzyme inhibition and endocannabinoid levels in our previous studies (Carr et al., 2013; 2014).
2.5 Enzyme Assays
The activities of ChE, FAAH, and MAGL in the left forebrain were measured as previously described (Carr et al., 2011). ChE activity was measured spectrophotometrically using a modification (Chambers et al., 1988) of Ellman et al. (1961) with acetylthiocholine as the substrate (1 mM final concentration) and 5,5'-dithiobis (nitrobenzoic acid) as the chromogen. FAAH and MAGL activities were measured using either 50 µM AEA or 2-AG as substrates, respectively, and arachidonic acid-d8 as internal standard. The amount of arachidonic acid formed was determined by LC-MS as previously described (Xie at al., 2010). Protein concentrations of tissue extracts were quantified with the Folin phenol reagent using bovine serum albumin as a standard (Lowry et. al., 1951). Specific activities were calculated as nmole (ChE) or pmole (FAAH or MAGL) product produced min−1 mg protein−1.
2.6 Endocannabinoid and N-acylethanolamide Levels
The right forebrain was subjected to determination of the levels of 2-AG, AEA, OEA, and PEA as previously described (Carr et al., 2013) with some modifications as follows. Following homogenization in 1:1 v/v ethyl acetate:0.1 M potassium phosphate (pH 7.0) containing known amounts of deuterated internal standards (2-AGd8, AEA-d8, OEA-d4, and PEA-d4), the homogenate was centrifuged to obtain the organic layer. The organic fraction was dried under a stream of N2 and the residue was resolubilized in 1:1 v/v water:methanol (100 µl). A 10 µl aliquot of the resolubilized lipid was injected onto an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm), equipped with pre-column (2.1× 5 mm, 1.7µm), interfaced with a Thermo Quantum Access triple-quadrupole mass spectrometer. The mobile phase was a blend of solvent A (0.1% acetic acid in water) and solvent B (0.1% acetic acid in methanol). The flow rate was 0.2 mL/min and analytes were eluted with the following gradient program: 0 min (15% A, 85% B), 0.5 min (15% A, 85% B), 3 min (5% A, 95% B), 6.9 min (5% A, 95% B), 7 min (15% A, 85% B), 9 min (15% A, 85% B). Single reaction monitoring (SRM) of each analyte was as follows: PEA, [M+H]+ m/z 300.2>62.4; PEA-d4, [M+H]+ m/z 304.3>62.5; OEA, [M+H]+ m/z 326.1>62.4; OEA-d4, [M+H]+ m/z 330.3>65.0; 2AG, [M+H]+ m/z 379.2>287.1; 2AG-d8, [M+H]+ m/z 387.0>293.5; AEA, [M+H]+ m/z 348.2>91.3; AEA-d8, [M+H]+ m/z 356.2>62.3. Scan times were 0.1 s per SRM and scan width was 0.01 m/z. The source settings were as follows: spray voltage, +3000V; vaporizer temp, 400°C; capillary temp, 325°C; sheath gas pressure, 25 psi; aux gas pressure, 2 psi.
Endocannabinoids are quantified by measuring the area under each chromatographic peak and comparing it to the area under the chromatographic peak for the appropriate deuterated internal standard, followed by correction for the ionization efficiency of AEA, 2AG, OEA and PEA relative to their deuterated standards (and the isotopic purity of the internal standard) which was empirically determined. The endocannabinoid amounts are normalized on the brain wet weight and expressed as pmol/g brain (AEA, OEA, and PEA) or nmol/g brain (2-AG).
2.7. Statistical Analysis
Statistical analysis was performed using SAS statistical package (SAS Institute Inc., Cary, NC). The sphericity of the body weight gain data was initially tested by analysis of variance (ANOVA) using the general linear model with a repeated measures paradigm and was found to violate the assumption of sphericity. Therefore, subsequent analysis by ANOVA using the Mixed procedure (Littell et al., 1996) was conducted with a repeat measures paradigm with a Huynh-Feldt covariance structure (Huynh Feldt, 1970), followed by separation of means using least significant difference. The analysis identified significant differences in the main effects (sex, treatment, and day) and all possible interactions.
Enzyme specific activities and endocannabinoid/N-acylethanolamine concentrations were analyzed by ANOVA using the Mixed procedure to determine significant differences in sex, treatment, and any sex × treatment interactions. All analyses included litter and sex × treatment × litter as random effects. Mean separation was performed by Least Significant Difference (LSD).
Behavioral data were subjected to Shapiro-Wilk’s test to check the normality of the residuals and homoscedasticity of data and the data were not found to be sufficiently normally distributed. Accordingly, to determine statistical differences between the four treatment groups, a method similar to the non-parametric Friedman's test was conducted. Each sex was considered a block. For each outcome, the data was first ranked within each sex. An ANOVA using the GLM procedure was then conducted on the ranked data with treatment and sex as the explanatory variables. If treatment was found to have a significant effect, differences in least squares means with Tukey adjusted p-values were used to make pairwise comparisons among the treatment levels. The criterion for significance was set at p < 0.05 for all parameters.
3. Results
No signs of overt toxicity or cholinergic hyperstimulation, such as lacrimation, diarrhea, or tremors, were observed following CPF exposure. With respect to weight gain in females (Figure 1A) and males (Figure 1B), there was no significant overall effect of sex but there was a significant overall effect of day (p<0.0001) and a significant sex × day interaction (p<0.0125). However, there was no significant overall effect of treatment or the presence of any significant interactions involving treatment. The lack of effects on weight gain observed in the 0.5 mg/kg and 1.0 mg/kg treatment groups agree with our previous observations (Carr et al., 2011; 2014).
Figure 1.
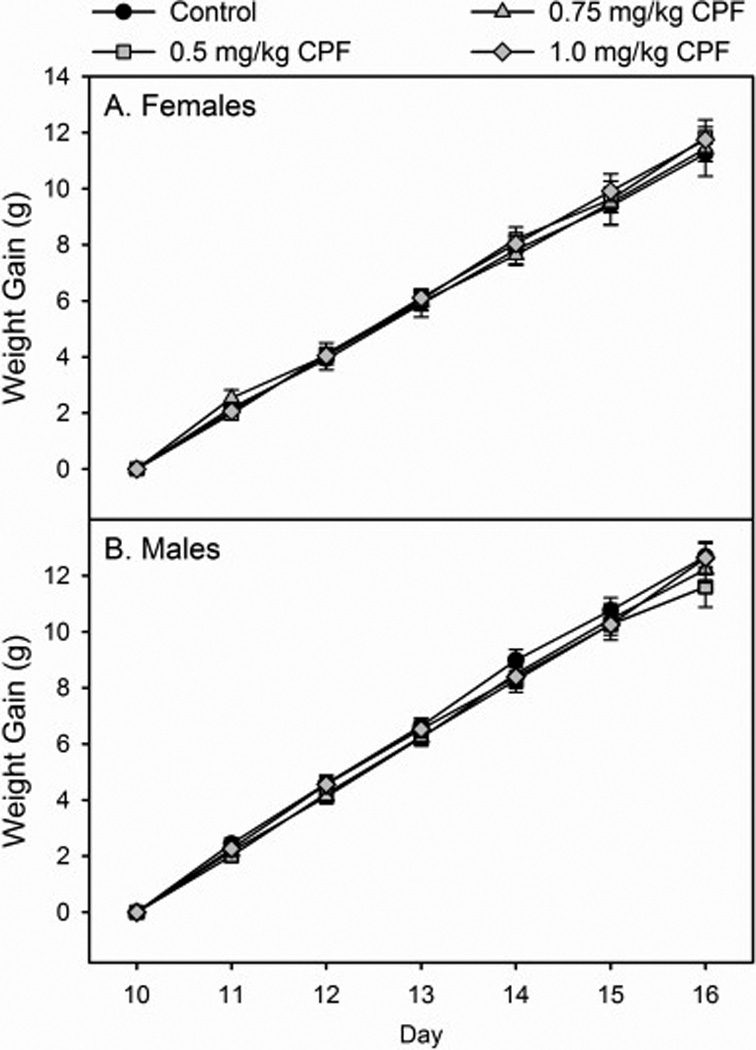
Rates of weight gain of (A) female and (B) male rat pups exposed daily from postnatal day 10 through 16 to either corn oil (control), 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as weight ± SE (n =13–23).
With respect to enzyme inhibition following developmental exposure to CPF, there was no significant overall effect of sex and no significant sex × treatment interaction with either ChE, MAGL, or FAAH. Therefore, male and female data were pooled for analysis for each enzyme. There was a significant effect of treatment for ChE (p<0.0001), MAGL (p<0.0062), and FAAH (p<0.0001). With respect to ChE, significant inhibition of activity (19%) was observed in the high dosage group but no significant inhibition was present in the low or medium dosage groups (Figure 2A). With respect to MAGL, significant inhibition of activity (12%) was observed in the high dosage group but no significant inhibition was present in the low or medium dosage groups (Figure 2B). With respect to FAAH, significant inhibition of activity was observed in the low (24%), medium (33%), and high (61%) dosage groups (Figure 2C). For the low and high dosages, these data are similar to levels of inhibition obtained previously (Carr et al., 2013; 2014).
Figure 2.
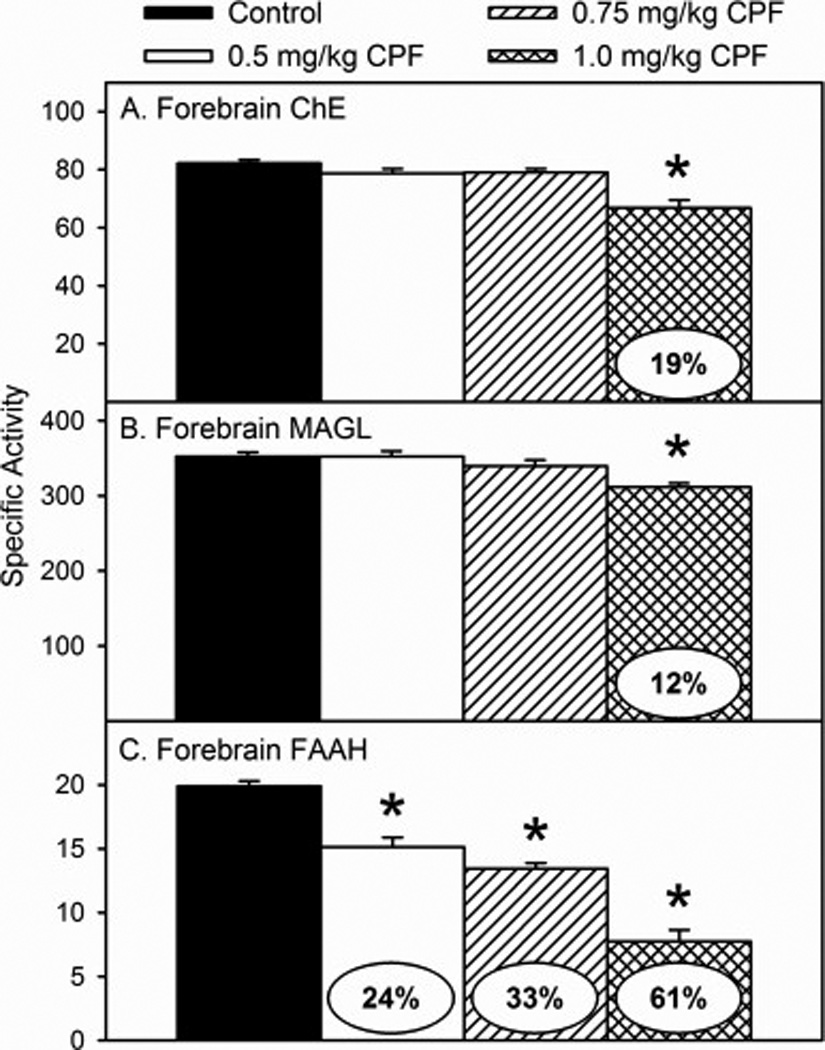
Specific activity of (A) cholinesterase (ChE), (B) monoacylglycerol lipase (MAGL), and (C) fatty acid amide hydrolase (FAAH) in the forebrain of rat pups at 12 h after last administration following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as nmole (ChE) or pmole (MAGL and FAAH) product min−1 mg protein−1 ± SE (n = 6–12). Percent inhibition for each treatment group as compared to its respective control is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from control.
With respect to endocannabinoid levels following developmental exposure to CPF, there was no significant overall effect of sex and no significant sex × treatment interaction with either AEA or 2-AG. Therefore, male and female data for each parameter were pooled for analysis. There was a significant effect of treatment for AEA (p<0.0001) and 2-AG (p<0.0008). With respect to AEA, there was a significant increase in the levels in the low (30%), medium (51%), and high (68%) dosage groups (Figure 3A). With respect to 2-AG, no effects were observed in the low dosage group but there was a significant increase in the levels in the medium (17%) and high (37%) dosage groups (Figure 3B). For the low and high dosages, these levels of increased AEA and 2-AG are similar to levels obtained previously (Carr et al., 2013; 2014).
Figure 3.
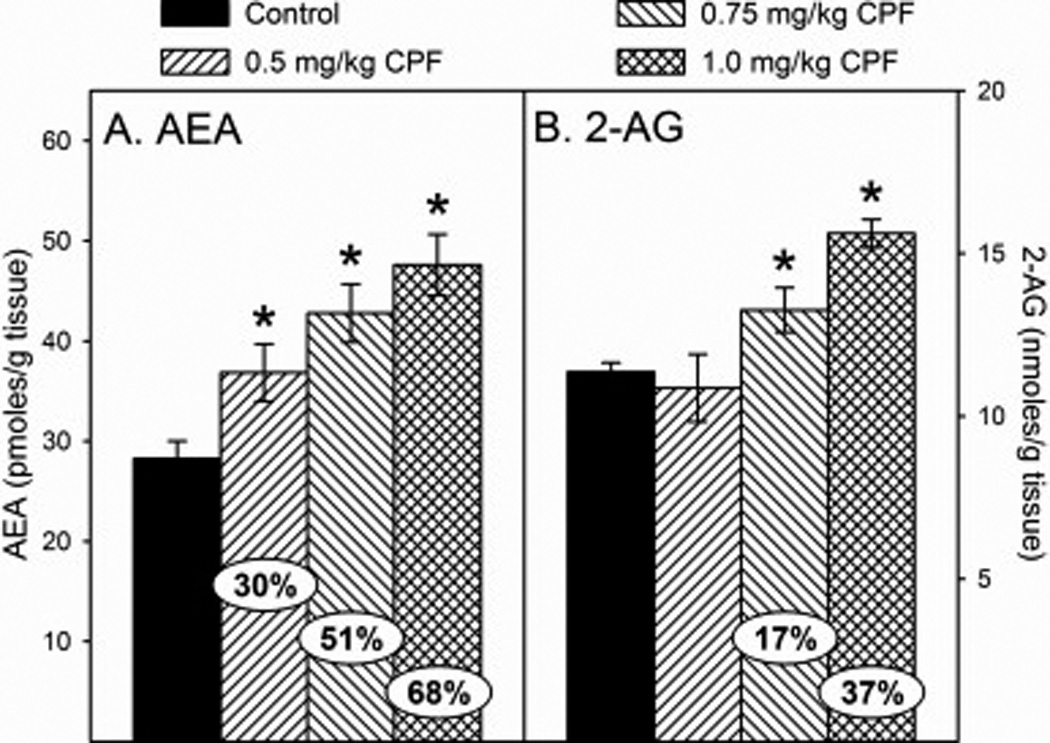
Levels of (A) arachidonoylethanolamide (AEA) and (B) 2-arachidonylglycerol (2-AG) in the forebrain of rat pups at 12 h after last administration following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as either pmoles/ g tissue (AEA) or nmoles/g tissue (2-AG) ± SE (n = 8–14). Percent change from control levels is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from the respective control.
With respect to the N-acylethanolamine levels following developmental exposure to CPF, there was no significant sex × treatment interaction but there was a significant effect of sex for both OEA (p<0.0002) and PEA (p<0.0017). Therefore, male and female data for each parameter were analyze separately. For OEA, there was a significant effect of treatment in females (p<0.0001) and males (p<0.0001) with OEA levels significantly increased in all treatment groups in both sexes. However, the level of increase appeared to be greater in females than in males with increases of 72% and 43%, respectively, in the low dosage group; 94% and 63%, respectively, in the medium dosage group; and 104% and 96%, respectively, in the high dosage group (Figures 4A and 4B). For PEA, there was a significant effect of treatment in females (p<0.0001) and males (p<0.0001) with PEA levels significantly increased in all treatment groups in both sexes. As with OEA, the level of increase of PEA appeared to be greater in females than in males with increases of 93% and 55%, respectively, in the low dosage group; 126% and 110%, respectively, in the medium dosage group; and 135% and 118%, respectively, in the high dosage group (Figures 5A and 5B).
Figure 4.
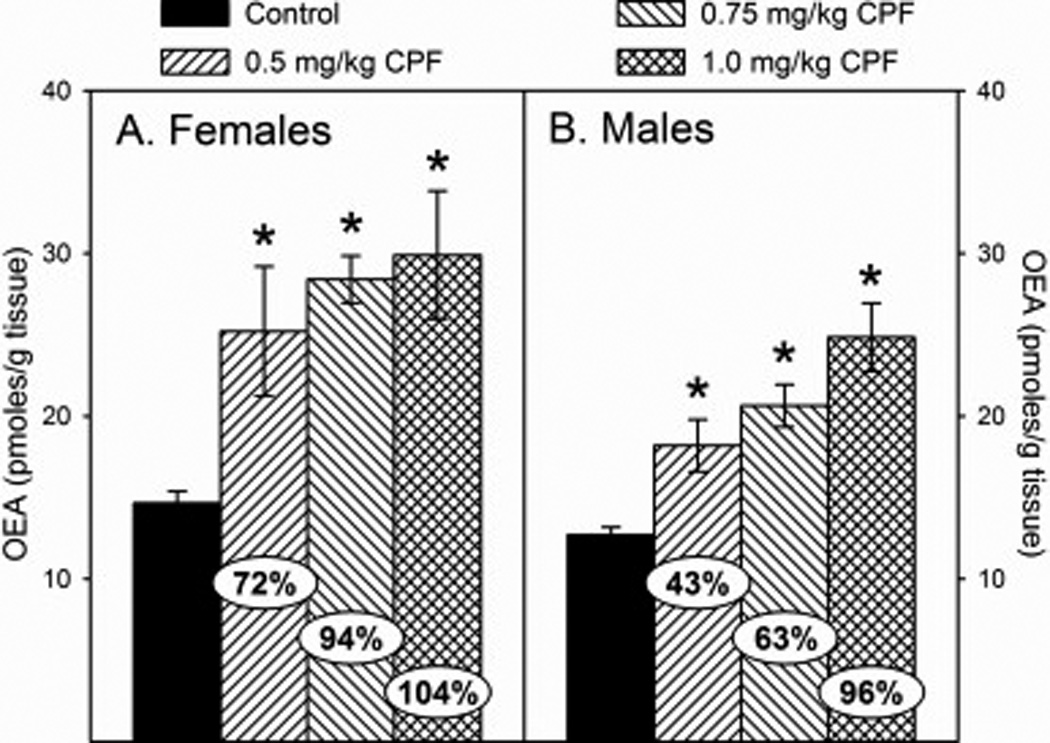
Levels of oleoylethanolamide (OEA) in the forebrain of (A) female and (B) male rat pups at 12 h after last administration following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as either pmoles/ g tissue ± SE (n = 4–7). Percent change from control levels is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from the respective control.
Figure 5.
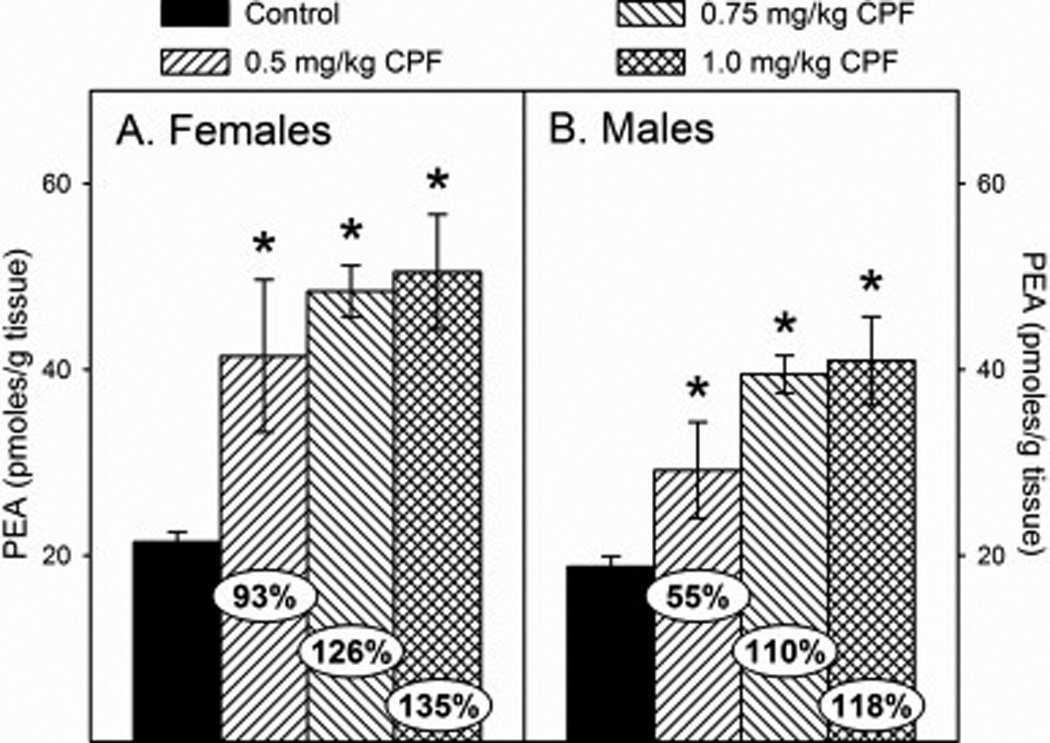
Levels of palmitoylethanolamide (PEA) in the forebrain of (A) female and (B) male rat pups at 12 h after last administration following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as either pmoles/ g tissue ± SE (n = 4–7). Percent change from control levels is presented in the oval overlaying the corresponding bar. Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from the respective control.
With respect to emergence behavior, there was an overall effect of sex (p<0.0027) and treatment (p<0.0044) but no significant sex×treatment interaction. This indicates that developmental exposure to CPF altered normal emergence behavior in a similar manner regardless of sex. However, even though not statistically significant, treated females appeared to remain in the cup for a slightly longer period of time prior to emergence than did treated males (Fig.6A and 6B). There was no observed dose-response effect on behavior; all dosage groups were statistically similar to one another, but all three dosages, low (p<0.0014), medium (p<0.0039), and high (p<0.0076), were significantly different from control.
Figure 6.
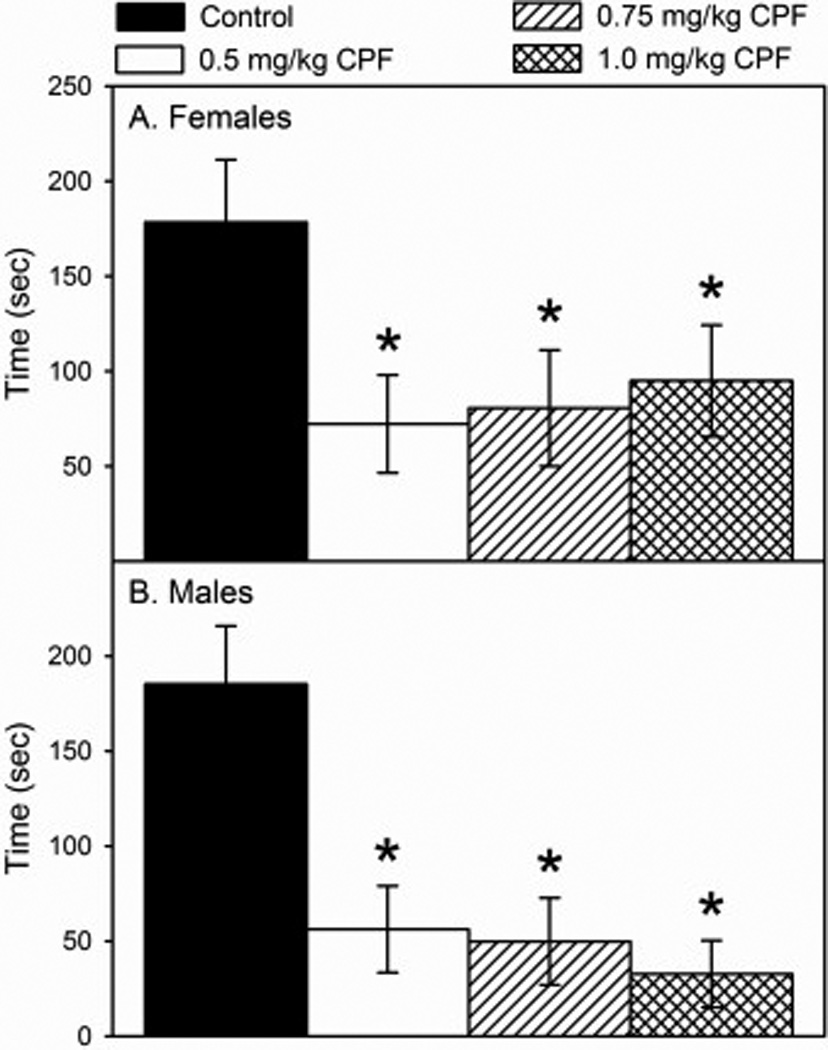
Time of emergence from a dark container into a novel aversive environment by (A) female and (B) male rats on postnatal day 25 following daily exposure from postnatal day 10 through 16 to either corn oil (control) or 0.5, 0.75, or 1.0 mg/kg chlorpyrifos (CPF). Values are expressed as time ± SE (n =13–23). Bars indicated with an asterisk (*) are statistically significant (p ≤ 0.05) from control.
4. Discussion
In the emergence test, the fact that the control rats preferred to remain in the safe area when placed in the novel environment indicated that the novel environment was an aversive one. It is very likely that the increased amount of light in this environment played a role. Rats are nocturnal and prefer dark areas but they do like to explore novel areas. The time it takes a rat to emerge from a dark area into a novel well-illuminated area is a measure of how it perceives its surrounding environment and how it reacts to that environment. The longer it takes for a rat to emerge is suggestive of a higher level of anxiety induced by the novel environment. The emergence test used in our study is the initial component that is measured in the light/dark test and this component has been reported to be a measure of anxiety-related behavior (Arrant et al., 2013). Previous studies employing the emergence test have demonstrated that the classical anxiolytic drug diazepam decreases the latency to emerge in Sprague Dawley rats (Pare et al., 2010) and the classical anxiolytic drug chlordiazepoxide decreases the latency to emerge in BALB/c mice (Lalonde et al., 2010). Our results suggest that the CPF-treated rats had reduced levels of anxiety compared to control rats.
Previous studies have demonstrated that developmental CPF exposure can disrupt behavior in tasks associated with anxiety. Anxiolytic responses were observed in the elevated plus maze behavioral test following postnatal exposure to CPF (Aldridge et al., 2005; Ricceri et al., 2006); however, gestational exposure resulted in either anxiogenic responses (Braquenier et al, 2010) or had no effect (Icenogle et al., 2004). Furthermore, both gestational and postnatal exposures resulted in altered parameters associated with social interactions suggesting changes in social anxiety (Ricceri et al., 2003; Venerosi et al., 2006; 2008; 2010; 2015; De Felice et al., 2014). Thus, our data are consistent with previous work demonstrating that developmental CPF exposure can disrupt behaviors that can be influenced by anxiety levels. Importantly, however, the lowest dosages which produced effects in the previous studies were either 1.0 mg/kg or greater which causes significant ChE inhibition as demonstrated in Figure 2A. Our study included dosages of CPF that did not inhibit ChE. Based on our biochemical data, the elevation of AEA, PEA, and/or OEA induced by the developmental exposure to CPF could play a role in the changes in behavior observed in the emergence test 9 days later
The lack of ChE inhibition observed in the medium dosage group agrees with Zheng et al. (2000) who reported that the brain No Observed Effect Level (NOEL) for significant change in ChE activity following repeated developmental exposure was 0.75 mg/kg. No significant inhibition was present with the lower dosage (0.5 mg/kg) and 19% inhibition was present with 1.0 mg/kg. Thus, our data indicate that the decreased anxiety-like behavior observed in preadolescent rats exposed developmentally to CPF occurs at dosages that either induce low levels of brain ChE inhibition or do not induce any inhibition. This suggests that the behavioral outcome is the result of the CPF exposure impacting a non-cholinergic target. In this study, all of our biochemical data was determined on PND16 after 7 days of treatment. It is possible that significant ChE inhibition could have been present during the earlier ages following exposure to the low dosages used in this study. However, Zheng et al. (2000) reported that the NOEL for significant alteration in brain ChE activity following acute oral exposure to CPF was 1.5 mg/kg on PND7. This suggests that none of our dosages at or below 1 mg/kg would likely have induced brain ChE inhibition following initial exposure on PND10. What is not clear is whether ChE inhibition is present after repeated exposures at the earlier ages such as PND11 or PND12. Future studies will be conducted to investigate this possibility.
At all three dosages of CPF, there was significant inhibition of FAAH activity but little effect on MAGL activity. Based on this and our previous studies, the inhibition of FAAH activity between 0.5–1.0 mg/kg is dose-related. There was also a dose-related increase in the levels of AEA and 2-AG. The significant increase in 2-AG in the medium dosage group was not accompanied by inhibition of MAGL. Our previous work demonstrated that in preweanling rat brain, only ~45% of 2-AG hydrolysis is due to the action of MAGL (Carr et al., 2011). This is in contrast to that observed in adult mice which is ~85% (Blankman et al., 2007). Other enzymes, including FAAH, have previously been reported to degrade 2-AG in vitro (Blankman et al., 2007) and it is possible that the inhibition of these enzymes could have disrupted normal 2-AG metabolism. While there were dose-related changes in the biochemical parameters measured in this study, there was not a dose-related response in emergence behavior. This suggests that all three dosages utilized in the present study are above the NOEL for the alteration of emergence behavior in preadolescent rats following developmental CPF exposure.
It is well documented that increasing AEA levels in adult brain, either by genetic deletion or pharmacological inhibition of FAAH activity, leads to anxiolytic behavior in multiple tests of anxiety (Kathuria et al., 2003; Patel and Hillard, 2006; Naidu et al., 2007; Moreira et al., 2008; Kinsey et al., 2011). As stated earlier, OEA and PEA are also substrates for FAAH and genetic deletion or inhibition of FAAH activity increases their levels (Cravatt et al., 1996; Fegley et al., 2005). OEA and PEA both exhibit antidepressant effects when administered prior to conducting animal tests of anxiety/depression (Crupi et al., 2013; Yu et al., 2011; 2015). In these studies, the dosages of FAAH inhibitor given to adult rodents were designed to produce a high level of FAAH inhibition in the brain and the testing occurred shortly after administration; however, little work has been done to investigate the persistent effects of developmental exposure to an inhibitor of FAAH activity. Exposure to the FAAH inhibitor URB597 during adolescence altered the levels of the CB1 receptor in adult rat brain (Marco et al., 2009) and led to increased anhedonia in adults (Macri et al., 2012). Gestational exposure to URB597 led to subtle behavioral deficits in the offspring after they reached adult ages (Wu et al., 2014). However, the dosages used in those developmental studies were also designed to produce substantial FAAH inhibition. In contrast, the highest dosage of CPF utilized in the present study only moderately inhibited FAAH activity (61%) with even lower inhibition at the lower two dosages. Based on our previous studies using the highest dosage of CPF (1.0 mg/kg), restoration of AEA levels to control levels occurred within 2 days following the last administration (Carr et al., 2013). Thus, at the time of behavioral testing in the current study (9 days following the last administration), the CPF-induced reduction in the activity of FAAH and elevated AEA levels are likely not a direct factor in the observed behavioral outcome. Currently, it is unclear if OEA or PEA levels follow a similar pattern of recovery and future studies will determine this. However, based on the work of Fegley et al. (2005), the patterns of recovery of AEA, OEA, and PEA levels following a single administration of URB597 in adult rats were very similar to each other suggesting that the pattern of recovery of OEA and PEA to control levels may not be that different from that of AEA in our repeated CPF exposure model.
One possible mechanism to the observed behavioral outcome is that the elevated AEA levels that occur during the exposure period induce changes in the endocannabinoid system that result in persistent negative effects on normal neurological processes controling the response to an aversive novel environment. Previous work demonstrated the long term effects caused by developmental exposure to the FAAH inhibitor URB597 (Marco et al., 2009; Macri et al., 2012; Wu et al., 2014), making this a plausible hypothesis. However, this mechanism does not take the elevated OEA and PEA levels into consideration. OEA and PEA do not activate the CB1 receptor, rather they are agonists to the peroxisome proliferator activated receptor alpha (PPAR-α; Fu et a1., 2003 and Lo Verme et al., 2005). There is, however, a connection between the endocannabinoid system and PPAR-α with respect to cognitive function. Previous studies have reported memory enhancement in adult male rats following administration of a FAAH inhibitor and abrogation of the enhancement by pretreatment with a PPAR-α antagonist (Mazzola et al., 2009). In addition, the memory enhancement was also blocked by pretreatment with a CB1 antagonist suggesting a synergism between PPAR-α and CB1. At this time, the role of PPAR-α in anxiety-like behavior, if any, is not clear. However, when considering the mechanistic basis for the persistent effects of developmental CPF exposure on anxiety-like behavior, the potential role of elevated levels of OEA and PEA, in addition to the elevated levels of AEA, should be considered as possibly exerting negative effects on the developing brain.
In conclusion, our previous findings indicated that developmental exposure to low levels of CPF during the preweanling exposure period alters endocannabinoid metabolism resulting in the accumulation of the endocannabinoids in the brain. Here, we demonstrate that this exposure paradigm also leads to accumulation of two non-cannabinoid N-acylethanolamides. Moreover, when tested 9 days after cessation of CPF treatment, this exposure disrupted anxiety-like behavior in preadolescent rats. Future studies will employ multiple behavioral tests to provide a more complete and reliable picture of the effect that low level developmental CPF exposure has on the anxiety state of an animal. To date, our data demonstrate that exposure to CPF, at dosages that do not yield significant inhibition of ChE, can induce biochemical changes in the endocannabinoid system and cause persistent disruption of behavior. The fact that behavioral effects have been demonstrated at dosages lower than those previously reported could contribute to the risk assessment of CPF. Nevertheless, additional studies are required to determine whether there is a mechanistic link between the neurochemical effects and the behavioral effects prior to considering the endocannabinoid system as a mechanistic target for use as an endpoint for risk assessment.
Highlights.
Repeated developmental chlorpyrifos exposure alters brain endocannabinoid signaling.
Preweanling exposure to chlorpyrifos inhibits FAAH and increases AEA, PEA, and OEA levels
Preweanling exposure to chlorpyrifos alters anxiety-like behavior in juveniles
These effects occur in the absence of brain ChE inhibition.
Acknowledgments
The authors acknowledge the statistical expertise of Dr. Robert Wills. Research was supported by the Mississippi Agricultural and Forestry Experiment Station (MAFES), the College of Veterinary Medicine, Mississippi State University, and NIH R15ES023162. LC-MS/MS analyses was supported by R15ES015348-02. This paper is MAFES publication #12709 and Center for Environmental Health Sciences publication #150.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice: a review. Anim. Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, Quandt SA. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environ. Health Perspect. 2007;115:1254–1260. doi: 10.1289/ehp.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrant AE, Schramm-Sapyta NL, Kuhn CM. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013;256:119–127. doi: 10.1016/j.bbr.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braquenier JB, Quertemont E, Tirelli E, Plumier JC. Anxiety in adult female mice following perinatal exposure to chlorpyrifos. Neurotoxicol. Teratol. 2010;32:234–239. doi: 10.1016/j.ntt.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Carr RL, Borazjani A, Ross MK. Effect of developmental chlorpyrifos exposure on endocannabinoid metabolizing enzymes in the brain of juvenile rats. Toxicol. Sci. 2011;122:112–120. doi: 10.1093/toxsci/kfr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Adams AL, Kepler DR, Ward AB, Ross MK. Induction of endocannabinoid levels in juvenile rat brain following developmental chlorpyrifos exposure. Toxicol Sci. 2013;135:193–201. doi: 10.1093/toxsci/kft126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Graves CA, Mangum LC, Nail CA, Ross MK. Low level chlorpyrifos exposure increases anandamide accumulation in juvenile rat brain in the absence of brain cholinesterase inhibition. Neurotoxicology. 2014;43:82–89. doi: 10.1016/j.neuro.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW. Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Commun. 1988;3:85–92. [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev. Cogn. Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Crupi R, Paterniti I, Ahmad A, Campolo M, Esposito E, Cuzzocrea S. Effects of palmitoylethanolamide and luteolin in an animal model of anxiety/depression. CNS Neurol Disord Drug Targets. 2013;12:989–1001. doi: 10.2174/18715273113129990084. [DOI] [PubMed] [Google Scholar]
- De Felice A, Venerosi A, Ricceri L, Sabbioni M, Scattoni ML, Chiarotti F, Calamandrei G. Sex-dimorphic effects of gestational exposure to the organophosphate insecticide chlorpyrifos on social investigation in mice. Neurotoxicol. Teratol. 2014;46:32–39. doi: 10.1016/j.ntt.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ. Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Grube A, Donaldson D, Kiely T, Wu L. Pesticides industry sales and usage, 2006 and 2007 market estimates. Washington, DC: United States Environmental Protection Agency EPA 733-R-11-001; 2011. [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurement designs have exact F–distributions. J. Am. Statist. Assoc. 1970;65:1582–1589. [Google Scholar]
- Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol. Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, O’Neal ST, Long JZ, Cravatt BF, Lichtman AH. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav. 2011;98:21–27. doi: 10.1016/j.pbb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children's pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ. Health Perspect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods. 2008;171:48–52. doi: 10.1016/j.jneumeth.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. The relation between open-field and emergence tests in a hyperactive mouse model. Neuropharmacology. 2009;57:722–724. doi: 10.1016/j.neuropharm.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Relations between open-field, elevated plus-maze, and emergence tests in C57BL/6J and BALB/c mice injected with GABA- and 5HT-anxiolytic agents. Fundam. Clin. Pharmacol. 2010;24:365–376. doi: 10.1111/j.1472-8206.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Liu J, Parsons L, Pope C. Comparative effects of parathion and chlorpyrifos on extracellular endocannabinoid levels in rat hippocampus: influence on cholinergic toxicity. Toxicol. Appl. Pharmacol. 2013;272:608–615. doi: 10.1016/j.taap.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Parsons L, Pope C. Comparative effects of parathion and chlorpyrifos on endocannabinoid and endocannabinoid-like lipid metabolites in rat striatum. Neurotoxicology. 2015;50:20–27. doi: 10.1016/j.neuro.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Macri S, Ceci C, Canese R, Laviola G. Prenatal stress and peripubertal stimulation of the endocannabinoid system differentially regulate emotional responses and brain metabolism in mice. PLoS One. 2012;7:e41821. doi: 10.1371/journal.pone.0041821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Rubino T, Adriani W, Viveros M-P, Parolaro D, Laviola G. Long-term consequences of URB597 administration during adolescence on cannabinoid CB1 receptor binding in brain areas. Brain Res. 2009;1257:25–31. doi: 10.1016/j.brainres.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ. Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-α nuclear receptors. Learn. Mem. 2009;16:332–337. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoiddegrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Nallapaneni A, Liu J, Karanth S, Pope C. Pharmacological enhancement of endocannabinoids signaling reduces the cholinergic toxicity of diisopropylfluorophosphate. Neurotoxicology. 2008;29:1037–1043. doi: 10.1016/j.neuro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Blankman JL, Simon GM, Fujioka K, Issa RS, Ward AM, Cravatt BF, Casida JE. Activation of the endocannabinoid system by organophosphorus nerve agents. Nat. Chem. Biol. 2008;4:373–378. doi: 10.1038/nchembio.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura DK, Casida JE. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J. Agric. Food Chem. 2011;59:2808–2815. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J. Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt S, Kluczynski J. The emergence test: effects of psychotropic drugs on neophobic disposition in Wistar Kyoto (WKY) and Sprague Dawley rats. Prog. Neuropsychopharmacol. Biol Psychiatry. 2010;25:1615–1628. doi: 10.1016/s0278-5846(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE. Fatty acid amide hydrolase inhibition by neurotoxic rganophosphorus pesticides. Toxicol Appl Pharmacol. 2001;173:48–55. doi: 10.1006/taap.2001.9175. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol. Appl. Pharmacol. 2006;211:78–83. doi: 10.1016/j.taap.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Segall Y, Nomura DK, Casida JE. Selective inhibitors of fatty acid amide hydrolase relative to neuropathy target esterase and acetylcholinesterase: toxicological implications. Toxicol. Appl. Pharmacol. 2002;179:57–63. doi: 10.1006/taap.2001.9342. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. USA. 2012;109:7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol. Appl. Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol. Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE. Long-Term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environ. Health. Perspect. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ. Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: Similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ. Health Perspect. 2007;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol. 2008a;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull. 2008b;77:404–111. doi: 10.1016/j.brainresbull.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Calamandrei G, Ricceri L. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol. Teratol. 2006;28:466–471. doi: 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L, Calamandrei G. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol. Teratol. 2008;30:468–474. doi: 10.1016/j.ntt.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Rungi A, Sanghez V, Calamandrei G. Gestational exposure to the organophosphate chlorpyrifos alters social-emotional behavior and impairs responsiveness to the serotonin transporter inhibitor fluvoxamine in mice. Psychopharmacology. 2010;208:99–107. doi: 10.1007/s00213-009-1713-2. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Tait S, Stecca L, Chiarotti F, De Felice A, Cometa MF, Volpe MT, Calamandrei G8, Ricceri L. Effects of maternal chlorpyrifos diet on social investigation and brain neuroendocrine markers in the offspring - a mouse study. Environ. Health. 2015;14:32. doi: 10.1186/s12940-015-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT. Role of endogenous cannabinoids in cognition and emotionality. Mini Rev. Med. Chem. 2005;5:659–670. doi: 10.2174/1389557054368763. [DOI] [PubMed] [Google Scholar]
- Wu CS, Morgan D, Jew CP, Haskins C, Andrews MJ, Leishman E, Spencer CM, Czyzyk T, Bradshaw H, Mackie K, Lu HC. Long-term consequences of perinatal fatty acid amino hydrolase inhibition. Br. J. Pharmacol. 2014;171:1420–1434. doi: 10.1111/bph.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Borazjani A, Hatfield MJ, Edwards CC, Potter PM, Ross MK. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: role of carboxylesterases 1 and 2. Chem Res Toxicol. 2010;23:1890–1904. doi: 10.1021/tx1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HL, Deng XQ, Li YJ, Li YC, Quan ZS, Sun XY. N-palmitoylethanolamide, an endocannabinoid, exhibits antidepressant effects in the forced swim test and the tail suspension test in mice. Pharmacol. Rep. 2011;63:834–839. doi: 10.1016/s1734-1140(11)70596-5. [DOI] [PubMed] [Google Scholar]
- Yu HL, Sun LP, Li MM, Quan ZS. Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neurosci Lett. 2015;593:24–28. doi: 10.1016/j.neulet.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Olivier K, Won YK, Pope CN. Comparative cholinergic neurotoxicity of oral chlorpyrifos exposures in preweanling and adult rats. Toxicol. Sci. 2000;55:124–132. doi: 10.1093/toxsci/55.1.124. [DOI] [PubMed] [Google Scholar]


