Abstract
Scope
Reduced expression of tumor suppressor genes (TSG) increases the susceptibility to breast cancer. However, only a small percentage of breast tumors is related to family history and mutational inactivation of TSG. Epigenetics refers to non-mutational events that alter gene expression. Endocrine disruptors found in foods and drinking water may disrupt epigenetically hormonal regulation and increase breast cancer risk. This review centers on the working hypothesis that agonists of the aromatic hydrocarbon receptor (AHR); bisphenol A (BPA); and arsenic compounds, induce in TSG epigenetic signatures that mirror those often seen in sporadic breast tumors. Conversely, it is hypothesized that bioactive food components that target epigenetic mechanisms protect against sporadic breast cancer induced by these disruptors.
Methods and results
This review highlights 1) overlaps between epigenetic signatures placed in TSG by AHR-ligands, BPA, and arsenic with epigenetic alterations associated with sporadic breast tumorigenesis; and 2) potential opportunities for prevention of sporadic breast cancer with food components that target the epigenetic machinery.
Conclusions
Characterizing the overlap between epigenetic signatures elicited in TSG by endocrine disruptors with those observed in sporadic breast tumors may afford new strategies for breast cancer prevention with specific bioactive food components or diet.
Keywords: Epigenetics, Endocrine Disruptors, Tumor Suppressor Genes, Breast Cancer, Food Components, Cancer Prevention
1 Introduction
Sporadic breast tumors represent the vast majority of breast cancer cases; are not related to germline mutations in tumor suppressor genes (TSG); and usually occur later in life [1, 2]. Epigenetics refers to changes in gene expression without changes in the DNA sequence. These include alterations in DNA methylation, histone posttranslational modifications, recruitment of chromatin remodeling factors, and expression of micro (miR) and long (lncR) non-coding RNA [3]. Importantly, epigenetic modifications such as CpG methylation may be conserved through cycles of cell division and transmitted to cell progenies. The accumulation of epigenetic changes in TSG may contribute to the “cancer epigenome” in the same individual or subsequent generations even after removal of the stimuli. On the other hand, epigenetic changes are potentially reversible and, thus, offer vast opportunities for cancer therapy [4].
Sporadic tumors in which TSG are silenced often have a phenotype that mirrors that of hereditary tumors in which the same TSG is silenced through mutation. This is the case of the breast cancer susceptibility gene, BRCA-1 whose repression through CpG methylation in sporadic breast tumors confers a “BRCAness” tumor phenotype similar to that generally seen in BRCA-1 mutation carriers [5]. Therefore, the main objective of this review was to develop a working hypothesis that endocrine disruptors induce in TSG epigenetic signatures that mirror those often seen in sporadic breast tumors. To develop this hypothesis, we focused on agonists of the aromatic hydrocarbon receptor (AHR) [2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), polycyclic aromatic hydrocarbons (PAH), polychlorinated biphenyls, and phthalates] [6]; bisphenol A (BPA) [7], and arsenic compounds[8] because they are known endocrine disruptors and ubiquitous in the environment, foods, and drinking water. Conversely, we hypothesized that food components that target the epigenetic machinery protect against alterations in TSG and mammary tumorigenesis associated with exposure to these xenobiotics. We preceded the presentation of our literature results for each xenobiotic with examples of epigenetic disruption in sporadic breast cancers of tumor suppressor proteins, miR, and lncR.
2 Methodology
We conducted a systematic review of the literature published in PubMed combining the search terms “tumor suppressor genes”, “breast”, “cancer”, and “epigenetic”, which yielded 442 articles since 1997. We also consulted the TSGene 2.0 Database (available at http://bioinfo.mc.vanderbilt.edu/TSGene/), which at the time of the access, listed 329 literature records related to breast cancer [9]. For PubMed searches of studies related to non-coding TSG, we used the terms: “breast”, “cancer”, “methylation”, and “microRNA” or “long non-coding RNA”. We followed up with a PubMed search of studies reporting on “breast”, “cancer”, “epigenetic” and “AHR’, “BPA” or “arsenic/arsenite”. Finally, we searched for studies reporting on prevention by food components of epigenetic signatures placed in TSG by AHR-ligands, BPA, and arsenic compounds in preclinical models and breast tumors. For comparison, we included examples of studies related to other endocrine-responsive tissues to further validate the role of xenobiotics as epigenetic disruptors of TSG, and prevention of sporadic tumorigenesis with food components.
3 Results
3.1 Mechanisms of epigenetic disruption of TSG in sporadic breast cancer
3.1.1 Tumor suppressor proteins
The BRCA-1 gene is perhaps one of the best examples of a breast cancer susceptibility gene often silenced in sporadic tumors. The BRCA-1 protein is involved in transcriptional control [10, 11] and repair of DNA damage [12]. Although mutations in BRCA-1 confer a high probability (55–65%) of developing breast cancer by age 70, germline BRCA-1 mutations account for only a small fraction (5%–10%) of all female breast cancers, and an estimated 5%–20% of male breast tumors [13–15]. Interestingly, most breast cancers that are categorized as sporadic, have low or undetectable BRCA-1 expression in the absence of BRCA-1 mutations [16–20]. The extent of BRCA-1 DNA methylation in sporadic breast tumors varies from ~10 to 85% based on tumor type with higher DNA methylation usually found in more invasive, compared to lobulo-alveolar, breast tumors [21, 22]. The coincident reduced expression, and increased CpG methylation, of BRCA-1 have also been described in earlier-onset and high-grade ovarian tumors [23–26].
The loss of BRCA-1 expression in breast tumors is almost invariably associated with reduced expression of estrogen receptor (ER)-α [27]. Familial and sporadic breast tumors with low BRCA-1 expression cluster with the basal-like and triple-negative (TNBC) phenotype with reduced expression of ERα, progesterone receptor (PR), and epidermal growth factor receptor-2 (HER-2) [5]. Interestingly, BRCA-1-mutation and sporadic TNBC with hypermethylated BRCA-1 tend to be refractory to endocrine therapies based on antagonists of the ERα (i.e., tamoxifen) [12]. One mechanism contributing to antiestrogen resistance is CpG hypermethylation of ESR1 (ERα) [28, 29], which has been documented in ~40% of breast cancer cases [30, 31], and especially, in TNBC [32]. Similarly, resistance of mammary tumors to antiprogestins has been correlated with loss of PR expression accompanied with higher expression of DNA methyltransferase (DNMT)-1 and DNMT-3b [33]. Conversely, inhibitors of DNMT [34] and histone deacetylase (HDAC) [35] enzymes were shown to favor re-expression of the ERα and PR, and restore breast cancer cells responsiveness, respectively to tamoxifen [36] and antiprogestin [33] treatment. These observations support the hypothesis that epigenetic dysregulation of tumor suppressor (i.e. BRCA-1) and hormone receptor (i.e. ESR1, PGR, ERBB2) genes combined with increased expression of DNMT contribute to sporadic breast tumorigenesis.
Posttranslational modifications of histones contribute to epigenetic dysregulation of TSG. For example, CpG hypermethylation of RASSF1A, PEMT, SFRP, and RKIP in breast tumors [9] has been linked to increased association of these genes with histone-3 trimethylated at lysine-9 (H3K9me3) and H3K27me3. These are repressive histone marks placed respectively by SUV/SET/G9 [37] and polycomb-2 enhancer of zeste-2 protein (EZH2) [38] methylases, which are associated with loss of active acetylated histone marks (i.e. H3K9Ac on PEMT). Conversely the combination of demethylating agents (5-aza-2’deoxycytidine) and inhibitors (i.e. GSK146) of histone methyltransferase EZH2 have been shown to restore expression of TSG and exert inhibitory effects on cell proliferation [39]. Therefore, the contribution of both DNA methylation and histone modifications should be considered when developing models of epigenetic disruption of TSG in breast tissue [40]. This concept may be extended to other endocrine-responsive tissues such as endometrium in which tumor development and resistance to progestin therapies have been linked in ~65–85% of the cases to concurrent DNA hypermethylation of PGR and its association with repressive H3K27me3 mark [41].
Loss of cell cycle checkpoints compromises fidelity of DNA replication prior to cell division. One such checkpoints is p16, a cyclin-dependent kinase (CDK) inhibitor of cyclin D1 (CCND1) [42, 43]. Unlike the tumor suppressor p53, whose gene is inactivated by mutations in ~50% of all human cancers, p16INK4a is often silenced in breast tumors through aberrant CpG promoter methylation. Epigenetic repression of p16INK4a usually occurs at the early stages of sporadic breast cancer development [44], and is linked to overexpression of oncogenic CCND1. Similarly, reduced expression of p21 due to hypermethylation of the p21/CIP1/WAF1 gene is an epigenetic alteration related to loss of cell cycle control that is observed in a large fraction (~80%) of breast tumors [45]. The hypermethylation of p21CIP1/WAF1 and p16INK4 have been correlated to shortening of telomeres in the breast tumor grades II and III. Telomeres are short DNA sequences present in many copies at the ends of eukaryotic chromosomes. They are essential for maintaining genomic integrity and stability [46]. Epigenetic repression of the glutathione-S-transferase-Pi (GSTP1) in luminal progenitor cells, is an event that has been causally linked to genomic instability and the pathogenesis of luminal-A, luminal-B, and HER-2-enriched, breast tumors [47]. Notably, reduced expression of GSTP1 has been reported in ~ 35% of breast tumor biopsies (74/215 cases) [48] in conjunction with hypermethylation of TSG (i.e. BRCA-2, WIT-1) and increasing grade of ductal carcinoma in situ (DCIS) [49].
3.1.2 miR and lncR
The development of sporadic breast tumors has been related, at least in part, to epigenetic dysregulation of tumor suppressor miR that bind to 3’-untranslated region (UTR) of mRNA encoding for factors that promote cancer processes [50]. For example, by targeting the 3’-UTR of FOSL1 (FRA-1), the miR-34a/c prevents migration and invasion processes. However, expression of miR-34a/c is significantly reduced through CpG hypermethylation in metastatic breast cancer cells, and human primary breast tumors [51]. Similarly, silencing of miR-122 has been related to loss of tumor suppressor functions targeting the 3’UTR of IGF1R, thus leading to increased expression of insulin-like growth factor-1 receptor (IGF1R) and activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/ mammalian target of rapamycin (mTOR)/p70S6K proliferative pathway [52]. In general, the development of sporadic breast cancer has been correlated to epigenetic repression of miR that activate apoptosis (miR-9-3) [53]; or inhibit proliferation (miR-148a, miR-152) [54], invasion (miR-125b) [55], metastasis (miR-126, miR-31) [56, 57]; and chemoresistance (miR-149) [58].
The concept of “BRCAness” between familial and sporadic breast tumors may be applicable to miR expression signatures. For example, silencing of members of the let-7 family (let-7a-3, let-7c, let-7e-3p) has been correlated with the development of high grade hormone receptor-negative tumors [59–61], metastasis [62], and poor outcome [63, 64]. Similarly, expression of tumor suppressor let-7a was markedly reduced in BRCA-1 mutation carriers. These findings suggested that profiling the non-coding RNA signatures of hereditary breast cancers [65] may provide biomarkers for the prediction of sporadic breast tumors that develop as a consequence of silencing of miR with tumor suppressor functions [66].
Risk factors for sporadic breast cancer include abnormalities in the expression of miR genes that regulate expression of DNMT. For instance, the reduced expression and CpG hypermethylation of BRCA-1 in sporadic breast tumors has been linked to overexpression of DNMT-3b, [67], an enzyme that is in involved in de novo DNA methylation. DNMT-3b expression is under the negative control of miR-203, whose gene, however, has been shown to be silenced through CpG hypermethylated in a subset of breast cancer cell lines [68]. Therefore, the loss of post-transcriptional repression of DNMT due to silencing of tumor suppressor miR represents a mechanism that may amplify epigenetic dysregulation of TSG.
Long non-coding RNA comprise approximately 80% of all non-coding RNA [69]. Although studies of epigenetic regulation of lncR in breast cancer are somewhat limited, a recent report indicated that in breast tumors DNA methylation in lncR was more frequent than that in protein-coding genes, and associated with increased levels of H3K27me3. Interestingly, intergenic lncR comprised ~50% of the aberrantly methylated non-coding RNA promoters [70]. Therefore, silencing of lncR may help distinguishing between breast cancer patients from healthy controls. For example, the lncR ENSG00000232821 next to TWST1, which encodes for a transcription factor involved in differentiation, was reported to be hypermethylated in breast tumors. Other lncR thought to have breast tumor suppressor roles include H19, whose aberrant hypermethylation has been described in invasive breast cancers compared with healthy breast tissue [71]. Similarly, aberrant promoter methylation of LINC00472 was associated with decreases survival in patients with grade II breast cancer [72]. In breast cancer cells, the transcriptional repression via CpG methylation of the lncR LED was found to compromise the function of p53 in control of cell cycle arrest [73]. Furthermore, the epigenetic repression via CpG methylation of “host” lncR may lead to aberrant silencing of nested miR. For example, promoter hypermethylation and silencing of LOC554202 may lead to concurrent repression of miR-31, which is transcribed from within the intronic sequence of LOC554202. This phenomenon has been observed in breast tumors and breast cancer cell lines of the basal-like subtype [65].
3.2 Epigenetic disruptors and breast cancer
3.2.1 AHR agonists
Agonists of the AHR are ubiquitous in the environment and include industrial xenobiotics, dietary compounds, metabolites of fatty acids, and photoproducts generated in the skin from ultraviolet radiation [74]. Studies have associated induction and/or constitutive overexpression of AHR with activation of cancer processes such as proliferation, epithelial-to-mesenchymal transition (EMT), DNA damage, inflammation, migration, angiogenesis, and metastasis; and inhibition of apoptosis [75, 76] (Figure 1). Increased AHR expression has been documented in DCIS; invasive ductal breast tumors; in conjunction with loss of p53 expression; and to a lower extent, in invasive lobular carcinomas [77]. Interestingly, some studies suggested a protective effect of AHR activation during gestation against mammary tumorigenesis later in life [78], or even discounted a role for the AHR in induction of proliferation, migration, invasion, and estrogen-dependent tumorigenesis [79]. These apparently conflicting results may be due to differences in ER status, type of AHR agonist, and timing, duration, and dose of exposure. For example, in reproductive organs, studies with AHR agonists reported antiestrogenic effects in the presence of estrogens, and estrogenic effects in the absence of estrogens [80].
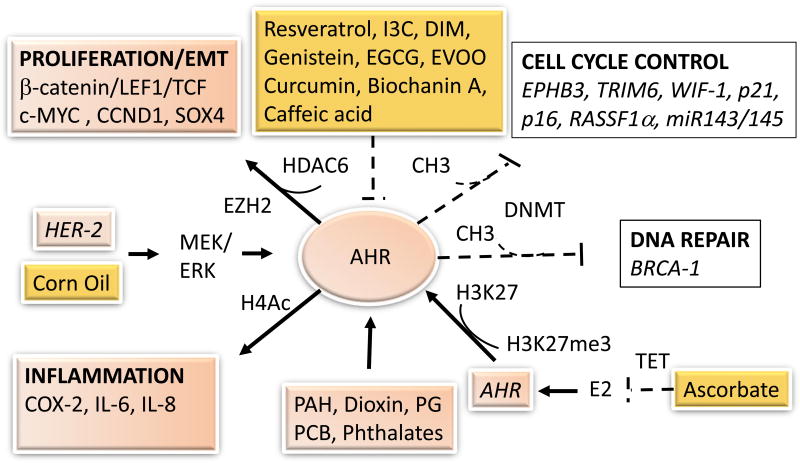
Figure 1.
Epigenetic disruption by AhR agonists and breast cancer prevention with food components. Exposure to environmental polycyclic aromatic hydrocarbons (PAH), dioxins, polychlorinated biphenyls (PCB), metabolites of fatty acids (i.e. prostaglandins, PG) activate (black solid arrows) the aromatic hydrocarbon receptor (AHR) leading to activation of proliferation, epithelial-to-mesenchymal transition (EMT), and inflammation, while disrupting cell cycle control and DNA repair tumor suppressor genes through the action of DNA methyltransferase (DNMT) enzymes. Some bioactive food components prevent (dashed back lines) AHR-induced, whereas corn oil, estrogens (E2) or overexpression of epidermal growth factor receptor-2 (HER-2) cooperate with the AHR to induce cancer processes. EVOO, extra-virgin olive oil.
Epigenetic studies with variant human mammary epithelial cells reported that activation of the AHR was paralleled by induction of c-MYC, and EZH2; and associated with repression of miR-143/145and hypermethylation of p53-binding sites in EPHB3 and TRIM6. These data suggested that AHR activation may inhibit cell cycle control via epigenetic deregulation of p53-target genes [40]. HER-2 overexpression occurs in 20–30% of breast cancers, and it has been shown to induce AHR expression via the mitogen-activated protein kinase-kinase (MEK)/extracellular-signal-regulated kinases (ERK) signaling pathways, leading to subsequent activation by the AHR without exogenous ligands, of expression of proinflammatory interleukin (IL)-6 and IL-8 [81]. The overexpression of AHR in MCF-7 cells exposed long-term to estrogen was attributed to reduced association of the AHR with H3K27me3 [82].
The involvement of epigenetic processes in AHR-induced tumorigenesis has been corroborated by evidence that activation of the AHR increased the expression of histone deacetylase-6 (HDAC-6) and subsequent formation of β-catenin/LEF1/TCF transcription complexes, which in turn, transactivated the c-MYC oncogene [83]. The aggressive proliferation of AHR-overexpressing mammary epithelial cells (i.e. MCF10AT1) was assisted by epigenetic repression via CpG methylation of WIF-1, which encodes an inhibitor of the Wnt pathway [84]. Conversely, depletion of AHR in ERα-negative breast cancer cells (MDA-MB-231) reduced expression of factors involved in cell growth (MUC1, IL-8), tryptophan metabolism (KYNU), multi-drug resistance (ABCC3), cell migration and invasion (S100A4), and angiogenesis (VEGFA) [85]. Our in vitro studies illustrated that the BRCA-1 gene was a direct target for epigenetic repression by the AHR [86, 87]. These repressive effects were mediated by increased occupancy of the activated AHR and HDAC-1, and reduced association of the histone acetyltransferase (HAT) p300, SRC-1, and acetylated H4 (H4Ac), with BRCA-1. Other epigenetic alterations associated with AHR-mediated silencing of BRCA-1 included deacetylation of H3K9; increased levels of H3K9me3, DNMT-1, DNMT-3a, DNMT-3b, and methyl-binding protein (MBD)-2; and CpG hypermethylation [88, 89]. Interestingly, the pattern of BRCA-1 CpG methylation induced in AHR-treated MCF-7 cells, which harbor wild-type and hypomethylated BRCA-1 gene, was similar to the one detected in human sporadic breast tumors [4] lending support to the hypothesis that epigenetic changes induced by the AHR may play an important role in sporadic breast tumorigenesis.
In animal models, the maternal activation of the AHR impaired mammary differentiation; increased mammary terminal end bud (TEB) formation; and predisposed to chemically-induced mammary tumorigenesis in female offspring [90]. TEB are undifferentiated structures equivalent to human lobules type-1 usually found in breast tumors of BRCA-1 mutation carriers [91]. In contrast, the knockdown of the AHR in the rodent mammary gland reduced the formation of TEB [92]. Other animal studies reported persistent impairment of mammary gland morphology in offspring as a result of gestational exposure to AHR agonists [93, 94]. Based on the information both human BRCA-1 and rat Brca-1 harbor binding sites (5’-GCGTG’3’) for the AHR, we extended our studies of BRCA-1 regulation to offspring of Sprague-Dawley rats treated during gestation with the AHR agonist TCDD, which increased the number of TEB in mammary tissue of offspring. These morphological changes were paralleled by greater occupancy of Brca-1 by DNMT-1; and increased Brca-1 hypermethylation and expression of Ccnd1 [95]. In a follow-up study with postpubertal Sprague-Dawley rats, we found that the AHR agonist and carcinogen 7,12-dimethyl-benzo(a)anthracene (DMBA) induced mammary tumors with reduced BRCA-1 and ERα; tumors had higher Brca-1 CpG methylation; increased expression of AHR and Cyp1b1; and higher Ccnd1 [96].
Turning to examples of human exposure, prenatal levels of AHR-activating compounds have been correlated with delayed initiation of breast development in girls [97]. In human breast tumors, we found that TNBC had higher basal AHR expression and BRCA-1 promoter CpG methylation compared to luminal-A, luminal-B, and HER-2-positive breast cancer tissue [96]. In human UACC-3199 cells, which harbor wild-type, but constitutively hypermethylated, BRCA-1 [17], we confirmed that low BRCA-1 expression was mirrored by constitutive high AHR expression; conversely, the treatment with the AHR antagonist α-naphthoflavone (αNF) partially rescued BRCA-1 and ERα expression, further highlighting the potential for AHR antagonists in reactivation of BRCA-1 in ERα-negative breast cancer cells [96].
In contrast to the large body of evidence that attributes to the AHR a causative role in sporadic breast tumorigenesis, some studies reported that activation of the AHR induced proteolytic degradation of the ERα in breast cancer MCF-7 cells [98], and hampered cell proliferation and androgen receptor (AR) expression in LNCaP prostate cancer cells [99]. The latter results were in disagreement with those of other reports indicating that the forced decrease in AHR expression through siRNA reduced by 50% growth of androgen-independent prostate cancer C4-2 cells compared to AR-positive LNCaP cells [100]. Therefore, further studies should investigate how cell context may influence the differential effects of AHR activation and/or overexpression on epigenetic control of cell proliferation in breast tissue.
3.2.2 BPA
BPA is an anthropogenic compound used in plasticizers. The leaching of BPA into food and drink containers has been attributed to its widespread detection in human urine and plasma. Estimates suggested that ~90% of total human exposure to BPA originated from foods [101]. The exposure to BPA has been correlated with increased risk of tumors of the breast and prostate [102]. Epigenetic effects of BPA have been demonstrated through decreased CpG methylation in the Agouti gene model [103]. At the cellular level, BPA can trigger changes in gene expression through nuclear pathways. BPA contains several phenolic groups and behaves as a “weak” estrogen, with 1000 to 2000-fold less affinity for the ERα. Through binding to ERα, it was shown to activate expression of proliferating cell nuclear antigen (PCNA) and cyclin A2 (CCNA2) [104–106] (Figure 2). In high-risk donor breast and T47-D (ERα-positive) epithelial cell lines, BPA (0.1 µM for 7 d) selectively increased ERα/ERβ ratio along with activation of cyclin D3 (CCND3), cdk6, CCNA, cdk2, and PCNA [107]. The induction of cell proliferation in MCF-7 cells was accompanied by activation of STAT3 [108] and CCND1, and inhibition of genes known to induce to apoptosis [109].
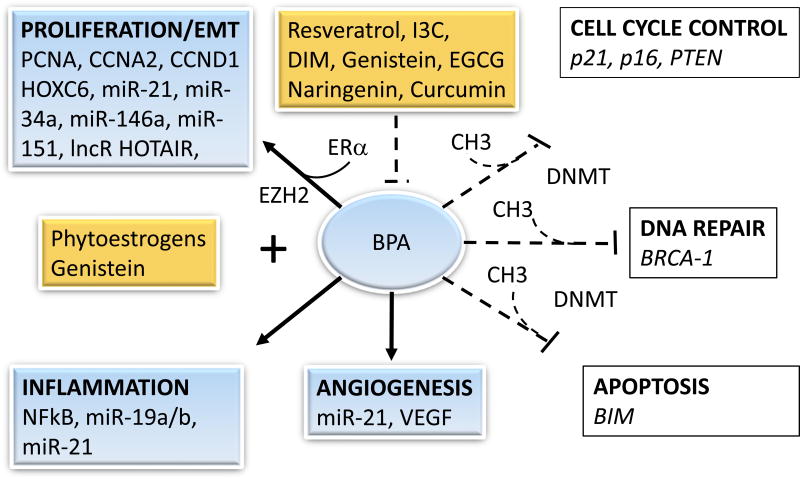
Figure 2.
Epigenetic disruption by BPA and breast cancer prevention with food components. Exposure to BPA activates (black solid arrows) factors the induce proliferation, epithelial-to-mesenchymal transition (EMT), inflammation, and angiogenesis, while silencing through DNA methyltransferases (DNMT) the expression of cell cycle control, apoptosis, and DNA repair tumor suppressor genes. Some bioactive food components prevent (dashed back lines) BPA-induced changes, whereas phytoestrogens (i.e. genistein) under certain conditions, may cooperate with BPA to induce cancer.
Bis-phenol A may induce proliferation through non-nuclear pathways involving activation of G-protein coupled receptor (GPER) [110] and ERK1/2/MAPK/Akt signals [111]. In non-cancerous human high-risk donor breast epithelial cell cells, the treatment with BPA antagonized the antiestrogenic effects of tamoxifen through activation of the PI3K/Akt/mTOR cascade [112]. MCF-7 cells exposed to BPA had increased expression of CCND1, CCNA2, and B-cell CLL/Lymphoma-2 (Bcl-2) at doses as low as 10 pM [113]. BPA-induced resistance to chemotherapeutic agents was confirmed in ERα-negative (MDA-MB-468) breast cancer cells [114]. At doses ranging from 1 to 10 µM, BPA promoted capillary permeability and angiogenesis through upregulation of vascular endothelial growth factor (VEGF) expression [115]. In ERα-negative MDA-MB-231 cells, BPA stimulated migration and invasion through activator protein-1 (AP-1), nuclear factor kappa-light-chain-enhancer in B cells (NFkB), SRC oncogene, and ERK2 pathways [116]; and EMT through inhibition of FOXA1 and CDH1 (E-cadherin) [117]. Repression of E-cadherin and stimulation of proliferation were observed in response to BPA, respectively in human embryonic stem [118] and normal human mammary endothelial cells [119]. These cumulative findings indicated that at levels detected in humans, BPA may elicit pleiotropic effects associated with breast cancer.
The breast tumor-inducing effects of BPA may be amplified by defects in expression of TSG. For example, primary breast cells derived from a BRCA-1 mutation carrier produced more spherical masses in collagen and invasion when cultured in the presence of BPA [120]. Hence, women who are BRCA mutation carriers, i.e. have one mutated copy, or harbor one BRCA-1 allele epigenetically-silenced, when exposed to BPA may be at greater risk of developing breast cancer. In support of this notion, primary breast cells from BRCA-1 mutation patients were shown to form an increased number of invasive masses subsequent to BPA treatment in culture [120]. Similarly, BPA administration to Brca-1 mutant mice was found to stimulate hyperplasia compared to control ([121].
Studies with rodent models illustrated that irrespective of timing of exposure, BPA altered mammary gland development and increased cancer risk. For example, the perinatal exposure to BPA increased the number of TEB [122] and ductal hyperplasia in offspring [123]. Moreover, the gestational, prepubertal, and pubertal exposure to BPA increased susceptibility to the AHR carcinogen, DMBA [124–128]. The prepubertal exposure to BPA induced cell proliferation and reduced p21 expression in mammary glands of Sprague-Dawley rats at postnatal day 50 [129]. The prenatal exposure to BPA was also shown to increase tumor susceptibility to nitroso-carcinogens [130], and repress immunoregulatory cytokines (IL-1, IL-2) in mammary gland of offspring [131]. In adult female rodents, BPA increased the number and size of the acini and ducts with hyperplasia of the lining epithelial cells [132]. Similar to the carcinogenic effects observed in rodent models, in nonhuman primates (rhesus monkey), the gestational exposure to BPA augmented density of mammary buds in the offspring [133].
In support of an epigenetic hypothesis for the breast cancer effects of BPA, in vitro studies with human mammary epithelial cells documented that low dose exposure (<~0.2 µM) to BPA increased DNA methylation of BRCA-1 and p16INK4 [120]. In MCF-7 cells, the BPA treatment increased the expression of HOXC6, which is commonly upregulated in breast and prostate tumors through H3K4me3, MLL-histone methylases, and acetylation of histones associated with HOXC6 [134]. In the non-tumorigenic breast epithelial MCF-10F cell line, BPA induced silencing through hypermethylation of proapoptotic BCL2L11 (BIM) [119]. Studies with primary human breast epithelial cells showed that at environmentally relevant doses (4 nM), BPA induced nuclear internalization of the ERα and altered DNA methylation of LAMP3 [135] further confirming its potential for epigenetic disruption.
In rodent models, the prenatal exposure to BPA induced H3K4me3 at the transcription initiation site of LALBA (alpha-lactalbumin) in the mammary gland of female offspring coincident with increased manifestation of DCIS [136]. The in utero exposure to BPA increased expression of EZH2 and H3me3 in the adult mammary gland [137]. These cumulative data supported the notion that maternal exposure to BPA may increase breast cancer risk in the offspring [138].
Changes in expression of non-coding RNA may contribute to the procarcinogenic effects of BPA. The induction of proliferation in MCF-7 cells treated with BPA was correlated to stimulation of oncogenic miR-21 [139], miR-19a and miR-19b, and repression of miR-19-related downstream targets including PTEN [140]. Through activation of the ERα in breast MCF-7 cells and in rat mammary tissue, BPA was found to increase expression of the lncR hox transcript antisense RNA (HOTAIR) [141], and EZH-2 [142], which have been related to epigenetic silencing and promotion of breast cancer. The in utero exposure to BPA induced in uterine tissue of offspring DNA hypomethylation of Hoxa10 leading to increased binding of the ERα to the Hoxa10 promoter [143] and on a genome-wide scale [144]. The treatment of human placental cell lines with BPA strongly induced miR-146a, whose abnormal expression has been linked to the development of TNBC as well as the development of cervical tumors [145, 146]. In rat models, the neonatal exposure to BPA induced the hypermethylation of Esr1 in testis [147], and Nsbp1 in prostate gland [148] further highlighting the contribution of epigenetic disruption in BPA-induced tumorigenesis in endocrine-responsive tissues.
3.2.3 Arsenic
Whereas chronic arsenic exposure through consumption of certain foods (i.e. rice and grains) [149, 150] and geologically contaminated water has been correlated to increased incidence of prostate cancer [151] and other malignancies [8], its impact on breast and ovarian cancer remains unclear. Common human exposure to arsenic includes inorganic trivalent arsenite (AsIII) and pentavalent arsenate (AsV). However, only AsIII has potent estrogen-like activities in connection with its affinity for the ligand-binding domain of the ERα and ability to stimulate cell growth and expression of estrogen responsive genes (i.e. PRG) [152] (Figure 3). Because AsV can be enzymatically converted to AsIII, it may provide a reservoir of ERα-disrupting arsenic that can generate monomethylated and dimethylated metabolites, which are more toxic than the inorganic parent compounds [reviewed in 153]. Therefore, total exposure to arsenic compounds should be considered when assessing breast cancer risks. Also, sex-specific patterns of deregulation of endocrine pathways related to arsenic-contaminated drinking water suggested thresholds for total arsenic exposure may be different between males and females [154].
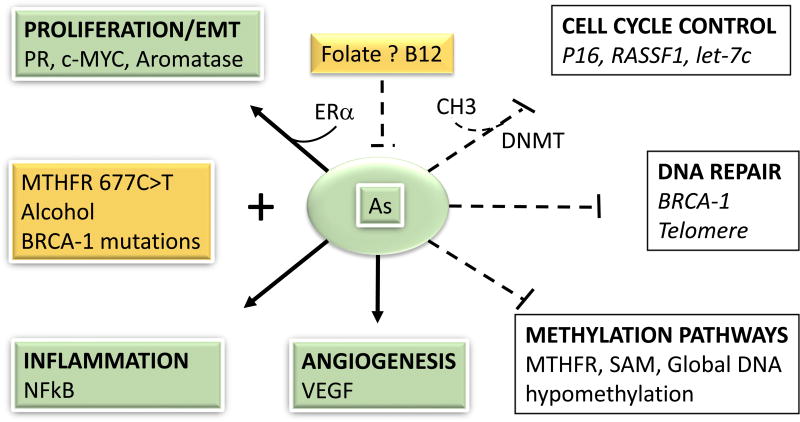
Figure 3.
Epigenetic disruption by arsenic and breast cancer prevention with food components. Exposure to arsenic (As) activates (black solid arrows) factors the induce proliferation, epithelial-to-mesenchymal transition (EMT), inflammation, and angiogenesis, while silencing through DNA methyltransferases (DNMT), the expression of cell cycle control and DNA repair tumor suppressor genes. Arsenic may deplete the pool of methyl groups (i.e. SAM) and induce global DNA hypomethylation. Some bioactive food components (i.e. folate, B12) may prevent (dashed back lines) As-induced changes, whereas others (i.e. alcohol) under certain conditions (i.e. carriers of BRCA-1 mutations or polymorphisms in the methyl-tetrahydrofolate reductase = MTHFR) may cooperate with As to induce cancer.
At the cellular level, AsIII was shown to inhibit DNA mismatch repair leading to genomic instability [155, also reviewed in 156]. Similarly, a positive association was reported in humans between inorganic arsenic exposure in utero and oxidative/methylated DNA damage in offspring [157]. In a spontaneous mammary-tumor model (C3H/St mice), arsenic trioxide (As2O3) abolished the anticancer effects of selenium and increased tumor growth rates and tumor multiplicity [158]. In human fibroblasts, As2O3 treatment was shown to disrupt the normal function of the Fanconi/BRCA-1 DNA repair pathway and increase genomic instability [159]. Perhaps not surprisingly, women carrying certain BRCA-1 mutations (5382insC, C61G, 4153delA) were found to be at higher risk (OR ~1.25–1.7) of breast tumorigenesis associated with increased serum arsenic (~4–6 µg/L) [160].
With respect to timing of exposure, studies with a rodent model (Sprague-Dawley) demonstrated that in utero exposure to AsIII induced an increase in the number of mammosphere-forming cells in the mammary gland of prepubertal offspring. In the postpubertal gland of offspring, the in utero exposure to AsIII stimulated branching of epithelial cells and density, as well as overexpression of ERα, which persisted into adulthood [161]. Other investigations concluded that AsIII was a “complete” transplacental carcinogen producing maternal dose-dependent induction of tumors of the adrenal gland, ovary, and uterus in female mice offspring [162]. Studies that examined in CD-1 mice the postnatal effects of AsIII exposure from two weeks prior to breeding through pregnancy, lactation, after weaning, and into adulthood observed an increase in the adult incidence of tumors at doses (6 to 24 ppm in drinking water) considerably lower than those generally utilized for in utero studies [163]. The same investigators proposed that monitoring of “whole” life, rather than acute, exposure may more accurately model the risk of breast cancer in humans.
Interactions between dose and duration of exposure may impact on breast cancer risk via a biphasic mode. At low-doses (0.01–1 µM), arsenic compounds increased proliferation of non-tumorigenic MCF-10A (As2O3), and breast cancer MCF-7 (AsIII) cells [164, 165]. Conversely, at higher concentrations (~5–10 µM), these compounds exerted apoptotic effects [164–166] associated with accumulation of caspase enzymes, p21, growth arrest DNA-damage-inducible-α GADD45 [167], and miR-238 [168]. These data pointed to chronic exposure as potentially important for transformation of normal breast epithelial cells, or growth of preneoplastic cells, as confirmed in prostate tissue in which AsIII triggered the transition to a steroid hormone-independent phenotype [169].
Interactions between ER status and arsenic exposure may influence the breast cancer response. In ERα-positive (i.e. T47D, MCF-7) breast cancer cells, the treatment with AsIII or As2O3 reduced the expression of ERα [170, 171]. Conversely, the treatment of ERα-negative (but ERβ positive) breast cancer MDA-MB-231 cells with As2O3 induced re-expression of ERα mediated by demethylation of the ERα promoter, lower expression of DMNT-1 and DNMT-3a, and partial dissociation of DNMT-1 from ESR1 [172]. The mechanisms responsible for these differential effects of arsenic in ER-positive vs negative breast cells remain largely unknown. Importantly, in the case of ER-positive tumors, the disruption of ERα expression by arsenic may compromise the effectiveness of antiestrogen therapies.
Arsenic detoxification uses methyl groups from S-adenosyl-homocysteine (SAM), and as a result, it affects the pool of methyl groups available for DNA methylation. [153]. Methyl donors include folate, methionine, and choline. Studies with ERα-positive MCF-7 breast cancer cells showed that AsIII treatment decreased methyl-tetrahydrofolate reductase (MTHFR) levels in a concentration-dependent fashion [166]. The reduction in MTHFR may compromise the production of methionine from homocysteine, lower SAM levels, and contribute to global DNA hypomethylation [173, 174]. Arsenic exposure through drinking was correlated with changes in global methylation of peripheral blood mononuclear cell DNA in adult populations [175]. On the other hand, preclinical [176, 177] and human [178] studies showed that arsenic induced gene-specific methylation (i.e. p16INK4, RASSF1), and a decrease in telomere length associated with genomic instability [179]. Additionally, treatment with AsIII was reported to induce cancer stem cell-like properties involving epigenetic silencing of let-7c via Ras/NF-kB pathways [180]. The maternal exposure to arsenic was also shown to alter DNA methylation in placenta [181]. Taken together, these data suggested that arsenic exposure may increase epigenetically breast cancer risk through repression of TSG involved in DNA repair and cell cycle control. In keeping with this hypothesis, our group found that in MCF-7 cells As2O3 (0.5–2.0 µM) and AsIII (1.0–8.0 µM) reduced in a dose-dependent fashion BRCA-1 expression. Also, AsIII interfered with normal development of endocrine tissues in X. tropicalis females (data not shown). These preliminary, albeit important, data suggest further studies are needed to clarify the epigenetic impact of arsenic compounds on TSG, and potential of dietary arsenic mimetics, for breast cancer prevention.
3.3 Prevention of epigenetic disruption with food components
This section summarizes the results of studies that reported on prevention of biochemical and epigenetic alterations commonly observed in breast tumors and associated with exposure to AHR-agonists (Figure 1), BPA (Figure 2), and arsenic (Figure 3) compounds.
3.3.1 AHR agonists
Ascorbate
In female ACI rats, ascorbate was found to prevent estrogen-induced mammary tumors and decrease oxidative stress. Specifically, vitamin C attenuated estrogen-induced markers of oxidative stress (i.e. 8-iso-prostane F2α) while inducing expression of the antioxidant enzymes superoxide dismutase and glutathione peroxidase in mammary gland tissue. Vitamin C protected against estrogen-induced mammary tumorigenesis, and increased tumor latency. The anticancer effects of ascorbate mimicked those of αNF, an inhibitor of the AHR, suggesting it could be useful for the prevention of AHR-mediated breast tumorigenesis [182]. A rodent study that evaluated the combined efficacy of α-tocopherol, selenium, and ascorbic acid on development of mammary tumors induced with DMBA, concluded that animals receiving the supplementation had lower tumor incidence and multiplicity [183]. Other studies provided evidence ascorbate may antagonize tumor progression [184] through activation of apoptosis-inducing factor-1-dependent cell death pathways [185].
Ascorbate may impact epigenetic regulation through stimulation of the catalytic activity of the Ten eleven translocation (TET) dioxygenase enzymes, and oxidation of 5-methylcytosine (5hmc) [186] leading to demethylation and activation of TSG. The knock-out of TET functions, or overexpression of oncogenic miR-22 that represses TET expression, was shown to repress expression of miR-200 due to reduced 5hmc levels leading to increased expression of EMT genes (BM1I, ZEB1/2) [187]. Therefore, through activation of TET enzymes ascorbate may reduce the risk of cancer in mammary tissues.
Resveratrol
The phytoalexin resveratrol has received attention as a cancer preventative and antagonist of the AHR [74]. In cell culture experiments with MCF-10A cells, resveratrol (1 to 5 µM) protected against PAH-induced DNA damage by suppressing expression of CYP1A1 and CYP1B1 [188]. In studies with Sprague-Dawley rats, the supplementation in the diet starting at birth and with serum levels approaching 2.0 µM, suppressed DMBA-induced mammary cancer [189]. These protective effects of resveratrol against DMBA-induced tumorigenesis were also seen when the supplementation started later in life (45 days of age) [190], and have been attributed, at least in part, to suppression of DMBA-induced NF-kB and PTGS2 (cyclooxygenase-2). In MCF-7 cells, at doses (1.0 µM) that approximated those achieved (2.4 µM) in human serum during pharmacokinetic studies [191], resveratrol antagonized AHR-dependent repression of BRCA-1 transcription; deacetylation of H4 associated with BRCA-1; and BRCA-1 CpG hypermethylation while reducing BRCA-1 association of AHR, MBD-2, DNMT-1, and H3K9me3to [88, 89]. In mammary tissue of Sprague-Dawley rat female offspring, we found that the maternal supplementation with resveratrol antagonized AHR-mediated downregulation of BRCA-1 and Brca-1 hypermethylation [95]. Conversely, resveratrol increased occupancy of Brca-1 by the AHR repressor while lowering the recruitment of DNMT-1, the number of TEB, and expression of Ccnd1.
Other epigenetic changes may account for the preventative effects of resveratrol. For example, the chronic supplementation (12 wk) of resveratrol to women at increased risk of breast cancer reduced the DNA methylation of RASSF1a in dose-dependent fashion (50 mg > 5 mg twice daily) in mammary ductoscopy specimens, and reduced prostaglandin E2 levels in nipple aspirates [192]. Epigenetic prevention of breast cancer by resveratrol has also been linked to demethylation of the ESR1 and sensitization to antiestrogen therapy. In cultured MDA-MB-468 cells, which are TNBC, resveratrol reduced acetylation of the oncogenic transcription factor STAT3 while increasing expression of ERα [193]. In the same study, resveratrol in combination with tamoxifen prevented growth of MDA-MD-468 tumor xenografts. Therefore, resveratrol may hold promise for epigenetic targeting of ERα-negative/AHR overexpressing breast tumors. Breast cancer protective effects of resveratrol have also been attributed to activation of tumor suppressor miR-10a/b, miR-129, miR-204, and miR-489 [194]. However, the same study documented activation by resveratrol of oncogenic miR-21. These data were in contrast with those of other reports documenting resveratrol lowered expression of miR-21 [195]. More studies are needed to clarify the impact of resveratrol on expression on non-coding RNA and relationships with breast cancer risk.
Genistein
The effects of genistein on AHR-mediated pathways may depend on cell context [196]. In Sprague-Dawley rats, the perinatal exposure from conception, through day 21, to post-partum, was correlated with enhanced mammary gland differentiation and reduced DMBA-induced mammary tumorigenesis [197]. Similarly, the prepubertal treatment with genistein antagonized DMBA-induced mammary adenocarcinomas [198] and increased Brca-1 expression [199]. The preventative effects of genistein against breast cancer have been related to inhibition of DNMT-1 leading to demethylation in TSG including BRCA-1 [200], ATM, APC, PTEN, and SERPINB5 [201]; enrichment of chromatin activators (H3Ac and H3K4me3) in the promoters of p16INK4 and p21CIP1/WAF1 [202]; and increased responsiveness to antiestrogen therapy [203].
Cruciferous indoles and isothiocyanates
Indol-3-carbinol (I3C) originates from enzymatic conversion of glucobracissin by myrosinase. Under acidic conditions, I3C undergoes rapid self-condensation to produce 3,3’-diindolylmethane (DIM). Studies concluded that I3C preferentially targeted ERα-positive breast cancer cells through the AHR and repressed proliferation [204]. The growth inhibition was dependent on BRCA-1 and BRCA-2 expression [205]. In combination with tamoxifen, I3C enhanced protection against post-initiation of mammary tumors with DMBA [206]. Similarly, DIM was reported to bind the AHR and inhibit DMBA-induced mammary tumorigenesis [207]. These data established a strong link between breast cancer prevention by I3C and DIM under conditions of AHR activation. Epigenetic prevention in prostate cancer cells by DIM, but not I3C, has been attributed to inhibition of HDAC activity accompanied by downregulation of HDAC-2, and upregulation of p21, expression [208]. Opposing effects on HDAC activity were also documented by our group in breast cancer MCF-7 cells in which the treatment with DIM (10 µM) abrogated TCDD-dependent activation of the AHR, and increased the association of AcH4, with PTGS2 (COX-2) [209]. Anti-inflammatory properties of I3C and DIM were related to inhibition of HDAC activities [210]. DIM may prevent breast cancer through activation of miR-212/132, which in turn suppress the expression of metastatic SOX4 [211]. Interestingly, a recent study reported that in the absence of estrogen and at concentrations commonly used in cell culture (10 µM), DIM enhanced growth of MCF-7 and T47D breast cancer cells through binding to the ERα. Conversely, at higher levels (50 µM), DIM inhibited proliferation [212]. Therefore, further studies are needed to study the bioactivity of I3C and DIM at physiological and supplemental doses and impact on epigenetic processes.
Sulforaphane (SFN) is an isothiocyanate found in cruciferous vegetables. Studies documented that SFN protected against DMBA-induced mammary tumorigenesis [213], and in MDA-MB-231 breast cancer cells and tumor xenografts it upregulated the tumor suppressor miR-140 [214]. In combination with green tea polyphenols (GTP), it reduced DNA methylation of ERα, sensitized cells to antiestrogen therapy [215], and demethylated p21CIP1/WAF1 [216]. Also, SFN was reported to induce demethylation and expression of PTEN and RARβ2 [217], and inhibit HDAC activity [218]. Surprisingly, when supplemented to B6129SF1 mice during the perinatal period, it enhanced dibenzo[def,p]chrysene (a strong AHR ligand)-dependent morbidity and lung tumorigenesis in offspring [219]. The mechanisms responsible for these pro-tumorigenic effects of DIM have not been clarified.
Green teat polyphenols include green tea catechins such as (−)-epigallocatechin-3-gallate (EGCG). In Sprague-Dawley rats treated with DMBA, green tea reduced by 87% tumors invading the ducts [220] and preserved E-cadherin expression in DCIS lesions. These effects were attributed to EGCG, which prevented EMT, and the in vitro formation of branching structures in the presence of increased AHR expression [221]. EGCG represents ~50% of the GTP found in green tea and is an established antagonist of the AHR. It was shown to inhibit DNMT, and reactivate p16 expression (20 µM) through CpG demethylation of p16INK4a [222]. In ERα-negative MDA-MB-231 breast cancer cells, GTP and SFN modified the chromatin structure of ESR1 through enrichment of transcriptionally active markers (i.e. H3K9Ac) [215]. This was accompanied by reduced association of ESR1 with SUV39H1 [3]. The combination of EGCG (20 µM) and SFN (5 µM), but not EGCG alone, was shown to reactivate ERα expression by reducing ESR1 methylation, and [216]. These studies suggested that associations of SFN and GTP may be epigenetically more effective for breast cancer treatment than the individual compounds.
Other food components and diet
Epigenetic protection against AHR-dependent mammary tumorigenesis has been suggested for curcumin, which in MCF-7 cells repressed AHR-dependent activation of CYP1A1 and CYP1B1 [223], and increased global levels of H3K18Ac and H4K16Ac [224]. These protective effects of curcumin were confirmed in hepatocellular carcinoma cells in which it inhibited phthalate-dependent expression and activation of the AHR, migration, invasion, and EMT [225]. Similarly, antagonistic effects on the AHR were described in vitro for kaempferol, quercetin, and luteolin [196], and in the DMBA-mammary tumor model for biochanin A [197]. In a French-Canadian cohort of women carriers of a BRCA-1 mutation, a significant reduction in incidence (~80%) of breast cancer was documented for subjects who consumed the highest intake quintile of fruits and vegetables [226]. It remains unknown if these protective effects of fruits and vegetables were related to epigenetic modulation of cancer processes. In the Nurses’ Health Study, longer telomeres were observed in groups with greater adherence to the Mediterranean diet, a dietary pattern that is rich in fruits and vegetables, and monounsaturated fatty acids [227]. The intake of extra virgin olive oil, one of the main sources of fat in the Mediterranean diet, was linked to activation of GSTP1 [228]. Interestingly, extra virgin olive oil was found to inhibit cancer-related global DNA hypomethylation [229]. Similarly, extracts from licorice, which is an herb commonly used by Mediterranean populations, were found to antagonize AHR-induced cell proliferation through induction of expression of p53 and p27. Caffeic acid was shown to demethylate miR-148 and abrogate cancer stem cell properties in MDA-MB-231 breast cancer cells [230]. These anticancer effects of caffeic acid were attributed to inhibition of AHR transactivation and nuclear localization [231]. Overall, many food components exert protective effects against epigenetic disruption induced by AHR agonists. Future studies are needed to elucidate in humans the dose- and stage of life-dependent effects of individual food components and association for breast cancer prevention.
3.3.2 BPA
Genistein
The maternal supplementation with genistein at levels consumed by populations on high-soy diet was shown to modify coat color of heterozygous viable yellow agouti (Avy/a) offspring toward pseudo-Agouti. This phenotypic change was correlated to increased methylation of CpG sites in a retrotransposon upstream of the transcription start site of the Agouti gene [232]. The DNA methylation induced by genistein during early embryonic development persisted into adulthood. In the same mouse model, the maternal dietary supplementation with the methyl donor folate and genistein, prevented the aberrant transplacental hypomethylating effects of BPA [103]. Although genistein is not a methyl donor, it could epigenetically oppose tumorigenesis by antagonizing the genome-wide hypomethylating effects of BPA and other xenoestrogens, while preventing gene-specific DNA hypermethylation and histone deacetylation in TSG [233]. Genistein may protect against BPA-induced breast tumorigenesis through activation of BRCA-1 [234] and tumor suppressor miR-574-3p [235]; inhibition of oncogenic lncR HOTAIR [236], miR-34a [237], and miR-151 [238]; and modulation of DNA methylation patterns [239]. The preventative effects of genistein against BPA-induced tumorigenesis may extend to other endocrine responsive tissues such as ovary [240]. On the other hand, some reports illustrated that BPA plus genistein exerted additive effects on proliferative endpoints and expression of estrogen-target genes in MCF-7 breast cancer cells [104]. Also, genistein was shown to induce in a dose-dependent fashion (1–10 µM) VEGF secretion in MCF-7 cells [115]. Therefore, studies should clarify in preclinical models and humans populations how the combination of BPA and phytoestrogens may impact the epigenome of the breast and cancer risk.
Other food components
Flavonoids have been proposed as preventatives against breast cancer [reviewed in 241]. For example, naringenin, a citrus flavanone, and curcumin, were shown to antagonize BPA-induced proliferation of ERα-positive MCF-7 cells respectively by activating caspase-3 [242] and repressing expression of oncogenic miR19a/b [137]. The latter is a negative regulator of PTEN. Similarly, in ERα-positive ovarian BG-1 cancer cells, resveratrol inhibited BPA-induced proliferation through repression of ERα and CCND1 [243]. The epigenetic changes associated with these preventative effects of resveratrol in ovarian tissue await further investigation.
3.3.3. Arsenic
Folate
Folate, methionine, choline, betaine, and various B vitamins (B2, B6, and B12) are food components that regulate the pool of available methyl groups. Gestational studies of arsenic exposure in the CD-1 mouse model indicated that AsIII induced low fetal body weight. The cotreatment with folate did not prevent these effects, but rather, induced in the offspring large changes in DNA methylation affecting CpG methylation of genes involved in Wnt-signaling [244]. The exposure to inorganic arsenic may be particularly detrimental during gestation as folate is necessary for the development of the placenta and epigenetic programming of the fetus [244]. Studies have yielded contrasting results about the effects of folate supplementation on the risk of breast cancer in women. For example, at doses higher than ~850 mg of total folate equivalents/d (DFE, 1 mg food folate=0.6 mg of supplemental folic acid), folate intake in postmenopausal women was associated with a 30% increase in the risk of breast cancer [245]. On the other hand, a study conducted also with postmenopausal women who consumed ~1,300 mg of DFE/d [246] reported a 22% reduction in the incidence of ERα-negative breast tumors. One mechanism that may influence the response to folate and arsenic is interactions with polymorphisms in the MTHFR (i.e. 677C>T), which lower the enzymatic activity of MTHFR. Women carrying mutated BRCA-1 and the MTHFR 677TT polymorphism are at higher risk of breast and ovarian cancer compared to women expressing the wildtype MTHFR allele (677CC) [247]. By repressing MTHFR expression [166] and BRCA-1 function in DNA repair [159], arsenic may direct folate to DNA synthesis rather than DNA methylation, and potentiate cancer cell growth. Interestingly, breast tumors from BRCA-1 mutation (Ser1841Asn) carriers were found to have increased expression of folate receptor-1 (FOLR-1), a member of the folate receptor family [248]. Protein members of the FOLR family have a high affinity for folic acid, and mediate delivery of 5-MTHF to the interior of cells [249]. Whereas expression of FOLR-1 was nearly undetectable in normal cells, it increased dramatically in breast cancer and its knockout restored sensitivity to treatment with doxorubicin [250].
B12 and alcohol
Intake of B vitamins may influence the epigenetic response to arsenic. The combined action of folate and B12 may protect the one-carbon metabolic pathway and reduce arsenic-induced mutagenic DNA breaks and tissue damage [251]. On the other hand, alcohol consumption was found to exert a negative effect on breast folate availability. This was associated with p16INK4 hypermethylation, which was enhanced in conjunction with genetic variations in methyl-transferase reductase and methylenetetrahydrofolate dehydrogenase [252]. Therefore, interactions between environmental arsenic exposure and alcohol consumption may interfere with normal folate and B12 metabolism and influence breast cancer risk through hypermethylation of TSG.
4 Conclusions
In spite of considerable progress in early cancer detection and treatment, excluding skin cancer, breast cancer remains the most common malignancy in women residing in the U.S. Because most breast cancer cases are sporadic, discovering the epigenetic mechanisms that regulate tumor development may offer new targets for prevention and treatment. These opportunities may extend to mutation carriers (e.g. BRCA-1), for whom epigenetic silencing of the wild-type allele may contribute to loss of heterozygosity and breast tumor development. Environmental pollutants, foods, and drinking water are sources of xenobiotics including agonists of the AHR (PAH, dioxin, phthalates, PCB), BPA, and arsenic which may contribute epigenetically to dysregulation of TSG and breast cancer. Conversely, some dietary compounds and patterns show promise for the prevention of breast cancers associated with these exposures. The breast cancer effects respectively of epigenetic disruptors and dietary compounds in breast tissue are influenced by complex interactions involving genotype, and timing, dose, and type (individual compound vs associations) of exposure. Future studies are needed to isolate potentially harmful interactions between epigenetic disruptors and food components in particular under supplementation regimens.
Acknowledgments
Authors wish to acknowledge the support (DFR and CRP) from the Native American Cancer Prevention Program (NACP) at The Northern Arizona University (U54CA143925) and The University of Arizona (U54CA143924); Arizona Biomedical Research Commission (QSR14082995) (to DFR and OIS); the US Department of Defense Breast Cancer Program (BC134119) (DFR and OIS); NIH Initiative to Maximize Student Diversity Program, 2R25GM056931-13 (CRP); and National Science Foundation Undergraduate Research Mentoring Program, DBI-1041255, Northern Arizona University.
Abbreviations
- AHR
aromatic hydrocarbon receptor
- αNF
αnaphthoflavone
- AP-1
activator protein-1
- AR
androgen receptor
- AsIII
arsenite
- AsV
arsenate
- As2O3
arsenic trioxide
- Bcl-2
B-cell CLL/Lymphoma-2
- BPA
bisphenol A
- CDK
cyclin-dependent kinase
- CCNA2
cyclin A2
- CCND3
cyclin D3
- CCND1
cyclin A1
- DCIS
ductal carcinoma in situ
- DFE
dietary folate equivalent
- DIM
3,3’-diindolylmethane
- DMBA
7,12-dimethylbenz[a]anthracene
- DNMT
DNA methyltransferase
- EGCG
(−)-epigallocatechin-3-gallate
- EMT
epithelial-to-mesenchymal transition
- ERK
extracellular-signal-regulated kinases
- ER
estrogen receptor
- EZH2
polycomb-2 protein enhancer of zeste
- FOLR-1
folate receptor-1
- GPER
G-protein coupled receptor
- GTP
green tea polyphenols
- GSTP1
glutathione-S-transferase-P1
- HDAC
histone deacetylase
- HAT
histone acetyl transferase
- HER-2
epidermal growth factor receptor-2
- H3K9Ac
H3 acetylated at lysine-9
- H3K9me3
histone-3 trimethylated at lysine-9
- H3K27me3
histone-3 trimethylated at lysine-27
- H4Ac
acetylated histone-4
- HOTAIR
hox transcript antisense RNA
- IGFR1
insulin-like growth factor-1 receptor
- I3C
indole-3-carbinol
- IL
interleukin
- MBD-2
methyl-binding protein-2
- MEK
mitogen-activated protein kinase
- MTHFR
methyl-tetrahydrofolate reductase
- 5-MTHF
5-methyltetrahydrofolate
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor of kappa light polypeptide gene enhancer in B-cells
- PAH
polycyclic aromatic hydrocarbons
- PCNA
proliferating cell nuclear antigen
- PI3K
phosphatidylinositol 3-kinase
- PR
progesterone receptor
- SAM
S-adenosyl-homocysteine
- SFN
sulforaphane
- TEB
terminal end bud
- TET
ten eleven translocation
- TNBC
triple-negative breast cancer
- TSG
tumor suppressor gene
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
Footnotes
Authors contributions
D.F.R., O.S.I, and C.P.R. contributed to the conception and writing of the article. K.D.D., J.T.G., S.A.R., provided technical support.
Conflict of interest
Authors have no conflict of interest.
References
- 1.Levy-Lahad E, Friedman E. Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer. 2007;96:11–5. doi: 10.1038/sj.bjc.6603535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476:163–9. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baylin SB, Jones PA. A decade of exploring the cancer epigenome – biological and translational implications. Nat. Rev. Cancer. 2011;11:726–34. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–98. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 5.Lips EH, Mulder L, Oonk A, van der Kolk LE, et al. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br. J. Cancer. 2013;108:2172–7. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–94. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins S, Betancourt AM, Wang J, Lamartiniere CA. Endocrine-active chemicals in mammary cancer causation and prevention. J Steroid Biochem Mol Biol. 2012;129:191–200. doi: 10.1016/j.jsbmb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Esquinas E, Pollan M, Umans JG, Francesconi KA, et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol. Biomarkers Prev. 2013;22:1944–1953. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M, Kim P, Mitra R, Zhao J, Zhao Z. TSGene 2.0: an updated literature-based knowledgebase for tumor suppressor genes. Nucleic Acids. Res. 2016;44:D1023–31. doi: 10.1093/nar/gkv1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene. 2006;25:5854–63. doi: 10.1038/sj.onc.1209872. [DOI] [PubMed] [Google Scholar]
- 11.Parvin JD. Overview of history and progress in BRCA1 research: the first BRCA1 decade. Cancer Biol. Ther. 2004;3:505–8. doi: 10.4161/cbt.3.6.839. [DOI] [PubMed] [Google Scholar]
- 12.Murphy CG, Moynahan ME. BRCA gene structure and function in tumor suppression: a repair-centric perspective. Cancer J. 2010;16:39–47. doi: 10.1097/PPO.0b013e3181cf0204. [DOI] [PubMed] [Google Scholar]
- 13.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 14.Ford D, Easton DF, Stratton M, Narod S, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1998;62:676–89. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 16.Magdinier F, Ribieras S, Lenoir GM, Frappart L, Dante R. Down-regulation of BRCA1 in human sporadic breast cancer; analysis of DNA methylation patterns of the putative promoter region. Oncogene. 1998;17:3169–76. doi: 10.1038/sj.onc.1202248. [DOI] [PubMed] [Google Scholar]
- 17.Rice JC, Massey-Brown KS, Futscher BW. Aberrant methylation of the BRCA1 CpG island promoter is associated with decreased BRCA1 mRNA in sporadic breast cancer cells. Oncogene. 1998;17:1807–12. doi: 10.1038/sj.onc.1202086. [DOI] [PubMed] [Google Scholar]
- 18.Seery LT, Knowlden JM, Gee JM, Robertson JF, et al. BRCA1 expression levels predict distant metastasis of sporadic breast cancers. Int. J. Cancer. 1999;84:258–62. doi: 10.1002/(sici)1097-0215(19990621)84:3<258::aid-ijc10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Thompson ME, Jensen RA, Obermiller PS, Page DL, et al. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat. Genet. 1995;9:444–50. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- 20.Wilson CA, Ramos L, Villaseñor MR, Handers KH, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat. Genet. 1999;21:236–40. doi: 10.1038/6029. [DOI] [PubMed] [Google Scholar]
- 21.Dobrovic A, Simpfendorfer D. Methylation of the BRCA1 gene in sporadic breast cancer. Cancer Res. 1997;57:3347–50. [PubMed] [Google Scholar]
- 22.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saldanha SN, Tollefsbol TO. Pathway modulations and epigenetic alterations in ovarian tumorbiogenesis. J. Cell. Physiol. 2014;229:393–406. doi: 10.1002/jcp.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101:403–10. doi: 10.1016/j.ygyno.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;23:6–212. doi: 10.1186/1471-2407-6-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruscito I, Dimitrova D, Vasconcelos I, Gellhaus K, et al. BRCA1 gene promoter methylation status in high-grade serous ovarian cancer patients--a study of the tumour Bank ovarian cancer (TOC) and ovarian cancer diagnosis consortium (OVCAD) Eur J Cancer. 2014;50:2090–8. doi: 10.1016/j.ejca.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Hosey AM, Gorski JJ, Murray MM, Quinn JA, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J. Natl. Cancer Inst. 2007;99:1683–94. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King MC, Wieand S, Hale K, Lee M, et al. National Surgical Adjuvant Breast and Bowel Project. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–6. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 29.Nass SJ, Herman JG, Gabrielson E, Iversen PW, et al. Aberrant methylation of the estrogen receptor and E-cadherin 5' CpG islands increases with malignant progression in human breast cancer. Cancer Res. 2000;60:4346–8. [PubMed] [Google Scholar]
- 30.Ramos EA, Camargo AA, Braun K, Slowik R, et al. Simultaneous CXCL12 and ESR1 CpG island hypermethylation correlates with poor prognosis in sporadic breast cancer. BMC Cancer. 2010;28:10–23. doi: 10.1186/1471-2407-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei M, Xu J, Dignam J, Nanda R, et al. Estrogen receptor alpha, BRCA1, and FANCF promoter methylation occur in distinct subsets of sporadic breast cancers. Breast Cancer Res. Treat. 2008;111:113–20. doi: 10.1007/s10549-007-9766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prabhu JS, Wahi K, Korlimarla A, Correa M, et al. The epigenetic silencing of the estrogen receptor (ER) by hypermethylation of the ESR1 promoter is seen predominantly in triple-negative breast cancers in Indian women. Tumour Biol. 2012;33:315–23. doi: 10.1007/s13277-012-0343-1. [DOI] [PubMed] [Google Scholar]
- 33.Wargon V, Fernandez SV, Goin M, Giulianelli S, Russo J, Lanari C. Hypermethylation of the progesterone receptor A in constitutive antiprogestin-resistant mouse mammary carcinomas. Breast Cancer Res Treat. 2011;126:319–32. doi: 10.1007/s10549-010-0908-x. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Phillips DL, Ferguson AT, Nelson WG, et al. Synergistic activation of functional estrogen receptor (ER)-alpha by DNA methyltransferase and histone deacetylase inhibition in human ER-alpha-negative breast cancer cells. Cancer Res. 2001;61:7025–9. [PubMed] [Google Scholar]
- 35.Yang X, Ferguson AT, Nass SJ, Phillips DL, et al. Transcriptional activation of estrogen receptor alpha in human breast cancer cells by histone deacetylase inhibition. Cancer Res. 2000;60:6890–4. [PubMed] [Google Scholar]
- 36.Jang ER, Lim SJ, Lee ES, Jeong G, et al. The histone deacetylase inhibitor trichostatin A sensitizes estrogen receptor alpha-negative breast cancer cells to tamoxifen. Oncogene. 2004;23:1724–36. doi: 10.1038/sj.onc.1207315. [DOI] [PubMed] [Google Scholar]
- 37.Stewart MD, Li J, Wong J. Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment. Mol Cell Biol. 2005;25:2525–38. doi: 10.1128/MCB.25.7.2525-2538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Jia Y, Liu X, Winters C, et al. Systematic dissection of the mechanisms underlying progesterone receptor downregulation in endometrial cancer. Oncotarget. 2014;5:9783–97. doi: 10.18632/oncotarget.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshima H, Wakabayashi M, Hattori N, Yamashita S, Ushijima T. Identification of coexistence of DNA methylation and H3K27me3 specifically in cancer cells as a promising target for epigenetic therapy. Carcinogenesis. 2015;36:192–201. doi: 10.1093/carcin/bgu238. [DOI] [PubMed] [Google Scholar]
- 40.Locke WJ, Zotenko E, Stirzaker C, Robinson MD, et al. Coordinated epigenetic remodelling of transcriptional networks occurs during early breast carcinogenesis. Clin Epigenetics. 2015;7:52. doi: 10.1186/s13148-015-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nie J, Xishi Liu, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod. Sci. 2010;17:995–1005. doi: 10.1177/1933719110377118. [DOI] [PubMed] [Google Scholar]
- 42.Liu T, Niu Y, Feng Y, Niu R, et al. Methylation of CpG islands of p16(INK4a) and cyclinD1 overexpression associated with progression of intraductal proliferative lesions of the breast. Hum. Pathol. 2008;39:1637–46. doi: 10.1016/j.humpath.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Tang L, Xie R, Nie W, et al. p16 promoter hypermethylation associated with increased breast cancer risk. Mol. Med. Rep. 2012;6:904–8. doi: 10.3892/mmr.2012.1001. [DOI] [PubMed] [Google Scholar]
- 44.Vallian S, Sedaghat M, Nassiri I, Frazmand A. Methylation status of p16 INK4A tumor suppressor gene in Iranian patients with sporadic breast cancer. J Cancer Res Clin Oncol. 2009;135:991–6. doi: 10.1007/s00432-008-0534-8. [DOI] [PubMed] [Google Scholar]
- 45.Askari M, Sobti RC, Nikbakht M, Sharma SC. Aberrant promoter hypermethylation of p21 (WAF1/CIP1) gene and its impact on expression and role of polymorphism in the risk of breast cancer. Mol. Cell. Biochem. 2013;382:19–26. doi: 10.1007/s11010-013-1696-5. [DOI] [PubMed] [Google Scholar]
- 46.Radpour R, Barekati Z, Haghighi MM, Kohler C, et al. Correlation of telomere length shortening with promoter methylation profile of p16/Rb and p53/p21 pathways in breast cancer. Mod. Pathol. 2010;23:763–72. doi: 10.1038/modpathol.2009.195. [DOI] [PubMed] [Google Scholar]
- 47.Miyake T, Nakayama T, Naoi Y, Yamamoto N, et al. GSTP1 expression predicts poor pathological complete response to neoadjuvant chemotherapy in ER-negative breast cancer. Cancer Sci. 2012;103:913–20. doi: 10.1111/j.1349-7006.2012.02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saxena A, Dhillon VS, Shahid M, Khalil HS, et al. GSTP1 methylation and polymorphism increase the risk of breast cancer and the effects of diet and lifestyle in breast cancer patients. Exp. Ther. Med. 2012;4:1097–1103. doi: 10.3892/etm.2012.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moelans CB, Verschuur-Maes AH, van Diest PJ. Frequent promoter hypermethylation of BRCA2, CDH13, MSH6, PAX5, PAX6 and WT1 in ductal carcinoma in situ and invasive breast cancer. J Pathol. 2011;225:222–31. doi: 10.1002/path.2930. [DOI] [PubMed] [Google Scholar]
- 50.Sandhu R, Rivenbark AG, Mackler RM, Livasy CA, Coleman WB. Dysregulation of microRNA expression drives aberrant DNA hypermethylation in basal-like breast cancer. Int J Oncol. 2014;44:563–72. doi: 10.3892/ijo.2013.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang S, Li Y, Gao J, Zhang T, et al. MicroRNA-34 suppresses breast cancer invasion and metastasis by directly targeting Fra-1. Oncogene. 2013;32:4294–303. doi: 10.1038/onc.2012.432. [DOI] [PubMed] [Google Scholar]
- 52.Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS One. 2012;7:e47053. doi: 10.1371/journal.pone.0047053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu PY, Deatherage DE, Rodriguez BA, Liyanarachchi S, et al. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Res. 2009;69:5936–45. doi: 10.1158/0008-5472.CAN-08-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q, Jiang Y, Yin Y, Li Q, et al. A regulatory circuit of miR-148a/152 and DNMT1 in modulating cell transformation and tumor angiogenesis through IGF-IR and IRS1. J Mol Cell Biol. 2013;5:3–13. doi: 10.1093/jmcb/mjs049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Yan LX, Wu QN, Du ZM, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–62. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Yang P, Sun T, Li D, et al. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–94. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 2012;11:5. doi: 10.1186/1476-4598-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He DX, Gu XT, Li YR, Jiang, et al. Methylation-regulated miR-149 modulates chemoresistance by targeting GlcNAc N-deacetylase/N-sulfotransferase-1 in human breast cancer. FEBS J. 2014;281:4718–30. doi: 10.1111/febs.13012. [DOI] [PubMed] [Google Scholar]
- 59.Lu L, Katsaros D, Zhu Y, Hoffman A, et al. Let-7a regulation of insulin-like growth factors in breast cancer. Breast Cancer Res Treat. 2011;126:687–94. doi: 10.1007/s10549-010-1168-5. [DOI] [PubMed] [Google Scholar]
- 60.Vrba L, Muñoz-Rodríguez JL, Stampfer MR, Futscher BW. miRNA gene promoters are frequent targets of aberrant DNA methylation in human breast cancer. PLoS One. 2013;8:e54398. doi: 10.1371/journal.pone.0054398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aure MR, Leivonen SK, Fleischer T, Zhu Q, et al. Individual and combined effects of DNA methylation and copy number alterations on miRNA expression in breast tumors. Genome Biol. 2013;14:R126. doi: 10.1186/gb-2013-14-11-r126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yun J, Frankenberger CA, Kuo WL, Boelens MC, et al. Signalling pathway for RKIP and Let-7 regulates and predicts metastatic breast cancer. EMBO J. 2011;30:4500–14. doi: 10.1038/emboj.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li XX, Gao SY, Wang PY, Zhou X, Li YJ, et al. Reduced expression levels of let-7c in human breast cancer patients. Oncol Lett. 2015;9:207–1212. doi: 10.3892/ol.2015.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bailey ST, Westerling T, Brown M. Loss of estrogen-regulated microRNA expression increases HER2 signaling and is prognostic of poor outcome in luminal breast cancer. Cancer Res. 2015;75:436–45. doi: 10.1158/0008-5472.CAN-14-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erturk E, Cecener G, Egeli U, Tunca B, et al. Expression status of let-7a and miR-335 among breast tumors in patients with and without germ-line BRCA mutations. Mol Cell Biochem. 2014;395:77–88. doi: 10.1007/s11010-014-2113-4. [DOI] [PubMed] [Google Scholar]
- 66.Pinto R, Pilato B, Ottini L, Lambo R, et al. Different methylation and microRNA expression pattern in male and female familial breast cancer. J Cell Physiol. 2013;228:1264–9. doi: 10.1002/jcp.24281. [DOI] [PubMed] [Google Scholar]
- 67.Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer. 2007;43:210–9. doi: 10.1016/j.ejca.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Sandhu R, Rivenbark AG, Coleman WB. Loss of post-transcriptional regulation of DNMT3b by microRNAs: a possible molecular mechanism for the hypermethylation defect observed in a subset of breast cancer cell lines. Int J Oncol. 2012;41:721–32. doi: 10.3892/ijo.2012.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cui P, Lin Q, Ding F, Xin C, et al. A comparison between ribo-minus RNA-sequencing and polyA-selected RNA-sequencing. Genomics. 2010;96:259–65. doi: 10.1016/j.ygeno.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Zhang Y, Li S, Lu J, et al. Genome-wide DNA methylome analysis reveals epigenetically dysregulated non-coding RNAs in human breast cancer. Sci Rep. 2015;5:8790. doi: 10.1038/srep08790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrow TM, Barault L, Ellsworth RE, Harris HR, et al. Aberrant methylation of imprinted genes is associated with negative hormone receptor status in invasive breast cancer. Int J Cancer. 2015;137:537–47. doi: 10.1002/ijc.29419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen Y, Wang Z, Loo LW, Ni Y, et al. LINC00472 expression is regulated by promoter methylation and associated with disease-free survival in patients with grade 2 breast cancer. Breast Cancer Res Treat. 2015;154:473–82. doi: 10.1007/s10549-015-3632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Léveillé N, Melo CA, Rooijers K, Díaz-Lagares A, et al. Genome-wide profiling of p53-regulated enhancer RNAs uncovers a subset of enhancers controlled by a lncRNA. Nat Commun. 2015;6:6520. doi: 10.1038/ncomms7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 75.Brooks J, Eltom SE. Malignant transformation of mammary epithelial cells by ectopic overexpression of the aryl hydrocarbon receptor. Curr Cancer Drug Targets. 2011;11:654–69. doi: 10.2174/156800911795655967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlezinger JJ, Liu D, Farago M, Seldin DC, et al. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–87. doi: 10.1515/BC.2006.145. [DOI] [PubMed] [Google Scholar]
- 77.Wang T, Gavin HM, Arlt VM, Lawrence BP, et al. Aryl hydrocarbon receptor activation during pregnancy, and in adult nulliparous mice, delays the subsequent development of DMBA-induced mammary tumors. Int J Cancer. 2011;128:1509–23. doi: 10.1002/ijc.25493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spink BC, Bennett JA, Lostritto N, Cole JR, Spink DC. Expression of the aryl hydrocarbon receptor is not required for the proliferation, migration, invasion, or estrogen-dependent tumorigenesis of MCF-7 breast cancer cells. Mol Carcinog. 2013;52:544–54. doi: 10.1002/mc.21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brunnberg S, Andersson P, Lindstam M, Paulson I, et al. The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure--effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 80.Li ZD, Wang K, Yang XW, Zhuang ZG, et al. Expression of aryl hydrocarbon receptor in relation to p53 status and clinicopathological parameters in breast cancer. Int J Clin Exp Pathol. 2014;7:7931–7. [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao S, Ohara S, Kanno Y, Midorikawa Y, et al. HER2 overexpression-mediated inflammatory signaling enhances mammosphere formation through up-regulation of aryl hydrocarbon receptor transcription. Cancer Lett. 2013;330:41–8. doi: 10.1016/j.canlet.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 82.Englert NA, Turesky RJ, Han W, Bessette EE, et al. Genetic and epigenetic regulation of AHR gene expression in MCF-7 breast cancer cells: role of the proximal promoter GC-rich region. Biochem Pharmacol. 2012;84:722–35. doi: 10.1016/j.bcp.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsieh TH, Tsai CF, Hsu CY, Kuo PL, et al. Phthalates induce proliferation and invasiveness of estrogen receptor-negative breast cancer through the AhR/HDAC6/c-Myc signaling pathway. FASEB J. 2012;26:778–87. doi: 10.1096/fj.11-191742. [DOI] [PubMed] [Google Scholar]
- 84.Wu D, Wong P, Li W, Vogel CF, Matsumura F. Suppression of WIF-1 through promoter hypermethylation causes accelerated proliferation of the aryl hydrocarbon receptor (AHR) overexpressing MCF10AT1 breast cancer cells. Toxicology. 2011;285:97–103. doi: 10.1016/j.tox.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 85.Goode G, Pratap S, Eltom SE. Depletion of the aryl hydrocarbon receptor in MDA-MB-231 human breast cancer cells altered the expression of genes in key regulatory pathways of cancer. PLoS One. 2014;9:e100103. doi: 10.1371/journal.pone.0100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, et al. An estrogen receptor-alpha/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors:repressive effects of p53 on BRCA-1 transcription. Neoplasia. 2005;7:873–82. doi: 10.1593/neo.05256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hockings JK, Thorne PA, Kemp MQ, Morgan SS, et al. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 2006;66:2224–32. doi: 10.1158/0008-5472.CAN-05-1619. [DOI] [PubMed] [Google Scholar]
- 88.Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 2010;140:1607–14. doi: 10.3945/jn.110.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papoutsis AJ, Borg JL, Selmin OI, Romagnolo DF. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor-ligand TCDD are prevented by resveratrol in MCF-7 cells. J. Nutr. Biochem. 2012;23:1324–32. doi: 10.1016/j.jnutbio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 90.Brown NM, Manzolillo PA, Zhang JX, Wang J, Lamartiniere CA. Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis. 1998;19:1623–1629. doi: 10.1093/carcin/19.9.1623. [DOI] [PubMed] [Google Scholar]
- 91.Russo J, Lynch H, Russo IH. Mammary gland architecture as a determining factor in the susceptibility of the human breast to cancer. Breast J. 2001;7:278–291. doi: 10.1046/j.1524-4741.2001.21033.x. [DOI] [PubMed] [Google Scholar]
- 92.Hushka LJ, Williams JS, Greenlee WF. Characterization of 2,3,7,8 tetrachlorodibenzofuran-dependent suppression and AH receptor pathway gene expression in the developing mouse mammary gland. Toxicol. Appl. Pharmacol. 1998;152:200–210. doi: 10.1006/taap.1998.8508. [DOI] [PubMed] [Google Scholar]
- 93.Lewis BC, Hudgins S, Lewis A, Schorr K, et al. In utero and lactational treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin impairs mammary gland differentiation but does not block the response to exogenous estrogen in the postpubertal female rat. Toxicol. Sci. 2001;62:46–53. doi: 10.1093/toxsci/62.1.46. [DOI] [PubMed] [Google Scholar]
- 94.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol. Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 95.Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol. Carcinog. 2015;54:261–9. doi: 10.1002/mc.22095. [DOI] [PubMed] [Google Scholar]
- 96.Romagnolo DF, Papoutsis AJ, Laukaitis C, Selmin OI. Constitutive expression of AhR and BRCA-1 promoter CpG hypermethylation as biomarkers of ERα-negative breast tumorigenesis. BMC Cancer. 2015;15:1026. doi: 10.1186/s12885-015-2044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leijs MM, Koppe JG, Olie K, van Aalderen WM. Delayed initiation of breast development in girls with higher prenatal dioxin exposure; a longitudinal cohort study. Chemosphere. 2008;6:999–1004. doi: 10.1016/j.chemosphere.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed S, Wang A, Celius T, Matthews J. Zinc finger nuclease-mediated knockout of AHR or ARNT in human breast cancer cells abolishes basal and ligand-dependent regulation of CYP1B1 and differentially affects estrogen receptor α transactivation. Toxicol Sci. 2014;138:89–103. doi: 10.1093/toxsci/kft274. [DOI] [PubMed] [Google Scholar]
- 99.Morrow D, Qin C, Smith R, 3rd, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J Steroid Biochem Mol Biol. 2004;88:27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 100.Tran C, Richmond O, Aaron L, Powell JB. Inhibition of constitutive aryl hydrocarbon receptor (AhR) signaling attenuates androgen independent signaling and growth in (C4-2) prostate cancer cells. Biochem Pharmacol. 2013;85:753–62. doi: 10.1016/j.bcp.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geens T, Aerts D, Berthot C, Bourguignon JP, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–40. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- 102.Seachrist DD, Bonk KW, Ho SM, Prins GS. A review of the carcinogenic potential of bisphenol A. Reprod Toxicol. 2016;59:167–82. doi: 10.1016/j.reprotox.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Katchy A, Pinto C, Jonsson P, Nguyen-Vu T, et al. Coexposure to phytoestrogens and bisphenol a mimics estrogenic effects in an additive manner. Toxicol. Sci. 2014;138:21–35. doi: 10.1093/toxsci/kft271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sengupta S, Obiorah I, Maximov PY, Curpan R, Jordan VC. Molecular mechanism ofaction of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–78. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee HR, Hwang KA, Park MA, Yi BR, et al. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int. J. Mol. Med. 2012;29:883–90. doi: 10.3892/ijmm.2012.903. [DOI] [PubMed] [Google Scholar]
- 107.Dairkee SH, Luciani-Torres MG, Moore DH, Goodson WH., III Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis. 2013;34:703–712. doi: 10.1093/carcin/bgs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang W, Fang Y, Shi X, Zhang M, et al. Effect of bisphenol A on the EGFR-STAT3 pathway in MCF-7 breast cancer cells. Mol Med Rep. 2012;5:41–7. doi: 10.3892/mmr.2011.583. [DOI] [PubMed] [Google Scholar]
- 109.Diel P, Olff S, Schmidt S, Michna H. Effects of the environmental estrogens bisphenol A, o,p'-DDT, p-tert-octylphenol and coumestrol on apoptosis induction, cell proliferation and the expression of estrogen sensitive molecular parameters in the human breast cancer cell line MCF-7. J. Steroid. Biochem. Mol. Biol. 2002;80:61–70. doi: 10.1016/s0960-0760(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 110.Pupo M, Pisano A, Lappano R, Santolla MF, et al. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ Health Perspect. 2012;120:1177–82. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song H, Zhang T, Yang P, Li M, et al. Low doses of bisphenol A stimulate the proliferation of breast cancer cells via ERK1/2/ERRγ signals. Toxicol In Vitro. 2015;30:521–8. doi: 10.1016/j.tiv.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 112.Goodson WH, 3rd, Luciani MG, Sayeed SA, Jaffee IM, et al. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis. 2011;32:1724–33. doi: 10.1093/carcin/bgr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mlynarcikova A, Macho L, Fickova M. Bisphenol A alone or in combination with estradiol modulates cell cycle- and apoptosis-related proteins and genes in MCF7 cells. Endocr Regul. 2013;47:189–99. doi: 10.4149/endo_2013_04_189. [DOI] [PubMed] [Google Scholar]
- 114.Lapensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and –negative breast cancer cells. Environ Health Perspect. 2009;117:175–80. doi: 10.1289/ehp.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buteau-Lozano H, Velasco G, Cristofari M, Balaguer P, Perrot-Applanat M. Xenoestrogens modulate vascular endothelial growth factor secretion in breast cancer cells through an estrogen receptor-dependent mechanism. J. Endocrinol. 2008;196:399–412. doi: 10.1677/JOE-07-0198. [DOI] [PubMed] [Google Scholar]
- 116.Castillo Sanchez R, Gomez R, Perez Salazar E. Bisphenol A Induces Migration through a GPER-, FAK-, Src-, and ERK2-Dependent Pathway in MDA-MB-231 Breast Cancer Cells. Chem Res Toxicol. 2016;29:285–95. doi: 10.1021/acs.chemrestox.5b00457. [DOI] [PubMed] [Google Scholar]
- 117.Zhang XL, Wang HS, Liu N, Ge LC. Bisphenol A stimulates the epithelial mesenchymal transition of estrogen negative breast cancer cells via FOXA1 signals. Arch Biochem Biophys. 2015;585:10–6. doi: 10.1016/j.abb.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Yang L, Luo L, Ji W, Gong C, et al. Effect of low dose bisphenol A on the early differentiation of human embryonic stem cells into mammary epithelial cells. Toxicol Lett. 2013;218:187–93. doi: 10.1016/j.toxlet.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 119.Qin XY, Fukuda T, Yang L, Zaha H, et al. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol. Ther. 2012;13:296–306. doi: 10.4161/cbt.18942. [DOI] [PubMed] [Google Scholar]
- 120.Fernandez SV, Huang Y, Snider KE, Zhou Y, et al. Expression and DNA methylation changes in human breast epithelial cells after bisphenol A exposure. Int. J. Oncol. 2012;41:369–377. doi: 10.3892/ijo.2012.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones LP, Sampson A, Kang HJ, Kim HJ, et al. Loss of BRCA1 leads to an increased sensitivity to Bisphenol A. Toxicol Lett. 2010;199:261–8. doi: 10.1016/j.toxlet.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, et al. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol. Endocrinol. 2011;25:1915–23. doi: 10.1210/me.2011-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–9. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weber Lozada K, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol. Reprod. 2011;85:490–7. doi: 10.1095/biolreprod.110.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the Endocrine Disruptor Bisphenol A Alters Susceptibility for Mammary Cancer. Horm. Mol. Biol. Clin. Investig. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moral R, Wang R, Russo IH, Lamartiniere CA, et al. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008;196:101–12. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 127.Wang D, Gao H, Bandyopadhyay A, Wu A, et al. Pubertal bisphenol A exposure alters murine mammary stem cell function leading to early neoplasia in regenerated glands. Cancer Prev Res. 2014;7:445–55. doi: 10.1158/1940-6207.CAPR-13-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Jenkins S, Lamartiniere CA. Cell proliferation and apoptosis in rat mammary glands following combinational exposure to bisphenol A and genistein. BMC Cancer. 2014;14:379. doi: 10.1186/1471-2407-14-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Betancourt AM, Wang J, Jenkins S, Mobley J, et al. Altered carcinogenesis and proteome in mammary glands of rats after prepubertal exposures to the hormonally active chemicals bisphenol a and genistein. J Nutr. 2012:1382S–8S. doi: 10.3945/jn.111.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Muñoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ. Health Perspect. 2007;115:80–6. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fischer C, Mamillapalli R, Goetz LG, Jorgenson E, et al. Bisphenol A (BPA) Exposure In Utero Leads to Immunoregulatory Cytokine Dysregulation in the Mouse Mammary Gland: A Potential Mechanism Programming Breast Cancer Risk. Horm Cancer. 2016 Feb 24; doi: 10.1007/s12672-016-0254-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ibrahim MA, Elbakry RH, Bayomy NA. Effect of bisphenol A on morphology, apoptosis and proliferation in the resting mammary gland of the adult albino rat. Int J Exp Pathol. 2016 Feb 15; doi: 10.1111/iep.12164. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, et al. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc. Natl. Acad. Sci. USA. 2012;109:8190–5. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hussain I, Bhan A, Ansari KI, Deb P, et al. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms. 2015;1849:697–708. doi: 10.1016/j.bbagrm.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weng YI, Hsu PY, Liyanarachchi S, Liu J, et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol. Appl. Pharmacol. 2010;248:111–121. doi: 10.1016/j.taap.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dhimolea E, Wadia PR, Murray TJ, Settles ML, et al. Prenatal exposure to BPA alters the epigenome of the rat mammary gland and increases the propensity to neoplastic development. PLoS One. 2014;9:e99800. doi: 10.1371/journal.pone.0099800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–55. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401. doi: 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tilghman SL, Bratton MR, Segar HC, Martin EC, et al. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One. 2012;7:e32754. doi: 10.1371/journal.pone.0032754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X, Xie W, Xie C, Huang C, et al. Curcumin modulates miR 19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res. 2014;28:1553–60. doi: 10.1002/ptr.5167. [DOI] [PubMed] [Google Scholar]
- 141.Bhan A, Hussain I, Ansari KI, Bobzean SAM, et al. Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J. Steroid Biochem. Mol. Biol. 2014;141:160–170. doi: 10.1016/j.jsbmb.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhan A, Hussain I, Ansari KI, Bobzean SAM, et al. Histone Methyltransferase EZH2 Is Transcriptionally Induced by Estradiol as Well as Estrogenic Endocrine Disruptors Bisphenol-A and Diethylstilbestrol. J. Mol. Biol. 2014;426:3426–3441. doi: 10.1016/j.jmb.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 143.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol—A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gertz J, Reddy TE, Varley KE, Garabedian MJ, Myers RM. Genistein and bisphenol A exposure cause estrogen receptor 1 to bind thousands of sites in a cell type-specific manner. Genome Res. 2012;22:2153–62. doi: 10.1101/gr.135681.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Avissar-Whiting M, Veiga KR, Uhl KM, Maccani MA, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol. 2010;4:401–6. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.M'hamed IF, Privat M, Ponelle F, Penault-Llorca F, et al. Identification of miR-10b, miR-26a, miR-146a and miR-153 as potential triple-negative breast cancer biomarkers. Cell. Oncol. (Dordr) 2015;38:433–42. doi: 10.1007/s13402-015-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289:74–82. doi: 10.1016/j.tox.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 148.Tang WY, Morey LM, Cheung YY, Birch L, et al. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology. 2012;153:42–55. doi: 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ayotte JD, Belaval M, Olson SA, Burow KR, et al. Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci. Total Environ. 2014 doi: 10.1016/j.scitotenv.2014.02.057. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 150.Sorg TJ, Chen AS, Wang L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res. 2014;48:156–169. doi: 10.1016/j.watres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 151.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–91. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 152.Stoica A, Pentecost E, Martin MB. Effects of arsenite on estrogen receptor-alpha expression and activity in MCF-7 breast cancer cells. Endocrinology. 2000;141:3595–3602. doi: 10.1210/endo.141.10.7704. [DOI] [PubMed] [Google Scholar]
- 153.Ren X, McHale CM, Skibola CF, Smith AH, et al. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ. Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Muñoz A, Chervona Y, Hall M, Kluz T, et al. Sex-specific patterns and deregulation of endocrine pathways in the gene expression profiles of Bangladeshi adults exposed to arsenic contaminated drinking water. Toxicol Appl Pharmacol. 2015;284:330–8. doi: 10.1016/j.taap.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tong D, Ortega J, Kim C, Huang J, Gu L, Li GM. Arsenic Inhibits DNA Mismatch Repair by Promoting EGFR Expression and PCNA Phosphorylation. J Biol Chem. 2015;290:14536–41. doi: 10.1074/jbc.M115.641399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bhattacharjee P, Banerjee M, Giri AK. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ Int. 2013;53:29–40. doi: 10.1016/j.envint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 157.Chou WC, Chung YT, Chen HY, Wang CJ, et al. Maternal arsenic exposure and DNA damage biomarkers, and the associations with birth outcomes in a general population from Taiwan. PLoS One. 2014;9:e86398. doi: 10.1371/journal.pone.0086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schrauzer GN, White DA, McGinness JE, Schneider CJ, Bell LJ. Arsenic and cancer: effects of joint administration of arsenite and selenite on the genesis of mammary adenocarcinoma in inbred female C3H/St mice. Bioinorg Chem. 1978;9:245–253. doi: 10.1016/s0006-3061(78)80005-2. [DOI] [PubMed] [Google Scholar]
- 159.Peremarti J, Ramos F, Marcos R, Hernandez A. Arsenic exposure disrupts the normal function of the FA/BRCA repair pathway. Toxicol Sci. 2014;142:93–104. doi: 10.1093/toxsci/kfu159. [DOI] [PubMed] [Google Scholar]
- 160.Muszyńska M, Jaworska-Bieniek K, Durda k, Sukiennicki G, et al. Arsenic (As) and breast cancer risk. Her. Cancer in Clin. Pract. 2012;10(Suppl 4):A8. [Google Scholar]
- 161.Parodi DA, Greenfield M, Evans C, Chichura A, et al. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod Toxicol. 2015;54:66–75. doi: 10.1016/j.reprotox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol. 2004;198:377–84. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 163.Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of "whole-life" exposure to inorganic arsenic in CD1 mice. Toxicol Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu Y, Hock JM, Sullivan C, Fang G, et al. Activation of the p38 MAPK/Akt/ERK1/2 signal pathways is required for the protein stabilization of CDC6 and cyclin D1 in low-dose arsenite-induced cell proliferation. J. Cell. Biochem. 2010;111:546–1555. doi: 10.1002/jcb.22886. [DOI] [PubMed] [Google Scholar]
- 165.Xu Y, Tokar EJ, Waalkes MP. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch. Toxicol. 2014;88:263–274. doi: 10.1007/s00204-013-1131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ruiz-Ramos R, Lopez-Carrillo L, Albores A, Hernandez-Ramirez RU, Cebrian ME. Sodium arsenite alters cell cycle and MTHFR, MT1/2, and c-Myc protein levels in MCF-7 cells. Toxicol. Appl. Pharmacol. 2009;241:269–274. doi: 10.1016/j.taap.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 167.Ling YH, Jiang JD, Holland JF, Perez-Soler R. Arsenic trioxide produces polymerization of microtubules and mitotic arrest before apoptosis in human tumor cell lines. Mol. Pharmacol. 2002;62:529–538. doi: 10.1124/mol.62.3.529. [DOI] [PubMed] [Google Scholar]
- 168.Wang Y, Wang L, Yin C, An B, et al. Arsenic trioxide inhibits breast cancer cell growth via microRNA-328/hERG pathway in MCF-7 cells. Mol. Med. Rep. 2015;12:1233–1238. doi: 10.3892/mmr.2015.3558. [DOI] [PubMed] [Google Scholar]
- 169.Benbrahim-Tallaa L, Webber MM, Waalkes MP. Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environ Health Perspect. 2005;113:1134–9. doi: 10.1289/ehp.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nakareangrit W, Thiantanawat A, Visitnonthachai D, Watcharasit P, Satayavivad J. Sodium arsenite inhibited genomic estrogen signaling but induced pERalpha (Ser118) via MAPK pathway in breast cancer cells. Environ. Toxicol. 2015 doi: 10.1002/tox.22122. [DOI] [PubMed] [Google Scholar]
- 171.Chen GC, Guan LS, Hu WL, Wang ZY. Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Res. 2002;22:633–638. [PubMed] [Google Scholar]
- 172.Du J, Zhou N, Liu H, Jiang F, et al. Arsenic induces functional re-expression of estrogen receptor alpha by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS One. 2012;7:e35957. doi: 10.1371/journal.pone.0035957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ding L, Saunders RJ, Drobná Z, Walton FS, et al. Methylation of Arsenic by Recombinant Human Wild-Type Arsenic (+3 Oxidation State) Methyltransferase and its Methionine 287 Threonine (M287T) Polymorph: Role of Glutathione. Toxicol Appl Pharmacol. 2012;264:121–130. doi: 10.1016/j.taap.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wlodarczyk BJ, Zhu H, Finnell RH. Mthfr gene ablation enhances susceptibility to arsenic prenatal toxicity. Toxicol. Appl. Pharmacol. 2014;275:22–27. doi: 10.1016/j.taap.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Niedzwiecki MM, Hall MN, Liu X, Oka J, et al. A dose-response study of arsenic exposure and global methylation of peripheral blood mononuclear cell DNA in Bangladeshi adults. Environ Health Perspect. 2013;121:1306–12. doi: 10.1289/ehp.1206421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci. 2006;91:372–81. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- 177.Ramirez T, Brocher J, Stopper H, Hock R. Sodium arsenite modulates histone acetylation, histone deacetylase activity and HMGN protein dynamics in human cells. Chromosoma. 2008;117:147–57. doi: 10.1007/s00412-007-0133-5. [DOI] [PubMed] [Google Scholar]
- 178.Lu G, Xu H, Chang D, Wu Z, et al. Arsenic exposure is associated with DNA hypermethylation of the tumor suppressor gene p16. J. Occup. Med. Toxicol. 2014;9:42. doi: 10.1186/s12995-014-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang TC, Schmitt MT, Mumford JL. Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis. 2003;24:1811–7. doi: 10.1093/carcin/bgg141. [DOI] [PubMed] [Google Scholar]
- 180.Jiang R, Li Y, Zhang A, Wang B, et al. The acquisition of cancer stem cell-like properties and neoplastic transformation of human keratinocytes induced by arsenite involves epigenetic silencing of let-7c via Ras/NF-κB. Toxicol Lett. 2014;227:91–8. doi: 10.1016/j.toxlet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 181.Cardenas A, Houseman EA, Baccarelli AA, Quamruzzaman Q, et al. In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics. 2015;10:1054–63. doi: 10.1080/15592294.2015.1105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mense SM, Singh B, Remotti F, Liu X, Bhat HK. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009;30:1202–8. doi: 10.1093/carcin/bgp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dias MF, Sousa E, Cabrita S, Patrício J, Oliveira CF. Chemoprevention of DMBA-Induced Mammary Tumors in Rats by a Combined Regimen of Alpha-Tocopherol, Selenium, and Ascorbic Acid. Breast J. 2000;6:14–19. doi: 10.1046/j.1524-4741.2000.98071.x. [DOI] [PubMed] [Google Scholar]
- 184.Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010;70:5749–58. doi: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- 185.Hong SW, Jin DH, Hahm ES, Yim SH, et al. Ascorbate (vitamin C) induces cell death through the apoptosis-inducing factor in human breast cancer cells. Oncol. Rep. 2007;18:811–5. [PubMed] [Google Scholar]
- 186.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martínez JA, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–6. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Song SJ, Poliseno L, Song MS, Ala U, et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell. 2013;154:311–24. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leung HY, Yung LH, Shi G, Lu AL, Leung LK. The red wine polyphenol resveratrol reduces polycyclic aromatic hydrocarbon-induced DNA damage in MCF-10A cells. Br J Nutr. 2009;102:1462–8. doi: 10.1017/S0007114509990481. [DOI] [PubMed] [Google Scholar]
- 189.Whitsett TG, Jr, Lamartiniere CA. Genistein and resveratrol: mammary cancer chemoprevention and mechanisms of action in the rat. Expert Rev Anticancer Ther. 2006;6:1699–706. doi: 10.1586/14737140.6.12.1699. [DOI] [PubMed] [Google Scholar]
- 190.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–54. [PubMed] [Google Scholar]
- 191.Boocock DJ, Faust GE, Patel KR, Schinas AM, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 192.Zhu W, Qin W, Zhang K, Rottinghaus GE, et al. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr Cancer. 2012;64:393–400. doi: 10.1080/01635581.2012.654926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee H, Zhang P, Herrmann A, et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc. Natl. Acad. Sci. USA. 2012;109:7765–9. doi: 10.1073/pnas.1205132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Qin W, Zhang K, Clarke K, Weiland T, Sauter ER. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr Cancer. 2014;66:270–7. doi: 10.1080/01635581.2014.868910. [DOI] [PubMed] [Google Scholar]
- 195.Tili E, Michaille JJ, Alder H, et al. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFβ signaling pathway in SW480 cells. Biochem. Pharmacol. 2010;80:2057–65. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang S, Qin C, Safe SH. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: effects of structure and cell context. Environ Health Perspect. 2003;111:1877–82. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fritz WA, Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein down-regulates androgen and estrogen receptor expression in the rat prostate. Mol Cell Endocrinol. 2002;186:89–99. doi: 10.1016/s0303-7207(01)00663-3. [DOI] [PubMed] [Google Scholar]
- 198.Murrill WB, Brown NM, Zhang JX, Manzolillo PA, et al. Prepubertal genistein exposure suppresses mammary cancer and enhances gland differentiation in rats. Carcinogenesis. 1996;17:1451–7. doi: 10.1093/carcin/17.7.1451. [DOI] [PubMed] [Google Scholar]
- 199.Cabanes A, Wang M, Olivo S, de Assis S, et al. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–8. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 200.Bosviel R, Dumollard E, Déchelotte P, Bignon YJ, Bernard-Gallon D. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? OMICS. 2012;16:235–44. doi: 10.1089/omi.2011.0105. [DOI] [PubMed] [Google Scholar]
- 201.Xie Q, Bai Q, Zou LY, Zhang QY, Zhou Y, et al. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer. 2014;53:422–31. doi: 10.1002/gcc.22154. [DOI] [PubMed] [Google Scholar]
- 202.Li Y, Chen H, Hardy TM, Tollefsbol TO. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS One. 2013;8:e54369. doi: 10.1371/journal.pone.0054369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li Y, Meeran SM, Patel SN, Chen H, et al. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol. Cancer. 2013;12:9. doi: 10.1186/1476-4598-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Caruso JA, Campana R, Wei C, Su CH, et al. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERα-positive breast cancer cells. Cell Cycle. 2014;13:2587–99. doi: 10.4161/15384101.2015.942210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br. J. Cancer. 2006;94:407–26. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Malejka-Giganti D, Parkin DR, Bennett KK, Lu Y. Suppression of mammary gland carcinogenesis by post-initiation treatment of rats with tamoxifen or indole-3-carbinol or their combination. Eur J Cancer Prev. 2007;16:130–41. doi: 10.1097/01.cej.0000228401.14988.50. [DOI] [PubMed] [Google Scholar]
- 207.Chen I, McDougal A, Wang F, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenic and antitumorigenic activity of diindolylmethane. Carcinogenesis. 1998;19:1631–9. doi: 10.1093/carcin/19.9.1631. [DOI] [PubMed] [Google Scholar]
- 208.Beaver LM, Yu TW, Sokolowski EI, Williams DE, et al. 3,3'-Diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012;263:345–51. doi: 10.1016/j.taap.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3'-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Busbee PB, Nagarkatti M, Nagarkatti PS. Natural indoles, indole-3-carbinol and 3,3'-diindolymethane, inhibit T cell activation by staphylococcal enterotoxin B through epigenetic regulation involving HDAC expression. Toxicol Appl Pharmacol. 2014;274:7–16. doi: 10.1016/j.taap.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hanieh H. Aryl hydrocarbon receptor-microRNA-212/132 axis in human breast cancer suppresses metastasis by targeting SOX4. Mol Cancer. 2015;14:172. doi: 10.1186/s12943-015-0443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Marques M, Laflamme L, Benassou I, Cissokho C, et al. Low levels of 3,3'-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol. BMC Cancer. 2014;14:524. doi: 10.1186/1471-2407-14-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Q, Yao Y, Eades G, Liu Z, et al. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene. 2014;33:2589–600. doi: 10.1038/onc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Meeran SM, Patel SN, Li Y, Shukla S, Tollefsbol TO. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One. 2012;7:e37748. doi: 10.1371/journal.pone.0037748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sinha S, Shukla S, Khan S, Tollefsbol TO, Meeran SM. Epigenetic reactivation of p21CIP1/WAF1 and KLOTHO by a combination of bioactive dietary supplements is partially ERα-dependent in ERα-negative human breast cancer cells. Mol. Cell. Endocrinol. 2015;406:102–14. doi: 10.1016/j.mce.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lubecka-Pietruszewska K, Kaufman-Szymczyk A, Stefanska B, Cebula-Obrzut B, et al. Sulforaphane Alone and in Combination with Clofarabine Epigenetically Regulates the Expression of DNA Methylation-Silenced Tumour Suppressor Genes in Human Breast Cancer Cells. J Nutrigenet Nutrigenomics. 2015;8:91–101. doi: 10.1159/000439111. [DOI] [PubMed] [Google Scholar]
- 218.Nian H, Delage B, Ho E, Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009;50:213–21. doi: 10.1002/em.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shorey LE, Madeen EP, Atwell LL, Ho E, et al. Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol Appl Pharmacol. 2013;270:60–9. doi: 10.1016/j.taap.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kavanagh KT, Hafer LJ, Kim DW, Mann KK, et al. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001;82:387–98. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 221.Belguise K, Guo S, Yang S, Rogers AE, et al. Green tea polyphenols reverse cooperation between c-Rel and CK2 that induces the aryl hydrocarbon receptor, slug, and an invasive phenotype. Cancer Res. 2007;67:11742–50. doi: 10.1158/0008-5472.CAN-07-2730. [DOI] [PubMed] [Google Scholar]
- 222.Fang MZ, Wang Y, Ai N, Hou Z, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 223.Choi H, Chun YS, Shin YJ, Ye SK, et al. Curcumin attenuates cytochrome P450 induction in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin by ROS-dependently degrading AhR and ARNT. Cancer Sci. 2008;99:2518–24. doi: 10.1111/j.1349-7006.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Collins HM, Abdelghany MK, Messmer M, Yue B. Differential effects of garcinol and curcumin on histone and p53 modifications in tumour cells. BMC Cancer. 2013;13:37. doi: 10.1186/1471-2407-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tsai CF, Hsieh TH, Lee JN, Hsu CY. Curcumin Suppresses Phthalate-Induced Metastasis and the Proportion of Cancer Stem Cell (CSC)-like Cells via the Inhibition of AhR/ERK/SK1 Signaling in Hepatocellular Carcinoma. J Agric Food Chem. 2015;63:10388–98. doi: 10.1021/acs.jafc.5b04415. [DOI] [PubMed] [Google Scholar]
- 226.Ghadirian P, Narod S, Fafard E, Costa M, et al. Breast cancer risk in relation to the joint effect of BRCA mutations and diet diversity. Breast Cancer Res. Treat. 2009;117:417–22. doi: 10.1007/s10549-008-0292-y. [DOI] [PubMed] [Google Scholar]
- 227.Crous-Bou M, Fung TT, Prescott J, Julin B, et al. Mediterranean diet and telomere length in Nurses' Health Study: population based cohort study. BMJ. 2014;349:g667. doi: 10.1136/bmj.g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Manzanares MA, Solanas M, Moral R, Escrich R, et al. Dietary extra-virgin olive oil and corn oil differentially modulate the mRNA expression of xenobiotic-metabolizing enzymes in the liver and in the mammary gland in a rat chemically induced breast cancer model. Eur. J. Cancer Prev. 2014 Apr 9; doi: 10.1097/CEJ.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 229.Rodríguez-Miguel C, Moral R, Escrich R, Vela E, et al. The Role of Dietary Extra Virgin Olive Oil and Corn Oil on the Alteration of Epigenetic Patterns in the Rat DMBA-Induced Breast Cancer Model. PLoS One. 2015 Sep 24;10(9):e0138980. doi: 10.1371/journal.pone.0138980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Li Y, Jiang F, Chen L, Yang Y, et al. Blockage of TGFβ-SMAD2 by demethylation-activated miR-148a is involved in caffeic acid-induced inhibition of cancer stem cell-like properties in vitro and in vivo. FEBS Open Bio. 2015;5:466–75. doi: 10.1016/j.fob.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim HG, Han EH, Im JH, Lee EJ, et al. Caffeic acid phenethyl ester inhibits 3-MC-induced CYP1A1 expression through induction of hypoxia-inducible factor-1α. Biochem Biophys Res Commun. 2015;465:562–8. doi: 10.1016/j.bbrc.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 232.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kikuno N, Shiina H, Urakami S, Kawamoto K, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–60. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- 234.de Assis S, Warri A, Benitez C, Helferich W, Hilakivi-Clarke L. Protective effects of prepubertal genistein exposure on mammary tumorigenesis are dependent on BRCA1 expression. Cancer Prev. Res. (Phila) 2011;4:1436–48. doi: 10.1158/1940-6207.CAPR-10-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS One. 2013;8:e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Chen J, Lin C, Yong W, Ye Y, Huang Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem. 2015;35:722–8. doi: 10.1159/000369732. [DOI] [PubMed] [Google Scholar]
- 237.Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, Shahryari V, Saini S, Hirata H, Ueno K, Chang I, Tanaka Y, Tabatabai ZL, Enokida H, Nakagawa M, Dahiya R. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS One. 2012;7(8):e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Day JK, Bauer AM, DesBordes C, Zhuang Y, et al. Genistein alters methylation patterns in mice. J. Nutr. 2002;132:2419S–2423S. doi: 10.1093/jn/132.8.2419S. [DOI] [PubMed] [Google Scholar]
- 240.Hwang KA, Park MA, Kang NH, Yi BR, et al. Anticancer effect of genistein on BG-1 ovarian cancer growth induced by 17 β-estradiol or bisphenol A via the suppression of the crosstalk between estrogen receptor α and insulin-like growth factor-1 receptor signaling pathways. Toxicol. Appl. Pharmacol. 2013;272:637–46. doi: 10.1016/j.taap.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 241.Romagnolo DF, Selmin OI. Flavonoids and cancer prevention: a review of the evidence. J. Nutr. Gerontol. Geriatr. 2012;31:206–38. doi: 10.1080/21551197.2012.702534. [DOI] [PubMed] [Google Scholar]
- 242.Bulzomi P, Bolli A, Galluzzo P, Acconcia F, et al. The naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. IUBMB Life. 2012;64:690–6. doi: 10.1002/iub.1049. [DOI] [PubMed] [Google Scholar]
- 243.Kang NH, Hwang KA, Lee HR, et al. Resveratrol regulates the cell viability promoted by 17β-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor α and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem Toxicol. 2013;59:373–9. doi: 10.1016/j.fct.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 244.Tsang V, Fry RC, Niculescu MD, Rager JE, et al. The epigenetic effects of a high prenatal folate Intake in male mouse fetuses exposed in utero to arsenic. Toxicol. Appl. Pharmacol. 2012;264:439–450. doi: 10.1016/j.taap.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, Johnson KA, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2006;83:895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- 246.Maruti SS, Ulrich CM, White E. Folate and one-carbon metabolism nutrients from supplements and diet in relation to breast cancer risk. Am. J. Clin. Nutr. 2009;89:624–33. doi: 10.3945/ajcn.2008.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jakubowska A, Gronwald J, Menkiszak J, Gorski B, et al. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res. Treat. 2007;104:299–308. doi: 10.1007/s10549-006-9417-3. [DOI] [PubMed] [Google Scholar]
- 248.Crugliano T, Quaresima B, Gaspari M, Faniello MC, et al. Specific changes in the proteomic pattern produced by the BRCA1-Ser1841Asn missense mutation. Int. J. Biochem. Cell Biol. 2007;39:220–6. doi: 10.1016/j.biocel.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 249.Campbell IG, Jones TA, Foulkes WD, Trowsdale J, et al. Folatebinding protein is a marker for ovarian cancer. Cancer Res. 1991;51:5329–5338. [PubMed] [Google Scholar]
- 250.Jhaveri MS, Rait AS, Chung KN, Trepel JB, Chang EH. Antisense oligonucleotides targeted to the human alpha folate receptor inhibit breast cancer cell growth and sensitize the cells to doxorubicin treatment. Mol. Cancer Therapeutics. 2004;3:1505–1512. [PubMed] [Google Scholar]
- 251.Acharyya N, Deb B, Chattopadhyay S, Maiti S. Arsenic-Induced Antioxidant Depletion, Oxidative DNA Breakage, and Tissue Damages are Prevented by the Combined Action of Folate and Vitamin B12. Biol Trace Elem Res. 2015;168:122–32. doi: 10.1007/s12011-015-0324-5. [DOI] [PubMed] [Google Scholar]
- 252.Llanos AA, Marian C, Brasky TM, Dumitrescu RG, et al. Associations between genetic variation in one-carbon metabolism and LINE-1 DNA methylation in histologically normal breast tissues. Epigenetics. 2015;10:727–35. doi: 10.1080/15592294.2015.1062205. [DOI] [PMC free article] [PubMed] [Google Scholar]