Abstract
Painful peripheral neuropathy is a severe side effect in oxaliplatin therapy that compromises cancer patients' quality of life. However, its underlying pathogenic mechanisms remain largely unknown. Here, we found that intraperitoneal consecutive administration of oxaliplatin significantly increased excitability of small diameter dorsal root ganglion neurons and induced thermal hyperalgesia in rats. Furthermore, the CX3CL1 expression was significantly increased after oxaliplatin treatment, and intrathecal injection of a neutralizing antibody against CX3CL1 markedly attenuated the enhanced excitability of dorsal root ganglion neurons and thermal hyperalgesia. Importantly, the upregulated CX3CL1 is mediated by the NF-κB signaling pathway, as inhibition of NF-κB p65 activation with pyrrolidine dithiocarbamate or p65 siRNA inhibited the upregulation of CX3CL1, the enhanced excitability of dorsal root ganglion neurons, and thermal hyperalgesia induced by oxaliplatin. Further studies with chromatin immunoprecipitation found that oxaliplatin treatment increased the recruitment of NF-κB p65 to the CX3Cl1 promoter region. Our results suggest that upregulation of CX3CL1 in dorsal root ganglion mediated by NF-κB activation contributes to the peripheral sensitization and chronic pain induced by oxaliplatin administration.
Keywords: Oxaliplatin, CX3CL1, NF-κB, dorsal root ganglion, chronic pain
Introduction
Oxaliplatin is a third-generation platinum-based chemotherapeutic agent widely used for therapy of various types of cancers.1 The application of oxaliplatin is often associated with a dose-limiting acute or chronic pain syndrome2 that compromises cancer patients' quality of life and hampers optimistic use of oxaliplatin in clinical treatment.3,4 Although many therapies have been investigated for preventing or minimizing oxaliplatin chemotherapy-induced painful neuropathy, there is no well-accepted proven therapy.5 Hence, investigating the underlying pathogenic mechanisms remains a high research priority.
Accumulating evidence has demonstrated that chemokines play important roles in chemotherapy-induced chronic pain.6,7 For example, CCL2 expression in the dorsal root ganglion (DRG) or CXCL12 expression in the spinal cord is critical for chronic pain following paclitaxel or vincristine treatment, respectively.6,7 Recently, CX3CL1 has been identified to mediate painful responses in chemotherapy drug-induced pain models.8–10 Upregulation of CX3CL1 in spinal dorsal horn contributed to oxaliplatin-induced acute pain.8 In addition, activation of CX3CL1/CX3CR1 signaling in DRG plays a pivotal role in paclitaxel-induced painful peripheral neuropathy.10 It is well-known that the hyperexcitability of DRG neurons contributes to neuropathic and inflammatory pain.11,12 However, whether CX3CL1 mediates oxaliplatin-induced chronic pain via regulating the excitability of DRG has not been elucidated.
Studies showed that nuclear factor-κB (NF-κB), as a critical transcriptional factor, played critical roles in the induction and development of chronic pain.13–15 Activation of NF-κB participated in nerve injury or inflammation-induced pathological pain.16,17 Recent studies showed that NF-κB is also involved in chemotherapy-induced chronic pain.8,9 However, whether activation of NF-κB p65 in DRG is involved in oxaliplatin-induced chronic pain is still unclear. Moreover, NF-κB can modulate the expression of proinflammatory factors such as CXCL10 and CCL2 during neuroinflammation.18 Inhibition of p65 activation reduced the upregulation of CXCL10 and CCL2 in sciatic nerves and DRG.18 Whether NF-κB regulates CX3CL1 expression at the transcription level in DRG remains unclear.
In the present study, we examined whether NF-κB activation-mediated upregulation of CX3CL1 in the DRG participated in oxaliplatin-induced peripheral sensitization and chronic pain. Our novel understanding of the mechanism of oxaliplatin-induced chronic pain in cancer therapy could partly enlighten a new therapeutic strategy to prevent pain hypersensitivity.
Materials and methods
Animals and surgery
Male Sprague-Dawley rats (220–250 g body weight) were supplied by the Institute of Experimental Animals of Zhengzhou University. All rats were housed in separated cages with ad libitum food and water. The room was kept at 25℃ and 50% to 60% humidity under a 12/12 h light/dark cycle. Rats were randomly divided into different experimental groups. All experimental procedures were approved by the Institutional Animal Care Committee of Zhengzhou University and were carried out in accordance with the guidelines of the National Institutes of Health Guide for the care and use of laboratory animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
For intrathecal injection of drugs, a polyethylene-10 (PE-10) catheter was implanted as described by our previous study.19 In brief, rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg), and a sterile catheter filled with saline was inserted into subarachnoid space of the rat through the L5/L6 intervertebral space and gently advanced caudally to the spinal lumbar enlargement level. Then, the catheter was fixed under the skin with paravertebral muscles and sutured to the head of the rats. After they completely recovered from anesthesia, the correct placement of the catheter was confirmed by observing the behavior of dragging or paralysis in bilateral hind limbs following the injection of 2% lidocaine (10 μl). Finally, rats with catheter prolapse, infection, or neurological deficit were excluded from the following experiments.
Drugs and administration
Oxaliplatin was purchased from Sigma (USA) and dissolved in 5% glucose/H2O as a stock solution of 1 mg/ml, as reported in a previous study.8 Rats were intraperitoneally administered oxaliplatin at 4 mg/kg once per day for five consecutive days to induce thermal hyperalgesia. The control rats were intraperitoneally injected with an equivalent volume of 5% glucose/H2O. Intrathecal injection of isotype IgG (10 μg in 10 μl, R&D system, USA), a neutralizing antibody against CX3CL1 (10 μg in 10 μl, Torrey Pines Bio Labs, USA), ammonium pyrrolidine dithiocarbamate (PDTC; 200 ng in 10 μl, Sigma, USA), scramble siRNA (50 μg in 15 μl, Ribobio, China), or NF-κB p65 small interfering RNA (siRNA; 50 μg in 15 μl, Ribobio, China) was performed 30 min before oxaliplatin administration.
Behavioral test
Thermal hyperalgesia was tested using a plantar test (7370, Ugo Basile Plantar Test Apparatus, Italy) according to the method described by Hargreaves et al.20 Briefly, a radiant heat source (set at an intensity of 55℃) with a 10 mm aperture beneath a glass floor was aimed at the plantar surface of the hind paw of rats. Three measurements of rat withdrawal latency were taken for each hind paw in each test session. The hind paw of each rat was tested alternately with greater than 5 min intervals between the consecutive tests. Three measurements of withdrawal latency per side were averaged for the result of each test. A 20 s cutoff was set to prevent tissue damage. The experimenter who conducted the behavioral test was blinded to the intervention.
Culture of DRG neurons and electrophysiological recordings
DRG neurons were dissociated using enzyme digestion as previously described with minor modifications.21 In brief, L4 and L5 DRGs were excised, freed from their connective tissue sheaths, and broken into pieces with a pair of sclerotic scissors in DMEM/F12 medium (GIBCO, USA) under low temperature (in a mixture of ice and water). After enzymatic and mechanical dissociation, DRG neurons were seeded on cover slips coated with Poly-L-Lysine (Sigma-Aldrich, USA) in a humidified atmosphere (5% CO2, 37℃) and used for patch-clamp investigation 4 h after plating.
Whole-cell patch-clamp recordings from acutely dissociated DRG neurons were performed using an EPC-10 amplifier and the PULSE program (HEKA Electronics, Lambrecht, Germany). Patch pipettes were fabricated from borosilicate glass capillaries (Sutter Instruments, Novato, CA) using a Sutter P-87 puller (Sutter Instruments, Novato, CA). After being filled with an internal solution, the recording patch pipette had a resistance of 4 to 8 MΩ. The action potentials of DRG neurons were recorded on small-size DRG neurons, which were defined by the diameter (≤30 μm) and Cm (≤28.3 pF), as other studies described in literatures.21,22 The external solution contained the following (in mM): 140 NaCl, 3 KCl, 1 MgCl2, 1 CaCl2, and 10 HEPES, pH adjusted to 7.3 with NaOH. The pipette solution contained the following (in mM): 140 KCl, 0.5 EGTA, 5 MgATP, and 5 HEPES, pH 7.3 with KOH. By using current-clamp recording, the small-size DRG neurons were held at 0 pA, and first, the firing threshold-rheobase of the cell was measured by injecting a series of 100 ms depolarizing current in 5 pA steps from 0 pA, which could elicit the first action potential. Second, to determine the firing properties of DRG neurons, a large depolarizing current (500 ms, 2.0-fold rheobase) was applied to elicit the cell producing sufficient firing.
RNA extraction and quantitative polymerase chain reaction
Total RNA was extracted from the rat L4 and L5 DRG tissues with Trizol reagent (Invitrogen). Reverse transcription was performed using oligo-dT primer and M-MLV reverse transcriptase (Promega, USA) according to the manufacturer's protocol, with minor modification as described elsewhere.8 The cDNA was amplified using the following primers: CX3CL1 forward, 5′-CTCCAGCCATCCAGCCATG-3′; CX3CL1 reverse, 5′-CATTTCGTCATGCCGAGGTG-3′; β-actin forward, 5′-AGGGAAATCGTGCGTGACAT-3′; β-actin reverse, 5′-GAACCGCTCATTGCCGATAG-3′; NF-κB p65 forward, 5′-CTCACCGGCCTCATCCACAT-3′; and NF-κB p65 reverse, 5′-TGGCTAATGGCTTGCTCCAG-3′. Real-time quantitative polymerase chain reaction (PCR) was performed using SYBR Green quantitative PCR SuperMix (Invitrogen) and the ABI PRISM 7500 Sequence Detection System (USA). The reactions were set up according to the manufacturer's protocol. The PCR amplifications were performed at 95℃ for 3 min followed by 40 cycles of thermal cycling (10 s at 95℃, 20 s at 58℃, and 10 s at 72℃). The melting curves were performed to validate the utility and specificity of each PCR product. The relative expression ratio of mRNA in the DRG tissues was evaluated using the Comparative CT Method (2 −ΔΔCT).
Western blot
Western blot analysis was performed according to the manufacturer's protocol with minor modification as described elsewhere.8 Six rats were randomly selected from each group and deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) at different time points. The L4 and L5 DRGs were immediately removed and homogenized on ice in 15 mmol/l Tris containing a cocktail of proteinase inhibitors and phosphatase inhibitors. Protein samples were separated by gel electrophoresis (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred onto a polyvinylidene difluoride membrane. The blots were placed in the block buffer for 1 h at room temperature and incubated overnight at 4℃ with primary antibodies against CX3CL1 (1:500, R&D Systerms, USA), NF-κB p65 (1:1000, Abcam, USA), phosphorylated NF-κB p65 (Ser311; 1:1000, CST, USA), and GAPDH (1:20000, Sigma, USA). The blots were then incubated with horseradish peroxidase-conjugated secondary antibody. Electro-Chemi-Luminescence (ECL) (Pierce, USA) was used to detect the immune complex. The band was exposed by Chemiluminescence and Fluorescence Imaging System (G:BOX XT4, Syngene, UK) and quantified with a computer-assisted imaging analysis system (NIH ImageJ).
Immunohistochemistry
For immunohistochemistry analysis, six rats were randomly selected from the oxaliplatin group. The rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused through the ascending aorta with saline (4℃) followed by 4% paraformaldehyde (4℃, pH: 7.4). After perfusion, L4 and L5 DRGs were removed and postfixed in the same fixative for 2 h and then placed in 30% sucrose overnight. Cryostat DRG sections (16 μm) were cut and processed for immunohistochemistry with 3% donkey serum in 0.3% Triton X-100 for 1 h at room temperature and then incubated with primary antibodies (anti-CX3CL1 antibody, 1:500, R&D Systems; anti-phosphorylated NF-κB p65 (Ser311) polyclonal antibody, 1:300, Cell Signaling Technology; anti-neuroflament-200 (NF-200) antibody, a marker for myelinated A-fibers, 1:200, Chemicon; isolectin B4 (IB4)-conjugated FITC, a marker for unmyelinated C-fibers, 1:25, Sigma; anti-GFAP antibody, a marker for satellite glial cell, 1:200, Chemicon) overnight at 4℃ according to the method described by Xu et al.23 After washing with 0.1 M phosphate buffer solution (4℃, pH: 7.4), all of the above sections were incubated with a mixture of fluoresceinisothiocyanat- and Cy3-conjugated secondary antibodies (1:400; Jackson Immuno Research, USA) for 1 h at room temperature, except IB4-treated DRG sections, which were only incubated with Cy3-conjugated secondary antibody. After washing with 0.1 M phosphate buffer solution (4℃, pH: 7.4), the stained sections were examined with a Leica (Leica, Solms, Germany) fluorescence microscope, and the images were captured with a Leica camera (DFC350 FX).
siRNA preparation, transfection, and screening
Specific siRNAs were used to knockdown the expression of NF-κB p65. According to a previous screening test, the siRNA with the nucleotide sequences of 5′-GCAUCCAGACCAACAAUAAdTdT-3′ (sense) and 3′-dTdTCGUAGGUCUGGUUGUUAUU-5′(antisense) showed significant efficacy to prevent the expression of the NF-κB p65 subunit in a spinal cord in vivo;9 this siRNA targeting rat Rela (NF-κB p65) gene was designed and synthesized by Ribobio (China) for the subsequent experiments in vivo.
Chromatin immunoprecipitation assays
Chromatin immunoprecipitation (ChIP) assays were performed using the CHIP Assay kit (Thermo, USA) following the manufacturer's protocol as described elsewhere.9 The rats' L4 to L5 DRGs were placed in 1% formaldehyde and sonicated on ice for 2 min. After DNA was fragmented and sheared with micrococcal nuclease, aliquots were kept for input DNA as control or subjected to ChIP with 1 μg of either the rabbit p65 antibody (Abcam, USA) or rabbit control IgG (Cell Signaling Technology, USA). Following incubation with precleared chromatin solution overnight, the complexes of antibody and DNA were captured, washed, and eluted, and the cross-link was reversed. The DNA purified from the complexes, and the input fractions were resuspended in nuclease-free water, and quantitative real-time PCR or semiquantitative PCR was conducted as described in the above methods. The relative ChIP/input ration was calculated. The primers (5′-GCTGCCCTGACCATAAAT-3′ and 5′-AGCTGTACGGCACTCACC-3′) were designed according to the description of Li et al.9 to amplify a −1941/−1931 region that contained a NF-κB binding site and was related to the transcription start site of rat CX3CL1 promoter.
Statistical analysis
All data were expressed as the means ± SEM and analyzed with SPSS 13.0 (SPSS, USA). Western blot, quantitative PCR, and electrophysiological data were analyzed via two-way analysis of variance followed by a Tukey post hoc test. For behavioral tests, one-way or two-way analysis of variance with repeated-measures followed by a Tukey post hoc test was carried out. The criterion for statistical significance was P < 0.05. While no power analysis was performed, the sample size was determined according to our and peers' previous publications on painful behavior and pertinent molecular studies.
Results
CX3CL1 upregulation contributed to thermal hyperalgesia following oxaliplatin administration via enhancing DRG neuronal hyperexcitability
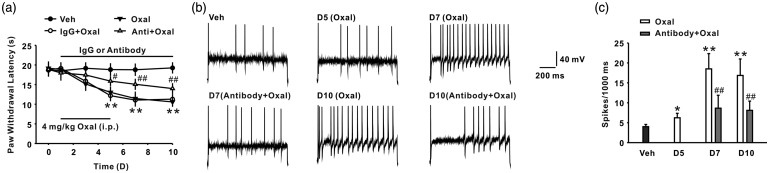
In the present study, we first observed the paw withdrawal latency in the rats following oxaliplatin administration (4 mg/kg for five consecutive days, i.p.). The results revealed that oxaliplatin treatment could induce thermal hyperalgesia from day 5, and this was maintained to the end of the experiment (day 10) (n = 8/group; Figure 1(a)). Next, we examined the expression of CX3CL1 in DRG after oxaliplatin treatment. Oxaliplatin application (i.p.) increased CX3CL1 expression of mRNA and protein levels in DRG significantly from day 5, and this was sustained till day 10 after treatment (n = 6/group; Figure 1(b) and (c)). Immunohistochemistry results further showed that CX3CL1 expression was exclusively located in IB4- and NF-200-positive cells but not in GFAP-positive cells in DRG after oxaliplatin treatment on day 7 (n = 6/group; Figure 1(d)). In addition, consecutive intrathecal administration of neutralizing antibody against CX3CL1 at a dose of 10 μg/10 μl for 10 days significantly attenuates oxaliplatin-induced thermal hyperalgesia (n = 8/group; Figure 2(a)). It is well-known that neuronal hyperexcitability played an essential role in the development of chronic pain, and thermal hyperalgesia is mediated by small diameter DRG neurons.24,25 To illustrate whether oxaliplatin-induced CX3CL1 upregulation regulate DRG neurons-mediated peripheral sensitization, we first observed the change of action potentials in the small diameter DRG neurons ( ≤ 30 μm) of rats following oxaliplatin treatment. The results showed that the spike number of small diameter DRG neurons was significantly increased on days 5, 7, and 10 following oxaliplatin treatment (i.p.) (n = 22/group; Figure 2(b) and (c)). Furthermore, consecutive intrathecal injection of anti-CX3CL1 neutralizing antibody (10 μg/10 μl for 10 days) prevented the increase in the spike number of small diameter DRG neurons on day 7 following oxaliplatin treatment (n = 22/group; Figure 2(b) and (c)). These results suggested that increased CX3CL1 in DRG enhanced the excitability of DRG neurons and contributed to chronic pain induced by oxaliplatin treatment. However, the mechanism of CX3CL1 upregulation in DRG mediated by oxaliplatin treatment is still unclear.
Figure 1.
Consecutive administration of oxaliplatin-induced thermal hyperalgesia and increased the expression of CX3CL1 in DRG. Consecutive intraperitoneal injection of oxaliplatin (oxal, 4 mg/kg for five days) produced significant thermal hyperalgesia (a), which started on day 5 and lasted for at least 10 days (n = 8 in each group; **P < 0.01 versus corresponding vehicle (veh) group). (b) to (c) Representative histogram and blots showed upregulation of CX3CL1 in mRNA and protein level induced by oxaliplatin treatment (n = 6 in each group; **P < 0.01 versus the veh group). (d) Double-staining results show that CX3CL1 + (red) cells were colocalized with NF200 + (green) cells and IB4 + (green) cells but not with GFAP + (green) cells in the oxaliplatin group (n = 6). Scale bar: 50 µm.
Figure 2.
Treatment with oxaliplatin-induced neuronal hyperexcitability in DRG. (a) Continuous intrathecal delivery of neutralizing antibody against CX3CL1 (10 μg/10 μl for 10 days) attenuates thermal hyperalgesia induced by oxaliplatin (n = 8 in each group; **P < 0.01 versus veh group; #P < 0.05, ##P < 0.01 versus corresponding IgG + oxal group). (b) Representative traces of action potential discharges of DRG neurons acutely dissociated from the vehicle group, the oxaliplatin group, and the anti-CX3CL1 neutralizing antibody + oxaliplatin group. (c) Intrathecal administration of neutralizing antibody against CX3CL1 (10 μg/10 μl for 10 days) significantly inhibited the increase in the AP spikes number of DRG neurons following oxaliplatin treatment (n = 22 in each group; *P < 0.05, **P < 0.01 versus veh group; ##P < 0.01 versus the corresponding oxal group).
Activation of NF-κB contributes to neuronal hyperexcitability in DRG and thermal hyperalgesia induced by oxaliplatin
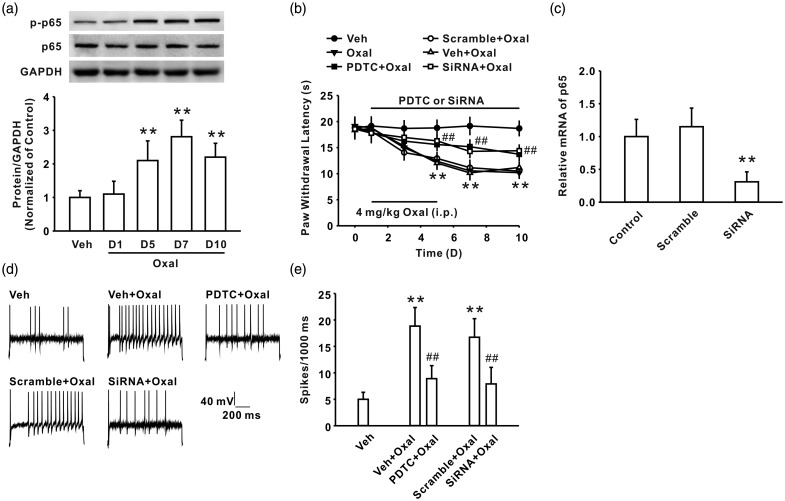
It is well-known that NF-κB, as an important transcriptional factor, via regulating the expression of many cytokines and chemokines, contributes to neuropathic pain induced by nerve injury and inflammation.26,27 A peer's study further indicated that NF-κB activation in the spinal cord played an important role in oxaliplatin-induced acute pain.8 To determine whether NF-κB signaling in DRG participated in oxaliplatin-induced chronic pain and peripheral sensitization, we first investigated the expression of phosphorylated NF-κB p65 in DRG following oxaliplatin treatment. The result showed that the expression phosphorylated p65 at Ser311 was significantly upregulated in DRG after oxaliplatin treatment (n = 6/group; Figure 3(a)). Behavioral study further indicated that continuous administration of NF-κB p65 inhibitor PDTC (200 ng/10 µl for 10 days, i.t.) ameliorated thermal hyperalgesia induced by oxaliplatin (n = 8/group; Figure 3(b)). To further confirm the role of NF-κB p65 in DRG in thermal hyperalgesia, we intrathecally injected siRNA targeting NF-κB p65 (50 μg/15 μl for 10 days), which is validated for the deletion of spinal cord CX3CL1.8 The results showed that the mRNA level of NF-κB p65 was downregulated by intrathecal injection of NF-κB p65 siRNA significantly in DRG (n = 6/group; Figure 3(c)), which indicated the efficiency of NF-κB p65 siRNA. Importantly, NF-κB p65 siRNA (i.t.) also significantly inhibited thermal hyperalgesia induced by oxaliplatin treatment (n = 8/group; Figure 3(b)). Next, we examined the role of NF-κB p65 in oxaliplatin-induced neuronal hyperexcitability in small DRG neurons. The results showed that continuous administration of PDTC (i.t.) or NF-κB p65 siRNA (i.t.) reduced the increased spike numbers in small DRG neurons on day 7 following oxaliplatin treatment (n = 22/group; Figure 3(d) and (e)). Taken together, these findings suggested that NF-κB p65 might be involved in oxaliplatin-induced thermal hyperalgesia via increasing DRG neuronal excitability.
Figure 3.
Increased phosphorylation of NF-κB p65 is involved in the thermal hyperalgesia induced by oxaliplatin. (a) The expression of phosphorylated p65 at Ser311 was significantly upregulated after oxaliplatin treatment (n = 6 in each group; **P < 0.01 versus veh group). (b) Continuous injection of p65 inhibitor PDTC (200 ng/10 µl for 10 days, i.t.) or p65 siRNA (50 μg/15 μl for 10 days, i.t.) reduced thermal hyperalgesia in oxaliplatin-treated rats (n = 8 in each group; **P < 0.01 versus veh group; ##P < 0.01 versus the corresponding veh + oxal group or scramble + oxal group). (c) Intrathecal injection of NF-κB p65 siRNA downregulated the mRNA level of NF-κB p65 in DRG (**P < 0.01 versus the corresponding scramble group). (d) Representative traces of action potential discharges of DRG neuron acutely dissociated from vehicle group, vehicle + oxaliplatin group, PDTC + oxaliplatin group, scramble + oxaliplatin group, and siRNA + oxaliplatin group. (e) The increased AP frequency in DRG neuron of oxaliplatin-treated rats was significantly inhibited by intrathecal injection of p65 inhibitor PDTC (200 ng/10 µl for 10 days, i.t.) or p65 siRNA (50 μg/15 μl for 10 days, i.t.) compared with the corresponding vehicle or scramble group (n = 22 in each group; **P < 0.01 versus veh group, ##P < 0.01 versus the corresponding veh+oxal group or scramble+oxal group).
NF-κB modulated CX3CL1 upregulation in DRG via enhancing the recruitment of NF-κB p65 to the CX3CL1 gene promoter region
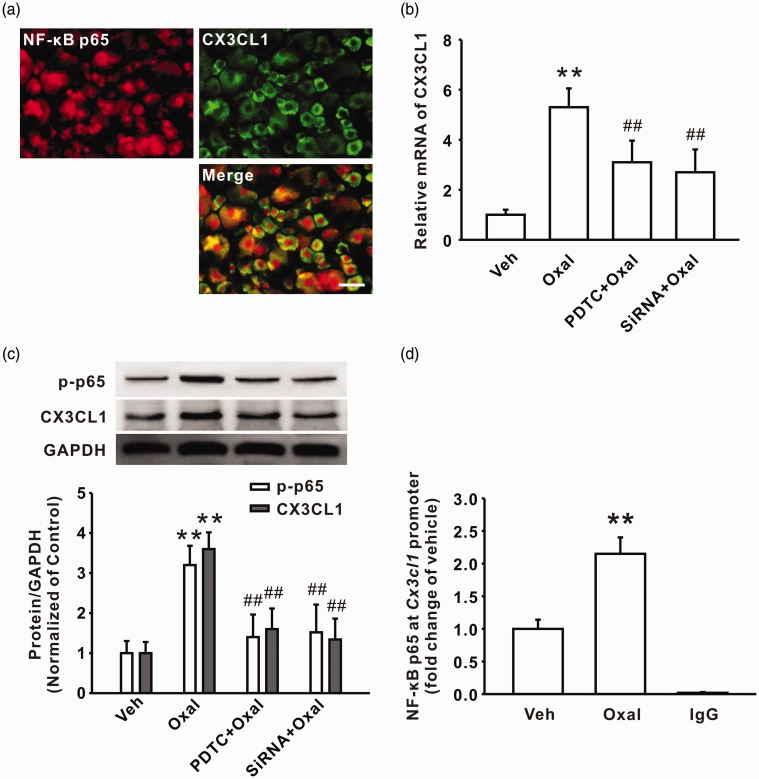
Upon activation of the NF-κB signaling pathway, NF-κB p65 can bind to a target gene promoter and facilitate the expression of target genes.28 To determine whether NF-κB p65-modulated expression of CX3CL1 in DRG contributed to oxaliplatin-induced persistent pain, we first investigated the location of NF-κB p65 and CX3CL1 in DRG cells; double immunostaining results showed that the activation of NF-κB p65 was localized in the CX3CL1-positive cells following oxaliplatin treatment on day 7 (n = 6/group; Figure 4(a)). In addition, intrathecal injection of NF-κB inhibitor PDTC or specific p65 siRNA reduced the upregulation of CX3CL1 in mRNA and protein levels in DRG following oxaliplatin treatment (n = 6/group; Figure 4(b) and (c)). Next, we examined whether activated NF-κB p65 can bind to the CX3CL1 gene and mediate CX3CL1 protein expression. Analysis from TFSEARCH and the jaspar database first showed that the position −1941/−1931 of the CX3CL1 gene is a potent-binding site for NF-κB p65. Then, ChIP-PCR assay was performed to examine the interaction of p65 and CX3CL1 gene. The results showed that the recruitment of p-p65 to the CX3CL1 gene promoter (a 162-bp fragment from −2029 to −1867) was significantly increased after oxaliplatin treatment on day 7 (n = 6/group; Figure 4(d)). These results suggested that upregulation of CX3CL1 in DRG induced by consecutive administration of oxaliplatin is dependent on the NF-κB p65 signaling pathway.
Figure 4.
Administration of oxaliplatin upregulated the expression of CX3CL1 via increasing the recruitment of NF-κB p65 to the CX3CL1 gene promoter region. (a) Double-staining result shows that NF-κB p65 + (red) cells were colocalized with CX3CL1 + (green) cells in the oxaliplatin group (n = 6). Scale bar: 50 µm. (b) Intrathecal administration of PDTC (200 ng/10 µl for 10 days) or p65 siRNA (50 μg/15 μl for 10 days) decreased the upregulation of CX3CL1 mRNA in DRG of oxaliplatin-treated rats on day 7 (n = 6 in each group; **P < 0.01 versus corresponding veh group, ##P < 0.01 versus oxal group). (c) Intrathecal injection of PDTC or p65 siRNA reduced the upregulation of p-p65 and CX3CL1 protein levels in DRG of oxaliplatin-treated rats on day 7 (n = 6 in each group; **P < 0.01 versus corresponding veh group, ##P < 0.01 versus oxal group). (d) A quantitative real-time polymerase chain reaction showed the enhanced recruitment of NF-κB p65 to the CX3CL1 gene promoter following oxaliplatin treatment (n = 6 in each group; **P < 0.01 versus veh group).
Discussion
In the present study, we provide evidence that CX3CL1 upregulation mediated by NF-κB p65 activation in DRG contributes to the peripheral sensitization and chronic pain induced by treatment with the chemotherapeutic drug oxaliplatin. Thermal hyperalgesia is a well-known marker of oxaliplatin neurotoxicity.29 Here, we observed the paw withdrawal latency of oxaliplatin-treated rats and found that intraperitoneal administration of oxaliplatin (4 mg/kg) for five consecutive days in rats also markedly induced thermal hyperalgesia. In addition, the neuronal excitability of small diameter DRG neurons was enhanced following administration of oxaliplatin. These results indicate that neuronal hyperexcitability-mediated peripheral sensitization is involved in the genesis of oxaliplatin-induced chronic pain. We further found that oxaliplatin application upregulated the expression of CX3CL1 in DRG neurons, and intrathecal administration of anti-CX3CL1 neutralizing antibody significantly attenuated thermal hyperalgesia following oxaliplatin treatment. In line with our findings, other studies have shown that CX3CL1 upregulation in rat DRG was involved in inflammatory pain30 or disc herniation-induced pathological pain.31 In addition, the result that neutralization of CX3CL1 activity prevented the increase of action potential firing in DRG neurons following oxaliplatin treatment suggested that DRG neuronal hyperexcitability mediated by CX3CL1 might be a major reason for oxaliplatin-induced chronic pain.
It is well-known that cytokines or chemokines synthesized by DRG neurons modulate the properties and number of ion channels in nociceptive sensory neurons.32,33 A peer's study showed that treatment of cultured DRG neurons with TNF-α enhanced the tetrodotoxin-resistant sodium currents.34 Therefore, in the present study, DRG neuronal hyperexcitability probably arises from the effect of CX3CL1 signaling on the function of ion channels. Importantly, another study showed that the increase of CX3CL1 also contributes to the chemotherapeutic paclitaxel-induced chronic pain via enhancing DRG neuronal excitability.10 Together with the present study, we hypothesize that CX3CL1 might be a common molecule in chemotherapy-induced chronic pain.
As an important transcription factor, NF-κB is originally known to regulate inflammation and immune responses35 and is now believed to be implicated in synaptic plasticity, learning, memory formation, neurotransmission, and neuroprotection in the nervous system.36–39 Recent studies have shown that NF-κB was also involved in the induction and maintenance of acute and chronic pain following nerve inflammation or injury.18,40,41 For example, NF-κB activation in DRG-mediated chronic pain induced by an inflammatory response.18 Consistent with these findings, we further found that NF-κB was markedly activated in DRG of oxalipatin-induced chronic pain rats. In support of our findings, peer studies have found that oxaliplatin induced NF-κB activation in several cell lines.42,43 Moreover, the present study showed that inhibition of NF-κB p65 activation by application of PDTC or siRNA ameliorated the thermal hyperalgesia following consecutive oxaliplatin treatment. Studies showed that NF-κB p65 activation mediated cytokine or chemokine expression,8,18 enhancement of neuronal excitability that contributed to chronic pain.44 In the present study, we also observed colocalization of NF-κB p65 and CX3CL1 in DRG cells, and intrathecal injection of PDTC or siRNA attenuated the CX3CL1 upregulation induced by oxaliplatin treatment. In line with our findings, Li et al.9 have reported that intrathecal injection of PDTC or siRNA decreased the spinal CX3CL1 upregulation induced by paclitaxel treatment. However, we cannot exclude the possibility that the intrathecal dosing scheme used in the present study affects the expression of CX3CL1 in the spinal cord. Furthermore, our data unequivocally showed that p-p65 directly bound to the CX3CL1 promoter region upregulated CX3CL1 expression in DRG. In support of our findings, recent studies have revealed that CX3CL1 expression was upregulated by increased NF-κB p65 binding and H4 acetylation in the CX3CL1 promoter region, thus contributing to oxaliplatin-induced acute pain and paclitaxel-induced mechanical allodynia.8,9 Studies showed that NF-κB, via mediating chemokines synthesis, regulated many channels' expression. For example, chemokine CXCL1 mediated by NF-κB activation modulates neuronal excitability of DRG neurons by increasing sodium currents, potassium currents, and the function of TRPV1 channels.45–47 Therefore, it is possible that the upregulation of CX3CL1 in DRG mediated by NF-κB p65 activation enhances neuronal excitability and contributes to the chronic pain following oxaliplatin treatment.
Author Contributions
Jing Wang, Xin-Sheng Zhang, Rong Tao, Lin-Xia Song, and Ling-Jie Xia conceived of the project and designed the experiments. Jing Wang, Xin-Sheng Zhang, Lin Liu, Song-He Ma, Ying-Hai Jiang, and Jie Zhang carried out the experiments. Jing Wang, Xin-Sheng Zhang, Rong Tao, and Lin-Xia Song analyzed the data and prepared the figures. Lin-Xia Song and Ling-Jie Xia supervised the overall experiment. Jing Wang, Xin-Sheng Zhang, Lin-Xia Song, and Ling-Jie Xia revised the manuscript. All authors read and approved the final manuscript. Jing Wang and Xin-Sheng Zhang contributed equally to this work.
Declaration of Conflicting Interests
The author(s) declared no conflict of interests with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Henan Science and Technology Plan Projects (201304042) and Henan Science and Technology Innovation Talents Project (201602229).
References
- 1.Culy CR, Clemett D, Wiseman LR. Oxaliplatin. A review of its pharmacological properties and clinical efficacy in metastatic colorectal cancer and its potential in other malignancies. Drugs 2000; 60: 895–924. [DOI] [PubMed] [Google Scholar]
- 2.Park SB, Krishnan AV, Lin CSY, et al. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem 2008; 15: 3081–3094. [DOI] [PubMed] [Google Scholar]
- 3.Brouwers EE, Huitema AD, Boogerd W, et al. Persistent neuropathy after treatment with cisplatin and oxaliplatin. Acta Oncol 2009; 48: 832–841. [DOI] [PubMed] [Google Scholar]
- 4.Xiao WH, Bennett GJ. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 2012; 153: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm 2014; 71: 19–25. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Luan S, OuYang H, et al. Upregulation of CCL2 via ATF3/c-Jun interaction mediated the Bortezomib-induced peripheral neuropathy. Brain Behav Immun 2016; 53: 96–104. [DOI] [PubMed] [Google Scholar]
- 7.Xu T, Zhang XL, Ou-Yang HD, et al. Epigenetic upregulation of CXCL12 expression mediates anti-tubulin chemotherapeutics-induced neuropathic pain. Pain 2017; 158: 637–648. [DOI] [PubMed] [Google Scholar]
- 8.Huang ZZ, Li D, Ou-Yang HD, et al. Cerebrospinal fluid oxaliplatin contributes to the acute pain induced by systemic administration of oxaliplatin. Anesthesiology 2016; 124: 1109–1121. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Huang ZZ, Ling YZ, et al. Up-regulation of CX3CL1 via nuclear factor-kappa B-dependent histone acetylation is involved in paclitaxel-induced peripheral neuropathy. Anesthesiology 2015; 122: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 10.Huang ZZ, Li D, Liu CC, et al. CX3CL1-mediated macrophage activation contributed to paclitaxel-induced DRG neuronal apoptosis and painful peripheral neuropathy. Brain Behav Immun 2014; 40: 155–165. [DOI] [PubMed] [Google Scholar]
- 11.Dib-Hajj SD, Cummins TR, Black JA, et al. Sodium channels in normal and pathological pain. Annu Rev Neurosci 2010; 33: 325–347. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Q, Fang D, Cai J, et al. Enhanced excitability of small dorsal root ganglion neurons in rats with bone cancer pain. Mol Pain 2012; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross-Huot MC, Laferriere A, Khorashadi M, et al. Glycemia-dependent nuclear factor kappaB activation contributes to mechanical allodynia in rats with chronic postischemia pain. Anesthesiology 2013; 119: 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowles RD, Mata BA, Bell RD, et al. In vivo luminescence imaging of NF-kappaB activity and serum cytokine levels predict pain sensitivities in a rodent model of osteoarthritis. Arthritis Rheumatol 2014; 66: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo JG, Zhao XL, Xu WC, et al. Activation of spinal NF-kappaB/p65 contributes to peripheral inflammation and hyperalgesia in rat adjuvant-induced arthritis. Arthritis Rheumatol 2014; 66: 896–906. [DOI] [PubMed] [Google Scholar]
- 16.Zhao LX, Jiang BC, Wu XB, et al. Ligustilide attenuates inflammatory pain via inhibition of NFkappaB-mediated chemokines production in spinal astrocytes. Eur J Neurosci 2014; 39: 1391–1402. [DOI] [PubMed] [Google Scholar]
- 17.Chu LW, Chen JY, Wu PC, et al. Atorvastatin prevents neuroinflammation in chronic constriction injury rats through nuclear NFkappaB downregulation in the dorsal root ganglion and spinal cord. ACS Chem Neurosci 2015; 6: 889–898. [DOI] [PubMed] [Google Scholar]
- 18.Fu ES, Zhang YP, Sagen J, et al. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain 2010; 148: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Su YM, Li D, et al. TNF-alpha-mediated JNK activation in the dorsal root ganglion neurons contributes to Bortezomib-induced peripheral neuropathy. Brain Behav Immun 2014; 38: 185–191. [DOI] [PubMed] [Google Scholar]
- 20.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 21.Huang ZJ, Song XJ. Differing alterations of sodium currents in small dorsal root ganglion neurons after ganglion compression and peripheral nerve injury. Mol Pain 2008; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Q, Fang D, Liu M, et al. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain 2013; 154: 434–448. [DOI] [PubMed] [Google Scholar]
- 23.Xu JT, Tu HY, Xin WJ, et al. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol 2007; 206: 269–279. [DOI] [PubMed] [Google Scholar]
- 24.Coderre TJ, Katz J. Peripheral and central hyperexcitability: differential signs and symptoms in persistent pain. Behav Brain Sci 1997; 20: 404–419. [DOI] [PubMed] [Google Scholar]
- 25.Carlton SM, Du J, Tan HY, et al. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 2009; 147: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC, et al. Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-kappaB. Chem Biol Interact 2015; 237: 9–17. [DOI] [PubMed] [Google Scholar]
- 27.Yin Q, Fan Q, Zhao Y, et al. Spinal NF-kappaB and chemokine ligand 5 expression during spinal glial cell activation in a neuropathic pain model. PloS One 2015; 10: e0115120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan F, Anderson DE, Barnitz RA, et al. Ribosomal protein S3: a KH domain subunit in NF-kappaB complexes that mediates selective gene regulation. Cell 2007; 131: 927–939. [DOI] [PubMed] [Google Scholar]
- 29.Attal N, Bouhassira D, Gautron M, et al. Thermal hyperalgesia as a marker of oxaliplatin neurotoxicity: a prospective quantified sensory assessment study. Pain 2009; 144: 245–252. [DOI] [PubMed] [Google Scholar]
- 30.Souza GR, Talbot J, Lotufo CM, et al. Fractalkine mediates inflammatory pain through activation of satellite glial cells. Proc Natl Acad Sci U S A 2013; 110: 11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobsen DP, Moen A, Haugen F, et al. Hyperexcitability in Spinal WDR neurons following experimental disc herniation is associated with upregulation of fractalkine and its receptor in nucleus pulposus and the dorsal root ganglion. Int J Inflam 2016; 2016: 6519408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RJ, Jung H, Bhangoo SK, et al. Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol 2009; 194: 417–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang F, Sun W, Yang Y, et al. SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation. J Neuroinflammation 2015; 12: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X, Gereau RWT. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 2006; 26: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994; 12: 141–179. [DOI] [PubMed] [Google Scholar]
- 36.Meffert MK, Chang JM, Wiltgen BJ, et al. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci 2003; 6: 1072–1078. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez H, Hale VA, Dolcet X, et al. NF-kappaB signalling regulates the growth of neural processes in the developing PNS and CNS. Development 2005; 132: 1713–1726. [DOI] [PubMed] [Google Scholar]
- 38.Fridmacher V, Kaltschmidt B, Goudeau B, et al. Forebrain-specific neuronal inhibition of nuclear factor-kappaB activity leads to loss of neuroprotection. J Neurosci 2003; 23: 9403–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol 2009; 1: a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu ES, Zhang YP, Sagen J, et al. Transgenic glial nuclear factor-kappa B inhibition decreases formalin pain in mice. Neuroreport 2007; 18: 713–717. [DOI] [PubMed] [Google Scholar]
- 41.Niederberger E, Schmidtko A, Gao W, et al. Impaired acute and inflammatory nociception in mice lacking the p50 subunit of NF-kappa B. Eur J Pharmacol 2007; 559: 55–60. [DOI] [PubMed] [Google Scholar]
- 42.Almendro V, Ametller E, Garcia-Recio S, et al. The role of MMP7 and its cross-talk with the FAS/FASL system during the acquisition of chemoresistance to oxaliplatin. PloS One 2009; 4: e4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson C, Purcell C, Seaton A, et al. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther 2008; 327: 746–759. [DOI] [PubMed] [Google Scholar]
- 44.Schafers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett 2008; 437: 188–193. [DOI] [PubMed] [Google Scholar]
- 45.Wang JG, Strong JA, Xie W, et al. The chemokine CXCL1/growth related oncogene increases sodium currents and neuronal excitability in small diameter sensory neurons. Mol Pain 2008; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang RH, Strong JA, Zhang JM. NF-kappaB mediated enhancement of potassium currents by the chemokine CXCL1/growth related oncogene in small diameter rat sensory neurons. Mol Pain 2009; 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong F, Du YR, Xie W, et al. Increased function of the TRPV1 channel in small sensory neurons after local inflammation or in vitro exposure to the pro-inflammatory cytokine GRO/KC. Neurosci Bull 2012; 28: 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]