Abstract
Background. Cardiovascular complications contribute to the high morbidity and mortality rate among children with anorexia nervosa (AN). Advances in cardiac imaging permit a more comprehensive assessment of myocardial performance in children that could not be previously obtained with conventional imaging. Myocardial strain analysis is an emerging quantitative echocardiographic technique to characterize global and regional ventricular function in children. Objective. To assess global and regional left ventricular (LV0 function in children newly diagnosed with AN with conventional and quantitative 2-dimensional speckle tracking echocardiographic (2DSTE)–derived strain imaging. Materials. In a cross-sectional study of 30 patients with AN (DSM-5) and 14 age-, sex-, and race-matched healthy children, markers of cardiovascular risk, conventional and 2DSTE measures of LV function, and structure were evaluated and compared. The AN cohort was further stratified by behavioral patterns (restrict, exercise, or purge). Results. Conventional measures and LV global strain were similar between controls and children with AN. A subgroup of AN children with purging behavior had LV remodeling characterized by significantly decreased LV mass index. Regional ventricular function at the apex, as measured by strain, was also decreased in all AN patients. Percent change from ideal body weight, body mass index Z-score, electrolyte profiles, heart rate, and blood pressure were similar. Conclusions. Subclinical regional ventricular dysfunction is present in children with AN. Ventricular remodeling exists in a subgroup of children with AN in association with purging behavior. Future studies may utilize strain imaging to identify those AN patients who are at an increased risk for developing significant cardiac dysfunction.
Keywords: adolescent medicine, cardiology, general pediatrics, echocardiography, eating disorders
Introduction
Cardiovascular complications significantly contribute to the high morbidity and mortality rate in adolescents with anorexia nervosa (AN).1-3 Sensitive methods for detecting cardiac dysfunction in these patients, prior to the appearance of overt symptoms, could be used to influence clinical decision making and improve long-term cardiovascular outcomes. Currently, the best predictor of cardiac complications associated with AN is the degree of weight loss and the chronicity of the illness, factors that do not allow for early clinical intervention.1,4,5 If conventional clinical symptoms of cardiac dysfunction are not present early in course of the disease (ie, bradycardia and/or hypotension), most newly diagnosed AN patients will not undergo a thorough cardiac evaluation.1-5 In the event that newly diagnosed patients even receive an echocardiogram as part of the initial cardiac evaluation, conventional imaging may not detect subtle alterations in myocardial function, which may affect the management and prognosis of the patient.6
Two-dimensional speckle tracking echocardiographic (2DSTE)–derived strain imaging is an emerging reproducible method to quantitatively characterize left ventricular (LV) function in children and has been shown to have a greater sensitivity for measuring global and regional LV performance than conventional echocardiography.6,7 This modality has not yet been applied to assess cardiac function in adolescent with AN. Therefore, the aim of this study was to measure clinical markers of cardiovascular risk and assess and compare LV structure and function in AN and healthy adolescents using noninvasive quantitative echocardiography.
Methods
Study Design and Study Population
Using a cross-sectional study approach, we studied a group of adolescents with AN (DSM-5 classification8) referred to the Eating Disorders Program at Goryeb Children’s Hospital (Morristown, NJ) between January and December 2015. Patients were eligible for inclusion if they were 12 to 18 years of age and had complete echocardiographic and laboratory evaluations performed during the study period. AN patients were further subclassified according to the DSM-5 subtypes as having excessive exercise, purging, and/or restrictive behaviors.8 Patients were excluded if they were pregnant, had heart disease, had diabetes, or had a history of smoking and substance and/or alcohol abuse. A cohort of 14 normal healthy, age-, sex-, and race-matched pediatric cohort was used as a control group from a study performed at Saint Louis Children’s Hospital, Saint Louis, MO.9 The institutional review boards at both institutions approved the study.
Assessment of Cardiac Structure and Function
LV Structure
A transthoracic M-mode, 2D and Doppler echocardiographic examination was performed with a commercially available ultrasound imaging system. Using M-mode imaging in the parasternal short-axis view, LV mass index (LVMI; Devereux formula) was calculated, and LVMI was indexed to height2.7 (g/m2.7).10 The 95th percentiles for LVMI for children older than 12 years of age (>40 g/height2.7 in females and >45 g/height2.7 in males) were used as cutoff values to categorize LV structure (geometry).11
LV Function
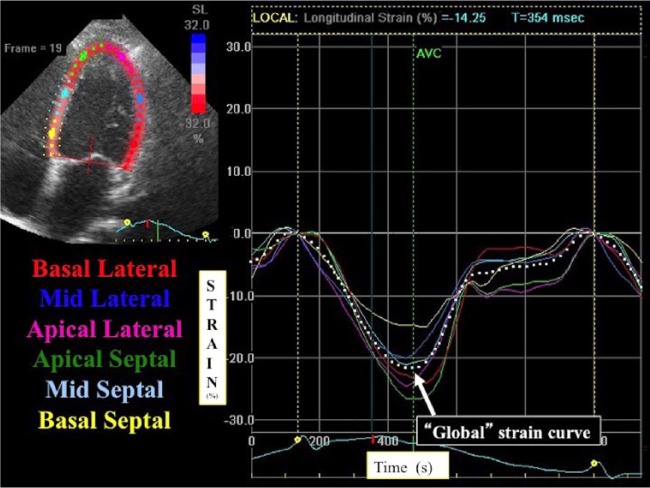
Conventional measures (LV fractional shortening [SF] and biplane ejection fraction [EF]) were measured at the time of diagnosis according to the American Society of Echocardiography guidelines.10 Myocardial mechanics were analyzed by the quantification of LV global and segmental longitudinal strain (% GLS and %SLS). Strain describes the fractional changes in the dimension of a myocardial fiber/segment. Echocardiographic images of the 3 LV apical views of the heart (apical 4-, 3-, and 2-chamber) were acquired by a single experienced cardiac sonographer. LV SLS values at the apical, midventricular, and basal levels were generated, and LV GLS was calculated as an average of the segments from the 3 views of the heart using a validated protocol10,12 (Figure 1). The higher the magnitude of strain (%) the better the LV function. A single observer, who was blinded to the patients’ clinical and metabolic values, analyzed the strain imaging. Our echocardiography laboratory has previously demonstrated high reproducibility of strain measurements.13
Figure 1.
Global and regional strain imaging. Two-dimensional speckle tracking echocardiography (2DSTE)–derived strain imaging of the left ventricle apical 4-chamber view. Regional strain is graphically presented by 6 different color-coded curves in the longitudinal direction (along the heart wall). The peak of the average curve of all the segments (the white dotted curve) is considered the global longitudinal strain. The higher the magnitude of strain, the better the function.
Statistical Analysis
Continuous variables of strain imaging were tested for normality using the Kolmogorov-Smirnov test and a histogram illustration of the data. The t test and the Wilcoxon rank sum test were used for data with normal and nonnormal distribution, respectively. Analysis of variance was used to compare the changes in SLS values at the apical, midventricular, and basal levels of the myocardium. Bivariate analyses and backward stepwise multiple linear regression analyses were used to determine which variables best predicted changes in LV structure and function for the entire cohort of AN (divided by subtype behavior) and healthy subjects. To predict LV structure and function, body mass index (BMI) Z-score, percentage change from ideal body weight, heart rate, and systolic blood pressure were used as independent variables. The maximum R2 from K-fold cross-validation was used for the multiple linear regression analyses. A P value <.05 was considered statistically significant. The statistical analysis was performed using SPSS version 14.0 (SPSS, Inc, Chicago, IL).
Results
Thirty AN patients with a median age of 13 (interquartile range = 12-15) were included in the study. Of the 30 patients, 23 (77%) displayed excessive exercise behavior, 5 (17%) had purging behavior, and all patients displayed restrictive behavior. Table 1 displays the demographic and clinical characteristics of the study populations.
Table 1.
Clinical and Demographic Characteristics in Patients With Anorexia Nervosa With and Without Purging Behaviora.
| Pediatric Controls |
Anorexia Nervosa |
P Value |
P Value |
||
|---|---|---|---|---|---|
| Healthy (n = 14) | No Purging Behavior (n = 25) | Purging Behavior (n = 5) | Control Patients Versus AN Patients | AN Patients: Purge Versus No Purge | |
| Age (years) | 15 (13, 17) | 13 (12, 15) | 14 (13, 15) | .15 | .23 |
| Gender (female) | 8 (57%) | 22 (88%) | 3 (60%) | .62 | .73 |
| Race (Caucasian) | 12 (86%) | 23 (92%) | 2 (40%) | .23 | .25 |
| Body mass index (mg/kg2) | 20 (17, 23) | 16 (15, 19) | 15 (13, 18) | .02 | .01 |
| BMI Z-score | −0.12 (−0.46, −0.15) | −0.44 (−1.63, −0.11) | −1.01 (−1.83, −0.15) | .002 | .001 |
| % Change in ideal body weight | NA | 85 (83, 03) | 81 (75, 85) | NA | .12 |
| Systolic blood pressure (mm Hg) | 109 (98, 120) | 97 (93, 101) | 90 (86, 95) | .09 | .19 |
| Diastolic blood pressure (mm Hg) | 68 (57, 67) | 60 (58, 64) | 60 (57, 64) | .12 | .82 |
| Amenorrhea | NA | 20 (80%) | 1 (20%) | NA | .13 |
| Potassium | 4.1 (3.2, 4.6) | 3.8 (3.6, 4.0) | 4 (3.7, 4.2) | .34 | .22 |
| White blood cell count | 6.4 (5.6, 7.2) | 6.1 (4.1, 7.8) | 5.5 (4.6, 6.1) | .45 | .45 |
Abbreviations: AN, anorexia nervosa; BMI, body mass index.
Data presented as median (interquartile range) or n (percentage).
Assessment of Cardiac Structure and Function
LV Structure
The results of analyses of the measurements of LV structure are shown in Table 2. A subgroup of AN with purging behavior (n = 5) had LV remodeling characterized by significantly decreased LVMI. There was no difference in age between the AN patients with normal and abnormal cardiac geometry. All the healthy patients had normal LV geometry. Backward stepwise multiple linear regression analyses retained only BMI Z-score as a predictor for LVMI (P < .001; R2 = 0.44).
Table 2.
Echocardiographic Assessment of Left Ventricular Structure and Function in Anorexia Nervosa Patients With and Without Purging Behaviora.
| Pediatric Controls |
Anorexia Nervosa |
P Value |
P Value |
||
|---|---|---|---|---|---|
| Healthy (n = 14) | No Purging Behavior (n = 25) | Purging Behavior (n = 5) | Control Patients Versus AN Patients | AN Patients: Purge Versus No Purge | |
| LV morphology | |||||
| LV mass index (g/height2.7) | 23.6 (18.9, 29.3) | 24.2 (21.2, 28.7) | 18 (16.2, 23.4) | .32 | .003 |
| Pericardial effusions (yes) | NA | 4 (8%) | 1 (20%) | NA | .35 |
| LV function | |||||
| Conventional | |||||
| Ejection fraction (%) | 62 (58, 70) | 68 (63, 69) | 65 (61, 68) | .65 | .56 |
| Shortening fraction (%) | 37 (32, 40) | 38 (34,40) | 37 (34,41) | .82 | .91 |
| Quantitative | |||||
| Global longitudinal strain (%) | −22 (−18, −24) | −23 (−22, −26) | −22 (−20, −26) | .23 | .34 |
| Segmental longitudinal strain (%) | |||||
| Basal | −22 (−19, −23) | −22 (−19, −24) | −22 (−17, −23) | .23 | .57 |
| Midventricular | −23 (−20, −26) | −24 (−21, −27) | −22 (−20, −26) | .65 | .72 |
| Apical | −26 (−22, −29) | −26 (−23, −32) | −21 (−19, −29) | .12 | .001 |
Abbreviations: AN, anorexia nervosa; LV, left ventricular.
Data presented as median (interquartile range) or n (percentage).
Global LV function
Conventional echocardiographic measures (EF and SF) were similar between patients newly diagnosed with AN and healthy age-matched controls (Table 2), irrespective of their subtype behaviors. Similarly, GLS was similar between all AN patients and the controls, irrespective of their subtype behaviors.
Regional LV function
Healthy controls displayed an apex-to-base (highest to lowest) SLS gradient (P < .001). AN patients with excessive exercise behavior (n = 23) also displayed a similar apex-to-base SLS gradient (P < .001) when compared with AN patients without excessive exercise behavior. Patients with purging behavior (n = 5) had significant decreased apical SLS values (Table 2 and Figure 2) and did not exhibit the same apex-to-base SLS gradient. There were no differences in electrolyte profiles between the AN patients with purging behavior and those without purging behavior. Backward stepwise multiple linear regression analyses for the cohort of AN who had purging behavior, AN without purging behavior, and control patients retained BMI Z-score as the only predictor for apical LS and altered SLS gradient (P < .01; R2 = 0.25).
Figure 2.
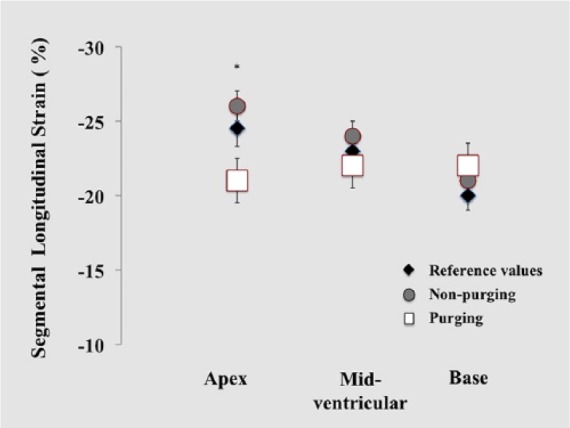
Regional strain imaging in AN patients with purging behavior. Comparison of regional LV regional longitudinal strain between pediatric control values (black diamond), anorexia nervosa (AN) patients who do not exhibit purging behavior (grey circle), and AN patients who have purging behavior (white square). *In adolescents newly diagnosed AN and have purging behavior, the apical strain (white squares) are significantly lower (P = .001) than those AN patients who did not have a purging behavior (gray circles), altering the physiological apex-to-base gradient.
Discussion
In this retrospective pilot study, LV performance was assessed in patients with AN using conventional and emerging quantitative echocardiography. The main findings of this study are the following: (1) ventricular remodeling is present in a subgroup of AN adolescents in association with purging behavior and (2) apical segmental strain, a quantitative measure of regional LV function, was also decreased in patients with new-onset AN and purging behavior.
There is a growing awareness of the cardiac manifestations associated with AN,1-5,14 but clinicians lack longitudinal markers of myocardial adaptation to eating disorders. This is the first study to characterize LV function in patients with AN, or any eating disorder, with 2DSTE-derived strain imaging. Previous studies have only focused on conventional echocardiography, which often only assess global function and may not detect subtle alterations in regional myocardial function.1,4,5 In this study, we did not observe any differences in the global measures of LV function, conventional or quantitative, between the patients newly diagnosed with AN and healthy controls, irrespective of their subtype behaviors. Interestingly, the patients with specific purging behaviors had decreased regional apical SLS that altered the physiological apex-to-base gradient (highest to lowest strain gradient).12 The mechanism is not completely clear, but purging behavior may alter the torsional mechanisms and decrease the electric excitation of cardiac motion that begins at the apex and has a slower transit time to the base.12 This observation has the potential to discern subclinical changes in myocardial function in patients with specific behaviors earlier than convectional measures, vital signs changes, or even changes in electrolyte profile.
The major limitation of this pilot study is its retrospective statistical analysis interpretation imposed by small sample and effect size comparisons. Nonetheless, the preliminary observations and associated limitations are less as important as the recognition that strain imaging is an emerging reproducible modality that is feasible in patients newly diagnosed with AN, may be more sensitive than conventional measures, and should be considered in the assessment of clinical changes in myocardial function in this population and those with other eating disorders. Future work in a large full-scale prospective study is needed to understand the effect that strain imaging will have on patient management strategies for cardiac health in this population.
Conclusion
Two-dimensional speckle tracking echocardiography–derived strain imaging represents an emerging modality that may be clinically useful in the assessment of global and regional LV function in adolescents newly diagnosed with AN. Ventricular remodeling and altered regional function is present in a subgroup of adolescents with AN in association with purging behavior. Future studies may utilize these novel methods to identify those AN patients who are at an increased risk for developing significant cardiac dysfunction.
Author Contributions
RM: Contributed to conception and design; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AP: Contributed to conception and design; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
JS: Contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MG: Contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
LS: Contributed to conception and design; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AH: Contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
GKS: Contributed to analysis; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
PTL: Contributed to conception and design; contributed to analysis; drafted the manuscript; critically revised the manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support for this study was provided by the Foundation for the Morristown Medical Center and Goryeb Children’s Hospital.
References
- 1. Di Cola G, Jacoangeli F, Jacoangeli F, Lombardo M, Iellamo F. Cardiovascular disorders in anorexia nervosa and potential therapeutic targets. Intern Emerg Med. 2014;9:717-721. [DOI] [PubMed] [Google Scholar]
- 2. Rosen DS. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240-1253. [DOI] [PubMed] [Google Scholar]
- 3. Mehler PS, Krantz MJ, Sachs KV. Treatments of medical complications of anorexia nervosa and bulimia nervosa. J Eat Disord. 2015;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casiero D, Frishman WH. Cardiovascular complications of eating disorders. Cardiol Rev. 2006;14:227-231. [DOI] [PubMed] [Google Scholar]
- 5. Olivares JL, Vázquez M, Fleta J, Moreno LA, Pérez-González JM, Bueno M. Cardiac findings in adolescents with anorexia nervosa at diagnosis and after weight restoration. Eur J Pediatr. 2005;164:383-386. [DOI] [PubMed] [Google Scholar]
- 6. Sanchez AA, Levy PT, Sekarski TJ, et al. Markers of cardiovascular risk, insulin resistance, and ventricular dysfunction and remodeling in obese adolescents. J Pediatr. 2015;160:650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levy PT, Sanchez Mejia AA, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 9. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for chamber quantification. J Am Soc Echocardiogr. 2015;28:1-39.e14. [DOI] [PubMed] [Google Scholar]
- 10. Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr. 2009;22:709-714. [DOI] [PubMed] [Google Scholar]
- 11. Levy PT, Machefsky AM, Sanchez AA, et al. Reference ranges of left ventricular strain measures by two-dimensional speckle tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2016;29:209-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh GK, Vitola BE, Holland MR, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;16:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sachs KV, Harnke B, Mehler PS, Krantz MJ. Cardiovascular complications of anorexia nervosa: A systematic review. Int J Eat Disord. 2016;49:238-48. [DOI] [PubMed] [Google Scholar]