Abstract
apoC-III is often assumed to retard the intravascular processing of triglyceride-rich lipoproteins (TRLs) by inhibiting LPL, but that view is based largely on studies of free LPL. We now recognize that intravascular LPL is neither free nor loosely bound, but instead is tightly bound to glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) on endothelial cells. Here, we revisited the effects of apoC-III on LPL, focusing on apoC-III’s capacity to affect the activity of GPIHBP1-bound LPL. We found that TRLs from APOC3 transgenic mice bound normally to GPIHBP1-bound LPL on cultured cells in vitro and to heart capillaries in vivo. However, the triglycerides in apoC-III–enriched TRLs were hydrolyzed more slowly by free LPL, and the inhibitory effect of apoC-III on triglyceride lipolysis was exaggerated when LPL was bound to GPIHBP1 on the surface of agarose beads. Also, recombinant apoC-III reduced triglyceride hydrolysis by free LPL only modestly, but the inhibitory effect was greater when the LPL was bound to GPIHBP1. A mutant apoC-III associated with low plasma triglyceride levels (p.A23T) displayed a reduced capacity to inhibit free and GPIHBP1-bound LPL. Our results show that apoC-III potently inhibits triglyceride hydrolysis when LPL is bound to GPIHBP1.
Keywords: glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1, lipoprotein lipase, hypertriglyceridemia
Genome-wide association studies uncovered an association between plasma triglyceride levels and coronary disease risk with the apoA5/A4/C3/A1 gene cluster (1). A specific role for apoC-III in regulating triglyceride levels was established with the discovery of loss-of-function mutations in APOC3. Pollin et al. (2) showed that 5% of an Amish population are heterozygous for an APOC3 nonsense mutation (R19X) and have half-normal levels of apoC-III, reduced plasma triglyceride levels, and reduced amounts of coronary atherosclerosis. Several years later, three additional APOC3 loss-of-function mutations were identified [two splice site mutations (IVS2+1G>A and IVS3+1G>T) and a missense mutation (p.A23T)]; all were associated with lower plasma triglyceride levels and reduced coronary disease risk (3, 4).
The mechanisms by which apoC-III influences plasma triglyceride levels are not clearly defined. Early in vitro studies, some dating to the 1970s, proposed that apoC-III directly inhibits the activity of LPL (5–7). Other studies with human APOC3 transgenic mice suggested that increased apoC-III expression inhibits the clearance of both VLDL and remnant lipoproteins (8, 9). Subsequent studies revealed that apoC-III overexpression markedly increased plasma triglyceride levels in the setting of apoE deficiency (where remnant clearance is markedly defective) (10); those findings implied that apoC-III overexpression impairs the processing of large, triglyceride-rich lipoproteins (TRLs).
Several groups have characterized the effects of apoC-III on LPL’s catalytic activity. In studies with artificial substrates (e.g., triglyceride emulsion particles), physiological levels of apoC-III [estimated to be ∼2 μM on TRLs (11)] inhibited LPL activity when apoC-II was absent (12, 13). However, when apoC-II was present, higher concentrations of apoC-III were required to inhibit LPL activity (12–15). In studies with more physiologic substrates (e.g., VLDL), apoC-III has been reported to have no effect on LPL activity (8); to have only minor effects on LPL activity (9); or to inhibit LPL activity (13, 15). In all of these studies, the effects of apoC-III were examined when LPL was free in solution. While it is most straightforward to examine lipolysis with “free LPL,” the physiologic relevance of these studies is open to question. LPL in capillaries is not loosely bound to the endothelial cell glycocalyx, as was once assumed (16), but instead is tightly bound to glycosylphosphatidylinositol-anchored HDL-binding protein 1 (GPIHBP1) on the surface of capillary endothelial cells (17). The GPIHBP1–LPL complex also mediates the margination of TRLs along capillary endothelial cells (18).
Very recent studies have demonstrated that GPIHBP1 is more than simply a binding site for LPL; GPIHBP1 also affects LPL activity. Mysling and coworkers (19, 20) showed that the binding of LPL to GPIHBP1 preserves LPL’s catalytic activity by preventing the unfolding of LPL’s hydrolase domain. The studies by Mysling and coworkers (19, 20) underscored the importance of considering the effects of GPIHBP1 when trying to understand mechanisms for the lipolytic processing of TRLs.
In the current study, we sought to define the effects of apoC-III on the processing of TRLs by the GPIHBP1–LPL complex. We sought to examine: 1) whether increased expression of apoC-III affects GPIHBP1 expression in capillaries or the amount of LPL within capillaries; 2) whether apoC-III would affect the ability of TRLs to bind to the GPIHBP1–LPL complex on cultured cells or the capacity of TRLs to marginate along heart capillaries in vivo; and 3) the effects of apoC-III on triglyceride hydrolysis by free and GPIHBP1-bound LPL.
MATERIALS AND METHODS
Genetically modified mice
Gpihbp1−/− mice have been described previously (21). APOC3 transgenic mice [B6; CBA-Tg(APOC3)3707Bres/J] were purchased from the Jackson Laboratory. All mice were fed rodent chow and housed in a specific pathogen-free barrier facility with a 12 h light–dark cycle. All studies were approved by University of California Los Angeles’ Animal Research Committee.
Protein expression
Bovine LPL was a gift from Dr. André Bensadoun (Cornell University). Human LPL harboring a carboxyl-terminal V5–His tag was expressed in Chinese hamster ovary (CHO) cells (22). Soluble human GPIHBP1 with a carboxyl-terminal epitope tag for monoclonal antibody 11A12 was expressed in Drosophila S2 cells as described (23). Wild-type and mutant (p.A23T) human apoC-III were expressed using the pET23a expression vector in Escherichia coli BL21(DE3) cells (24). ApoC-III proteins were purified as described (13), and further purified by size-exclusion chromatography on a Superdex 75 10/30 column (GE Healthcare) equilibrated with 20 mM sodium phosphate buffer and 6 M urea (pH 7.4).
Isolation and labeling of TRLs
TRLs were isolated from the plasma of Gpihbp1−/− and APOC3 transgenic mice by ultracentrifugation. In brief, EDTA-plasma was overlaid with PBS and ultracentrifuged in a Beckman TLA-100.3 rotor at 424,000 g at 10°C for 2 h. The d < 1.006 g/dl lipoproteins were collected, placed in PBS, and refloated with a second round of ultracentrifugation. The apolipoproteins of the TRLs were labeled with an infrared (IR) dye with the DyLight NHS-ester labeling kit (ThermoFisher Scientific). To separate IR-labeled TRLs from unincorporated dye, samples were applied to an Econo-Pac 10DG gel-filtration column (Bio-Rad) equilibrated with PBS. TRLs were also labeled with the fluorescent dye, Alexa Fluor 555, with a kit from Molecular Probes (ThermoFisher Scientific). Labeled TRLs were separated from unincorporated dye by gel filtration as described earlier.
Cell culture studies
CHO pgsA-745 cells [CHO cells with a reduced capacity to produce sulfated proteoglycans (25)] (4 × 106 cells) were electroporated (with an Amaxa nucleofector from Lonza) with either no vector, a GFP control plasmid (Lonza), or mouse Gpihbp1 (4 μg DNA per cuvette) (21). Transfected cells were seeded (1 × 106 per well) in 24-well plates or on fibronectin-treated (10 μg/ml) coverslips in 24-well plates. After 24 h, the cells were washed with PBS/Ca/Mg containing 0.5% BSA (PBS-BSA) and incubated with 300 μl of cold PBS-BSA with or without 1 μg bovine LPL at 4°C for 1 h. The cells were washed with cold PBS-BSA buffer and incubated with cold PBS/Ca/Mg containing 6% BSA and 0.5 mg triglyceride per milliliter of either IRDye800-labeled or Alexa555-labeled TRLs and incubated at 4°C for 1 h. Unbound lipoproteins were removed by washing with cold PBS/Ca/Mg and the cells were processed for either IR scanning or fluorescence microscopy.
For cells incubated with IRDye-labeled TRLs, the amounts of bound lipoproteins were quantified by IR laser scanning on an Odyssey scanner (LI-COR). For fluorescence microscopy, the cells on coverslips were fixed for 15 min with 3% paraformaldehyde, blocked for 1 h at room temperature with 10% FBS in PBS/Ca/Mg, and incubated overnight at 4°C with a mouse monoclonal antibody against bovine LPL (5D2, 20 μg/ml) (26). The cells were washed to remove unbound antibody, followed by a 30 min incubation at room temperature with Alexa488-labeled donkey anti-mouse IgG (ThermoFisher Scientific; 1:200). After removing unbound secondary antibody, the cells were incubated with Alexa647-labeled antibody 11A12 (3 μg/ml), an antibody against mouse GPIHBP1 (27). Nuclei were stained with DAPI and the samples mounted in ProLong Gold antifade. Images were recorded on an Axiovert 200M microscope (Zeiss) with 40× magnification and processed with Zen 2010 software. The exposure conditions for each coverslip were identical.
Measurement of TRL binding to isolated perfused mouse hearts
Mice were anesthetized with ketamine/xylazine and injected intravenously (via the inferior vena cava) with 50 μl of PBS containing 5 mM tetrahydrolipstatin dissolved in 5% DMSO. After 2 min, mice were perfused with 15 ml of modified Tyrode’s buffer [136 mM NaCl, 5.4 mM KCl, 0.33 mM NaH2PO4, 1 mM MgCl2, and 10 mM HEPES (pH 7.4)] followed by removal of the heart and cannulation of the aorta with a blunt 25 gauge needle. The hearts were submerged in 20 ml of modified Tyrode’s buffer, flushed with 3 ml of modified Tyrode’s buffer, and perfused with 1 ml of PBS containing 100 μg of IRDye800-labeled TRLs and 50 μg of IRDye680-labeled antibody 11A12. After 5 min, unbound proteins were removed by perfusion with 5 ml of modified Tyrode’s buffer, followed by 3 ml of 3% paraformaldehyde in PBS. Hearts were embedded in OCT compound and 10 μm cryosections were scanned with an Odyssey IR scanner (LI-COR). The IR signal intensities were normalized to tissue area, as determined by ImageJ software (28).
Heparin-mediated release of LPL from mouse hearts
Anesthetized mice were perfused with 15 ml of modified Tyrode’s buffer followed by removal of the heart and cannulation of the aorta as described earlier. Hearts were perfused with 5 ml of modified Tyrode’s buffer supplemented with heparin (100 U/ml). The perfusate was collected dropwise into microcentrifuge tubes (0.9 ml/tube) containing 100 μl of 2 M Tris, 50 mM deoxycholate, and 1% sodium dodecyl sulfate (pH 8.5) (to stabilize LPL). The first three fractions were pooled and LPL activity was determined. Samples (50 μl) were added to 150 μl of substrate solution containing [3H]triolein that had been incorporated into Intralipid (0.5 μCi [3H]triolein/mg triglyceride) and activity was measured as described (29).
Release of LPL into the plasma with an intravenous injection of heparin
APOC3 transgenic mice were anesthetized with isoflurane and injected intravenously with 50 units of heparin (Fresenius Kabi) or an equivalent volume (50 μl) of normal saline. Blood samples were collected for triglyceride measurements over the subsequent 4 h.
SDS-PAGE and Western blotting
For the analysis of TRL apolipoproteins, samples were delipidated before SDS-PAGE. Delipidation was performed by adding 100 μl of acetone:ethanol (1:1) per milligram of TRL triglycerides and incubating on ice for 15 min followed by centrifugation at 13,300 g for 15 min at 4°C. Pelleted apolipoproteins were dissolved in loading buffer (NuPAGE; Invitrogen) under reducing conditions and heated at 90°C for 10 min. For LPL in heparin perfusates, 500 μl aliquots were precipitated in 10% TCA and incubated on ice for 15 min followed by centrifugation at 13,300 g for 15 min at 4°C. TCA precipitates were dissolved in loading buffer supplemented with 0.5 M Tris (pH 8.5) under reducing conditions and heated at 90°C for 10 min. All samples were size-fractionated on 12% Bis-Tris gels (Novex) in MES buffer (ThermoFisher Scientific). For Western blot analysis, samples were transferred to nitrocellulose membranes and then incubated in blocking buffer (LI-COR) for either 1 h at room temperature or overnight at 4°C, followed by incubation with primary antibodies in fresh blocking buffer containing 0.2% Tween 20 for 1 h at room temperature. Antibody dilutions were as follows: anti-apoC-III (33A-G2b, Academy Biomed, 1:500); anti-apoB [2G11 (30), 10 μg/ml]; and anti-LPL [a goat polyclonal antibody against mouse LPL (31), 20 μg/ml]. Membranes were washed in PBS with 0.1% Tween 20 and incubated with IRDye680- or IRDye800-labeled secondary antibodies (LI-COR; 1:2,000) in blocking buffer with 0.2% Tween 20 for 30 min at room temperature. After washing, membranes were scanned with an Odyssey IR scanner (LI-COR).
Immobilization of LPL on GPIHBP1 on agarose beads or on a cobalt resin
The GPIHBP1 antibody, 11A12, was covalently linked to agarose beads with the AminoLink Plus system (ThermoFisher Scientific). The beads (300 μl per microfuge tube) were blocked by incubating with 500 μl of PBS/Ca/Mg containing 10% FBS at room temperature for 1 h with constant rotation. The beads were then washed three times with PBS containing 0.2% nonidet P-40 (NP40) (PBS-NP40). Bovine LPL (1 μg) and 150 μl of concentrated medium from Drosophila S2 cells expressing human GPIHBP1 harboring the 11A12 epitope were added to the beads (final volume, 500 μl per tube in ice-cold PBS-NP40). The beads were then incubated for 1 h at 4°C with constant rotation. Multiple tubes of GPIHBP1–LPL beads were generated, washed with PBS-NP40, pooled, and then aliquoted to separate tubes to ensure that equal amounts of LPL–GPIHBP1 complexes were present in each tube.
In other studies, LPL harboring a carboxyl-terminal V5–His tag was immobilized on HisPur cobalt resin (Thermo Scientific). The cobalt resin was washed with a buffer containing 50 mM sodium phosphate, 300 mM NaCl, and 10 mM imidazole (pH 7.4). Medium from CHO cells expressing LPL with the V5–His tag and an equivalent volume of equilibration buffer were added to the resin and incubated at 4°C for 2 h with constant rotation. The resin was then washed three times with equilibration buffer and distributed to microfuge tubes to ensure equal amounts of immobilized LPL. Finally, TRLs (1.0 mg triglyceride/ml) in PBS/Ca/Mg containing 6% BSA (500 μl final volume) were added to the resin and incubated for 1–4 h at room temperature with constant rotation. In some experiments 10 mM EDTA was added to release LPL from the cobalt resin.
Measurement of LPL catalytic activity
Bovine LPL (82.5 ng) in ice-cold PBS with 0.01% Triton X-100 (w/v) was incubated with increasing amounts of TRLs in PBS/Ca/Mg containing 6% BSA (150 μl incubation volume) at room temperature for 2 h in 96-well microtiter plates (Costar 3915) with gentle agitation (150 rpm). Alternatively, 100 ng of bovine LPL was added to 1 mg triglyceride per milliliter of TRLs in microfuge tubes (500 μl incubation volume) diluted in PBS/Ca/Mg and 6% BSA and incubated for 1–2 h at room temperature with constant rotation. In one experiment, 400 nM bovine LPL was preincubated in PBS containing 2 mg/ml BSA with or without 2 μM soluble human GPIHBP1 on ice. After 10 min, aliquots of the preincubated LPL (100 ng) were added to TRLs. The reactions were stopped by adding 50 mM tetrahydrolipstatin, and free fatty levels were measured with the NEFA HR(2) kit (Wako Chemicals). Briefly, reaction solutions (5 μl) were added to microtiter plates (Fisher 12565501) containing 150 μl NEFA HR(2) reagent A and incubated for 15 min at room temperature on an orbital shaker (150 rpm). Next, NEFA HR(2) reagent B (75 μl) was added and incubated for 30 min. The absorbances at 560 and 670 nm were measured on a SpectraMax 190 (Molecular Devices) and fatty acid levels quantified based on a NEFA standard curve.
Blood collection and plasma triglyceride measurements
Blood was collected from anesthetized mice by retro-orbital puncture with heparinized capillary tubes (Kimble Chase). Plasma samples were separated from red blood cells by centrifugation (14,000 g for 30 s) and stored at −80°C. Plasma triglycerides were quantified using a commercial kit (Sigma, TR0100).
Statistical analysis
All statistical analyses were performed with GraphPad Prism software. Differences in levels of TRL margination and LPL activities were analyzed by unpaired two-tailed Student’s t-test.
RESULTS
ApoC-III does not inhibit the binding of TRLs to the GPIHBP1–LPL complex on cells
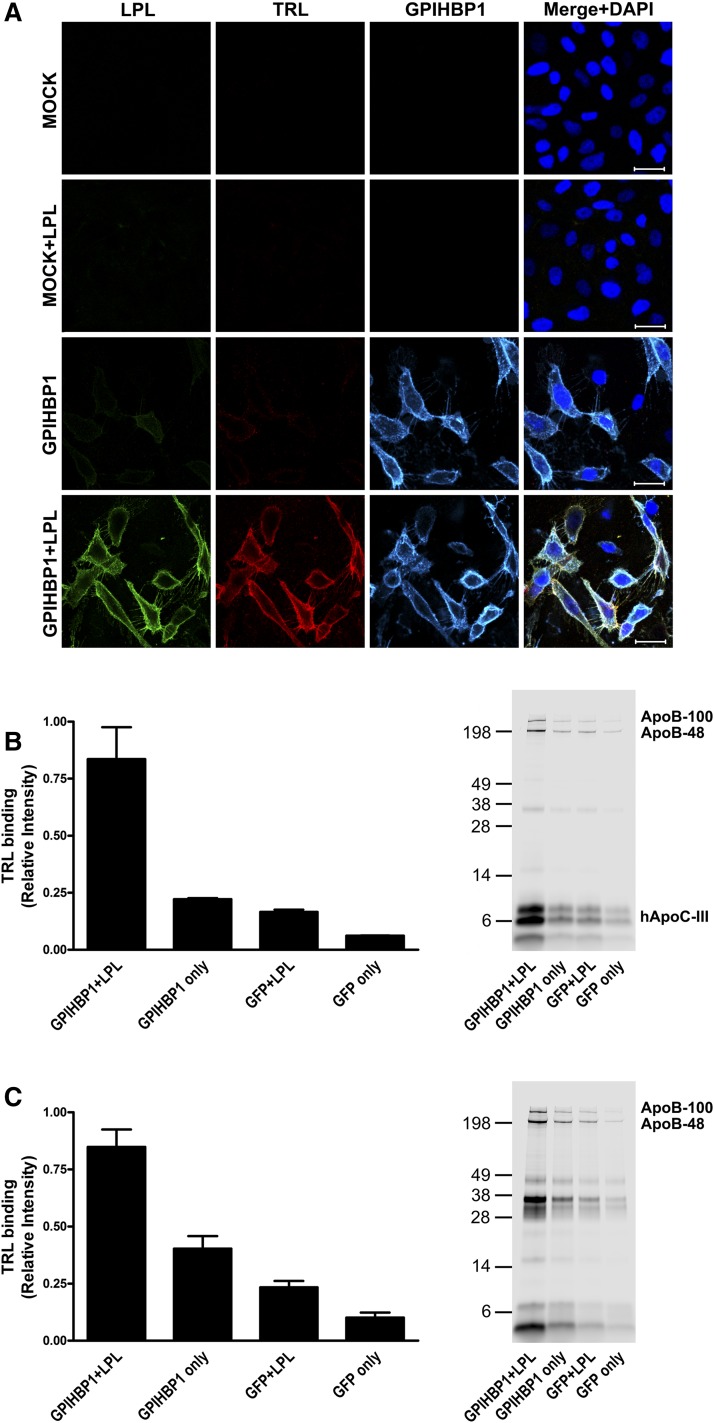
Earlier studies showed that the GPIHBP1–LPL complex mediates TRL binding to the surface of cultured cells and to capillary endothelial cells in vivo (18). In the current studies, we began by testing whether apoC-III–enriched TRLs from APOC3 transgenic mice (32) (“apoC-III–TRLs”) are capable of binding to the GPIHBP1–LPL complex on cultured cells. In these studies, we first added bovine LPL to GPIHBP1-expressing CHO cells and then incubated washed cells with Alexa555-labeled apoC-III–TRLs. We found that apoC-III–TRLs bound to GPIHBP1-transfected cells that had been loaded with bovine LPL, but there was no binding to nontransfected cells or to GPIHBP1-expressing cells that had not been loaded with LPL (Fig. 1A). To further explore the impact of apoC-III on the binding of TRLs to the GPIHBP1–LPL complex, we analyzed binding of TRLs that had been labeled with an IR dye (IRDye800). This approach allowed us to compare binding of apoC-III–TRLs and “normal TRLs” (i.e., TRLs with normal amounts of apoC-III; isolated from the plasma of Gpihbp1−/− mice) to the GPIHBP1–LPL complex. Gpihbp1−/− mice have severe hypertriglyceridemia (21), with plasma triglyceride levels of 2,000–4,000 mg/dl, similar to the triglyceride levels in the APOC3 transgenic mice used in this study. We found that apoC-III overexpression had little effect on the binding of TRLs to the GPIHBP1–LPL complex on cultured cells (Fig. 1B, C). This was the case regardless of whether the binding data were normalized to the area of the tissue culture well or to levels of GPIHBP1 expression. Also, the amounts of apoB protein bound to the GPIHBP1–LPL complex on the surface of cells were similar with apoC-III–TRLs and normal TRLs (Fig. 1B, C).
Fig. 1.
ApoC-III does not block the binding of TRLs to the GPIHBP1–LPL complex on cultured cells. The binding of TRLs to the GPIHBP1–LPL complex on CHO pgsA-745 cells was determined by fluorescence microscopy and quantitative IR imaging. A: Fluorescence microscopy images showing apoC-III–TRLs binding to the GPIHBP1–LPL complex. Fluorescently-labeled apoC-III–TRLs were incubated with nontransfected (MOCK) or GPIHBP1-transfected cells (GPIHBP1) in the presence or absence of bovine LPL (see Materials and Methods). Binding of TRLs was detected by confocal fluorescence microscopy; LPL (green), TRLs (red), and GPIHBP1 (cyan). Nuclei were stained with DAPI (blue). Scale bar, 30 μm. B: Bar graph depicting binding of apoC-III–TRLs to cells and SDS-PAGE of TRL–apolipoproteins. IRDye800-labeled apoC-III–TRLs were added to GPIHBP1- or GFP-transfected CHO pgsA-745 cells in the presence or absence of bovine LPL (see Materials and Methods). TRL binding was measured by IR laser scanning (n = 3 studies). C: Bar graph depicting the binding of normal TRLs to cells (n = 3 studies) and SDS-PAGE of TRL–apolipoproteins. The binding of TRLs (normalized to GPIHBP1 expression) was 1.54 ± 0.16 (K counts/mm2) for normal TRLs versus 1.71 ± 0.20 (K counts/mm2) for apoC-III–TRLs.
ApoC-III does not reduce intravascular GPIHBP1 or LPL levels in the heart
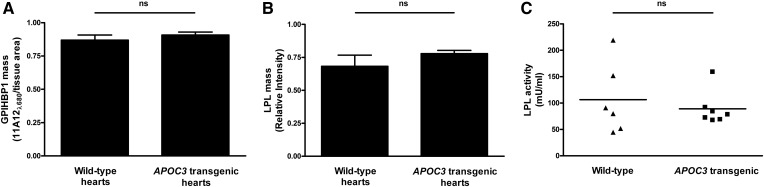
To determine whether apoC-III overexpression in vivo alters the expression of GPIHBP1 in capillaries, we perfused hearts of wild-type and APOC3 transgenic mice with an IRDye680-labeled GPIHBP1-specific antibody (11A12) and then quantified antibody binding by IR laser scanning. To assess amounts of GPIHBP1-bound LPL, hearts were perfused with heparin, and LPL mass and activity in the perfusate were measured. The amounts of intravascular GPIHBP1 (Fig. 2A) and heparin-releasable LPL mass (Fig. 2B) were similar in wild-type and APOC3 transgenic mice. Recent in vitro studies suggested that apoC-III might render LPL more susceptible to inactivation (13), but we found that the catalytic activity of heparin-released LPL was similar in hearts of wild-type and APOC3 transgenic mice (Fig. 2C).
Fig. 2.
Intravascular GPIHBP1 expression as well as intravascular LPL mass and activity are not perturbed in hearts of APOC3 transgenic mice. Isolated hearts from wild-type and APOC3 transgenic mice were perfused with an IRDye680-labeled anti-GPIHBP1 monoclonal antibody (11A12) to assess amounts of GPIHBP1 in capillaries. To assess intravascular LPL mass and activity, hearts were perfused with heparin (100 U/ml), and the perfusate was collected and analyzed. A: Bar graph comparing GPIHBP1 levels in hearts from wild-type and APOC3 transgenic mice. The results are expressed as the amount of IRDye680–11A12 signal/tissue area. Mean ± SEM is shown (eight sections per heart, n = 7–14 hearts per group). B: Bar graph comparing LPL mass (as measured by Western blotting) in perfusates from hearts of wild-type and APOC3 transgenic mice. Mean ± SEM is shown (n = 6–7 hearts per group). C: Plot comparing LPL activity (as measured by [3H]triolein hydrolysis) in perfusates from hearts of wild-type and APOC3 transgenic mice (n = 6–7 hearts per group). ns, no significant difference.
ApoC-III does not affect the margination of TRLs in isolated perfused hearts
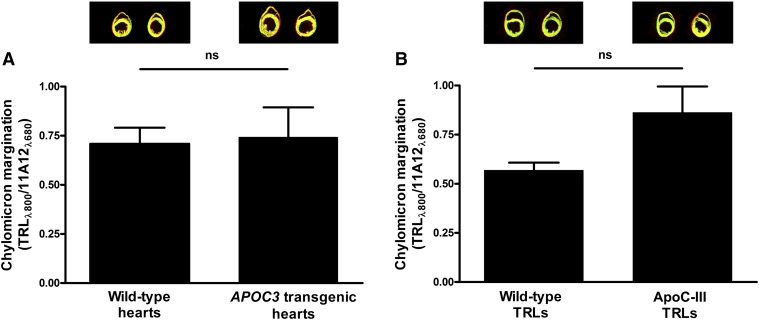
Because the margination of TRLs along heart capillaries depends on GPIHBP1-bound LPL, and because we found normal amounts of GPIHBP1 and LPL in hearts of APOC3 transgenic mice, we suspected that TRL margination along heart capillaries would not be reduced in APOC3 transgenic mice. To test this possibility, we perfused hearts of wild-type and APOC3 transgenic mice with IRDye800-labeled normal TRLs (from Gpihbp1−/− mice) and then measured TRL binding by IR scanning. TRL binding to isolated hearts of wild-type and APOC3 transgenic mice was similar (Fig. 3A). This was the case regardless of whether TRL binding was normalized to the area of the tissue section or to the expression of GPIHBP1 (assessed with an IRDye680-labeled GPIHBP1-specific monoclonal antibody, 11A12) (Fig. 3A). We also considered the possibility that apoC-III–enriched TRLs from APOC3 transgenic mice might lack the capacity to marginate along heart capillaries of wild-type mice. This was not the case; the margination of apoC-III–TRLs along heart capillaries was not reduced, compared with normal TRLs (Fig. 3B).
Fig. 3.
The margination of TRLs in hearts from APOC3 transgenic mice is normal. A: Bar graph comparing the margination of normal TRLs in hearts of wild-type and APOC3 transgenic mice. IRDye800-labeled normal TRLs and IRDye680-labeled antibody 11A12 were injected into isolated, perfused hearts from wild-type (n = 8) and APOC3 transgenic (n = 4) mice. TRL binding was measured by IR scanning (eight sections per heart). Results (mean ± SEM) are expressed relative to GPIHBP1 expression (as judged from the IRDye680 signal). B: Bar graph depicting the binding of normal TRLs and apoC-III–TRLs to isolated, perfused hearts of wild-type mice. TRL binding and GPIHBP1 levels were measured as described in (A) (eight sections per heart, n = 3 hearts per group). ns, no significant difference.
ApoC-III–enriched TRLs are suboptimal substrates for immobilized LPL
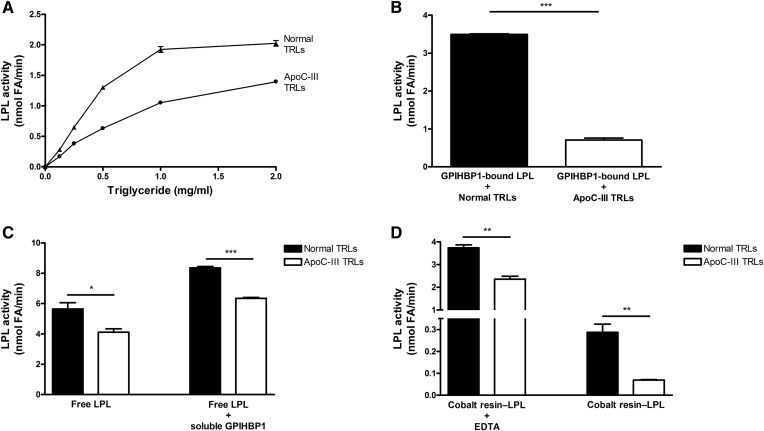
We next tested to determine whether the enrichment of TRLs with apoC-III affected triglyceride hydrolysis by free LPL. Bovine LPL was incubated with apoC-III–TRLs and normal TRLs, and the release of FFAs was measured. With increasing amounts of TRL-triglyceride, we observed increasing amounts of FFA release from both apoC-III–TRLs and normal TRLs, but the release of FFAs from apoC-III–TRLs was lower (Fig. 4A). At 1.0 mg triglyceride per milliliter, FFA release from apoC-III–TRLs was 45% lower than FFA release from normal TRLs. When we assessed triglyceride hydrolysis in the setting of GPIHBP1-bound LPL (with the GPIHBP1 immobilized on agarose beads), the reduction in the processing of apoC-III–TRLs was exaggerated (i.e., the release of FFAs from apoC-III–TRLs was ∼80% lower than FFA release from normal TRLs) (Fig. 4B). To test whether the exaggerated inhibition was due to GPIHBP1 binding, we preincubated LPL in the presence or absence of soluble GPIHBP1 and then added either normal TRLs or apoC-III–TRLs. When LPL was bound to soluble GPIHBP1, we observed higher levels of LPL activity, consistent with the fact that GPIHBP1 stabilizes the structural integrity and catalytic activity of LPL (19). However, we did not observe an exaggerated inhibition of LPL by apoC-III with “free LPL–GPIHBP1 complexes” (i.e., LPL bound to soluble GPIHBP1 that was free in solution) (Fig. 4C). Those findings suggested that exaggerated inhibition of LPL by apoC-III occurs only when the LPL is immobilized. To explore this idea, we immobilized V5–His-tagged human LPL on a cobalt resin. When the LPL was immobilized on the cobalt resin, the amount of triglyceride hydrolysis with apoC-III–TRLs was lower than with normal TRLs. Once again, the effect on triglyceride hydrolysis was less impressive with free LPL (when the LPL was released from the resin with EDTA) (Fig. 4D). In control studies, we demonstrated that EDTA was effective in releasing LPL from the cobalt resin and that EDTA could not account for the increased catalytic activity of free LPL (supplemental Fig. S1).
Fig. 4.
ApoC-III–enriched TRLs are suboptimal substrates for LPL, particularly when LPL is immobilized on GPIHBP1. A: LPL-mediated triglyceride hydrolysis with normal TRLs and apoC-III–TRLs. Increasing amounts of normal TRLs (closed triangles) and apoC-III–TRLs (closed circles) were added to bovine LPL (10 nM), and LPL activity was measured. B: A comparison of the processing of normal TRLs and apoC-III–TRLs by GPIHBP1-bound LPL. Normal TRLs and apoC-III–TRLs were incubated with bovine LPL that was bound to GPIHBP1 on agarose beads. C: Processing of normal TRLs and apoC-III–TRLs by free LPL and free LPL–GPIHBP1 complexes (i.e., LPL bound to soluble GPIHBP1). LPL was incubated on ice for 10 min with or without soluble GPIHBP1. Aliquots of those samples were then mixed with normal TRLs or apoC-III–TRLs and LPL activity was measured. D: A comparison of the processing of normal TRLs or apoC-III–TRLs by V5–His-tagged LPL immobilized on cobalt resin, both in the presence and absence of EDTA. The LPL was incubated in microtiter plates in (A) and in microfuge tubes in (B–D). LPL activity was determined by measuring the production of FFAs (see Materials and Methods). Mean ± SEM (n = 3 experiments). *P < 0.05; **P < 0.01; ***P < 0.001.
Because the inhibitory effect of apoC-III was exaggerated when LPL was immobilized (either on agarose beads or on the cobalt resin), we suspected that the release of LPL from GPIHBP1 in APOC3 transgenic mice would increase triglyceride hydrolysis and lower plasma triglyceride levels. Indeed, the plasma triglyceride levels in APOC3 transgenic mice fell by ∼65% after an injection of heparin, consistent with increased TRL processing by LPL (supplemental Fig. S2).
ApoC-III directly inhibits the metabolism of TRLs by LPL
The differences in the amounts of FFA released from apoC-III–TRLs and normal TRLs were consistent, but one could argue that these differences were secondary to other compositional differences in these lipoproteins—aside from the different amounts of apoC-III. To exclude this possibility, we tested the effects of recombinant apoC-III on the lipolytic processing of normal TRLs by free and GPIHBP1-bound LPL. In the setting of free LPL, supplementing normal TRLs with recombinant apoC-III reduced triglyceride hydrolysis, but the inhibitory effect of apoC-III was exaggerated when LPL was bound to GPIHBP1 on agarose beads (Fig. 5A). To determine whether this effect was due to reduced amounts of apoC-II, we added recombinant apoC-III to TRLs and then reisolated the TRLs by flotation. We did not observe any difference in apoC-II content on reisolated TRLs—even at apoC-III concentrations that had substantial effects on lipolysis (supplemental Fig. S3).
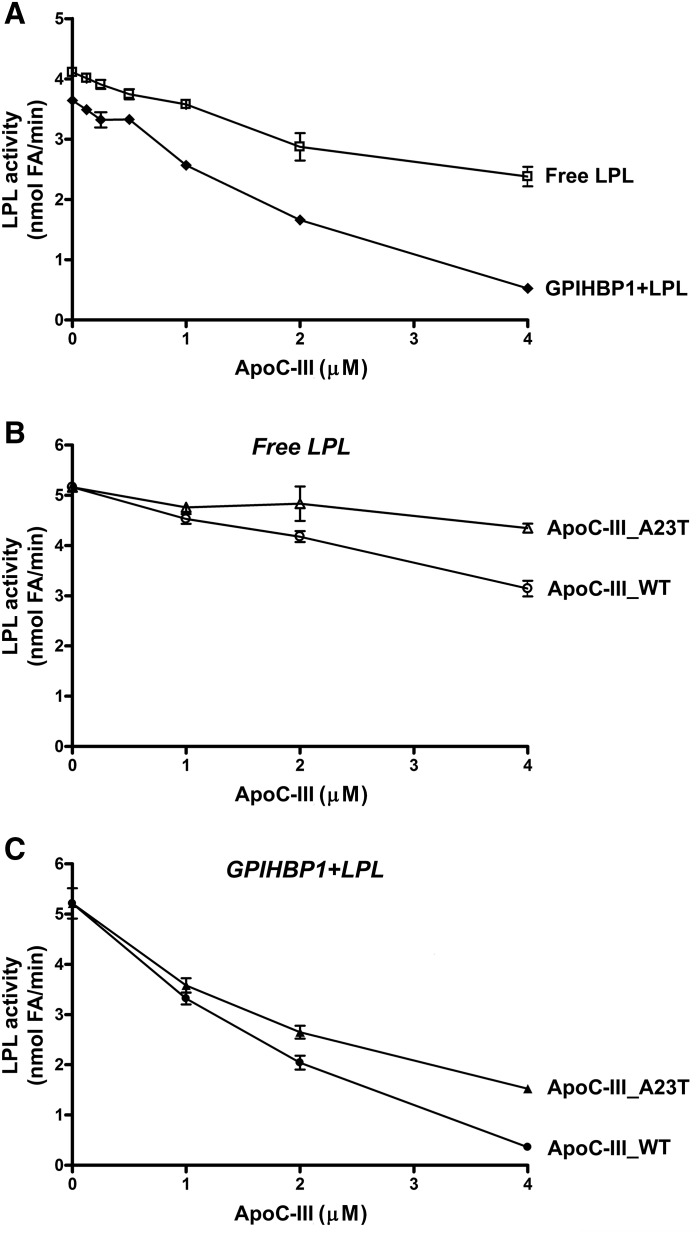
Fig. 5.
ApoC-III inhibits LPL-mediated processing of TRLs. A: ApoC-III is a potent inhibitor of LPL catalytic activity when LPL is bound to GPIHBP1. Increasing amounts of apoC-III were added to normal TRLs (1 mg triglyceride per milliliter) with free LPL (open squares) or with GPIHBP1-bound LPL (closed diamonds). After 2 h, LPL activity was determined by measuring the production of FFAs. Mean ± SEM (n = 3 experiments). B: Inhibition of LPL activity by wild-type apoC-III and apoC-III–A23T. Increasing amounts of wild-type apoC-III (open circles) and apoC-III–A23T (open triangles) were added to normal TRLs (1 mg triglyceride per milliliter) and LPL that was free in solution. After 2 h, amounts of FFAs produced were measured. C: ApoC-III–A23T inhibits the activity of GPIHBP1-bound LPL less than wild-type apoC-III. Increasing amounts of wild-type apoC-III (closed circles) or apoC-III–A23T (closed triangles) were added to normal TRLs (1 mg triglyceride per milliliter) along with GPIHBP1-bound LPL. After 2 h, the production of FFAs produced was measured. For (B) and (C), the mean and range for duplicate samples are shown.
An apoC-III variant containing a p.A23T substitution is associated with lower plasma triglyceride levels and reduced coronary disease risk (3, 4, 24). This effect was proposed to be independent of LPL (24), but we suspected that the mutant apoC-III might simply have a reduced capacity to inhibit LPL-mediated TRL processing. Indeed, apoC-III–A23T was less effective than wild-type apoC-III in inhibiting triglyceride hydrolysis with free LPL (Fig. 5B). The inhibitory effects of both wild-type apoC-III and apoC-III–A23T were greater in the setting of GPIHBP1-bound LPL (Fig. 5C).
DISCUSSION
For years, LPL was thought to be loosely bound to the HSPGs within the glycocalyx lining of blood vessels (16), but more recent studies have shown that LPL is tightly bound to GPIHBP1 on capillary endothelial cells (17). The GPIHBP1–LPL complex on endothelial cells serves as a platform for the margination of TRLs in the circulation (33) and for LPL-mediated hydrolysis of the triglycerides within TRLs (18). These new insights into intravascular lipolysis necessitate a fresh look at the biochemical properties of intravascular LPL and the causes of hypertriglyceridemia. In the current studies, we focused on the effects of apoC-III, with the goal of gaining further insights into the hypertriglyceridemia elicited by overexpression of apoC-III. Initially, we suspected that apoC-III overexpression would interfere with the binding of TRLs to the GPIHBP1–LPL complex, either indirectly by reducing amounts of GPIHBP1 or LPL on capillaries or more directly by altering the composition of TRLs such that the TRLs lacked the capacity to bind to the GPIHBP1–LPL complex. We found no evidence for either scenario. Increased apoC-III expression was not associated with reduced GPIHBP1 expression in heart capillaries, nor did it reduce amounts of heparin-releasable intravascular LPL. Also, apoC-III–TRLs readily bound to the GPIHBP1–LPL complex on cultured cells, and the margination of apoC-III–TRLs along the GPIHBP1–LPL complex in heart capillaries was not reduced. However, when we investigated the next step in intravascular lipolysis, namely hydrolysis of TRL triglycerides by LPL, we found that apoC-III had noteworthy effects. The triglycerides in apoC-III–TRLs were hydrolyzed by free LPL at a slower rate than triglycerides in normal TRLs, and this inhibitory effect was exaggerated when the LPL that was bound to GPIHBP1 immobilized on agarose beads—or when LPL was immobilized on a cobalt resin. We observed similar findings, namely exaggerated inhibition of triglyceride hydrolysis by GPIHBP1-bound LPL compared with free LPL, when we supplemented normal TRLs with recombinant apoC-III. We observed a ∼50% decrease in the activity of GPIHBP1-bound LPL activity with 2 μM apoC-III and ∼25% inhibition at 1 μM apoC-III. apoC-III levels in TRLs from normolipidemic and familial hypertriglyceridemic patients are reported to be 2.2 ± 0.6 μM and 25.9 ± 3.4 μM, respectively (11). Thus, increasing amounts of apoC-III retard TRL processing by reducing triglyceride hydrolysis by the GPIHBP1–LPL complex, rather than by interfering with TRL binding to the complex. A mutant apoC-III harboring an A23T amino acid substitution inhibited LPL-mediated triglyceride hydrolysis, but to a lesser degree than wild-type apoC-III. Once again, the effects of wild-type apoC-III and apoC-III–A23T on triglyceride hydrolysis were exaggerated when LPL was bound to GPIHBP1 on agarose beads. An earlier study by Liu et al. (24) found that the A23T substitution did not alter apoC-III’s ability to inhibit LPL activity. The explanation for the inability of Liu et al. to detect an effect of the mutant apoC-III on lipolysis is not clear, but it might relate to the fact that they used a different LPL activity assay using Intralipid rather than TRLs as substrates.
The exaggerated inhibition of triglyceride hydrolysis by GPIHBP1-bound LPL in the setting of increased amounts of apoC-III was unexpected. GPIHBP1 is known to preserve the catalytic activity of LPL (19), and for that reason we suspected that GPIHBP1 might protect LPL activity from inhibition by apoC-III. To our surprise, we observed the opposite: inhibition of TRL processing by apoC-III was exaggerated when LPL was immobilized—either on GPIHBP1 or on a cobalt resin. We suspect that the inhibitory effects by apoC-III on lipolysis relate to its ability to block the interaction between LPL and TRL triglycerides. Approximately 3% of the triglycerides in TRLs reside on the surface of TRLs (34). It is unclear whether LPL hydrolyzes only the triglycerides on the surface of TRLs, or whether LPL is capable of reaching the triglycerides within the core of the particle. It is possible that immobilized LPL has a more limited ability to access the core triglycerides, and that the relatively greater inhibition on the activity of immobilized LPL by apoC-III simply relates to the ability of apoC-III to block the access of LPL to surface triglycerides.
We suspect that reduced lipolytic processing of apoC-III–TRLs by LPL that is immobilized on capillaries helps to explain the hypertriglyceridemia in APOC3 transgenic mice. In our mouse colony, the plasma triglyceride levels in chow-fed APOC3 transgenic mice frequently exceed 2,500 mg/dl, similar to the plasma triglyceride levels in Gpihbp1−/− mice (where LPL-mediated TRL processing is unequivocally defective) (21). It is conceivable that this same mechanism is relevant to some cases of human hypertriglyceridemia (where TRLs are often enriched in apoC-III) (11).
While the impaired processing of apoC-III–enriched TRLs by the GPIHBP1–LPL complex is likely relevant to the hypertriglyceridemia in APOC3 transgenic mice, we would caution against extrapolating our results to the triglyceride-lowering effects of reduced apoC-III expression. Recent studies with genetically modified mice have shown that reducing apoC-III expression with an antisense oligonucleotide lowers plasma triglycerides by accelerating the hepatic clearance of remnants by LDLR and LRP1, rather than by accelerating LPL-mediated TRL processing in peripheral tissues (35). Also, lowering apoC-III expression in genetically modified mice with reduced expression of LPL appeared to lower plasma lipid levels primarily by increasing remnant clearance in the liver (35). It is therefore likely that increased remnant clearance underlies the lower plasma triglyceride levels in humans with APOC3 loss-of-function mutations (2–4). Homozygous apoC-III deficiency in humans is associated with low plasma triglyceride levels and a profound blunting of the usual postprandial increase in plasma triglycerides after an oral fat load (36). It is very likely that the absence of apoC-III blunts the postprandial increase in plasma triglycerides by accelerating remnant clearance, although it is conceivable that more rapid processing of TRLs by the GPIHBP1–LPL complex contributes to the triglyceride-lowering effect.
Supplementary Material
Acknowledgments
The authors thank Dr. André Bensadoun (Cornell University) for purified bovine LPL.
Footnotes
Abbreviations:
- IR
- infrared
- GPIHBP1
- glycosylphosphatidylinositol-anchored HDL-binding protein 1
- NP40
- nonidet P-40
- TRL
- triglyceride-rich lipoprotein
This work was supported by Foundation for the National Institutes of Health Grant P01 HL090553, Fondation Leducq Transatlantic Network Grant 12CVD04, American Heart Association Grant 15POST22960055, and the Wenner-Gren Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Lai C. Q., Parnell L. D., and Ordovas J. M.. 2005. The APOA1/C3/A4/A5 gene cluster, lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 16: 153–166. [DOI] [PubMed] [Google Scholar]
- 2.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crosby J., Peloso G. M., Auer P. L., Crosslin D. R., Stitziel N. O., Lange L. A., Lu Y., Tang Z. Z., Zhang H., Hindy G., et al. ; TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. 2014. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjærg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371: 32–41. [DOI] [PubMed] [Google Scholar]
- 5.Brown W. V., and Baginsky M. L.. 1972. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem. Biophys. Res. Commun. 46: 375–382. [DOI] [PubMed] [Google Scholar]
- 6.Havel R. J., Kane J. P., and Kashyap M. L.. 1973. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J. Clin. Invest. 52: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss R. M., Herbert P. N., Levy R. I., and Fredrickson D. S.. 1973. Further observations on the activation and inhibition of lipoprotein lipase by apolipoproteins. Circ. Res. 33: 403–411. [DOI] [PubMed] [Google Scholar]
- 8.Aalto-Setälä K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., and Breslow J. L.. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Silva H. V., Lauer S. J., Wang J., Simonet W. S., Weisgraber K. H., Mahley R. W., and Taylor J. M.. 1994. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J. Biol. Chem. 269: 2324–2335. [PubMed] [Google Scholar]
- 10.Ebara T., Ramakrishnan R., Steiner G., and Shachter N. S.. 1997. Chylomicronemia due to apolipoprotein CIII overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J. Clin. Invest. 99: 2672–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., and Cohn J. S.. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. [PubMed] [Google Scholar]
- 12.Liu H., Talmud P. J., Lins L., Brasseur R., Olivecrona G., Peelman F., Vandekerckhove J., Rosseneu M., and Labeur C.. 2000. Characterization of recombinant wild type and site-directed mutations of apolipoprotein C-III: lipid binding, displacement of ApoE, and inhibition of lipoprotein lipase. Biochemistry. 39: 9201–9212. [DOI] [PubMed] [Google Scholar]
- 13.Larsson M., Vorrsjo E., Talmud P., Lookene A., and Olivecrona G.. 2013. Apolipoproteins C-I and C–III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 288: 33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson R. L., Tajima S., Yamamura T., Yokoyama S., and Yamamoto A.. 1986. Comparison of apolipoprotein C-II-deficient triacylglycerol-rich lipoproteins and trioleoylglycerol/phosphatidylcholine-stabilized particles as substrates for lipoprotein lipase. Biochim. Biophys. Acta. 875: 211–219. [DOI] [PubMed] [Google Scholar]
- 15.Wang C. S., McConathy W. J., Kloer H. U., and Alaupovic P.. 1985. Modulation of lipoprotein lipase activity by apolipoproteins. Effect of apolipoprotein C-III. J. Clin. Invest. 75: 384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg I. J. 1996. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 37: 693–707. [PubMed] [Google Scholar]
- 17.Davies B. S., Beigneux A. P., Barnes R. H., Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulbourne C. N., Gin P., Tatar A., Nobumori C., Hoenger A., Jiang H., Grovenor C. R., Adeyo O., Esko J. D., Goldberg I. J., et al. 2014. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 19: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mysling S., Kristensen K. K., Larsson M., Beigneux A. P., Gårdsvoll H., Fong L. G., Bensadouen A., Jørgensen T. J., Young S. G., and Ploug M.. 2016. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. Elife. 5: e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mysling S., Kristensen K. K., Larsson M., Kovrov O., Bensadouen A., Jørgensen T. J., Olivecrona G., Young S. G., and Ploug M.. 2016. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. Elife. 5: e20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigneux A. P., Davies B. S., Gin P., Weinstein M. M., Farber E., Qiao X., Peale F., Bunting S., Walzem R. L., Wong J. S., et al. 2007. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 5: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigneux A. P., Gin P., Davies B. S., Weinstein M. M., Bensadoun A., Fong L. G., and Young S. G.. 2009. Highly conserved cysteines within the Ly6 domain of GPIHBP1 are crucial for the binding of lipoprotein lipase. J. Biol. Chem. 284: 30240–30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beigneux A. P., Fong L. G., Bensadoun A., Davies B. S., Oberer M., Gardsvoll H., Ploug M., and Young S. G.. 2015. GPIHBP1 missense mutations often cause multimerization of GPIHBP1 and thereby prevent lipoprotein lipase binding. Circ. Res. 116: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu H., Labeur C., Xu C. F., Ferrell R., Lins L., Brasseur R., Rosseneu M., Weiss K. M., Humphries S. E., and Talmud P. J.. 2000. Characterization of the lipid-binding properties and lipoprotein lipase inhibition of a novel apolipoprotein C–III variant Ala23Thr. J. Lipid Res. 41: 1760–1771. [PubMed] [Google Scholar]
- 25.Esko J. D., Stewart T. E., and Taylor W. H.. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA. 82: 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang S. F., Reich B., Brunzell J. D., and Will H.. 1998. Detailed characterization of the binding site of the lipoprotein lipase-specific monoclonal antibody 5D2. J. Lipid Res. 39: 2350–2359. [PubMed] [Google Scholar]
- 27.Gin P., Beigneux A. P., Voss C., Davies B. S., Beckstead J. A., Ryan R. O., Bensadoun A., Fong L. G., and Young S. G.. 2011. Binding preferences for GPIHBP1, a glycosylphosphatidylinositol-anchored protein of capillary endothelial cells. Arterioscler. Thromb. Vasc. Biol. 31: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider C. A., Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bengtsson-Olivecrona G., and Olivecrona T.. 1992. Assay of lipoprotein lipase and hepatic lipase. In Converse C. A. and Skinner E. R., editors. Oxford University Press, New York: 169–185. [Google Scholar]
- 30.Nguyen A. T., Braschi S., Geoffrion M., Fong L. G., Crooke R. M., Graham M. J., Young S. G., and Milne R.. 2006. A mouse monoclonal antibody specific for mouse apoB48 and apoB100 produced by immunizing “apoB39-only” mice with mouse apoB48. Biochim. Biophys. Acta. 1761: 182–185. [DOI] [PubMed] [Google Scholar]
- 31.Page S., Judson A., Melford K., and Bensadoun A.. 2006. Interaction of lipoprotein lipase and receptor-associated protein. J. Biol. Chem. 281: 13931–13938. [DOI] [PubMed] [Google Scholar]
- 32.Ito Y., Azrolan N., O’Connell A., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 33.Cryer A. 1989. The role of the endothelium in myocardial lipoprotein dynamics. Mol. Cell. Biochem. 88: 7–15. [DOI] [PubMed] [Google Scholar]
- 34.Miller K. W., and Small D. M.. 1983. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J. Biol. Chem. 258: 13772–13784. [PubMed] [Google Scholar]
- 35.Gordts P. L., Nock R., Son N. H., Ramms B., Lew I., Gonzales J. C., Thacker B. E., Basu D., Lee R. G., Mullick A. E., et al. 2016. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Invest. 126: 2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saleheen D., Natarajan P., Armean I. M., Zhao W., Rasheed A., Khetarpal S. A., Won H. H., Karczewski K. J., O’Donnell-Luria A. H., Samocha K. E., et al. 2017. Human knockouts and phenotypic analysis in a cohort with a high rate of consanguinity. Nature. 544: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.