Abstract
We aimed to determine the risk factors associated with the depletion of large HDL particles and enrichment of small HDL particles observed in adolescents with T2D. Four groups of adolescents were recruited: 1) lean insulin-sensitive (L-IS), normal BMI and no insulin resistance; 2) lean insulin-resistant (L-IR), normal BMI but insulin resistance (fasting insulin levels ≥ 25 mU/ml and homeostatic model assessment of insulin resistance ≥ 6); 3) obese insulin-sensitive (O-IS), BMI ≥ 95th percentile and no insulin resistance; and 4) obese insulin-resistant (O-IR), BMI ≥ 95th percentile and insulin resistance. Plasma was separated by using gel-filtration chromatography to assess the HDL subspecies profile and compared with that of obese adolescents with T2D (O-T2D). Large HDL subspecies were significantly lower across groups from L-IS > L-IR > O-IS > O-IR > O-T2D (P < 0.0001); small HDL particles were higher from L-IS to O-T2D (P < 0.0001); and medium-sized particles did not differ across groups. The contributions of obesity, insulin resistance, and diabetes to HDL subspecies profile were between 23% and 28%, 1% and 10%, and 4% and 9%, respectively. Obesity is the major risk factor associated with the altered HDL subspecies profile previously reported in adolescents with T2D, with smaller contributions from insulin resistance and diabetes.
Keywords: high density lipoprotein, insulin resistance, diabetes, phospholipids
The Framingham Heart study first established the positive association between HDL-cholesterol (HDL-C) and protection against CVD (1). HDLs are considered atheroprotective because of their ability to remove cholesterol from artery wall macrophages (2), reduce oxidative stress (3), and stimulate vasodilatory nitric oxide production in endothelial cells (4). As a result, one strategy aimed at reducing CVD risk, after targeting lowering of LDL cholesterol (LDL-C), has focused on raising HDL-C. Unfortunately, recent pharmacological intervention trials that raised HDL-C levels modestly by niacin treatment and more substantially by inhibition of cholesteryl ester transfer protein have largely been unsuccessful in reducing CVD endpoints in most patients (5–9).
We and others have shown that “HDL” exists not as a single entity (as defined by the HDL-C concentration), but rather as a diverse group of subspecies distinguished by protein and lipid composition (10–13), an important observation masked by the clinical lipid measurement HDL-C. Additionally, we have previously demonstrated that, compared with healthy lean adolescent males, adolescents with T2D have an altered HDL subspecies profile characterized by a depletion of large HDL particles and corresponding increases in small ones (14). We also found that this HDL subspecies profile is associated with higher pulse wave velocity, a noninvasive measure of atherosclerosis and CVD (15, 16), suggesting that it may confer a higher risk for vascular disease. These findings, taken together with previous clinical reports (17–22), indicate that the pattern of HDL subspecies may be a more robust biomarker of atherosclerosis burden compared with HDL-C. Indeed, we found no association between HDL-C and arterial stiffness in our study (14). Thus, raising HDL-C indiscriminately without considering the type of HDL particles may explain the failure of many of the recent HDL drug trials (5–9).
In the current study, we sought to explore independent risk factors that contribute to the altered HDL subspecies profile observed in adolescents with T2D. Using a well-phenotyped population of adolescents, we aimed to examine the independent influence of obesity, insulin resistance, and glucose on this HDL subspecies profile.
MATERIALS AND METHODS
Study population
Participants were recruited in the Landmarks in the Progression to Type 2 Diabetes Study (DK59183), a school-based study of children in grades 5–12 conducted in Cincinnati, OH, from 2001 to 2007 (23). Postpubertal males (older than age 16 years) were randomly selected from the parent study if they fell into one of the following prespecified groups: lean insulin-sensitive (L-IS), lean insulin-resistant (L-IR), obese insulin-sensitive (O-IS), or obese insulin-resistant (O-IR). Lean for this study was defined as a BMI between the 25th and 75th percentiles using age/sex growth curves from the Centers for Disease Control and Prevention. Obese was defined as a BMI ≥ 95th percentile for age/sex based on the definition of obesity by the Centers for Disease Control and Prevention. Insulin-sensitive was defined as a fasting insulin level <18 mU/ml (Esoterix Laboratory), and insulin-resistant was defined as fasting insulin level ≥ 25 mU/ml [a value >2 SD higher than the mean for normal weight (BMI <85th percentile) age-, race-, sex-, and puberty-matched youth (23)], plus a homeostatic model assessment of insulin resistance (HOMA-IR) value of ≥6, a value consistent with insulin resistance based on prior work in postpubertal adolescents (24). HOMA-IR was calculated as glucose (mg/dl) × insulin (mU/l)/405. All O-IR participants had a 2 h oral glucose tolerance test to ensure they had no evidence of T2D by the American Diabetes Association criteria (fasting blood glucose <100 mg/dl and 2 h oral glucose tolerance glucose value <200 mg/dl) (25). Participants with T2D [defined by American Diabetes Association criteria (25)] were used as a reference group and were recruited from an ongoing study designed to investigate the effects of diabetes on lipoprotein composition and function (HL118132). T2D participants also had a BMI ≥ 95th percentile for age/sex and negative islet cell antibody (glutamic acid decarboxylase, insulin autoantibodies, and islet antigen-2 titers).
Males were only included in this study given known differences in lipoproteins by sex and timing of menstrual cycle (26). Height was measured to the closest 0.1 cm on a wall-mounted stadiometer, and weight was measured on an electronic scale with both measurements obtained twice and averaged. BMI was calculated in kilograms per square meter. The protocol was reviewed and approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. Written informed consent was obtained from the parent/legal guardian, and assent was obtained from the participant.
Clinical measurements
Measurements of total cholesterol, HDL-C, and triglycerides (TGs) were performed on fasting samples at the time of patient recruitment in the Clinical Laboratory at Cincinnati Children’s Hospital Medical Center by using a Roche Modular P analyzer (Roche, Indianapolis, IN). Determination of HDL-C was performed after precipitation of apo B containing particles by dextran sulfate and magnesium (14). LDL-C was calculated by using the Friedewald equation if TGs were <350 mg/dl and was measured directly otherwise. Glucose was measured by using a Hitachi model 704 glucose analyzer (Roche Hitachi, Indianapolis, IN), and insulin was measured by RIA with an antiinsulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St Louis, MO) and a double-antibody method to separate bound from free tracer. HOMA-IR was calculated as glucose (mg/dl) × insulin (mU/l)/405.
HDL subspecies
For all participant groups, 370 μl of previously frozen plasma (−80°C) was applied to three Superdex 200 Increase columns (10/300, GE Healthcare) arranged in series (27). We have previously shown that the subspecies profile does not differ among fresh versus frozen plasma samples (28). To relate gel filtration fractions to traditional density-centric lipoprotein definitions (LDL, VLDL, and HDL), we use the presence of apoB, the core constituent of LDL, as the key distinguisher, which appears in fractions 15−19. Fractions 20–30 were considered HDL because their diameters are consistent with density-isolated HDL and because of the abundance of the major HDL protein, apoA-I [details have been published (27)]. Choline-containing phospholipid and total cholesterol were measured in each fraction with kits from Wako (Richmond, VA) and Pointe Scientific (Canton, MI), respectively. Interassay coefficients of variation for these measurements in our laboratory are between 6% and 8% and 8% and 10%, respectively.
HDL subspecies were quantified by NMR spectroscopy at LabCorp (Morrisville, NC; formerly LipoScience) using an optimized version (LP4) of NMR LipoProfile (29). Seven HDL subspecies with the following estimated diameters were measured by this method: H7 (12.0 nm), H6 (10.8 nm), H5 (10.3 nm), H4 (9.5 nm), H3 (8.7 nm), H2 (7.8 nm), and H1 (7.4 nm), where H7 to H5 are considered large HDL particles, H4 medium-size HDL particles, and H3 to H1 small HDL particles. As previously described, mean HDL size is calculated as the sum of the diameter of each subspecies multiplied by its relative mass percentage as estimated from the amplitude of its lipid methyl NMR signal (29). Analyses were conducted both on plasma stored frozen at −80°C and on individual gel-filtration fractions. To obtain enough material for the latter measurements, four gel-filtration separations were conducted sequentially under identical conditions by using 370 μl of the same freshly thawed plasma specimen. Each separation was performed by using three Superdex 200 Increase columns arranged in series (10/300, GE Healthcare) and eluted with buffer containing 50 mM sodium phosphate, 120 mM KCl, 5 mM EDTA, and 1 mM CaCl2 at pH 7.4. The 1.5 ml HDL fractions (fractions 21–26) from the four runs were pooled (1.5 × 4) to give 6 ml for each fraction. After concentration down to 150 μl by centrifugal ultrafiltration and dilution in the same buffer to give the 400 μl required for NMR analysis, each fraction was analyzed by NMR for HDL subspecies composition.
Statistics.
SAS (Version 9.4, SAS Institute, Cary NC) was used to examine distributions of the dependent variables and associations between independent and dependent variables. Histograms and quantile plots were examined, as well as skewness and kurtosis. The majority of the phospholipid- and cholesterol-containing fractions (dependent variables) showed no significant deviation from normality, and therefore parametric tests were used for analysis. In this case, the differences across the four or five groups (as appropriate) were analyzed by using one-way ANOVA. When the overall ANOVA was statistically significant, individual group differences were tested by using a Student–Newman–Kuels correction to adjust for the multiple comparisons. For a few fractions where there was a deviation from normality, phospholipid fractions 15, 16, 29, and 30 and cholesterol fractions 15, 16, and 27–30. In these cases, a Kruskal-Wallis test for the group comparison and a Bonferroni correction was applied to adjust for multiple testing of individual group differences. For consistency, means are shown in the plots. To evaluate the relationship between risk factors of interest (obesity, insulin resistance, and diabetes) and phospholipid or cholesterol content within each lipoprotein fraction, we used Pearson or Spearman correlation coefficients, as appropriate (see fractions above). These analyses were repeated within each race group (Caucasian and African-American). Finally, we used regression analysis to examine the relative contribution of obesity, insulin resistance, and diabetes to the differences in HDL subspecies. A partial r-square was used to evaluate the percent of variation explained.
RESULTS
The mean age of the study cohort was 17.8 ± 1.2 years (Table 1). Weight, BMI, fasting insulin, total cholesterol, and LDL-C were highest in the O-IR group, and HDL-C was lowest. Despite comparable BMI, the O-T2D reference group exhibited higher total cholesterol, LDL-C, and HDL-C compared with O-IR. These trends persisted when Caucasian and African-American males were evaluated separately. Hemoglobin A1c for the T2D group was 7.1 ± 1.9%.
TABLE 1.
Characteristics of the study population
| L-IS, n = 20 | L-IR, n = 20 | O-IS, n = 20 | O-IR, n = 20 | T2D. n = 12 | P | |
| Age (years) | 18.2 ± 1.2 | 17.6 ± 1.6 | 17.9 ± 1.0 | 17.6 ± 1.0 | 18.4 ± 2.9 | 0.52 |
| Race, n (% Caucasian) | 6 (30) | 10 (50) | 10 (50) | 10 (50) | 6 (50) | 0.64 |
| Weight (kg) | 66.9 ± 5.8c | 69.3 ± 7.5c | 97.4 ± 8.9b | 120.5 ± 22.4a | 118.8 ± 24.6a | <0.001 |
| BMI (kg/m2) | 21.9 ± 1.3c | 22.1 ± 1.6c | 30.8 ± 2.9b | 38.0 ± 6.3a | 38.2 ± 6.0a | <0.001 |
| Fasting insulin (mU/ml) | 4.5 ± 1.2c | 42.5 ± 16.3b | 8.1 ± 1.9b | 73.2 ± 21.8a | Na | <0.001 |
| Fasting glucose (mg/dl) | 70 ± 8b | 82 ± 18b | 80 ± 9b | 81 ± 8b | 176 ± 92a | <0.001 |
| 2 h OGTT glucose (mg/dl) | NA | NA | 81 ± 18 | 84 ± 20 | NA | 0.628 |
| Total cholesterol (mg/dl) | 139 ± 15b | 146 ± 36b | 146 ± 27b | 159 ± 29b | 196 ± 22a | <0.001 |
| HDL-C (mg/dl) | 46 ± 6a | 42 ± 12ab | 40 ± 10ab | 35 ± 8b | 42 ± 8a | 0.020 |
| LDL-C (mg/dl) | 79 ± 13b | 74 ± 29b | 92 ± 24b | 98 ± 25b | 125 ± 29a | <0.001 |
| TGs (mg/dl) | 67 ± 20b | 159 ± 171ab | 69 ± 32b | 136 ± 81ab | 222 ± 136a | <0.001 |
Data are presented as mean ± SD or number (%). NA indicates value was not obtained. P values are from ANOVA for a five-group comparison, except insulin where four-group ANOVA was used and 2 h oral glucose tolerance test (OGTT) where unpaired t-test was used. If the ANOVA was significant, between-group differences were tested and are marked by superscripts. Superscripts marked “a” indicate values are significant different from “b” or “c” at P < 0.05. Those marked as “ab” are not different from “a” and not different from “b.”
We assessed lipoprotein subspecies by size using gel-filtration chromatography, a method optimized to increase particle resolution in the HDL size range (14, 28). Plasma typically shows three distinct peaks when fractions are plotted based on phospholipid and cholesterol content. Fractions 15–19 contain VLDL/LDL-sized particles and fractions 20–30 encompass HDL-sized particles, with fractions 27–30 representing relatively small, lipid-poor species.
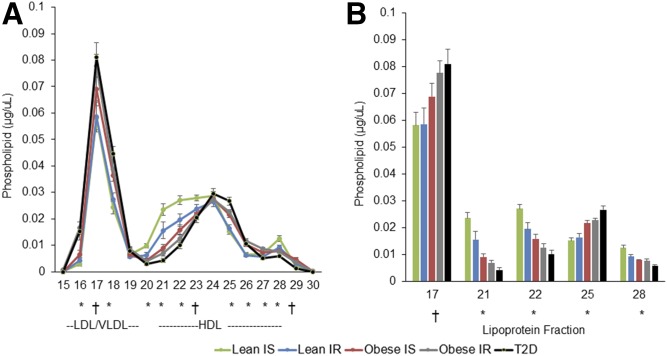
In fractions containing large HDL subspecies (fractions 20–23), there were clear differences in phospholipid content across groups (Fig. 1A; P < 0.001). L-IS showed the most phospholipid in fractions 20–23, which was progressively and significantly lower from L-IS > L-IR > O-IS > O-IR > O-T2D. The inverse relationship was seen for small HDL particles (fraction 25), where the O-T2D group had the most phospholipid. Less distinct, but significant, group differences were seen in lipid-poor subspecies (fraction 28 for phospholipid). No differences were seen in fraction 24 containing medium-sized HDL subspecies. These trends persisted when Caucasians and African-American males were evaluated separately (data not shown).
Fig. 1.
Phospholipid across lipoprotein fractions. Phospholipid concentration in serum fractionated by gel-filtration chromatography (A) and phospholipid concentration in specific LDL/VLDL and HDL fractions (B). Data are presented as mean and SE. * P < 0.001; † P < 0.01 by ANOVA or Kruskal-Wallis, as appropriate, for the five-group comparison.
Cholesterol content across fractions mirrored the phospholipid findings (supplemental Fig. S1) for large and medium HDL subspecies, but not for small HDL subspecies. O-IS and O-IR were indistinguishable in fraction 25 by cholesterol content.
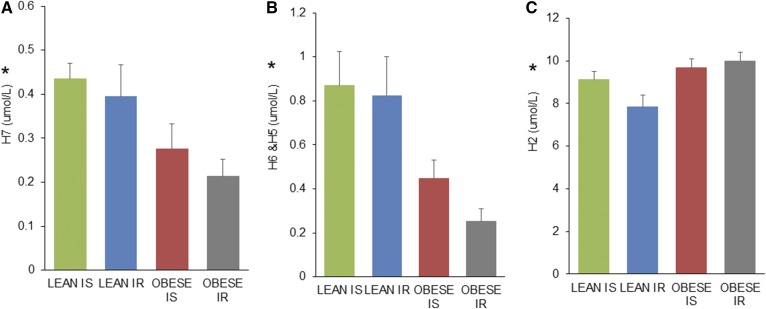
Given the distinct group differences in HDL sizes by gel-filtration chromatography, we set out to confirm these results using an orthogonal sizing method. We analyzed plasma by NMR spectroscopy using the LabCorp LP4 algorithm, an alternative method that examines lipoprotein particle number and size. The T2D group was not included in these analyses due to lack of sample available. Table 2 shows how our gel-filtration fractions relate to NMR spectroscopy. Fraction 21 contains large HDL particles consistent in size with H7 particles by NMR spectroscopy; fraction 22 contains a combination of H6 and H5 particles; and fraction 25 contains mostly H2 particles. Figure 2 shows the same trend across groups as observed by gel filtration lower for NMR-measured large HDL particles (H7 and H6+H5). Although levels of small HDL particles (H2) were not strictly reversed across the four groups as they were by gel-filtration phospholipid content, levels were higher for obese compared with lean subjects. The mean concentrations across the HDL size range as well as total particle number for the four groups are shown in supplemental Table S1. Although the overall size distribution of particles differed across groups, there was no significant difference in the total HDL particle number across groups.
TABLE 2.
HDL subclass concentration in gel-filtration fractions by LP4 deconvolution
| FPLC fraction | H7 (µmol/l) | H6 (µmol/l) | H5 (µmol/l) | H4 (µmol/l) | H3 (µmol/l) | H2 (µmol/l) | H1 (µmol/l) | HDL-P (µmol/l) | HDL size (nm) |
| 21 | 4.48 | 1.32 | 0 | 0 | 0 | 0 | 0.55 | 6.4 | 11.75 |
| 22 | 0 | 8.45 | 5.53 | 0 | 0 | 0 | 0 | 14.0 | 10.63 |
| 23 | 0 | 0 | 0 | 13.57 | 2.15 | 1.25 | 0 | 17.0 | 9.37 |
| 24 | 0 | 0 | 0 | 3.75 | 12.98 | 7.01 | 0 | 23.7 | 8.73 |
| 25 | 0 | 0 | 0.11 | 0 | 1.57 | 17.70 | 1.09 | 20.5 | 7.99 |
| 26 | 0 | 0 | 0.30 | 0 | 0 | 6.68 | 4.04 | 11.0 | 7.88 |
FPLC, fast-protein liquid chromatography; HDL-P, HDL particle number. Bold in the table indicates the major HDL subspecies contained in each gel filtration fraction.
Fig. 2.
HDL subspecies concentrations across groups by NMR spectrospcopy where (A) is large H7 subspecies, (B) is mean of large H6 and H5 subspecies, and (C) is small H2 subspecies. Green is L-IS, blue is L-IR, red is O-IS, and gray is O-IR. Data are presented as mean and SD. * P < 0.05 by ANOVA for the four-group comparison.
We also assessed the association between obesity (measured as BMI), insulin resistance (HOMA-IR), and diabetes (fasting glucose) with HDL-C and HDL subspecies. For these analyses, all participants were included, except for the correlations with HOMA-IR, where the O-T2D group was excluded because some participants reported taking exogenous insulin.
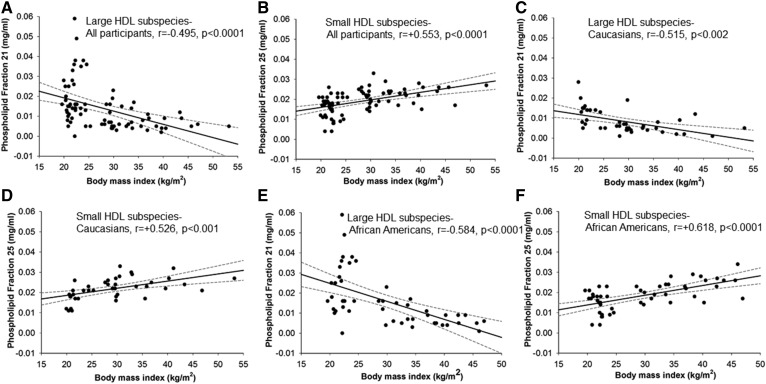
There was a strong inverse relationship between BMI and the phospholipid content in large HDL particles (Fig. 3A) in fractions 21 and 22 (fraction 21 is shown). Pearson correlation coefficients (r values) were −0.495 and −0.476, respectively, with P < 0.0001, suggesting that a higher BMI is associated with less choline-containing phospholipid content in large HDL subspecies. The opposite relationship was seen for small HDL subspecies, where a higher BMI is associated with higher phospholipid in small HDL subspecies (Fig. 3B, r value = 0.553, P < 0.0001). Significant correlations were also observed when Caucasian and African-American males were evaluated separately (Fig. 3C–F). When the relationship between BMI and cholesterol content within fractions 21, 22, and 25 was examined, correlation coefficients were comparable for large HDL subspecies (fractions 21 and 22, r = −0.472, r = −0.478, P < 0.0001), but lower for small HDL subspecies (compared with those observed between BMI and phospholipid content, correlation with cholesterol in fraction 25 was r = 0.386, P < 0.001). The correlation coefficient between BMI and HDL-C was r = −0.300, P = 0.003.
Fig. 3.
A, B: Relationship between the phospholipid content in fractions containing large HDL subspecies (fraction 21) and small HDL subspecies (fraction 25) and BMI for all participants. C–F: Also shown are correlations by race group. Pearson correlation coefficients are shown.
To evaluate the relationship between insulin resistance and HDL subspecies, we used HOMA-IR levels. HOMA-IR was significantly and inversely correlated with large HDL subspecies (phospholipid in fractions 21 and 22), with correlations of r = −0.352 and −0.404, respectively, both P < 0.001 (supplemental Fig. S2). The inverse relationship was observed between HOMA-IR and small HDL subspecies by phospholipid in fraction 25 (r = 0.279, P = 0.012). Correlations between HOMA-IR and cholesterol content in fractions were lower than those observed for phospholipid (fraction 21 = −0.331, fraction 22 = −0.359, and fraction 25 = 0.189). The correlation coefficient between HOMA-IR and HDL-C was r = 0.333, P = 0.003.
Fasting glucose was also inversely associated with phospholipid content in large HDL subspecies (supplemental Fig. S2, fractions 21 and 22, r = −0.248 and −0.308, respectively, both P < 0.03)and fasting glucose was positively correlated with phospholipids in small HDL subspecies (phospholipid in fraction 25, r = 0.299, P = 0.007). Corresponding correlations between glucose and cholesterol content in fractions 21, 22, and 25 were r = 0.227, r = 0.288, and r = 0.076, respectively. It should be noted, however, that these relationships were largely driven by a few individuals with elevated glucose values. The correlation coefficient between glucose and HDL-C was not significant.
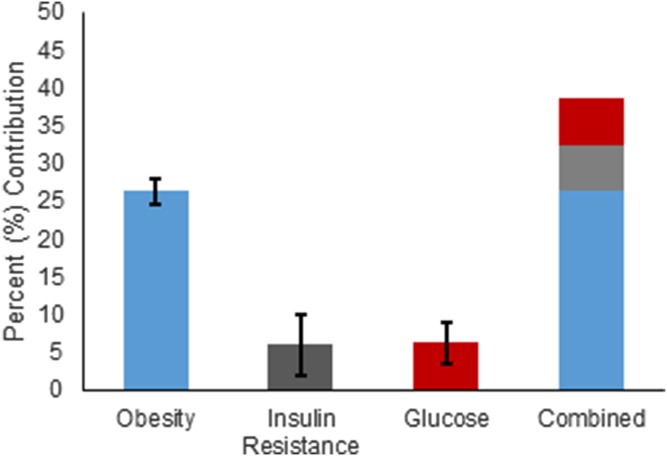
Finally, we examined the relative contribution of obesity, insulin resistance, and diabetes to phospholipid content in fractions 21, 22, and 25. Partial r-square values for obesity were 28, 23, and 28% for the three fractions, respectively. For insulin resistance, partial r-square values were 7, 10, and 1%, and for glucose, partial r square values were 4, 6, and 9% (Fig. 4). Thus, in total, these risk factors explained about 39% of the variance in the phospholipid content in large and small HDL subspecies.
Fig. 4.
Contribution of risk factors to the phospholipid content in large and small HDL subspecies. Data are presented as mean and SD from fractions 21, 22, and 25 combined.
DISCUSSION
We used gel-filtration chromatography to separate lipoproteins by size among four highly selected participant groups differentiated by their insulin resistance and obesity status. Our goal was to examine the major risk factors that contribute to the altered HDL subspecies profile observed in O-T2D. We made three key observations: 1) as metabolic disease worsens, there are less large HDL subspecies confirmed by two independent sizing methods; 2) there was a stronger association between phospholipid content versus cholesterol content with disease phenotype; and 3) obesity is the major risk factor associated with this profile. Each of these will be discussed in depth below.
As the metabolic disease state worsened from L-IS > L-IR > O-IS > O-IR > O-T2D, there was a remarkably consistent lowering in large HDL subspecies noted by gel-filtration chromatography. Specifically, there was less phospholipid and cholesterol content in fractions 21 and 22 (fractions containing large HDL particles). We also noted the opposite trend in small HDL subspecies by gel filtration where LIS < L-IR < O-IS < O-IR < O-T2D. The trend for lower concentrations of large HDL subspecies were also seen by NMR spectroscopy, suggesting that with insulin resistance and obesity, there are a lower number of large HDL particles. NMR agreed with gel filtration in showing that small HDL levels were higher in obesity, but the trend relating insulin resistance to higher small HDL in lean adolescents was not mirrored by NMR. Reasons for this discrepancy are not apparent, but may indicate greater sensitivity in the gel-filtration method versus NMR spectroscopy.
The altered HDL profile with metabolic disease, characterized by fewer large particles and more small particles, without a change in total particle number by NMR suggests alterated HDL metabolism, as described in previous work (18, 20–22, 30). We hypothesize that obesity, insulin resistance, and diabetes may alter one or more of the following processes, which could result in the observed findings. LCAT is an HDL-associated enzyme that esterifies free cholesterol to cholesterol esters in HDL, expanding the HDL size from a nascent particle to a disc and eventually a larger sphere. In insulin resistance and T2D, diminished activity or decreased ability to interact with LCAT (with higher activity) could explain fewer large HDL particles and the enrichment of small particles. Both have been described in diabetes and insulin resistance (31–33). Second, there could be increased lipid transfer or catabolism of large particles with metabolic disease [higher phospholipid transfer protein activity (34, 35), lipase, or phospholipase A2 activity (36)], resulting in smaller HDL particles at the expense of large ones. Lastly, it is possible that HDL metabolism is not altered, but, instead, there is less production of larger particles into circulation with metabolic disease (37). These pathways are areas of future research in these patient populations.
We found that the phospholipid content of the particles better differentiated the groups compared with the cholesterol content across fractions. This may be because cholesterol is more readily exchanged among HDL particles (in exchange for TG via cholesteryl ester transfer protein) compared with phospholipid. Thus, the phospholipid content of HDL may have the potential to be used as a biomarker of disease. Unfortunately, the gel-filtration method as currently performed is labor-intensive and not suited for clinical use. Therefore, we continue to work to develop tools to rapidly identify and quantitate the particles that elute in fractions 21, 22, and 25.
Whereas the cholesterol content across HDL subspecies was less sensitive than corresponding phospholipid content, both were more sensitive than total HDL-C, the current clinical lipid measurement used to quantify total cholesterol across all HDL particles and for detecting associations with risk factors. Although HDL-C concentration across groups paralleled the findings in large HDL subspecies (lower across groups), the HDL-C number lacked sensitivity to detect the enrichment of small atherogenic HDL with metabolic disease. This supports the notion that particle sizes provide more valuable information about the metabolic disease state of an individual. Indeed, prior work, including studies from our group, has shown that the types/size of HDL particles are strongly correlated with measurements of arterial thickness and stiffness and are better predictors of cardiovascular risk (38–44).
We observed that obesity explained the most variance in the HDL subspecies profile. Our previous work has shown that the atherogenic HDL profile seen in both obese and T2D adolescents is reversed by weight-loss surgery, specifically vertical sleeve gastrectomy (VSG) (28). One year after VSG, adolescents reversed their profile toward lean adolescents (gain of larger HDL particles, loss of small HDL particles). In that study, the individual contributions of obesity, insulin resistance, and glucose (diabetes) could not be assessed because BMI decreased by 32%, HOMA-IR decreased by ∼70%, and fasting glucose decreased by ∼20% with surgery. Additionally, weight-loss surgery is associated with altered gut physiology, which probably influences HDL metabolism (45). Therefore, we could not conclude whether the improved HDL subspecies profile was a result of improved metabolic disease or the surgery. Here, we show that, although insulin resistance and glucose are associated with an altered HDL subspecies profile, obesity is the predominant risk factor associated with lowering of large HDL subspecies and a rise in small HDL subspecies. This suggests that weight loss, even without weight-loss surgery, may have a beneficial effect in altering the HDL profile toward that seen in L-IS. Future studies are needed to establish this and determine whether this translates into decreased cardiovascular risk.
Limitations of the current study included a lack of female participants, use of a reference group that was recruited from a different study, and the inability to account for important confounders, such as alcohol or smoking. We also lacked additional measures of adiposity, such as waist circumference and gold-standard measurements of insulin resistance. Additionally, since our methodology did not include a proteomic or lipidomic analysis, we cannot rule out compositional differences among particles with metabolic disease. Finally, from our data, we cannot conclude that other sized VLDL/LDL particles are not different across groups, because our technique is not optimized for this range. However, strengths of the study include a well-phenotyped cohort of young adolescents and the ability to separate obesity and insulin resistance.
We found that metabolic disease, largely driven by obesity, alters the HDL subspecies profile in a distinct way, resulting in loss of large HDL and enrichment of small HDL. Although future work is needed to determine how these alterations specifically influence cardiovascular outcomes, our work highlights the necessity of understanding the molecular basis of HDL subspeciation and the lack of information provided by current clinical measurements of HDL-C. If these subspecies profiles indeed impinge on disease risk, it may be possible to manipulate particular metabolic pathways to favorably alter subspecies profiles.
Supplementary Material
Footnotes
Abbreviations:
- HDL-C
- HDL cholesterol
- HOMA-IR
- homeostatic model assessment of insulin resistance
- LDL-C
- LDL cholesterol
- L-IR
- lean insulin-resistant
- L-IS
- lean insulin-sensitive
- O-IR
- obese insulin-resistant
- O-IS
- obese insulin-sensitive
- O-T2D
- obese adolescents with T2D
- TG
- triglyceride
This work was supported by National Institutes of Health Grants K23HL118132 (A.S.S.), R01HL67093 (W.S.D.), R01HL104136 (W.S.D.), UL1TR000077 (Cincinnati Children’s), and DK59183 (L.M.D.); The Central Society for Clinical and Translational Research (A.S.S.); and the Cincinnati Pediatric Diabetes and Obesity Center at Cincinnati Children’s Hospital Medical Center (A.S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Cincinnati Children’s Hospital Medical Center. The authors have no conflicts of interest.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Castelli W. P., Garrison R. J., Wilson P. W., Abbott R. D., Kalousdian S., and Kannel W. B.. 1986. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 256: 2835–2838. [PubMed] [Google Scholar]
- 2.Cuchel M., and Rader D. J.. 2006. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 113: 2548–2555. [DOI] [PubMed] [Google Scholar]
- 3.Quinn M. T., Parthasarathy S., Fong L. G., and Steinberg D.. 1987. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA. 84: 2995–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuvin J. T., Harati N. A., Pandian N. G., Bojar R. M., and Khabbaz K. R.. 2002. Postoperative cardiac tamponade in the modern surgical era. Ann. Thorac. Surg. 74: 1148–1153. [DOI] [PubMed] [Google Scholar]
- 5.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 6.Landray M. J., Haynes R., Hopewell J. C., Parish S., Aung T., Tomson J., Wallendszus K., Craig M., Jiang L., Collins R., et al. 2014. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 8.Lincoff A. M., Nicholls S. J., Riesmeyer J. S., Barter P. J., Brewer H. B., Fox K. A. A., Gibson C. M., Granger C., Menon V., Montalescot G., et al. 2017. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N. Engl. J. Med. 376: 1933–1942. [DOI] [PubMed] [Google Scholar]
- 9.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., and Weintraub W.; AIM-HIGH Investigators . 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255– 2267 [Erratum. 2012. N. Engl. J. Med. 367: 189.] [DOI] [PubMed] [Google Scholar]
- 10.Cheung M. C., Vaisar T., Han X., Heinecke J. W., and Albers J. J.. 2010. Phospholipid transfer protein in human plasma associates with proteins linked to immunity and inflammation. Biochemistry. 49: 7314–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro G. R., and Fielding C. J.. 1988. Early incorporation of cell-derived cholesterol into prebBeta-migrating high-density lipoprotein. Biochemistry. 27: 25–29. [DOI] [PubMed] [Google Scholar]
- 12.Shah A. S., Tan L., Long J. L., and Davidson W. S.. 2013. The proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 54: 2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiflett A. M., Bishop J. R., Pahwa A., and Hajduk S. L.. 2005. Human high density lipoproteins are platforms for the assembly of multi-component innate immune complexes. J. Biol. Chem. 280: 32578–32585. [DOI] [PubMed] [Google Scholar]
- 14.Gordon S. M., Davidson W. S., Urbina E. M., Dolan L. M., Heink A., Zang H., Lu L. J., and Shah A. S.. 2013. The effects of type 2 diabetes on lipoprotein composition and arterial stiffness in male youth. Diabetes. 62: 2958–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent S., Cockcroft J., Van Bortel L., Boutouyrie P., Giannattasio C., Hayoz D., Pannier B., Vlachopoulos C., Wilkinson I., Struijker-Boudier H.; European Network for Non-invasive Investigation of Large Arteries . 2006. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J. 27: 2588–2605. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell G. F., Hwang S. J., Vasan R. S., Larson M. G., Pencina M. J., Hamburg N. M., Vita J. A., Levy D., and Benjamin E. J.. 2010. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 121: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appachi S., Kelly K. R., Schauer P. R., Kirwan J. P., Hazen S., Gupta M., and Kashyap S. R.. 2011. Reduced cardiovascular risk following bariatric surgeries is related to a partial recovery from “adiposopathy”. Obes. Surg. 21: 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goff D. C. Jr., D’Agostino R. B. Jr., Haffner S. M., and Otvos J. D.. 2005. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the Insulin Resistance Atherosclerosis Study. Metabolism. 54: 264–270. [DOI] [PubMed] [Google Scholar]
- 19.Burns S. F., and Arslanian S. A.. 2009. Waist circumference, atherogenic lipoproteins, and vascular smooth muscle biomarkers in children. J. Clin. Endocrinol. Metab. 94: 4914–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magge S. N., Prasad D., Koren D., Gallagher P. R., Mohler E. R. III, Stettler N., Levitt Katz L. E., and Rader D. J.. 2012. Prediabetic obese adolescents have a more atherogenic lipoprotein profile compared with normoglycemic obese peers. J. Pediatr. 161: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garvey W. T., Kwon S., Zheng D., Shaughnessy S., Wallace P., Hutto A., Pugh K., Jenkins A. J., Klein R. L., and Liao Y.. 2003. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 52: 453–462. [DOI] [PubMed] [Google Scholar]
- 22.Burns S. F., Lee S., and Arslanian S. A.. 2009. In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care. 32: 2087–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolan L. M., Bean J., D’Alessio D., Cohen R. M., Morrison J. A., Goodman E., and Daniels S. R.. 2005. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J. Pediatr. 146: 751–758. [DOI] [PubMed] [Google Scholar]
- 24.Kurtoǧlu S., Hatipoǧlu N., Mazicioǧlu M., Kendirici M., Keskin M., and Kondolot M.. 2010. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J. Clin. Res. Pediatr. Endocrinol. 2: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Diabetes Association. 2014. Standards of medical care in diabetes–2014. Diabetes Care. 37 (Suppl. 1): S14–S80. [DOI] [PubMed] [Google Scholar]
- 26.Johnson J. L., Slentz C. A., Duscha B. D., Samsa G. P., McCartney J. S., Houmard J. A., and Kraus W. E.. 2004. Gender and racial differences in lipoprotein subclass distributions: the STRRIDE study. Atherosclerosis. 176: 371–377. [DOI] [PubMed] [Google Scholar]
- 27.Gordon S. M., Deng J., Lu L. J., and Davidson W. S.. 2010. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J. Proteome Res. 9: 5239–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson W. S., Inge T. H., Sexmith H., Heink A., Elder D., Hui D. Y., Melchior J. T., Kelesidis T., and Shah A. S.. 2017. Weight loss surgery in adolescents corrects high-density lipoprotein subspecies and their function. Int. J. Obes. (Lond.) 41: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeyarajah E. J., Cromwell W. C., and Otvos J. D.. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 26: 847–870. [DOI] [PubMed] [Google Scholar]
- 30.Borggreve S. E., De Vries R., and Dullaart R. P.. 2003. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur. J. Clin. Invest. 33: 1051–1069. [DOI] [PubMed] [Google Scholar]
- 31.Nakhjavani M., Asgharani F., Khalilzadeh O., Esteghamati A., Ghaneei A., Morteza A., and Anvari M.. 2011. Oxidized low-density lipoprotein is negatively correlated with lecithin-cholesterol acyltransferase activity in type 2 diabetes mellitus. Am. J. Med. Sci. 341: 92–95. [DOI] [PubMed] [Google Scholar]
- 32.Kappelle P. J., de Boer J. F., Perton F. G., Annema W., de Vries R., Dullaart R. P., and Tietge U. J.. 2012. Increased LCAT activity and hyperglycaemia decrease the antioxidative functionality of HDL. Eur. J. Clin. Invest. 42: 487–495. [DOI] [PubMed] [Google Scholar]
- 33.de Vries R., Borggreve S. E., and Dullaart R. P.. 2003. Role of lipases, lecithin:cholesterol acyltransferase and cholesteryl ester transfer protein in abnormal high density lipoprotein metabolism in insulin resistance and type 2 diabetes mellitus. Clin. Lab. 49: 601–613. [PubMed] [Google Scholar]
- 34.Dullaart R. P., De Vries R., Scheek L., Borggreve S. E., Van Gent T., Dallinga-Thie G. M., Ito M., Nagano M., Sluiter W. J., Hattori H., et al. 2004. Type 2 diabetes mellitus is associated with differential effects on plasma cholesteryl ester transfer protein and phospholipid transfer protein activities and concentrations. Scand. J. Clin. Lab. Invest. 64: 205–215. [DOI] [PubMed] [Google Scholar]
- 35.van Tol A. 2002. Phospholipid transfer protein. Curr. Opin. Lipidol. 13: 135–139. [DOI] [PubMed] [Google Scholar]
- 36.Tietge U. J., Maugeais C., Cain W., Grass D., Glick J. M., de Beer F. C., and Rader D. J.. 2000. Overexpression of secretory phospholipase A(2) causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J. Biol. Chem. 275: 10077–10084. [DOI] [PubMed] [Google Scholar]
- 37.Mendivil C. O., Furtado J., Morton A. M., Wang L., and Sacks F. M.. 2016. Novel pathways of apolipoprotein A-I metabolism in high-density lipoprotein of different sizes in humans. Arterioscler. Thromb. Vasc. Biol. 36: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake G. J., Otvos J. D., Rifai N., and Ridker P. M.. 2002. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 106: 1930–1937. [DOI] [PubMed] [Google Scholar]
- 39.Cromwell W. C., Otvos J. D., Keyes M. J., Pencina M. J., Sullivan L., Vasan R. S., Wilson P. W., and D’Agostino R. B.. 2007. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study - implications for LDL management. J. Clin. Lipidol. 1: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Harchaoui K., van der Steeg W. A., Stroes E. S., Kuivenhoven J. A., Otvos J. D., Wareham N. J., Hutten B. A., Kastelein J. J., Khaw K. T., and Boekholdt S. M.. 2007. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 49: 547–553. [DOI] [PubMed] [Google Scholar]
- 41.Mora S., Szklo M., Otvos J. D., Greenland P., Psaty B. M., Goff D. C. Jr., O’Leary D. H., Saad M. F., Tsai M. Y., and Sharrett A. R.. 2007. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis. 192: 211–217. [DOI] [PubMed] [Google Scholar]
- 42.Mackey R. H., Greenland P., Goff D. C. Jr., Lloyd-Jones D., Sibley C. T., and Mora S.. 2012. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J. Am. Coll. Cardiol. 60: 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mora S., Glynn R. J., and Ridker P. M.. 2013. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 128: 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah A. S., Davidson W. S., Gao Z., Dolan L. M., Kimball T. R., and Urbina E. M.. 2016. Superiority of lipoprotein particle number to detect associations with arterial thickness and stiffness in obese youth with and without prediabetes. J. Clin. Lipidol. 10: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osto E., Doytcheva P., Corteville C., Bueter M., Dorig C., Stivala S., Buhmann H., Colin S., Rohrer L., Hasballa R., et al. 2015. Rapid and body weight-independent improvement of endothelial and high-density lipoprotein function after Roux-en-Y gastric bypass: role of glucagon-like peptide-1. Circulation. 131: 871–881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.