Abstract
Loeffler endocarditis is a rare restrictive cardiomyopathy caused by abnormal endomyocardial infiltration of eosinophils, with subsequent tissue damage from degranulation, eventually leading to fibrosis. Although an uncommon entity, it is still a disease with significant morbidity and mortality. Often identified only at late stages, treatment options are limited once fibrosis occurs, usually requiring heart failure medications or surgical intervention. We present a unique case of a woman with remote history of hypereosinophilic syndrome, attributed to treatment of rheumatoid arthritis with infliximab, who presented with symptoms of heart failure refractory to medical management and was found to have Loeffler endocarditis. The severe progression of the disease required surgical intervention with endocardial stripping to treat the right-sided diastolic heart failure.
Keywords: Loeffler endocarditis, eosinophilic cardiomyopathy, hypereosinophilic syndrome, endocardial stripping
Introduction
Loeffler endocarditis describes a rare restrictive cardiomyopathy associated with eosinophilia in which there is impaired ventricular filling and diastolic dysfunction due to endomyocardial fibrosis. First described by Loeffler in 1936, the process involves the abnormal infiltration of eosinophils into the endomyocardium, with subsequent tissue damage from degranulation, eventually leading to fibrosis.1 Predisposition for this condition can occur from any eosinophilic state, including drug reaction, parasitic infection, eosinophilic leukemia, and most commonly, hypereosinophilic syndrome (HES), a myeloproliferative disorder marked by persistent peripheral eosinophilia (>1.5 × 109/L) and end-organ damage.2 Although an uncommon entity, the disease causes significant morbidity and mortality. Diagnosis often only occurs once the patient has become symptomatic from fibrosis, at which point management options are limited, usually requiring treatment with heart failure medications or surgical intervention.3 We report a case of a 58-year-old woman who presented with clinical signs and symptoms of heart failure. The patient was found to have a primarily right-sided restrictive cardiomyopathy due to Loeffler endocarditis and underwent successful endocardial stripping. This case demonstrates the efficacy of endocardectomy for the treatment of an unusual case of right-sided heart failure.
Case Description
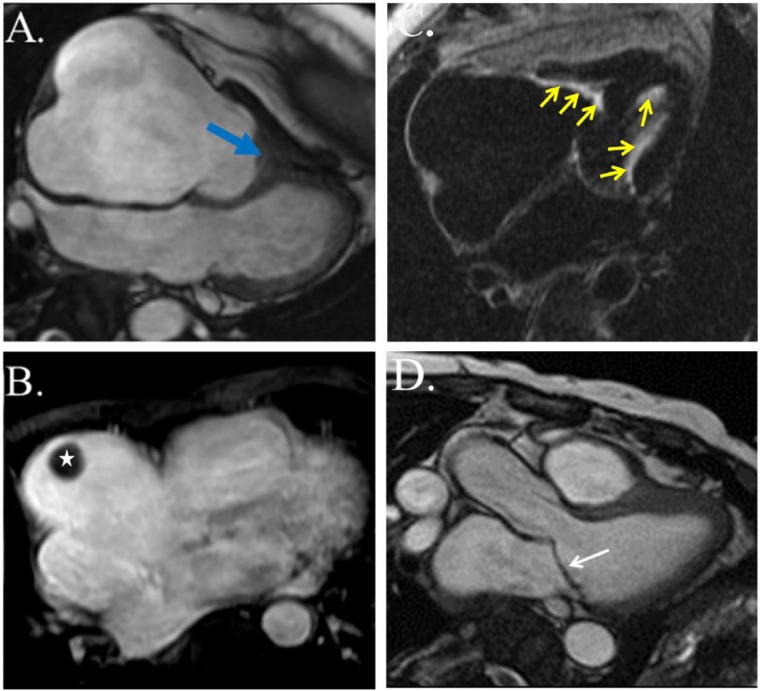
A 58-year-old woman presented in the outpatient setting with progressively worsening shortness of breath and bilateral lower extremity edema. The patient had a past medical history significant for HES with myocarditis 9 years prior, presumed to be secondary to infliximab used in the management of rheumatoid arthritis. She reportedly had significant hypereosinophilia at that time and was found to have acute myocarditis with mild pericardial and pleural effusions. The anti–tumor necrosis factor agent was thought to be the responsible due to its chronological relation to disease development. She was treated successfully with a course of steroids and discontinuation of the alleged culprit medication. Gene testing was not done. The patient had no other documented recurrences or residual symptoms of the disease until the development of dyspnea months prior to current presentation. Eosinophil counts at that time were within normal limits. Electrocardiogram showed first-degree atrioventricular block and left atrial enlargement, as well as nonspecific ST and T-wave changes (Figure 1). Transthoracic echocardiogram (TTE) showed no left ventricular (LV) dysfunction but was notable for thickened mitral valve (MV) leaflets (Figure 2), as well as severe right atrial (RA) enlargement with poor visualization of the right ventricle (RV). As seen in Figure 3, cardiac magnetic resonance imaging (CMR) showed diffuse endocardial late gadolinium enhancement consistent with fibrosis (Figure 3C), obliteration of the RV apex, bowing of the interventricular septum toward the left in diastole compatible with increased right-heart filling pressure pressures (Figure 3A), severe RA enlargement with thrombus (Figure 3B), and MV leaflet obliteration (Figure 3D). Left ventricular size and function were normal. Despite aggressive diuresis with increasing doses of furosemide, the patient’s heart failure symptoms continued to become progressively more debilitating. To decrease RV filling pressures, the patient underwent RV endocardial stripping (endocardectomy), which involves decortication of the fibrosed tissue. The fibrotic peel in the RV was resected resulting in damage to the subvalvular area which required tricuspid valve replacement. Examination of the MV showed involvement of the leaflets in the Loeffler process, prompting MV replacement. Pathology showed degenerative disease with obliteration of the mitral leaflets. Postoperatively, the patient was found to be in complete heart block due to damage to the conduction system, so a permanent pacemaker was placed. Echocardiography and right-heart catheterization 6 months postoperatively showed marked improvement in RA and RV size, with normalization of RV diastolic pressure. At her 1-year postoperative visit, the patient had symptomatically improved with no complaints of shortness of breath, dyspnea on exertion, or other heart failure symptoms.
Figure 1.
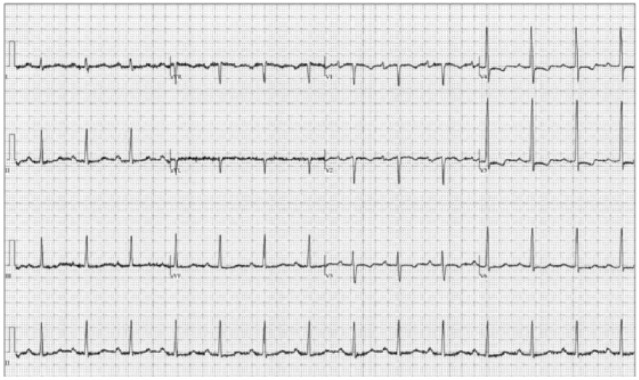
Electrocardiogram: normal sinus rhythm with first-degree atrioventricular block, left atrial enlargement, and nonspecific ST and T-wave changes.
Figure 2.
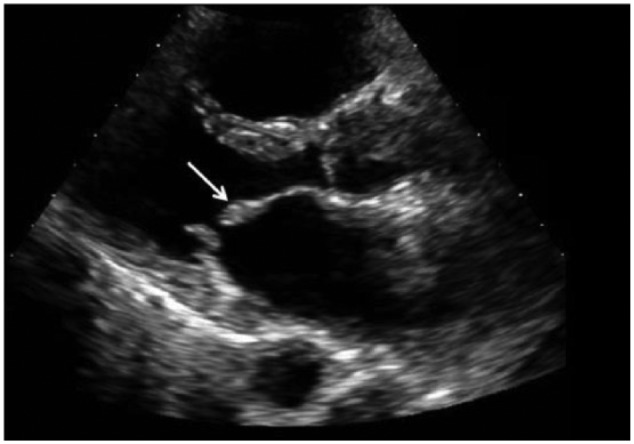
Transthoracic echocardiogram: parasternal long-axis view highlights a thickened anterior mitral valve leaflet (white arrow).
Figure 3.
Cardiac magnetic resonance. (A) Four-chamber view (balanced steady-state free precession image) in early diastole demonstrates obliteration of the right ventricular apex (blue arrow) and severe dilation of the right atrium. The interventricular septum bows toward the left ventricle due to elevated right ventricular filling pressures. (B) Axial contrast-enhanced (T1-weighted fat-suppressed) image shows a structure with dark signal intensity in the right atrium (white star) compatible with thrombus. (C) Late gadolinium enhancement imaging reveals bright signal intensity along the right and left ventricular endocardium consistent with endomyocardial fibrosis (yellow arrows). (D) Anterior mitral valve leaflet obliteration (white arrow).
Discussion
The pathogenesis of Loeffler endocarditis has been fairly well described. In the early phase of the process, patients usually present with systemic illness in the setting of hypereosinophilia, including fevers, chest pain, diaphoresis, and often acute myocarditis, although the presentation may sometimes be subtle. It is at this point where the initial insult occurs, with eosinophilic infiltration of cardiac tissue.3 The release of major basic proteins and reactive oxygen species from activated eosinophils causes endothelial and myocyte injury.3,4 Over time, this leads to fibrosis, resulting in restrictive cardiomyopathy.3 These patients are prone to thrombus formation as well due to cationic protein release from the eosinophils binding to the anionic endothelial protein thrombomodulin, leading to localized hypercoagulability. Electrocardiogram findings are nonspecific and can include left atrial enlargement, first-degree atrioventricular block, and nonspecific ST and T-wave changes as noted in this patient, as well as LV hypertrophy and premature ventricular contractions, thus decreasing their diagnostic utility.2,3 Similarly, TTE findings are often unremarkable as well, but may show endomyocardial obliteration and ventricular thrombus formation.3 However, recent publications have helped establish CMR as an effective tool for accurately diagnosing and monitoring Loeffler endocarditis at each stage of disease, largely due to its ability to characterize endomyocardial tissue involvement with greater detail, which can help guide treatment.2,3,5 During the acute myocarditis phase, CMR may demonstrate increased T2 signal and late gadolinium enhancement consistent with edema and inflammation, whereas in the thrombotic stage, CMR may show thrombi at the ventricular apices. Finally, once in the chronic stage late gadolinium enhancement is seen, consistent with fibrosis,2,3,5 as was observed in this patient (Figure 3). It is important to highlight here that multiple stages can exist simultaneously.
A key aspect of this patient’s history is the remote history of HES, a multi-organ system disease process in which there is a significant elevation of circulating eosinophils, with invasion into tissues where they are not normally present, and subsequent organ damage. The initial presentation can be variable depending on which system is affected; however, cardiac involvement is common and dangerous as complications such as Loeffler endocarditis may have a slow, indolent course.2,3 Serial examinations with CMR may be beneficial in closely monitoring progression of disease, by showing increased fibrotic tissue and worsening contractile function. Hypereosinophilia secondary to infliximab or other immunomodulators is also uncommon, with few case reports in the literature, and none that the authors are aware of leading to cardiac involvement. The mechanism of this hypereosinophilia is unclear but appears to be a drug reaction driven by inflammatory processes.6–8
Although an uncommon entity, Loeffler endocarditis is still a disease with significant morbidity and mortality, with a reported 35% to 50% 2-year mortality in those with advanced fibrosis, largely due to heart failure and thromboembolism.5,9 Even more problematic is that fibrotic eosinophilic endocardial disease is often not noted until postmortem due to the rapid clinical deterioration of those affected, and thus, the true rate may be even higher. A reason for this delayed diagnosis is that the severity of acute myocarditis is directly related to the severity of initial eosinophilia, and thus, patients with low to moderate levels of eosinophilia may develop only low-grade endomyocarditis that may not be clinically apparent. Eventually, however, this inflammation still leads to progressive fibrosis.5 In the case of this patient, the initial cardiac insult was mild, and the patient was not diagnosed until very late into the fibrotic process when she became symptomatic from severe heart failure. Treatment options largely depend on the level of progression of the disease. There is some evidence which supports the use of steroids, interferon, hydroxyurea, and other immunosuppressant or cytotoxic medications during the early acute myocarditis stage. However, once fibrosis occurs, management becomes more difficult, usually requiring diuretics and medications for afterload reduction. In severe refractory cases, such as this one, surgical intervention may be required with endocardectomy. Of note, the endomyocardial damage associated with Loeffler endocarditis is usually associated with the apical endocardium, particularly of the LV.4 There is little in the literature about management of predominantly right-sided endocarditis of Loeffler type causing right-sided heart failure as was noted in this patient.10
Loeffler endocarditis is a lethal disease which requires prompt diagnosis and treatment. Patients with a history of hypereosinophilia should be closely monitored (ideally with serial imaging such as TTE or CMR) for the development of thromobosis and fibrotic, restrictive cardiomyopathy. If diagnosed early, treatment with steroids and cytotoxic medications can be beneficial; however, if diagnosed later in the disease processes, once fibrotic disease develops, surgical intervention may be required to prevent morbidity and mortality.
Footnotes
Peer review:Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 496 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AA and ST wrote the first draft of the manuscript. AA, ST, SGS, and RJ contributed to the writing of the manuscript, agree with manuscript results and conclusions, jointly developed the structure and arguments for the paper, and made critical revisions and approved final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Spry CJ, Tai PC, Davies J. The cardiotoxicity of eosinophils. Postgrad Med J. 1983;59:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ogbogu P, Rosing DR, Horne MK., III Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mankad R, Bonnichsen C, Mankad S. Hypereosinophilic syndrome: cardiac diagnosis and management. Heart. 2016;102:100–106. [DOI] [PubMed] [Google Scholar]
- 4. Çetin S, Heper G, Vural MG, Hazirolan T. Loeffler endocarditis: silent right ventricular myocardium. Wien Klin Wochenschr. 2016;128:513–515. [DOI] [PubMed] [Google Scholar]
- 5. Kleinfeldt T, Hueseyin I, Nienaber CA. Hypereosinophilic syndrome: a rare case of Loeffler’s endocarditis documented in cardiac MRI. Int J Cardiol. 2011;149:e30–e32. [DOI] [PubMed] [Google Scholar]
- 6. Cancelliere N, Barranco P, Vidaurrazaga C, Benito DM, Quirce S. Subacute prurigo and eosinophilia in a patient with rheumatoid arthritis receiving infliximab and etanercept. J Investig Allergol Clin Immunol. 2011;21:248. [PubMed] [Google Scholar]
- 7. Malisiewicz B, Murer C, Pachlopnic Schmid J, French LE, Schmid-Grendelmeier P, Navarini AA. Eosinophilia during psoriasis treatment with TNF antagonists. Dermatology. 2012;223:311–315. [DOI] [PubMed] [Google Scholar]
- 8. Bessissow T, Renard M, Hoffman J, Vermeire S, Rutgeerts P, Van Assche G. Review article: non-malignant haematological complications of anti-tumour necrosis factor alpha therapy. Aliment Pharmacol Ther. 2012;36:312–323. [DOI] [PubMed] [Google Scholar]
- 9. Ramrakha P, Hill J. Chapter 14. Cardiovascular disease in less-developed countries. In: Ramrakha PS, ed. Oxford Handbook of Cardiology. 2nd ed. New York, NY: Oxford University Press; 2012:668–669. [Google Scholar]
- 10. Beedupalli J, Modi K. Early-stage Loeffler’s endocarditis with isolated right ventricular involvement: management, long-term follow-up, and review of literature. Echocardiography. 2016;33:1422–1427. [DOI] [PubMed] [Google Scholar]