Abstract
Objectives/Hypothesis
No biomarkers are used to estimate the prognosis in oral cavity squamous cell carcinoma (OSCC). In our previously published work, we have reported the prognostic value of CD44 and hypoxia inducible factor (HIF)−1α in patients with stage I disease.
Study Design
In this study, we tested our previous observations in a larger cohort. We also studied the predictive value of common lymphatic endothelial and vascular endothelial receptor (CLEVER)−1 in this material.
Methods
CD44, HIF1α, and CLEVER‐1 were immunohistochemically analyzed in paraffin‐embedded tissue material of stage I OSCC patients treated at three Finnish university hospitals. Microscopy results were correlated with OSCC outcome.
Results
As in our pilot study, the CD44lowHIF1αhigh signature was associated with poorer disease‐free survival. Clear correlations between CLEVER‐1 expression and clinical outcome were not evident.
Conclusion
Our results suggest that immunohistochemistry of CD44 and HIF1α may be useful in identification of patients with poor prognoses. These parameters could be used to select the optimal treatment modalities for stage I OSCC patients.
Level of Evidence
2b.
Keywords: Biomarkers, CD44, CLEVER‐1, HIF1α, OSCC
INTRODUCTION
About 300,000 people are diagnosed with oral cavity squamous cell carcinoma (OSCC) worldwide each year.1 In the Finnish population, this translates to roughly 250 newly diagnosed cases of OSCC, in a total population of 5.4 million, according to data from the NORDCAN registry.2 The most widely studied etiological factors in OSCC include tobacco and alcohol. The human papilloma virus status and p16 expression as prognostic markers in SCC have gained attention in oropharyngeal tumors,3 but the role in OSCC is not clear.4 In SCC of the oral cavity, several immunohistochemical biomarkers for clinical decision making have been studied, but none are yet widely used. The modern gold standard of care includes surgical resection of the primary tumor, with sufficient margins combined with sentinel node biopsy or elective neck dissection levels I–III.5
CD44, the most important cell surface receptor for extracellular hyaluronan, mediates cell adhesion to its surroundings. Its functional role in head and neck cancer includes interaction with the epidermal growth factor receptor (EGFR), which influences EGFR signaling into the cytoplasm.6 Hypoxia inducible factor‐1α is a master transcription factor that is stabilized in hypoxic conditions, after which it translocates into the nucleus to interact with hypoxia responsive elements.7 A recent meta‐analysis on the prognostic significance of HIF1α in oral cavity cancer has shown HIF1α up‐regulation to be associated with poorer overall survival.8
Common lymphatic endothelial and vascular endothelial receptor (CLEVER‐1)/Stabilin‐1 is a scavenger receptor expressed by lymphatic and inflamed vascular endothelium and a subset of type 2 polarized macrophages.9 CLEVER‐1 expression has been reported in several tumor types such as head and neck cancer and breast cancer.10 Macrophage polarization to type 2 has been shown to be correlated with tumor aggressiveness in cervical lymph nodes (LN) of OSCC patients.11 Recent observations in a mouse model indicate CLEVER‐1 to be important in supporting tumor growth.12
We have previously reported on the predictive value of combined tumor cell expression of HIF1α and CD44 in stage I OSCC. The expression of these markers allowed us to define patients with dramatically different clinical outcomes in a small pilot study with 35 tissue specimens.13 In our pilot study, up to 75% of the patients with a CD44low/HIF1αhigh signature experienced a local recurrence or metastasis within 5 years after primary therapy.
Our primary objective in this work was to validate our previous results with HIF1α and CD44 in a larger sample, with more emphasis also on clinical parameters such as tumor size, grade, and smoking status. Our secondary objective in this study was to evaluate if CLEVER‐1 expression correlates with cancer‐specific outcome in stage I OSCC patients.
MATERIALS AND METHODS
Patients and Samples
This is a population‐based study conducted with archive material from three university hospital districts in Finland, covering a population of 2.7 million inhabitants. Tissue specimens have been collected at Oulu University Hospital, Turku University Hospital, and Tampere University Hospital as a part of normal patient care. Patients who were diagnosed with stage I oral cavity squamous cell carcinoma (C01‐06 in the International Classification of Diseases, 10th Revision, classification) during the years 2000 through 2009 were included in the study. For Turku University Hospital, samples collected in 2000 through 2004 were omitted because the material has been previously published in the earlier study.13 Clinical parameters were traced from patient records, and formalin‐fixed paraffin‐embedded tissue specimens were collected at the hospital archives. The patients whose clinical data were not retrievable were excluded from all analyses.
Ethical Considerations
Ethical permission for the study was admitted by the ethical committee of Turku University Hospital, Turku, Finland, and by the Finnish National Supervisory Authority for Welfare and Health (Valvira). Tissue specimens and clinical data have been collected in the archives and have not influenced patient care.
Immunohistochemistry
Five micrometer tissue sections were cut from representative tissue blocks and mounted on coated objective slides. Paraffin was removed with xylene, and samples were rehydrated with descending ethanol. Epitope retrieval was performed by boiling in citrate for 5 minutes (CD44 and HIF1α stainings) or Proteinase K (CLEVER‐1 stainings) pretreatment for 5 minutes at +37°C. Vectastain ABC antimouse and antirat kits (Vector laboratories, Burlingame, CA) were used according to the manufacturer's recommendations. Primary antibodies were used at the following concentrations: anti‐CD44 (mouse antihuman, Hermes‐3 clone14) at 0.20 µg/ml, anti‐HIF1α (mouse antihuman, H1alpha67 clone, Thermo Fisher Scientific, Waltham, MA) at 1:25, and anti‐CLEVER‐1 (2–7 clone15) at 1:10. Diaminobenzidine (Dako, Glostrup, Denmark) was used as a chromogen and Mayer's hematoxylin for counterstaining.
Microscopic Evaluation
All slides were scanned with a Pannoramic 250 slide scanner (3DHistec, Budapest, Hungary), and digital files were viewed on CaseViewer v1.3 software (3DHistec). Hypoxia inducible factor‐1α–stained samples were graded as negative/low, intermediate, or high in histologically cancerous areas (Fig. 1). CLEVER‐1 positive vessels and macrophages were separately counted in peritumoral areas (Fig. 1). Exact CLEVER‐1 positive macrophage densities were calculated. CLEVER‐1 positive flat‐walled vessel densities were determined semiquantitatively as missing, scarce, or abundant.
Figure 1.
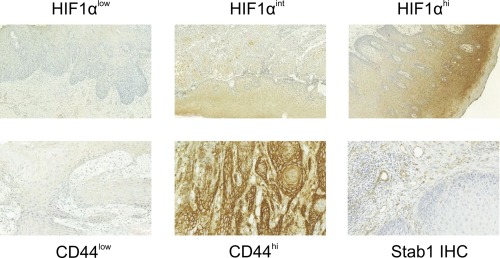
Figure Legends: Example images of immunohistochemistry. Whole‐slide scans were zoomed in to 20× magnification. The different staining patterns of HIF1α and CD44 are displayed, along with common lymphatic endothelial and vascular endothelial receptor staining (Stab‐1).
HIF = hypoxia inducible factor; Stab = stabilin.
Representative JPEG‐quality captions of CD44 stained samples were exported from the CaseViewer v1.3 software (3DHistec) for quantitative analysis (Fig. 1). CD44 staining intensity was graded on a scale of 1 to 20 with the ImmunoMembrane plugin for ImageJ16 (Supp. Fig. S1). Optimal cutoff value determination was assisted by receiver operating characteristic (ROC) analysis, with the intent to stratify patients as CD44low or CD44high.
Statistical Analyses
All analyses were performed on SPSS Statistics 21 (IBM Corp., Armonk, NY). Relationships among the patient's immunohistochemical score, clinicopathological variables, and prognoses were evaluated. Receiver operating characteristic curves were constructed for continuous covariates, with a cancer recurrence (local or metastatic) as the explanatory variable.
Optimal cutoff points for tumor diameter, operation margin, age, and immunohistochemical scores were sought manually, assisted by ROC analyses, for univariate and multivariate survival analyses. In survival statistics, disease‐free survival (DFS) and disease‐specific survival (DSS) were analyzed over 60 months after primary treatment.
Cox proportional hazards regression model and the Kaplan‐Meier method with log‐rank tests were used for univariate analyses. Multivariate analyses were performed with the Cox proportional hazards regression model. The covariates that reached statistical significance in multivariate analyses of clinical parameters were included in multivariate analyses with the biomarkers. Results were considered statistically significant when P < 0.05.
RESULTS
Similar to our pilot study,13 more than half of the patients in this study were female. Of those patients, whose smoking status was retrievable, 14% reported to be current smokers and 43% reported to have no smoking history. The most common tumor site for patients in this study was the tongue (Table 1).
Table 1.
Clinical Features of the Patients in this Study.
| n | % | ||
|---|---|---|---|
| Gender | Male | 78 | 45.6 |
| Female | 93 | 54.4 | |
| Center | Turku | 58 | 33.9 |
| Tampere | 48 | 28.1 | |
| Oulu | 65 | 38.0 | |
| Grade | 1 | 86 | 50.3 |
| 2 | 63 | 36.8 | |
| 3 | 9 | 5.3 | |
| 4 | 0 | 0.0 | |
| Data missing | 13 | 7.4 | |
| Smoking status | Non‐smoker | 51 | 29.8 |
| Previous smoker | 50 | 29.2 | |
| Current smoker | 17 | 9.9 | |
| Data missing | 53 | 31.0 | |
| Tumor site | Tongue | 123 | 71.9 |
| Floor of mouth | 21 | 12.3 | |
| Upper gingiva | 3 | 1.8 | |
| Lower gingiva | 14 | 8.2 | |
| Hard palate | 2 | 1.2 | |
| Soft palate | 3 | 1.8 | |
| Cheek mucosa | 5 | 2.9 | |
| Median | Range | ||
| Age at diagnosis (years) | 65 | (23‐91) | |
| Tumor diameter (mm) | 12 | (2‐40) | |
| Operation margin (mm) | 4 | (0‐17) | |
| Follow‐up time (months) | 55 | (0‐168) |
All but eight patients were primarily treated surgically. Surgical margins with at least 5‐mm healthy tissue were considered sufficient. The primary treatment of some patients was supplemented with elective neck dissection (44 patients) or sentinel node biopsy (17 patients). Twenty‐eight patients received secondary treatment, mostly due to insufficient surgical margins in the primary surgery (Supp. Table SI).
After primary treatment, patients were routinely followed by a specialist for 60 months, when a clinical examination and medical imaging on demand were performed. With 171 patients in this study, 21 local recurrences, 36 neck metastases, and nine distant metastases were observed. Treatment results were similar in all centers (P = 0,70; P = 0,69; and P = 0,69 respectively, Fisher's exact test). Eighteen of the recurrent diseases were treated surgically, one with radiotherapy, three with chemoradiation, and 26 with combined therapy. Three patients had only palliative treatment. Within 5 years from primary treatment, 24 patients died of reasons attributable to OSCC (Fig. 2).
Figure 2.
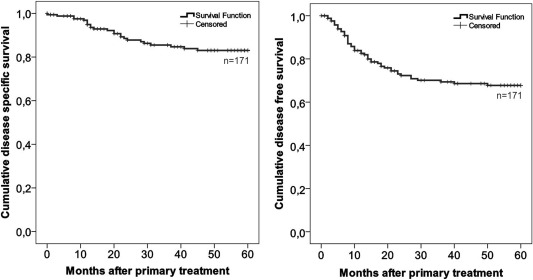
Kaplan‐Meier analyses of disease‐free and disease‐specific survival of all patients in this study.
Correlations Between Clinical Parameters and Outcome
Correlations between gender, age, smoking status, grade, tumor size, operation margin, and sentinel node biopsy (SNB) or elective neck dissection (END) with cancer recurrence (DFS) or cancer‐associated mortality (DSS) were analyzed with the Cox proportional hazards model in univariate and multivariate analyses. Where correlations were found, Kaplan‐Meier curves were also constructed. Smoking status was analyzed in the univariate analyses, but it was omitted from the multivariate analyses because the categories are not clearly hierarchic (current vs. never vs. previous smoking) and data was not retrievable for 31% of the patients.
Tumor size was a predictive factor of poor DFS and DSS among patients with stage I OSCC, when all cancer recurrences and cancer‐associated deaths within 60 months after primary treatment were taken into account (log‐rank P = 0.01 and 0.04, respectively) (Supp. Fig. S2). Among patients with cT1 OSCC, those whose tumors exceeded 15 mm in diameter had a poorer DFS and DSS (P = 0.02 and P = 0.04, respectively, in multivariate analyses) (Table 2B).
Table 2.
Correlations Between Clinical Features and Disease‐Free or Disease‐Specific Survival.
| A. Results From Univariate Cox Proportional Hazards Model. | |||||
|---|---|---|---|---|---|
| Disease free survival | Disease specific survival | ||||
| Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | ||
| Univariate analyses | Gender: Men vs. women | 0.986 | 0.995 | 0.748 | 1.142 |
| Age: Over 60 vs. under 60 years | 0.292 | 0.736 | 0.32 | 1.538 | |
| Smoking: Ever vs. never | 0.363 | 0.741 | 0.643 | 0.808 | |
| Smoking: Current vs. previous | 0.421 | 1.496 | 0.674 | 1.347 | |
| Grade:1 vs. 2 or 3 | 0.124 | 0.631 | 0.033 | 0.371 | |
| Tumor size over 15mm vs. under 15mm | 0.019 | 2.055 | 0.051 | 2.277 | |
| Operation margin over 5mm vs. under 5mm | 0.573 | 0.812 | 0.846 | 1.097 | |
| SNB or END: yes vs. no | 0.098 | 0.577 | 0.615 | 0.798 | |
| B. Results From Multivariate Cox Proportional Hazards Model. | |||||
|---|---|---|---|---|---|
| Disease free survival | Disease specific survival | ||||
| Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | ||
| Multivariate analyses | Gender: Men vs. women | 0.759 | 1.11 | 0.884 | 0.932 |
| Age: Over 60 vs. under 60 years | 0.16 | 1.676 | 0.128 | 2.409 | |
| Grade:1 vs. 2 or 3 | 0.397 | 0.754 | 0.14 | 0.491 | |
| Tumor size over 15mm vs. under 15mm | 0.023 | 2.214 | 0.037 | 2.659 | |
| Operation margin over 5mm vs. under 5mm | 0.496 | 0.758 | 0.943 | 1.038 | |
| SNB or END: yes vs. no | 0.304 | 0.694 | 0.936 | 0.962 | |
END = elective neck dissection; SNB = sentinel node biopsy.
A statistically significant correlation was seen between advancing tumor grade and poorer DSS in univariate analyses (log‐rank P = 0.03) (Supp. Fig. S2). A nonsignificant trend was observed between advancing tumor grade and poor DFS within 5 years from primary treatment (log‐rank P = 0.09) (Supp. Fig. S2). Patients treated primarily with SNB or END had a trend to better DFS (P = 0.09), but this did not affect DSS (P = 0.61) (Supp. Fig. S3).
Correlations between gender, age, or smoking status and DFS or DSS were not seen in univariate analyses (Tables 2A, IIB).
Correlations Between Biomarkers and Clinical Outcome
In order to improve reproducibility in grading of CD44 analyses, virtual microscopy was implemented. We found analyses done with the ImmunoMembrane plugin for ImageJ to yield in results similar to those of an experienced microscopist (Supp. Fig. S1). Results from microscopic evaluations are summarized in the supplements (Supp. Table SII).
CD44 score was categorized to high versus low by manually testing different cutoff points. An optimal cutoff was found at 12 points (scale 0–20 points).
Statistically borderline‐significant trends were observed between low CD44 expression or high HIF1α expression and decreased DFS in univariate analyses and multivariate analyses with tumor size (hazard ratio [HR] = 1.984, P = 0.144 and HR=1.856, P = 0.13, respectively, in multivariate analyses). Thus, low CD44 score or high HIF1α score were risk factors for cancer recurrence or OSCC‐associated death. Clear correlations between CLEVER‐1 expression on tumor‐associated macrophages or flat‐walled vessels and DFS or DSS were not seen (Tables 3A, IIIB).
Table 3.
Correlations Between Disease‐Free or Disease‐Specific Survival and Immunohistochemical Scores.
| A. Univariate Cox Proportional Hazards Model Analyses of Immunohistochemical Scores | |||||
|---|---|---|---|---|---|
| Disease free survival | Disease specific survival | ||||
| Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | ||
| Univariate analyses | HIF1α high vs. low | 0.216 | 1.569 | 0.911 | 0.946 |
| CD44 low vs. high | 0.154 | 1.718 | 0.146 | 2.12 | |
| Stab1 Mφ low vs. high | 0.777 | 0.915 | 0.187 | 1.917 | |
| Stab1 vessels low vs. high | 0.303 | 0.701 | 0.326 | 0.6 | |
| B. Three Separate Multivariate Analyses of Immunohistochemical Scores. | |||||
|---|---|---|---|---|---|
| Disease free survival | Disease specific survival | ||||
| Variables in the Equation | Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | |
| Multivariate analyses | HIF1α high vs. low | 0.059 | 2.24 | 0.931 | 1.048 |
| CD44 low vs. high | 0.135 | 1.769 | 0.145 | 2.137 | |
| Stab1 Mφ low vs. high | 0.299 | 0.678 | 0.167 | 0.449 | |
| Stab1 vessels low vs. high | 0.32 | 0.69 | 0.2 | 0.475 | |
| Variables in the Equation | Disease free survival | Disease specific survival | |||
| Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | ||
| Tumor size over 15 mm | 0.186 | 1.741 | 0.324 | 1.782 | |
| HIF1α high vs. low | 0.129 | 1.856 | 0.234 | 1.952 | |
| CD44 low vs. high | 0.114 | 1.984 | 0.997 | 0.998 | |
| Variables in the Equation | Disease free survival | Disease specific survival | |||
| Cox P‐value | Hazard Ratio | Cox P‐value | Hazard Ratio | ||
| Tumor size over 15 mm | 0.187 | 1.727 | 0.251 | 1.934 | |
| HIFhighCD44low vs. others | 0.038 | 1.915 | 0.461 | 1.396 | |
Tumor size (cutoff at 15 mm) was included in some analyses because it was the only examined clinical parameter that yielded a significant P value in previous analyses.
HIF = hypoxia inducible factor; Stab = stabilin.
We stratified patients according to CD44 and HIF1α score into CD44lowHIF1αhigh, CD44lowHIF1αlow, CD44highHIF1αhigh, and CD44highHIF1αlow strata. Because low CD44 grade and high HIF1α grade were associated with elevated risk, patients with CD44lowHIF1αlow or CD44highHIF1αhigh signature were processed together as intermediate risk patients. Improved DFS in patients with CD44highHIF1αlow tumors was observed, whereas those with CD44lowHIF1αhigh tumors had the worst DFS. Five‐year cancer recurrence rates of CD44lowHIF1αhigh, intermediate risk, and CD44highHIF1αlow tumors were 53%, 30%, and 19%, respectively (Fig. 3) (P = 0.03 in log‐rank). Disease‐specific mortality rates were 33%, 11%, and 15%, respectively (Fig. 3) (P = 0.08 in log‐rank).The clinical parameters that reached statistical significance in multivariate analyses were analyzed together with immunohistochemical markers. Tumor size was analyzed with CD44 and HIF1α independently, or with the combination of CD44 and HIF1α. In these analyses, a significant P value was only seen between the combination of CD44 and HIF1α and DFS (P = 0.04) (Table 3B).
Figure 3.
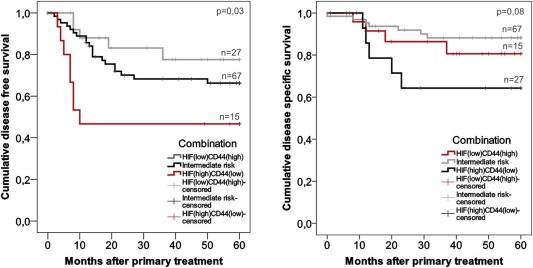
Kaplan‐Meier analyses of disease‐free and disease‐specific survival. The impact of CD44 and HIF1α was analyzed. CD44highHIF1αhigh and CD44lowHIF1αlow patients were grouped as an intermediate risk group. P values: log‐rank test.
HIF = hypoxia inducible factor.
DISCUSSION
Oral cavity cancer is a growing problem due to steadily rising incidence levels. Despite recent advances in imaging techniques, identification of patients with high risk of metastasis and worse prognoses from all the patients with cN0 OSCC at the time of diagnoses remains difficult.17 Whereas different cancer therapy modalities such as chemoradiotherapy, surgical techniques, and emerging new immunotherapeutics exist, the key to successful patient care lies in a standardized way to select the correct treatment modality for each patient. Most patients do well with standard treatment, but some of the patients would need more aggressive treatment from the beginning. At the moment, we do not have tools to separate these groups.
Our previous results indicated CD44 and HIF1α to be highly applicable biomarkers for stage I OSCC in a small pilot study.13 In the current study, we have validated our previous results in a larger series of patient samples collected in three Finnish tertiary cancer treatment centers. Although we did not get as clear black‐and‐white results in this work as we did in our pilot study, we could still identify the patient population with only 47% DFS, which is unacceptably poor for stage I disease. These 14% of the patients had high HIF1α‐ and low CD44‐expressing tumors, and they were at the largest risk of cancer recurrence. These two biomarkers might provide new opportunities for clinicians in recurrence risk evaluation and therapy planning. Patients with CD44lowHIF1αhigh tumors should be candidates to receive more aggressive initial cancer therapy.
The microscopy analyses in this study were partially assisted by virtual microscopy, where immunohistochemically stained tissue samples are digitized and analyses are assisted by computer software.16 This method improves the reproducibility of grading, and we presume that it might ease the implementation of new immunohistochemical biomarkers, particularly in smaller cancer units due to improved opportunities of virtual case‐by‐case consultation. At the moment, this method is not reliable in analyses where the identification of tissue structures is necessary, such as CLEVER‐1 in our case, but it can be used in clear cytoplasmic cell stainings on definite areas, such as CD44 expression of cancer cells in this study. Computer‐assisted analysis is thought to reduce operator‐dependent and operator‐independent variation.
Alternative biomarkers for clinical prognosis of OSCC have been studied. Epidermal growth factor receptor (EGFR), proliferation marker Ki67, and tumor suppressor protein p53 are most widely studied, but the results are heterogeneous and none of the studied prognostic markers are in routine clinical use.18 Most of the markers studied earlier have been reported only once. In this study, we have wanted to avoid typical pitfalls of immunohistochemical studies by combining the material of three university hospitals for validation this series, by using standardized virtual microscopy as much as possible, and finally by performing multivariate analyses to confirm the significance of immunohistochemical results.
As more knowledge considering cancer of the head and neck is gathering, it has become evident that this is not a consistent disease group. First of all, the nature of the disease is dependent on the site of the tumor.19 Secondly, the nature of the tumor changes as the tumor grows, and therefore it is not surprising that uniform molecular markers for the prognoses have not been found. For example, CD44 as a cell adhesion molecule in normal conditions is shown to be a predictor of good prognoses in our study, but the very same CD44 has also been associated with poor prognoses in more advanced stages and even recognized as a cancer stem cell marker.20 Therefore, we need to limit our analyses to reasonable subgroups when unraveling the prognostic markers for carcinoma of the head and neck.
Among clinically T1‐classified OSCC, tumor diameter showed to be of predictive importance. Particularly tumors whose size exceeded 15 mm in diameter had a high probability of recurrence. In this series, we had three tumors exceeding 20 mm in pathological analyses, although they were clinically classified as T1, which may affect this result. This raises the question of the optimal cutoff value between T1 and T2 in a prognostic sense. Evidently our material is too limited to make any deeper conclusions considering this classification, but it is important to remember that tumor size is not a categorical variable in the real life. Also, tumor grade correlated with disease‐specific survival, as we saw in our pilot study.13 There are several studies showing the predictive value of tumor grade, but in general it is considered to be too subjective and unreliable, and therefore it is not used for treatment decision making in the clinic.21
This patient population was collected between 2000 and 2009. Sentinel node biopsy or END were implemented to routine treatment of T1 OSCC during this interval, and therefore more than half of these patients have still had only local surgery as their primary treatment modality. Controlling the neck improved disease‐free survival, as suspected, but it did not have an effect on disease‐specific survival. This reflects an effective follow‐up protocol and active secondary treatment when any recurrences are detected. This is also consistent with the studies showing no survival advantage for END patients in comparison to watchful waiting in clinically N0 neck in T1‐2 head and neck cancer.22, 23
CONCLUSION
In conclusion, current clinical protocol without predictive biomarkers either leads to overtreatment of 70% to 80% of these patients, or inversely to under treatment of 20% to 30% of the stage I OSCC patients, depending on which treatment protocol we choose to use. In many other cancer types, an immunohistochemical panel is used to determine the optimal treatment protocol, but there are no such panels in head and neck carcinoma. We believe that combination of CD44 and HIF1α could be used as diagnostic aid to find the patients with poor prognoses, who would benefit from more aggressive initial treatment. Both CD44 and HIF1α are well known, and antibodies are easily available to any immunohistochemistry laboratory. Clinical implementation of this panel still requires a prospective study, which we are planning to conduct.
Supporting information
Supp. Fig. S1. Virtual microscopy analyses. CD44 staining intensity was analyzed with the ImmunoMembrane plugin in ImageJ. For each sample, two representative images were uploaded and automatically scored. Sites of complete staining are marked red and sites of incomplete staining are marked green. The sample displayed was scored to 14 points.
Supp. Fig. S2. Kaplan‐Meier analyses of disease free and disease specific survival of the patients in this study with respect to tumor grade and tumor diameter. p‐values: log‐rank test.
Supp. Fig. S3. Kaplan‐Meier analyses of disease free or disease specific survival of patients after elective neck dissection or sentinel node biopsy was compared to those patients, whose neck status was not surgically determined. p‐values: log‐rank test.
Supp. Table SI. Treatment modalities and outcome. The patients were primarily treated surgically. Secondary treatment was given when surgical healthy tissue margins were insufficient. CRT=chemoradiotherapy.
Supp. Table SII. Distributions of immunohistochemical scores in the study. HIF1α and CLEVER‐1 (Stab1) stainings were manually graded. CD44 stainings were graded by virtual microscopy and divided to CD44high or CD44low at an optimal cutoff of 12 points (scale 0–20) by manual testing.
Acknowledgments
We thank Sari Mäki for expert technical help and Kristiina Aalto and Tero Vahlberg for biostatistical consultation. We thank the Kirsti and Tor Johansson Foundation for funding.
Funding: This work has been funded by the Emil Aaltonen Foundation, Ida Montin Foundation, Kirsti and Tor Johansson Foundation, University of Turku, and Turku University Hospital. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. doi:10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2. Engholm G, Ferlay J, Christensen N, et al. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol 2010;49:725–736. doi:10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 3. Melkane AE, Auperin A, Saulnier P, et al. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head Neck 2014;36:257–265. doi:10.1002/hed.23302. [DOI] [PubMed] [Google Scholar]
- 4. Sand L, Jalouli J. Viruses and oral cancer. Is there a link? Microbes Infect 2014;16:371–378. doi:10.1016/j.micinf.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 5. Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta‐analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node‐negative neck. Oral Oncol 2011;47:320–324. doi:10.1016/j.oraloncology.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 6. Wang SJ, Bourguignon LY. Role of hyaluronan‐mediated CD44 signaling in head and neck squamous cell carcinoma progression and chemoresistance. Am J Pathol 2010;178:956–963. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21356346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005. ;2005:re12. doi:10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 8. Gong L, Zhang W, Zhou J, et al. Prognostic value of HIFs expression in head and neck cancer: a systematic review. PLoS One 2013;8:e75094. doi:10.1371/journal.pone.0075094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goerdt S, Bhardwaj R, Sorg C. Inducible expression of MS‐1 high‐molecular‐weight protein by endothelial cells of continuous origin and by dendritic cells/macrophages in vivo and in vitro. Am J Pathol 1993;142:1409–1422. Available at: http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=8494045. [PMC free article] [PubMed] [Google Scholar]
- 10. Irjala H, Alanen K, Grenman R, Heikkila P, Joensuu H, Jalkanen S. Mannose receptor (MR) and common lymphatic endothelial and vascular endothelial receptor (CLEVER)−1 Direct the binding of cancer cells to the lymph vessel endothelium. Cancer Res 2003;63:4671–4676. [PubMed] [Google Scholar]
- 11. Wehrhan F, Buttner‐Herold M, Hyckel P, et al. Increased malignancy of oral squamous cell carcinomas (oscc) is associated with macrophage polarization in regional lymph nodes: an immunohistochemical study. BMC Cancer 2014;14:522. doi:10.1186/1471-2407-14-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karikoski M, Marttila‐Ichihara F, Elima K, et al. Clever‐1/stabilin‐1 controls cancer growth and metastasis. Clin Cancer Res 2014;20:6452–6464. doi:10.1158/1078-0432.CCR-14-1236. [DOI] [PubMed] [Google Scholar]
- 13. Dunkel J, Vaittinen S, Grenman R, Kinnunen I, Irjala H. Prognostic markers in stage I oral cavity squamous cell carcinoma. Laryngoscope 2013;123:2435–41. doi:10.1002/lary.23888. [DOI] [PubMed] [Google Scholar]
- 14. Jalkanen S, Bargatze RF, de los Toyos J, Butcher EC. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85–95‐kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol 1987;105:983–990. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2114763&tool=pmcentrez&rendertype=abstract. Accessed June 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Algars A, Irjala H, Vaittinen S, et al. Type and location of tumor‐infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 2012;131:864–873. doi:10.1002/ijc.26457. [DOI] [PubMed] [Google Scholar]
- 16. Tuominen VJ, Tolonen TT, Isola J. ImmunoMembrane: a publicly available web application for digital image analysis of HER2 immunohistochemistry. Histopathology 2012;60:758–767. doi:10.1111/j.1365-2559.2011.04142.x. [DOI] [PubMed] [Google Scholar]
- 17. Psychogios G, Mantsopoulos K, Bohr C, Koch M, Zenk J, Iro H. Incidence of occult cervical metastasis in head and neck carcinomas: development over time. J Surg Oncol 2013;107:384–387. doi:10.1002/jso.23221. [DOI] [PubMed] [Google Scholar]
- 18. Soland TM, Brusevold IJ. Prognostic molecular markers in cancer: quo vadis? Histopathology 2013;63:297–308. doi:10.1111/his.12184. [DOI] [PubMed] [Google Scholar]
- 19. Kokko L‐L, Hurme S, Maula S‐M, et al. Significance of site‐specific prognosis of cancer stem cell marker CD44 in head and neck squamous‐cell carcinoma. Oral Oncol 2011;47:510–516. doi:10.1016/j.oraloncology.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 20. Joshua B, Kaplan MJ, Doweck I, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck 2012;34:42–49. doi:10.1002/hed.21699. [DOI] [PubMed] [Google Scholar]
- 21. Woolgar JA. Histopathological prognosticators in oral and oropharyngeal squamous cell carcinoma. Oral Oncol 2006;42:229–239. doi:10.1016/j.oraloncology.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22. Canis M, Pluquett S, Ihler F, Matthias C, Kron M, Steiner W. Impact of elective neck dissection vs observation on regional recurrence and survival in cN0‐staged patients with squamous cell carcinomas of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg 2012;138:650–655. doi:10.1001/archoto.2012.1026. [DOI] [PubMed] [Google Scholar]
- 23. Boscke R, Cakir BD, Hoffmann AS, Wiegand S, Quetz J, Meyer JE. Outcome after elective neck dissection and observation for the treatment of the clinically node‐negative neck (cN0) in squamous cell carcinoma of the oropharynx. Eur Arch Otorhinolaryngol 2014;271:567–574. doi:10.1007/s00405-013-2545-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Fig. S1. Virtual microscopy analyses. CD44 staining intensity was analyzed with the ImmunoMembrane plugin in ImageJ. For each sample, two representative images were uploaded and automatically scored. Sites of complete staining are marked red and sites of incomplete staining are marked green. The sample displayed was scored to 14 points.
Supp. Fig. S2. Kaplan‐Meier analyses of disease free and disease specific survival of the patients in this study with respect to tumor grade and tumor diameter. p‐values: log‐rank test.
Supp. Fig. S3. Kaplan‐Meier analyses of disease free or disease specific survival of patients after elective neck dissection or sentinel node biopsy was compared to those patients, whose neck status was not surgically determined. p‐values: log‐rank test.
Supp. Table SI. Treatment modalities and outcome. The patients were primarily treated surgically. Secondary treatment was given when surgical healthy tissue margins were insufficient. CRT=chemoradiotherapy.
Supp. Table SII. Distributions of immunohistochemical scores in the study. HIF1α and CLEVER‐1 (Stab1) stainings were manually graded. CD44 stainings were graded by virtual microscopy and divided to CD44high or CD44low at an optimal cutoff of 12 points (scale 0–20) by manual testing.


