Abstract
Objectives:
The aim of this clinical assessment was to ascertain whether a 70-year-old Canadian patient, who had no history of out-of-country travel, had contracted a Babesia infection.
Methods:
The adult human male developed constitutional symptoms, which included sweats, chills, and immobilizing fatigue, and was screened for human babesiosis. Subsequent testing included a complete Babesia panel that consisted of B. microti immunoflourescent antibody IgM and IgG, B. duncani immunofluorescent antibody IgM and IgG, Babesia PCR, and Babesia fluorescent in situ hybridization (FISH) test.
Results:
Both the IgM serology and the molecular FISH RNA probe were positive for B. duncani; all tests for B. microti were negative. Based on clinical symptoms and laboratory tests, the patient was diagnosed with human babesiosis. Interestingly, the patient’s wife also was confirmed positive using serological and molecular testing.
Conclusions:
This is the first report of a locally acquired case of human babesiosis in Canada caused by Babesia duncani. The geographical distribution of B. duncani in North America is much greater than previously anticipated, especially north of the Canada-United States border. Since the patient was bitten by a blacklegged tick, Ixodes scapularis, a carrier of multiple zoonotic pathogens, the author suggests that this tick species is a vector of B. duncani. Health-care providers must be aware that B. duncani is present in Canada, and poses a public health risk.
Keywords: Human babesiosis, Babesia duncani, case presentation, parasitemia, babesial piroplasm, Canada
Introduction
Babesiosis is a zoonosis caused by a malaria-like parasite typically transmitted by ixodid (hard-bodied) ticks (Acari: Ixodidae). Worldwide, there are over 100 Babesia species (Piroplasmida: Babesiidae) and, in North America, at least 4 Babesia species (i.e., Babesia bovis, Babesia canis, Babesia duncani, and Babesia microti) will cause human babesiosis.1 Dating back to 1991, the archetypes of B. duncani isolates (WA1, CA5) originated from human patients in Washington state and California.2 B. duncani is morphologically similar to, but molecularly and physiologically distinct from B. microti, a species that is common in central and eastern North America.2 Babesia piroplasms are pleomorphic with diverse stages consisting of gametocytes (polymorphous), trophozoites (round, oval, pear-shaped, amoeboid), and merozoites (paired pear-shaped forms); the latter may exhibit a “Maltese cross” tetrad formation.2 Pathologically, Babesia invades red blood cells, and will lyse them, and also has white blood cell involvement.1
Babesia can be detected by blood smear, serology, fluorescent in situ hybridization (FISH) test, or nucleic acid–based tests, such as quantitative polymerase chain reaction (qPCR) and reverse transcription polymerase chain reaction (RT-PCR).3 The blood smear depends on microscopic examination, which is labor intensive, difficult to process, and requires special taxonomic expertise.3 With certain subsets of patients, such as neonates, elderly patients, or splenectomized and immunocompromised patients, human babesiosis may be severe or fatal.4
Elsewhere, in Canada, human babesiosis, caused by B. microti, was recorded in southeastern Manitoba.5 Although the patient did not recall a tick bite, researchers purported that a blacklegged tick, Ixodes scapularis, was the vector of B. microti. Anderson et al.6 isolated B. microti from white-footed mice, Peromyscus leucopus; however, this small mammal is not indigenous to Manitoba. Therefore, the zoonotic reservoir of B. microti is likely another vertebrate, such as the deer mouse, Peromyscus maniculatus. It is noteworthy that both P. leucopus and P. maniculatus are competent reservoirs of B. microti and, likewise, the Lyme disease bacterium, Borrelia burgdorferi, a common spirochetal co-infection. Of epidemiological significance, the blacklegged tick is a vector of at least 10 tick-borne pathogens. Pertinent to the present case, the attached I. scapularis nymph (Figure 1) was likely the vector of B. duncani.
Figure 1.

Blacklegged tick, Ixodes scapularis, unfed nymph. This tick species is a vector of at least 10 tick-borne pathogens.
Photo credits: Elizabeth Spears.
Case presentation
An adult human male, age 70, living in southern Ontario developed profound fatigue, night sweats, chills, malaise, fractured sleep, fever, increased thirst, body aches, mild headaches, joint pain, and loss of concentration 3 months following a tick bite. Of significance, the patient had been bitten by a tick while hiking locally, and it went unnoticed until the replete nymph (3.2 mm) dropped off in bed one morning. An erythematous macular rash developed at the bite site and lasted for 3 days (Figure 2). A tick expert identified and confirmed the tick as an I. scapularis nymph and, when it was tested for B. burgdorferi, was found to be negative. Of note, the patient and his wife had not had any out-of-province travel. At initial presentation, the primary physician attributed the symptoms to “old age.” Subsequently, another physician made a diagnosis of fibromyalgia, while a third physician diagnosed chronic fatigue syndrome. When the patient consulted the fourth physician, he had a fever of 39.4°C, and Babesia testing was authorized. Since I. scapularis ticks are carriers of Babesia infections, blood was sent to a Clinical Laboratory Improvement Amendments (CLIA)-approved laboratory specializing in tick-borne diseases. For thorough screening, the complete Babesia panel (B. duncani immunofluorescent IgM and IgG; B. microti immunofluorescent IgM and IgG; Babesia PCR; and B. duncani fluorescent in situ hybridization (FISH) RNA probe; B. microti FISH RNA probe) was used. Each of the individual serology tests and molecular assays for B. microti and B. duncani were run separately. Initially, the lab uses part of the 18S rDNA gene as the PCR target for the Babesia PCR test. This step is followed by PCR confirmation to differentiate either B. duncani or B. microti using specific RNA probes within the amplified amplicon target. Wilson et al.7 provide the algorithm for the sensitive and species-specific digital droplet PCR assays that detect and distinguish between B. duncani and B. microti within the internal transcribed spacer (ITS) regions of the nuclear ribosomal RNAs. The serology was positive for IgM antibodies against B. duncani and the FISH RNA probe was positive for B. duncani, which, collectively, confirms the diagnosis. All testing for B. microti was negative. The patient had never received a blood transfusion and, hence, this medical fact rules out this portal of transmission.
Figure 2.
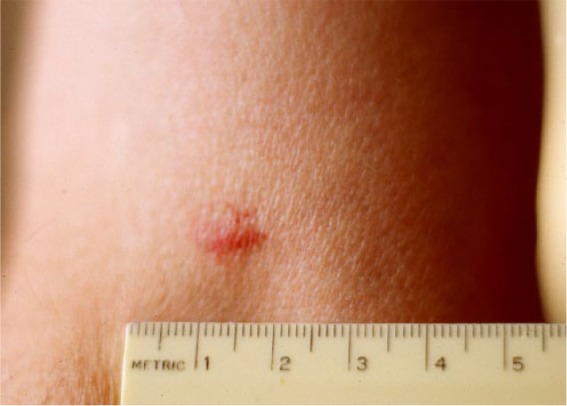
An erythematous macular lesion with diffuse borders (5 mm × 10 mm) on upper right arm 1.5 h after a replete Ixodes scapularis nymph (3.2 mm) dropped off. The rash is a hypersensitivity reaction to tick salivary compounds and, possibly, zoonotic pathogens transmitted during the blood meal.
Routine lab results were unremarkable except for the following: a low free T3: 2.3 (3.1–6.2) pmol/L, low sodium: 134 (135–145) mmol/L, and high amylase: 183 (30–110) µ/L. The liver function enzymes were gamma glutamyl transferase: 13 (14–62) µ/L, alanine aminotransferase: 24 (<50) µ/L, and aspartate aminotransferase: 31 (<35) µ/L. The high amylase reading is not uncommon for Babesia patients.
The patient was started on the standard treatment (atovaquone/proguanil and azithromycin), but quickly ran into drug intolerance. The patient developed urticarial-like rashes and upper quadrant pain. Since the half-life of atovaquone is 2.2–3.2 days (DrugBank), the treatment regimen for this patient was switched to atovaquone/proguanil (250 mg/100 mg) daily, pulsed 2 days on and 2 days off, without azithromycin. This change helped ameliorate inflammation and upper quadrant pain induced by babesial biotoxins and drug sensitivities. Fatigue and upper quadrant pain waxed and waned, and lifted gradually.
Because Babesia typically has a 4-month life cycle, 5 months of pulsed antimicrobials were administered. Since B. duncani can be recalcitrant and last for months or years, the patient is being monitored on an ongoing basis. Notably, the patient has a clinical diagnosis, which is reinforced and confirmed with serological and molecular testing, and has responded positively to Babesia treatment.
Discussion
A locally acquired case of human babesiosis, caused by B. duncani, is reported in Canada for the first time. The patient had characteristic symptoms (i.e. chills, sweats, episodes of profound fatigue) associated with human babesiosis and had positive IgM serology and positive FISH RNA probe testing for B. duncani indicating active parasitemia.
Of note, the patient’s wife had positive IgM and IgG and, likewise, positive FISH RNA testing for B. duncani; the Babesia PCR was negative. Both the patient and his wife tested negative by Babesia PCR, which suggests low parasitemia. The patient’s wife has never had a blood transfusion or an alternate sexual partner. Although sexual transmission of B. duncani was considered, it was more likely that the patient’s wife, who is post-menopausal, acquired infection by a tick bite that went unnoticed. Notably, only 14% of patients remember a tick bite.8 A patient, who is co-infected with tick-transmitted pathogens, may have acquired different pathogens either by a single co-infected tick or, sequentially, via ticks that each harbored a different pathogen. Piroplasms can be persistent in cells,9–11 and hosts can be asymptomatic carriers.
When someone is diagnosed with moderate to severe Lyme disease, Babesia should be suspected in patients as a possible co-infection, especially if there has been a poor response to standard antibiotic treatment.11 When human babesiosis is a concurrent infection with Lyme disease, symptoms are typically exacerbated.
Even though the antiquity of B. duncani is unknown, B. microti dates back 20–30 million years.12 This intraerythrocytic hemoparasite was recently detected in a fossilized Amblyomma sp. nymph that was believed to be groomed from a monkey; this engorged nymph and its blood meal were preserved in amber located in a Dominican Republic cave. Some authors claim that patients must visit an endemic area to contract human babesiosis.5 However, Neotropical and southern temperate songbirds annually introduce bird-feeding ticks during northward spring migration and widely disperse these ticks across Canada.13 Hersh et al.14 reported a triple infection (Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi) in an I. scapularis nymph collected from a Veery, Catharus fuscescens, a Neotropical, ground-foraging songbird. This microbial triple infection reveals the capability of passerine migrants to transport bird-feeding ticks long distances and widely disperse Babesia-infected ticks, including I. scapularis, into new geographical areas. Since the patient was bitten by an I. scapularis nymph, and this tick species is a carrier of multiple tick-borne pathogens, the author suggests that I. scapularis is a vector of B. duncani. Moreover, B. duncani is morphologically similar to B. microti.2 Biogeographically, B. duncani is currently reported throughout continental United States.
The zoonotic wildlife reservoir of B. duncani has not been confirmed in North America. Hornok et al.15 reported four Babesia species in three ixodid bat tick species collected in Hungary and Romania. One of these species, Ixodes vespertilionis, is known to parasitize humans. Not only are certain passerines reservoir hosts, chiropterans may also be zoonotic reservoirs of Babesia species.
Babesia hemoparasites can unknowingly be transmitted to humans via blood transfusion. Although human babesiosis is currently not a nationally notifiable disease in Canada, it is in the United States. In Canada, the Canadian Blood Services conducted a large seroprevalence survey for Babesia and found nil Babesia antibody-positive donors. Apparently, Health Canada does not have an approved blood donor screening assay for Babesia piroplasms. Because blood donors can be asymptomatic carriers of B. duncani and B. microti, and the majority of people don’t remember a tick bite, Canadian Blood Services needs to routinely monitor intake blood donors to prevent the risk of babesial transmission. The only mitigating strategy to protect transfusion recipients is to screen the blood supply using validated molecular tests to detect Babesia species and apply indefinite deferral for any donors who report a history of human babesiosis.
During pregnancy, a mother can transmit Babesia to the fetus congenitally. Cornett et al.16 documented vertical transmission in a neonate whose blood had antibodies to B. microti. In this particular case, B. microti DNA was amplified from the placental tissue to confirm congenital infection, and definitively establishes that this babesial piroplasm can be transplacentally transmitted. Although it is unusual, neonatal babesiosis may cause morbidity and may be mistaken for other infections.17
In conclusion, we report a locally acquired case of human babesiosis caused by B. duncani in Canada. The patient was not only diagnosed clinically, he was confirmed positive using serological and molecular methodologies. Biogeographically, the distribution of B. duncani in North America is much greater than previously thought. Since this patient had no history of out-of-country travel, and had not received a blood transfusion, he must have acquired B. duncani locally in southern Ontario. Since B. duncani and B. microti are often co-infections with B. burgdorferi, Lyme disease patients should also be screened for these Babesia piroplasms. Because wild birds widely disperse Babesia-infected ticks, especially I. scapularis, people do not have to frequent an endemic area to contract human babesiosis. Health-care professionals must be aware that Canadian patients who have been bitten by a tick or had a blood transfusion may be infected with B. duncani.
Acknowledgments
The author would like to thank Catherine M. Scott and Lance A. Durden for technical support for this study. He is indebted to Elizabeth Sears for taking a specialized, high-density photograph of a tick and to Amanda Green for computer graphics.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported, in part, by Lyme Ontario.
Informed consent: Verbal informed consent was obtained from the patient for his anonymized information to be published in this article.
References
- 1. Akel T, Mobarakai N. Hematologic manifestations of babesiosis. Ann Clin Microbiol Antimicrob 2017; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conrad PA, Kjemtrup AM, Carreno RA, et al. Description of Babesia duncani n. sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol 2006; 36: 779–789. [DOI] [PubMed] [Google Scholar]
- 3. Akoolo L, Schlachter S, Khan R, et al. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiol 2017; 17: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am 2008; 22: 469–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bullard JM, Ahsanuddin AN, Perry AM, et al. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol 2014; 25: e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson JF, Mintz ED, Gadbaw JJ, et al. Babesia microti, human babesiosis, and Borrelia burgdorferi in Connecticut. J Clin Microbiol 1991; 29: 2779–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson M, Glaser KC, Adams-Fish D, et al. Development of droplet digital PCR for the detection of Babesia microti and Babesia duncani. Exp Parasitol 2015; 149: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berger BW. Dermatologic manifestations of Lyme disease. Rev Infect Dis 1989; 11 (Suppl 6): S1475–S1481. http://www.jstor.org/stable/4455358. [DOI] [PubMed] [Google Scholar]
- 9. Krause PJ, Spielman A, Telford SR, et al. Persistent parasitemia after acute babesiosis. N Eng J Med 1998; 339: 160–165. [DOI] [PubMed] [Google Scholar]
- 10. Allred DR. Babesiosis: persistence in the face of adversity. Trends Parasitol 2003; 19: 51–55. [DOI] [PubMed] [Google Scholar]
- 11. Oleson CV, Sivalingam JJ, O’Neill BJ, et al. Transverse myelitis secondary to coexistent Lyme disease and babesiosis. J Spinal Cord Med 2003; 26: 168–171. [DOI] [PubMed] [Google Scholar]
- 12. Poinar G., Jr. Fossilized mammalian erythrocytes associated with a tick reveal ancient piroplasms. J Med Entomol 2017; 54: 895–900. DOI: 10.1093/jme/tjw247. [DOI] [PubMed] [Google Scholar]
- 13. Scott JD, Anderson JF, Durden LA. Widespread dispersal of Borrelia burgdorferi-infected ticks collected from songbirds across Canada. J Parasitol 2012; 98: 49–59. [DOI] [PubMed] [Google Scholar]
- 14. Hersh MH, Ostfeld RS, McHenry DJ, et al. Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014; 9: e99348 DOI: 10.1371/journal.pone.0099348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hornok S, Szöke K, Kováts D, et al. DNA of piroplasms of ruminants and dogs in ixodid bat ticks. PLoS ONE 2016; 11: e0167735 DOI: 10.1371/journal.pone.0167735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cornett JK, Malhotra A, Hart D. Vertical transmission of babesiosis from a pregnant, splenectomized mother to her neonate. Infect Dis Clin Pract 2012; 20: 408–410. [Google Scholar]
- 17. Krause PJ, Vannier E. Transplacental transmission of human babesiosis. Infect Dis Clin Pract 2012; 20: 365–367. [Google Scholar]


