Abstract
Purpose
Neurocognitive impairment is frequently observed among acute lymphoblastic leukemia (ALL) survivors within the domains of intelligence, attention, processing speed, working memory, learning, and memory. However, few have investigated treatment-induced changes in neurocognitive function during the first months of treatment. Additionally, dysfunction during treatment may be preceded by changes in biomarkers measured within cerebrospinal fluid (CSF). Identification of acute declines in neurocognitive function, as well as predictive genotypes or biomarkers, could guide therapeutic trials of protective interventions.
Methods
This study collects CSF while prospectively assessing neurocognitive functioning (working memory, executive function, learning, processing speed, and attention) of ALL patients using the Cogstate computerized battery at six time points during and after the 2 years of leukemia treatment on a Dana-Farber Cancer Institute ALL Consortium trial.
Results
Baseline data collected during the first 3 weeks of induction chemotherapy indicate reliable data as all subjects (N = 34) completed Cogstate baseline testing, while completion and performance checks indicate that 100 % of subjects completed testing and complied with test requirements. The majority (85 %) exhibited normal function compared with age peers. Preliminary analysis of CSF biomarkers (folate, homocysteine, 8-isoprostane, and myelin basic protein) similarly reveals values at baseline within expected normal ranges.
Conclusions
The first month of induction therapy for ALL is a reliable baseline for detecting treatment-induced changes in neurocognitive functioning. Consequently, serial data collection might identify subgroups of ALL patients at increased risk for neurocognitive decline, warranting proactive interventions to improve their level of functioning both during treatment and into survivorship.
Keywords: Neurocognitive, Acute lymphoblastic leukemia, Neurotoxicity, Methotrexate, Late effects, Biomarkers
Background
Curative treatment for children with acute lymphoblastic leukemia (ALL), either with cranial irradiation or with chemotherapy only, frequently leads to measurable neurocognitive deficits among survivors, including the domains of attention, processing speed, executive functioning, and quality of life [1–20]. Furthermore, additional research spanning 2 to 32 years off treatment has identified medical and demographic variables, such as young age at diagnosis, intensity of CNS-directed therapies, length of follow-up, and gender as salient risk factors [21–28]. In specific, a longitudinal assessment of neurocognitive function among ALL survivors that began during treatment, at the end of treatment, and 2 years into survivorship found that survivors as a group performed within age expectations on several measures; however, a significant increase in learning problems was identified between the assessments while on treatment and survivorship, while survivors continued to demonstrate attention impairments that significantly and negatively impact real world functioning [29]. Consequently, there remain subgroups of ALL survivors that are at increased risk for neurocognitive declines and it is imperative to more clearly elucidate these cohorts.
Preclinical and translational studies [30–35] have expanded our understanding of the pathophysiology underlying cognitive decline and are leading to the development of protective interventions. However, while there is a moderate body of literature documenting late cognitive effects, observed years after the conclusion of therapy, relatively little is known about neurocognitive changes that occur early during leukemia therapy, when interrupting the pathophysiologic processes might prevent further chemotherapy-induced cognitive decline. Relevant questions that remain to be answered include the following: what proportion of children with ALL exhibit abnormalities while on therapy; at what point in therapy do these deficits become manifest; and how do deficits evolve during and after the completion of therapy.
Cogstate, a battery of computer-based tests selected specifically to assess domains of neurocognitive function that have been previously found to be impaired among childhood leukemia survivors, may be particularly well suited for the purpose of detecting treatment-induced neurocognitive decline and may also utilize the same subtests across the age groups. Although Cogstate is new relative to “pencil and paper” neuropsychological instruments for assessing changes in pediatric neurocognitive functioning, there is an extensive body of literature [36–45] illustrating that it has construct validity (i.e., correlation with conventional neuropsychological tests) and criterion validity (i.e., ability to correctly identify subjects with known impairment) [40, 41], absence of cultural bias [36–39], and absence of practice effects [42–44]. Notably, this battery of tests is highly sensitive to subtle changes in functioning induced by of neuroactive compounds [45]. The Cogstate normative dataset was last updated in February 2016, and the pediatric normative data are drawn from a database of over 55,000 healthy children, though not all Cogstate tests and all ages are included in that database. For pediatric age groups in the 4 to 17 year range, separate age bins were created for each individual year given maturational changes in cognition over the course of child and adolescent developments. The pediatric normative sample for subjects aged 4 to 17 years is based on a healthy population of children and adolescents enrolled in a series of dedicated normative studies as well as other research and academic studies and are clustered into gender and year-based intervals. The normative sample was collected on the basis of aggregated data from many studies, with participants recruited from countries in North and South America, Europe, Asia, and Australia.
This pilot study was conducted to demonstrate the feasibility of using Cogstate during treatment for leukemia. We sought to establish that a time point during the first month of treatment can be a valid baseline from which to detect treatment-induced changes by demonstrating that patients exhibit function similar to the normative population at this time point, despite the recent diagnosis of ALL. This early time point was chosen because it precedes the majority of neurotoxic therapy in the post-remission consolidation phases, specifically high-dose intravenous methotrexate and repeated intensive CNS-directed therapy, such as intrathecal Methotrexate.
In addition, we analyzed biomarkers in cerebrospinal fluid (CSF) thought to relate to the pathophysiology of treatment-induced cognitive decline. Our longitudinal study will determine whether changes in these biomarkers will track with declines in cognitive function over the course of treatment for ALL. For that reason, we sought to establish baseline values for these biomarkers in CSF collected from the same population of children undergoing therapy for ALL.
Design and methods
Subjects
Children between the ages of 5–21 years with ALL, enrolled on, or treated according to Dana-Farber Cancer Institute (DFCI) ALL Consortium protocol 11-001 “Randomized Trial of IV SC-PEG asparaginase and IV Oncaspar in Children with Acute Lymphoblastic Leukemia or Lymphoblastic Lymphoma” were eligible for a companion study “Serial Neurocognitive Screening of Children and Adolescents During Treatment for Acute Lymphoblastic Leukemia (ALL) on the DFCI ALL Consortium Study 11-001.” Patients known to have any of the following conditions were excluded: active meningitis, poorly controlled seizures, neurodevelopmental disorder (e.g. autism), congenital condition associated with intellectual disability (e.g., trisomy 21), or serious concomitant systemic disorders (including active infections) that would compromise the patient’s ability to complete the study. The institutional review boards of the treating institutions approved both clinical protocols. Patients provided informed consent over the age of 18 and by younger subjects’ guardians. Written assent was provided if age appropriate, following institutional guidelines.
The Cogstate battery
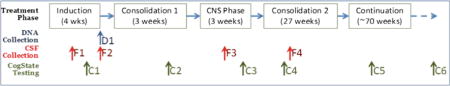
Cogstate is a battery of computer-based tests (Table 1) selected specifically to assess domains of neurocognitive function that have been previously found to be impaired among childhood leukemia survivors. Participants completed each 20– 25 min computerized neurocognitive evaluation (Table 2) supervised by a research team member. To reduce participant burden and facilitate data collection, all baseline assessments were carried out using a laptop computer at the bedside for those receiving inpatient care during the first 4 weeks of medical treatment. The timing of all planned Cogstate assessments and CSF collections is indicated in Table 2. The baseline assessment (C1) was conducted within the first month after initiation of leukemia therapy, once the patients were medically stabilized. This baseline assessment specifically precedes the more neurotoxic therapy in post-remission consolidation phases, including high-dose intravenous methotrexate and repeated intrathecal chemotherapy.
Table 1.
Cogstate subtest names, task descriptions and cognitive domains measured
| Test | Description | Time (min) |
|---|---|---|
| Detection (DET) Psychomotor Function | The Detection test is a measure of psychomotor function and uses a well-validated simple reaction time paradigm with playing card stimuli. The subject is asked to press the Yes key as soon as the card in the center of the screen turns face up. The software measures the speed and accuracy of each response. | 3 |
| Identification (IDN) Attention | The Identification test is a measure of visual attention and uses a well-validated choice reaction time paradigm with playing card stimuli. In this test, the playing cards are all either red or black. The subject responds by pressing the Red key when the card is red and Black when it is black. The software measures the speed and accuracy of each response. | 3 |
| One Back (ONB) Working Memory | The One Back test is a measure of working memory and uses a well-validated n-back paradigm with playing card stimuli. The subject is asked whether the card displayed in the center of the screen is the same as the card presented immediately previously. The subject responds by accordingly pressing the Yes or No key. The software measures the speed and accuracy of each response. | 4 |
| Groton Maze Learning (GML) Test Executive Function | The Groton Maze Learning test is a measure of problem solving and reasoning and uses a well-validated maze-learning paradigm. In this test, the subject is shown a grid of boxes on a computer screen. A pathway is hidden among these locations. Each box represents move locations, and the grid refers to the box array. Subjects are required to find the hidden pathway guided by search rules. These rules are as follows: do not move diagonally, do not move more than one box (i.e., do not jump), and do not move back on the pathway. At each step, only the most recently selected box is shown. Feedback is given with visual and auditory cues (green check marks and red crosses) to indicate whether the selected box is correct or incorrect. The software records each move as an error or as a correct move. | 7 |
| Continuous Paired Associate Learning (CPAL) Paired Associate Learning | The Continuous Paired Associate Learning test is a measure of visual associate memory and uses a well-validated paired associate learning paradigm in which the subject must learn the locations of a number of amoeba-like shapes on the computer screen. This test consists of a single amoeboid shape displayed in the center of the screen surrounded by a number of blue-filled circles. In the exposure phase of the test, all of the to-be-remembered pattern-location associations are presented on the computer screen simultaneously. After a 5-s delay, a pattern is shown in the central location and this signals that the subject should touch the location in the periphery that contains the same pattern. This process continues until the participant has acknowledged all of the pattern-location associations. The learning phase begins with the same test display presented during the exposure phase except that now all of the peripheral locations are shown as blue spheres. One of the patterns presented in the exposure phase is presented in the center location. With the presentation of this pattern, the subject is required to select the peripheral location where an identical pattern is hidden beneath the blue sphere. This process continues until the correct location of each pattern is found. Finding the correct location for all patterns in the set is defined as a learning trial. The software records each move as an error or as a correct move. | 7 |
Table 2.
Cogstate assessment schedule during ALL therapy
| ||
|---|---|---|
| Cognitive Assessment and Medical Therapy Schedule | Notable CNS-toxic therapy | |
| C1 | Less than 4 weeks from the start of therapy | |
| C2 | Week 3 of Consolidation 1 | High-dose intravenous methotrexate |
| C3 | Week 3 of CNS phase | 4 doses of intrathecal chemotherapy within 2 weeks |
| C4 | In Consolidation 2; week 3 of a cycle | |
| C5 | In Continuation, approximately 15 months post-diagnosis; week 3 of a cycle | |
| C6 | 1 year after completion of protocol therapy | |
D DNA collection, F CSF collection, C Cogstate testing
Cerebrospinal fluid biomarkers
CSF collection for biomarkers was an optional research study for participants enrolled on DFCI protocol 11-001. For participants who consented to the collection of these samples, CSF was collected prior to the administration of intrathecal chemotherapy in a volume equal to the volume of chemotherapy to be administered. Two to 3 mL were placed on ice immediately after collection and centrifuged within 60 min in order to remove cellular elements. The supernatant was stored at −70 and shipped on dry ice for analysis. Samples for this pilot analysis were collected at the time of initial diagnosis, day 18 of induction phase, and at the end of induction (day 32). Homocysteine, folate, S-adenosyl-methionine and S-adenosyl-homocysteine, and homocysteic acid and homocysteine sulfinic acid were measured by HPLC, as previously described. ELISA was used to measure 8-isoprostane, a marker of oxidative stress (Cayman Chemical, Ann Arbor, MI) tau protein (Invitrogen, Carlsbad, CA) and myelin basic protein (LifeSpan Biosciences, Seattle, WA).
Statistical analyses
To demonstrate the feasibility of using Cogstate during treatment for leukemia, we assessed the willingness of participants to consent to the study, as well as the ability of the participants to successfully perform Cogstate. Successful performance was defined as completing each test in a manner that complied with test requirements. Performance check criteria were specified a priori in order to identify scores that indicated either the participant did not understand the test instructions or the participant was not cooperative. Performance check criteria are derived statistically such that when trained and supervised appropriately, the relevant study population will achieve the said criterion for the respective task 90 % of the time when they are demonstrating the appropriate level of effort.
We assessed the proportion of children evidencing impaired cognition at baseline. Although the criteria for impaired test performance varies, scores of 2 or more standard deviations (SDs) below the population mean are commonly interpreted as within the impaired range based upon the normal curve distribution. As such, cognitive impairment was defined as test performance of 2 or more SDs below the population mean. This more conservative approach also minimized the risk of type 1 error given the number of tests included. When using cognitive assessment as a screening tool for further assessment, there might be a greater willingness to tolerate type 1 error in order to ensure that no child with potential cognitive difficulties is missed. As Fig. 1 illustrates, the distribution of Cogstate scores is predominantly considered to be within normal limits as defined by ±1 standard deviation around the population mean.
Fig. 1.
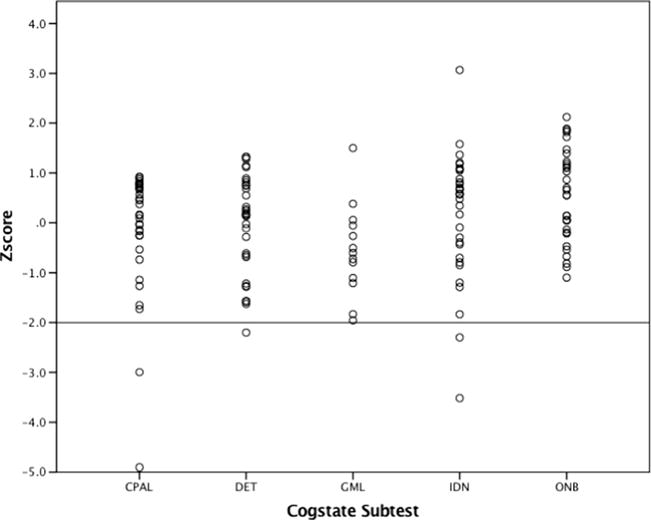
Distribution of Cogstate scores across all five subtests at baseline
Descriptive statistics were employed for patient characteristics and baseline Cogstate performance. Analysis of variance was used to test for a time effect on CSF biomarker levels, with post hoc unpaired t tests to compare later time points with CSF collected at initial diagnosis, prior to any chemotherapy.
Results
Eighty percent of patients with ALL who were approached consented to participate in the companion neurocognitive testing protocol. Between January 2013 and February 2016, 34 patients enrolled at four sites in the DFCI ALL Consortium. The median age was 9 years (range 5–19), 72 % of the participants were male and 61 % were white (black = 8 %, Asian = 6 %, mixed = 14 %, other = 11 %). Sixty percent were non-Hispanic. These 34 patients were significantly older than the median patient enrolled on DFCI 11-001 (median = 4.2 years, n = 240, P < 0.001), due to the eligibility requirement of age ≥5 for Cogstate testing. There were no other statistically significant differences between the demographic characteristics of these 34 patients and the larger cohort of 240 patients. Insurance type was utilized as a proxy for socio-economic status; 58 % of the participants having a private health insurance. Participants were classified after induction by risk level (standard risk = 38.9 %, high risk = 55.6 %, very high risk = 5.6 %) and 97 % of them were right handed. All enrolled subjects completed Cogstate testing during the first 3 weeks of induction chemotherapy. Completion and performance checks indicate that 100 % of subjects completed the battery of tests and complied with test requirements.
Analyses of overall performance reveal that 29 of 34 subjects (85.3 %) performed within the non-impaired range at baseline on all 5 subtests, while 5 subjects (14.7 %) performed within the impaired range (≤2 SD) on 1 of the 5 subtests compared with age peers. Specifically, the distribution of scores on the five Cogstate subtests indicates performances solidly within normal limits (±1 SD) for the majority of subjects at baseline: Continuous Paired Associate Learning (CPAL) mean z-score = −0.153, SD = 1.27; Detection (DET) mean z-score = −0.005, SD = 0.995; Groton Maze Learning (GML) mean z-score = −0.548, SD = 0.923; Identification (IND) mean z-score = 0.161, SD = 1.28; and One Back (ONB) mean z-score = −0.538, SD = 0.921 (Fig. 1). Further investigation into the distribution of scores reveals a similar pattern of impairment when the criteria are set more liberally. Specifically, the percentages of children evidencing impairment were as follows: 12.5 % on CPAL, 12.5 % on DET, 6.3 % on GML, 9.4 % on IND, and 0 % on ONB when the threshold is 1.5 SD and 18.8 % on CPAL, 21.9 % on DET, 12.5%on GML, 15.6%on IND, and 3.1 %on ONB when the threshold is 1 SD (Fig. 1). Furthermore, an examination of the distribution reveals that performance was most frequently impaired on the Detection (processing speed), Identification (attention), and Continuous Paired Associate Learning (associate learning) subtests (Fig. 2).
Fig. 2.
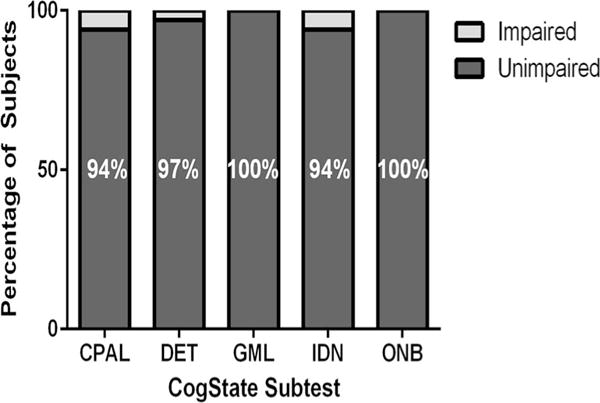
Percentage of subjects who are non-impaired/impaired on each subtest as defined by performance ≤ 2SD below the mean. Distribution of Cogstate scores at baseline assessment
As of February 2016, over 900 CSF samples have been received from patients treated on or according to DFCI ALL protocol 11-001, including 260 samples collected during the initial phase (induction) of therapy. At baseline (Fig. 3), all biomarkers approximate the published normal ranges (shown as green dotted lines). Total CSF folate decreases significantly by day 18 (P < 0.0001, two-tailed paired t test) and remains below normal at the end of induction (P < 0.001). None of the other tested biomarkers changed significantly during the month of induction therapy.
Fig. 3.
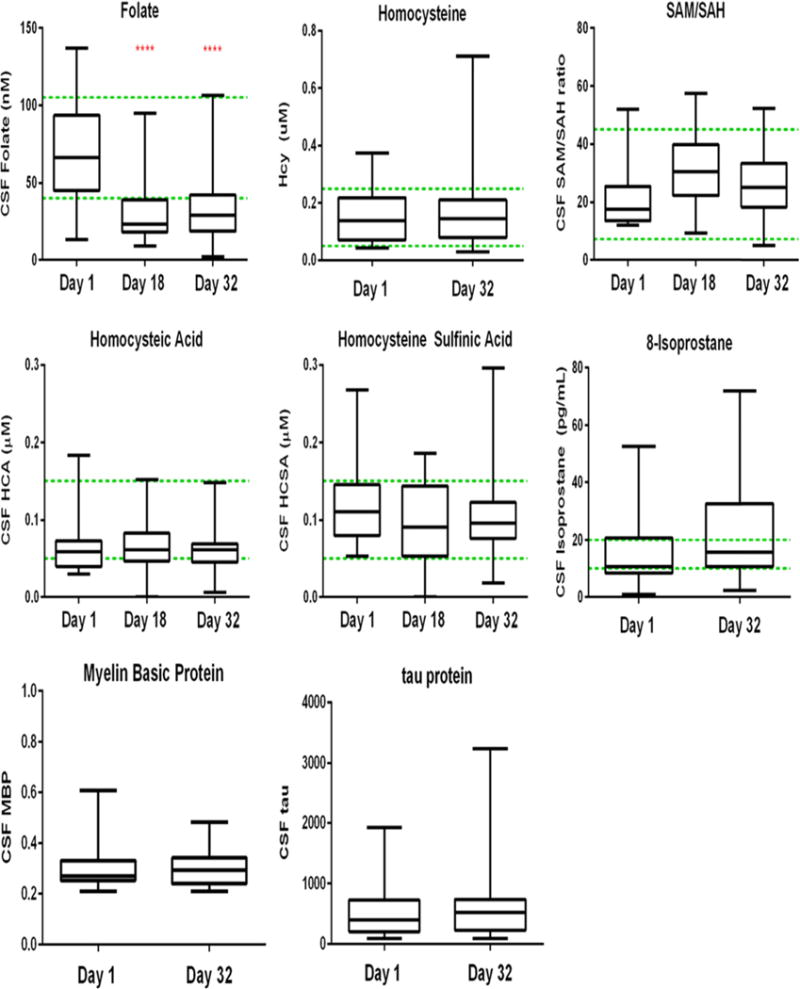
Preliminary analysis of CSF biomarkers (folate, homocysteine, 8-isoprostane and myelin basic protein) during induction therapy
Discussion
We have demonstrated that neurocognitive testing of patients receiving treatment for ALL is feasible—with a high participation rate among those approached and 100 % completion by those enrolled. This finding of high participation and even higher completion is particularly promising given the traditionally poor compliance with neuropsychological testing seen in many US cooperative group studies. Additionally, the computer-based format is engaging to children and adolescents as well as being both focused in domain assessment and brief in duration. Furthermore, serial testing can be performed across the wide age range eligibility by trained non-psychologists using the same assessment instrument without having to switch test measures based upon the age of the subject. Lastly, serial assessments using Cogstate also benefit significantly from the ability of the software to change the stimuli within each test, thereby minimizing any practice effect from repeated exposure, which is not possible with paper and pencil test measures.
These findings provide evidence that the first month of induction therapy for ALL is a reliable baseline for detecting treatment-induced changes in neurocognitive functioning. Despite a recent diagnosis of ALL, the majority of subjects in this pilot study were generally not impaired compared to age peers. Similarly, analysis of CSF biomarkers demonstrates that samples collected during the induction phase are within expected norms, indicating a reliable baseline for detecting treatment-induced changes. The one exception is the total CSF folate, which decreased from pretreatment to day 18 of induction. Given that cytarabine is given intrathecally on day 1 and no intrathecal methotrexate is given prior to day 18, it is not immediately apparent why this decrease in folate occurred. However, the absence of an increase in CSF homocysteine in this same time period suggests that there is no significant change in cellular folate physiology.
The overall goal of this study is to characterize treatment-induced changes in cognitive functioning early during the treatment of children with ALL. This study is designed to test the hypothesis that subclinical changes in cognitive function early in treatment are predictive of cognitive deficits among survivors. Analysis of genetic polymorphisms in candidate genes [37] will detect those patients with inherited susceptibility to neurotoxicity. Moreover, the correlation of treatment-induced changes in neurocognitive functioning with CSF biomarker changes may point to targetable pathophysiologic processes. Consequently, these data will hopefully support a clinical trial of pharmacologic and/or behavioral interventions proactively administered to at-risk subgroups to prevent deterioration of neurocognitive function among children with ALL. As an example, the NMDA antagonist memantine is a potentially beneficial intervention that has shown promise in our preclinical model [18]. Thus, early identification of patients at risk for cognitive decline is an essential first step in the design of such preventive intervention trials. In conclusion, it is our intention and hope that this study, once completed, will allow us to describe subclinical changes in neurocognitive functioning, early in ALL therapy, when an intervention might prevent further decline.
Acknowledgments
Patients were enrolled at the following sites: Columbia University Medical Center, New York, NY; The Children’s Hospital at Montefiore, Bronx, NY; Dana-Farber Cancer Institute/Boston Children’s Hospital, Boston, NY; and Hasbro Children’s Hospital, Providence, RI. This work was supported in part by NIH/NCI R21-CA187226.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest, with the exception of Brian Harel who was an employee and a stockholder of Cogstate until February 2016.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Peterson CC, Johnson CE, Ramirez LY, Huestis S, Pai ALH, Demaree HA, et al. A meta-analysis of the neuropsychological sequelae of chemotherapy-only treatment for pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:99–104. doi: 10.1002/pbc.21544. [DOI] [PubMed] [Google Scholar]
- 2.Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wefel J, Schagen S. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267–275. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 4.Waber DP, Queally JT, Catania L, Robaey P, Romero I, Adams H, et al. Neuropsychological outcomes of standard risk and high risk patients treated for acute lymphoblastic leukemia on Dana-Farber ALL consortium protocol 95-01 at 5 years post-diagnosis. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102:881–893. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buizer AI, de Sonneville LM, Veerman AJ, Buizer AI, de Sonneville LMJ, Veerman AJP. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: a critical review of the literature. Pediatr Blood Cancer. 2009;52:447–454. doi: 10.1002/pbc.21869. [DOI] [PubMed] [Google Scholar]
- 7.Lofstad GE, Reinfjell T, Hestad K, Diseth TH, Lofstad GE, Reinfjell T, et al. Cognitive outcome in children and adolescents treated for acute lymphoblastic leukaemia with chemotherapy only. Acta Paediatr. 2009;98:180–186. doi: 10.1111/j.1651-2227.2008.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krull KR, Hockenberry MJ, Miketova P, Carey M, Moore IM. Chemotherapy-related changes in central nervous system phospholipids and neurocognitive function in childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:535–540. doi: 10.3109/10428194.2012.717080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ficek K, Blamek S, Sygula D, Miszczyk L, Sonta-Jakimczyk D, Tarnawski R. Evaluation of the late effects of CNS prophylactic treatment in childhood acute lymphoblastic leukemia (ALL) using magnetic resonance spectroscopy. Acta Neurochir Suppl. 2010;106:195–197. doi: 10.1007/978-3-211-98811-4_36. [DOI] [PubMed] [Google Scholar]
- 10.Krull KR, Bhojwani D, Conklin HM, Pei D, Cheng C, Reddick WE, et al. Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2182–2188. doi: 10.1200/JCO.2012.46.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankovic M, Brouwers P, Valsecchi MG, et al. International Study Group on Psychosocial Aspects of Childhood Cancer: association of 1800 cGy cranial irradiation with intellectual function in children with acute lymphoblastic leukaemia. ISPACC Lancet. 1994;344:224–227. doi: 10.1016/s0140-6736(94)92997-1. [DOI] [PubMed] [Google Scholar]
- 12.Langer T, Martus P, Ottensmeier H, et al. CNS late effects after ALL therapy in childhood. Part III: neuropsychological performance in long-term survivors of childhood ALL: impairments of concentration, attention and memory. Med Pediatr Oncol. 2002;38:320–328. doi: 10.1002/mpo.10055. [DOI] [PubMed] [Google Scholar]
- 13.Cheung YT, Krull KR. Neurocognitive outcomes in long term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: a systematic review. Neurosci Biobehav Rev. 2015;53:108–120. doi: 10.1016/j.neubiorev.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iyer NS, Balsamo LM, Bracken MB, et al. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126:346–353. doi: 10.1182/blood-2015-02-627414. [DOI] [PubMed] [Google Scholar]
- 15.Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer. 2006;106:2067–2075. doi: 10.1002/cncr.21820. [DOI] [PubMed] [Google Scholar]
- 16.Kunin-Batson A, Kadan-Lottick N, Neglia JP. The contribution of neurocognitive functioning to quality of life after childhood acute lymphoblastic leukemia. Psychooncology. 2014;23:692–699. doi: 10.1002/pon.3470. [DOI] [PubMed] [Google Scholar]
- 17.Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE, et al. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. J Pediatr Oncol. 2009;34:317–327. doi: 10.1093/jpepsy/jsn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadan-Lottick NS, Brouwers P, Breiger D, Kaleita T, Dziura J, Northrup V, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashford J, Schoffstall C, Reddick WE, Leone C, Laningham FH, Glass JO, et al. Attention and working memory abilities in children treated for acute lymphoblastic leukemia. Cancer. 2010;116:4638–4645. doi: 10.1002/cncr.25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297–304. doi: 10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, et al. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effects of treatment intensity. Pediatr Blood Cancer. 2005;45:281–290. doi: 10.1002/pbc.20397. [DOI] [PubMed] [Google Scholar]
- 22.Conklin HM, Krull KR, Reddick WE, et al. Cognitive outcomes following chemotherapy treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104:1386–1395. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buizer AI, de Sonneville LM, van de Heuevel-Eibrink MM, et al. Visoumotor control in survivors of acute lymphoblastic leukemia treated with chemotherapy only. J Int Neuropsychol Soc. 2005;11:554–565. doi: 10.1017/S1355617705050666. [DOI] [PubMed] [Google Scholar]
- 24.Kingma A, van Dommelen RI, Mooyaart EL, et al. Slight cognitive impairment and magnetic resonance imaging abnormalities but normal school levels in children treated for acute lymphoblastic leukemia with chemotherapy only. J Pediatr. 2001;139:413–420. doi: 10.1067/mpd.2001.117066. [DOI] [PubMed] [Google Scholar]
- 25.von der Weid N, Mosimann I, Hirt A, et al. Intellectual outcome in children and adolescents with acute lymphoblastic leukemia treated with chemotherapy along: age- and sex-related differences. Eur J Cancer. 2003;39:359–365. doi: 10.1016/s0959-8049(02)00260-5. [DOI] [PubMed] [Google Scholar]
- 26.Jain N, Brouwers P, Okcu MF, et al. Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;115:4238–4245. doi: 10.1002/cncr.24464. [DOI] [PubMed] [Google Scholar]
- 27.Harila MJ, Winqvist S, Lanning M, Bloigu R, Harila-Saari AH, Harila MJ, et al. Progressive neurocognitive impairment in young adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2009;53:156–161. doi: 10.1002/pbc.21992. [DOI] [PubMed] [Google Scholar]
- 28.Waber DP, McCabe M, Sebree M, Forbes PW, Adams H, Alyman C, et al. Neuropsychological outcomes of a randomized trial of prednisone versus dexamethasone in acute lymphoblastic leukemia: Findings from Dana-Farber Cancer Institute All Consortium Protocol 00-01. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacola LM, Krull KR, Pui C-H, Pei D, Cheng C, Reddick WE, et al. Longitudinal assessment of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia treated on a contemporary chemotherapy protocol. J Clin Oncol. 2016;34:1239–1247. doi: 10.1200/JCO.2015.64.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole PD, Vijayanathan V, Ali NF, Wagshul ME, Tanenbaum EJ, Price J, et al. Memantine protects rats treated with intrathecal methotrexate from developing spatial memory deficits. Clin Cancer Res. 2013;19:4446–4454. doi: 10.1158/1078-0432.CCR-13-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Vijayanathan V, Gulinello M, Cole PD. Intrathecal methotrexate induces focal cognitive deficits and increases cerebro-spinal fluid homocysteine. Pharmacol Biochem Behav. 2010;95:428–433. doi: 10.1016/j.pbb.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Vijayanathan V, Gulinello ME, Cole PD. Systemic methotrexate induces spatial memory deficits and depletes cerebrospinal fluid folate in rats. Pharmacol Biochem Behav. 2010;94:454–463. doi: 10.1016/j.pbb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Vijayanathan V, Ali N, Gulinello M, Cole PD. Persistent cognitive deficits, induced by intrathecal methotrexate, are associated with elevated CSF concentrations of excitotoxic glutamate analogs and can be reversed by an NMDA antagonist. Behav Brain Res. 2011;225:491–497. doi: 10.1016/j.bbr.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Cole PD, Beckwith KA, Vijayanathan V, Roychowdhury S, Smith AK, Kamen BA. Folate homeostasis in cerebrospinal fluid during therapy for acute lymphoblastic leukemia. Pediatr Neurol. 2009;40:34–41. doi: 10.1016/j.pediatrneurol.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, Silverman LB, et al. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2015;33:2205–2211. doi: 10.1200/JCO.2014.59.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology. 2010;24:667. doi: 10.1037/a0019312. [DOI] [PubMed] [Google Scholar]
- 37.Cairney S, Clough A, Jaragba M, Maruff P. Cognitive impairment in aboriginal people with heavy episodic patterns of alcohol use. Addiction. 2007;102:909–915. doi: 10.1111/j.1360-0443.2007.01840.x. [DOI] [PubMed] [Google Scholar]
- 38.Dingwall KM, Cairney S. Psychological and cognitive assessment of indigenous Australians. Aust N Z J Psychiatry. 2010;44:20–30. doi: 10.3109/00048670903393670. [DOI] [PubMed] [Google Scholar]
- 39.Lewis MS, Dingwall KM, Berkhout N, Sayers S, Maruff P, Cairney S. Assessment of cognition in an adolescent indigenous population. Aust Psychol. 2010;45:123–131. [Google Scholar]
- 40.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 41.Pietrzak RH, Olver J, Norman T, Piskulic D, Maruff P, Snyder PJ. A comparison of the CogState schizophrenia battery and the measurement and treatment research to improve cognition in schizophrenia (MATRICS) battery in assessing cognitive impairment in chronic schizophrenia. J Clin Exp Neuropsychol. 2009;31:848–859. doi: 10.1080/13803390802592458. [DOI] [PubMed] [Google Scholar]
- 42.Kral TV, Heo M, Whiteford LM, Faith MS. Effects on cognitive performance of eating compared with omitting breakfast in elementary schoolchildren. J Dev Behav Pediatr. 2012;33:9–16. doi: 10.1097/DBP.0b013e31823f2f35. [DOI] [PubMed] [Google Scholar]
- 43.Mollica CM, Maruff P, Collie A, Vance A. Repeated assessment of cognition in children and the measurement of performance change. Child Neuropsychol. 2005;11:303–310. doi: 10.1080/092970490911306. [DOI] [PubMed] [Google Scholar]
- 44.Falleti MG, Maruff P, Collie A, Darby DG. Practice effects associated with the repeated assessment of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–1112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- 45.Snyder AM, Maruff P, Pietrzak RH, Cromer JR, Snyder PJ. Effect of treatment with stimulant medication on nonverbal executive function and visuomotor speed in children with attention deficit/hyperactivity disorder (ADHD) Child Neuropsychol. 2008;14:211–226. doi: 10.1080/09297040701220005. [DOI] [PubMed] [Google Scholar]


