Abstract
Familial hypercholesterolemia (FH) is a common genetic disorder that causes elevated LDL cholesterol levels from birth. Untreated FH accelerates atherosclerosis and predisposes individuals to premature coronary artery disease (CAD) in adulthood. Mendelian randomization studies have demonstrated that LDL cholesterol has both a causal and cumulative effect on the risk of CAD. This supports clinical recommendations that children with FH commence pharmacological treatment from the age of 8 to 10 years, to reduce the burden of hypercholesterolemia. Worldwide, the majority of children with FH remain undiagnosed. Recent evidence suggests that the frequency of FH is at least 1 in 250 and this constitutes a public health issue. We review and identify the knowns and unknowns concerning the detection and management of pediatric FH that impact on the developing model of care for this condition.
Keywords: cholesterol, dyslipidemias, lipoproteins, statins, LDL cholesterol, adolescents, children, screening
INTRODUCTION
Familial hypercholesterolemia (FH) is a codominant condition with a prevalence between 1:200 and 1:350 (1–6), caused by defects in genes affecting the LDL pathway (7, 8). Individuals with FH have markedly elevated LDL cholesterol from birth predisposing them to accelerated atherosclerosis and premature coronary artery disease (CAD) as adults (9, 10).
In the first section of this article, we briefly review the rationale for identifying and treating FH in childhood. In the second section, we extensively review the knowns and unknowns relevant to subject.
FH and childhood atherosclerosis
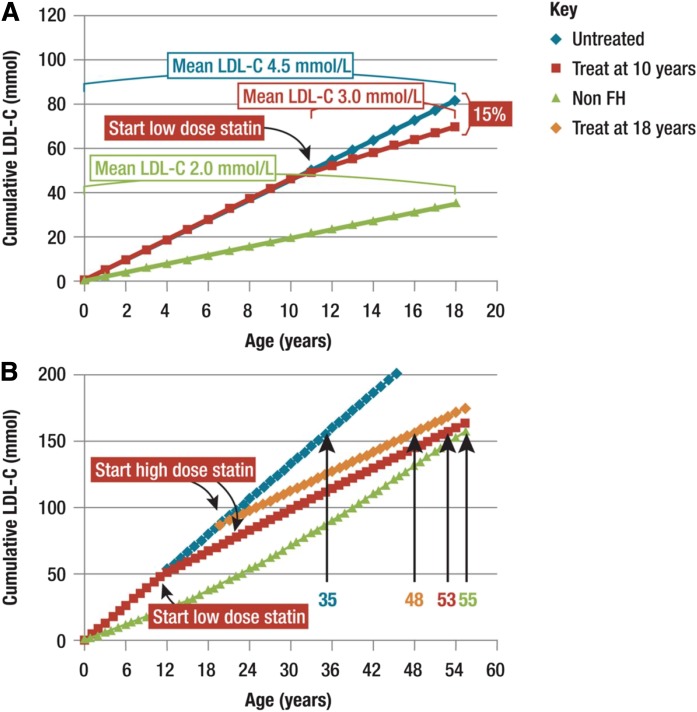
Atherosclerosis begins from conception, with evidence that in utero exposure to maternal high cholesterol impacts on arterial biology in the fetus (11, 12). Autopsy and imaging studies demonstrate that the atherosclerotic process begins in childhood and progresses in direct proportion to plasma LDL cholesterol levels (13, 14). Early signs of atherosclerosis in children with FH include elevated markers of vascular inflammation (15, 16), endothelial dysfunction (17, 18), increased pulse wave velocity (19), and increased carotid intima media thickness (CIMT) (20, 21). Early treatment with statins can reduce the progression of CIMT (22–24). This strengthens the case for early treatment of children with FH to reduce the impact on the cumulative life-burden of LDL cholesterol (Fig. 1) (25). This notion is supported by Mendelian randomization (MR) data indicating that exposure to a 1 mmol/l change in LDL cholesterol is associated with a 50% change in coronary heart disease (CHD) risk (26). However, while individuals with FH who are treated from a young age can have a normal life expectancy, FH remains largely under-diagnosed, especially in the young (25, 27).
Fig. 1.
Impact of statin treatment on cholesterol burden in FH. Early initiation of statin treatment reduces the LDL cholesterol (LDL-C) burden in subjects with FH. A: Cumulative LDL cholesterol burden by the age of 18 years is 15% lower in FH subjects treated with low dose statin from the age of 10 years (70 mmol) than in untreated FH subjects (80 mmol). B: Cumulative LDL cholesterol burden of a 55-year-old non-FH subject is 160 mmol. In an untreated FH subject, this is attained by age 35 years, but is delayed in FH patients treated from the age of 18 years (48 years), and further delayed in those treated from the age of 10 years (53 years). For calculation of the LDL cholesterol burden, the following assumed mean LDL cholesterol values were used. Non-FH subjects: 2.0 mmol/l for the age range of 0–15 years; 2.5 mmol/l for 15–24 years; 3.0 mmol/l for 25–34 years; 3.5 mmol/l for 35–44 years; and 3.5 mmol/l for 45–54 years. FH subjects: 4.5 mmol/l in untreated FH patients; 3 mmol/l in FH patients treated during the age range of 10–18 years; and 2.5 mmol/l in FH patients with treatment started at the age of 18 years. Reproduced from Wiegman et al. (25).
Clinical care of FH
Several guidelines and models of care have been published for clinical services (25, 27–33). Components include screening and detection, diagnosis, risk stratification, treatment, integration of clinical care, patient/family support, registries, and research and audit. However, there are several gaps in knowledge that need to be addressed.
DETECTION OF CHILDREN WITH FH
Current knowledge and practice.
FH fulfills classical criteria justifying screening for a condition (34). Screening may be divided into universal or selective, and as opportunistic and systematic within each subcategory. Cascade testing is the method most commonly employed to screen the population for FH in countries around the world; but used alone, it fails to identify sufficient index cases (35). Recent studies propose a combination of universal and reverse cascade testing approaches, identifying the index case in childhood and proceeding to screen the child’s parents and close relatives (5, 36).
Suggestions for future inquiries.
The optimal processes and pathways for the organization of childhood screening for FH.
Universal screening by phenotypic and genotypic approaches
Phenotypic strategy.
Current knowledge and practice.
Total or LDL cholesterol level measured between 1 and 9 years of age best discriminates between individuals with and without FH in the general population (37). A pilot study confirmed the feasibility and acceptability of screening for FH with a total cholesterol level at immunization in children aged 1–2 years (38).
In the US, universal screening of children aged 9–11 years for hypercholesterolemia has been proposed. This was part of an integrated guideline to improve cardiovascular (CV) health in young people and not directed at identifying children with FH. Uptake of this recommendation has been limited (39, 40). However, by focusing the screening on diagnosing FH and setting a higher cholesterol threshold (e.g., LDL cholesterol ≥4 mmol/l or ≥160 mg/dl), one can markedly reduce the number of false positives and only recall individuals with probable FH, who are likely to require pharmacological intervention to lower LDL cholesterol. Once an index case has been identified by a universal screening approach and the diagnosis confirmed, reverse cascade testing can identify other family members (5, 37).
Suggestions for future inquiries.
i) The acceptability of universal screening by children/adolescents and parents and community perceptions. ii) The efficacy and cost-effectiveness of a phenotypic approach to universal screening of children for FH. iii) The feasibility and acceptability of alternative approaches to universal screening (e.g., university entry).
Genotypic strategy.
Current knowledge and practice.
The feasibility and efficacy of child-parent screening for FH has been demonstrated in a prospective study of children aged 1–2 years, who had a total cholesterol level measured at the time of an immunization, with subsequent testing for mutations. Using a cholesterol cut-off of the 95th percentile plus a FH mutation or a cut-off of the 99th percentile without a FH mutation for every 1,000 children screened, eight individuals (four children and four parents) were identified as having FH, of whom 80% had a FH mutation detected (5).
Futema et al. (36) evaluated this child-parent screening strategy in the Avon Longitudinal Study of Parents and Children. Using a two-stage model that included biochemical screening (total cholesterol) followed by next generation sequencing (NGS) of FH genes for all screen-positive samples, a total cholesterol cut-off of the 99th percentile resulted in a similar FH detection rate of 83% and a false-positive rate of 0.8%. The authors propose that including a NGS step for all screen-positive samples would eliminate false positive cases and improve the screening strategy for children with FH.
Slovenia is the only country that has successfully implemented a universal screening program for FH to date (41). Children are screened at 5 years of age and those with fasting total cholesterol >6 mmol/l without a family history of premature CV complications or total cholesterol >5 mmol/l with a positive family history have a repeat lipid profile and targeted NGS. A pathogenic FH mutation can be detected in 57% of participants with a positive biochemical screen.
Elevated cholesterol may be due not only to monogenic gene variants but also to polygenic gene variants, which may explain why mutations are often not detected in patients with phenotypic FH (42). Conversely, some patients with a pathogenic FH gene variant do not have hypercholesterolemia (5), but may still be at increased risk of CAD (6).
A recent Australian study demonstrated that universal screening of children for FH is likely to be acceptable to the general public and general practitioners (GPs) (43).
Suggestions for future inquiries.
i) The cost-effectiveness of an integrated universal screening approach to FH in children coupled with reverse cascade screening of close relatives. ii) The contribution that polygenic gene variants make to the apparent phenotypic diagnosis of FH. iii) The role of protective modifier genes in masking the effect of pathogenic variants. iv) The natural history of children with pathogenic FH gene variants without elevated LDL cholesterol. v) The natural history of children with high LDL cholesterol levels without FH.
Selective screening
Cascade testing (systematic).
Current knowledge and practice.
The codominant inheritance of FH supports cascade testing, where individuals considered at increased risk of FH owing to a family member having the condition are invited for testing; each first-degree relative of an index case has a 50% chance of having FH. Cascade testing can be performed by either genetic testing, if a family mutation has been established, or by LDL cholesterol levels (44). An average of two and up to eight new cases with FH may be identified for each newly diagnosed index case (44–46). Cascade testing is efficacious and cost-effective (45, 47, 48), particularly in adults (49, 50). Children with FH are mostly diagnosed by forward cascade testing following a diagnosis of a parent. In reverse cascade testing, parents are screened following diagnosis of a child.
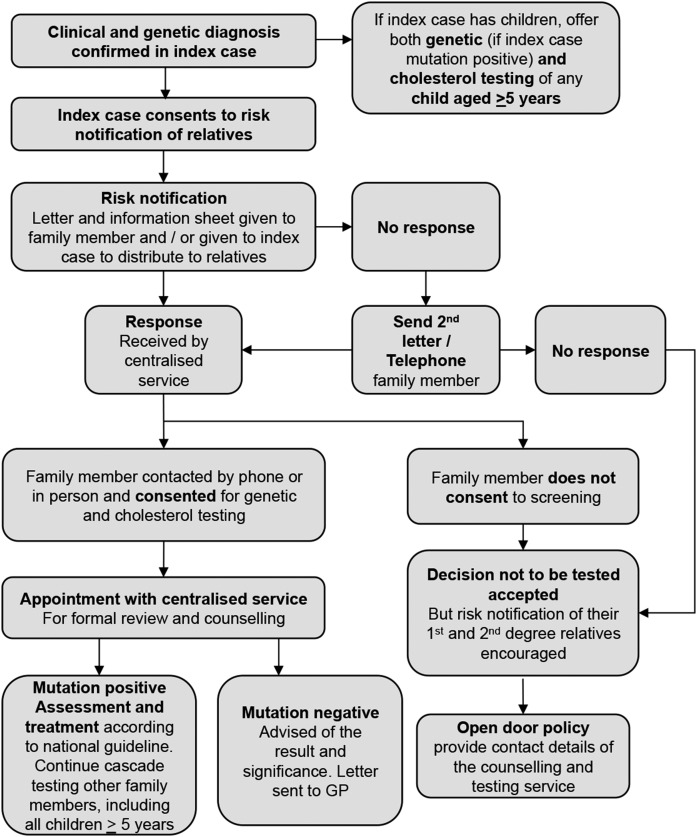
The cascade screening program in The Netherlands has been the most successful worldwide, with up to 70% of all individuals with FH identified by 2014 (27, 46). This may be difficult to replicate in other countries. In Australia, a centralized cascade-screening program was established in 2007 (Fig. 2), but after almost 10 years, only 13% of adults and 4% of children <16 years of age were detected (J. Pang, personal communication). While many economic analyses report it to be cost-effective (45, 47, 48), in practice and with the exception of the Netherlands, cascade screening has been relatively ineffective in identifying the majority of individuals with FH in the community (51). This is predominantly owing to the failure to detect an adequate number of index cases in the community (35).
Fig. 2.
Protocol for genetic cascade screening in Western Australia. Adapted from Bell and Watts (123).
Suggestions for future inquiries.
i) The effectiveness of cascade testing in the community via primary care. ii) The integration of centralized and primary care screening strategies.
Opportunistic.
Current knowledge and practice.
Selective screening of children for hypercholesterolemia was first recommended in 1992, based on a family history of premature atherosclerotic CV disease or hypercholesterolemia (52). Unfortunately, family history failed to identify the majority of children with FH (39, 53, 54), which led to the recommendation of a universal lipid screening program for children aged 9–11 years and young people aged 17–21 years (55).
Laboratory reporting systems have been developed to highlight individuals with cholesterol results suggestive of FH. The addition of interpretive comments and a phone call to the referring GP can increase the likelihood of a diagnosis of FH (56, 57). Electronic medical records and utilization of a computerized algorithm may be useful in detecting and diagnosing FH in primary care (58, 59).
Suggestions for future inquiries.
i) The role of allied health groups in identifying patients with FH, e.g., pharmacists, school nurses, and coronary rehabilitation nurses. ii) The effectiveness of screening strategies for children in primary care settings.
DIAGNOSIS
Current knowledge and practice.
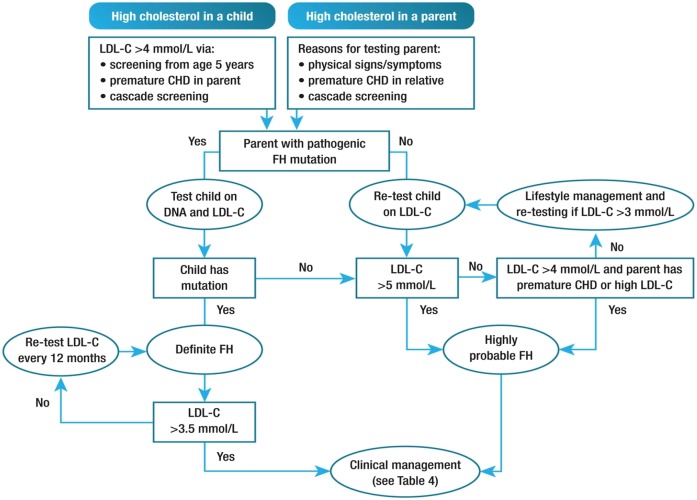
The Dutch Lipid Clinic Network criteria are not valid in children, but Simon Broome criteria have specific LDL cholesterol cut-offs for this age group. Any child with a LDL cholesterol level ≥5 mmol/l on two successive occasions has a high probability of FH (25). An LDL cholesterol level ≥4 mmol/l in a child with a family history of premature CHD in a close relative and/or baseline high cholesterol in one parent indicates a high probability of FH (8, 25). If the parent has a genetic diagnosis of FH, an LDL cholesterol ≥3.5 mmol/l in the child suggests FH (60, 61).
The diagnosis of FH in children usually follows cascade testing. Children over 5 years of age should be offered testing when a parent (or close relative in absence of parent) is identified with FH (25, 30). As a first screening test, a nonfasting lipid profile is sufficient (62), but LDL cholesterol levels should be measured at least twice over 3 months in a fasting state to confirm the diagnosis and secondary causes of hypercholesterolemia must be excluded (31, 63). Detection of a pathogenic mutation in a child is the gold standard for the diagnosis of FH (Fig. 3) (25). Sitosterolemia, a very rare disorder, may mimic FH in childhood.
Fig. 3.
Potential strategy for diagnosis of FH in children and adolescents. Premature CHD is defined as a coronary event before age 55 years in men and age 60 years in women. Definite FH is defined as genetic confirmation of at least one FH-causing genetic mutation. Close relative is defined as first or second degree. Highly probable FH is based on clinical presentation (i.e., phenotypic FH), either an elevated LDL cholesterol (LDL-C) level ≥5 mmol/l in a child after dietary intervention or an LDL cholesterol level ≥4 mmol/l in a child with a family history of premature CHD in close relatives and/or baseline high cholesterol in one parent. Cascade screening from an index case with a FH-causing mutation may identify a child with elevated LDL cholesterol levels ≥3.5 mmol/l. Reproduced from Wiegman et al. (25).
The diagnosis of homozygous FH (hoFH) can be made genetically, by identifying two pathogenic FH mutations, or phenotypically, in those with an untreated LDL cholesterol of >13 mmol/l together with the presence of cutaneous or tendon xanthomas before age 10 or elevated LDL cholesterol levels in both parents consistent with a diagnosis of heterozygous FH (64). There can, however, be a significant variation in LDL cholesterol levels in patients with hoFH, and recent reports have highlighted the variability in the expression of the clinical phenotype related to the presence of other modifier genes, gene-gene and gene-environment interactions, and epigenetic influences (4, 64, 65).
Suggestions for future inquiries.
The definition of the diagnostic criteria for FH in children in diverse populations, including country-, gender-, and age-specific cholesterol thresholds.
RISK STRATIFICATION
Current knowledge and practice.
The risk of early CAD in individuals with FH is associated with the lifelong exposure to elevated LDL cholesterol levels. In addition, the presence of a FH mutation triples this risk at any LDL cholesterol level (6, 66). However, while individuals with FH who are untreated have a 20-fold increased risk of premature CHD, such an outcome is not inevitable (29). A variety of predictor variables have been proposed, including a combination of clinical and biochemical markers (67), genetic risk scoring systems (68), markers of inflammation (15), and different forms of imaging, such as endothelial function (69), CIMT (70), and coronary artery calcium scores (71). To date, none of these predictor variables has proven to be sufficiently robust to allow widespread use in clinical practice. Intuitively, traditional CV risk factors and a history of premature CHD in a parent increase the risk in the child and, thus, form part of the routine risk assessment for children with FH.
Suggestions for future inquiries.
i) The optimal form of CV imaging for children with FH. A simple to use, accurate, and noninvasive form of coronary artery imaging that indicates early atherosclerosis is needed. ii) The development of a life-time risk prediction model in children. iii) The biomarkers of CV risk.
MANAGEMENT
Current knowledge and practice.
Early treatment of FH with statins from childhood reduces the lifetime burden of LDL cholesterol and the rate of development of atherosclerosis (17, 22, 23, 25).
However, as a long-term prospective randomized controlled study comparing treatment of children with LDL cholesterol-lowering therapy or placebo is unlikely to ever be conducted, MR studies or “natures randomized controlled trials” can help fill evidence gaps (26). MR studies have demonstrated that several single nucleotide polymorphisms associated with lower LDL cholesterol levels are associated with a lower risk of CHD (72–74). However, while meta-analyses of statin trials have shown a 23% reduction in the risk of CHD for every millimole per liter lowering of LDL cholesterol (75, 76), meta-analyses of MR studies reveal a corresponding 50% reduction in the risk of CHD (72). This 2-fold difference in relative risk can be explained by the fact that the LDL cholesterol-lowering effect in MR studies commences from the time of conception, rather than when a statin is prescribed in later adult life. MR studies also suggest that the benefit of lowering LDL cholesterol levels is independent of the mechanism of action, with a similar magnitude of effect in genes coding for targets of statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies (mAbs) (26). MR studies offer a rapid and powerful approach to answering clinically relevant questions, especially in children and young people, but are limited by the fact that they fail to recognize adverse events related to medications.
Suggestions for future inquiries.
i) The individual variation in the natural history of atherosclerosis to inform the timing and intensity of interventions to lower LDL cholesterol. ii) The risk/benefit of lifelong FH care for children, including assessment of age-appropriate treatment goals and long-term physical and psychological side effects of treatment.
Diet and lifestyle measures.
Current knowledge and practice.
Adult FH registry data demonstrate that many FH patients are overweight or obese, smoke, and have type 2 diabetes, all preventable causes of CAD. Thus, because it is easier to learn good habits than to break bad habits, all children with FH should receive advice on lifestyle modifications, incorporating a healthy diet low in saturated and trans-fat (77), regular exercise, and avoidance of cigarette smoking, including environmental tobacco smoke exposure (25, 78). While lifestyle interventions alone are rarely sufficient to lower LDL cholesterol levels to recommended targets, establishing these in childhood reduces the likelihood of developing additional CV risk factors, meaning they should be promoted for all children with FH (31, 55).
Suggestions for future inquiries.
i) The effective methods of primordial prevention of non-cholesterol CV risk factors. ii) The effectiveness of different heart-healthy and culturally specific diets in childhood FH, including the role of specific nutrients and nutraceuticals.
Nutraceutical supplements.
Current knowledge and practice.
Plant sterols and stanols are naturally occurring compounds that compete with and inhibit the absorption of cholesterol in the small intestine. A daily dose of 1.5–3 g in children with FH is safe, palatable, and reduces LDL cholesterol levels by 9–19% (79, 80), but does not restore endothelial function (81). Trials in adults have failed to demonstrate improvements in CV events, making it difficult to routinely recommend these supplements in children.
Many other supplements have been trialed in children with FH, including psyllium husk (82), fish oil (83), rapeseed oil (84), soy protein (85), policosanols (86), and red-yeast rice extract (86), but none can be recommended at this time (87). Berberine (88) and Armolipid Plus (89) have been trialed in adults only.
Suggestions for future inquiries.
The efficacy, acceptability, and safety of nutraceuticals in children with FH, used alone or in combination with a low-dose low-frequency statin.
Pharmacotherapy.
Current knowledge and practice.
Pharmacotherapy is almost always required and statins are the most commonly used as a first line agent (25). Statins lower LDL cholesterol levels by up to 50% in children with FH, with most of the effect occurring at lower doses. Each subsequent doubling of dose achieves a further 6–7% reduction in LDL cholesterol levels (90).
Most guidelines recommend commencing treatment in boys and girls with FH from 8 years of age, although the decision to treat must be made in partnership with the child and family and be guided by the family history of CV events and the level of LDL cholesterol (25, 31). Treatment should be initiated at the lowest dose using the least potent statin and titrated up every 6–8 weeks, depending on the LDL cholesterol response. Guidelines suggest a target LDL cholesterol level of 3.5 mmol/l or a 50% reduction in LDL cholesterol from pretreatment levels (7, 25, 31). Following commencement of a statin or an increase in dose, a lipid profile and liver function tests should be repeated after 6–8 weeks, which equates to the maximal LDL cholesterol-lowering effect. Once LDL cholesterol targets have been achieved, a lipid profile and liver function tests should be repeated every 6–12 months throughout childhood, to confirm adherence and monitor for side effects of medication (31).
While there remains an ongoing debate regarding the magnitude of statin side effects in adults, particularly muscle-related complaints (91), side effects are rare in children and, if they do occur, are usually in the early stages of treatment and resolve spontaneously. There are limited long-term data for children treated with statins, but a recent study has provided reassuring results (23). In a 10 year follow-up trial of statins started in childhood, compliance was high and only 1.5% stopped statin therapy. There were no serious adverse events, such as hepatitis or rhabdomyolysis (23, 92). While further long-term studies are required, children and families should be reassured that current evidence suggests that statins have an excellent safety profile in children (25).
For children who fail to achieve LDL cholesterol targets, the addition of ezetimibe or a bile acid sequestrant may be considered (25, 31). Ezetimibe is a selective cholesterol absorption inhibitor that reduces LDL cholesterol levels by up to 20% when used as monotherapy or in combination with a statin (93, 94). Ezetimibe is approved for use from the age of 10 years in the USA and Europe and appears to be safe and well-tolerated. Bile acid sequestrants reduce LDL cholesterol levels by up to 10%, but their use is frequently limited by gastrointestinal side effects. The best-tolerated agent is colesevelam, which is approved from the age of 10 years in the USA, but not in Europe (95).
mAbs to PCSK9 (alirocumab and evolocumab) lower LDL cholesterol and are being used increasingly in adults with and without FH to achieve very low LDL cholesterol targets. It has been proposed that treatment of children with FH could potentially be delayed until adulthood, at which time a more aggressive cholesterol lowering approach could be initiated with PCSK9 mAbs (96, 97). Formal prospective studies of PCSK9 mAbs in children with FH are currently underway (ClinicalTrials.gov: NCT02392559, NCT02890992, and NCT02624869).
Before a statin is commenced in any female of childbearing age, specific advice must be given regarding contraceptive choices and this should be discussed at each outpatient review (31). All women planning pregnancy should be advised to discontinue statins 3 months before conception. However, while a range of congenital abnormalities have been reported in infants following in utero exposure (98), for women who do fall pregnant while taking a statin, they can be reassured that the likelihood of complications is small (99, 100). For pregnant women who need to continue cholesterol-lowering therapy, bile acid sequestrants are the only safe oral agent. In women with severe CAD or hoFH, LDL-apheresis (LDL-A) can be safely continued during pregnancy (98).
Suggestions for future inquiries.
i) The thresholds for commencing treatment in children with FH and a mutation, compared with those who have no mutation identified. ii) The long-term safety of statins and other cholesterol-lowering drug therapies in children. iii) Data on statin intolerance in children. iv) The role of new pharmacological agents (e.g., bempedoic acid, PCSK9 mAbs) in managing childhood FH. v) The impact of withholding statins and ezetimibe during pregnancy and lactation on CV outcomes of children born with FH. vi) Pregnancy outcomes, both CHD in mothers and CAD risk in offspring.
hoFH.
Current knowledge and practice.
All children with hoFH should be cared for in a specialist pediatric center (64). Following diagnosis, treatment should be commenced with a statin and ezetimibe without delay. This combination therapy usually results in a 30–40% reduction in LDL cholesterol levels. Additional treatment with LDL-A should be commenced as soon as possible, ideally by age 5 years (64). Maintaining good quality of life is important during apheresis and can be improved by reducing the frequency of the procedure with the use of new biologics (101). Injectable mipomersen, an antisense RNA therapy, is approved in the USA from 12 years of age and oral lomitapide, a microsomal triglyceride transfer protein inhibitor, from 18 years. Both agents target the hepatic production of apo-B-containing lipoproteins (102). Both agents have been reported to cause fatty liver disease in adults. (25) PCSK9 mAbs can lower the frequency of apheresis in adults (103, 104).
Liver transplantation, alone or in combination with heart transplantation, is an option that cures the patient of their molecular defect, but replaces one serious disease with the need to take life-long immunosuppressant medication and the associated risks.
Suggestions for future inquiries.
i) The role of PCSK9 mAbs in decreasing the requirement for or frequency of LDL-A in children. ii) Trialling improved methods for administering LDL-A in children. iii) International registry data on outcomes of liver transplantation in hoFH. iv) The outcome of trials of gene therapy (AAV-8 LDL-R gene transfer therapy) for hoFH. v) The treatment of hoFH in remote regions and low-income countries (105). vi) Psychological studies and support for families of children with hoFH.
Patient perspectives.
Current knowledge and practice.
Every individual has a self-perception on risk and preferences on care for FH. The family history of a coronary event impacts greatly on patients’ perceptions and awareness (106). Providing crisp and comprehensible information to children and families about FH and the risk of CV events is essential (107). Adolescence is a particularly challenging period when parents are making important decisions on their offspring’s behalf. However, adolescents must be given the opportunity to see their treating physician alone, without their parent, for at least a portion of each clinic visit, allowing the doctor an opportunity to learn about the priorities of the young person and to address sensitive issues such as sexual health/contraception and smoking (108). Patients with FH need to engage in a life-long treatment and unless they genuinely understand and believe in the benefits, medium- to long-term adherence is unlikely (109). FH support groups led by other FH-affected individuals, including those of the same generation should be encouraged and are a powerful way to encourage patient engagement by providing meaningful education and support for individuals and families.
Suggestions for future inquiries.
i) Approaches for involving children and adolescents in their care and shared decision making. ii) The most effective tools for education and support: social media (Instagram, Facebook, Twitter). iii) Quality of life studies to provide a better understanding of the child’s or adolescent’s perception of living with FH.
INTEGRATION OF CARE AND SERVICES
Current knowledge and practice.
Optimal care of children and adolescents with FH requires a multidisciplinary framework integrated across primary care, pediatric specialist, and adult services (31). All children should be seen in a pediatric clinic at least yearly. Alternatively, a pediatrician should join the adult service to jointly review children (31). Family clinics may also be effective and convenient for patients. Access to a pediatric dietician, nurse specialist, genetics counselor, social worker, and clinical psychologist is essential. Shared care with the family GP is also recommended. Pathways for transition of adolescents from pediatric to adult services are essential (110).
Suggestions for future inquiries.
i) The effectiveness of family clinics compared with traditional clinics. This will improve services for children and families. ii) The specific role of allied health personnel in the care of pediatric FH. This may improve awareness and adherence with care and self-management later in adolescence. iii) The optimal strategies for integrating primary and specialist services. This may allow greater continuity of care of patients with FH. iv) The optimal pathways for transitional care. This is important to ensure continuity of care for adolescents with FH.
PATIENT SUPPORT GROUPS AND NETWORKS
Current knowledge and practice.
Patient support groups and networks have a critical role in improving the care of children with FH. Empowering patients raises the awareness of FH in the community and improves collaboration between patient groups and the medical/scientific world (111). Patients and families are central for developing research programs (8). Patient advocacy groups can effectively lobby for improved care, including better access to and reimbursement for essential medications.
Suggestions for future inquiries.
i) The optimal strategies for recruiting and running family support groups and networks, including the role of social media. ii) Exploring strategies for promoting effective advocacy.
REGISTRIES AND CODIFICATION
Current knowledge and practice.
Registries facilitate research and education and lead to better health outcomes for patients (112). Several FH registries have been established or are in development (111, 113–120). The longest existing pediatric registry started in the The Netherlands in 1989 and has provided much of the information on which current knowledge is based (60, 121). A pediatric FH register was also established in the United Kingdom in 2012 (122). As with registries, codification of FH is essential for health service research and funding. An ICD-10 code for FH was granted in the US in 2016.
Suggestions for future inquiries.
i) The definition and harmonization of data elements for international registries. ii) The use of pediatric registries in defining the long-term natural history of FH. iii) The development of a global network of inter-operable registries of children with FH. iv) The value of linking pregnancy registries with pediatric registries. v) Obtaining standardized codification of FH worldwide.
RESEARCH AND AUDIT
In this work, we have identified many unknowns in pediatric FH. These are summarized in Table 1, a research agenda for the future.
TABLE 1.
Some suggestions for future needs in pediatric FH
| Basic Science | Population Science | Life Course | Clinical Research | Patient-Centric Research | Model of Care |
| Interaction of main FH gene effects and modifier genes. | Risk-benefit and cost effectiveness of universal screening of children with reverse cascade testing, compared with cascade (forward) screening methods. | Natural history of atherosclerosis in relation to timing and intensity of intervention to lower LDL cholesterol. | Trials of new pharmacological agents and nutraceuticals. | Assessment of community perceptions of FH screening strategies. | Optimal processes and pathways for organization of screening and follow-up. |
| Create a FH genetic biobank with long-term goal of linkage of registry to biobank. | Use of registries to understand natural history and impact of treatments. | Risk-benefit of lifelong FH care, including assessment of treatment goals and long term side effects of treatment. | Role of imaging for assessing of atherosclerosis. | Assessment of patient and family perceptions of different aspects of care. | Optimal pathways for transitional care. |
| Influence of genotype on response to cholesterol-lowering medications. | Country-specific LDL cholesterol and apoB cut-off levels to diagnose FH. | Better understanding of the impact of pregnancy on natural history of FH in mothers and offspring. | Long-term safety of statins and other cholesterol lowering therapies. | Role of decision aids (i.e., tools to help children and adolescents think through screening and treatment decisions). | Integration of centralized FH services with primary care. |
| Impact of maternal hypercholesterolemia on programming arterial biology of fetus. | Long-term outcomes of children with pathogenic gene variants with normal LDL cholesterol. | Outcomes of liver transplantation on children with severe hoFH. | Effectiveness of culturally diverse heart-healthy diets | Most effective tools for education and support (e.g., social media). | Effectiveness of family based FH clinics compared with separate adult and pediatric clinics. |
| Discovery of new monogenic causes of FH phenotype. | Biomarkers of arterial health in children. | Psychological impact of diagnosis of FH. | Role of allied health personnel (e.g., pharmacist, nurse) and peer mentors with FH. | ||
| Methods for enhancing shared decision making with families. | Enhancing literacy and understanding of FH. | Collaboration with patient support groups and networks. |
For more suggestions and context see text. Adapted from Gidding et al. (8).
CONCLUSIONS
Children treated for FH can avoid premature atherosclerosis and should have a normal life expectancy. However, despite progress in care, several evidence gaps need to be filled. This can improve existing models of care for children with FH, which need to be pragmatic and context specific. Aiming for optimal models of care will ultimately change the natural history of this common and life-threatening condition.
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- CIMT
- carotid intima media thickness
- CV
- cardiovascular
- FH
- familial hypercholesterolemia
- GP
- general practitioner
- hoFH
- homozygous familial hypercholesterolemia
- LDL-A
- LDL-apheresis
- mAb
- monoclonal antibody
- MR
- Mendelian randomization
- NGS
- next generation sequencing
- PCSK9
- proprotein convertase subtilisin/kexin type 9
G.F.W. has received honoraria for advisory boards and lectures from Amgen, Sanofi, Regeneron, Kowa, and Merck Sharp & Dohme. S.S.G. is a consultant for Regnxbio. All other authors declare no potential conflicts of interest.
REFERENCES
- 1.Pang J., Martin A. C., Mori T. A., Beilin L. J., and Watts G. F.. 2016. Prevalence of familial hypercholesterolemia in adolescents: potential value of universal screening? J. Pediatr. 170: 315–316. [DOI] [PubMed] [Google Scholar]
- 2.Benn M., Watts G. F., Tybjaerg-Hansen A., and Nordestgaard B. G.. 2012. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J. Clin. Endocrinol. Metab. 97: 3956–3964. [DOI] [PubMed] [Google Scholar]
- 3.Watts G. F., Shaw J. E., Pang J., Magliano D. J., Jennings G. L., and Carrington M. J.. 2015. Prevalence and treatment of familial hypercholesterolaemia in Australian communities. Int. J. Cardiol. 185: 69–71. [DOI] [PubMed] [Google Scholar]
- 4.Sjouke B., Kusters D. M., Kindt I., Besseling J., Defesche J. C., Sijbrands E. J., Roeters van Lennep J. E., Stalenhoef A. F., Wiegman A., de Graaf J., et al. 2015. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur. Heart J. 36: 560–565. [DOI] [PubMed] [Google Scholar]
- 5.Wald D. S., Bestwick J. P., Morris J. K., Whyte K., Jenkins L., and Wald N. J.. 2016. Child-parent familial hypercholesterolemia screening in primary care. N. Engl. J. Med. 375: 1628–1637. [DOI] [PubMed] [Google Scholar]
- 6.Khera A. V., Won H. H., Peloso G. M., Lawson K. S., Bartz T. M., Deng X., van Leeuwen E. M., Natarajan P., Emdin C. A., Bick A. G., et al. 2016. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J. Am. Coll. Cardiol. 67: 2578–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watts G. F., Gidding S., Wierzbicki A. S., Toth P. P., Alonso R., Brown W. V., Bruckert E., Defesche J., Lin K. K., Livingston M., et al. 2015. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Eur. J. Prev. Cardiol. 22: 849–854. [DOI] [PubMed] [Google Scholar]
- 8.Gidding S. S., Champagne M. A., de Ferranti S. D., Defesche J., Ito M. K., Knowles J. W., McCrindle B., Raal F., Rader D., Santos R. D., et al. 2015. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 132: 2167–2192. [DOI] [PubMed] [Google Scholar]
- 9.Slack J. 1969. Risks of ischaemic heart-disease in familial hyperlipoproteinaemic states. Lancet. 2: 1380–1382. [DOI] [PubMed] [Google Scholar]
- 10.Stone N. J., Levy R. I., Fredrickson D. S., and Verter J.. 1974. Coronary artery disease in 116 kindred with familial type II hyperlipoproteinemia. Circulation. 49: 476–488. [DOI] [PubMed] [Google Scholar]
- 11.Alkemade F. E., Gittenberger-de Groot A. C., Schiel A. E., VanMunsteren J. C., Hogers B., van Vliet L. S., Poelmann R. E., Havekes L. M., Willems van Dijk K., and DeRuiter M. C.. 2007. Intrauterine exposure to maternal atherosclerotic risk factors increases the susceptibility to atherosclerosis in adult life. Arterioscler. Thromb. Vasc. Biol. 27: 2228–2235. [DOI] [PubMed] [Google Scholar]
- 12.Napoli C., Glass C. K., Witztum J. L., Deutsch R., D’Armiento F. P., and Palinski W.. 1999. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: fate of early lesions in children (FELIC) study. Lancet. 354: 1234–1241. [DOI] [PubMed] [Google Scholar]
- 13.McGill H. C. Jr., and McMahan C. A.. 1998. Determinants of atherosclerosis in the young. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) research group. Am. J. Cardiol. 82: 30T–36T. [DOI] [PubMed] [Google Scholar]
- 14.Newman W. P. III, Freedman D. S., Voors A. W., Gard P. D., Srinivasan S. R., Cresanta J. L., Williamson G. D., Webber L. S., and Berenson G. S.. 1986. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N. Engl. J. Med. 314: 138–144. [DOI] [PubMed] [Google Scholar]
- 15.Narverud I., Retterstol K., Iversen P. O., Halvorsen B., Ueland T., Ulven S. M., Ose L., Aukrust P., Veierod M. B., and Holven K. B.. 2014. Markers of atherosclerotic development in children with familial hypercholesterolemia: a literature review. Atherosclerosis. 235: 299–309. [DOI] [PubMed] [Google Scholar]
- 16.Ueland T., Vissers M. N., Wiegman A., Rodenburg J., Hutten B., Gullestad L., Ose L., Rifai N., Ridker P. M., Kastelein J. J., et al. 2006. Increased inflammatory markers in children with familial hypercholesterolaemia. Eur. J. Clin. Invest. 36: 147–152. [DOI] [PubMed] [Google Scholar]
- 17.de Jongh S., Lilien M. R., Bakker H. D., Hutten B. A., Kastelein J. J., and Stroes E. S.. 2002. Family history of cardiovascular events and endothelial dysfunction in children with familial hypercholesterolemia. Atherosclerosis. 163: 193–197. [DOI] [PubMed] [Google Scholar]
- 18.Vlahos A. P., Naka K. K., Bechlioulis A., Theoharis P., Vakalis K., Moutzouri E., Miltiadous G., Michalis L. K., Siamopoulou-Mavridou A., Elisaf M., et al. 2014. Endothelial dysfunction, but not structural atherosclerosis, is evident early in children with heterozygous familial hypercholesterolemia. Pediatr. Cardiol. 35: 63–70. [DOI] [PubMed] [Google Scholar]
- 19.Riggio S., Mandraffino G., Sardo M. A., Iudicello R., Camarda N., Imbalzano E., Alibrandi A., Saitta C., Carerj S., Arrigo T., et al. 2010. Pulse wave velocity and augmentation index, but not intima-media thickness, are early indicators of vascular damage in hypercholesterolemic children. Eur. J. Clin. Invest. 40: 250–257. [DOI] [PubMed] [Google Scholar]
- 20.Wiegman A., de Groot E., Hutten B. A., Rodenburg J., Gort J., Bakker H. D., Sijbrands E. J., and Kastelein J. J.. 2004. Arterial intima-media thickness in children heterozygous for familial hypercholesterolaemia. Lancet. 363: 369–370. [DOI] [PubMed] [Google Scholar]
- 21.Kusters D. M., Wiegman A., Kastelein J. J., and Hutten B. A.. 2014. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ. Res. 114: 307–310. [DOI] [PubMed] [Google Scholar]
- 22.Wiegman A., Hutten B. A., de Groot E., Rodenburg J., Bakker H. D., Buller H. R., Sijbrands E. J., and Kastelein J. J.. 2004. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 292: 331–337. [DOI] [PubMed] [Google Scholar]
- 23.Kusters D. M., Avis H. J., de Groot E., Wijburg F. A., Kastelein J. J., Wiegman A., and Hutten B. A.. 2014. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA. 312: 1055–1057. [DOI] [PubMed] [Google Scholar]
- 24.Braamskamp M. J. A. M., Langslet G., McCrindle B. W., Cassiman D. M., Francis G. A., Gagne C., Gaudet D., Morrison K. M., Wiegman A., Turner T., et al. Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: The CHARON study. Circulation. Epub ahead of print. June 7, 2017; doi:10.1161/CIRCULATIONAHA.116.025158. [DOI] [PubMed] [Google Scholar]
- 25.Wiegman A., Gidding S. S., Watts G. F., Chapman M. J., Ginsberg H. N., Cuchel M., Ose L., Averna M., Boileau C., Boren J., et al. 2015. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur. Heart J. 36: 2425–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ference B. A. 2015. Mendelian randomization studies: using naturally randomized genetic data to fill evidence gaps. Curr. Opin. Lipidol. 26: 566–571. [DOI] [PubMed] [Google Scholar]
- 27.Nordestgaard B. G., Chapman M. J., Humphries S. E., Ginsberg H. N., Masana L., Descamps O. S., Wiklund O., Hegele R. A., Raal F. J., Defesche J. C., et al. 2013. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34: 3478–3490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts G. F., Gidding S., Wierzbicki A. S., Toth P. P., Alonso R., Brown W. V., Bruckert E., Defesche J., Lin K. K., Livingston M., et al. 2014. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. J. Clin. Lipidol. 8: 148–172. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg A. C., Hopkins P. N., Toth P. P., Ballantyne C. M., Rader D. J., Robinson J. G., Daniels S. R., Gidding S. S., de Ferranti S. D., Ito M. K., et al. 2011. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 5: 133–140. [DOI] [PubMed] [Google Scholar]
- 30.Watts G. F., Sullivan D. R., Poplawski N., van Bockxmeer F., Hamilton-Craig I., Clifton P. M., O’Brien R., Bishop W., George P., Barter P. J., et al. 2011. Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler. Suppl. 12: 221–263. [DOI] [PubMed] [Google Scholar]
- 31.Martin A. C., Coakley J., Forbes D. A., Sullivan D. R., and Watts G. F.. 2013. Familial hypercholesterolaemia in children and adolescents: a new paediatric model of care. J. Paediatr. Child Health. 49: E263–E272. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Clinical Excellence and the National Collaborating Centre for Primary Care. NICE clinical guideline 71. Identification and management of familial hypercholesterolaemia. Accessed June 25 2017, at www.nice.org.uk/nicemedia/pdf/CG071NICEGuideline.pdf. [Google Scholar]
- 33.Descamps O. S., Tenoutasse S., Stephenne X., Gies I., Beauloye V., Lebrethon M. C., De Beaufort C., De Waele K., Scheen A., Rietzschel E., et al. 2011. Management of familial hypercholesterolemia in children and young adults: consensus paper developed by a panel of lipidologists, cardiologists, paediatricians, nutritionists, gastroenterologists, general practitioners and a patient organization. Atherosclerosis. 218: 272–280. [DOI] [PubMed] [Google Scholar]
- 34.Wilson J. M. G., and Jungner G.. 1968. Principles and Practice of Screening for Disease. World Health Organization, Geneva. [Google Scholar]
- 35.Morris J. K., Wald D. S., and Wald N. J.. 2012. The evaluation of cascade testing for familial hypercholesterolemia. Am. J. Med. Genet. A. 158A: 78–84. [DOI] [PubMed] [Google Scholar]
- 36.Futema M., Cooper J. A., Charakida M., Boustred C., Sattar N., Deanfield J., Lawlor D. A., Timpson N. J., Humphries S. E., and Hingorani A. D.. 2017. Screening for familial hypercholesterolaemia in childhood: Avon Longitudinal Study of Parents and Children (ALSPAC). Atherosclerosis. 260: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wald D. S., Bestwick J. P., and Wald N. J.. 2007. Child-parent screening for familial hypercholesterolaemia: screening strategy based on a meta-analysis. BMJ. 335: 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wald D. S., Kasturiratne A., Godoy A., Ma L., Bestwick J. P., Brewer N., and Wald N. J.. 2011. Child-parent screening for familial hypercholesterolemia. J. Pediatr. 159: 865–867. [DOI] [PubMed] [Google Scholar]
- 39.Dixon D. B., Kornblum A. P., Steffen L. M., Zhou X., and Steinberger J.. 2014. Implementation of lipid screening guidelines in children by primary pediatric providers. J. Pediatr. 164: 572–576. [DOI] [PubMed] [Google Scholar]
- 40.de Ferranti S. D., Rodday A. M., Parsons S. K., Cull W. L., O’Connor K. G., Daniels S. R., and Leslie L. K.. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J. Pediatr. Epub ahead of print. February 13, 2017; doi:10.1016/j.jpeds.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 41.Klančar G., Groselj U., Kovac J., Bratanic N., Bratina N., Trebusak Podkrajsek K., and Battelino T.. 2015. Universal screening for familial hypercholesterolemia in children. J. Am. Coll. Cardiol. 66: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 42.Talmud P. J., Shah S., Whittall R., Futema M., Howard P., Cooper J. A., Harrison S. C., Li K., Drenos F., Karpe F., et al. 2013. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 381: 1293–1301. [DOI] [PubMed] [Google Scholar]
- 43.Lister K., Molster C., Martin A. C., Bowman F., Buaskis A., Cho A., Vickery A., Garton-Smith J., Kirke A., and Watts G. F.. 2017. Screening for familial hypercholestrolaemia: engaging the public in policy direction (Abstract in 15th World Congress on Public Health. Melbourne, Australia, April 3–7, 2017). [Google Scholar]
- 44.Bell D. A., Pang J., Burrows S., Bates T. R., van Bockxmeer F. M., Hooper A. J., O’Leary P., Burnett J. R., and Watts G. F.. 2015. Effectiveness of genetic cascade screening for familial hypercholesterolaemia using a centrally co-ordinated clinical service: an Australian experience. Atherosclerosis. 239: 93–100. [DOI] [PubMed] [Google Scholar]
- 45.Wonderling D., Umans-Eckenhausen M. A., Marks D., Defesche J. C., Kastelein J. J., and Thorogood M.. 2004. Cost-effectiveness analysis of the genetic screening program for familial hypercholesterolemia in The Netherlands. Semin. Vasc. Med. 4: 97–104. [DOI] [PubMed] [Google Scholar]
- 46.Umans-Eckenhausen M. A., Defesche J. C., Sijbrands E. J., Scheerder R. L., and Kastelein J. J.. 2001. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet. 357: 165–168. [DOI] [PubMed] [Google Scholar]
- 47.Marks D., Wonderling D., Thorogood M., Lambert H., Humphries S. E., and Neil H. A.. 2002. Cost effectiveness analysis of different approaches of screening for familial hypercholesterolaemia. BMJ. 324: 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ademi Z., Watts G. F., Pang J., Sijbrands E. J., van Bockxmeer F. M., O’Leary P., Geelhoed E., and Liew D.. 2014. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolemia. J. Clin. Lipidol. 8: 390–400. [DOI] [PubMed] [Google Scholar]
- 49.Ademi Z., Watts G. F., Juniper A., and Liew D.. 2013. A systematic review of economic evaluations of the detection and treatment of familial hypercholesterolemia. Int. J. Cardiol. 167: 2391–2396. [DOI] [PubMed] [Google Scholar]
- 50.Norman R., Watts G. F., Weintraub W., and Gidding S. S.. 2016. Challenges in the health economics of familial hypercholesterolemia. Curr. Opin. Lipidol. 27: 563–569. [DOI] [PubMed] [Google Scholar]
- 51.Martin A. C., Bell D. A., Brett T., and Watts G. F.. 2017. Beyond cascade screening: detection of familial hypercholesterolaemia at childhood immunization and other strategies. Curr. Opin. Lipidol. [DOI] [PubMed] [Google Scholar]
- 52.1992. National cholesterol education program (NCEP): highlights of the report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 89: 495–501. [PubMed] [Google Scholar]
- 53.Griffin T. C., Christoffel K. K., Binns H. J., and McGuire P. A.. 1989. Family history evaluation as a predictive screen for childhood hypercholesterolemia. Pediatric Practice Research Group. Pediatrics. 84: 365–373. [PubMed] [Google Scholar]
- 54.Ritchie S. K., Murphy E. C., Ice C., Cottrell L. A., Minor V., Elliott E., and Neal W.. 2010. Universal versus targeted blood cholesterol screening among youth: the CARDIAC project. Pediatrics. 126: 260–265. [DOI] [PubMed] [Google Scholar]
- 55.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. 2011. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 128 (Suppl. 5): S213–S256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell D. A., Bender R., Hooper A. J., McMahon J., Edwards G., van Bockxmeer F. M., Watts G. F., and Burnett J. R.. 2013. Impact of interpretative commenting on lipid profiles in people at high risk of familial hypercholesterolaemia. Clin. Chim. Acta. 422: 21–25. [DOI] [PubMed] [Google Scholar]
- 57.Bell D. A., Hooper A. J., Edwards G., Southwell L., Pang J., van Bockxmeer F. M., Watts G. F., and Burnett J. R.. 2014. Detecting familial hypercholesterolaemia in the community: impact of a telephone call from a chemical pathologist to the requesting general practitioner. Atherosclerosis. 234: 469–472. [DOI] [PubMed] [Google Scholar]
- 58.Vickery A. W., Bell D., Garton-Smith J., Kirke A. B., Pang J., and Watts G. F.. 2014. Optimising the detection and management of familial hypercholesterolaemia: central role of primary care and its integration with specialist services. Heart Lung Circ. 23: 1158–1164. [DOI] [PubMed] [Google Scholar]
- 59.Troeung L., Arnold-Reed D., Chan She Ping-Delfos W., Watts G. F., Pang J., Lugonja M., Bulsara M., Mortley D., James M., and Brett T.. 2016. A new electronic screening tool for identifying risk of familial hypercholesterolaemia in general practice. Heart. 102: 855–861. [DOI] [PubMed] [Google Scholar]
- 60.Wiegman A., Rodenburg J., de Jongh S., Defesche J. C., Bakker H. D., Kastelein J. J., and Sijbrands E. J.. 2003. Family history and cardiovascular risk in familial hypercholesterolemia: data in more than 1000 children. Circulation. 107: 1473–1478. [DOI] [PubMed] [Google Scholar]
- 61.Starr B., Hadfield S. G., Hutten B. A., Lansberg P. J., Leren T. P., Damgaard D., Neil H. A., and Humphries S. E.. 2008. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin. Chem. Lab. Med. 46: 791–803. [DOI] [PubMed] [Google Scholar]
- 62.Nordestgaard B. G., Langsted A., Mora S., Kolovou G., Baum H., Bruckert E., Watts G. F., Sypniewska G., Wiklund O., Boren J., et al. 2016. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 37: 1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniels S. R., Gidding S. S., and de Ferranti S. D.. 2011. Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 5: S30–S37. [DOI] [PubMed] [Google Scholar]
- 64.Cuchel M., Bruckert E., Ginsberg H. N., Raal F. J., Santos R. D., Hegele R. A., Kuivenhoven J. A., Nordestgaard B. G., Descamps O. S., Steinhagen-Thiessen E., et al. 2014. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 35: 2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raal F. J., Sjouke B., Hovingh G. K., and Isaac B. F.. 2016. Phenotype diversity among patients with homozygous familial hypercholesterolemia: a cohort study. Atherosclerosis. 248: 238–244. [DOI] [PubMed] [Google Scholar]
- 66.Perak A. M., Ning H., de Ferranti S. D., Gooding H. C., Wilkins J. T., and Lloyd-Jones D. M.. 2016. Long-term risk of atherosclerotic cardiovascular disease in US adults with the familial hypercholesterolemia phenotype. Circulation. 134: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pérez de Isla L., Alonso R., Mata N., Fernandez-Perez C., Muniz O., Diaz-Diaz J. L., Saltijeral A., Fuentes-Jimenez F., de Andres R., Zambon D., et al. 2017. Predicting cardiovascular events in familial hypercholesterolemia: the SAFEHEART registry (Spanish Familial Hypercholesterolemia Cohort Study). Circulation. 135: 2133–2144. [DOI] [PubMed] [Google Scholar]
- 68.Paquette M., Chong M., Theriault S., Dufour R., Pare G., and Baass A.. 2017. Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. J. Clin. Lipidol. 11: 725–732.e5. [DOI] [PubMed] [Google Scholar]
- 69.Sorensen K. E., Celermajer D. S., Georgakopoulos D., Hatcher G., Betteridge D. J., and Deanfield J. E.. 1994. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J. Clin. Invest. 93: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urbina E. M., Williams R. V., Alpert B. S., Collins R. T., Daniels S. R., Hayman L., Jacobson M., Mahoney L., Mietus-Snyder M., Rocchini A., et al. 2009. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 54: 919–950. [DOI] [PubMed] [Google Scholar]
- 71.Gidding S. S., McMahan C. A., McGill H. C., Colangelo L. A., Schreiner P. J., Williams O. D., and Liu K.. 2006. Prediction of coronary artery calcium in young adults using the pathobiological determinants of atherosclerosis in youth (PDAY) risk score: the CARDIA study. Arch. Intern. Med. 166: 2341–2347. [DOI] [PubMed] [Google Scholar]
- 72.Ference B. A., Yoo W., Alesh I., Mahajan N., Mirowska K. K., Mewada A., Kahn J., Afonso L., Williams K. A. Sr , and Flack J. M.. 2012. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J. Am. Coll. Cardiol. 60: 2631–2639. [DOI] [PubMed] [Google Scholar]
- 73.Schunkert H., Konig I. R., Kathiresan S., Reilly M. P., Assimes T. L., Holm H., Preuss M., Stewart A. F., Barbalic M., Gieger C., et al. 2011. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43: 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deloukas P., Kanoni S., Willenborg C., Farrall M., Assimes T. L., Thompson J. R., Ingelsson E., Saleheen D., Erdmann J., Goldstein B. A., et al. 2013. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 45: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 76.Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sacks F. M., Lichtenstein A. H., Wu J. H. Y., Appel L. J., Creager M. A., Kris-Etherton P. M., Miller M., Rimm E. B., Rudel L. L., Robinson J. G., et al. 2017. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 136: e1–e23. [DOI] [PubMed] [Google Scholar]
- 78.de Ferranti S. D. 2015. Familial hypercholesterolemia in children and adolescents: a clinical perspective. J. Clin. Lipidol. 9: S11–S19. [DOI] [PubMed] [Google Scholar]
- 79.Garoufi A., Vorre S., Soldatou A., Tsentidis C., Kossiva L., Drakatos A., Marmarinos A., and Gourgiotis D.. 2014. Plant sterols-enriched diet decreases small, dense LDL-cholesterol levels in children with hypercholesterolemia: a prospective study. Ital. J. Pediatr. 40: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amundsen A. L., Ose L., Nenseter M. S., and Ntanios F. Y.. 2002. Plant sterol ester-enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am. J. Clin. Nutr. 76: 338–344. [DOI] [PubMed] [Google Scholar]
- 81.Jakulj L., Vissers M. N., Rodenburg J., Wiegman A., Trip M. D., and Kastelein J. J.. 2006. Plant stanols do not restore endothelial function in pre-pubertal children with familial hypercholesterolemia despite reduction of low-density lipoprotein cholesterol levels. J. Pediatr. 148: 495–500. [DOI] [PubMed] [Google Scholar]
- 82.Davidson M. H., Dugan L. D., Burns J. H., Sugimoto D., Story K., and Drennan K.. 1996. A psyllium-enriched cereal for the treatment of hypercholesterolemia in children: a controlled, double-blind, crossover study. Am. J. Clin. Nutr. 63: 96–102. [DOI] [PubMed] [Google Scholar]
- 83.Engler M. M., Engler M. B., Malloy M. J., Paul S. M., Kulkarni K. R., and Mietus-Snyder M. L.. 2005. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the EARLY study). Am. J. Cardiol. 95: 869–871. [DOI] [PubMed] [Google Scholar]
- 84.Gulesserian T., and Widhalm K.. 2002. Effect of a rapeseed oil substituting diet on serum lipids and lipoproteins in children and adolescents with familial hypercholesterolemia. J. Am. Coll. Nutr. 21: 103–108. [DOI] [PubMed] [Google Scholar]
- 85.Widhalm K., Brazda G., Schneider B., and Kohl S.. 1993. Effect of soy protein diet versus standard low fat, low cholesterol diet on lipid and lipoprotein levels in children with familial or polygenic hypercholesterolemia. J. Pediatr. 123: 30–34. [DOI] [PubMed] [Google Scholar]
- 86.Guardamagna O., Abello F., Baracco V., Stasiowska B., and Martino F.. 2011. The treatment of hypercholesterolemic children: efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 21: 424–429. [DOI] [PubMed] [Google Scholar]
- 87.Malhotra A., Shafiq N., Arora A., Singh M., Kumar R., and Malhotra S.. 2014. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. CD001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong W., Wei J., Abidi P., Lin M., Inaba S., Li C., Wang Y., Wang Z., Si S., Pan H., et al. 2004. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 89.Ruscica M., Gomaraschi M., Mombelli G., Macchi C., Bosisio R., Pazzucconi F., Pavanello C., Calabresi L., Arnoldi A., Sirtori C. R., et al. 2014. Nutraceutical approach to moderate cardiometabolic risk: results of a randomized, double-blind and crossover study with Armolipid Plus. J. Clin. Lipidol. 8: 61–68. [DOI] [PubMed] [Google Scholar]
- 90.Reiner Z., Catapano A. L., De Backer G., Graham I., Taskinen M. R., Wiklund O., Agewall S., Alegria E., Chapman M. J., Durrington P., et al. 2011. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 32: 1769–1818. [DOI] [PubMed] [Google Scholar]
- 91.Collins R., Reith C., Emberson J., Armitage J., Baigent C., Blackwell L., Blumenthal R., Danesh J., Smith G. D., DeMets D., et al. 2016. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 388: 2532–2561. [DOI] [PubMed] [Google Scholar]
- 92.Braamskamp M. J., Kusters D. M., Avis H. J., Smets E. M., Wijburg F. A., Kastelein J. J., Wiegman A., and Hutten B. A.. 2015. Long-term statin treatment in children with familial hypercholesterolemia: more insight into tolerability and adherence. Paediatr. Drugs. 17: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Araujo M. B., and Pacce M. S.. 2016. A 10-year experience using combined lipid-lowering pharmacotherapy in children and adolescents. J. Pediatr. Endocrinol. Metab. 29: 1285–1291. [DOI] [PubMed] [Google Scholar]
- 94.Kusters D. M., Caceres M., Coll M., Cuffie C., Gagne C., Jacobson M. S., Kwiterovich P. O., Lee R., Lowe R. S., Massaad R., et al. 2015. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J. Pediatr. 166: 1377–1384.e1-e3. [DOI] [PubMed] [Google Scholar]
- 95.Stein E. A., Marais A. D., Szamosi T., Raal F. J., Schurr D., Urbina E. M., Hopkins P. N., Karki S., Xu J., Misir S., and Melino M.. 2010. Colesevelam hydrochloride: efficacy and safety in pediatric subjects with heterozygous familial hypercholesterolemia. J. Pediatr. 156: 231–236.e1-e3. [DOI] [PubMed] [Google Scholar]
- 96.Vuorio A., Watts G. F., and Kovanen P. T.. 2016. Initiation of PCSK9 inhibition in patients with heterozygous familial hypercholesterolaemia entering adulthood: a new design for living with a high-risk condition? Eur. Heart J. 37: 1353–1356. [DOI] [PubMed] [Google Scholar]
- 97.Langslet G. 2016. Replacing statins with PCSK9-inhibitors and delaying treatment until 18 years of age in patients with familial hypercholesterolaemia is not a good idea. Eur. Heart J. 37: 1357–1359. [DOI] [PubMed] [Google Scholar]
- 98.Thorogood M., Seed M., and De Mott K.. 2009. Management of fertility in women with familial hypercholesterolaemia: summary of NICE guidance. BJOG. 116: 478–479. [DOI] [PubMed] [Google Scholar]
- 99.Bateman B. T., Hernandez-Diaz S., Fischer M. A., Seely E. W., Ecker J. L., Franklin J. M., Desai R. J., Allen-Coleman C., Mogun H., Avorn J., et al. 2015. Statins and congenital malformations: cohort study. BMJ. 350: h1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winterfeld U., Allignol A., Panchaud A., Rothuizen L. E., Merlob P., Cuppers-Maarschalkerweerd B., Vial T., Stephens S., Clementi M., De Santis M., et al. 2013. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG. 120: 463–471. [DOI] [PubMed] [Google Scholar]
- 101.Stefanutti C., Julius U., Watts G. F., Harada-Shiba M., Cossu M., Schettler V. J., De Silvestro G., Soran H., Van Lennep J. R., Pisciotta L., et al. Toward an international consensus-integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. Epub ahead of print. April 25, 2017; doi:10.1016/j.jacl.2017.04.114. [DOI] [PubMed] [Google Scholar]
- 102.Page M. M., Bell D. A., Hooper A. J., Watts G. F., and Burnett J. R.. 2014. Lipoprotein apheresis and new therapies for severe familial hypercholesterolemia in adults and children. Best Pract. Res. Clin. Endocrinol. Metab. 28: 387–403. [DOI] [PubMed] [Google Scholar]
- 103.Raal F. J., Hovingh G. K., Blom D., Santos R. D., Harada-Shiba M., Bruckert E., Couture P., Soran H., Watts G. F., Kurtz C., et al. 2017. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 5: 280–290. [DOI] [PubMed] [Google Scholar]
- 104.Moriarty P. M., Parhofer K. G., Babirak S. P., Cornier M. A., Duell P. B., Hohenstein B., Leebmann J., Ramlow W., Schettler V., Simha V., et al. 2016. Alirocumab in patients with heterozygous familial hypercholesterolaemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur. Heart J. 37: 3588–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khoo K. L., Page M. M., Liew Y. M., Defesche J. C., and Watts G. F.. 2016. Ten years of lipoprotein apheresis for familial hypercholesterolemia in Malaysia: a creative approach by a cardiologist in a developing country. J. Clin. Lipidol. 10: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 106.Claassen L., Henneman L., van der Weijden T., Marteau T. M., and Timmermans D. R.. 2012. Being at risk for cardiovascular disease: perceptions and preventive behavior in people with and without a known genetic predisposition. Psychol. Health Med. 17: 511–521. [DOI] [PubMed] [Google Scholar]
- 107.Hardcastle S. J., Legge E., Laundy C. S., Egan S. J., French R., Watts G. F., and Hagger M. S.. 2015. Patients’ perceptions and experiences of familial hypercholesterolemia, cascade genetic screening and treatment. Int. J. Behav. Med. 22: 92–100. [DOI] [PubMed] [Google Scholar]
- 108.Michaud P. A., Suris J. C., and Viner R.. 2004. The adolescent with a chronic condition. Part II: healthcare provision. Arch. Dis. Child. 89: 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ting H. H., Brito J. P., and Montori V. M.. 2014. Shared decision making: science and action. Circ. Cardiovasc. Qual. Outcomes. 7: 323–327. [DOI] [PubMed] [Google Scholar]
- 110.Viner R. 2001. Barriers and good practice in transition from paediatric to adult care. J. R. Soc. Med. 94(Suppl. 40): 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vallejo-Vaz A. J., Kondapally Seshasai S. R., Cole D., Hovingh G. K., Kastelein J. J., Mata P., Raal F. J., Santos R. D., Soran H., Watts G. F., et al. 2015. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis. 243: 257–259. [DOI] [PubMed] [Google Scholar]
- 112.Pang J., Lansberg P. J., and Watts G. F.. 2016. International developments in the care of familial hypercholesterolemia: where now and where to next? J. Atheroscler. Thromb. 23: 505–519. [DOI] [PubMed] [Google Scholar]
- 113.O’Brien E. C., Roe M. T., Fraulo E. S., Peterson E. D., Ballantyne C. M., Genest J., Gidding S. S., Hammond E., Hemphill L. C., Hudgins L. C., et al. 2014. Rationale and design of the familial hypercholesterolemia foundation Cascade Screening for Awareness and Detection of Familial Hypercholesterolemia registry. Am. Heart J. 167: 342–349.e17. [DOI] [PubMed] [Google Scholar]
- 114.1999. Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis. 142: 105–112. [PubMed] [Google Scholar]
- 115.Mata N., Alonso R., Badimon L., Padro T., Fuentes F., Muniz O., Perez-Jimenez F., Lopez-Miranda J., Diaz J. L., Vidal J. I., et al. 2011. Clinical characteristics and evaluation of LDL-cholesterol treatment of the Spanish Familial Hypercholesterolemia Longitudinal Cohort Study (SAFEHEART). Lipids Health Dis. 10: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Farnier M., Bruckert E., Boileau C., and Krempf M.. 2013. [Diagnostic and treatment of familial hypercholesterolemia (FH) in adult: guidelines from the New French Society of Atherosclerosis (NSFA)]. Presse Med. 42: 930–950. French. [DOI] [PubMed] [Google Scholar]
- 117.Hammond E., Watts G. F., Rubinstein Y., Farid W., Livingston M., Knowles J. W., Lochmuller H., Bellgard M., and Dawkins H. J.. 2013. Role of international registries in enhancing the care of familial hypercholesterolaemia. Int. J. Evid.-Based Healthc. 11: 134–139. [DOI] [PubMed] [Google Scholar]
- 118.Bellgard M. I., Walker C. E., Napier K. R., Lamont L., Hunter A. A., Render L., Radochonski M., Pang J., Pedrotti A., Sullivan D. R., et al. Design of the Familial Hypercholesterolaemia Australasia Network Registry: creating opportunities for greater international collaboration. J. Atheroscler. Thromb. Epub ahead of print. March 24, 2017; doi:10.5551/jat.37507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Genest J., Hegele R. A., Bergeron J., Brophy J., Carpentier A., Couture P., Davignon J., Dufour R., Frohlich J., Gaudet D., et al. 2014. Canadian Cardiovascular Society position statement on familial hypercholesterolemia. Can. J. Cardiol. 30: 1471–1481. [DOI] [PubMed] [Google Scholar]
- 120.Bamimore M. A., Zaid A., Banerjee Y., Al-Sarraf A., Abifadel M., Seidah N. G., Al-Waili K., Al-Rasadi K., and Awan Z.. 2015. Familial hypercholesterolemia mutations in the Middle Eastern and North African region: a need for a national registry. J. Clin. Lipidol. 9: 187–194. [DOI] [PubMed] [Google Scholar]
- 121.van der Graaf A., Avis H. J., Kusters D. M., Vissers M. N., Hutten B. A., Defesche J. C., Huijgen R., Fouchier S. W., Wijburg F. A., Kastelein J. J., et al. 2011. Molecular basis of autosomal dominant hypercholesterolemia: assessment in a large cohort of hypercholesterolemic children. Circulation. 123: 1167–1173. [DOI] [PubMed] [Google Scholar]
- 122.Ramaswami U., Cooper J., and Humphries S. E.. 2017. The UK Paediatric Familial Hypercholesterolaemia Register: preliminary data. Arch. Dis. Child. 102: 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bell D. A., and Watts G. F.. 2016. Progress in the care of familial hypercholesterolaemia: 2016. Med. J. Aust. 205: 232–236. [DOI] [PubMed] [Google Scholar]