Abstract
Background
Cataract is a leading cause of blindness worldwide. Cataract surgery is commonly performed but can result in postoperative inflammation of the eye. Inadequately controlled inflammation increases the risk of complications. Non‐steroidal anti‐inflammatory drugs (NSAIDs) and corticosteroids are used to prevent and reduce inflammation following cataract surgery, but these two drug classes work by different mechanisms. Corticosteroids are effective, but NSAIDs may provide an additional benefit to reduce inflammation when given in combination with corticosteroids. A comparison of NSAIDs to corticosteroids alone or combination therapy with these two anti‐inflammatory agents will help to determine the role of NSAIDs in controlling inflammation after routine cataract surgery.
Objectives
To evaluate the comparative effectiveness of topical NSAIDs (alone or in combination with topical corticosteroids) versus topical corticosteroids alone in controlling intraocular inflammation after uncomplicated phacoemulsification. To assess postoperative best‐corrected visual acuity (BCVA), patient‐reported discomfort, symptoms, or complications (such as elevation of IOP), and cost‐effectiveness with the use of postoperative NSAIDs or corticosteroids.
Search methods
To identify studies relevant to this review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Eyes and Vision Trials Register (2016, Issue 12), MEDLINE Ovid (1946 to December 2016), Embase Ovid (1947 to 16 December 2016), PubMed (1948 to December 2016), LILACS (Latin American and Caribbean Health Sciences Literature Database) (1982 to 16 December 2016), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 17 June 2013), ClinicalTrials.gov (www.clinicaltrials.gov; searched December 2016), and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en; searched December 2016).
Selection criteria
We included randomized controlled trials (RCTs) in which participants were undergoing phacoemulsification for uncomplicated cataract extraction. We included both trials in which topical NSAIDs were compared with topical corticosteroids and trials in which combination therapy (topical NSAIDs and corticosteroids) was compared with topical corticosteroids alone. The primary outcomes for this review were inflammation and best‐corrected visual acuity (BCVA).
Data collection and analysis
Two review authors independently screened the full‐text articles, extracted data from included trials, and assessed included trials for risk of bias according to Cochrane standards. The two review authors resolved any disagreements by discussion. We graded the certainty of the evidence using GRADE.
Main results
This review included 48 RCTs conducted in 17 different countries and two ongoing studies. Ten included studies had a trial registry record. Fifteen studies compared an NSAID with a corticosteroid alone, and 19 studies compared a combination of an NSAID plus a corticosteroid with a corticosteroid alone. Fourteen other studies had more than two study arms. Overall, we judged the studies to be at unclear risk of bias.
NSAIDs alone versus corticosteroids alone
None of the included studies reported postoperative intraocular inflammation in terms of cells and flare as a dichotomous variable. Inflammation was reported as a continuous variable in seven studies. There was moderate‐certainty evidence of no difference in mean cell value in the participants receiving an NSAID compared with the participants receiving a corticosteroid (mean difference (MD) ‐0.60, 95% confidence interval (CI) ‐2.19 to 0.99), and there was low‐certainty evidence that the mean flare value was lower in the group receiving NSAIDs (MD ‐13.74, 95% CI ‐21.45 to ‐6.04). Only one study reported on corneal edema at one week postoperatively and there was uncertainty as to whether the risk of edema was higher or lower in the group that received NSAIDs (risk ratio (RR) 0.77, 95% CI 0.26 to 2.29). No included studies reported BCVA as a dichotomous outcome and no study reported time to cessation of treatment. None of the included studies reported the proportion of eyes with cystoid macular edema (CME) at one week postoperatively. Based on four RCTs that reported CME at one month, we found low‐certainty evidence that participants treated with an NSAID alone had a lower risk of developing CME compared with those treated with a corticosteroid alone (RR 0.26, 95% CI 0.17 to 0.41). No studies reported on other adverse events or economic outcomes.
NSAIDs plus corticosteroids versus corticosteroids alone
No study described intraocular inflammation in terms of cells and flare as a dichotomous variable and there was not enough continuous data for anterior chamber cell and flare to perform a meta‐analysis. One study reported presence of corneal edema at various times. Postoperative treatment with neither a combination treatment with a NSAID plus corticosteroid or with corticosteroid alone was favored (RR 1.07, 95% CI 0.98 to 1.16). We judged this study to have high risk of reporting bias, and the certainty of the evidence was downgraded to moderate. No included study reported the proportion of participants with BCVA better than 20/40 at one week postoperatively or reported time to cessation of treatment. Only one included study reported on the presence of CME at one week after surgery and one study reported on CME at two weeks after surgery. After combining findings from these two studies, we estimated with low‐certainty evidence that there was a lower risk of CME in the group that received NSAIDs plus corticosteroids (RR 0.17, 95% CI 0.03 to 0.97). Seven RCTs reported the proportion of participants with CME at one month postoperatively; however there was low‐certainty evidence of a lower risk of CME in participants receiving an NSAID plus a corticosteroid compared with those receiving a corticosteroid alone (RR 0.50, 95% CI 0.23 to 1.06). The few adverse events reported were due to phacoemulsification rather than the eye drops.
Authors' conclusions
We found insufficient evidence from this review to inform practice for treatment of postoperative inflammation after uncomplicated phacoemulsification. Based on the RCTs included in this review, we could not conclude the equivalence or superiority of NSAIDs with or without corticosteroids versus corticosteroids alone. There may be some risk reduction of CME in the NSAID‐alone group and the combination of NSAID plus corticosteroid group. Future RCTs on these interventions should standardize the type of medication used, dosing, and treatment regimen; data should be collected and presented using the Standardization of Uveitis Nomenclature (SUN) outcome measures so that dichotomous outcomes can be analyzed.
Keywords: Humans; Phacoemulsification; Phacoemulsification/adverse effects; Adrenal Cortex Hormones; Adrenal Cortex Hormones/therapeutic use; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Cataract Extraction; Cataract Extraction/adverse effects; Cataract Extraction/methods; Corneal Edema; Corneal Edema/etiology; Corneal Edema/prevention & control; Drug Therapy, Combination; Eye Diseases; Eye Diseases/etiology; Eye Diseases/prevention & control; Inflammation; Inflammation/etiology; Inflammation/prevention & control; Macular Edema; Macular Edema/etiology; Macular Edema/prevention & control; Postoperative Complications; Postoperative Complications/etiology; Postoperative Complications/prevention & control; Randomized Controlled Trials as Topic; Visual Acuity
Plain language summary
Non‐steroidal anti‐inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery
Review aim The aim of this review was to find out if topical non‐steroidal anti‐inflammatory drugs (NSAIDs) (alone or taken in combination with topical corticosteroids) or topical corticosteroids alone are better for controlling eye inflammation after cataract surgery. Cochrane review authors collected and analyzed all relevant studies to answer this question and found 48 studies.
Key messages It is unclear whether NSAIDs or corticosteroids are better at treating eye inflammation after cataract surgery. There were many combinations of drugs and dosing regimens in the included studies. The majority of the studies did not provide data on inflammation.
What was studied in this review? Cataract surgery is one of the most commonly performed eye surgeries. Eye inflammation is common after cataract surgery. If left untreated, this inflammation can cause many complications. NSAIDs or corticosteroids are typically used to control swelling after cataract surgery. These drugs work differently, so comparing their effects is necessary. Cochrane review authors compared the effectiveness of NSAIDs (alone or in combination with corticosteroids) versus corticosteroids alone for controlling swelling after cataract surgery.
Main results We included 48 randomized controlled trials from 17 different countries. Fifteen studies compared an NSAID with a corticosteroid. Nineteen studies compared an NSAID plus corticosteroid versus a corticosteroid alone. Fourteen other studies had more than two study arms, with different combinations of NSAIDs and corticosteroids.
In comparing participants who received an NSAID with those who received a corticosteroid:
• it was unclear whether the number of cells, which were a sign of inflammation inside the eye, was higher or lower
• there was less flare (another sign of inflammation inside the eye, in which a beam of light becomes visible passing through the eye fluids, like the beam of a searchlight) in the back of the eye in the group that received only an NSAID
• it was unclear whether there was a higher instance of swelling of the cornea, the clear window at the front of the eye which becomes misty if swollen, one month after surgery
• there was a lower risk of developing cystoid macular edema (fluid and swelling in a part of the eye called the macula, the central part of the retina, a light‐sensitive membrane at the back of the eye which is responsible for detailed vision and if so effected can make vision clouded and distorted) in the group that received only an NSAID
The included studies in this comparison did not provide enough information to look into sharpness of vision, how long participants needed treatment, side effects of the medications, or cost.
In comparing participants who received a combination of an NSAID plus a corticosteroid compared with those who received a corticosteroid alone:
• there was a higher instance of corneal edema in the group that received a combination of the two types of medications
• there was a lower risk of developing cystoid macular edema one week after surgery in the group that received a combination of the two types of medications
The included studies did not provide enough information to look into the amount of cells in the back of the eye, sharpness of vision, how long participants needed treatment, side effects of the medications or cost.
This review compared many different types of drugs, dosing, and treatments. We tried to look at all types of anti‐inflammatory agents in this review. NSAIDs considered in this review were indomethacin, ketorolac, nepafenac, diclofenac, bromfenac, flurbiprofen, and pranoprofen. Corticosteroids included in this review were dexamethasone, prednisolone acetate, betamethasone, rimexolone, fluorometholone, and loteprednol. A future review with different outcomes may be more effective in determining whether NSAIDs or corticosteroids are better at treating swelling after cataract surgery.
How up to date is this review? Cochrane review authors searched for studies that had been published up to 16 December 2016.
Summary of findings
Summary of findings for the main comparison. Summary of findings for NSAIDs versus corticosteroids.
| NSAIDs compared with corticosteroids for controlling inflammation after uncomplicated cataract surgery | ||||||
|
Patient or population: people who received phacoemulsification Intervention: NSAID Comparison: corticosteroid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroid | NSAID | |||||
|
Intraocular inflammation measured by anterior chamber cell and flare 1 week |
Cell values | — | 174 (3 RCTs) |
⊕⊕⊕⊝1 moderate | The cell values presented were measured using a Kowa cell meter. 2 additional studies measured cells using a slit lamp, but we were unable to combine these data as the studies used different scales to report the number of cells. | |
| The mean cell value ranged from 3.7 to 5.8. | The mean cell value was0.24 cells lower (1.65 lower to 1.16 higher). | |||||
| Flare values | — | 365 (5 RCTs) |
⊕⊕⊝⊝1,2 low | The flare values presented were measured using a Kowa cell meter. There was high statistical heterogeneity among the included studies (I2 = 92%). | ||
| The mean flare ranged from 15.65 to 48.3 photons/ms. | The mean flare was 13.74 photons/ms lower (21.47 lower to 6 lower). | |||||
|
Intraocular inflammation measured by proportion of participants with corneal edema 1 week |
133 per 1000 |
103 per 1000 (35 to 305) |
RR 0.77 (0.26 to 2.29) | 114 (1 RCT) |
⊕⊕⊝⊝3,4 low | |
|
Proportion of participants with best‐corrected visual acuity of 20/40 1 week |
None of the included studies reported on this outcome. | |||||
|
Proportion of participants with cystoid macular edema 1 week |
521 per 1000 |
135 per 1000 (89 to 214) |
RR 0.26 (0.17 to 0.41) | 291 (4 RCTs) | ⊕⊕⊝⊝1,5 low | None of the included studies reported the proportion of participants with cystoid macular edema at 1 week, our intended outcome of interest. The data shown here are for 4 studies that reported on the presence of macular edema at 1 month. |
| Time to cessation of treatment | None of the included studies reported on this outcome. | |||||
| Adverse events | None of the included studies reported on this outcome. | |||||
| Economic outcomes | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1We downgraded the evidence due to risk of bias: the studies included in this meta‐analysis were poorly reported. 2We downgraded the certainty of the evidence due to inconsistency: the I2 for this estimate was 92%. 3We downgraded the certainty of the evidence because the study was unmasked: participants and outcome assessor were not masked, and there was high risk of performance and detection bias. 4We downgraded the certainty of the evidence due to imprecision. 5We downgraded the certainty of the evidence due to indirectness: the time point at which the study data were reported was one month rather than one week, and two of the studies, which made up 30% of the weight, used a weak corticosteroid in their comparison.
Summary of findings 2. Summary of findings for NSAIDs plus corticosteroids versus corticosteroids alone.
| NSAIDs plus corticosteroids compared with corticosteroids alone for controlling inflammation after uncomplicated cataract surgery | ||||||
|
Patient or population: people who received phacoemulsification Intervention: NSAID plus corticosteroid Comparison: corticosteroid alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Corticosteroid alone | NSAID plus corticosteroid | |||||
|
Intraocular inflammation measured by anterior chamber cell and flare 1 week |
None of the included studies reported on this outcome. | |||||
|
Intraocular inflammation measured by proportion of participants with corneal edema 1 week |
912 per 1000 |
976 per 1000 (894 to 1000) |
RR 1.07 (0.98 to 1.16) | 138 (1 RCT) | ⊕⊕⊕⊝1 moderate | |
|
Proportion of participants with best‐corrected visual acuity of 20/40 1 week |
None of the included studies reported on this outcome. | |||||
|
Proportion of participants with cystoid macular edema 1 week |
47 per 1000 | 8 per 1000 (1 to 46) | RR 0.17 (0.03 to 0.97) | 220 (2 RCTs) | ⊕⊕⊝⊝2,3 low | 7 additional studies (including 1213 participants) reported on the presence of cystoid macular edema at 1 month postoperatively. The meta‐analysis showed that the group that received a combination of NSAID plus corticosteroid had a lower risk of macular edema at 1 month compared with the group that received a corticosteroid only, however there was uncertainty in the measurement (RR 0.50, 95% CI 0.23 to 1.06) |
| Time to cessation of treatment | None of the included studies reported on this outcome. | |||||
| Adverse events | See comment | — | — | — | Only 2 studies reported on adverse events. 1 reported that there were no adverse events related to NSAID use, but that 1 participant randomized to NSAIDs plus corticosteroid had heterogeneous retinal detachment as a complication of cataract surgery. Another study used the COMTOL questionnaire to ask participants about the frequency and severity of side effects; 3 of the top 5 most commonly reported side effects were markers of ocular discomfort: burning, redness, and blurred vision. The adverse events reported in this study were not separated by intervention group. | |
| Economic outcomes | None of the included studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COMTOL: Comparison of Ophthalmic Medications for Tolerability; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||
1We downgraded the certainty of the evidence due to high risk of reporting bias: the study reported collecting data for certain time points, but results were not reported. 2We downgraded the certainty of the evidence due to inconsistency based on the heterogeneity of types, doses, and regimens of NSAIDs and corticosteroids used. 3We downgraded the certainty of the evidence due to imprecision: there was a small number of events for this outcome.
Background
Description of the condition
Cataract is a leading cause of blindness worldwide. It is the leading cause of visual impairment in the United States, affecting approximately 20.5 million Americans older than 40 years, and is expected to increase to 30.1 million by 2020. Cataract progresses with age, therefore the number of surgeries is expected to increase more than four‐fold from 8 million annually to 35 million as the population grows older (Congdon 2004).
Presently, surgery is the only curative therapy. Cataract surgery is one of the most commonly performed surgical procedures worldwide and one of the most successful. Following cataract surgery, inflammation occurs in the eye. The severity of postoperative inflammation varies and in most cases is self limiting. Anti‐inflammatory agents may hasten resolution and decrease patient discomfort following surgery. In theory, any agent that blocks the action of inflammatory mediators can control postoperative inflammation. Non‐steroidal anti‐inflammatory drugs (NSAIDs) and corticosteroids are used to prevent and reduce inflammation following cataract surgery. NSAIDs and corticosteroids work by different mechanisms to decrease inflammation (Hirneiss 2005).
Description of the intervention
Despite advances in cataract surgery, postoperative inflammation continues to cause patient discomfort, delay recovery for surgery and, in some cases, result in suboptimal vision. Inadequately controlled inflammation increases the risk of complications such as anterior or posterior synechiae (where the iris adheres to either the cornea or the lens capsule), corneal edema, progression of pre‐existing glaucoma, post‐operative pain or cystoid macular edema (CME) (Colin 2007; Flach 1988; McColgin 2000).
How the intervention might work
Corticosteroids are well known to have anti‐inflammatory action. Despite their effectiveness, corticosteroids can cause ocular side effects such as inhibition of corneal wound healing, elevation of intraocular pressure (IOP), increased likelihood of infection, and complications in the presence of herpes virus infection (Lane 2007). Due to these potential significant side effects, cataract surgeons have been interested in alternatives to corticosteroids. NSAIDs are commercially available, in topical and systemic formulations, for ocular indications. Topically applied NSAIDs are commonly used in the management and prevention of non‐infectious ocular inflammation. They have been shown to be effective against chronic CME following cataract surgery (Sivaprasad 2012). NSAIDs may provide an additional benefit to reduce inflammation when given in conjunction with corticosteroids.
Corticosteroids inhibit the action of phospholipase‐A2 and consequently inhibit the release of arachidonic acid (Vane 1996). Arachidonic acid is metabolized to leukotrienes and prostaglandins, both of which mediate the inflammatory response (Needleman 1986). Corticosteroids also down‐regulate genes that encode cytokines, chemokines, adhesion molecules, inflammatory enzymes, receptors, and proteins (Barnes 2006).
NSAIDs inhibit the enzyme cyclo‐oxygenase and consequently inhibit prostaglandin production (Vane 1998). They are known for their anti‐inflammatory, antipyretic, and analgesic effects. Topical ocular NSAIDs first became available in the 1990s and are used for the inhibition of intraoperative miosis, management of postoperative inflammation, treatment of allergic conditions, and control of pain after excimer laser procedures (Guidera 2001). Reports from several studies have indicated that NSAIDs decrease the incidence of postoperative CME apparent on fluorescein angiography (Rossetti 1996; Solomon 1995), as well as improve postoperative visual acuity (Flach 1987; Flach 1991; Sivaprasad 2012).
Topical ocular NSAIDs can have side effects such as post‐cataract surgery atonic mydriasis, contact dermatitis, delayed wound healing (including slowed corneal re‐epithelialization), and corneal melting (Flach 2002; Guidera 2001). Rare, systemic adverse reactions include asthma, gastrointestinal irritation and ulceration, inhibition of platelet function, and renal dysfunction (Sharir 1997).
Why it is important to do this review
Both topical NSAIDs and corticosteroids are used to control postoperative inflammation following cataract surgery. The most effective treatment or combination of treatments for controlling inflammation following cataract surgery has not been established.
A comparison of the effectiveness and safety of NSAIDs and corticosteroids alone and their combined use will help elucidate the role of NSAIDs in controlling inflammation after routine cataract surgery (DeCroos 2008).
Objectives
To evaluate the comparative effectiveness of topical NSAIDs (alone or in combination with topical corticosteroids) versus topical corticosteroids to control intraocular inflammation after uncomplicated phacoemulsification for cataract extraction. To assess postoperative best‐corrected visual acuity (BCVA), patient‐reported discomfort, symptoms, or complications (such as elevation of IOP), and cost‐effectiveness with the use of postoperative NSAIDs or corticosteroids.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) in which the medications of interest were compared. We had planned to include quasi‐RCTs if there were few RCTs identified, however as we identified an abundance of RCTs, we chose to focus our review on those trials. We considered methods of treatment allocation such as alternation or based date of birth, Social Security number, and medical record number to be quasi‐random methods of allocation and thus did not included studies that used these methods.
Types of participants
We included trials in which participants underwent phacoemulsification only for cataract extraction. We excluded trials that used other types of cataract surgery, such as manual small‐incision cataract surgery, extracapsular cataract extraction, and intracapsular cataract extraction. The focus of this review was the control of inflammation after uncomplicated cataract surgery, however we did not exclude studies in which participants may have experienced a complication after randomization.
Types of interventions
We included trials in which topical NSAIDs were compared with topical corticosteroids and trials in which combination therapy (topical NSAIDs and corticosteroids) was compared with topical corticosteroids alone. We did not include dosing studies. We did not include studies that compared only one type of topical anti‐inflammatory agent with placebo, since administering inactive drops may be construed as being unethical following an inflammation‐inducing procedure such as cataract surgery.
Types of outcome measures
Primary outcomes
The primary outcomes for this review were intraocular inflammation and BCVA, defined as:
the proportion of participants with intraocular inflammation at one‐week after surgery;
the proportion of participants with BCVA of 20/40 or better at one‐week after surgery.
Intraocular inflammation is assessed clinically based on the presence of corneal edema, as well as anterior chamber cells and flare (Hirneiss 2005; Jabs 2005; Snyder 2000). Corneal edema may be classified as 0 (none), 1 (moderate), 2 (marked), and 3 (severe). We sought data for anterior chamber cells (Table 3) and flare (Table 4) assessed by slit‐lamp examination as described by the Standardization of Uveitis Nomenclature (SUN) working group criteria (Jabs 2005). For the purposes of this review, we considered corneal edema grades above 0 and anterior chamber cells and flare grades above 1 as indication of intraocular inflammation.
1. The SUN Working Group Grading Scheme for Anterior Chamber Cells.
| Cells/Grade | Cells per field |
| 0 | Less than 1 |
| 0.5+ | 1 to 5 cells |
| 1+ | 6 to 15 cells |
| 2+ | 16 to 25 cells |
| 3+ | 26 to 50 cells |
| 4+ | More than 50 cells |
2. The SUN Working Group Grading Scheme for Anterior Chamber Flare.
| Flare/Grade | Description |
| 0 | None to trace |
| 1+ | Faint |
| 2+ | Moderate (iris and lens details clear) |
| 3+ | Marked (iris and lens details hazy) |
| 4+ | Intense (fibrin or plastic aqueous) |
We also considered intraocular inflammation measured and defined in other ways in included studies. Although our protocol called for meta‐analysis on anterior chamber cell and flare as dichotomous outcomes (Gonzales 2013), we performed meta‐analyses on continuous cell and flare data as this was how this information was reported in many of the included studies.
The primary time point was one‐week after surgery, however we also included data reported at time points up to one month after surgery.
Secondary outcomes
The proportion of participants with CME, as measured by ocular coherence tomography or fluorescein angiography, at one‐week after surgery. (Cystoid macular edema is frequently used as a surrogate for active intraocular inflammation.) We used definitions of CME as defined by the study authors, for example, any dye leakage or dye accumulation captured by fluorescein angiography.
Time to cessation of treatment for inflammation.
Adverse events
We aimed to summarize the proportion of participants who reported self‐rated ocular discomfort at one day, one week, and one month after surgery. An example of a scale used to rate ocular discomfort is a 0‐to‐3‐point scale within one hour after self‐instillation of the topical agent, as described by Donnenfeld 2007 and shown in Table 5.
3. Rating scale to determine the degree of ocular discomfort.
| Grade | Degree of ocular discomfort | Description |
| 0 | None | Absent |
| 1 | Mild | You experience ocular discomfort, but it does not interfere at all with your completion of daily tasks. |
| 2 | Moderate | You experience ocular discomfort and it slows you down, but you are able to carry out work of a light or sedentary nature (e.g. light house work, office work). |
| 3 | Severe | Your experience of ocular discomfort makes you completely unable to carry out any work activities. |
We investigated the proportion of participants with complications such as elevated IOP (greater than 21 mm Hg), corneal melting, ocular surface toxicity defined as punctate epithelial erosion or tear film instability, delayed wound healing (greater than two weeks), wound infection, or endophthalmitis.
Economic outcomes
We planned to document economic outcomes reported from the included trials, namely whether topical NSAIDs alone or in combination with topical corticosteroids provide a significant benefit in primary or secondary outcome measures that would warrant their use as well as cost, however none of the included studies reported a cost comparison.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomized controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 16 December 2016.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 16 December 2016) (Appendix 1)
MEDLINE Ovid (1946 to 16 December 2016) (Appendix 2)
Embase.com (1947 to 16 December 2016) (Appendix 3)
PubMed (1948 to 16 December 2016) (Appendix 4)
LILACS (Latin American and Caribbean Health Science Information database (1982 to 16 December 2016) (Appendix 5)
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com; last searched 17 June 2013) (Appendix 6)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 16 December 2016) (Appendix 7)
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp; searched 16 December 2016) (Appendix 8)
Searching other resources
We searched reference lists of trials included in the review to identify any additional candidate studies. We searched the Web of Science Citation Index in December 2016 to find potentially eligible studies that had cited the included trials. We did not search conference proceedings for this review.
Data collection and analysis
Selection of studies
Two review authors independently screened titles and abstracts retrieved from the searches. We classified each abstract as reporting a study eligible for inclusion, unsure, or definitely ineligible. We retrieved full‐text articles corresponding to abstracts classified as eligible for inclusion or unsure. Two review authors then independently screened each full‐text article to determine final eligibility for inclusion in the review. We classified each study as eligible for inclusion, exclusion, or awaiting assessment. We documented excluded studies and reported the reasons for exclusion. We contacted study authors of reports from studies classified as awaiting assessment for information that would allow us to either include or exclude the study from the review. We allowed study authors four weeks to respond. When we received no response after the allotted time period, we classified the study based on available information. Disagreements between the two review authors were resolved by discussion or by arbitration from a third review author if the two authors could not come to an agreement. We did not mask the names of authors, institutions, or journals when reviewing reports from studies during screening.
Data extraction and management
Two review authors independently extracted data from the included trials. We resolved discrepancies and disagreements by discussion. We contacted trial authors to obtain unreported outcome data, allowing them four weeks for a response. Whenever we did not receive a response within four weeks, we reported this and extracted available data. One review author entered data into Review Manager 5 (Review Manager 5 2014), and a second review author checked the data for inaccuracies.
Assessment of risk of bias in included studies
Two review authors independently assessed the included trials for risk of bias. Domains assessed for each included trial are listed below and described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed each trial for each 'Risk of bias' domain as being "low," "high," or "unclear" risk of bias and provide descriptions to support our assessments. Disagreements were resolved by discussion.
We assessed risk of bias for the following domains.
-
Selection bias
Sequence generation. We considered use of methods such as random number tables and computer‐based algorithms to confer "low" risk of bias.
Allocation concealment before randomization. We considered methods such as sequentially numbered, opaque envelopes or central randomization to result in "low" risk of bias.
-
Performance bias
We considered masking of participants and personnel (including any treating physician and other trial staff) to provide "low" risk of bias.
-
Detection bias
Masking (blinding) of outcome assessors. We examined masking of outcome assessors for the primary and secondary outcomes.
-
Attrition bias
Incomplete outcome data. We examined reasons for incomplete data or losses to follow‐up for the primary and secondary outcomes. We also assessed methods used in the trial to account for incomplete data in analyses.
-
Reporting bias
Selective outcome reporting. Whenever the protocol or other reports of methods in the included trial were available, we compared the outcomes specified a prior with those reported for evidence of selective outcome reporting.
-
Other sources of bias
We extracted data on other sources of bias such as source of funding.
Measures of treatment effect
For outcome measures reported as dichotomous data (proportions of participants with intraocular inflammation, BCVA of 20/40 or better, CME, ocular discomfort, and complications), we calculated risk ratios (RR) with 95% confidence intervals (CI). For outcome measures reported as continuous data (time to cessation of treatment and cost analysis), we calculated mean differences (MD) with 95% CIs.
Unit of analysis issues
We expected that most of the included trials would have assigned randomization based on participant, either one or both eyes to the same intervention. We considered the participant to be a cluster when both eyes of a single participant were randomized. We applied the methods described in the Cochrane Handbook for Systematic Reviews of Interventions for analyzing cluster‐randomized trials (Section 16.3) (Higgins 2011b). We sought statistical support from the Cochrane Eyes and Vision Group Editorial Base for analysis of data from trials with both eyes of participants randomized to the same intervention. If some participants in a trial had more than one eye included, for example one eye randomized to one treatment arm and one eye randomized to another treatment arm, we considered the non‐independence of eyes within the same person.
Dealing with missing data
We attempted to contact the authors of reports from included trials for unclear or unreported information needed to assess risk of bias and to analyze outcomes. In the event of unsuccessful contact, we planned to conduct sensitivity analyses and discuss the implications. We planned to (a) assume all participants with missing data in the treatment group had the worse outcome, and (b) no participant with missing data in the other treatment group had the worse outcome. We used methods to handle missing data in the included trials described in the attrition bias section of the 'Risk of bias' tables in the Characteristics of included studies tables.
Assessment of heterogeneity
We examined whether effect estimates among the included trials differed by characteristics of participants and methodology. We examined the forest plots of the primary outcomes and the characteristics of the studies for consistency across studies by considering the value of the I2 statistic. We considered I2 values greater than 50% to represent substantial statistical heterogeneity.
Assessment of reporting biases
We assessed selective reporting of outcomes and analysis as described in the Assessment of risk of bias in included studies section. We assessed potential publication bias by examining the symmetry of funnel plots.
Data synthesis
We conducted meta‐analyses using random‐effects models, unless there were fewer than three trials that contributed outcome data for a meta‐analysis, in which case we used a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analyses to investigate heterogeneity of type, dose, and timing of interventions across trials. We examined consistency in effect estimates in two subgroup comparisons: studies that compared an NSAID with a corticosteroid and studies that compared a combination of an NSAID plus a corticosteroid with corticosteroid treatment alone. We were unable to conduct subgroup analyses using dose and timing of interventions because there were too many different dosing schedules and combinations of treatments to define meaningful subgroups. We had planned to conduct a subgroup analysis for participants who were currently on systemic immunosuppression at the time of cataract surgery or who had received periocular corticosteroid injection less than six weeks prior to cataract surgery compared with participants who were not on immunosuppression and had not received periocular corticosteroid injections, but required data were reported from too few included studies.
Sensitivity analysis
We planned to conduct sensitivity analyses by excluding trials that were judged to be at high risk of bias on domains of allocation and detection bias, but none of the studies included in the meta‐analyses was judged to have high risk of bias for these domains. We performed a sensitivity analysis in which we excluded a study that was industry funded. We also had planned to perform a sensitivity analysis excluding any unpublished studies, but all of the studies included in our meta‐analyses were published journal articles. Sensitivity analyses for incomplete outcome data were described in the Dealing with missing data section.
'Summary of findings' table
We prepared a 'Summary of findings' table for each comparison to summarize the results of our analyses (Table 1, Table 2). We used the GRADE classification system to assess the certainty of the evidence for each outcome based on five factors: study limitations (risk of bias), inconsistency of effect, imprecision, indirectness, and publication bias (GRADEpro 2014). The main outcomes presented in the 'Summary of findings' tables are:
intraocular inflammation measured by anterior chamber cell and flare at one week;
intraocular inflammation measured by proportion of participants with corneal edema at one week;
proportion of participants with BCVA of 20/40 at one week;
proportion of participants with CME at one week;
time to cessation of treatment;
adverse events; and
economic outcomes.
Results
Description of studies
Results of the search
The initial electronic search performed in June 2013 identified 4886 potentially relevant unique references and 386 registry records. We revised the search strategy slightly and conducted updated searches in April and December 2016, identifying an additional 1409 references and 135 registry records. We reviewed 6816 total records (6295 unique references and 521 unique registry records) (Figure 1). We assessed 126 full‐text reports and identified 48 studies that fulfilled our inclusion criteria. These 48 studies were published in 60 reports. We excluded 50 studies from 52 full‐text reports. We categorized eight studies as Studies awaiting classification, either because they appeared to fulfill the inclusion criteria but the registry record provided insufficient detail or lacked data, or because the study was published in a language other than English and we have yet to identify someone to assist with determining the eligibility. Two studies identified as ongoing are currently recruiting participants or have just completed data collection. Details of these studies are reported in the Ongoing studies section. We have reported details of the included studies in the Characteristics of included studies table.
1.
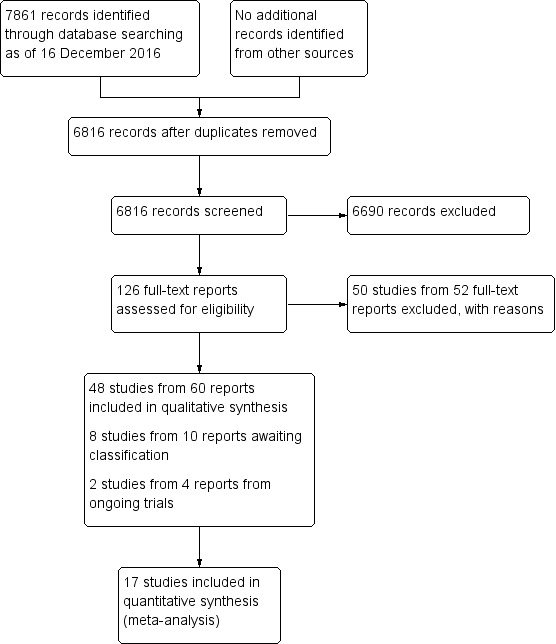
Study flow diagram.
Included studies
We included 48 studies in this review. Details and results from the studies were published in 60 reports and included over 5000 eyes. All included studies were parallel‐group RCTs. Some studies had participants with more than one eye included; in this studies both eyes may be randomized to the same intervention group or one eye randomized to one treatment arm and one eye randomized to another treatment arm. We observed that the analyses reported in these studies did not take into account the non‐independence of eyes and outcomes by using paired‐eye analysis methods. The appropriate use of paired‐eye analysis methods may have resulted in different findings in the individual studies, and therefore may have effected our findings; however, we are unable to assess the effect without the raw data from each included study with this problem.
Types of participants
We included studies that enrolled participants who received phacoemulsification for cataract. Some studies included a group of participants who received other types of cataract surgery, in which case we used only the data from the participants who underwent phacoemulsification. The studies were conducted in 17 different countries: three in Brazil (Ticly 2014; Tzelikis 2015; Zanetti 2012), two in Canada (Almeida 2008; Almeida 2012), four in China (Chen 2015; Li 2011; Wang 2013; Zhang 2008), one in Cuba (Ruiz Rodríguez 2011), one in Egypt (Elsawy 2013), one in France (Adam 2005), two in Germany (Hessemer 1996; Schmitt 1995), two in Greece (Chatziralli 2011; Moschos 2012), one in India (Sahu 2015), one in Iran (Entezari 2016), seven in Japan (Asano 2008; Endo 2010; Kato 1998; Miyake 2007; Miyake 2011; Miyanaga 2009; Shimazaki 1996), one in Mexico (Cervantes‐Coste 2009), one in South Korea (Jung 2015), two in Sweden (Laurell 2002; Zaczek 2014), one in Thailand (Trinavarat 2003), two in Turkey (Dal 2014; Yavas 2007), and seven in the United States (Duong 2014; el‐Harazi 1998; Holzer 2002; Mathys 2010; Singh 2012; Solomon 2001; Wittpenn 2008). Investigators of nine studies did not report in which country the trial had been conducted (Bucci 2001; Demco 1997; Donnenfeld 2006; Guzey 2000; McColgin 1999; Mulet 2001; Ostrov 1997; Roberts 1995; Voudouri 2002).
Types of interventions
Fifteen studies compared an NSAID to a corticosteroid (Asano 2008; Bucci 2001; Demco 1997; Duong 2014; Endo 2010; Guzey 2000; Holzer 2002; Kato 1998; Laurell 2002; Miyake 2007; Miyake 2011; Roberts 1995; Solomon 2001; Voudouri 2002; Wang 2013). Nineteen studies compared a combination of an NSAID plus a corticosteroid with a corticosteroid only (Adam 2005; Almeida 2008; Cervantes‐Coste 2009; Chatziralli 2011; Chen 2015; Dal 2014; Elsawy 2013; Entezari 2016; Li 2011; Mathys 2010; McColgin 1999; Moschos 2012; Ruiz Rodríguez 2011; Shimazaki 1996; Singh 2012; Ticly 2014; Wittpenn 2008; Zaczek 2014; Zhang 2008). Fourteen other studies had three or more arms and had such combinations as an NSAID alone versus a corticosteroid alone versus a combination (Hessemer 1996; Miyanaga 2009; Schmitt 1995); multiple NSAIDs compared with each other and versus a corticosteroid (el‐Harazi 1998; Mulet 2001; Zanetti 2012); an NSAID versus a corticosteroid versus a different corticosteroid (Ostrov 1997; Trinavarat 2003); and multiple combinations of an NSAID plus a corticosteroid against each other with a corticosteroid‐only arm in addition (Almeida 2012; Donnenfeld 2006; Jung 2015; Sahu 2015; Tzelikis 2015; Yavas 2007). NSAIDs given in the studies were indomethacin, ketorolac, nepafenac, diclofenac, bromfenac, flurbiprofen, and pranoprofen. Corticosteroids given in the trials were dexamethasone, prednisolone acetate, betamethasone, rimexolone, fluorometholone, and loteprednol etabonate.
Types of outcomes
The most common primary outcomes reported were flare and cell measurements, visual acuity, IOP measurements, presence of macular edema, and macular thickness and macular volume measurements. The majority of the studies that reported on ocular inflammation using cell and flare measurements in the anterior chamber provided data in a continuous format (mean number of cells, mean photons per milliseconds of flare, or sum of cells and flare), rather than the dichotomous format we expected for this review. The disparity between the format of the expected data and the reported data is shown in Table 6. There were few studies that reported other outcome data in a dichotomous format, stating the "proportion of participants" with a certain outcome, but there were a much larger number of studies that provided outcome data in continuous format. Besides inflammation measured by anterior chamber cells and flare, this situation was also seen with BCVA outcomes (visual acuity reported as a mean for each group rather than a proportion having better than a certain measure) and CME outcomes (mean or change in OCT measures rather than the proportion with CME). Some of the studies collected data on the outcomes we intended to include, but these data were reported only in figures from which we were unable to extract the numbers needed for a meta‐analysis; these studies are noted in the table. For the first primary outcome of this review, the proportion of participants with intraocular inflammation measured by presence of macular edema, only 4/48 (8%) of the included studies reported data (Chatziralli 2011; Singh 2012; Trinavarat 2003 and Tzelikis 2015). Of the four studies, three reported the data in a format that we could not use for meta‐analysis (data was presented either in a figure only, at time points that were not of interest in this review, or used a different definition of macular edema). The other measure of intraocular inflammation that we had planned as our primary outcome was the proportion of participants with anterior chamber cells and flare above grade 1 using the SUN scale, and only 3/48 (6%) studies reported the data in this dichotomous format. We were unable to use these data in any meta‐analysis because in one study, the time point at which these outcomes were measured did not fit our planned time point (Ruiz Rodríguez 2011), and in the two other studies the authors used their own grading scales instead of using the SUN scale (Ostrov 1997); therefore, we were unable to perform a meta‐analysis for this outcome. For the comparison of NSAIDs versus corticosteroids, 12 studies reported measures of inflammation of either the mean number of cells, the mean flare, both separately, or a sum of cells and flare. We were able to include 6 of these in meta‐analyses (Asano 2008; Bucci 2001; Holzer 2002; Miyake 2007; Miyake 2011 and Roberts 1995). Reasons that data from some studies were not usable in meta‐analysis included the studies using their own scale rather than the SUN scale, studies missing data or not reporting all data needed to perform a meta‐analysis, studies reporting data at time points that were not of interest for this review, or studies providing data in the figure only. The second part of our primary outcome was the proportion of participants with BCVA of 20/40 or better at one‐week after surgery, but none of the included studies included BCVA in a dichotomous format. More than half of the included studies provided visual acuity outcomes as continuous measures, such as mean BCVA at a time point or mean BCVA at a time point.
4. Outcomes in included studies: Expected outcomes compared with reported outcomes.
| Primary Outcomes | Secondary outcomes | |||||||||
| Study | Expected outcome: Proportion of participants with corneal edema >grade 0 | Expected outcome: Proportion of participants with anterior chamber cells and flare >grade 1 | Reported outcome: Cells and flare as a continuous measure | Expected outcome: Proportion of participants with BCVA of 20/40 or better | Reported outcome: Visual acuity as a continuous measure | Expected outcome: Proportion of participants with CME | Reported outcome: OCT outcomes or FFA outcomes measured continuously (macular thickness, total macular volume, macular cube volume, fluorescein leakage,etc) | Expected outcome: Time to cessation of treatment for inflammation | Expected outcome: Adverse events | Expected outcome: Economic outcomes (cost) |
| Studies comparing an NSAID to a corticosteroid | ||||||||||
| Asano 2008 | Yes | Yes | Yes | |||||||
| Bucci 2001 | Yes | |||||||||
| Demco 1997 | Yes | Yes | Yes | |||||||
| Duong 2014 | Yes | Yes | ||||||||
| Endo 2010 | Data in figure only | Yes | Yes | |||||||
| Guzey 2000 | Yes | Yes | ||||||||
| Holzer 2002 | Yes | Yes | ||||||||
| Kato 1998 | Partial | |||||||||
| Laurell 2002 | Yes | Yes | ||||||||
| Miyake 2007 | Yes | Yes | Yes | Yes | ||||||
| Miyake 2011 | Yes | Yes | Yes | Yes | ||||||
| Roberts 1995 | Yes | |||||||||
| Solomon 2001 | Yes | Yes | ||||||||
| Voudouri 2002 | Yes | |||||||||
| Wang 2013 | Yes | Yes | ||||||||
| Studies comparing an NSAID plus a corticosteroid to a corticosteroid alone | ||||||||||
| Adam 2005 | ||||||||||
| Almeida 2008 | ||||||||||
| Cervantes‐Coste 2009 | Yes | |||||||||
| Chatziralli 2011 | Data in figure only | Yes | ||||||||
| Chen 2015 | Yes | |||||||||
| Dal 2014 | Yes | |||||||||
| Elsawy 2013 | Yes | Yes | ||||||||
| Entezari 2016 | Yes | Yes | Yes | |||||||
| Li 2011 | Yes | Yes | Yes | |||||||
| Mathys 2010 | Yes | Yes | ||||||||
| McColgin 1999 | Yes | Yes | ||||||||
| Moschos 2012 | Yes | Yes | Yes | |||||||
| Ruiz Rodríguez 2011 | Partial | Yes | ||||||||
| Shimazaki 1996 | Data in figure only | Yes | ||||||||
| Singh 2012 | Partial | Yes | ||||||||
| Ticly 2014 | Yes | Yes | Yes | |||||||
| Wittpenn 2008 | Yes | Yes | Yes | Yes | ||||||
| Zaczek 2014 | Data in figure only | Yes | Yes | Yes | Yes | |||||
| Zhang 2008 | Yes | Yes | ||||||||
| Studies with other combinations of NSAIDs and corticosteroids | ||||||||||
| Almeida 2012 | Yes | |||||||||
| Donnenfeld 2006 | Yes | Yes | Yes | Yes | ||||||
| el‐Harazi 1998 | Yes | |||||||||
| Hessemer 1996 | Data in figure only | Yes | Yes | |||||||
| Jung 2015 | Yes | Yes | Yes | |||||||
| Miyanaga 2009 | Yes | Yes | Yes | Yes | ||||||
| Mulet 2001 | ||||||||||
| Ostrov 1997 | Yes | Yes | Yes | |||||||
| Sahu 2015 | Yes | Yes | Yes | |||||||
| Schmitt 1995 | Data in figure only | |||||||||
| Trinavarat 2003 | Yes | Yes | Yes | Yes | ||||||
| Tzelikis 2015 | Yes | Yes | Yes | |||||||
| Yavas 2007 | Yes | Yes | ||||||||
| Zanetti 2012 | Yes | |||||||||
Partial refers to studies missing data or not reporting all data needed to perform a meta‐analysis (such as reporting means without standard deviations) and studies reporting data at time points that were not of interest for this review.
Excluded studies
We excluded 50 studies from the review after assessing the full‐text reports. The reasons for exclusion are detailed in the Characteristics of excluded studies tables. We excluded nine studies because participants received a type of cataract surgery other than phacoemulsification (such as extracapsular cataract extraction or intracapsular cataract extraction) (Abelson 1989; Hessemer 1994; Knopf 1970; Othenin‐Girard 1994; Smerdon 1986; Sourdille 1993; Suharwardy 1994; Szymanski 1994; Tunc 1999). In six other studies, the type of cataract surgery was unclearly reported (EUCTR2015‐003296‐30‐FI; Ferreira 2006; Kraff 1990; McDonald 1998; Tauber 2006; Yung 2007). Four studies included a combination of participants who received various types of cataract surgery and did not report outcomes separately for phacoemulsification (Barequet 2002; Farooq 2003; Missotten 2001; Raju 2005). We excluded eight studies because comparisons differed from those specified for this review, for example an NSAID plus a corticosteroid versus a placebo or an NSAID plus a corticosteroid versus an NSAID alone (EUCTR2009‐017031‐18‐NL; ISRCTN02628492; Luo 2013; NCT00407017; NCT00698724; NCT01193504; Nishino 2009; Ramakrishnan 2015); in one study the comparison was unclear (NCT00433225). Six other studies did not include an either NSAID arm or a corticosteroid arm (Burde 1972; Chang 2016; Corbett 1993; Hosseini 2016; Meconi 1998; Pollack 2017). Seven studies either were not randomized or we could not tell whether they were randomized (Hossain 2010; Liou 1991; Miyake 1998; Miyake 2000; Ozkurt 2003; Turan‐Vural 2013; Waseem 2009). Two were letters to the editor (Barequet 2004; Rocha 2009), one had no comparison group (Carenini 1993), one modeled existing trial data (Mullins 2016), two studies reported on a mix of surgeries for various eye conditions (Maheshwari 1995; Yasuda 2016), one was a dosage study (NCT00758199), and one used oral rather than topical corticosteroids (Sabiston 1987). We excluded one other study because the report was not intended to assess vision outcomes (Bahar 2007).
Risk of bias in included studies
Only one of the included studies had low risk of bias in all categories (Zanetti 2012). We judged seven studies to be at unclear risk of bias across all domains; four of these studies had been reported only in abstracts and provided limited detail on study characteristics (Bucci 2001; Dal 2014; Guzey 2000; Kato 1998; Miyanaga 2009; Mulet 2001; Voudouri 2002). The majority of the studies (26 of 48) were judged to be at unclear risk of bias in five or more domains. Figure 2 and Figure 3 summarize the 'Risk of bias' judgements across studies and for each individual study, respectively.
2.
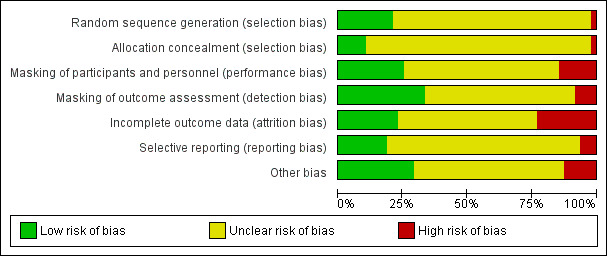
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
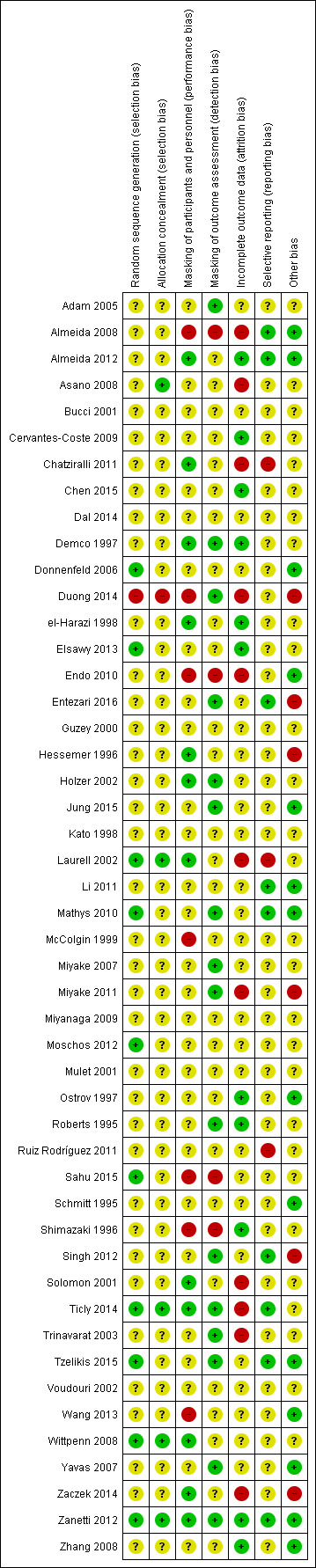
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Though all included studies were reported to be randomized, reports from 36 studies did not describe either how the randomization was performed or how allocation was concealed before randomization. We judged these studies to be at unclear risk for selection bias (Adam 2005; Almeida 2008; Almeida 2012; Bucci 2001; Cervantes‐Coste 2009; Chatziralli 2011; Chen 2015; Dal 2014; Demco 1997; el‐Harazi 1998; Endo 2010; Entezari 2016; Guzey 2000; Hessemer 1996; Holzer 2002; Jung 2015; Kato 1998; Li 2011; McColgin 1999; Miyake 2007; Miyake 2011; Miyanaga 2009; Mulet 2001; Ostrov 1997; Roberts 1995; Ruiz Rodríguez 2011; Schmitt 1995; Shimazaki 1996; Singh 2012; Solomon 2001; Trinavarat 2003; Voudouri 2002; Wang 2013; Yavas 2007; Zaczek 2014; Zhang 2008). Reports from six studies described on how the randomization sequence was generated but did not report allocation concealment (Donnenfeld 2006; Elsawy 2013; Mathys 2010; Moschos 2012; Sahu 2015; Tzelikis 2015). Investigators from only four studies reported both how the randomization sequence was generated and how allocation was concealed (Laurell 2002; Ticly 2014; Wittpenn 2008; Zanetti 2012). One study described allocation concealment but not sequence generation (Asano 2008). We judged one study to have a high risk of selection bias: Duong 2014 reported randomizing participants to an intervention group based on which week of the month they were going to receive surgery. Since it was planned in advance which groups the participants would be assigned to, allocation was not concealed, creating a high risk of selection bias in this study.
Masking (performance bias and detection bias)
Many of the included studies did not report masking of participants and personnel or masking of outcome assessors and were therefore judged to have an unclear risk of both performance and detection bias (Asano 2008; Bucci 2001; Cervantes‐Coste 2009; Chen 2015; Dal 2014; Donnenfeld 2006; Elsawy 2013; Guzey 2000; Kato 1998; Li 2011; Miyanaga 2009; Moschos 2012; Mulet 2001; Ostrov 1997; Ruiz Rodríguez 2011; Schmitt 1995; Voudouri 2002; Zhang 2008). We judged four studies to have a high risk of both performance bias and detection bias because they were reported to be non‐masked, open‐label trials (Almeida 2008; Endo 2010; Sahu 2015; Shimazaki 1996). We judged four studies to have a low risk of both performance bias and detection bias because they reported masking for participants, personnel, and outcome assessors (Demco 1997; Holzer 2002; Ticly 2014; Zanetti 2012). Some of the included studies reported that participants and surgeons were masked, but did not report on masking for outcome assessors; we judged these studies to have a low risk of performance bias but an unclear risk of detection bias (Almeida 2012; Chatziralli 2011; el‐Harazi 1998; Hessemer 1996; Laurell 2002; Solomon 2001; Wittpenn 2008; Zaczek 2014). Conversely, we judged other studies to have an unclear risk of performance bias because they did not report whether participants and physicians were masked, but a low risk of detection bias because they reported that outcome assessors were masked (Adam 2005; Entezari 2016; Jung 2015; Mathys 2010; Miyake 2007; Miyake 2011; Roberts 1995; Singh 2012; Trinavarat 2003; Tzelikis 2015; Yavas 2007). We judged one study that reported that participants were not masked, although the surgeons and evaluating ophthalmologist were, to be at high risk for performance bias and low risk for detection bias (Duong 2014). We judged two studies that were self reported "single‐masked" studies to be at high risk of performance bias and unclear risk of detection bias due to participants having knowledge of their assigned study medication, but unclear reporting on whether the physicians, surgeons, or outcome assessors were masked (McColgin 1999; Wang 2013).
Incomplete outcome data
Eleven studies were judged to have a high risk of attrition bias because some or all participants who did not complete the study were not included in the data analysis (Almeida 2008; Asano 2008; Chatziralli 2011; Duong 2014; Endo 2010; Laurell 2002; Miyake 2011; Solomon 2001; Ticly 2014; Trinavarat 2003; Zaczek 2014). We evaluated 11 studies as having a low risk of attrition bias (Almeida 2012; Cervantes‐Coste 2009; Chen 2015; Demco 1997; el‐Harazi 1998; Elsawy 2013; Ostrov 1997; Roberts 1995; Shimazaki 1996; Zanetti 2012; Zhang 2008). These studies reported that all participants completed all of the follow‐up visits (no missing data) or that missing data were handled appropriately by using an intention‐to‐treat analysis. The other 26 studies did not report on the degree of missing data or how this information was handled and were judged to have an unclear risk of attrition bias (Adam 2005; Bucci 2001; Dal 2014; Donnenfeld 2006; Entezari 2016; Guzey 2000; Hessemer 1996; Holzer 2002; Jung 2015; Kato 1998; Li 2011; Mathys 2010; McColgin 1999; Miyake 2007; Miyanaga 2009; Moschos 2012; Mulet 2001; Ruiz Rodríguez 2011; Sahu 2015; Schmitt 1995; Singh 2012; Tzelikis 2015; Voudouri 2002; Wang 2013; Wittpenn 2008; Yavas 2007).
Selective reporting
For the majority of included studies we could not judge whether there was selective outcome reporting because a protocol was either not cited or not available (Adam 2005; Asano 2008; Bucci 2001; Cervantes‐Coste 2009; Chen 2015; Dal 2014; Demco 1997; Donnenfeld 2006; Duong 2014; el‐Harazi 1998; Elsawy 2013; Endo 2010; Guzey 2000; Hessemer 1996; Holzer 2002; Jung 2015; Kato 1998; McColgin 1999; Miyake 2007; Miyake 2011; Miyanaga 2009; Moschos 2012; Mulet 2001; Ostrov 1997; Roberts 1995; Sahu 2015; Schmitt 1995; Shimazaki 1996; Solomon 2001; Trinavarat 2003; Voudouri 2002; Wang 2013; Wittpenn 2008; Yavas 2007; Zaczek 2014; Zhang 2008). We judged three studies to have a high risk of reporting bias: in Laurell 2002 and Ruiz Rodríguez 2011 some outcomes were not reported at all follow‐up time points. In Chatziralli 2011 some participants continued treatment longer than others, but the results for the continued treatment were not reported. In nine studies, the reported outcomes matched the outcomes described in the trial registry, protocol, or methods section of the study reports (Almeida 2008; Almeida 2012; Entezari 2016; Li 2011; Mathys 2010; Singh 2012; Ticly 2014; Tzelikis 2015; Zanetti 2012).
Other potential sources of bias
For 13 studies we did not identify any other potential sources of bias (Almeida 2008; Almeida 2012; Donnenfeld 2006; Endo 2010; Jung 2015; Li 2011; Mathys 2010; Ostrov 1997; Schmitt 1995; Tzelikis 2015; Wang 2013; Yavas 2007; Zanetti 2012; Zhang 2008). We judged 22 studies to have an unclear risk of bias due to funding sources or declarations of interest or both not being reported (Adam 2005; Asano 2008; Bucci 2001; Cervantes‐Coste 2009; Chatziralli 2011; Chen 2015; Dal 2014; Elsawy 2013; Guzey 2000; Kato 1998; Laurell 2002; McColgin 1999; Miyake 2007; Miyanaga 2009; Moschos 2012; Mulet 2001; Ruiz Rodríguez 2011; Sahu 2015; Shimazaki 1996; Ticly 2014; Trinavarat 2003; Voudouri 2002; Wittpenn 2008). We judged some of these studies to have unclear risk of bias also because some participants had both eyes enrolled and randomized, but it was unclear whether the authors had taken into account the non‐independence of eyes in the analysis. We judged four studies to have a high risk of bias because the authors were employees or consultants to the pharmaceutical company that manufactured one of the study medications, or the company provided the medications used in the study (Hessemer 1996; Miyake 2011; Singh 2012; Zaczek 2014). Five studies were judged to have unclear risk of bias because although it was reported that funding was provided by a pharmaceutical company that manufactured that study drug, the authors were not employees or consultants of the companies and reported no conflicts of interest (Demco 1997; el‐Harazi 1998; Holzer 2002; Roberts 1995; Solomon 2001). We judged two studies to have a high risk of bias because of unusual protocols when treatment with the assigned study drug appeared not to work. In Duong 2014, participants who did not respond to the study drug were given a "rescue medication" of the other medication class. Entezari 2016 reported that "the frequency of the corticosteroid drops was adjusted for each eye."
Effects of interventions
NSAIDs versus corticosteroids
Primary outcomes
Intraocular inflammation at one week after surgery
Inflammation measured by anterior chamber cell and flare
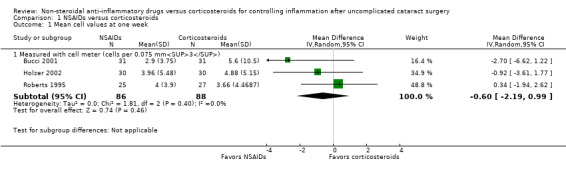
None of the included studies that compared treatment with an NSAID to treatment with a corticosteroid reported inflammation as a dichotomous outcome in the format of the percentage with anterior chamber cell and flare classifications of 1 or higher according to the SUN working group criteria (Jabs 2005). However, several of the included studies reported mean cell and flare measurements. As a modification to the published protocol, we performed a meta‐analysis on the mean cell values at one week (Analysis 1.1, Figure 4) and the mean flare values at one week (Analysis 1.2, Figure 5), though we noted that the data for these outcomes were skewed, which may affect our analysis and interpretation of the data. Three studies reported the mean cell data measured using a cell meter (Bucci 2001; Holzer 2002; Roberts 1995). There was uncertainty as to whether treatment with an NSAID or treatment with a corticosteroid resulted in less inflammation marked by fewer cells in the anterior chamber (MD ‐0.60, 95% CI ‐2.19 to 0.99). We downgraded the certainty of the evidence for this outcome once and judged it to be moderate due to risk of bias because the included studies were all poorly reported. We did not downgrade for imprecision because a difference of approximately two cells in the anterior chamber is not clinically significant. Two other studies reported on mean cell data measured using a slit lamp rather than the cell and flare meter, however they used different scales to grade the number of cells and could not be combined in a meta‐analysis (Guzey 2000; Holzer 2002). Five studies reported the mean flare data obtained from a flare meter (in photons/millisecond) at one week postoperatively (Asano 2008; Bucci 2001; Miyake 2007; Miyake 2011; Roberts 1995). The meta‐analysis showed that the mean flare was 13.74 photons/ms lower in the NSAID group compared with the corticosteroid group (MD ‐13.74, 95% CI ‐21.47 to ‐6.00), indicating less intraocular inflammation for those who received NSAIDs, but we noted that very high statistical heterogeneity among the studies included in this analysis (I2 = 92%). We judged the certainty of the evidence for this outcome to be low; we downgraded for this inconsistency and because of potential risk of bias due to poor reporting of the studies included in this meta‐analysis. As with the cell measurements, the same studies also reported mean flare value measured using a slit lamp rather than a flare meter (Guzey 2000; Holzer 2002), but we again did not combine these in a meta‐analysis. Holzer 2002 reported on the mean flare data recorded by a flare meter as well, but the scale used to report the outcome was unclear. Three studies reported on the sum of cells and flare, but because one used its own scale (Demco 1997), and one did not report standard deviations (Duong 2014), the data could not be analyzed with the third study (Roberts 1995).
1.1. Analysis.
Comparison 1 NSAIDs versus corticosteroids, Outcome 1 Mean cell values at one week.
4.
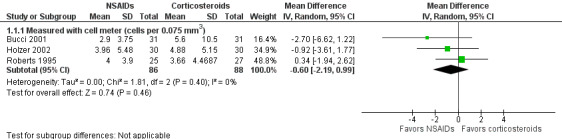
Forest plot of comparison: 1 NSAIDs versus corticosteroids, outcome: 1.1 Mean cell values at one week.
1.2. Analysis.
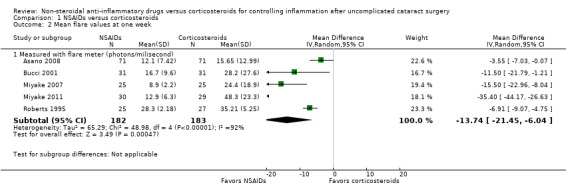
Comparison 1 NSAIDs versus corticosteroids, Outcome 2 Mean flare values at one week.
5.
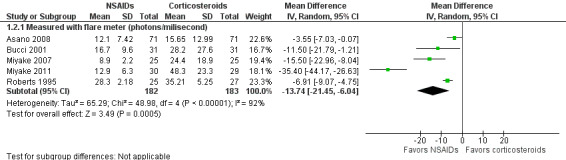
Forest plot of comparison: 1 NSAIDs versus corticosteroids, outcome: 1.2 Mean flare values at one week.
Inflammation defined by presence of corneal edema
Only one study that compared an NSAID with a corticosteroid reported on the presence of corneal edema at one week. In the NSAID group, 4 of 39 participants (10.3%) and, in the corticosteroid group, 10 out of 75 participants (13.3%) showed signs of corneal edema (Trinavarat 2003). There was insufficient evidence to conclude that one treatment group had a lower proportion of corneal edema at one week post‐surgery than the other (RR 0.77, 95% CI 0.26 to 2.29). We judged the certainty of the evidence for this outcome to be low, downgraded one level each due to imprecision and high risk of performance and detection bias; this was reported as an unmasked study.
BCVA of 20/40 or better at one week after surgery
Though some included studies that compared treatment with an NSAID with treatment with a corticosteroid reported the mean BCVA for treatment groups at one week follow‐up, none of the included studies reported BCVA as a dichotomous variable that reported on the proportion of participants with BCVA of 20/40 or better at one‐week after surgery.
Secondary outcomes
Cystoid macular edema at one week after surgery
None of the included studies reported the presence of CME at one week after surgery, but several studies reported this outcome at other time points up to one month postoperatively. Miyake 2007 reported that at two weeks' post‐cataract surgery none of the participants who had received an NSAID had CME, while four participants who had received a corticosteroid had CME (0% versus 16%). However, this study was relatively small, and there was statistical uncertainty as to whether the NSAID treatment was favored (RR 0.11, 95% CI 0.01 to 1.96). Another study reported on the proportion of participants that had a CME during the trial follow‐up period, but did not report the time point at which the outcome was observed (Wang 2013). In this study, none of the participants in the NSAID group had CME, while seven in the corticosteroid group had CME. This study showed that participants who received an NSAID had a lower risk of developing CME, but the 95% confidence interval crossed the null value and there was uncertainty in the measurement (RR 0.07, 95% CI 0.00 to 1.16).
We included four studies in our meta‐analysis of the proportion of participants with CME by one month postoperatively. Asano 2008, Miyake 2007, and Miyake 2011 reported this outcome at five weeks' postoperatively, while Miyanaga 2009 reported at one month postoperatively. Participants who were treated with an NSAID had a lower risk of CME at one month compared with participants who were treated with a corticosteroid (RR 0.26, 95% CI 0.17 to 0.41; Analysis 1.3). Another study with three arms that compared two different NSAIDs with a corticosteroid reported that no participants had CME at one month postoperatively. We performed a sensitivity analysis removing one study (Miyake 2011) in which the authors were paid consultants the manufacturer one the study drugs. Removing this data from the meta‐analysis did not change the conclusions; participants treated with an NSAID had less risk of developing CME compared with participants treated with a corticosteroid (RR 0.30, 95% CI 0.18 to 0.49). Based on our sensitivity analysis, we did not downgrade the certainty of the evidence based on the potential risk of bias from the influence of the manufacturer of the study drugs, however we did downgrade based on risk of bias for the studies in this analysis, as they were poorly reported, details of masking and analysis were unclear, and in one there was a high risk of attrition bias. We also downgraded the evidence one level due to indirectness, because our outcome of interest was CME at one week, and the data we were able to combine was for one month. Additionally, two of the studies included in the meta‐analysis used fluorometholone as the corticosteroid in the comparison, which is a very weak corticosteroid and therefore a weak comparator. These two studies contributed 30% of the weight of the meta‐analysis, which also contributed to our decision to downgrade for indirectness. The certainty of the evidence for the outcome of CME at one month postoperatively was low.
1.3. Analysis.
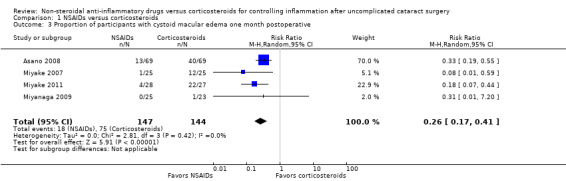
Comparison 1 NSAIDs versus corticosteroids, Outcome 3 Proportion of participants with cystoid macular edema one month postoperative.
Time to cessation of treatment
None of the included studies that compared an NSAID with a corticosteroid reported on the time to cessation of treatment.
Adverse events
None of the studies in this comparison reported on adverse events.
Economic outcomes
None of the studies in this comparison reported on economic outcomes.
Combination of NSAIDs plus corticosteroids versus corticosteroids alone
Primary outcomes
Intraocular inflammation at one week after surgery
Inflammation measured by anterior chamber cell and flare
None of the included studies that compared a combination of NSAID plus a corticosteroid with a corticosteroid alone reported inflammation by anterior chamber cell and flare as a dichotomous outcome. There was not enough continuous data for anterior chamber cell and flare to perform a meta‐analysis.
Inflammation defined by presence of corneal edema
Only one study that compared the combination of an NSAID plus a corticosteroid versus a corticosteroid alone reported on the proportion of participants with corneal edema each week up to one month postoperatively (Chatziralli 2011). At each of the four time points, there was statistical uncertainty as to which group had a lower risk of developing corneal edema (RR 1.07, 95% CI 0.98 to 1.16 at one week; RR 0.52, 95% CI 0.24 to 1.14 at two weeks; RR 0.97, 95% CI 0.20 to 4.65 at three weeks; and RR 2.92, 95% CI 0.12 to 70.35 at four weeks). We reported the one‐week data in our Table 2, and downgraded one level to moderate due to concerns about reporting bias: the study investigators said they collected data at certain postoperative time points, but no all results were reported. Another study reported that no participant in either the NSAID plus corticosteroid group or the corticosteroid‐alone group presented with corneal edema at four weeks (Moschos 2012).
BCVA of 20/40 or better at one week after surgery
None of the included studies that compared a combination of an NSAID plus a corticosteroid with a corticosteroid alone reported on the proportion of participants that had BCVA of 20/40 or better one week after surgery. Some included studies did report the mean BCVA for each treatment group at one week after surgery; however, this was not an outcome of interest for this review.
Secondary outcomes
Cystoid macular edema at one week after surgery
Only one of the included studies reported presence of CME at one week after surgery (Chen 2015), but several studies reported this outcome at later follow‐up time points. One study reported on the incidence of CME at two weeks after surgery (Donnenfeld 2006). We combined the one‐week data from Chen 2015 with the two‐week data from Donnenfeld 2006; the combined estimates favored the combination of NSAID plus corticosteroid over corticosteroid alone (RR 0.17, 95% CI 0.03 to 0.97; Figure 6, Analysis 2.1). We graded the certainty of the evidence for this outcome as low; we downgraded one level each for inconsistency and imprecision. We included seven studies in the meta‐analysis for the proportion of participants who had CME at one month postoperatively (Jung 2015; Li 2011; Miyanaga 2009; Moschos 2012; Ticly 2014; Wittpenn 2008; Zaczek 2014), including one study that reported cystoid macular edema at a three‐week examination and another study that reported at five weeks post‐operatively (Zaczek 2014 and Ticly 2014, respectively). This analysis favored the combination of an NSAID plus a corticosteroid over a corticosteroid alone for an approximately 50% lower risk of CME present at one month after surgery; however there was statistical uncertainty (RR 0.50, 95% CI 0.23 to 1.06; Analysis 2.2). Two of the studies included in the analysis reported that no participant in either study arm had CME at one month after surgery.
6.
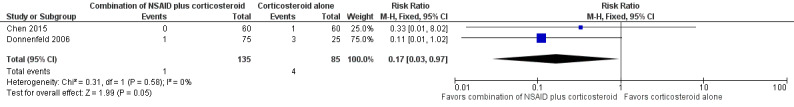
Forest plot of comparison: 2 NSAIDs plus corticosteroids versus corticosteroids alone, outcome: 2.1 Proportion of participants with cystoid macular edema at one week.
2.1. Analysis.
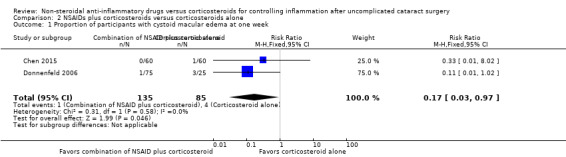
Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 1 Proportion of participants with cystoid macular edema at one week.
2.2. Analysis.
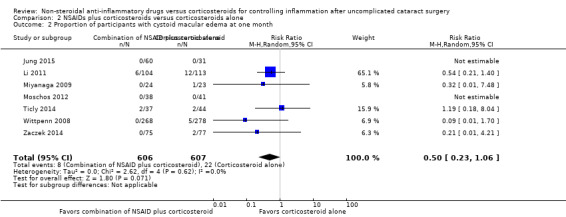
Comparison 2 NSAIDs plus corticosteroids versus corticosteroids alone, Outcome 2 Proportion of participants with cystoid macular edema at one month.
Time to cessation of treatment
None of the included studies that compared a combination of an NSAID plus a corticosteroid with a corticosteroid alone reported time to cessation of treatment.
Adverse events
Only a few of the studies in this comparison reported adverse events associated with the eye drops used after cataract surgery. Sahu 2015 reported that no participants in that study reported any adverse effects related to topical NSAID use and that the only clinically significant adverse event was associated with a complication of the surgery rather than the NSAID eye drops. This participant affected had a heterogeneous retinal detachment at four weeks that subsequently was managed with pars plana vitrectomy. An epiretinal membrane was detected at four weeks after surgery in another participant. Almeida 2012 used the Comparison of Ophthalmic Medications for Tolerability Questionnaire (COMTOL) to ascertain the frequency and severity of side effects. The investigators reported that there was no significant difference between the three study groups for all side effects, and presented only the overall number of participants with selected adverse events. Three of the five most commonly reported side effects were markers of ocular discomfort: burning (29%), redness (18%), and blurred vision (12%).
Economic outcomes
None of the studies in this comparison reported on economic outcomes.
Discussion
Summary of main results
NSAIDs alone versus corticosteroids alone
Though we had pre‐specified the use of dichotomous outcomes, none of the included studies that compared NSAIDs alone with corticosteroids alone reported postoperative ocular inflammation in terms of cells and flare as a dichotomous variables; to make use of data provided in the included studies, we examined data for cell and flare measurements that had been reported as continuous measures. Only one study reported the other indicator of intraocular inflammation, presence of corneal edema at one week postoperatively: no statistically significant difference was found between the NSAID group and the corticosteroid group. None of the included studies reported BCVA after phacoemulsification or time to cessation of treatment of inflammation. None of the included studies reported on the proportion of eyes with CME at one week postoperatively; however, six studies reported the presence of CME at other time points, including four RCTs involving a total of 291 participants that reported CME at one month. We found low‐certainty evidence that participants treated with an NSAID had a lower risk of CME compared with those treated with a corticosteroid (RR 0.26, 95% CI 0.17 to 0.41). One of the studies had authors who were paid consultants of the company that manufactured the study drugs. We performed a sensitivity analysis to examine the effect of this potential risk of bias on the outcome estimate. Excluding data from this study did not change the conclusions: participants treated with an NSAID still had a lower risk of developing CME compared with participants treated with a corticosteroid (RR 0.30, 95% CI 0.18 to 0.49). None of the studies in this comparison reported on adverse events or economic outcomes. A variety of NSAIDs were used in these studies including diclofenac, nepafenac, and bromfenac. The studies also varied in the corticosteroid that was used and in their dosing schedules. Follow‐up after phacoemulsification ranged from five to eight weeks. In general, the studies were poorly reported so that many of the 'Risk of bias' domains were judged to be unclear. Only one included study was judged to be at low risk of bias in all domains.
NSAIDs plus corticosteroids versus corticosteroids alone
Again, ocular inflammation in terms of cells and flare were not reported as a dichotomous variable in the studies that compared a combination of an NSAID plus a corticosteroid with a corticosteroid alone. One study with high risk of reporting bias reported corneal edema at various postoperative time points. At each time point, neither treatment with a combination of NSAID plus corticosteroid nor corticosteroid alone was favored. No study reported on BCVA at one week postoperatively or time to cessation of treatment. Only one included study reported on the presence of CME at one week after surgery, and another study reported CME at two weeks after surgery. The combined analysis included 220 participants and estimated a lower risk of CME in the group receiving NSAIDs plus corticosteroids (RR 0.17, 95% CI 0.03 to 0.97), however we found this to be weak evidence based on inconsistency and imprecision of the estimate. At one‐month follow‐up, seven RCTs reported the proportion of participants with CME; the combined data did not support a significant risk reduction (RR 0.50, 95% CI 0.23 to 1.06). In general, the studies were poorly reported. Two studies in this comparison reported adverse events: one noted that adverse events were complications of surgery rather than a result of study treatments, and the other found ocular side effects such as burning, redness, and blurred vision that occurred at about the same rates in each study arm. None of the studies in this comparison reported on economic outcomes. None of the studies were judged to be at low risk of bias in all domains.
Overall completeness and applicability of evidence
We included a relatively large number of studies in both intervention comparisons examined in this review; the studies have a wide global range.
The included studies compared NSAIDs and corticosteroids after uncomplicated phacoemulsification. The results of this review may therefore be less applicable in parts of the world where other cataract surgery techniques, including manual extracapsular cataract extraction, are more commonly utilized.
The primary aim of this review was to assess the effectiveness of topical NSAIDs, alone or in combination with topical corticosteroids, versus topical corticosteroids alone, in controlling intraocular inflammation after uncomplicated phacoemulsification. No studies reported anterior chamber inflammation as a dichotomous variable, therefore no conclusion can be made about this topic. We were able to perform meta‐analysis of continuous cell and flare data for the comparison of NSAIDs versus corticosteroids, however, we noted that the data was skewed. The evidence was also sparse for corneal edema, however there did not appear to be any significant effect of NSAIDs this outcome. There have been more reports of the proportion of eyes with postoperative CME, mainly at time points beyond two weeks after surgery. However, due to the heterogeneity of types, doses, and regimens of NSAIDs and corticosteroids used in the studies, we could only make moderate‐certainty conclusions.
In order to perform meta‐analyses for this review, we chose to combine data from different time points, such as outcomes reported at "one month" with outcomes reported at 5 weeks. It is possible that extra days of treatment in one study compared with fewer days in another (for example, 35 days [5 weeks] of treatment versus 28‐31 days [one month] of treatment) may be significant, especially given that the presence or absence of some of our outcomes may be dose‐dependent and differ based on the amount of medication to which each eye was exposed.
This review included studies that used a variety of NSAIDs and corticosteroids. Fluorometholone is a low potency corticosteroid that was used in two studies that contributed to our meta‐analyses. This is a weak comparator and though it contributed about 30% of the weight of the data for our meta‐analysis on the risk of CME at one month postoperatively, the effect estimates may have been different had a stronger corticosteroid been used. In terms of the secondary objectives of this review, BCVA, patient‐reported discomfort, complications such as elevation of IOP, and cost‐effectiveness were rarely or never reported; no conclusions could be drawn regarding these topics.
Quality of the evidence
In general, we graded the evidence as being of low and moderate‐certainty. The reasons for downgrades included imprecision and heterogeneity in factors such as study design, medications used, and regimens studied. The outcome of mean anterior chamber flare was downgraded due to suspected publication bias. We suspected it may be possible that smaller studies that did not have statistically significant effects may never have been submitted for publication. Many of the included studies were also poorly reported, so that it was difficult to judge the degree of bias likely present in the various domains. In our certainty estimates, we downgraded for risk of bias when the analysis included poorly‐reported studies.
Potential biases in the review process
Due to the nature of the research question we posed in the review, and our goal of exploring the proportion of participants with anterior chamber inflammation at one week postoperative (cell or flare) as a dichotomous variable, the data regarding anterior chamber inflammation in all RCTs were not applicable. Defining the primary outcome as intraocular inflammation at one week of follow‐up also limited the applicability of data regarding CME, which were mostly reported at later time points.
Agreements and disagreements with other studies or reviews
A recent Cochrane review (Lim 2016) evaluated the proportion of people with a poor vision outcome due to CME at three months after cataract surgery, with poor vision defined as 6/9 or worse attributed to a diagnosis of CME. That study found low‐certainty evidence that people who received topical NSAIDs in combination with steroid may have had a lower risk of poor vision due to macular edema at three months after cataract surgery compared to those taking corticosteroids alone (RR 0.40, 95% CI 0.27 to 0.61). In that study, cataract surgery included manual extracapsular cataract extraction, manual small‐incision cataract surgery, phacoemulsification cataract surgery, and intracapsular cataract extraction.
Another review by Wielders 2015 reported a similar effect, though they reported odds ratios rather than risk ratios. The authors did not comment on the quality of the evidence in their conclusions.
A report by the American Academy of Ophthalmology (Kim 2015) concluded that NSAIDs reduced the incidence of CME, and may increase visual recovery, but found that the use of NSAIDs did not alter long‐term (three‐month) visual outcomes.
One review published in 2014 evaluated the incidence of CME after phacoemulsification alone and compared NSAIDs with corticosteroids alone (Kessel 2014). They evaluated inflammation within one week of surgery and macular edema at any time point. The authors reported greater risk of CME in the corticosteroid group when compared with the NSAID group as we do in this review, but with a stronger effect (RR 5.35, 95% CI 2.94 to 9.76). Kessel 2014 considered the evidence in their review to be of high‐certainty.
Authors' conclusions
Implications for practice.
The evidence for the treatment of postoperative inflammation after uncomplicated phacoemulsification is insufficient to inform practice. From the 48 trials included in this review, we could not ascertain the equivalence or superiority of non‐steroidal anti‐inflammatory drugs (NSAIDs) with or without corticosteroids versus corticosteroids for the treatment of postoperative inflammation; we were unable to make conclusions about our primary outcome. The amount of flare in the anterior chamber at one week postoperatively may be lower for those treated with an NSAID, but the evidence is weak. There may be some risk reduction of CME with NSAIDs alone (low‐certainty evidence) or in combination with corticosteroids (low‐certainty evidence) in the first month after phacoemulsification.
Implications for research.
Large, adequately powered, well‐designed, independently‐funded RCTs to compare NSAIDs with and without corticosteroids versus corticosteroids alone for the treatment of inflammation following uncomplicated phacoemulsification are needed. These studies should ideally standardize the type of medication used, dosing, and treatment regimen. Data should be collected and presented using the Standardization of Uveitis Nomenclature (SUN) outcome measures so that dichotomous outcomes can be analyzed. Reporting these outcomes as percentages rather than means will allow clinicians to determine whether there will be a difference in risk of inflammation when prescribing an NSAID, a corticosteroid, or a combination. This is preferable to a mean change in an inflammation measure, because an individual patient may have measures higher or lower than the mean. With meta‐analyses based on dichotomous outcomes, ophthalmologists could make clinical decisions on which medication to use based on the risk of inflammation rather than the expected mean change. Future meta‐analyses and systematic reviews may make use of continuous data for BCVA in order to elucidate the relationship of macular edema and BCVA.
Acknowledgements
We acknowledge John Gonzales, David Gritz, Roomasa Channa, Guilherme Quinto, and Alisa Kim for co‐authoring the protocol for this review and Swaroop Vedula for assisting with the protocol design and draft. We acknowledge Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), for developing the search strategy and executing the electronic searches. We acknowledge Sueko Matsumura, Reva Datar, Sarah Money, Cesar Ugarte, and Nan Guo for assistance with data extraction. We acknowledge Shuiqing Liu, Hsin‐wen Wu, Yu‐Tian Xiao, and Yuanxi Jia for help with screening and data extraction of Chinese language studies; Karin Rau and Jutta Scheffczik for help with screening and data extraction of German language studies; Jan Witowski for help with screening of Polish language studies; and Cesar Ugarte for help with screening and data extraction of Spanish language studies.
This work was undertaken in collaboration with the National Institute for Health and Care Excellence (NICE). The views expressed in this publication are those of the authors and not necessarily those of NICE.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cataract] explode all trees #2 MeSH descriptor: [Cataract Extraction] explode all trees #3 MeSH descriptor: [Phacoemulsification] explode all trees #4 MeSH descriptor: [Capsulorhexis] explode all trees #5 (Cataract* near/4 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant*)) #6 (lens* near/4 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant* or emulsif*)) #7 (Cataract Extraction* or Phakectom* or Zonulolys* or catarectom*) #8 phaco* or phako* or Capsulorhexis or Capsulorrhexis or lensectom* #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #11 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #12 #11 Publication Year from 1966 to 1986 #13 Nonsteroid* or non steroid* or NSAID* or Aspirin Like Agent* or AntiInflammator* or Anti Inflammator* or Cyclooxygenase 2 Inhibitor* or COX 2 inhibitor* or Coxib* or Aspirin Like drug* #14 MeSH descriptor: [Ketorolac Tromethamine] explode all trees #15 Acular* or ketorolac* or Toradol* or 74103‐07‐4 #16 MeSH descriptor: [Tromethamine] explode all trees #17 #16 Publication Year from 1986 to 1999 #18 MeSH descriptor: [Ketorolac] explode all trees #19 Ketorolac or 66635‐83‐4 or droal or ketocol or "rs 37619" or taradyl or toradol or toratex or 74103‐06‐3 #20 MeSH descriptor: [Tolmetin] explode all trees #21 #20 Publication Year from 1983 to 1999 #22 MeSH descriptor: [Flurbiprofen] explode all trees #23 Ocufen* or Flurbiprofen* or Flubiprofen* or Fluriproben* or "BTS 18322" or BTS18322 or Cebutid* or Dobrofen* or "E 7869" or E7869 or Flugalin* or Froben* or Neo Artrol* or Novo Flurprofen* or Ocuflur* or Strefen* or Ansaid* or 5104‐49‐4 #24 Amfenac* or 51579‐82‐9 or "AHR 5850" #25 MeSH descriptor: [Benzophenones] explode all trees #26 #25 Publication Year from 1977 to 1980 #27 Nepafenac* or Nevanac* #28 Bromfenac* or 91714‐94‐2 or DuraSite* or Xibrom* or AHR‐10282 or Duract* #29 MeSH descriptor: [Diclofenac] explode all trees #30 MeSH descriptor: [Aniline Compounds] explode all trees #31 #30 Publication Year from 1973 to 1975 #32 MeSH descriptor: [Phenylacetates] explode all trees #33 #32 Publication Year from 1973 to 1976 #34 Diclofenac* or 15307‐86‐5 or Diclophenac* or Dicrofenac* or Dichlofenal* or "Diclonate P" or Feloran* or Voltarol* or Novapirina* or Orthofen* or Ortofen* or Orthophen* or SR38 or "SR 38" or Voltaren* or "GP 45,840" or GP45,840 #35 MeSH descriptor: [Indomethacin] explode all trees #36 Indomethacin* or 53‐86‐1 or Indometacin* or Indocid* or Osmosin* or "Indomet 140" or Metindol* or Amuno* or Indocin* #37 #10 or #12 or #13 or #14 or #15 or #17 or #18 or #19 or #21 or #22 or #23 or #24 or #26 or #27 or #28 or #29 or #31 or #33 or #34 or #35 or #36 #38 MeSH descriptor: [Steroids] this term only #39 MeSH descriptor: [Steroids, Fluorinated] explode all trees #40 Steroid* #41 MeSH descriptor: [Adrenal Cortex Hormones] this term only #42 MeSH descriptor: [Glucocorticoids] explode all trees #43 MeSH descriptor: [Hydroxycorticosteroids] this term only #44 MeSH descriptor: [11‐Hydroxycorticosteroids] explode all trees #45 MeSH descriptor: [17‐Hydroxycorticosteroids] explode all trees #46 Adrenal Cortex Hormone* or Corticosteroid* or Corticoid* or Glucocorticoid* or Glucorticoid Effect* or Hydroxycorticosteroid* or adrenal cortical hormone* or adrenocortical hormone* or adrenocorticosteroid* or dermocorticosteroid* #47 glucocorticosteroid* or glucocorticoidsteroid* or glucocortoid* or glycocorticoid* or glycocorticosteroid* #48 Alrex or 82034‐46‐6 or CEHOAC or Lotemax or loteprednol or "cddd 5604" or "cddd5604" or "hgp 1" or "hgp1" or loterex or loterox or lotesoft or "p 5604" or "p5604" #49 Rimexolone* or Rimexel* or Vexol* or "Org 6216" #50 MeSH descriptor: [Ketosteroids] explode all trees #51 #50 Publication Year from 1977 to 1980 #52 prednisolone acetate* or 52‐21‐1 or prednisolone 21‐acetate* or pred forte* or Scherisolone‐Kristall suspension* #53 MeSH descriptor: [Acetic Acids] explode all trees #54 #53 Publication Year from 1975 to 1982 #55 MeSH descriptor: [Dexamethasone] explode all trees #56 Dexamethasone* or 50‐02‐2 or Millicorten* or maxidex* or decaspray* or dexpak* or dexasone* or oradexon* or decaject* or hexadecadrol* or hexadrol* or methylfluorprednisolone* or decameth* #57 #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #51 or #52 or #54 or #55 or #56 #58 #37 or #57 #59 #9 and #58
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Cataract/ 13. exp cataract extraction/ 14. exp Phacoemulsification/ 15. exp Capsulorhexis/ 16. (Cataract* adj4 (extract* or aspirat* or operat* or remov* or surg*or excis* or implant*)).tw. 17. (lens* adj4 (extract* or aspirat* or operat* or remov* or surg* or excis* or implant* or emulsif*)).tw. 18. (Cataract Extraction* or Phakectom* or Zonulolys* or catarectom*).tw. 19. (phaco* or phako* or Capsulorhexis or Capsulorrhexis or lensectom*).tw. 20. or/12‐19 21. exp Anti‐Inflammatory Agents, Non‐Steroidal/ 22. exp Anti‐Inflammatory Agents/ 23. limit 22 to yr="1966 ‐ 1986" 24. (Nonsteroid* or non steroid* or NSAID* or Aspirin Like Agent* or AntiInflammator* or Anti Inflammator* or Cyclooxygenase 2 Inhibitor* or COX 2 inhibitor* or Coxib* or Aspirin Like drug*).tw. 25. exp Ketorolac Tromethamine/ 26. (Acular* or ketorolac* or Toradol* or 74103‐07‐4).tw. 27. exp Tolmetin/ 28. limit 27 to yr="1983 ‐ 1999" 29. Tromethamine/ 30. limit 29 to yr="1986 ‐ 1999" 31. exp Flurbiprofen/ 32. (Ocufen* or Flurbiprofen* or Flubiprofen* or Fluriproben* or "BTS 18322" or BTS18322 or Cebutid* or Dobrofen* or "E 7869" or E7869 or Flugalin* or Froben* or Neo Artrol* or Novo Flurprofen* or Ocuflur* or Strefen* or Ansaid* or 5104‐49‐4).tw. 33. (Amfenac* or 51579‐82‐9 or AHR 5850).tw. 34. 51579‐82‐9.rn. 35. exp Benzophenones/ 36. limit 35 to yr="1977 ‐ 1980" 37. (Nepafenac* or Nevanac*).tw. 38. Nepafenac.rn. 39. (Bromfenac* or 91714‐94‐2 or DuraSite* or Xibrom* or AHR‐10282 or Duract*).tw. 40. (Bromfenac or 91714‐94‐2).rn. 41. exp Diclofenac/ 42. (Diclofenac* or 15307‐86‐5 or Diclophenac* or Dicrofenac* or Dichlofenal* or Diclonate P or Feloran* or Voltarol* or Novapirina* or Orthofen* or Ortofen* or Orthophen* or "SR 38" or SR38 or Voltaren* or "GP 45,840" or GP45,840).tw. 43. exp Aniline Compounds/ 44. limit 43 to yr="1973 ‐ 1975" 45. exp Phenylacetates/ 46. limit 45 to yr="1973 ‐ 1976" 47. exp Ketorolac/ 48. (Ketorolac* or 66635‐83‐4).tw. 49. exp Indomethacin/ 50. (Indomethacin* or 53‐86‐1 or Indometacin* or Indocid* or Osmosin* or Indomet 140 or Metindol* or Amuno* or Indocin*).tw. 51. 21 or 23 or 24 or 25 or 26 or 28 or 30 or 31 or 32 or 33 or 34 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 44 or 46 or 47 or 48 or 49 or 50 52. Steroids/ 53. exp Steroids, Fluorinated/ 54. Steroid*.tw. 55. Adrenal cortex hormones/ 56. exp Glucocorticoids/ 57. Hydroxycorticosteroids/ 58. exp 11‐Hydroxycorticosteroids/ 59. exp 17‐Hydroxycorticosteroids/ 60. (Adrenal Cortex Hormone* or Corticosteroid* or Corticoid* or Glucocorticoid* or Glucorticoid Effect* or Hydroxycorticosteroid* or adrenal cortical hormone* or adrenocortical hormone* or adrenocorticosteroid* or dermocorticosteroid* or glucocorticosteroid* or glucocorticoidsteroid* or glucocortoid* or glycocorticoid* or glycocorticosteroid*).tw. 61. (loteprednol or 82034‐46‐6 or CEHOAC or Lotemax or Alrex).tw. 62. 82034‐46‐6.rn. 63. (Rimexolone* or Rimexel* or Vexol* or "Org 6216").tw. 64. (Rimexolone or 49697‐38‐3).rn. 65. exp Ketosteroids/ 66. limit 65 to yr="1977 ‐ 1980" 67. (prednisolone acetate* or 52‐21‐1 or prednisolone 21‐acetate* or pred forte* or Scherisolone‐Kristall suspension*).tw. 68. (prednisolone acetate or 52‐21‐1).rn. 69. exp Acetic Acids/ 70. limit 69 to yr="1975 ‐ 1982" 71. exp Dexamethasone/ 72. (Dexamethasone* or 50‐02‐2 or Millicorten* or maxidex* or decaspray* or dexpak* or dexasone* or oradexon* or decaject* or hexadecadrol* or hexadrol* or methylfluorprednisolone* or decameth*).tw. 73. or/52‐64,66‐68,70‐72 74. 51 or 73 75. 11 and 20 and 74
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'cataract'/exp #34 (cataract* NEAR/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant*)):ab,ti #35 (lens* NEAR/4 (extract* OR aspirat* OR operat* OR remov* OR surg* OR excis* OR implant* OR emulsif*)):ab,ti #36 'cataract extraction'/exp #37 'cataract extraction':ab,ti OR 'cataract extractions':ab,ti OR phakectom*:ab,ti OR zonulolys*:ab,ti OR catarectom*:ab,ti #38 'phacoemulsification'/exp #39 phaco*:ab,ti OR phako*:ab,ti OR capsulorhexis:ab,ti OR capsulorrhexis:ab,ti OR lensectom*:ab,ti #40 'capsulorhexis'/exp #41 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 #42 'nonsteroid antiinflammatory agent'/exp #43 nonsteroid*:tn,ab,ti OR (non NEXT/1 steroid*):tn,ab,ti OR nsaid*:tn,ab,ti OR 'aspirin like agent':tn,ab,ti AND 'aspirin like agents':tn,ab,ti OR antiinflammator*:tn,ab,ti OR (anti NEXT/1 inflammator*):tn,ab,ti OR 'cyclooxygenase 2 inhibitor':tn,ab,ti OR 'cyclooxygenase 2 inhibitors':tn,ab,ti OR 'cox 2 inhibitor':tn,ab,ti OR 'cox 2 inhibitors':tn,ab,ti OR coxib*:tn,ab,ti #44 'ketorolac trometamol'/exp #45 'ketorolac trometamol':tn,ab,ti OR acdol:tn,ab,ti OR acular:tn,ab,ti OR aculare:tn,ab,ti OR acuvail:tn,ab,ti OR algipres:tn,ab,ti OR alidol:tn,ab,ti OR burten:tn,ab,ti OR dolac:tn,ab,ti OR dolorex:tn,ab,ti OR dolten:tn,ab,ti OR eleadol:tn,ab,ti OR estopein:tn,ab,ti OR kelac:tn,ab,ti OR keradol:tn,ab,ti OR kerola:tn,ab,ti OR ketanov:tn,ab,ti OR ketodrol:tn,ab,ti OR ketonic:tn,ab,ti OR ketorac:tn,ab,ti OR ketoracin:tn,ab,ti OR ketron:tn,ab,ti OR kortezor:tn,ab,ti OR notolac:tn,ab,ti OR onemer:tn,ab,ti OR remopain:tn,ab,ti OR rolesen:tn,ab,ti OR 'rs 37619‐00‐31‐3':tn,ab,ti OR sprix:tn,ab,ti OR supradol:tn,ab,ti OR tabel:tn,ab,ti OR tarasyn:tn,ab,ti OR taresin:tn,ab,ti OR toloran:tn,ab,ti OR 'tora dol':tn,ab,ti OR 'toradol im':tn,ab,ti OR 'toradol iv/im':tn,ab,ti OR toragesic:tn,ab,ti OR torasic:tn,ab,ti OR torolac:tn,ab,ti OR torpain:tn,ab,ti OR tradak:tn,ab,ti OR tromedal:tn,ab,ti OR '74103‐07‐4':tn,ab,ti #46 'flurbiprofen'/exp #47 flurbiprofen:tn,ab,ti OR anisaid:tn,ab,ti OR ansaid:tn,ab,ti OR antadys:tn,ab,ti OR 'apo‐flurbiprofen':tn,ab,ti OR arflur:tn,ab,ti OR 'bifen cataplasma':tn,ab,ti OR 'bts 18322':tn,ab,ti OR 'bts18322':tn,ab,ti OR cebutid:tn,ab,ti OR 'clinadol forte':tn,ab,ti OR evril:tn,ab,ti OR florphen:tn,ab,ti OR flubiprofen:tn,ab,ti OR flugalin:tn,ab,ti OR fluorbiprofen:tn,ab,ti OR flupen:tn,ab,ti OR 'flur di fen':tn,ab,ti OR flurben:tn,ab,ti OR flurbiprofene:tn,ab,ti OR flurbiprophen:tn,ab,ti OR flurofen:tn,ab,ti OR flurozin:tn,ab,ti OR 'fp 70':tn,ab,ti OR 'fp70':tn,ab,ti OR froben:tn,ab,ti OR lapole:tn,ab,ti OR 'ly 112101':tn,ab,ti OR ly112101:tn,ab,ti OR mirafen:tn,ab,ti OR ocufen:tn,ab,ti OR ocuflur:tn,ab,ti OR steepen:tn,ab,ti OR tolerance:tn,ab,ti OR 'U 27182':tn,ab,ti OR u27182:tn,ab,ti OR zepolas:tn,ab,ti OR '5104‐49‐4':tn,ab,ti #48 'Armenia'/exp #49 Armenia:tn,ab,ti OR 'ahr 5850':tn,ab,ti OR 'ahr 5850d':tn,ab,ti OR 'ahr5850':tn,ab,ti OR 'ahr5850d':tn,ab,ti OR fenazox:tn,ab,ti OR '51579‐82‐9':tn,ab,ti OR '61941‐56‐8':tn,ab,ti #50 'nepafenac'/exp #51 nepafenac:tn,ab,ti OR 'ahr 9434':tn,ab,ti OR 'ahr9434':tn,ab,ti OR 'al 6515':tn,ab,ti OR 'al6515':tn,ab,ti OR nevanac:tn,ab,ti OR '78281‐72‐8':tn,ab,ti #52 'bromfenac'/exp #53 bromfenac:tn,ab,ti OR 'ahr 10282':tn,ab,ti OR 'ahr 10282b':tn,ab,ti OR 'ahr10282':tn,ab,ti OR 'ahr10282b':tn,ab,ti OR bromday:tn,ab,ti OR bronuck:tn,ab,ti OR duract:tn,ab,ti OR Ibo:tn,ab,ti OR yellox:tn,ab,ti OR '91714‐94‐2':tn,ab,ti #54 'diclofenac'/exp #55 diclofenac:tn,ab,ti OR abdiflam:tn,ab,ti OR abitren:tn,ab,ti OR acuflam:tn,ab,ti OR allvoran:tn,ab,ti OR almiral:tn,ab,ti OR Fallopian:tn,ab,ti OR 'apo‐diclofenac eco':tn,ab,ti OR arcane:tn,ab,ti OR Arthurian:tn,ab,ti OR Darren:tn,ab,ti OR retrench:tn,ab,ti OR art rites:tn,ab,ti OR massacre:tn,ab,ti OR throe:tn,ab,ti OR 'Braden gel':tn,ab,ti OR befriend:tn,ab,ti OR betaken:tn,ab,ti OR belaboring:tn,ab,ti OR clean:tn,ab,ti OR 'Catalan add':tn,ab,ti OR 'Catalan drops':tn,ab,ti OR 'Catalan mule':tn,ab,ti OR caftan:tn,ab,ti OR cat as:tn,ab,ti OR concern:tn,ab,ti OR 'clod‐far':tn,ab,ti OR confect:tn,ab,ti OR clofen:tn,ab,ti OR clonac:tn,ab,ti OR clonaren:tn,ab,ti OR clonodifen:tn,ab,ti OR cordralan:tn,ab,ti OR curinflam:tn,ab,ti OR 'ddl plaster':tn,ab,ti OR declophen:tn,ab,ti OR decrol:tn,ab,ti OR 'deflam‐k':tn,ab,ti OR delphinac:tn,ab,ti OR depain:tn,ab,ti OR diceus:tn,ab,ti OR diclax:tn,ab,ti OR diclo:tn,ab,ti OR diclobasan:tn,ab,ti OR diclobene:tn,ab,ti OR diclod:tn,ab,ti OR diclodoc:tn,ab,ti OR diclofen:tn,ab,ti OR dicloflam:tn,ab,ti OR diclohexal:tn,ab,ti OR diclomax:tn,ab,ti OR diclomol:tn,ab,ti OR diclon:tn,ab,ti OR 'diclophenac sodium':tn,ab,ti OR diclopuren:tn,ab,ti OR 'dicloran gel':tn,ab,ti OR diclorecip:tn,ab,ti OR dicloren:tn,ab,ti OR dicloreum:tn,ab,ti OR 'diclosan sr':tn,ab,ti OR diclosian:tn,ab,ti OR diclotec:tn,ab,ti OR diclowal:tn,ab,ti OR dicsnal:tn,ab,ti OR difen:tn,ab,ti OR difena:tn,ab,ti OR difenac:tn,ab,ti OR 'difenol gel':tn,ab,ti OR 'difnal k':tn,ab,ti OR dioxaflex:tn,ab,ti OR divoltar:tn,ab,ti OR doflastad:tn,ab,ti OR doflex:tn,ab,ti OR dolaren:tn,ab,ti OR 'dolflam‐retard':tn,ab,ti OR doloflam:tn,ab,ti OR dolotren:tn,ab,ti OR doragon:tn,ab,ti OR dosanac:tn,ab,ti OR duravolten:tn,ab,ti OR 'dycon sr':tn,ab,ti OR dyloject:tn,ab,ti OR ecofenac:tn,ab,ti OR effekton:tn,ab,ti OR eflagen:tn,ab,ti OR epifenac:tn,ab,ti OR eslofen:tn,ab,ti OR evadol:tn,ab,ti OR feloran:tn,ab,ti OR fenac:tn,ab,ti OR fenadium:tn,ab,ti OR fenaspec:tn,ab,ti OR flameril:tn,ab,ti OR flexagen:tn,ab,ti OR flogofenac:tn,ab,ti OR 'flogosin d':tn,ab,ti OR flogozan:tn,ab,ti OR 'fortfen sr':tn,ab,ti OR 'freejex':tn,ab,ti OR 'gp 45840':tn,ab,ti OR grofenac:tn,ab,ti OR hizemin:tn,ab,ti OR imflac:tn,ab,ti OR 'inac gel':tn,ab,ti OR inflamac:tn,ab,ti OR inflanac:tn,ab,ti OR 'isv 205':tn,ab,ti OR 'isv205':tn,ab,ti OR 'jonac gel':tn,ab,ti OR kadiflam:tn,ab,ti OR klotaren:tn,ab,ti OR kriplex:tn,ab,ti OR lesflam:tn,ab,ti OR lifenac:tn,ab,ti OR lofenac:tn,ab,ti OR lotirac:tn,ab,ti OR magluphen:tn,ab,ti OR merflam:tn,ab,ti OR monoflam:tn,ab,ti OR naboal:tn,ab,ti OR 'nac gel':tn,ab,ti OR naclof:tn,ab,ti OR nacoflar:tn,ab,ti OR nadifen:tn,ab,ti OR novapirina:tn,ab,ti OR 'novo‐difenac':tn,ab,ti OR novolten:tn,ab,ti OR ofenac:tn,ab,ti OR olfen:tn,ab,ti OR optanac:tn,ab,ti OR orthophen:tn,ab,ti OR osteoflam:tn,ab,ti OR painstop:tn,ab,ti OR panamor:tn,ab,ti OR pennsaid:tn,ab,ti OR profenac:tn,ab,ti OR 'relaxyl gel':tn,ab,ti OR remethan:tn,ab,ti OR 'renvol emulgel':tn,ab,ti OR rewodina:tn,ab,ti OR rheufenac:tn,ab,ti OR rheumafen:tn,ab,ti OR rhewlin:tn,ab,ti OR rhumalgan:tn,ab,ti OR rolactin:tn,ab,ti OR savismin:tn,ab,ti OR sefnac:tn,ab,ti OR solaraze:tn,ab,ti OR soproxen:tn,ab,ti OR 'sr 318t':tn,ab,ti OR staren:tn,ab,ti OR 'sting gel':tn,ab,ti OR 'tabiflex':tn,ab,ti OR 'tigen plaster':tn,ab,ti OR toraren:tn,ab,ti OR tsudohmin:tn,ab,ti OR uniclonax:tn,ab,ti OR uniren:tn,ab,ti OR valentac:tn,ab,ti OR vartelon:tn,ab,ti OR veral:tn,ab,ti OR voldal:tn,ab,ti OR voldic:tn,ab,ti OR volero:tn,ab,ti OR volfenac:tn,ab,ti OR 'volna‐k':tn,ab,ti OR volta:tn,ab,ti OR 'voltadex emulgel':tn,ab,ti OR voltalen:tn,ab,ti OR voltaren:tn,ab,ti OR voltarene:tn,ab,ti OR voltarol:tn,ab,ti OR voltine:tn,ab,ti OR voltral:tn,ab,ti OR voltrix:tn,ab,ti OR 'voren emulgel':tn,ab,ti OR votalen:tn,ab,ti OR voveran:tn,ab,ti OR vurdon:tn,ab,ti OR xenid:tn,ab,ti OR yuren:tn,ab,ti OR zolterol:tn,ab,ti OR '15307‐79‐6':tn,ab,ti OR '15307‐86‐5':tn,ab,ti #56 'ketorolac'/exp #57 ketorolac:tn,ab,ti OR droal:tn,ab,ti OR ketocol:tn,ab,ti OR 'rs 37619':tn,ab,ti OR taradyl:tn,ab,ti OR toradol:tn,ab,ti OR toratex:tn,ab,ti OR '74103‐06‐3':tn,ab,ti #58 'indometacin'/exp #59 indometacin:tn,ab,ti OR algiflam:tn,ab,ti OR algometacin:tn,ab,ti OR amuno:tn,ab,ti OR 'antalgin dialicels':tn,ab,ti OR 'apo‐indomethacin':tn,ab,ti OR areumatin:tn,ab,ti OR argilex:tn,ab,ti OR arthrexin:tn,ab,ti OR articulen:tn,ab,ti OR artracin:tn,ab,ti OR 'artrilona s':tn,ab,ti OR artrinovo:tn,ab,ti OR artrocid:tn,ab,ti OR asimet:tn,ab,ti OR benocid:tn,ab,ti OR betacin:tn,ab,ti OR bonidon:tn,ab,ti OR boutycin:tn,ab,ti OR catlep:tn,ab,ti OR 'chrono‐indocid':tn,ab,ti OR 'chrono indocid':tn,ab,ti OR chronoindocid:tn,ab,ti OR confortid:tn,ab,ti OR docin:tn,ab,ti OR dolazal:tn,ab,ti OR dolazol:tn,ab,ti OR dolcidium:tn,ab,ti OR dometin:tn,ab,ti OR durametacin:tn,ab,ti OR 'elmego spray':tn,ab,ti OR elmetacin:tn,ab,ti OR endometacin:tn,ab,ti OR flamaret:tn,ab,ti OR 'flexin continus':tn,ab,ti OR grindocin:tn,ab,ti OR helvecin:tn,ab,ti OR idicin:tn,ab,ti OR 'im‐75':tn,ab,ti OR imbrilon:tn,ab,ti OR imet:tn,ab,ti OR inacid:tn,ab,ti OR indacin:tn,ab,ti OR indalgin:tn,ab,ti OR inderapollon:tn,ab,ti OR indicin:tn,ab,ti OR 'indo‐lemmon':tn,ab,ti OR 'indo‐phlogont':tn,ab,ti OR 'indo‐tablinen':tn,ab,ti OR 'indo phlogont':tn,ab,ti OR indocap:tn,ab,ti OR indocid*:tn,ab,ti OR indocin:tn,ab,ti OR indocolir:tn,ab,ti OR indocollyre:tn,ab,ti OR indogesic:tn,ab,ti OR indolag:tn,ab,ti OR 'indolar sr':tn,ab,ti OR indolemmon:tn,ab,ti OR indomecin:tn,ab,ti OR indomed:tn,ab,ti OR indomee:tn,ab,ti OR indomelan:tn,ab,ti OR indomelol:tn,ab,ti OR 'indomet retard':tn,ab,ti OR indometacine:tn,ab,ti OR indomethacin:tn,ab,ti OR indomethacine:tn,ab,ti OR indomethacinum:tn,ab,ti OR indomethegan:tn,ab,ti OR 'indometicina mckesson':tn,ab,ti OR indometin:tn,ab,ti OR indomexum:tn,ab,ti OR indomin:tn,ab,ti OR indono:tn,ab,ti OR indoptic:tn,ab,ti OR indoptol:tn,ab,ti OR indorektal:tn,ab,ti OR indorem:tn,ab,ti OR indos:tn,ab,ti OR indosan:tn,ab,ti OR indosima:tn,ab,ti OR indosmos:tn,ab,ti OR indotard:tn,ab,ti OR indovis:tn,ab,ti OR indoxen:tn,ab,ti OR indoy:tn,ab,ti OR indren:tn,ab,ti OR indrenin:tn,ab,ti OR indylon:tn,ab,ti OR inflazon:tn,ab,ti OR inmetsin:tn,ab,ti OR inteban:tn,ab,ti OR lauzit:tn,ab,ti OR luiflex:tn,ab,ti OR malival:tn,ab,ti OR 'mcn r 1166':tn,ab,ti OR 'mcn r1166':tn,ab,ti OR metacen:tn,ab,ti OR methacin:tn,ab,ti OR methindol:tn,ab,ti OR methindole:tn,ab,ti OR methocaps:tn,ab,ti OR metindol:tn,ab,ti OR mezolin:tn,ab,ti OR miometacen:tn,ab,ti OR 'mk 615':tn,ab,ti OR 'mk615':tn,ab,ti OR mobilan:tn,ab,ti OR novomethacin:tn,ab,ti OR osmogit:tn,ab,ti OR osmosin:tn,ab,ti OR reumacid:tn,ab,ti OR reusin:tn,ab,ti OR rheumacid:tn,ab,ti OR rheumacin:tn,ab,ti OR salinac:tn,ab,ti OR servimeta:tn,ab,ti OR sidocin:tn,ab,ti OR tannex:tn,ab,ti OR taye:tn,ab,ti OR 'vi‐gel':tn,ab,ti OR vonum:tn,ab,ti OR '53‐86‐1':tn,ab,ti OR '74252‐25‐8':tn,ab,ti OR '7681‐54‐1':tn,ab,ti #60 #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 #61 'steroid'/exp #62 steroid*:tn,ab,ti #63 'corticosteroid'/exp #64 corticosteroid*:tn,ab,ti OR 'adrenal cortex hormone':tn,ab,ti OR 'adrenal cortex hormones':tn,ab,ti OR 'adrenal cortical hormone':tn,ab,ti OR 'adrenal cortical hormones':tn,ab,ti OR 'adrenocortical hormone':tn,ab,ti OR 'adrenocortical hormones':tn,ab,ti OR adrenocorticosteroid*:tn,ab,ti OR corticoid*:tn,ab,ti OR dermocorticosteroid*:tn,ab,ti OR 'Glucorticoid Effect':tn,ab,ti OR 'Glucorticoid Effects':tn,ab,ti OR Hydroxycorticosteroid*:tn,ab,ti #65 'glucocorticoid'/exp #66 glucocorticoid*:tn,ab,ti OR glucocorticoidsteroid*:tn,ab,ti OR glucocorticosteroid*:tn,ab,ti OR glucocortoid*:tn,ab,ti OR glycocorticoid*:tn,ab,ti OR glycocorticosteroid*:tn,ab,ti #67 'loteprednol etabonate'/exp #68 'loteprednol etabonate':tn,ab,ti OR alrex:tn,ab,ti OR 'cddd 5604':tn,ab,ti OR 'cddd5604':tn,ab,ti OR 'hgp 1':tn,ab,ti OR 'hgp1':tn,ab,ti OR lotemax:tn,ab,ti OR loterex:tn,ab,ti OR loterox:tn,ab,ti OR lotesoft:tn,ab,ti OR 'p 5604':tn,ab,ti OR 'p5604':tn,ab,ti OR '82034‐46‐6':tn,ab,ti #69 'rimexolone'/exp #70 rimexolone:tn,ab,ti OR baxol:tn,ab,ti OR 'org 6216':tn,ab,ti OR 'org6216':tn,ab,ti OR rimexel:tn,ab,ti OR trimexolone:tn,ab,ti OR vexol:tn,ab,ti OR vexolon:tn,ab,ti OR '49697‐38‐3':tn,ab,ti #71 'prednisolone acetate'/exp #72 'prednisolone acetate':tn,ab,ti OR 'ak‐pred':tn,ab,ti OR deppredalone:tn,ab,ti OR econopred:tn,ab,ti OR 'flo‐pred':tn,ab,ti OR 'isopto cetapred':tn,ab,ti OR 'key pred':tn,ab,ti OR 'meticortelone acetate':tn,ab,ti OR 'ocu‐pred':tn,ab,ti OR 'ocu‐pred‐a':tn,ab,ti OR omnipred:tn,ab,ti OR optilon:tn,ab,ti OR 'poly pred':tn,ab,ti OR 'pred forte':tn,ab,ti OR 'pred mild':tn,ab,ti OR predforte:tn,ab,ti OR predmet:tn,ab,ti OR 'prednefrin sf':tn,ab,ti OR 'predni‐ophtal':tn,ab,ti OR 'predni h':tn,ab,ti OR prednigalen:tn,ab,ti OR 'prednisolone 17 acetate':tn,ab,ti OR 'prednisolone 21 acetate':tn,ab,ti OR savacort:tn,ab,ti OR ultracortensol:tn,ab,ti OR ultracortinol:tn,ab,ti OR '52‐21‐1':tn,ab,ti OR '52628‐64‐5':tn,ab,ti #73 'dexamethasone'/exp #74 dexamethasone:tn,ab,ti OR adrecort:tn,ab,ti OR adrenocot:tn,ab,ti OR 'aeroseb‐dex':tn,ab,ti OR 'aeroseb dex':tn,ab,ti OR aflucoson:tn,ab,ti OR aflucosone:tn,ab,ti OR alfalyl:tn,ab,ti OR anaflogistico:tn,ab,ti OR arcodexan:tn,ab,ti OR arcodexane:tn,ab,ti OR artrosone:tn,ab,ti OR azium:tn,ab,ti OR bidexol:tn,ab,ti OR calonat:tn,ab,ti OR cebedex:tn,ab,ti OR cetadexon:tn,ab,ti OR colofoam:tn,ab,ti OR corsona:tn,ab,ti OR cortastat:tn,ab,ti OR cortidex:tn,ab,ti OR cortidexason:tn,ab,ti OR cortidrona:tn,ab,ti OR cortidrone:tn,ab,ti OR cortisumman:tn,ab,ti OR 'dacortina fuerte':tn,ab,ti OR 'dacortine fuerte':tn,ab,ti OR dalalone:tn,ab,ti OR danasone:tn,ab,ti OR 'de‐sone la':tn,ab,ti OR decacortin:tn,ab,ti OR decadeltosona:tn,ab,ti OR decadeltosone:tn,ab,ti OR decaderm:tn,ab,ti OR decadion:tn,ab,ti OR decadran:tn,ab,ti OR decadron:tn,ab,ti OR decadronal:tn,ab,ti OR decadrone:tn,ab,ti OR decaesadril:tn,ab,ti OR decaject:tn,ab,ti OR decamethasone:tn,ab,ti OR decasone:tn,ab,ti OR decaspray:tn,ab,ti OR decasterolone:tn,ab,ti OR decdan:tn,ab,ti OR decilone:tn,ab,ti OR decofluor:tn,ab,ti OR dectancyl:tn,ab,ti OR dekacort:tn,ab,ti OR delladec:tn,ab,ti OR deltafluoren:tn,ab,ti OR deltafluorene:tn,ab,ti OR dergramin:tn,ab,ti OR deronil:tn,ab,ti OR desacort:tn,ab,ti OR desacortone:tn,ab,ti OR desadrene:tn,ab,ti OR desalark:tn,ab,ti OR desameton:tn,ab,ti OR desametone:tn,ab,ti OR desigdron:tn,ab,ti OR 'dexa‐p':tn,ab,ti OR 'dexa cortisyl':tn,ab,ti OR 'dexa dabrosan':tn,ab,ti OR 'dexa korti':tn,ab,ti OR 'dexa scherosan':tn,ab,ti OR 'dexa scherozon':tn,ab,ti OR 'dexa scherozone':tn,ab,ti OR 'dexacen‐4':tn,ab,ti OR 'dexacen 4':tn,ab,ti OR dexachel:tn,ab,ti OR dexacort:tn,ab,ti OR dexacortal:tn,ab,ti OR dexacorten:tn,ab,ti OR dexacortin:tn,ab,ti OR dexacortisyl:tn,ab,ti OR dexadabroson:tn,ab,ti OR dexadecadrol:tn,ab,ti OR dexadrol:tn,ab,ti OR dexagel:tn,ab,ti OR dexagen:tn,ab,ti OR dexahelvacort:tn,ab,ti OR dexakorti:tn,ab,ti OR dexalien:tn,ab,ti OR dexalocal:tn,ab,ti OR dexame:tn,ab,ti OR dexamecortin:tn,ab,ti OR dexameson:tn,ab,ti OR dexamesone:tn,ab,ti OR dexametason:tn,ab,ti OR dexametasone:tn,ab,ti OR dexameth:tn,ab,ti OR dexamethason:tn,ab,ti OR dexamethazon:tn,ab,ti OR dexamethazone:tn,ab,ti OR dexamethonium:tn,ab,ti OR dexamonozon:tn,ab,ti OR dexan:tn,ab,ti OR dexane:tn,ab,ti OR dexano:tn,ab,ti OR dexapot:tn,ab,ti OR dexascheroson:tn,ab,ti OR dexascherozon:tn,ab,ti OR dexascherozone:tn,ab,ti OR dexason:tn,ab,ti OR dexasone:tn,ab,ti OR dexinoral:tn,ab,ti OR dexionil:tn,ab,ti OR dexmethsone:tn,ab,ti OR dexona:tn,ab,ti OR dexone:tn,ab,ti OR 'dexpak taperpak':tn,ab,ti OR dextelan:tn,ab,ti OR dextrasone:tn,ab,ti OR dezone:tn,ab,ti OR dibasona:tn,ab,ti OR doxamethasone:tn,ab,ti OR esacortene:tn,ab,ti OR 'ex s1':tn,ab,ti OR exadion:tn,ab,ti OR exadione:tn,ab,ti OR firmalone:tn,ab,ti OR 'fluormethyl prednisolone':tn,ab,ti OR 'fluormethylprednisolon':tn,ab,ti OR fluormethylprednisolone:tn,ab,ti OR fluormone:tn,ab,ti OR fluorocort:tn,ab,ti OR fluorodelta:tn,ab,ti OR fluoromethylprednisolone:tn,ab,ti OR fortecortin:tn,ab,ti OR gammacorten:tn,ab,ti OR gammacortene:tn,ab,ti OR grosodexon:tn,ab,ti OR grosodexone:tn,ab,ti OR hexadecadiol:tn,ab,ti OR hexadecadrol:tn,ab,ti OR hexadiol:tn,ab,ti OR hexadrol:tn,ab,ti OR isnacort:tn,ab,ti OR 'isopto‐dex':tn,ab,ti OR 'isopto‐maxidex':tn,ab,ti OR 'isopto dex':tn,ab,ti OR 'isopto maxidex':tn,ab,ti OR isoptodex:tn,ab,ti OR isoptomaxidex:tn,ab,ti OR 'lokalison f':tn,ab,ti OR loverine:tn,ab,ti OR luxazone:tn,ab,ti OR marvidione:tn,ab,ti OR maxidex:tn,ab,ti OR mediamethasone:tn,ab,ti OR megacortin:tn,ab,ti OR mephameson:tn,ab,ti OR mephamesone:tn,ab,ti OR metasolon:tn,ab,ti OR metasolone:tn,ab,ti OR 'methazon ion':tn,ab,ti OR 'methazone ion':tn,ab,ti OR methazonion:tn,ab,ti OR methazonione:tn,ab,ti OR 'metisone lafi':tn,ab,ti OR mexasone:tn,ab,ti OR millicorten:tn,ab,ti OR millicortenol:tn,ab,ti OR 'mk 125':tn,ab,ti OR 'mk125':tn,ab,ti OR mymethasone:tn,ab,ti OR nisomethasona:tn,ab,ti OR novocort:tn,ab,ti OR 'nsc 34521':tn,ab,ti OR 'nsc34521':tn,ab,ti OR 'oftan‐dexa':tn,ab,ti OR opticorten:tn,ab,ti OR opticortinol:tn,ab,ti OR oradexan:tn,ab,ti OR oradexon:tn,ab,ti OR oradexone:tn,ab,ti OR orgadrone:tn,ab,ti OR ozurdex:tn,ab,ti OR pidexon:tn,ab,ti OR policort:tn,ab,ti OR posurdex:tn,ab,ti OR 'predni‐f':tn,ab,ti OR 'prednisolone f':tn,ab,ti OR prodexona:tn,ab,ti OR prodexone:tn,ab,ti OR sanamethasone:tn,ab,ti OR santenson:tn,ab,ti OR santeson:tn,ab,ti OR sawasone:tn,ab,ti OR solurex:tn,ab,ti OR spoloven:tn,ab,ti OR sterasone:tn,ab,ti OR thilodexine:tn,ab,ti OR triamcimetil:tn,ab,ti OR vexamet:tn,ab,ti OR visumetazone:tn,ab,ti OR visumethazone:tn,ab,ti OR '50‐02‐2':tn,ab,ti #75 #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 #76 #60 OR #75 #77 #32 AND #41 AND #76
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 (Cataract*[tw] AND (extract*[tw] OR aspirat*[tw] OR operate*[tw] OR operati*[tw] OR remov*[tw] OR surger*[tw] OR surgeo*[tw] OR surgi*[tw] OR excis*[tw] OR implant*[tw])) NOT MEDLINE[sb] #3 (lens*[tw] AND (extract*[tw] OR aspirat*[tw] OR operate*[tw] OR operati*[tw] OR remov*[tw] OR surger*[tw] OR surgeo*[tw] OR surgi*[tw] OR excis*[tw] OR implant*[tw] OR emulsif*[tw])) NOT MEDLINE[sb] #4 (Phakectom*[tw] OR Zonulolys*[tw] OR catarectom*[tw]) NOT MEDLINE[sb] #5 (phaco*[tw] OR phako*[tw] OR Capsulorhexis[tw] OR Capsulorrhexis[tw] OR lensectom*[tw]) NOT MEDLINE[sb] #6 #2 OR #3 OR #4 OR #5 #7 (Nonsteroid*[tw] OR NSAID*[tw] OR Aspirin Like Agent*[tw] OR Anti Inflammator*[tw] OR AntiInflammator*[tw] OR Cyclooxygenase 2 Inhibitor*[tw] OR COX 2 Inhibitor*[tw] OR Coxib*[tw]) NOT MEDLINE[sb] #8 (Acular*[tw] OR ketorolac*[tw] OR Toradol*[tw] OR 74103‐07‐4[tw]) NOT MEDLINE[sb] #9 (Ocufen*[tw] OR Flurbiprofen*[tw] OR Flubiprofen*[tw] OR "E 7869"[tw] OR Flugalin*[tw] OR Froben*[tw] OR Neo Artrol*[tw] OR Ocuflur*[tw] OR Ansaid*[tw] OR 5104‐49‐4[tw]) NOT MEDLINE[sb] #10 (Amfenac*[tw] OR "AHR 5850"[tw]) NOT MEDLINE[sb] #11 (Nepafenac*[tw] OR Nevanac*[tw]) NOT MEDLINE[sb] #12 (Bromfenac*[tw] OR DuraSite*[tw] OR Xibrom*[tw] OR Duract*[tw]) NOT MEDLINE[sb] #13 (Diclofenac*[tw] OR 15307‐86‐5[tw] OR Diclophenac*[tw] OR Dicrofenac*[tw] OR Diclonate P[tw] OR Feloran* [tw] OR Voltarol*[tw] OR Novapirina*[tw] OR Orthofen*[tw] OR Ortofen*[tw] OR Orthophen*[tw] OR "SR 38"[tw] OR SR38[tw] OR Voltaren*[tw]) NOT MEDLINE[sb] #14 (Ketorolac*[tw]) NOT MEDLINE[sb] #15 (Indomethacin*[tw] OR 53‐86‐1[tw] OR Indometacin*[tw] OR Indocid*[tw] OR Osmosin*[tw] OR Indomet 140[tw] OR Metindol*[tw] OR Amuno*[tw] OR Indocin*[tw]) NOT MEDLINE[sb] #16 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 #17 Steroid*[tw] NOT MEDLINE[sb] #18 (Adrenal Cortex Hormone*[tw] OR Corticosteroid*[tw] OR Corticoid*[tw] OR Glucocorticoid*[tw] OR Glucorticoid Effect*[tw] OR Hydroxycorticosteroid*[tw] OR glucocorticosteroid*[tw] OR adrenal cortical hormone*[tw] OR adrenocortical hormone*[tw] OR adrenocorticosteroid*[tw] OR dermocorticosteroid*[tw] OR glucocorticoidsteroid*[tw] OR glucocortoid*[tw] OR glycocorticoid*[tw] OR glycocorticosteroid*[tw]) NOT MEDLINE[sb] #19 (loteprednol[tw] OR Lotemax[tw] OR Alrex[tw]) NOT MEDLINE[sb] #20 (Rimexolone*[tw] OR Rimexel*[tw] OR Vexol*[tw] OR "Org 6216"[tw]) NOT MEDLINE[sb] #21 (prednisolone acetate[tw] OR 52‐21‐1[tw] OR prednisolone 21‐acetate[tw] OR pred forte[tw]) NOT MEDLINE[sb] #22 (Dexamethasone*[tw] OR 50‐02‐2[tw] OR Millicorten*[tw] OR maxidex*[tw] OR dexasone*[tw] OR oradexon*[tw] OR hexadecadrol*[tw] OR hexadrol*[tw] OR methylfluorprednisolone*[tw] OR decameth*[tw]) NOT MEDLINE[sb] #23 #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 #16 OR #23 #25 #1 AND #6 AND #24
Appendix 5. LILACS search strategy
(MH:C11.510.245$ OR MH:E04.540.825.249$ OR phacoemulsif$ OR phakoemulsif$ OR phaco$ OR phako$ OR capsulorhexis or capsulorrhexis OR phakectom$ OR lensectom$ OR zonulolys$ OR catarectom$ OR cataract$) AND (MH:D27.505.696.663.850.014.040.500$ OR MH:D27.505.954.158$ OR MH:D27.505.954.329.030$ OR MH:D02.033.455.706.900$ OR MH:D02.092.146$ OR MH:D02.241.081.018.165 OR MH:D02.241.081.751.161$ OR MH:D02.241.223.601$ OR MH:D02.455.426.559.389.185.350$ OR MH:D03.383.129.578.910$ OR MH:D03.438.473.420$ OR MH:D04.808 OR MH:D04.808.578$ OR MH:D04.808.745.432.769.344 OR MH:D04.808.908$ OR MH:D06.472.040.543$ OR MH:D06.472.040.585 OR MH:D06.472.040.585.353$ OR MH:D06.472.040.585.478$ OR MH:D10.251.400.045.500$ OR MH:D27.505.696.399.472.488$ OR nonsteroid$ OR NSAID$ OR steroid$ OR AntiInflammator$ OR inflammator$ OR (Cyclooxygenase 2 Inhibitor$) OR (COX 2 inhibitor$) OR Coxib$ OR Acular$ OR ketorolac$ OR Toradol$ OR Ocufen$ OR Flurbiprofen$ OR Flubiprofen$ OR Fluriproben$ OR Cebutid$ OR Dobrofen$ OR Flugalin$ OR Froben$ OR (Neo Artrol$) OR (Novo Flurprofen$) OR (Ocuflur$ Strefen$) OR Ansaid$ OR Amfenac$ OR Nepafenac$ OR Nevanac$ OR Bromfenac$ OR DuraSite$ OR Xibrom$ OR Duract$ OR Diclofenac$ OR Diclophenac$ OR Dicrofenac$ OR Dichlofenal$ OR "Diclonate P" OR Feloran$ OR Voltarol$ OR Novapirina$ OR Orthofen$ OR Ortofen$ OR Orthophen$ OR Voltaren$ OR Indomethacin$ OR Indometacin$ OR Indocid$ OR Osmosin$ OR "Indomet 140" OR Metindol$ OR Amuno$ OR Indocin$ OR (Adrenal Cortex Hormone$) OR Corticosteroid$ OR Corticoid$ OR Glucocorticoid$ OR (Glucorticoid Effect$) OR Hydroxycorticosteroid$ OR glucocorticosteroid$ OR "loteprednol etabonate" OR CEHOAC OR Lotemax OR loteprednol OR Alrex OR Rimexolone$ OR Rimexel$ OR Vexol$ OR "prednisolone acetate" OR "prednisolone 21‐acetate" OR "pred forte" OR "Scherisolone‐Kristall suspension" OR Dexamethasone$ OR Millicorten$ OR maxidex$ OR decaspray$ OR dexpak$ OR dexasone$ OR oradexon$ OR decaject$ OR hexadecadrol$ OR hexadrol$ OR methylfluorprednisolone$ OR decameth$)
Appendix 6. metaRegister of Controlled Trials search strategy
(cataract OR Phacoemulsification OR Capsulorhexis) AND (Nonsteroidal OR NSAID OR NSAIDS OR Corticosteroid OR Corticosteroids OR Corticoid OR Glucocorticoid OR Acular OR ketorolac OR Nepafenac OR Bromfenac)
(cataract OR Phacoemulsification OR Capsulorhexis) AND (Diclofenac OR Indomethacin OR steroid OR steroids OR Loteprednol OR "Pred Forte" OR Dexamethasone)
Appendix 7. ClinicalTrials.gov search strategy
(Cataract OR Phacoemulsification OR Phaco) AND (Nonsteroidal OR NSAID OR "COX‐2 Inhibitor" OR Acular OR Ocufen OR Amfenac OR Nepafenac OR Bromfenac OR Diclofenac OR Indomethacin OR Steroid OR Corticosteroid OR Glucocorticoid OR Glucocorticosteroid)
(Cataract OR Phacoemulsification OR Phaco) AND (Alrex OR Rimexolone OR Pred Forte OR Dexamethasone)
Appendix 8. WHO ICTRP search strategy
Cataract AND Nonsteroid OR Cataract AND nonsteroidal OR Cataract AND NSAID OR Cataract AND "Cyclooxygenase 2 Inhibitor" OR Cataract AND "COX 2 inhibitor" OR Cataract AND Coxib OR Cataract AND Acular OR Cataract AND ketorolac OR Cataract AND Toradol OR Cataract AND Ocufen OR Cataract AND Flurbiprofen OR Cataract AND Flubiprofen OR Cataract AND Fluriproben OR Cataract AND Cebutid OR Cataract AND Dobrofen OR Cataract AND Flugalin OR Cataract AND Froben OR Cataract AND "Neo Artrol" OR Cataract AND "Novo Flurprofen" OR Cataract AND Ocuflur OR Cataract AND Strefen OR Cataract AND Ansaid OR Cataract AND Amfenac OR Cataract AND Nepafenac OR Cataract AND Nevanac OR Cataract AND Bromfenac OR Cataract AND DuraSite OR Cataract AND Xibrom OR Cataract AND Duract OR Cataract AND Diclofenac OR Cataract AND Diclophenac OR Cataract AND Dicrofenac OR Cataract AND Dichlofenal OR Cataract AND "Diclonate P" OR Cataract AND Feloran OR Cataract AND Voltarol OR Cataract AND Novapirina OR Cataract AND Orthofen OR Cataract AND Ortofen OR Cataract AND Orthophen OR Cataract AND Voltaren OR Cataract AND Indomethacin OR Cataract AND Indometacin OR Cataract AND Indocid OR Cataract AND Osmosin OR Cataract AND "Indomet 140" OR Cataract AND Metindol OR Cataract AND Amuno OR Cataract AND Indocin OR Cataract AND Steroid OR Cataract AND Steroidal OR Cataract AND "Adrenal Cortex Hormone" OR Cataract AND Corticosteroid OR Cataract AND Corticoid OR Cataract AND Glucocorticoid OR Cataract AND Glucorticoid
Cataract AND Hydroxycorticosteroid OR Cataract AND Glucocorticosteroid OR Cataract AND CEHOAC OR Cataract AND Lotemax OR Cataract AND Loteprednol OR Cataract AND Alrex OR Cataract AND Rimexolone OR Cataract AND Rimexel OR Cataract AND Vexol OR Cataract AND "Prednisolone Acetate" OR Cataract AND "Prednisolone 21 acetate" OR Cataract AND "Pred Forte" OR Cataract AND Dexamethasone OR Cataract AND Millicorten OR Cataract AND Maxidex OR Cataract AND Decaspray OR Cataract AND Dexpak OR Cataract AND Dexasone OR Cataract AND Oradexon OR Cataract AND Decaject OR Cataract AND Hexadecadrol OR Cataract AND Hexadrol OR Cataract AND Methylfluorprednisolone OR Cataract AND Decameth
Phaco* AND Nonsteroid OR Phaco* AND nonsteroidal OR Phaco* AND NSAID OR Phaco* AND "Cyclooxygenase 2 Inhibitor" OR Phaco* AND "COX 2 inhibitor" OR Phaco* AND Coxib OR Phaco* AND Acular OR Phaco* AND ketorolac OR Phaco* AND Toradol OR Phaco* AND Ocufen OR Phaco* AND Flurbiprofen OR Phaco* AND Flubiprofen OR Phaco* AND Fluriproben OR Phaco* AND Cebutid OR Phaco* AND Dobrofen OR Phaco* AND Flugalin OR Phaco* AND Froben OR Phaco* AND "Neo Artrol" OR Phaco* AND "Novo Flurprofen" OR Phaco* AND Ocuflur OR Phaco* AND Strefen OR Phaco* AND Ansaid OR Phaco* AND Amfenac OR Phaco* AND Nepafenac OR Phaco* AND Nevanac OR Phaco* AND Bromfenac OR Phaco* AND DuraSite OR Phaco* AND Xibrom OR Phaco* AND Duract OR Phaco* AND Diclofenac OR Phaco* AND Diclophenac OR Phaco* AND Dicrofenac OR Phaco* AND Dichlofenal OR Phaco* AND "Diclonate P" OR Phaco* AND Feloran OR Phaco* AND Voltarol OR Phaco* AND Novapirina OR Phaco* AND Orthofen OR Phaco* AND Ortofen OR Phaco* AND Orthophen OR Phaco* AND Voltaren OR Phaco* AND Indomethacin OR Phaco* AND Indometacin OR Phaco* AND Indocid OR Phaco* AND Osmosin OR Phaco* AND "Indomet 140" OR Phaco* AND Metindol OR Phaco* AND Amuno OR Phaco* AND Indocin OR Phaco* AND Steroid OR Phaco* AND Steroidal OR Phaco* AND "Adrenal Cortex Hormone" OR Phaco* AND Corticosteroid OR Phaco* AND Corticoid OR Phaco* AND Glucocorticoid OR Phaco* AND Glucorticoid
Phaco* AND Hydroxycorticosteroid OR Phaco* AND Glucocorticosteroid OR Phaco* AND CEHOAC OR Phaco* AND Lotemax OR Phaco* AND Loteprednol OR Phaco* AND Alrex OR Phaco* AND Rimexolone OR Phaco* AND Rimexel OR Phaco* AND Vexol OR Phaco* AND "Prednisolone Acetate" OR Phaco* AND "Prednisolone 21 acetate" OR Phaco* AND "Pred Forte" OR Phaco* AND Dexamethasone OR Phaco* AND Millicorten OR Phaco* AND Maxidex OR Phaco* AND Decaspray OR Phaco* AND Dexpak OR Phaco* AND Dexasone OR Phaco* AND Oradexon OR Phaco* AND Decaject OR Phaco* AND Hexadecadrol OR Phaco* AND Hexadrol OR Phaco* AND Methylfluorprednisolone OR Phaco* AND Decameth
Data and analyses
Comparison 1. NSAIDs versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean cell values at one week | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Measured with cell meter (cells per 0.075 mm3) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.19, 0.99] |
| 2 Mean flare values at one week | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Measured with flare meter (photons/milisecond) | 5 | 365 | Mean Difference (IV, Random, 95% CI) | ‐13.74 [‐21.45, ‐6.04] |
| 3 Proportion of participants with cystoid macular edema one month postoperative | 4 | 291 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.17, 0.41] |
Comparison 2. NSAIDs plus corticosteroids versus corticosteroids alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with cystoid macular edema at one week | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.03, 0.97] |
| 2 Proportion of participants with cystoid macular edema at one month | 7 | 1213 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.23, 1.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 2005.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 50 eyes of 42 participants Per group: NSAID plus corticosteroid: 25 eyes, corticosteroid only: 25 eyes Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: France Age: not reported Gender (per cent): not reported Inclusion criteria: participants scheduled for phacoemulsification Exclusion criteria: complicated surgeries and participants with OCT images with artifacts Equivalence of baseline characteristics: yes, “No significant preoperative difference was detected between the two groups for age, visual acuity and macular thickness.” |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: indomethacin plus dexamethasone postoperatively Corticosteroid: dexamethasone only postoperatively Other medications: all participants also received the antibiotic tobramycin postoperatively Length of follow‐up: Planned: not reported Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: macular thickness measured using OCT Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day before surgery, 1 week and 1 month after surgery |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not described. |
| Masking of outcome assessment (detection bias) | Low risk | “OCT examination and interpretation were done by an expert masked for treatment and exam timing.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | Unable to judge whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not provided; some participants had more than 1 eye included, but authors do not report on how correlation between eyes was handled in the analysis. |
Almeida 2008.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 106 eyes of 98 participants Per group: NSAID + corticosteroid: 53, corticosteroid alone: 53 Exclusions after randomization: 1 participant in the ketorolac group canceled surgery Number analyzed (total and per group): Total: at 1 week: 98; at 1 month: 80 Per group: at 1 week: NSAID + corticosteroid: 50, corticosteroid alone: 48; at 1 month: NSAID + corticosteroid: 38, corticosteroid alone: 42 Unit of analysis: eyes Losses to follow‐up: ketorolac group: total of 15 (1 canceled surgery, 3 ketorolac corneal toxicity, 1 Alzheimer’s disease‐related decompensation, 1 urgent orthopedic surgery, 9 missed postop. month appointment); control group: total of 11 (1 postoperative corneal edema, 1 intraoperative complication, 3 voluntarily withdrew from study to avoid extra follow‐up at 1 month, 1 CME detected at 1 week and not included in 1‐month follow‐up, 1 iritis, 1 Alzheimer’s disease‐related decompensation, 3 missed postop month appointment) How were missing data handled?: complete‐case analysis (those with missing data were not included) Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Canada Age: NSAID + corticosteroid mean age = 71.3 ± 10.3 (range 45 to 92); corticosteroid‐alone mean age = 72.4 ± 10.3 (45 to 92) Gender (per cent): NSAID + corticosteroid: 26 males (49.1%) and 27 females (50.9%); corticosteroid alone: 16 males (30.1%) and 37 females (69.8%) Inclusion criteria: first cataract eye surgery, receiving phacoemulsification with intraocular lens implantation Exclusion criteria: hypersensitivity to the NSAID drug class, aspirin/NSAID‐induced asthma, and pregnancy in the 3 trimester Equivalence of baseline characteristics: yes, “Overall, baseline demographics and clinical characteristics were similar in both groups.” |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: ketorolac tromethamine 0.5% 4 times a day beginning 2 days before surgery and for 29 days after surgery plus prednisolone acetate 1% 4 times a day for 1 week followed by twice a day for 1 week Corticosteroid: prednisolone acetate 1% 4 times a day for 1 week followed by twice a day for 1 week Other medications: all participants also received the antibiotic gatifloxacin 0.3% Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: difference in total macular volume (TMV) between 1 month and baseline measured using OCT Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: preoperatively, 7 days (range 5 days to 10 days), and 28 days (range 4 to 6 weeks) after surgery |
|
| Notes |
Type of study: published Funding sources: funded by a Queen’s University grant, Kingston, Ontario, Canada Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned” Study period: June 2006 to May 2007 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | High risk | This study was reported as non‐masked. |
| Masking of outcome assessment (detection bias) | High risk | This study was reported as non‐masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who dropped out were not included in the analysis. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
Almeida 2012.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 193 eyes of 193 participants Per group: not reported Exclusions after randomization: 31 participants did not complete the study Number analyzed (total and per group): Total: 162 eyes of 162 participants Per group: NSAID + corticosteroid 1: 54, NSAID + corticosteroid 2: 54, placebo + corticosteroid: 54 Unit of analysis: eyes Losses to follow‐up: none (participants were withdrawn because of side effects/rescheduled surgery) How were missing data handled?: intention‐to‐treat analysis Reported power calculation: yes, 80% Unusual study design?: none |
|
| Participants |
Country: Canada Age: 72.4 ± 8.2 Gender (per cent): 88 (54%) female, 74 (46%) male Inclusion criteria: 18 years or older, cataract, expected to have phacoemulsification with implantation of posterior chamber intraocular lens Exclusion criteria: pre‐existing retinal disease, previous uveitis, previous intraocular surgery, allergy or hypersensitivity to NSAIDs, complicated previous cataract surgery Equivalence of baseline characteristics: yes, "There were no differences in age, sex, or operative eye between the 3 groups." |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid 1: ketorolac 0.5% 4 times a day starting 1 day before surgery and continuing for 4 weeks plus prednisolone 1% 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week NSAID + corticosteroid 2: nepafenac 0.1% 4 times a day starting 1 day before surgery and continuing for 4 weeks plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Corticosteroid: placebo plus prednisolone Other medications: all participants also received the antibiotic gatifloxacin 0.3% Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: change in OCT macular cube central subfield thickness, macular cube volume, and average macular cube thickness Secondary outcomes, as defined in study reports: COMTOL HRQOL analysis Adverse events reported: yes Intervals at which outcomes assessed: day 0 or 1, 1 month |
|
| Notes |
Type of study: published Funding sources: Queen's University educational research grant Disclosures of interest: no financial or proprietary interest Study period: March 2010 to May 2011 Reported subgroup analyses: yes Trial registry #: NCT01395069 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “placebo, nepafenac, and ketorolac suspensions were supplied in identical generic drop bottles ... Bottles were labeled with study identification number, patient identification number, expiration date, and emergency contact information only.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Study is reported as double‐masked, but masking of outcome assessors was not described specifically. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “One hundred sixty‐two patients, 54 in each arm, made up the intent‐to‐treat data set” |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
Asano 2008.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: NSAID: 75 eyes of 75 participants, corticosteroid: 75 eyes of 75 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 142 eyes of 142 participants Per group: NSAID: 71 eyes of 71 participants, corticosteroid: 71 eyes of 71 participants Unit of analysis: individual (1 eye per participant) Losses to follow‐up: NSAID: total 4: 1 due to "complications", 3 due to "discontinuation proposal (there were patients who withdrew their consent during the course of this study)"; corticosteroid: total 4: 1 due to "complications", 2 due to "discontinuation proposal", 1 who "did not return to the hospital 2 weeks after surgery" How were missing data handled?: missing data were not included Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Japan Age: NSAID mean age = 66.07 ± 5.51; corticosteroid mean age = 66.23 ± 5.55 Gender (per cent): NSAID: 43.6% male, 56.3% female; corticosteroid: 45% male, 55% female Inclusion criteria: age 55 to 75 years, nuclear hardness of Emery‐Little grade IV or less, and surgery in 1 eye only Exclusion criteria: acute infection or inflammation within 1 month after initiation of the study, allergy to NSAIDs, steroids or fluorescein, history of eye trauma or intraocular disease other than cataract, pseudoexfoliation syndrome, uveitis, glaucoma, diabetes and related complications, kidney disease, asthma or chronic airway disease, uncontrolled hypertension, severe heart failure, myocardial infarction or cerebrovascular disorders, and intraoperative complications such as posterior capsule rupture, vitreous loss, retained lens nucleus, or lens fragments in the vitreous Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac 0.1% 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery Corticosteroid: topical betamethasone 0.1% 3 hours, 2 hours, 1 hour, and 30 minutes before surgery and 3 times a day for 8 weeks after surgery Length of follow‐up: Planned: 8 weeks Actual: 8 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, IOP and flare Secondary outcomes, as defined in study reports: incidence and severity of CME (measured by fluorescein angiography) Adverse events reported: no Intervals at which outcomes assessed: 1 and 3 days, 1, 2, 5, and 8 weeks after surgery |
|
| Notes |
Type of study: published Funding sources: none reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: April 2004 to September 2005 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | “The test drugs were assigned to patients at random after the controller validated that the assigned therapy was indistinguishable from the alternative therapy. The controller kept the assignment code until completion of the study. The controller created an emergency code, which was given to the principal investigator in an envelope. The investigator could open the envelope if severe adverse effects developed.” |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is described as double‐masked, but it is unclear who was masked — participants, surgeons, or outcomes assessors. |
| Masking of outcome assessment (detection bias) | Unclear risk | The study is described as double‐masked, but it is unclear who was masked — participants, surgeons, or outcomes assessors. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4 participants from each group were randomized but not included in the analysis; of these 3 in one group and 2 in the other group were not included due to withdrawing consent. It is not clear if this was due to the study drug or not. |
| Selective reporting (reporting bias) | Unclear risk | Though IOP was evaluated at every postsurgery visit, the incidence of CME was only evaluated at week 5, for reasons that are not clearly stated. |
| Other bias | Unclear risk | Though the authors report no conflict of interest, no funding source is provided. |
Bucci 2001.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 62 eyes Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: not reported Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac 4 times a day Corticosteroid: rimexolone 4 times a day Length of follow‐up: Planned: 2 weeks Actual: 2 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: cell and flare measure Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 2 weeks postoperatively |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there was any loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources were not reported. |
Cervantes‐Coste 2009.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: 30 eyes of 30 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 60 eyes of 60 participants Per group: 30 eyes of 30 participants Unit of analysis: participants Losses to follow‐up: “All patients completed the follow‐up visits over a 6‐week period” How were missing data handled?: N/A Reported power calculation: Yes, reported in Discussion section and appears to be post hoc ‐ “Statistical analysis showed that 46 patients per arm (80%) power were needed to show difference in FT [foveal thickness] between the two groups” Unusual study design?: no |
|
| Participants |
Country: Mexico Age: Mean age = 71.9 +/‐ 9.7 years (range 51 to 88) Gender (per cent): 36.6% male, 63.4% female Inclusion criteria: adults 40 years of age or older, regardless of race or gender, who were diagnosed with senile or metabolic cataract or both and were scheduled for surgery by phacoemulsification and IOL implantation inside the capsular bag, with a normal fundoscopy exam (if observance was possible) Exclusion criteria: pregnant or breastfeeding women; history of ocular inflammatory or infectious eye disease; treatment for eye infection within 30 days prior to inclusion in the study; alterations on the eye surface (including dry eye); history of ocular surgery or trauma or both; and knowledge or suspicion of allergy or hypersensitivity to the preservatives, steroids, topical NSAIDs, or any other component of the study medication. Other exclusion criteria were use of eye medications, including prostaglandin analogues; use of topical or systemic steroids within 30 days prior to inclusion in the study; and use of topical or systemic NSAIDs within 14 days prior to inclusion in the study. People with non‐controlled diabetes mellitus based on clinical history and blood glucose level (≥ 126 mg), proliferative diabetic retinopathy, and/or macular edema were excluded from the study. Preoperative mydriasis less than 6 mm prior to the study; synechiae; ocular alteration preventing adequate mydriasis such as iris atrophy; macular alteration documented by optical coherence tomography, including macular edema of any etiology, macular holes, epiretinal membrane, macular degeneration related to age, and central serous chorioretinopathy; and the use of contact lens in the eye involved during the study were also considered exclusion criteria. Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 0.1% 3 x daily 1 day prior to surgery and continued for 6 weeks plus dexamethasone treatment 4 x daily for 10 days after surgery Corticosteroid: dexamethasone treatment 4 x daily for 10 days after surgery Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 6 weeks Actual: 6 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: trans‐operative mydriasis and postoperative macular edema (“FT [foveal thickness] and TMV [total macular volume] at baseline”) measured using macular OCT and the "Fast Macular Thickness Map" scan Secondary outcomes, as defined in study reports: average foveal thickness and total macular volume in the control and nepafenac groups Adverse events reported: “There were no serious treatment‐related adverse events or toxicity related to the use of nepafenac 0.1%” Intervals at which outcomes assessed: 1 day, 2 weeks, 6 weeks after surgery |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: the authors have no conflicts of interest to disclose Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | “The identity of patients receiving preoperative mydriatic or preoperative mydriatic and nepafenac was concealed from the surgeons”. This is reported as a single‐masked study. Since surgeons were masked, we infer that participants were not masked, but it is unclear whether this would create bias. |
| Masking of outcome assessment (detection bias) | Unclear risk | This is reported as a single‐masked study and it is stated that surgeons were masked. It is unclear whether the outcomes assessors were the surgeons or other study personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All patients completed the follow‐up visits over a 6 week period” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
Chatziralli 2011.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 145 participants Per group: NSAID plus corticosteroid: 73; corticosteroid alone: 72 Exclusions after randomization: people who underwent vitrectomy due to PCR were excluded: 3 people in the NSAID plus corticosteroid group; 4 people in the corticosteroid alone group Number analyzed (total and per group): Total: 138 Per group: NSAID plus corticosteroid: 70; corticosteroid alone: 68 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Greece Age: NSAID plus corticosteroid mean age = 74.3 ± 7.3; corticosteroid alone = 74.0 ± 7.6 Gender (per cent): NSAID plus corticosteroid: 43 (61.4%) men, 27 (38.6%) women; corticosteroid alone: 40 (58.9%) men, 28 (41.1%) women Inclusion criteria: not reported Exclusion criteria: history of intraocular surgery on the eye to be operated; any previous episode of uveitis in the eye to be operated; severe systemic disease (heart failure of the New York Heart Association stage III of IV, end‐stage renal failure, pulmonary failure, receiving chemotherapy); and regular, systemic use of steroid or NSAID during the last 3 months Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: combination of ketorolac tromethamine 0.5%, 1 drop 3 times per day plus dexamethasone 0.1%, 1 drop 4 times per day for 28 days after phacoemulsification Corticosteroid: dexamethasone 0.1%, 1 drop 4 times per day for 28 days after phacoemulsification Other medications: all participants also received the antibiotic tobramycin 0.3% Length of follow‐up: Planned: 42 days Actual: 42 days “On day 35, patients who needed continuation of the treatment were once again evaluated.” “Irrespective of continuation, on day 42 all patients underwent fundoscopy and an Amsler grid test, so as to trace any suspicious signs for the development of clinically significant cystoid macular edema (CME)." |
|
| Outcomes |
Primary outcome, as defined in study reports: corneal edema, conjunctival hyperemia, anterior chamber or Tyndall reaction Secondary outcomes, as defined in study reports: BCVA Adverse events reported: no Intervals at which outcomes assessed: days 7, 14, 21, and 28 postoperation |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: 1 October 2009 to 10 January 2010 Reported subgroup analyses: no Trial registry #: NCT01103401 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “The study was masked to the patients, i.e. they received unmarked bottles so as to be unaware of which treatment they received”. “Each patient was independently assessed by 2 ophthalmologists at each visit” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Patients who underwent vitrectomy due to posterior capsule rupture were excluded” |
| Selective reporting (reporting bias) | High risk | “On day 35, patients who needed continuation of the treatment were once again evaluated as above” (results not shown) “All patients reported pain and ocular discomfort lower than 1/10 on the visual analog scale at all time points” (it is unclear whether this includes days 35 and 42) |
| Other bias | Unclear risk | Funding sources were not reported. |
Chen 2015.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 100 individuals, 120 eyes Per group: 50 individuals, 60 eyes Exclusions after randomization: not reported Number analyzed (total and per group): Total: 100 individuals, 120 eyes Per group: NSAID plus corticosteroid: 50 individuals, 60 eyes; corticosteroid alone: 50 individuals, 60 eyes Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: China Age: NSAID plus corticosteroid mean age = 72.08 ± 9.68, corticosteroid only mean age = 75.12 ± 9.42 Gender (per cent): NSAID plus corticosteroid: 30 (50%) women's eyes, 30 (50%) men's eyes; corticosteroid only: 28 (47%) women's eyes, 32 (53%) men's eyes Inclusion criteria: cataract surgery with phacoemulsification and implantation of posterior chamber lens from January 2013 to May 2015; normal heart, lung, kidney, and liver function Exclusion criteria: non‐infectious blepharitis; chronic conjunctivitis; non‐infectious keratitis; sclerotitis; glaucoma; uveitis; ocular bottom diseases; eye trauma history; lens nucleus level II‐III; tear break‐up time ≥ 10 seconds Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: bromfenac sodium 0.1% twice daily for 1 to 3 days preoperatively and 2 weeks postoperatively plus dexamethasone 4 times daily for 1 week postoperation and 3 times daily for another week Corticosteroid alone: dexamethasone 6 times daily for 1 week postoperation and 4 times daily for another week Other medication: all participants also received the antibiotics tobramycin 4 or 6 times daily for 1 week postoperation and 3 or 4 times daily for another week and levofloxacin 4 times daily for 1 to 3 days preoperatively Length of follow‐up: Planned: 2 weeks Actual: 2 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: inflammation score, IOP, cystoid macular edema measured with OCT Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1, 7, 14 days |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: none Study period: January 2013 to May 2015 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up and no missing data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; protocol was not available. |
| Other bias | Unclear risk | Funding sources were not reported. |
Dal 2014.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 58 eyes of 43 participants Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: not reported Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: not reported Unusual study design?: in some participants, both eyes of a single participant were included, and it is unclear if non‐independence of the eyes was taken into consideration in the analysis |
|
| Participants |
Country: Turkey Age: not reported Gender (per cent): not reported Inclusion criteria: people who underwent uneventful phacoemulsification surgery Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: topical dexamethasone 0.1% 6 times daily postoperatively, which was tapered and discontinued 6 weeks after surgery plus topical ketorolac 0.5% 4 times daily, beginning 2 days prior to surgery and discontinued 4 weeks after surgery Corticosteroid: topical dexamethasone 0.1% 6 times daily postoperatively, which was tapered and discontinued 6 weeks after surgery Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: macular thickness; mean foveal thickness; parafoveal and perifoveal thickness (all measured using OCT) Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: week 1, months 1 and 2 postoperatively |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were randomized, excluded, lost to follow‐up were not reported. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; protocol was not available. |
| Other bias | Unclear risk | Both eyes of some participants were included, and it is unclear whether non‐independence of the eyes was taken into consideration in the analysis; this study was published in abstract form and full‐text was not available. Declarations of interest were not reported. |
Demco 1997.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 116 eyes of 116 participants Per group: NSAID: 57 eyes of 57 participants, corticosteroid: 59 eyes of 59 participants Exclusions after randomization: not reported Number analyzed (total and per group): Total: ITT analysis: 116 eyes of 116 participants; per‐protocol analysis: 101 eyes of 101 participants Per group: ITT analysis: NSAID 57 eyes of 57 participants, corticosteroid: 59 eyes of 59 participants; per‐protocol analysis: NSAID 50 eyes of 50 participants, corticosteroid 51 eyes of 51 participants Unit of analysis (individuals vs eyes): eyes (1 eye per participant) Losses to follow‐up: protocol violations NSAID: 7 (3 failure to follow the appointment schedule, 1 intake of unacceptable concomitant medication, 3 non‐compliance with the study medication); corticosteroid: 8 (4 complicated surgery, 3 failure to follow the appointment schedule, 1 intake of unacceptable concomitant medication) How were missing data handled?: ITT analysis for the efficacy, safety, and tolerance parameters Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: not reported Age: NSAID mean = 74, corticosteroid mean = 72 Gender (per cent): NSAID: 26 (45.6%) men and 31 (54.3%) women; corticosteroid: 16 (27.1%) men and 43 (72.9%) women Inclusion criteria: at least 40 years old with visually disabling cataract and scheduled to undergo phacoemulsification with posterior chamber lens implication Exclusion criteria: intraocular pressure greater than 26 mm Hg with or without treatment, any ocular pathology like pseudoexfoliation or diabetic retinopathy, ocular medications other than topical beta‐blockers or artificial tear substitutes, previous intraocular surgery in the operative eye, signs of or history of intraocular inflammation or herpes infection in the operative eye, use of topical or systemic steroids in the 4 weeks preceding surgery, hypersensitivity to NSAIDs or any of the ingredients in the study medications, operative eye being the only useful eye, presence of insulin‐dependent diabetes mellitus or uncontrolled diabetes mellitus of any type, and the presence or likelihood of pregnancy or substance abuse Equivalence of baseline characteristics: “It was found that there was no statistically significant difference between the two groups except with relation to sex (chi‐squared test, p=0.039).” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% (1 drop 4 times daily) for the duration of the study Corticosteroid: prednisolone acetate 1.0% (1 drop 4 times daily) for the duration of the study Length of follow‐up: Planned: 12 to 16 days Actual: 12 to 16 days |
|
| Outcomes |
Primary outcome, as defined in study reports: sum of flare and cells Secondary outcomes, as defined in study reports: conjunctival hyperemia, visual acuity, slit‐lamp examination, applanation tonometry Adverse events reported: yes, “There were two probably drug‐related adverse events in the prednisolone group and none in the diclofenac group.” Intervals at which outcomes assessed: day 1, 5 to 8, and 12 to 16 postsurgery |
|
| Notes |
Type of study: published Funding sources: “This study was supported in part by a grant from CIBA Vision Ophthalmics, Bülach, Switzerland.” Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “To ensure masking of the groups, the patients, investigators and study personnel from the investigator site and CIBA Vision Ophthalmics were masked as to the study drug codes until the statistical analysis of the study data was completed.” |
| Masking of outcome assessment (detection bias) | Low risk | “To ensure masking of the groups, the patients, investigators and study personnel from the investigator site and CIBA Vision Ophthalmics were masked as to the study drug codes until the statistical analysis of the study data was completed.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An ITT analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | It is stated that visual acuity outcomes were collected, but they were not reported, for unknown reasons. |
| Other bias | Unclear risk | The study was funded in part by CIBA Vision, which makes diclofenac sodium (NSAID). |
Donnenfeld 2006.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 100 eyes of 100 participants Per group: 25 each Exclusions after randomization: not reported Number analyzed (total and per group): Total: 100 eyes of 100 participants Per group: 25 each Unit of analysis (individuals vs eyes): individuals Losses to follow‐up: unclear How were missing data handled?: unclear Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: not reported Age: 72.8 +/‐ 8.5 Gender (per cent): 55% female, 45% male Inclusion criteria: not reported Exclusion criteria: sensitivity to study medications, monocular status, previous intraocular surgery, diabetes mellitus, history of uveitis, iritis, or intraocular inflammation, use of NSAID during the study or the week before, pupils that dilated no more than 5.0 mm or required mechanical pupil stretching, pregnant, nursing, or planning a pregnancy Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 3 days preoperatively and 3 times every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week NSAID 2 plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 1 day preoperatively and every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week NSAID 3 plus corticosteroid: ketorolac tromethamine 0.4% every 15 minutes in the hour before surgery and 4 times daily for 3 weeks after surgery plus topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week Corticosteroid: vehicle every 15 minutes in the hour before surgery and topical prednisolone acetate 1% 4 times a day for 2 weeks after surgery then twice a day for 1 additional week Other medications: all participants also received topical gatifloxacin 0.3% 4 times daily for 3 days before cataract surgery and 1 week after surgery Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcomes, as defined in study reports: mydriasis at the onset of surgery and at the time of IOL insertion, inflammation, incidence of clinically significant CME at 2 weeks, participant discomfort requiring additional analgesia, UCVA, BCVA, surgical time, endothelial cell counts, corneal clarity, intraoperative surgical complications, and mean ultrasound and phacoemulsification times Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 2 weeks, 3 months |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: Drs Donnenfeld, Perry, and Wittpenn are consultants to Allergan Pharmaceuticals Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group assignment was based on a random‐number‐generated protocol that was created before initiation of the study. The process ensured the randomization of cataract density in the 4 groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether participants, personnel, or outcome assessors were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether participants, personnel, or outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were any missing data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective reporting. |
| Other bias | Low risk | No other sources of bias |
Duong 2014.
| Methods |
Study design: CCT Number randomized (total and per group): Total: 269 eyes of 269 participants Per group: bromfenac 0.9%: 138 eyes of 138 participants; prednisolone acetate 1%: 135 eyes of 135 participants Exclusions after randomization: not reported Number analyzed (total and per group): Total: 222 eyes of 222 participants Per group: bromfenac 0.9%: 113, prednisolone acetate 1%: 109 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: bromfenac 0.9%: 25, prednisolone acetate 1%: 26 How were missing data handled?: data from participants lost to follow‐up were not included in the analysis Reported power calculation: yes Unusual study design?: “Patients not responding to the respective group’s postoperative regimen were given rescue medication, that is, if the degree of anterior chamber inflammation did not improve clinically or if the SOIS remained the same in subsequent clinic visits, the patient in the NSAID group (group I) was given a steroid, and the patient in the steroid group (group II) was given an NSAID”; “Patients were excluded from the study if the FT (foveal thickness) and TMV (total macular volumes) were 2 SDs from the baseline OCT value(s)” |
|
| Participants |
Country: United States Age: bromfenac mean age = 71.8 ± 8.5, prednisolone mean age = 71.1 ± 10.4 Gender (per cent): bromfenac: 53 (46.9%) male and 60 (53.1%) female; prednisolone: 42 (38.5%) male and 67 (61.5%) female Inclusion criteria: people with visually significant cataracts which were defined as follows: BCVA worse than 20/50; cataracts affecting the activities of daily living; symptomatic, e.g. glares and halos, myopic shift; and/or monocular diplopia. The Lens Opacities Classification System II was used to classify the opacity. Greater than 98% of the operated cataracts were of the age‐related type, nuclear sclerosis with/without cortical changes, to include mature and hard cataracts. The remaining cataracts were of the posterior subcapsular cataract and traumatic types. Specific to this study, type II diabetic patients with nonproliferative diabetic retinopathy without a history of macular edema or photocoagulation therapy were included in this study. Exclusion criteria: proliferative diabetic retinopathy, persistent macular edema secondary to diabetic retinopathy, epiretinal membrane, pre‐existing anterior uveitis, exfoliation syndrome, and exudative macular degeneration Equivalence of baseline characteristics: yes, “No significant differences were observed in age, visual acuity, operation time, and macular edema” |
|
| Interventions |
Comparison: NSAID vs corticosteroid NSAID: bromfenac 0.09%, 1 drop in operated eye every day starting 3 days before surgery and continuing for a total of 14 days Corticosteroid: prednisolone acetate 1%, 1 drop in the operated eye 4 times a day for 7 days followed by a tapering dose totaling 14 days of treatment Other medications: besifloxacin 0.6%, 1 drop in the operated eye twice a day for 2 days before surgery and 7 days after surgery Length of follow‐up: Planned: 2 months Actual: 82 (73%) of participants in the bromfenac group and 83 (76%) of participants in the prednisolone group returned for their 2‐month postoperative OCT measures |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, degree of anterior chamber inflammation, prevalence of macular edema using foveal thickness and mean thickness of the fovea using OCT (postoperative macular changes falling outside of 2 SDs were considered to have cystoid macular edema) Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months |
|
| Notes |
Type of study: published Funding sources: “This study was not funded by any government or nongovernment agencies. Ample pharmaceutical agents were made available to all the patients throughout the study to ensure that patients enrolled in the study did not have to pay out of their pocket” Disclosures of interest: “All participants (ophthalmologists and optometrist) in this study do not have any financial and proprietary interests in any of the products included in this study. None of the authors and personnel involved in this study have any competing interests with any of the products mentioned throughout this article.” Study period: 4 April 2011 to 31 August 2011 Reported subgroup analyses: yes, participants with diabetes Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients undergoing cataract surgery on the even week of the month, that is, the second and fourth week of the month, were placed in group I, whereas those scheduled for the odd week of the month, that is, first, third, and fifth (when applicable) week of the month, were placed in group II. The nursing staff at the surgery center was given a study schedule indicating which medications were to be given preoperatively and postoperatively" |
| Allocation concealment (selection bias) | High risk | It was planned in advance to which groups participants would be assigned. |
| Masking of participants and personnel (performance bias) | High risk | “single‐masked” study; “the surgical counselor/scheduler, the operating surgeon, and the evaluating ophthalmologist were 'blinded' about which group the patients will be placed into. This information was known only to the primary author and the nursing staff at the surgery center.”; participants were not masked. |
| Masking of outcome assessment (detection bias) | Low risk | “single‐masked” study; “the surgical counselor/scheduler, the operating surgeon, and the evaluating ophthalmologist were 'blinded' about which group the patients will be placed into. This information was known only to the primary author and the nursing staff at the surgery center.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is reported that 51 participants were lost to follow‐up, and the data from these participants were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | “Patients not responding to the respective group’s postoperative regimen were given rescue medication, that is, if the degree of anterior chamber inflammation did not improve clinically or if the SOIS remained the same in subsequent clinic visits, the patient in the NSAID group (group I) was given a steroid, and the patient in the steroid group (group II) was given an NSAID”; “Patients were excluded from the study if the FT and TMV were 2 SDs from the baseline OCT value(s)” |
el‐Harazi 1998.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: 20 participants per group Exclusions after randomization: 2; people were excluded from the study if they had any postoperative complication affecting accurate measurements of the flare and cells Number analyzed (total and per group): Total: 58 Per group: NSAID group 1: 19; NSAID group 2: 19; corticosteroid group: 20 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: United States Age: NSAID group 1 mean = 68.1 (range 32 to 85); NSAID group 2 mean = 70.2 (range 48 to 81); corticosteroid group mean = 71.1 (range 49 to 86) Gender (per cent): NSAID group 1: 47.3% men, 52.7% women; NSAID group 2: 57.9% men, 42.1% women; corticosteroid group: 55% men, 45% women Inclusion criteria: people who underwent phacoemulsification with intraocular lens implantation Exclusion criteria: evidence of acute or chronic ocular inflammation, previous surgery in the eye that was operated on, use of any systemic or topical corticosteroids or NSAIDs, known hypersensitivity to any of the study drugs, and lack of consent Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs NSAID vs corticosteroid NSAID 1: diclofenac sodium 0.1% one drop 4 times a day for 1 week, then 2 times a day for 3 weeks NSAID 2: ketorolac tromethamine 0.5% one drop 4 times a day for 1 week, then 2 times a day for 3 weeks Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: 28 days Actual: 28 days |
|
| Outcomes |
Primary outcome, as defined in study reports: flare, cells, and intraocular pressures Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: postoperative days 1, 7, and 28 |
|
| Notes |
Type of study: published Funding sources: Hermann Eye Fund, Research to Prevent Blindness and by an unrestricted grant from Allergan Inc. Disclosures of interest: none Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “Postoperatively, drops were supplied by the hospital pharmacy in identical 10‐mL bottles labeled with the drug code and the patient’s identification number. Both patients and study examiners were masked to the content of the bottles” |
| Masking of outcome assessment (detection bias) | Unclear risk | It is unclear whether outcomes assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Two patients were withdrawn from the study after randomization ... neither patient received study medications. Fifty‐eight patients completed the study” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective reporting. |
| Other bias | Unclear risk | The study was funded by an unrestricted grant from Allergan, the makers of one of the study drugs. |
Elsawy 2013.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 86 eyes of 70 participants Per group: NSAID group: 43 eyes of 35 participants; corticosteroid group: 43 eyes of 35 participants Exclusions after randomization: none Number analyzed (total and per group): Total: 86 eyes of 70 participants Per group: NSAID group: 43 eyes of 35 participants; corticosteroid group: 43 eyes of 35 participants Unit of analysis: eyes Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: some participants had both eyes included analyses did not take into account the non‐independence of eyes |
|
| Participants |
Country: Egypt Age: NSAID group: 13 aged 50 to 60, 14 aged 61 to 70, 6 aged > 70; corticosteroid group: 15 aged 50 to 60, 13 aged 61 to 70, 9 aged > 70 Gender (per cent): overall: 44 male and 26 female; NSAID group: 23 male and 12 female; steroid group: 21 male and 14 female Inclusion criteria: people selected for the study had at least 1 risk factor for CME (besides diabetic retinopathy), including history of retinal vein occlusion, presence of epiretinal membrane, or preoperative use of prostaglandin analogues eye drops Exclusion criteria: not reported Equivalence of baseline characteristics: yes, “There was non‐significant (P>0.05) difference between both studied groups regarding the enrollment data (Table 1).” |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID: topical ketorolac tromethamine 0.4% twice daily in addition to topical dexamethasone 0.1% 4 times daily for 12 weeks Corticosteroid: topical dexamethasone 0.1% 4 times daily for 12 weeks postoperatively Length of follow‐up: Planned: 12 weeks Actual: 12 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: clinical signs of CME; ocular coherent tomography Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 3, 6, and 12 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “The authors report no conflicts of interest in this work.” Study period: January 2011 to March 2012 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization process used four opaque envelopes in two containers. The first container had (1) for dexamethasone drops only, and (2) for combined drops, and the second container had the name of patients listed for cataract surgery on that day. Patients were randomized to one of the regimes by asking an independent person to choose one envelope from each container.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not described. Both eyes of some participants were included, and it is unclear whether non‐independence of the eyes was taken into consideration in the analysis. |
Endo 2010.
| Methods |
Study design: parallel RCT Number randomized (total and per group): Total: 75 eyes of 75 participants Per group: NSAID: 40; corticosteroid: 35 Exclusions after randomization: 3 people due to difficulty with the OCT measurement; 10 dropped out because of poor health (n = 8), posterior capsular rupture (n = 1), and epidemic keratoconjunctivitis (n = 1) Number analyzed (total and per group): Total: 62 eyes of 62 participants Per group: NSAID: 31; corticosteroid: 31 Unit of analysis (individuals vs eyes): 1 eye per individual Losses to follow‐up: 10 participants dropped out How were missing data handled?: not included in final results Reported power calculation: no Unusual study design?: the 2 groups had different drug administration schedules; “Considering the side‐effects of long‐term steroid administration, we substituted fluorometholone for betamethasone 1 week postoperatively because of ethical considerations” |
|
| Participants |
Country: Japan Age: 37 to 84 years Gender (per cent): 54.8% men and 45.2% women Inclusion criteria: people with diabetes who underwent small‐incision phacoemulsification with intraocular lens implantation at the facility between March 2005 and May 2007 Exclusion criteria: foveal thickness of 250 μm or more; severe diabetic retinopathy for which ocular surgery (including photocoagulation) was indicated; use of topical medications for glaucoma, uveitis, and other diseases that cause CME; ocular allergies to bromfenac (NSAID group) or steroids (corticosteroid group); use of systemic steroids or NSAIDs; and serious cardiac, cerebral, or renal disease Equivalence of baseline characteristics: yes, however there was a significant (P = 0.003) difference in glycated hemoglobin despite randomization |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: bromfenac sodium ophthalmic solution twice daily for 6 weeks Corticosteroid: steroidal solution (betamethasone sodium phosphate and neomycin) 4 times daily for 1 week followed by fluorometholone 4 times daily for 5 weeks; “Considering the side‐effects of long term steroid administration, we substituted fluorometholone for betamethasone 1 week postoperatively because of ethical considerations” Length of follow‐up: Planned: 6 weeks postoperatively Actual: 6 weeks postoperatively |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA, intraocular pressure, slit‐lamp biomicroscopy, anterior chamber flare, and average perifoveal thickness Secondary outcomes, as defined in study reports: not reported Adverse events reported: “No adverse events occurred in either group” Intervals at which outcomes assessed: preoperatively, and 1 day and 1, 2, 4, and 6 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “The authors have no financial interest in any aspect of this article.” Study period: enrollment March 2005 to May 2007 Reported subgroup analyses: yes; participants with non‐proliferative diabetic retinopathy in the NSAID group (n = 16) and the corticosteroid group (n = 11) were compared Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors describe using the “envelope method,” but it is unclear how the sequence was generated: “A prospective open‐label trial was conducted using the envelope method”. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | High risk | This was an open‐label trial. |
| Masking of outcome assessment (detection bias) | High risk | This was an open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Three patients were excluded because of difficulty with the OCT measurements. Ten patients (10 eyes) dropped out of the study because of poor health (eight patients), posterior capsular rupture (one patient) and epidemic keratoconjunctivitis (one patient).” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias. |
Entezari 2016.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 108 eyes of 108 participants Per group: steroid: 54, NSAID: 54 Exclusions after randomization: none Number analyzed (total and per group): Total: 108 eyes of 108 participants Per group: steroid: 54, NSAID: 54 Unit of analysis: eyes (1 eye per individual) Losses to follow‐up: none How were missing data handled?: not reported Reported power calculation: yes, 90% Unusual study design?: none |
|
| Participants |
Country: Iran Age: total: 68 ± 7 years; diclofenac group: 67 ± 8 years; corticosteroid group: 69 ± 6 years Gender (per cent): diclofenac group: 21 male (38.9%) and 33 female (61.1%); corticosteroid group: 27 male (50%) and 27 female (50%) Inclusion criteria: diabetic patients with non‐proliferative diabetic retinopathy who were candidates for phacoemulsification and IOL implantation Exclusion criteria: eyes with clinically significant macular edema based on the ETDRS or central macular thickness > 260 µm or both; eyes with other accompanying diseases affecting the macula or eyes with history of previous retinal laser photocoagulation or intraocular surgery; eyes with severe cataract that precludes performing acceptable quality optical coherence tomography Equivalence of baseline characteristics: no, “The groups were comparable regarding all the initial characteristics except for the baseline BCVA which was better in the case group (p = 0.036)” |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID: diclofenac sodium 0.1% every 6 hours from 1 week before surgery and up to 6 weeks after surgery plus corticosteroid drops for 1 month after surgery Corticosteroid: corticosteroid drops (not specified) for 1 month after surgery Additional medication: all participants also received antibiotics (unspecified) 4 times a day for 1 week after surgery Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA, OCT findings, IOP Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes, “None of the eyes developed drug‐related complications throughout the study.” Intervals at which outcomes assessed: preoperatively, day 1, 30, and 90 |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “None of the authors have any financial/conflicting interests to disclose.” Study period: March 2010 to June 2012 Reported subgroup analyses: no Trial registry number: NCT02306031 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study reports: “Before surgical intervention, all included eyes were randomly assigned to the case and control groups according to a permuted randomization,” but it remains unclear how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | The study reports: “Before surgical intervention, all included eyes were randomly assigned to the case and control groups according to a permuted randomization,” but it is unclear whether study personnel knew the permutation sequence. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported to be double‐masked, but details of masking or participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | “OCTs were performed by an experienced optometrist who was masked to the eyes before, and 1 and 3 months after the surgeries” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | High risk | Funding sources are not provided; “The frequency of the corticosteroid drops was adjusted for each eye”; and visual acuity at baseline was not equivalent between groups. |
Guzey 2000.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Unit of analysis: individuals (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design (any issues with study design)?: none |
|
| Participants |
Country: not reported Age: NSAID mean age = 58.8 ± 13.6; corticosteroid mean age = 60.7 ± 11.4 Gender (per cent): NSAID: 17 (56.7%) women and 13 (43.3%) men; corticosteroid: 14 (46.7%) women and 16 (53.3%) men Inclusion criteria: participants undergoing uncomplicated cataract lens implant surgery Exclusion criteria: severe systemic disorders (in particular diabetes), clinically significant ocular disease such as glaucoma, ocular hypertension, or dry eyes, history of intraocular surgery or recent extraocular surgery, history of hypersensitivity to components of the study drug, complicated surgery or significant complications on the first postoperative day, the use of topical or systemic NSAIDs, corticosteroids, or antibiotics within 14 days prior to surgery Equivalence of baseline characteristics: yes, “The baseline parameters were similar in the two study groups.” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac 0.5% one drop 5 times daily Corticosteroid: fluorometholone 0.1% one drop 5 times daily Other medications: all participants also received the antibiotic tobramycin 0.3% (1 drop 5 times daily) Length of follow‐up: Planned: not reported Actual: 14 days |
|
| Outcomes |
Primary outcome, as defined in study reports: anterior chamber flare and cells Secondary outcomes, as defined in study reports: symptoms of ocular discomfort, pinhole visual acuity, conjunctival hyperemia, local tolerance (burning/stinging and blurring of vision) Adverse events reported: yes, “During the study no adverse events requiring discontinuation from the study occurred.” Intervals at which outcomes assessed: postoperative day 1, 2, 3, 7, and 14 |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Investigators were masked, but it is not reported if participants were masked. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study is reported to be investigator‐masked, but details of masking are not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were any missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and declarations of interest were not reported. |
Hessemer 1996.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: 30 (5 treatment groups) Exclusions after randomization: “Six patients (4%) were excluded because of the following complications/adverse events: postoperative hyphema of unknown genesis (1 patient; group 2), corneal stromal edema after an ultrasonic time of more than 3 minutes of progressing brunescent cataract (1 patient each in group 1 and 5), traumatic wound rupture with required wound examination (1 patient; group 4) and two patients didn't come to follow up (group 2 and 3)" Number analyzed (total and per group): Total number: 144 eyes of 144 participants Per group: group 1: 29, group 2: 28, group 3: 29, group 4: 29, group:5: 29 Unit of analysis: individuals Losses to follow‐up: 2 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Germany Age: 39 to 88 years; group 1 mean age = 71 ± 1.9 years; group 2 mean age = 69.8 ± 1.9 years; group 3 mean age = 70.7 ± 1.7 years; group 4 mean age = 68.6 ± 1.9 years; group 5 mean age = 72.2 ± 8.9 years Gender (per cent): 82 (54.6%) women, 68 (45.3%) men Inclusion criteria: age‐related cataract, minimally inflammatory cataract surgery Exclusion criteria: glaucoma, deficiencies of the blood‐aqueous barrier, pseudoexfoliation syndrome, uveitis, ocular infections, cornea guttata, diabetes mellitus, diseases of the rheumatic form circle, local or systemic anti‐inflammatory drugs within the last 4 weeks Equivalence of baseline characteristics: unclear; age was not significantly different between groups (P = 0.69), but other characteristics were not mentioned |
|
| Interventions |
Comparison analyzed: NSAID vs NSAID plus corticosteroid vs corticosteroid alone NSAID 1 (group 1): preservative‐free diclofenac 0.1% eye drops pre‐ and postoperatively NSAID 2 (group 2): diclofenac 0.1% eye drops with preservative pre‐ and postoperatively NSAID 3 (group 3): preservative‐free diclofenac 0.1% only postoperatively NSAID plus corticosteroid (group 4): preservative‐free diclofenac 0.1% pre‐ and postoperatively in combination with dexamethasone‐21‐dihydrogenphosphate 0.1% eye drops (DEXA) Corticosteroid alone (group 5): dexamethasone 0.1% postoperatively Medication timing: Preoperatively: night before surgery, and on the day of surgery 3, 2, 1.5, 1, and ½ hours preoperatively Postoperatively: immediately after the surgery, during changing of bandages, and in the following night; they were also applied from day 1 to 7 postoperatively 5 times a day every 3 hours Length of follow‐up: Planned: 7 days postop Actual: 7 days postop |
|
| Outcomes |
Primary outcome, as defined in study reports: aqueous flare measured by laser flare‐cell meter FC‐1000 (photon counts/ms), postoperative IOP measured by Goldmann applanation tonometer (mm Hg) and BCVA Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes (postoperative hyphema of unknown genesis, corneal stromal edema after an ultrasonic time of over 3 minutes of progressing brunescent cataract, traumatic wound rupture with required wound examination) Intervals at which outcomes assessed: preoperative (day before surgery), about 6 hours postoperatively, 1, 3, 7 days postoperatively |
|
| Notes |
Type of study: published Funding sources: study medications were provided by the company “Dr. Mann Pharma, Berlin” Disclosures of interest: authors report no financial interest Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Low risk | Double‐blind study design. All eye drops were put into identical‐looking 1‐dose ampoules. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants were lost to follow‐up, but may have been included in data analysis. There is a discrepancy in the text, which states that participants have been excluded from the study, but the text underneath Table 1 states that the “mean values are from 30 patients per group” when there would have been fewer than 30. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | A pharmaceutical company provided study medications. |
Holzer 2002.
| Methods |
Study design: parallel RCT Number randomized (total and per group): Total: 60 Per group: NSAID: 30; corticosteroid: 30 Exclusions after randomization: Number analyzed (total and per group): Total: 59 Per group: NSAID: 30; corticosteroid: 29 Unit of analysis: individuals Losses to follow‐up: 1 participant in the corticosteroid group was excluded from the study because of a pre‐existing preoperative epithelial defect. 3 participants in the NSAID group and 1 in the corticosteroid group missed the 3‐day follow‐up, and 1 participant in each group missed the 7‐day follow‐up. How were missing data handled?: not directly addressed in text; possibly not included in results for those specific time points Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: United States Age: NSAID mean age = 67.3; corticosteroid mean age = 68.5 Gender (per cent): 35% men and 63% women Inclusion criteria: over 18 years of age, with a potential visual acuity of 20/40 or better in the fellow eye, had a visually significant cataract in the study eye, and were willing to comply with all postoperative instructions Exclusion criteria: pregnant, nursing, or of childbearing age and not using a medically acceptable form of birth control or had previous surgery in the eye, uveitis, insulin‐requiring diabetes, advanced glaucoma, a history of recent oral NSAID use, a known sensitivity to the study medications, or an ocular or systemic disease that could interfere with the study Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac tromethamine 0.5% ophthalmic solution 4 drops daily for 1 week and then 2 drops daily for the remainder of the study Corticosteroid: loteprednol etabonate 0.5% ophthalmic suspension 4 drops daily for 1 week and then 2 drops daily for the remainder of the study Length of follow‐up: Planned: 30 +/‐ 7 days postoperatively Actual: 30 +/‐ 7 days postoperatively |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, external examination, slit‐lamp examination including IOP measurement, and Kowa LFCM cell and flare measurement Secondary outcomes, as defined in study reports: not reported Adverse events reported: there were no adverse events in either group Intervals at which outcomes assessed: day 1, 4, 7, and 30 |
|
| Notes |
Type of study: published Funding sources: unrestricted grant from Allergan Inc. Disclosures of interest: “None of the authors has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | “The medications were given in identical bottles, and none of the patients, investigators, or technical staff knew which medication was given” |
| Masking of outcome assessment (detection bias) | Low risk | “The medications were given in identical bottles, and none of the patients, investigators, or technical staff knew which medication was given” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “One patient in the loteprednol group was excluded from the study because of a pre‐existing preoperative epithelial defect. Three patients in the ketorolac group and 1 in the loteprednol group missed the 3‐day follow‐up and one patient in each group missed the 7‐day follow‐up”. The authors do not address how the missing data were handled; implies that these measurements were not considered, but the percentage of missing data is small. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | This study is funded by an unrestricted grant from Allergan, which manufactures one of the study drugs. |
Jung 2015.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 91 eyes of 91 participants Per group: NSAID 1 plus corticosteroid: 28; NSAID 2 plus corticosteroid: 32; corticosteroid alone: 31 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 91 eyes of 91 participants Per group: NSAID 1 plus corticosteroid: 28; NSAID 2 plus corticosteroid: 32; corticosteroid alone: 31 Unit of analysis: eye (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: no |
|
| Participants |
Country: South Korea Age: total mean age = 66.9 ± 8.5 years (range 33 to 78 years); NSAID 1 plus corticosteroid mean age = 66.9 ± 11.1; NSAID 2 plus corticosteroid mean age = 67.5 ± 7.0; corticosteroid alone mean age = 66.8 ± 8.1 Gender (per cent): overall: 41 men (45.1%) and 50 women (54.9%); NSAID 1 plus corticosteroid: 13 men (46.4%) and 15 women (53.6%); NSAID 2 plus corticosteroid: 15 men (46.9%) and 17 women (53.1%); corticosteroid: 13 men (41.9%) and 18 women (58.1%) Inclusion criteria: males or non‐pregnant females aged between 20 and 80 years Exclusion criteria: poor general condition, including high blood pressure, poor blood glucose control, or renal failure; history of ocular trauma or disease; history of intraocular surgery; systemic or topical NSAIDs or corticosteroids use within 4 weeks of enrollment; known hypersensitivity to salicylates or other NSAIDs; and use of alpha‐1 adrenergic antagonist or other analogous systemic medications that could increase the tendency for miosis during the operation (intraoperative floppy iris syndrome) Equivalence of baseline characteristics: yes, “Overall, baseline demographics and clinical characteristics, including preoperative ocular surface status and macular thickness and volume, were similar among the groups.” |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid alone NSAID 1: 1 drop of bromfenac sodium hydrate ophthalmic solution 0.1% twice daily beginning 3 days before surgery and 2 drops at 20‐minute intervals 2 hours before surgery plus prednisolone acetate 1% applied 4 times daily for 4 weeks NSAID 2: 1 drop of ketorolac 0.45% ophthalmic solution twice‐daily beginning 1 day prior to cataract surgery and 2 drops at 20‐minute intervals 2 hours before surgery, with continued application twice‐daily throughout the first 2 postoperative weeks plus prednisolone acetate 1% applied 4 times daily for 4 weeks Corticosteroid: prednisolone acetate 1% eye drops applied 4 times daily for 4 weeks Other medications: all participants also received topical gatifloxacin 0.3% Length of follow‐up: Planned: 4 weeks Actual: 4 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: intraoperative change in pupil size, postoperative anterior chamber inflammation control, change in macular thickness and volume measured using OCT, and ocular surface status after operation Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: postoperative day 1, 7, and 28 |
|
| Notes |
Type of study: published Funding sources: "This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A1A2058907)." Disclosures of interest: “The authors have no financial conflicts of interest” Study period: November 2013 to June 2014 Reported subgroup analyses: yes Trial registry #: NRF 2013R1A1A2058907 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | “... and the study medications were masked to both the surgeon and examiners.” It is unclear whether participants were masked. |
| Masking of outcome assessment (detection bias) | Low risk | “... and the study medications were masked to both the surgeon and examiners.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
Kato 1998.
| Methods |
Study design: parallel‐group RCT (single center) Number randomized (total and per group): Total: 212 eyes of 108 participants Per group: group 1: 53; group 2: 50; group 3: 58; group 4: 51 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: eye Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: both eyes of some participants were independently assigned to interventions and analyzed without taking into account the non‐independence of eyes |
|
| Participants |
Country: Japan Age: mean 72.6 years, range 48 to 88 years Gender (per cent): not reported Inclusion criteria: not reported Exclusion criteria: any ocular complications such as uveitis, glaucoma, pigmentary retinopathy, or diabetic retinopathy, and intraoperative complications including iris damage, posterior capsular rupture, vitreous prolapse Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID 1 (group 1): polymethylmethacrylate lens and diclofenac sodium 3 times daily Corticosteroid 1 (group 2): polymethylmethacrylate lens and corticosteroid drops 3 times daily NSAID 2 (group 3): silicone lens and diclofenac sodium 3 times daily Corticosteroid 2 (group 4): silicone lens and corticosteroid drops 3 times daily Notes: study medication was initiated 1 day after the operation; all participants received dibekacin (Panimycin) 3 times daily Length of follow‐up: Planned: 90 days Actual: 90 days |
|
| Outcomes |
Primary outcome, as defined in study reports: anterior chamber flare Secondary outcomes, as defined in study reports: none Adverse events reported: yes Intervals at which outcomes assessed: day 1 and 3, week 1 and 2, and month 1 and 3 |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 1996 to January 1997 Reported subgroup analyses: not reported Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were excluded, lost to follow‐up, and analyzed were not reported. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | For some participants, both eyes were independently assigned to interventions and analyzed without taking into account the non‐independence of eyes; funding source and declarations of interest were not reported. |
Laurell 2002.
| Methods |
Study design: parallel RCT Number randomized (total and per group): Total: 180 eyes of 180 participants Per group: 60 eyes of 60 participants Exclusions after randomization: 9 Number analyzed (total and per group): Total: 180 eyes of 180 participants Per group: 60 eyes of 60 participants Unit of analysis (individuals vs eyes): individuals (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not included in results for specific observation times with missing data Reported power calculation: yes, 80% Unusual study design?: none |
|
| Participants |
Country: Sweden Age: 64 to 85 years of age Gender (per cent): NSAID: 33% women and 67% men; corticosteroid: 37% women and 63% men; saline: 32% women and 68% men Inclusion criteria: people with no other known eye disease, enrolled for cataract surgery Exclusion criteria: pseudoexfoliation syndrome, small pupils (< 5 mm after pharmacological dilation), dark brown iris, glaucoma, uveitis, diabetes, treatment with topical medications, or anti‐inflammatory drugs, eyes with a dark brown iris (due to being more prone postoperative inflammation) Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% 4 times daily for 1 week and then 2 times daily for 3 weeks Corticosteroid: postoperative treatment with dexamethasone phosphate 0.1% 4 times daily for 1 week and then 2 times daily for 3 weeks Control: saline 0.9% Length of follow‐up: Planned: 4 years Actual: 4 years |
|
| Outcomes |
Primary outcome, as defined in study reports: inflammatory response, visual acuity, intraocular pressure, capsulotomy rate, corneal reactions Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: “The inflammatory reaction in the anterior chamber was measured with laser flare photometry preoperatively and 1, 3, and 8 days, 2 and 4 weeks, 2 and 6 months, and 1, 2, and 4 years postoperatively” |
|
| Notes |
Type of study: published Funding sources: Alcon and Leiras provided drug, the pharmacy at St Göran’s Hospital, Stockholm provided masked bottles Disclosures of interest: “The authors have no proprietary interest in any of the products or equipment mentioned in this article” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was done by a computer in blocks of 6 participants (randomly permuted blocks, SAS/PLAN procedure). |
| Allocation concealment (selection bias) | Low risk | See above |
| Masking of participants and personnel (performance bias) | Low risk | “On the first day after surgery, the patients received three coded bottles containing one of the treatment solutions”. All bottles were white and non‐transparent. They were delivered from the pharmacy with identical labels except for the randomization number. |
| Masking of outcome assessment (detection bias) | Unclear risk | This study was reported as double‐masked, but it is unclear whether the outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The authors did not report data from withdrawn participants. |
| Selective reporting (reporting bias) | High risk | Not all follow‐up points were reported for rate of inflammatory symptoms in participants, striate keratopathy, or Nd:YAG. |
| Other bias | Unclear risk | Study drugs were provided by the respective pharmaceutical companies |
Li 2011.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 217 eyes of 217 participants Per group: NSAID: 104, corticosteroid: 113 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 217 eyes of 217 participants Per group: NSAID: 104, corticosteroid: 113 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: China Age: NSAID mean age = 72.2 ± 10.8, corticosteroid mean age = 71.6 ± 8.5 Gender (per cent): NSAID: 35 (33.7%) men and 69 (66.3%) women; corticosteroid: 46 (40.7%) men and 67 (59.3%) women Inclusion criteria: diagnosed with type II diabetes mellitus but without diabetic retinopathy and given phacoemulsification and intraocular lens implantation Exclusion criteria: diabetic retinopathy, age‐related macular degeneration, macular membrane, or retinal vascular disease, people with intraoperative complications Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac 1% for 4 weeks postoperatively and dexamethasone 4 times daily for 1 day preoperatively and 4 weeks postoperatively Corticosteroid: dexamethasone 4 times daily for 4 weeks postoperatively Other medications: all participants also received the topical antibiotic tobramycin Length of follow‐up: Planned: 4 weeks Actual: 4 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: cystoid macular edema incidence measured using OCT Secondary outcomes, as defined in study reports: central field retinal thickness and BCVA Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 and 4 weeks |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: January 2009 to December 2010 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Low risk | Published reports appear to include all expected outcomes. |
| Other bias | Low risk | No other sources of bias |
Mathys 2010.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 84 eyes of 84 participants Per group: NSAID plus corticosteroid: 42, corticosteroid alone: 42 Exclusions after randomization: 5; “Three subjects in the treatment group (NSAID plus corticosteroid) and two subjects in the control group (corticosteroid alone) had unreliable preoperative OCT scans because of dense posterior subcapsular cataracts" Number analyzed (total and per group): Total: 79 eyes of 79 participants Per group: NSAID plus corticosteroid: 39, corticosteroid alone: 40 Unit of analysis: individual (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: yes, 90% power Unusual study design?: none |
|
| Participants |
Country: United States Age: NSAID plus corticosteroid mean age = 74.0 ± 9.00 (range 51 to 90); corticosteroid alone mean age = 70.3 ± 8.0 (range 44 to 88) Gender (per cent): NSAID plus corticosteroid: 19 (47.5%) men and 21 (52.5%) women; corticosteroid alone: 18 (46.2%) men and 21 (53.9%) women Inclusion criteria: people planning to have cataract surgery by KLC at the Ambulatory Care Center, the University of North Carolina Hospitals Exclusion criteria: factors that could increase the risk of postcataract CME, including medically treated diabetes mellitus, history of uveitis, use of topical prostaglandin analogues for glaucoma, history of earlier intraocular surgery in the same eye, retinal vascular disease, and macular degeneration, abnormal preoperative OCT measurements Equivalence of baseline characteristics: no, “The mean age of patients in the treatment group was slightly greater than that in the control group, 73.95 and 70.33 years, respectively (P=0.0460)" |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 0.1% 3 times a day for 1 month plus prednisolone acetate 1% 4 times a day for 1 month Corticosteroid alone: prednisolone acetate 1% 4 times a day for 1 month Other medications: all participants also received the antibiotic moxifloxacin 0.5% 4 times a day for 10 days Length of follow‐up: Planned: not reported Actual: corticosteroid alone mean = 68.98 ± 13.98 days, NSAID plus corticosteroid mean = 73.31 ± 21.58 |
|
| Outcomes |
Primary outcome, as defined in study reports: changes of macular thickness in OCT Secondary outcomes, as defined in study reports: total macular volume and BCVA Adverse events reported: yes, “There were no adverse events reported by patients using nepafenac.” Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months |
|
| Notes |
Type of study: published Funding sources: “This work was supported in part by Research to Prevent Blindness, Inc., New York, NY.” Disclosures of interest: the authors report no financial interest Study period: not reported Reported subgroup analyses: no Trial registry #: NCT00494494 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomised according to even/odd subject identification number, using computer‐generated random numbers, to the control group (standard of care only) or to the treatment group (standard of care plus nepafenac).” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | It is unclear whether participants and personnel were masked; participants in the treatment group had an extra bottle of eye drops to use. |
| Masking of outcome assessment (detection bias) | Low risk | “Postoperative follow‐up was at 1 day, 1 week, 1 month, and 2 months. At the 2 months visits, technicians, who were masked to treatment, measured ETDRS BCVA, and OCT scans were performed.” “Experienced ophthalmic photographers, who were masked to treatment, obtained Stratus OCT (Carl Zeiss Meditec, Inc., San Francisco, CA, USA) scans using the fast macular thickness protocol." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants who were randomized were not analyzed due to unreliable preoperative OCT scans. It is unclear how incomplete outcome data were addressed. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
McColgin 1999.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: the study is ongoing: “Additional patients are being enrolled in the study in order to confirm these findings.” |
|
| Participants |
Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: people scheduled for cataract extraction Exclusion criteria: pre‐existing retinal disease, diabetes, previous intraocular surgery, dense cataract preventing preoperative retinal evaluation, or were unable to come to the site for follow‐up visits Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac sodium 0.1% 2 days prior to surgery and for 4 weeks following surgery plus postoperative topical steroid Corticosteroid: postoperative topical steroid only Length of follow‐up: Planned: 6 weeks Actual: not reported |
|
| Outcomes |
Primary outcome, as defined in study reports: presence of CME measured using OCT Secondary outcomes, as defined in study reports: visual acuity testing, Amsler grid testing, dilated fundus examination Adverse events reported: no Intervals at which outcomes assessed: postoperatively and 6 weeks |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | Participants in the NSAID group received eye drops to take at home, while participants in the steroid‐only group did not receive any medication to take at home. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and disclosures of interest were not provided; this is an ongoing study and initial results were reported. |
Miyake 2007.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 62 eyes of 62 participants Per group: NSAID: 31, corticosteroid: 31 Exclusions after randomization: 12 total, 6 eyes from each group; “The reasons for exclusion in the diclofenac group were as follows: one eye showed sensitivity to fluorescein sodium, three eyes presented insufficient pupil dilation, and two eyes had glaucoma. The causes in the fluorometholone group were as follows: Three eyes presented insufficient pupil dilation, one eye had uveitis, one subject had diabetes, and one subject had hypertension.” Number analyzed (total and per group): Total: 50 eyes of 50 participants Per group: NSAID: 25, corticosteroid: 25 Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Japan Age: NSAID mean age = 65.4 ± 7.0, corticosteroid mean age = 65.8 ± 7.1 Gender (per cent): NSAID: 13 (52%) men and 12 (48%) women, corticosteroid: 10 (40%) men and 15 (60%) women Inclusion criteria: between 50 and 70 years of age, subjected to unilateral surgery or to have 6 months’ span between surgeries in people with bilateral cataract Exclusion criteria: eyes encountering acute ocular infection or inflammation during the first month of the study; eyes showing sensitivity to diclofenac or fluorometholone; eyes showing sensitivity to fluorescein sodium; eyes with insufficient dilation; eyes with a history of other ocular surgeries; eyes with pseudoexfoliation syndrome; eyes with a history of trauma; eyes with uveitis, glaucoma, or other disorders; eyes with complication of diabetes and kidney disorders; people with uncontrollable hypertension; and eyes encountering rupture of the posterior capsule, vitreous loss, and other complications during a cataract/IOL implantation procedure Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: topical diclofenac 0.1% 4 times before surgery and 3 times daily for 5 weeks after surgery Corticosteroid: fluorometholone 0.1% 4 times before surgery and 3 times daily for 5 weeks after surgery Length of follow‐up: Planned: 5 weeks Actual: 5 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, intraocular pressure, blood pressure, laser Doppler flowmetry, flare, fluorescein fundus angiography Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: baseline and 2 days, 1, 2, 5 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: no disclosures of interest Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that “Each patient was randomly assigned to one of the two groups by one of the authors (SA), using the envelope method ...,” but it is unclear what the envelope method is. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported to be double‐masked, but details of masking or participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | “CME, captured by fluorescein angiography at 2 and 5 weeks after surgery, was analyzed by one of the authors (NK) in a masked fashion and graded with a method explained previously.” “Laser flarimetry was conducted to measure the amount of aqueous flare by one of the authors (SA) in a masked fashion.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
Miyake 2011.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 60 eyes of 60 participants Per group: NSAID: 30, corticosteroid: 30 Exclusions after randomization: “One patient was excluded because the patient wanted a bilateral procedure immediately after signing up for the study” Number analyzed (total and per group): Total: 59 eyes of 59 participants Per group: NSAID: 30, corticosteroid: 29 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 participant in the NSAID group had macular degeneration that precluded fluorescein fundus angiography and dropped out of the study, and another participant was dropped due to an unwillingness to attend the examination. 2 participants in the corticosteroid group dropped out, 1 because a humeral fracture prevented fluorescein fundus angiography and another due to posterior lens capsule rupture during surgery How were missing data handled?: all participants were included in the safety evaluation; it was not reported how losses to follow‐up were handled Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Japan Age: NSAID group: 16 (53.3%) were 18 to 64 years and 14 (46.7%) were ≥ 65 years, corticosteroid group: 12 (41.4%) were 18 to 64 years and 17 (58.6%) were ≥ 65 years Gender (per cent): NSAID group: 16 (53.5%) men and 14 (46.7%) women, corticosteroid group: 16 (55.2%) men and 13 (44.8%) women Inclusion criteria: people older than 20 years who had phacoemulsification cataract extraction and IOL implantation between October 2007 and April 2008 at Shohzankai Medical Foundation, Miyake Eye Hospital Exclusion criteria: people were excluded if they (1) had taken systemic, topical, or ointment steroidal agents within 14 days of surgery; (2) had an intraocular or periocular injection of steroidal agents within 90 days of surgery; (3) had taken systemic or topical NSAIDs within 7 days of surgery; (4) had a history of ophthalmic surgery (including laser surgery) or of ocular trauma that could affect the study results; (5) had pseudoexfoliation syndrome; (6) had a history of chronic or recurring ocular inflammation (e.g. uveitis or scleritis); (7) had diabetic retinopathy; (8) had an ocular anomaly (e.g. aniridia, congenital cataract); (9) had iris atrophy; (10) had disorders that would preclude improvement in visual function; (11) had macular edema; (12) had severe corneal epithelial disorder (e.g. corneal ulcer); (13) had no visual function in the contralateral eye; (14) were scheduled to have other ocular surgery from baseline to 5 weeks after cataract surgery; (15) had secondary IOL implantation; (16) were allergic to or might have been sensitive to NSAIDs, amfenac, or fluorometholone; (17) had a positive skin reaction to fluorescein; (18) had a tendency to bleed or were currently on anticoagulants; (19) had prostaglandin‐type treatment for glaucoma within 4 days of surgery; (20) had been included in a previous study of prostaglandin‐type antiglaucoma drugs; (21) had joined another clinical study within 30 days of the study; (22) had ocular infection; (23) had uncontrollable diabetes mellitus; (24) had severe liver, kidney, or heart disorder; (25) might have been pregnant or were currently breastfeeding; (26) had other factors determined to be unsuitable for the study. Equivalence of baseline characteristics?: yes, “There was no statistically significant difference in any [patient characteristics and surgical] parameter between the 2 groups.” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: nepafenac 0.1% 1 drop 3 times a day from 1 day before surgery until 5 weeks after surgery Corticosteriod: fluorometholone 0.1% 1 drop 3 times a day from 1 day before surgery until 5 weeks after surgery Length of follow‐up: Planned: 5 weeks Actual: 5 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, intraocular pressure, fundus examination, slit‐lamp examination, ocular coherence tomography, fluorescein angiography, laser flare‐cell meter Secondary outcomes, as defined in study reports: the study did not differentiate between primary and secondary outcomes Adverse events reported: yes Intervals at which outcomes assessed: 1 day, 1, 2, and 5 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: Drs Miyake and Numaga are consultants to Alcon Japan Ltd. Study period: surgery between October 2007 and April 2008 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This was reported as a double‐masked study, and it is reported that outcome assessors were masked. However, it is unclear whether participants and/or other study personnel were masked. |
| Masking of outcome assessment (detection bias) | Low risk | The presence and grade of CME was determined in a masked manner. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some participants were excluded from analyses when there were problems with the measurement; one participant was excluded due to surgical complications. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | High risk | Drs Miyake and Numaga are consultants to Alcon Japan Ltd., which manufacturers the study drugs |
Miyanaga 2009.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 72 eyes of 72 patients Per group: NSAID: 25, corticosteroid: 23, combined treatment: 24 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: not reported Losses to follow‐up: 1 participant in the corticosteroid group developed CME at 1 month after cataract surgery in the left eye and was withdrawn from the study How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Japan Age: NSAID group mean age = 73.6 (range 48 to 86), corticosteroid group mean age = 69.7 (range 41 to 83), combined group mean = 71.4 (range 46 to 86) Gender (per cent): NSAID group: 8 (32%) men and 17 (68%) women; corticosteroid group: 6 (26%) men and 17 (74%) women; combined group: 7 (29%) men and 17 (71%) women Inclusion criteria: people scheduled to undergo routine phacoemulsification combined with IOL implantation Exclusion criteria: people with corneal disease, glaucoma, uveitis, pseudoexfoliation syndrome, diabetes, or other pathologies that might affect treatment responses or evaluations; people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery Equivalence of baseline characteristics: yes, “There was no significant differences between groups in gender or age.” “There were no statistically significant differences between the groups in surgical data, such as the nucleus degrees of the lens, surgical time, phacoemulsification time, and amount of irrigation fluid.” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid vs NSAID plus corticosteroid NSAID: 0.1% bromfenac 2 times daily for 2 months Corticosteroid: 0.1% betamethasone 4 times daily for 1 month and then fluorometholone 4 times daily for 1 month NSAID plus corticosteroid: combined 0.1% bromfenac 2 times daily for 2 months plus 0.1% betamethasone 4 times daily for 1 month and then fluorometholone 4 times daily for 1 month Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: inflammatory reaction using laser flare cell photometry Secondary outcomes, as defined in study reports: BCVA, intraocular pressure, aqueous flare, corneal thickness measured using pachymetry and corneal endothelial cell count Adverse events reported: no Intervals at which outcomes assessed: 1, 2, and 3 days, 1 and 2 weeks, and 1 and 2 months postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: February to August 2006 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and conflicts of interest were not reported. |
Moschos 2012.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 79 eyes of 79 participants Per group: NSAID plus corticosteroid: 38, corticosteroid alone: 41 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individual (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: yes, “A post hoc power calculation was performed at the completion of the study (day 28), as this time point may reflect the clinical performance of diclofenac.” Unusual study design?: none |
|
| Participants |
Country: Greece Age: NSAID plus corticosteroid mean age = 76.68 ± 10.72; corticosteroid alone mean age = 76.71 ± 8.82 Gender (per cent): NSAID plus corticosteroid: 12 (31.6%) men and 26 (68.4%) women; corticosteroid alone: 15 (36.6%) men and 26 (63.4%) women Inclusion criteria: not reported Exclusion criteria: presence of corneal abnormalities; history of intraocular surgery; preoperative ECC < 1500 cells/mm2; history of uveitis, diabetes, and age‐related macular degeneration; regular, systemic use of steroid or NSAIDs during the previous 3 months; and intraoperative complication, such as posterior capsule rupture, vitreous loss, lost nucleus, zonule dehiscence, and wound leak Equivalence of baseline characteristics: yes, ”The groups had no statistically significant differences for age, gender, BCVA, ECC, CCT, and macular thickness.” |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac 0.1% 1 drop 3 times a day plus dexamethasone 0.1% 1 drop 4 times a day Corticosteroid: dexamethasone 0.1% 1 drop 4 times a day Other medications: all participants also received the antibiotic chloramphenicol 0.5% Length of follow‐up: Planned: 28 days Actual: 28 days |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA, macular thickness, endothelial cell density, central corneal thickness Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: postoperative days 1, 14, 28 |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “No competing financial interests exist” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random was done using a random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing outcome data and how they were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources are not reported. |
Mulet 2001.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 105 eyes of 105 participants Per group: 35 in each group Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individuals (1 eye per participant) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: not reported Age: mean age 68.61 ± 0.98 Gender (per cent): 45 males (42.9%), 60 females (57.1%) Inclusion criteria: people undergoing phacoemulsification and posterior chamber IOL Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs NSAID vs corticosteroid NSAID 1: diclofenac sodium 0.1% 4 times daily for 1 month thereafter 3 times daily for remainder NSAID 2: ketorolac 0.5% 4 times daily for 1 month thereafter 3 times daily for remainder Corticosteroid: dexamethasone 0.1% 4 times daily for 1 month thereafter 3 times daily for remainder Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: biotolerance (epithelial staining), comfort (0‐to‐3 subjective scale), and inflammatory response (flare, hyperemia, edema, and IOP) Secondary outcomes, as defined in study reports: primary and secondary outcomes were not differentiated Adverse events reported: no Intervals at which outcomes assessed: 5 postoperative visits; timing not reported |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This was a “double masked” study, but details of masking of participants and personnel were not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | This was a “double masked” study, but details of masking of outcome assessors were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear if there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources were not reported. |
Ostrov 1997.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 157 eyes of 157 participants Per group: NSAID: 57, corticosteroid group 1: 59, corticosteroid group 2: 41 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 155 eyes of 155 participants Per group: NSAID group: 56, corticosteroid group 1: 58, corticosteroid group 2: 41 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 6 participants discontinued treatment due to adverse events, 1 participant was withdrawn from the study because of surgical complications and 1 for noncompliance How were missing data handled?: data from the 8 participants who were withdrawn were included if the participant had a least 1 postoperative visit. The data from 1 participant in the prednisolone group and 1 participant in the dexamethasone group were excluded from the analysis Reported power calculation: no Unusual study design?: “To provide partial masking of the two agents — ketorolac in the form of a solution and prednisolone in the form of a suspension — dexamethasone, a glucocorticoid solution, was added as a third study drug.” |
|
| Participants |
Country: not reported Age: mean age ± SD: NSAID mean age = 67.3 ± 12.1 (range 26 to 85), corticosteroid group 1 mean age = 71.8 ± 9.7 (range 44 to 87), corticosteroid group 2 mean age = 69.5 ± 12.1 (range 30 to 98) Gender (per cent): NSAID group: 28 (50%) men and 28 (50%) women, corticosteroid group 1: 28 (48%) men and 30 (52%) women, corticosteroid group 2: 19 (46%) men and 22 (54%) women Inclusion criteria: adult candidates for routine cataract extraction and posterior chamber intraocular lens implantation, undergoing surgery in 1 eye only Exclusion criteria: people who had received any glucocorticoid or cyclooxygenase inhibitor within 1 week of surgery, people with known hypersensitivity to any study drug or fluorescein, serious uncontrolled systemic disease, women who were pregnant or lactating Equivalence of baseline characteristics: yes, “With the exception of iris color, no significant differences were found among the three treatment groups in demographic measures, baseline intraocular pressure, operative eye selection, or surgical procedure.” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid vs corticosteroid NSAID: 0.5% ketorolac sterile ophthalmic solution 3 times daily from 1 day before surgery to 4 weeks after surgery Corticosteroid 1: 1% prednisolone acetate sterile ophthalmic suspension 3 times daily from 1 day before surgery to 4 weeks after surgery Corticosteroid 2: 0.1% dexamethasone sodium phosphate sterile ophthalmic solution from 1 day before surgery to 4 weeks after surgery Length of follow‐up: Planned: 6 weeks Actual: 6 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: cells and flare in the anterior chamber, fluorescein leakage Secondary outcomes, as defined in study reports: other clinical signs of inflammation: edema of the lid; hyperemia, ciliary flush, and chemosis of the conjunctiva; superior edema, central edema, and Descemet's folds of the cornea; and the presence of hyphema and fibrin in the anterior chamber Adverse events reported: yes Intervals at which outcomes assessed: prior to surgery, day 1 or 2 and 5, week 2, 4, and 6 |
|
| Notes |
Type of study: published Funding sources: “This study was sponsored by a grant from Syntex Laboratories Inc., Palo Alto, California" Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study was described as partially masked due to the formulation of the study drugs, so participants and personnel could determine assignment to prednisolone, they could not distinguish between ketorolac and dexamethasone. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants were excluded only when they did not complete any study visits (n = 2). |
| Selective reporting (reporting bias) | Unclear risk | A protocol was mentioned but not cited, so it is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | Funded by Syntex Laboratories Inc., but they did not manufacture any of the study drugs. |
Roberts 1995.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 52 eyes of 52 participants Per group: NSAID: 25, corticosteroid: 27 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 52 eyes of 52 participants Per group: NSAID: 25, corticosteroid: 27 Unit of analysis: not reported Losses to follow‐up: none, “All patients enrolled completed the study.” How were missing data handled?: N/A Reported power calculation: yes, the study had power of at least 80% Unusual study design?: none |
|
| Participants |
Country: not reported Age: not reported Gender (per cent): not reported Inclusion criteria: participants undergoing phacoemulsification with posterior chamber intraocular lens implantation Exclusion criteria: history of systemic or ocular inflammation (iritis, uveitis), taking oral or topical ophthalmic corticosteroids or NSAIDs, if they had other ocular disease such as glaucoma, corneal disease, or diabetic retinopathy Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% 4 times per day Corticosteroid: prednisolone acetate 1% 4 times per day Length of follow‐up: Planned: 1 month Actual: 1 month |
|
| Outcomes |
Primary outcome, as defined in study reports: IOP, cell and flare measurements for postoperative inflammation Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 1 day, 1 week, 1 month postoperatively |
|
| Notes |
Type of study: published Funding sources: the investigation was supported in part by grants from Michael and Gina Ricciardi, Wantagh, NY, Leonard and Silva Marx, Mammaroneck, NY, and Research to Prevent Blindness, New York, NY Disclosures of interest: “The authors have no commercial or proprietary interest in any of the drugs discussed in the article. Dr. Roberts serves on the CIBA Vision Ophthalmics Physician Advisory Panel.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | The study is reported as double‐masked, but it is unclear who among participants, personnel, and outcome assessors was masked. |
| Masking of outcome assessment (detection bias) | Low risk | “Neither examiner knew in which of the study groups the patient was enrolled, nor did the examiners know the results of the other’s examination.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “All patients enrolled completed the study. No postoperative visits were missed.” |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | The study was funded in part by Ciba Vision Ophthalmics, the manufacturer of one of the study drugs. |
Ruiz Rodríguez 2011.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 40 eyes of 40 participants Per group: NSAID: 20, corticosteroid: 20 Exclusions after randomization: not reported Number analyzed (total and per group): Total: not reported Per group: not reported Unit of analysis: individuals (1 eye per individual) Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Cuba Age: NSAID mean age = 73 ± 9, corticosteroid mean age = 72 ± 8 Gender (per cent): NSAID: 45% men and 55% women, corticosteroid: 60% men and 40% women Inclusion criteria: people who underwent phacoemulsification for cataract Exclusion criteria: people who had ophthalmological or systemic disease, showed alterations in pupillary dilation Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID: diclofenac sodium 0.1% 1 drop every 4 hours 2 days prior to surgery, 1 drop every 30 minutes 2 hours prior to surgery, and 1 drop every 4 hours during the 1st week postoperatively plus prednisolone eye drops Corticosteroid: prednisolone eye drops Other medications: all participants also received routine treatment eye drops (phenylephrine and tropicamide, chloramphenicol) Length of follow‐up: Planned: 1 month Actual: 6 days |
|
| Outcomes |
Primary outcome, as defined in study reports: the presence of hyperemia conjunctival and cells in the anterior chamber Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: 24 hours, 6 days, 1 month |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: during 2010 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not described. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were incomplete outcome data or any missing data. |
| Selective reporting (reporting bias) | High risk | The study was planned for 1‐month follow‐up, but data from 1 month are not presented. |
| Other bias | Unclear risk | Funding sources were not reported. |
Sahu 2015.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: not reported Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: 120 eyes of 120 participants Per group: NSAID 1 plus corticosteroid: 33, NSAID 2 plus corticosteroid: 30, NSAID 3 plus corticosteroid: 31, corticosteroid alone: 26 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: India Age: overall mean age = 61.14 ± 9.76, NSAID 1 plus corticosteroid mean age = 63.48 ± 9.60, NSAID 2 plus corticosteroid mean age = 59.63 ± 8.96, NSAID 3 plus corticosteroid mean age = 60.42 ± 10.72, corticosteroid alone mean age = 60.77 ± 9.65 Gender (per cent): overall: 67 men (55.8%) and 53 women (44.2%); NSAID 1 plus corticosteroid: 19 men (57.6%) and 14 women (26.4%); NSAID 2 plus corticosteroid: 15 men (50%) and 15 women (50%); NSAID 3 plus corticosteroid: 18 men (58.1%) and 13 women (41.9%); corticosteroid alone: 15 men (57.7%) and 11 women (42.3%) Inclusion criteria: people with visually significant age‐related cataract who were 40 years or older of either sex having phacoemulsification with posterior chamber IOL implantation Exclusion criteria: prior ocular trauma or intraocular surgery; history of uveitis; eyes with corneal disease, pseudoexfoliation syndrome, or ocular infection; history of coexistent ocular disease such as glaucoma, optic atrophy, or ocular tumors; history of use of topical steroids, NSAIDs, or prostaglandins within 2 weeks of enrollment; eyes with complications from diabetic mellitus; uncontrolled hypertension; any connective tissue disorders, serious renal, hepatic, endocrine, pulmonary, cardiac, neurologic, rheumatic, psychiatric, or cerebral dysfunction; and ocular allergy to ketorolac, bromfenac, or nepafenac Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac 0.4% 3 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued NSAID 2 plus corticosteroid: bromfenac 0.09% 2 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued NSAID 3 plus corticosteroid: nepafenac 0.1% 3 times a day 1 day before surgery and 3 times at 30‐minute intervals before surgery plus prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued Corticosteroid: prednisolone acetate 1.0% 4 times a day for 7 days, 3 times a day for 3 days, twice a day for 3 days, once every day for 3 days, and then discontinued Other medication: all participants also received moxifloxacin 0.5% 6 times a day Length of follow‐up: Planned: 8 weeks Actual: 8 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity, IOP, laser flare photometry, and fundus examination Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: day 1, weeks 1, 2, 4, and 8 |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: all surgeries were performed between July 2013 and June 2014 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were randomly divided into 1 of 4 groups using a computer‐generated random‐number table” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment is not described. |
| Masking of participants and personnel (performance bias) | High risk | Not a masked study |
| Masking of outcome assessment (detection bias) | High risk | Not a masked study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there was any loss to follow‐up and how missing data were handled. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting; a protocol was not available. |
| Other bias | Unclear risk | Funding sources were not reported. |
Schmitt 1995.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 125 participants Per group: 25 Exclusions after randomization: none Number analyzed (total and per group): Total: 150 participants Per group: 25 Unit of analysis: individuals Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: no Unusual study design?: 125 people undergoing a phacoemulsification were randomized to 5 different therapy groups. 25 people undergoing the ECCE procedure were also included in the study because they showed higher postoperative flare values, but they were not randomized to 1 of the 5 groups |
|
| Participants |
Country: Germany Age: mean age 69 years Gender (per cent): not reported Inclusion criteria: age‐related cataract, stationary cataract extraction with posterior chamber lenses implantation Exclusion criteria: other diseases of the eye, diabetes mellitus, diseases of the rheumatic form circle, anti‐inflammatory general therapy Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid vs combinations of NSAIDs plus corticosteroids NSAID: flurbiprofen 0.03% (Ocuflur) Corticosteroid: prednisolone acetate 1% (Inflanefran forte) NSAID plus corticosteroid 1: prednisolone acetate 0.12% (Inflanefran), flurbiprofen 0.03% (Ocuflur) NSAID plus corticosteroid 2: prednisolone acetate 1% (Inflanefran forte), flurbiprofen 0.03% (Ocuflur) NSAId plus corticosteroid 3: prednisolone hemisuccinate 2.5%, flurbiprofen 0.03% (Ocuflur) Additional ECCE procedure (non‐randomised): fortified topical corticosteroid therapy with prednisolone acetate 1% (Inflanefran forte) each hour (25 people who were not randomized and undergoing another surgery procedure (ECCE) were subsequently included in the study because it was not possible to define a “fibrin border” with the 125 people who received the phacoemulsification) Length of follow‐up: Planned: 3 days Actual: 3 days |
|
| Outcomes |
Primary outcome, as defined in study reports: aqueous flare and aqueous cell counts measured by laser flare‐cell photometer FC‐1000 (photon counts/ms) Secondary outcomes, as defined in study reports: definition of a “fibrin border”: high postoperative flare values that will lead to a fibrin exudation within 24 hours, and if a fortified topical corticosteroid therapy prevents a fibrin exudation Adverse events reported: no Intervals at which outcomes assessed: preoperative, 1 and 3 days postoperative |
|
| Notes |
Type of study: published Funding sources: the laser flare‐cell photometer was provided by the company Pharm‐Allergan GmbH, Ettlingen; other funding sources were not mentioned Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there was any loss to follow‐up or missing data. |
| Selective reporting (reporting bias) | Unclear risk | The authors refer to a study protocol because they added to it 25 participants who underwent another surgery form (ECCE). It is not clear if they also changed the selection of outcomes. |
| Other bias | Low risk | No other sources of bias |
Shimazaki 1996.
| Methods |
Study design: intra‐individual RCT Number randomized (total and per group): Total: 36 eyes of 18 participants Per group: 18 eyes of 18 participants Exclusions after randomization: 2 eyes (1 individual) Number analyzed (total and per group): Total: 34 eyes of 17 participants Per group: 17 eyes of 17 participants Unit of analysis: eyes Losses to follow‐up: 1 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: analysis does not take account of paired‐eye design |
|
| Participants |
Country: Japan Age: 71 (57 to 85) Gender (per cent): 23.5% men, 76.5% women Inclusion criteria: bilateral age‐related cataract; underwent phacoemulsification and intraocular lens implantation in both eyes Exclusion criteria: diabetes mellitus, autoimmune diseases, history of chronic corneal or conjunctival diseases or both, and those who wore contact lenses Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: diclofenac sodium 0.1% plus dexamethasone Corticosteroid: dexamethasone Additional ECCE procedure (non‐randomized): fortified topical corticosteroid therapy with prednisolone acetate 1% (Inflanefran forte) each hour. (25 participants without randomization and with another surgery procedure (ECCE) were subsequently included in the study because it was not possible to define a “fibrin border” with the 125 participants who received phacoemulsification) Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: vital stainings, tear film break‐up time, and corneal sensitivity; tear function tests; inflammation in the anterior chamber; specular microscopy and corneal thickness; epithelial barrier function Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1 month |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | This was an unmasked study. |
| Masking of outcome assessment (detection bias) | High risk | This was an unmasked study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant was excluded from analysis due to posterior capsule rupture in 1 eye. There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources not reported. |
Singh 2012.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 263 eyes of 263 participants Per group: nepafenac plus prednisolone acetate: 133, vehicle plus prednisolone acetate: 130 Exclusions after randomization: not reported Number analyzed (total and per group): Total: intention to treat: 251, safety analysis: 253 Per group: nepafenac plus prednisolone acetate: 125, vehicle plus prednisolone acetate: 126 Unit of analysis: eyes (1 eye per individual) Losses to follow‐up: 3 serious events in the vehicle group led to participant discontinuation; 4 other participants discontinued study due to non‐serious adverse events How were missing data handled?: ITT analysis, last observation carried forward method was used to impute missing macular thickness and BCVA data; no imputations for missing data were made in the safety analysis Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: United States Age: nepafenac plus prednisolone acetate mean age = 66.6 ± 9.3, vehicle plus prednisolone acetate mean age = 66.4 ± 9.7 Gender (per cent): nepafenac plus prednisolone acetate: 42 male (33.6%), 83 female (66.4%); vehicle plus prednisolone acetate: 51 male (40.5%), 75 female (59.5%) Inclusion criteria: male and female diabetic (type 1 or type 2) patients of any race/ethnicity aged 18 years and older having an existing diagnosis of nonproliferative diabetic retinopathy that required cataract extraction with planned implantation of a posterior chamber intraocular lens (at least 50% of all enrolled participants were required to have moderate to severe nonproliferative diabetic retinopathy, as defined by the International Clinical Diabetic Retinopathy Disease Severity Scale) Exclusion criteria: no significant corneal staining scores at baseline and no history of dry eye syndrome; conditions that caused macular edema including pre‐existing histories of retinal vein occlusions, ocular surgeries, inflammatory eye diseases, ocular infections, congenital ocular anomalies, and ocular traumas; people with baseline cysts or the presence of macular traction and epiretinal membrane; people using concomitant medication such as topical or systemic NSAIDs and steroids which could have interfered with the assessment of the study outcome measures Equivalence of baseline characteristics: “The demographic and baseline characteristics of the patients in the nepafenac and vehicle groups were generally similar” |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac 3 times daily on the day prior to cataract surgery and for 90 days plus prednisolone acetate ophthalmic suspension four times daily for 2 weeks postsurgery Corticosteroid: vehicle 3 times daily on the day prior to cataract surgery and for 90 days plus prednisolone acetate ophthalmic suspension four times daily for 2 weeks postsurgery Length of follow‐up: Planned: 90 days Actual: 90 days |
|
| Outcomes |
Primary outcome, as defined in study reports: efficacy included the percentage of participants who developed macular edema (≥ 30% increase in central subfield macular thickness from baseline) and the percentage of participants with decreases of more than 5 letters in BCVA from day 7 to 90 Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: yes Intervals at which outcomes assessed: days 1, 7, 14, 30, 60, and 90 |
|
| Notes |
Type of study: published Funding sources: Alcon Research Disclosures of interest: RS, LA, GJJ, RPL, JL, HJR, KS, and TW are paid consultants for Alcon Research Ltd. (Fort Worth, TX). DS is an employee of Alcon Research Ltd. Medical writing support, which was funded by Alcon Research Ltd., was provided by Cullen T Vogelson and Usha Sivaprasad of Illuminated Research LLC (Fort Worth, TX) Study period: enrollment between November 2008 and July 2010 Reported subgroup analyses: yes, by nonproliferative diabetic retinopathy classification Trial Registry #: NCT00782717 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Unclear risk | Study is reported as double‐masked, but it is unclear whether this refers to participants, surgeons, or other study personnel. |
| Masking of outcome assessment (detection bias) | Low risk | “Morphological features, including intraretinal cysts, were analyzed by the reading center in a masked fashion” and "... study personnel at each investigational center who were responsible for administering the study medication on day 0 did not assess adverse events or the corneal staining results." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This study used an ITT analysis with a last observation carried forward method for macular thickness and BCVA data, however 12 participants who were enrolled were not included in the analysis, and the reason for this was not explained. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | High risk | Eight study authors are paid consultants to Alcon Japan Ltd., which manufacturers one of the study drugs. Alcon Japan Ltd. also provided funding for medical writing support. |
Solomon 2001.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 38 eyes of 38 participants Per group: NSAID: 18, corticosteroid: 20 Exclusions after randomization: 7 total: NSAID: 4, corticosteroid: 3 Number analyzed (total and per group): Total: 31 Per group: NSAID: 14, corticosteroid: 17 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 in the NSAID group How were missing data handled?: not included Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: United States Age: NSAID mean age = 70.6, corticosteroid mean age = 67.6 Gender (per cent): NSAID: 7 (50%) men and 7 (50%) women; corticosteroid: 6 (35.3%) men and 11 (64.7%) women Inclusion criteria: older than 18 years, had potential visual acuity of 20/40 or better in the fellow eye, had a visually significant cataract in the study eye, and were willing to comply with all postoperative instructions Exclusion criteria: pregnant, nursing, or of childbearing age and not using a medically acceptable form of birth control or if they had prior surgery in the eye or had uveitis, diabetes requiring insulin, advanced glaucoma, a history of recent oral NSAID use, a known sensitivity to the study medications, or an ocular or systemic disease that could interfere with the study Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: ketorolac tromethamine 0.5% 4 times a day for 1 week, then 2 times a day Corticosteroid: rimexolone 1% 4 times a day for 1 week, then 2 times a day Length of follow‐up: Planned: 30 days Actual: 30 days |
|
| Outcomes |
Primary outcome, as defined in study reports: ocular and systemic symptoms, Kowa cell and flare measurement, visual acuity, an external examination, a slit‐lamp examination, and IOP measurement Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: preoperative assessment then 1, 4, 7, and 30 days postoperative |
|
| Notes |
Type of study: published Funding sources: “Funded by an unrestricted grant from Allergan, Inc.” Disclosures of interest: “None of the authors has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Low risk | “Treatment was masked to both patient and investigator.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who did not complete the study were not included in the analysis (n=4 in the NSAID group (22%) and 3 in the corticosteroid group (15%). |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | This study was funded by an unrestricted grant from Allergan, which manufactures 1 of the study drugs. |
Ticly 2014.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 91 participants Per group: ketorolac plus prednisolone: 42, prednisolone: 49 Exclusions after randomization: “Five patients did not complete the trial in the placebo group: 1 patient withdrew because of adverse events (burning); 1 was excluded because of intraoperative complications; 1 refused to undergo fluorescein angiography; and 2 patients were discontinued because of protocol violations. Five patients did not complete the study in the ketorolac group: 4 patients were discontinued because of protocol violations and 1 was discontinued because of intraoperative complications.” Number analyzed (total and per group): Total: 81 Per group: ketorolac plus prednisolone: 37, prednisolone: 44 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: none How were missing data handled?: excluded Reported power calculation: yes, 80% power Unusual study design?: “Patients who were initially enrolled and randomized into the study were excluded if complications occurred during cataract surgery (e.g., posterior capsule rupture, vitreous loss, retained cortical material, or an IOL not placed in the capsular bag). Patients were also excluded if they did not follow instructions or if they did not show up for appointments” |
|
| Participants |
Country: Brazil Age: ketorolac plus prednisolone mean age = 67.1 ± 10.8, prednisolone alone mean age = 66.1 ± 8.7 Gender (per cent): ketorolac plus prednisolone: 21 male (56.8%) and 16 female (43.2%), prednisolone alone: 22 male (50%) and 22 female (50%) Inclusion criteria: people with nuclear cataract density of 2 and 3 determined by LOCS II (> 50 years old), with indication for cataract surgery with intraocular lens implantation under local anesthesia Exclusion criteria: diabetes, NSAID use, use of topical eye drops (including antiglaucoma drugs), history of uveitis, macular disease, pseudoexfoliation syndrome, congenital ocular abnormalities, cataract density of 1 and 4 determined by LOCS II, and previous intraocular surgery or injections into the eye Equivalence of baseline characteristics: yes, “Baselines demographics were similar in all groups. There were no differences regarding age (P = 0.617), preoperative BCVA (P = 0.153), or gender distribution (P = 0.656)” |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: ketorolac tromethamine 0.4% 4 times daily for 3 days before surgery and 5 weeks postoperatively and prednisolone acetate 1% 4 times daily Corticosteroid: hypromellose/dextran 70 as a placebo 4 times daily for 3 days before surgery plus prednisolone acetate 1% 4 times daily Other medications: all participants were also treated with gatifloxacin ophthalmic solution 0.3% 4 times daily for 3 days preoperatively and 4 times daily for 7 days postoperatively Length of follow‐up: Planned: 5 weeks Actual: 5 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: incidence of angiographic CME 5 weeks after surgery Secondary outcomes, as defined in study reports: mean change in BCVA, clinical CME incidence, IOP, and retinal thickness measured using OCT Adverse events reported: yes Intervals at which outcomes assessed: 1 day and 5 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “No competing financial interests exist.” Study period: February 2011 to March 2012 Reported subgroup analyses: no Trial registry #: NTC01542190 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was performed in a simple manner. Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. When a patient was included in the study, a pharmacist provided the patient with a small individual envelope, and after viewing the random number, the patient took the respective eye drop bottle” |
| Allocation concealment (selection bias) | Low risk | “The randomization was performed in a simple manner. Each of the 2 intervention groups received 50 different numbers from a random number table. These numbers were transferred to small individual envelopes and also affixed to one of the relabeled eye drop bottles. When a patient was included in the study, a pharmacist provided the patient with a small individual envelope, and after viewing the random number, the patient took the respective eye drop bottle” |
| Masking of participants and personnel (performance bias) | Low risk | “The surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients.” “All study participants were blinded to their treatment assignment” |
| Masking of outcome assessment (detection bias) | Low risk | “The surgeon and the ophthalmologist who collected the data were not aware of the group assignment of the patients.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants in the ketorolac plus prednisolone group (1 due to adverse event; 1 due to intraoperative complication; 1 refused to undergo fluorescein angiography; 2 protocol violations) and 5 participants in the prednisolone alone group (1 intraoperative complication; 4 protocol violations) did not complete the study and were excluded from the analysis. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Unclear risk | Funding sources were not reported. |
Trinavarat 2003.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 120 eyes of 102 participants Per group: NSAID: 40 eyes, corticosteroid 1: 39 eyes, corticosteroid 2: 41 eyes Exclusions after randomization: corticosteroid 1: 2 eyes, corticosteroid 2: 2 eyes Number analyzed (total and per group): Total: 116 Per group: NSAID: 40, corticosteroid 1: 37, corticosteroid 2: 39 Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: by day 28, corticosteroid group 1: 4, NSAID group: 3, corticosteroid group 2: 4 How were missing data handled?: excluded from the study Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Thailand Age: NSAID mean age = 61.8 ± 12.8; corticosteroid group 1 mean age = 63.3 ± 12.2; corticosteroid group 2 mean age = 63.2 ± 11.4 Gender (per cent): NSAID: 14 (35%) men and 26 (65%) women; corticosteroid group 1: 14 (37.8%) men and 23 (62.2%) women; corticosteroid group 2: 15 (42.9%) men and 20 (57.1%) women Inclusion criteria: eyes of non‐diabetic patients that had undergone phacoemulsification and intraocular lens implantation Exclusion criteria: glaucoma, hypersensitivity to the study drugs, using any corticosteroids or NSAIDs in the previous 2 months, previous intraocular surgery, and accompanying ocular disease or corneal lesions that interfered with intraocular examination Equivalence of baseline characteristics: yes, “From baseline data collect on post‐operative day 1 prior to the application of the drugs, there was no significant difference among groups in visual acuity, intraocular pressure, inflammation of conjunctiva and cornea, and number of cells and flare in the anterior chamber.” “There was no significant difference in demographic data among the 3 groups as shown in Table 1.” |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid vs corticosteroid NSAID: ketorolac tromethamine 0.5% 4 times a day for 28 days Corticosteroid 1: topical prednisolone acetate 1% 4 times a day for 28 days Corticosteroid 2: fluorometholone acetate 0.1% 4 times a day for 28 days Length of follow‐up: Planned: 28 days Actual: 28 days |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA, intraocular pressure, slit‐lamp biomicroscopy, grading of cells and flare in the anterior chamber, and ocular symptoms Secondary outcomes, as defined in study reports: not reported Adverse events reported: yes Intervals at which outcomes assessed: weekly |
|
| Notes |
Type of study: published Funding sources: the study drugs were provided by Allergan (Thailand) and Alcon Laboratories (Thailand) Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | This study is reported as investigator‐masked but it is unclear whether participants were also masked to which study medication they received. |
| Masking of outcome assessment (detection bias) | Low risk | “Baseline and four successive weekly ocular examinations were performed by a single ophthalmologist who was masked to allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who were lost to follow‐up were not included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | A protocol is mentioned but not cited, so it is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Conflicts of interest were not reported. |
Tzelikis 2015.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 150 eyes of 150 participants Per group: 50 Exclusions after randomization: 24 Number analyzed (total and per group): Total: 126 eyes of 126 participants Per group: ketorolac + corticosteroid group: 40, nepafenac + corticosteroid group: 41, corticosteroid only: 40 Unit of analysis (individuals vs eyes): eyes Losses to follow‐up: 8 How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Brazil Age: mean age 65.36 ± 7.3 years Gender (per cent): 44% men, 56% women Inclusion criteria: older than 40 years; age‐related cataract; a normal ophthalmological examination besides senile cataract Exclusion criteria: previous ocular surgery; central endothelial cell count < 2000 cells/mm2; glaucoma or IOP > 21 mm Hg; amblyopia; retinal abnormalities; steroid or immunosuppressive treatment, connective tissue diseases, or an allergy or hypersensitivity to NSAIDs; complicated cataract surgery (e.g. posterior capsule rupture, vitreous loss, or an IOL not placed in the capsular bag) Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs NSAID plus corticosteroid vs corticosteroid NSAID 1 plus corticosteroid: ketorolac tromethamine 0.4% 4 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 1% 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week NSAID 2 plus corticosteroid: nepafenac 0.1% 3 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Corticosteroid: artificial tear substitute 4 times a day for 2 days before surgery and for 4 weeks after surgery plus prednisolone 4 times a day for 1 week, 3 times a day for 1 week, 2 times a day for 1 week, and 1 time a day for 1 week Other medication: all participants received moxifloxacin 0.5% 4 times a day starting 2 days before surgery and for 7 days after surgery Length of follow‐up: Planned: 12 weeks Actual: 12 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: visual acuity and refraction; central corneal thickness and endothelial cell density; preoperative and postoperative thickness, values of the retinal layers; the percentage of participants in whom the postoperative checkup revealed a change in central subfields retinal thickness; the percentage of participants to be diagnosed with macular edema Secondary outcomes, as defined in study reports: not distinguished Adverse events reported: no Intervals at which outcomes assessed: 1, 4, 12 weeks |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: the authors report no competing interest Study period: recruitment between 4 June 2013 and 7 October 2013 Reported subgroup analyses: no Trial registry #: NCT02084576 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned in a 1:1:1 ratio to one of three treatment groups using a computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | "All investigators were masked with regard to treatment group." |
| Masking of outcome assessment (detection bias) | Low risk | "All investigators were masked with regard to treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear if there was attrition bias. The authors state that 8 participants were lost to follow‐up and 8 were discontinued due to protocol violations and that their data were not included in the results. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
Voudouri 2002.
| Methods |
Study design: RCT Number randomized (total and per group): Total: 100 eyes Per group: not reported Exclusions after randomization: not reported Number analyzed (total and per group): Total: 98 eyes of 98 participants Per group: not reported Unit of analysis: eyes Losses to follow‐up: 2 eyes How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: not reported Age: mean = 76.0 ± 6.4 years (range 50 to 81) Gender (per cent): 40 men (40.8%), 48 women (49.0%) Inclusion criteria: people scheduled for clear corneal phacoemulsification cataract surgery Exclusion criteria: not reported Equivalence of baseline characteristics: not reported |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: diclofenac sodium 0.1% Corticosteroid: prednisolone acetate 1.0% Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: postoperative discomfort, redness, BCVA, signs of inflammation, and intraocular pressure Secondary outcomes, as defined in study reports: not reported Adverse events reported: not reported Intervals at which outcomes assessed: not reported |
|
| Notes |
Type of study: published abstract Funding sources: not reported Disclosures of interest: not reported Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcomes assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants did not complete the study, but the reasons were not described. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Funding sources and declarations of interest were not reported. |
Wang 2013.
| Methods |
Study design: RCT Number randomized (total and per group): Total: 240 participants Per group: 60 Exclusions after randomization: none Number analyzed (total and per group): Total: 167 participants Per group: group 1: 40, group 2: 43, group 3: 43, group 4: 41 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: yes, only 167 (70%) completed the study How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: China Age: mean age = 73.4 ± 9.2 (range 46 to 92) Gender (per cent): 46.3% men and 53.8% women Inclusion criteria: people undergoing phacoemulsification with posterior chamber IOL Exclusion criteria: (1) people who were suffering from any ocular diseases that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy, or amblyopia; (2) people who were suffering from any systemic diseases that might affect treatment responses or evaluations, such as diabetes mellitus; (3) potentially pregnant women; (4) people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery and people with contraindication to oral steroids, such as those with peptic ulcer, cancer, or tuberculosis; (5) people who had surgical complications, such as posterior capsule rupture or hyphema, and (6) people with particular diseases that might affect surgery in the eyes, such as limitation of pupil dilation Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID 1 (group 1): ophthalmic bromfenac sodium 0.1% twice per day for 1 month postoperatively NSAID 2 (group 2): ophthalmic bromfenac sodium 0.1% twice per day for 2 months postoperatively Corticosteroid 1 (group 3): ophthalmic fluorometholone 0.1% 3 times a day for 1 month postoperatively Corticosteroid 2 (group 4): ophthalmic dexamethasone 0.1% 3 times a day for 1 month postoperatively Length of follow‐up: Planned: 2 months Actual: 2 months |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA Secondary outcomes, as defined in study reports: IOP, endothelial cell density, photon count value, and retinal foveal thickness Adverse events reported: yes (reported as no drug‐related adverse events) Intervals at which outcomes assessed: 1 day, 1 week, 1 and 2 months |
|
| Notes |
Type of study: published Funding sources: this study was supported by grants from Zhejiang Key Innovation Team Project of China (grant no. 2009R50039) and Zhejiang Key Laboratory Fund of China (no. 2011E10006) Disclosures of interest: not reported Study period: October 2010 to December 2011 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | High risk | “The drugs were applied topically to the assigned patients open‐labeled. The same physician served as the medical monitor and assigned one of the drugs to each patient." |
| Masking of outcome assessment (detection bias) | Unclear risk | “The drugs were applied topically to the assigned patients open‐labeled. The same physician served as the medical monitor and assigned one of the drugs to each patient. “ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
Wittpenn 2008.
| Methods |
Study design: RCT Number randomized (total and per group): Total: 578 participants Per group: not reported Exclusions after randomization: 32 participants did not undergo surgery: 18 decided not to participate in the study, 5 were lost to follow‐up, 5 were discontinued because of protocol violations, and 4 could not participate because of insurance issues Number analyzed (total and per group): Total: 546 participants Per group: NSAID + corticosteroid: 268, corticosteroid: 278 Unit of analysis: participants (1 eye per participant) Losses to follow‐up: 5 were lost to follow‐up prior to surgery, 11 were discontinued due to adverse events How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: United States Age: mean age = 70 Gender (per cent): 291 (53.3%) women and 255 (46.7%) men Inclusion criteria: as determined by the individual investigator, each eligible patient had 20/20 BCVA potential without any evidence of macular abnormality, including age‐related macular changes, epiretinal membranes, or other retinal‐vascular anomalies. People with systemic diseases were eligible only if there were no ocular manifestations of the disease (e.g. diabetic patients with normal retinal exams) Exclusion criteria: people initially enrolled and randomized into the study were subsequently disqualified if vitreous loss or capsular disruption/rupture occurred during surgery. Participants could also be exited from the study if, on postoperative day 1, the surgeon felt the amount of inflammation was greater than expected and, in his best clinical judgement, more aggressive anti‐inflammatory treatment was indicated Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: ketorolac 0.4% plus prednisolone 1% Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: 4 weeks Actual: 4 weeks; “Patients could also return for an additional visit between days 43 and 50 (week 6) at the discretion of the investigator” |
|
| Outcomes |
Primary outcome, as defined in study reports: incidence of CME Secondary outcomes, as defined in study reports: these included an assessment of retinal (foveal) thickness (based on OCT measurements), Snellen BCVA, and contrast sensitivity Adverse events reported: yes Intervals at which outcomes assessed: day 1, between days 8 and 12 (week 1), and between days 26 and 34 (week 4) |
|
| Notes |
Type of study: published Funding sources: this study was supported by an unrestricted educational grant from Allergan Inc., Irvine, CA Disclosures of interest: the authors indicate no financial conflict of interest. Drs Wittpenn, Silverstein, and Heier are consultants for Allergan Inc. Dr Heier is also a consultant for Ista Pharmaceuticals. Study period: 5 June 2005 to 10 August 2006 Reported subgroup analyses: no Trial Registry #: NCT00348244 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A central coordination center (IMEDS Inc, Riverside, California, USA; [M.E.]) generated the allocation sequence, enrolled participants, and assigned participants to their treatment groups.“ |
| Allocation concealment (selection bias) | Low risk | See above |
| Masking of participants and personnel (performance bias) | Low risk | “All investigators were masked with regard to treatment group. The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles.“ |
| Masking of outcome assessment (detection bias) | Unclear risk | “All investigators were masked with regard to treatment group. The patients and technical staff were unmasked because regulations prevented the medications from being repackaged into similar, unmarked bottles.“ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Unclear risk | Unrestricted educational grant from Allergan Inc. The authors declare no financial conflict of interest. The authors are also consultants for Allergan. |
Yavas 2007.
| Methods |
Study design: RCT Number randomized (total and per group): Total: 179 eyes of 179 participants Per group: not reported Exclusions after randomization: 10 Number analyzed (total and per group): Total: 179 participants Per group: group 1: 61, group 2: 60, group 3: 58 Unit of analysis: participants Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: Turkey Age: group 1 mean age = 65.28 ± 9.90, group 2 mean age = 62.25 ± 11.57, group 3 mean age = 64.78 ± 9.18 Gender (per cent): group 1: 54.1% men and 45.9% women; group 2: 60.0% men and 40.0% women; group 3: 63.8% men and 36.2% women Inclusion criteria: not reported Exclusion criteria: people with a history of intraocular surgery; any complication during cataract surgery; glaucoma; uveitis; vitreoretinal pathology; history of diabetes mellitus, hypertension, or cardiac disease; or topical or systemic drug use were excluded from the study Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid 1 (group 1): indomethacin 0.1% 4 times daily for 3 days preoperatively and 1 month postoperatively plus topical prednisolone acetate 1% 4 times daily for 1 month NSAID plus corticosteroid 2 (group 2): indomethacin 0.1% 4 times daily for 1 month postoperatively plus topical prednisolone acetate 1% 4 times daily for 1 month Corticosteroid (group 3): prednisolone acetate 1% 4 times daily for 1 month Other medications: all participants also received the antibiotic ofloxacin 0.3% 4 times daily for 1 week Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: BCVA, angiographic CME Secondary outcomes, as defined in study reports: not reported Adverse events reported: no Intervals at which outcomes assessed: 3 months |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Low risk | Fluorescein leakage to diagnose angiographic CME was evaluated by a masked observer. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear whether there were incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
Zaczek 2014.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 160 eyes of 160 participants Per group: 80 in each group Exclusions after randomization: 5 in the nepafenac group (1 cortical remnants in the anterior chamber on the postoperative day; 1 loose zonular fibers intraoperatively requiring a placement of a capsule tension ring; 1 improper dosing of study medication; 1 use of non‐allowed concomitant medicine; and 1 appearance of general health problems judged to be unrelated to the study treatments); 2 in the control group (participant decision). “Eyes with intraoperative difficulties such as loose zonular fibers, extended operating time, or residual cortical material or with complications such as posterior capsule rupture and vitreous loss were excluded from the study after randomization.” Number analyzed (total and per group): Total: 152 eyes of 152 participants Per group: NSAID plus corticosteroid: 75, corticosteroid alone: 77 Unit of analysis: individual (1 eye per individual) Losses to follow‐up: 1 in the control group How were missing data handled?: 8 were not included in the ITT analysis Reported power calculation: yes, 80% Unusual study design?: some exclusions after randomization based on operation difficulties |
|
| Participants |
Country: Sweden Age: NSAID plus corticosteroid mean age = 70.4 ± 7.4 (range 51.0 to 84.0), corticosteroid alone mean age = 68.3 ± 7.5 (range 51.0 to 83.0) Gender (per cent): NSAID plus corticosteroid: 27 males (36.0%) and 48 females (64.0%), corticosteroid alone: 27 males (35.1%) and 50 females (64.9%) Inclusion criteria: people between the ages of 45 and 85 years scheduled for cataract surgery under local anesthesia Exclusion criteria: people with small pupils (< 5.0 mm after pharmacologic dilation), dark brown irides, exfoliation syndrome, history of uveitis, glaucoma, macular degeneration, or any vision‐impairing eye disorder except cataract, diabetic patients, pregnant women, and people using any topical or systemic anti‐inflammatory treatment or with hypersensitivity to any of the given study treatments Equivalence of baseline characteristics: yes, there were no statistically significant differences in these parameters between the 2 groups |
|
| Interventions |
Comparison: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: nepafenac ophthalmic suspension 0.1% starting 2 days before surgery and given 30 minutes before surgery 3 times 5 minutes apart and dexamethasone 0.1% 3 times a day for 3 weeks after surgery Corticosteroid: dexamethasone 0.1% 3 times a day for 3 weeks after surgery and a placebo (dextran 70‐hypromellose (Tears Naturale II Polyquad)) starting 2 days before surgery and given 30 minutes before surgery 3 times 5 minutes apart Length of follow‐up: Planned: 6 weeks Actual: 6 weeks |
|
| Outcomes |
Primary outcome, as defined in study reports: change in total macular volume 6 weeks after cataract surgery compared with baseline, proportion of participants with macular swelling of at least 10 mm 6 weeks postoperatively compared with baseline Secondary outcomes, as defined in study reports: change in total macular volume 3 weeks postoperatively compared with baseline; proportion of participants with macular swelling of at least 10 mm 3 weeks postoperatively compared with baseline; visual acuity at 3 and 6 weeks; anterior segment inflammation measured with a laser flare meter at 1 day, 3 and 6 weeks; ocular pain sensation during surgery and 24 hours and 3 weeks postoperatively; ocular discomfort and photophobia 1 day postoperatively and during the 3‐week treatment course; and intraoperative pupil dilation measured with a caliper Adverse events reported: yes Intervals at which outcomes assessed: preoperative examination within 1 week of surgery and 1 day, 3 and 6 weeks postoperatively |
|
| Notes |
Type of study: published Funding sources: “Supported by Alcon Research Ltd, Fort Worth, Texas, USA, and S.A. Alcon‐Couvreur N.V., Puurs, Belgium, which produced and provided the masked eye drop bottles. Partially supported by Alcon, Inc., Sweden. Financial support was also provided through the regional agreement on Medical training and Clinical research (ALF) between Stockholm County Council and Karolinska Institutet (20120623). The statistical analyses were supported by G. Andersson Foundation.” Disclosures of interest: “No author has a financial or proprietary interest in any material or method mentioned.” Study period: not reported Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Masking of participants and personnel (performance bias) | Low risk | "double‐masked” study; “Nepafenac and placebo suspensions were supplied in identical bottles labeled with a protocol and a patient number so neither the investigators nor the patients were able to identify their contents.” |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not explicitly reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | An ITT analysis was used, but 8 participants were not included. |
| Selective reporting (reporting bias) | Unclear risk | This trial was registered at the European Clinical Trials Database, but the record could not be retrieved. |
| Other bias | High risk | The study was supported by Alcon Research Ltd., which manufactures the NSAID used. Alcon Research Ltd provided the masked eyedrop bottles |
Zanetti 2012.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 140 participants Per group: 35 per group Exclusions after randomization: none Number analyzed (total and per group): Total: 140 participants Per group: 35 per group Unit of analysis: eye Losses to follow‐up: none How were missing data handled?: N/A Reported power calculation: yes, 80% Unusual study design?: none |
|
| Participants |
Country: Brazil Age: ketorolac 0.4% mean age = 66 ± 8; nepafenac 0.1% mean age = 66 ± 7; prednisolone 1% mean age = 67 ± 8; placebo mean age = 67 ± 6 Gender (per cent): ketorolac 0.4%: 15 male (42.9%) and 20 female (57.1%); nepafenac 0.1%: 14 male (40%) and 21 female (60%); prednisolone 1%: 13 male (37.1%) and 22 female (62.9%); placebo: 12 male (34.3%) and 23 female (65.7%) Inclusion criteria: people with nuclear cataract density of 2 and 3 by LOCS II (> 50 years old), with indication for cataract surgery with intraocular lens implant, under local anesthesia Exclusion criteria: diabetes, hypertension, people using NSAID, alpha‐blocker, topical eye drops (including antiglaucoma drugs), history of uveitis, macular disease, pseudoexfoliation syndrome, congenital ocular abnormalities, cataract density of 1 and 4 by LOCS II, and previous intraocular surgery Equivalence of baseline characteristics: yes, “Baseline demographic and clinical characteristics were similar in all groups. There were no differences regarding ages (P = 0.930), neither in age‐related cataract density (P = 0.852), nor in gender distribution (P = 0.896), ultrasound time (P = 0.986) and surgical time (P = 0.666)” |
|
| Interventions |
Comparison: NSAID vs NSAID vs corticosteroid vs placebo NSAID 1: ketorolac tromethamine 0.4% 3 times daily for 2 days prior to surgery NSAID 2: nepafenac 0.1% 3 times daily for 2 days prior to surgery Corticosteroid: prednisolone acetate 1% 3 times daily for 2 days prior to surgery Placebo: carboxymethylcellulose sodium 0.5% 3 times daily for 2 days prior to surgery Other medications: gatifloxacin 4 times daily for 2 days prior to surgery Length of follow‐up: Planned: N/A Actual: N/A |
|
| Outcomes |
Primary outcome, as defined in study reports: number of participants with pupil ≥ 6 mm (vertical and horizontal diameters) at the end of the surgery Secondary outcomes, as defined in study reports: number of participants with pupil ≥ 6 mm (vertical and horizontal diameters) at the beginning of the surgery (prior to the corneal section) Adverse events reported: yes Intervals at which outcomes assessed: before and after surgery |
|
| Notes |
Type of study: published Funding sources: none Disclosures of interest: “None declared” Study period: eligible participants were recruited from March 2009 to March 2010 Reported subgroup analyses: no Trial Registry #: NCT00865540, Conep ‐ National Research Ethics Committee – Brazil – 0816.0.146.000‐10 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization was done in a blocking manner. Each of the four intervention groups received 35 different numbers from a random number table.” |
| Allocation concealment (selection bias) | Low risk | “These numbers were transferred to small individual envelopes and also fixed at one of the opaque eye drop bottles. It helped not only to randomize the patients but also to mask the treatment groups until data analysis. Four small envelopes, one of each intervention group, were sealed and placed into a larger envelope, totalizing 35 large envelopes containing four small individual envelopes in each one. This comprises the block length of four to assure that every four patients have received all four interventions. When a patient was included in the study, a pharmacist took for him a small individual envelope and after discovering the random number, she took the respective eye drop bottle. The surgeon and the ophthalmologist who collected the data did not know the randomized groups.” |
| Masking of participants and personnel (performance bias) | Low risk | “These eye drops were administered 48 hours before surgery by mask fashion, three times daily for two days prior to surgery”; “The surgeon and the ophthalmologist who collected the data did not know the randomized groups.” “The eye drop group was revealed to the researchers once recruitment, data collection, and statistical analyses were complete. All study participants were masked to treatment assignment.” |
| Masking of outcome assessment (detection bias) | Low risk | “The surgeon and the ophthalmologist who collected the data did not know the randomized groups." “The eye drop group was revealed to the researchers once recruitment, data collection, and statistical analyses were complete.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up and there were no missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The outcomes reported match the outcomes described in the trial registry. |
| Other bias | Low risk | No other sources of bias |
Zhang 2008.
| Methods |
Study design: parallel‐group RCT Number randomized (total and per group): Total: 220 eyes of 220 participants Per group: 110 Exclusions after randomization: not reported Number analyzed (total and per group): Total: 220 eyes of 220 participants Per group: 110 Unit of analysis: eyes Losses to follow‐up: not reported How were missing data handled?: not reported Reported power calculation: no Unusual study design?: none |
|
| Participants |
Country: China Age: mean age = 71 (range 55 to 87) Gender (per cent): 98 (44.5%) men and 122 (55.5%) women Inclusion criteria: cataract patient having uneventful phacoemulsification and IOL implantation Exclusion criteria: younger than 18 years of age; used steroid hormone or NSAID within 1 week; severe cornea dysfunction; severe cardiovascular, lung, liver, kidney failure; diabetes patients; glaucoma patients; pregnant or breastfeeding women Equivalence of baseline characteristics: yes |
|
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID plus corticosteroid: pranoprofen plus dexamethasone Corticosteroid: dexamethasone Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 3 months Actual: 3 months |
|
| Outcomes |
Primary outcome, as defined in study reports: the proportion of participants with intraocular inflammation Secondary outcomes, as defined in study reports: the proportion of participants with CME Adverse events reported: yes Intervals at which outcomes assessed: 1, 2 weeks, 1, 3 months after surgery |
|
| Notes |
Type of study: published Funding sources: not reported Disclosures of interest: not reported Study period: 2007 to 2008 Reported subgroup analyses: no Trial registry #: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants and personnel (performance bias) | Unclear risk | Masking of participants and personnel was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all randomized participants. |
| Selective reporting (reporting bias) | Unclear risk | It is unclear whether there was selective outcome reporting. |
| Other bias | Low risk | No other sources of bias |
BCVA: best‐corrected visual acuity CCT: central corneal thickness CME: cystoid macular edema COMTOL: Comparison of Ophthalmic Medications for Tolerability Questionnaire ECC: endothelial cell count ECCE: extracapsular cataract extraction ETDRS: Early Treatment Diabetic Retinopathy Study FT: foveal thickness HRQOL: health‐related quality of life IOL: intraocular lens IOP: intraocular pressure ITT: intention‐to‐treat LFCM: laser flare‐cell meter LOCS II: Lens Opacities Classification System, version II N/A: not applicable Nd:YAG: neodymium‐doped yttrium aluminium garnet NSAID: non‐steroidal anti‐inflammatory drug OCT: optical coherence tomography PCR: posterior capsule rupture RCT: randomized controlled trial SD: standard deviation TMV: total macular volume UCVA: uncorrected visual acuity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelson 1989 | Used extracapsular cataract extraction rather than phacoemulsification; oral NSAID and corticosteroid were used rather than topical drops |
| Bahar 2007 | Study not intended to assess vision outcomes |
| Barequet 2002 | Some participants received extracapsular cataract extraction and results were not separated |
| Barequet 2004 | Letter to the editor |
| Burde 1972 | No NSAID treatment arm, used intracapsular cataract extraction |
| Carenini 1993 | Only one treatment group; no comparison |
| Chang 2016 | Corticosteroids were not used |
| Corbett 1993 | No NSAID treatment arm; used extracapsular cataract extraction for some participants without separating out results |
| EUCTR2009‐017031‐18‐NL | Compared NSAID plus corticosteroid with placebo |
| EUCTR2015‐003296‐30‐FI | Unclear which type of cataract extraction surgery was used |
| Farooq 2003 | Multiple types of cataract surgery, results are not divided |
| Ferreira 2006 | Type of cataract surgery is unclear |
| Hessemer 1994 | Used extracapsular cataract extraction rather than phacoemulsification |
| Hossain 2010 | Unclear if groups are randomized |
| Hosseini 2016 | Corticosteroids were not used |
| ISRCTN02628492 | Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest |
| Knopf 1970 | Used extracapsular cataract extraction |
| Kraff 1990 | Does not specify type of cataract surgery |
| Liou 1991 | Unclear whether the study is randomized |
| Luo 2013 | Interventions do not fit criteria for review |
| Maheshwari 1995 | Participants had cataract, glaucoma, and strabismus surgery |
| McDonald 1998 | Unclear which type of surgery |
| Meconi 1998 | Corticosteroids were not used |
| Missotten 2001 | Multiple surgical techniques employed, outcomes are aggregated |
| Miyake 1998 | Unclear whether the study is randomized |
| Miyake 2000 | Unclear whether the study is randomized |
| Mullins 2016 | Not a clinical trial |
| NCT00407017 | Both study arms received NSAID plus corticosteroid |
| NCT00433225 | Comparisons unclear; trial record does not provide detail |
| NCT00698724 | Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest |
| NCT00758199 | Dosage study, both groups received a corticosteroid and a different dose of an NSAID |
| NCT01193504 | Study compares 2 corticosteroids, both study arms received NSAID plus corticosteroid |
| Nishino 2009 | Comparison (combined NSAID plus corticosteroid vs NSAID alone) was not one of the interventions of interest |
| Othenin‐Girard 1994 | Participants underwent extracapsular cataract extraction |
| Ozkurt 2003 | Could not confirm whether trial was randomized |
| Pollack 2017 | Corticosteroids were not used |
| Raju 2005 | Multiple types of surgeries, results are not divided |
| Ramakrishnan 2015 | Study compares 2 NSAIDs, both study arms received NSAID plus corticosteroid |
| Rocha 2009 | Letter to the editor |
| Sabiston 1987 | Not topical corticosteroids, surgery method unclear |
| Smerdon 1986 | Participants underwent extracapsular cataract extraction |
| Sourdille 1993 | Parrticipants underwent extracapsular cataract extraction |
| Suharwardy 1994 | Participants underwent extracapsular cataract extraction |
| Szymanski 1994 | Participants underwent extracapsular cataract extraction |
| Tauber 2006 | Type of cataract surgery is not specified |
| Tunc 1999 | Participants underwent extracapsular cataract extraction |
| Turan‐Vural 2013 | Quasi‐randomized trial |
| Waseem 2009 | Participants were not randomized |
| Yasuda 2016 | Multiple types of surgeries, phacoemulsification plus vitrectomy |
| Yung 2007 | Type of cataract surgery is not specified |
NSAID: non‐steroidal anti‐inflammatory drug
Characteristics of studies awaiting assessment [ordered by study ID]
ChiCTR‐TRC‐12002600.
| Methods |
Study design: parallel‐group RCT Number enrolled: 240 |
| Participants |
Country: China Condition: ocular inflammation and CME after phacoemulsification Gender: both Age: minimum 46, maximum 92 Inclusion criteria: people with age‐related cataract undergoing phacoemulsification with posterior chamber intraocular lens implantation Exclusion criteria: people with any ocular disease that might affect treatment responses or evaluations, such as corneal disease, glaucoma, uveitis, retinal detachment, optic neuropathy, or amblyopia; people with any systemic disease that might affect treatment responses or evaluations, such as diabetes mellitus; potentially pregnant women; people who had received systemic or topical anti‐inflammatory therapy within 1 month prior to surgery |
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID: bromfenac sodium Corticosteroid 1: fluorometholone Corticosteroid 2: dexamethasone 0.1% Length of follow‐up: Planned: not reported |
| Outcomes |
Outcomes: photon count value, retinal foveal thickness, intraocular pressure, BCVA, endothelial cell density Intervals at which outcomes assessed: not reported |
| Notes |
Funding source: Zhejiang Key Innovation Team Project of China (grant no. 2009R50039), Zhejiang Key Laboratory Fund of China (no. 2011E10006) Study period: not reported Trial registry #: ChiCTR‐TRC‐12002600 |
Coassin 2016.
| Methods |
Study design: parallel‐group RCT Number enrolled: 62 |
| Participants |
Country: Italy Condition: cataract, pseudoexfoliation syndrome Gender: both Age: 60 years and older Inclusion criteria: cataract, pseudoexfoliation syndrome Exclusion criteria: history of ocular inflammation or trauma, previous intraocular surgery, corneal haze, retinal vascular disease, diabetic retinopathy, variation of the foveal profile at OCT (macular edema, epiretinal membrane), moderate to severe age‐related macular degeneration |
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: bromfenac 0.09% twice a day for 2 weeks starting the day after surgery plus dexamethasone 0.1% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Corticosteroid: dexamethasone 0.1% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Other medications: all participants also received the antibiotic tobramycin 0.3% 4 times a day for the 1st week and twice a day for the 2nd week after surgery Length of follow‐up: Planned: 4 weeks |
| Outcomes |
Outcomes: change from baseline in anterior chamber inflammation measured by laser flare photometry, proportion of participants with central macular thickness greater than 300 μm, proportion of participants with BCVA equal to 20/20, proportion of participants with no ocular pain Intervals at which outcomes assessed: 1, 3 days, 1, 4 weeks |
| Notes |
Study period: November 2013 to October 2014 Trial registry #: NCT02137161 and EUCTR2013‐002066‐39‐IT |
Jirásková 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Czech. Awaiting translation. |
Liu 2016.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Chinese. Awaiting translation. |
Malik 2016.
| Methods |
Study design: parallel‐group RCT Number enrolled: 200 |
| Participants |
Country: India Condition: senile cataract Gender: both Age: 40 years and older Inclusion criteria: "indication for cataract extraction with implantation of the intraocular lens and uncomplicated cataract surgery" Exclusion criteria: "topical/inhaled/systemic steroid 14 days before surgery, topical/inhaled/systemic NSAIDs within 7 days of surgery, eyes with cells and flare and ocular pain in preoperative baseline examination, corneal opacity, suspected hypersensitivity to NSAIDs, chronic or recurrent inflammatory eye disease, diabetic patient, severe dry eye patient, cases in which there is intraoperative complications, glaucoma patient, patient having macular pathology" |
| Interventions |
Comparison analyzed: NSAIDs vs corticosteroid NSAIDs (3 groups): nepafenac 0.1%, bromfenac 0.09%, and ketorolac 0.5% Corticosteroid: prednisolone acetate 1% Length of follow‐up: Planned: one month |
| Outcomes |
Outcomes: "postoperative inflammation was evaluated subjectively by intraocular pressure, slit‐lamp assessment of signs of inflammation, including conjunctival hyperemia, ocular pain, and aqueous cells and flare." Intervals at which outcomes assessed: baseline, 1 week, 2 weeks, and one month |
| Notes |
Funding source: reported "nil" Study period: not reported Trial registry #: none reported |
NCT00366691.
| Methods |
Study design: parallel‐group RCT Number enrolled: 40 |
| Participants |
Country: United States Condition: not reported Gender: both Age: 18 years and older Inclusion criteria: person must have a visually significant age‐related cataract, in the planned operated eye, 18 years of age or older, the vision in the fellow, unoperated eye should have a potential visual acuity of 20/40 or better as determined by the principal investigator, person must desire cataract extraction, be willing and able to comply with scheduled visits and other study procedures Exclusion criteria: advanced glaucomatous damage, any abnormality preventing reliable applanation tonometry in operated eye, contact lens use during the active treatment portion of the trial in the operated eye, any concurrent infectious/non‐infectious conjunctivitis, keratitis or uveitis in either eye, any history of allergic hypersensitivity or poor tolerance to any component of the preparations used in this trial, pregnant or nursing mothers and females of childbearing potential not practicing a reliable and medically acceptable method of birth control, any clinically significant, serious, or severe medical or psychiatric condition, participation (or current participation) in any investigational drug or device trial within the previous 30 days prior to the start date of this trial, intraocular conventional surgery within the past 3 months or intraocular laser surgery within 1 month in the operated eye, required use of other topical medications during the active portion of the trial except prophylactic antibiotic, topical lid care, tear replacement solutions, or glaucoma medications, other ocular surgery at the time of the cataract extraction, use of topical or oral antiprostaglandins or corticosteroids as well as aspirin products (> 81 mg) during the active treatment portion of the trial. If patient wants to participate in the trial and can stop the medication, he/she can be enrolled after 7‐day washout period. |
| Interventions |
Comparison analyzed: NSAID vs corticosteroid NSAID + corticosteroid: ketorolac tromethamine 0.4% (Acular) Corticosteroid: loteprednol etabonate ophthalmic suspension 0.5% (Lotemax) Length of follow‐up: Planned: not reported |
| Outcomes |
Outcomes: not reported Intervals at which outcomes assessed: not reported |
| Notes |
Funding source: not reported Study period: February 2006 to September 2007 Trial registry #: NCT00366691 |
NCT00992355.
| Methods |
Study design: parallel‐group RCT Number enrolled: 97 |
| Participants |
Country: Greece Condition: cataracts Gender: both Age: 55 to 95 years Inclusion criteria: phacoemulsification (due to cataract), uneventful phacoemulsification surgery Exclusion criteria: disruption of the anterior lens capsule, age‐related macular degeneration, proliferative diabetic retinopathy |
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: ketorolac tromethamine 0.5% plus dexamethasone 0.1% Corticosteroid: dexamethasone Other medications: all participants also received the antibiotic tobramycin Length of follow‐up: Planned: 28 days |
| Outcomes |
Outcomes: corneal edema, conjunctival redness, anterior chamber reaction Intervals at which outcomes assessed: 28, 42 days |
| Notes |
Study period: January 2009 to April 2009 Trial registry #: NCT00992355 |
Stringa 1996.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article in Italian. Awaiting translation. |
BCVA: best‐corrected visual acuity CME: cystoid macular edema NSAID: non‐steroidal anti‐inflammatory drug OCT: optical coherence tomography RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01774474.
| Trial name or title | PRevention of Macular EDema after cataract surgery (PREMED) |
| Methods |
Study design: parallel‐group RCT Anticipated enrollment: 926 non‐diabetic and 209 diabetic patients |
| Participants |
Country: Austria, Belgium, Germany, Hungary, Netherlands, Portugal, Spain Condition: cystoid macular edema, cataract, diabetes mellitus Inclusion criteria: all people undergoing routine phacoemulsification (1 eye per person), willing and/or able to comply with the scheduled visits and other study procedures, able to communicate properly and understand instructions, accepting possible off‐label use of intravitreal bevacizumab and/or subconjunctival preservative‐free triamcinolone acetonide Exclusion criteria: general exclusion criteria for participation in this study are: age below 21 years old, participation in another clinical study, post‐traumatic cataract, combined surgery, functional monoculus, previous ocular surgery, progressive glaucoma with severe visual field defects, use of antiglaucomatous medication or steroid‐induced IOP elevation requiring IOP‐lowering treatment, IOP ≥ 25 mm Hg, history of any intraocular inflammation or uveitis, history of pseudoexfoliation syndrome, which is expected to cause perioperative complications, history of Fuchs' endothelial dystrophy or cornea guttata 3+, history of retinal vein occlusion, any macular pathology that might influence visual acuity other than diabetic macular edema, use of intravitreal bevacizumab or ranibizumab in the previous 6 weeks or intravitreal aflibercept in the previous 10 weeks, use of intra‐ or periocular corticosteroid injection in the previous 4 months, current use of topical NSAIDs or corticosteroids, use of systemic corticosteroids (≥ 20 mg prednisolone or equivalence), history of relevant adverse events including serious adverse events occurring after administration of NSAIDs, acetylsalicylic acid, sodium sulphite, corticosteroids, or bevacizumab, contraindications for use of topical NSAIDs, topical or subconjunctival corticosteroids, or intravitreal bevacizumab or related drugs. Non‐diabetic patients with a history of CME will be excluded from participation in the study. Additionally, diabetic patients will be excluded from participation in case of: macular edema with a central subfield macular thickness ≥ 450 µm, very severe NPDR or proliferative DR requiring panretinal photocoagulation or vitrectomy, vitreous hemorrhage present during preoperative visit(s), cerebrovascular accident, myocardial infarction, or other thromboembolic events in the previous 3 months, a history of recurrent thromboembolic events, a history of severe systemic bleeding in the previous 3 months, major surgery in the previous 3 months, history of glaucoma |
| Interventions |
Comparison analyzed: NSAID vs corticosteroid vs NSAID plus corticosteroid NSAID in non‐diabetics: bromfenac 0.9% Corticosteroid in non‐diabetics: dexamethasone 0.1% NSAID plus corticosteroid in non‐diabetics: bromfenac 0.9% plus dexamethasone 0.1% NSAID plus corticosteroid in diabetics: bromfenac 0.9% plus dexamethasone 0.1% NSAID plus 2 corticosteroids in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus triamcinolone acetonide NSAID plus corticosteroid plus anti‐VEGF in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus bevacizumab NSAID plus 2 corticosteroids plus anti‐VEGF in diabetics: bromfenac 0.9% plus dexamethasone 0.1% plus triamcinolone acetonide plus bevacizumab Length of follow‐up: Planned: 12 weeks |
| Outcomes |
Primary outcome, as defined in study reports: change in central subfield mean macular thickness as a measurement of efficacy; "The primary endpoint is the change in central subfield mean macular thickness in the 1 mm area (central subfield macular thickness, CSMT) as compared to baseline within the first 6 weeks postoperatively" Secondary outcomes, as defined in study reports: number of participants who develop clinically significant macular edema as a measurement of efficacy; "The secondary endpoint is the occurrence of postoperative clinically significant macular edema (CSME) within 12 weeks postoperatively". Other secondary outcome measures: change in corrected distance visual acuity as a measurement of efficacy, change in retinal thickness in the central inner circle (3 mm) as a measurement of efficacy, intraocular pressure as a measurement of safety, health‐related quality of life as a measurement of efficacy and tolerability, number of participants experiencing adverse events as a measurement of safety and tolerability, change in retinal thickness in the central outer circle (6 mm) as a measurement of efficacy, change in macular volume as a measurement of efficacy, vision‐related quality of life as a measurement of efficacy and tolerability, cost‐effectiveness Adverse events reported: yes Intervals at which outcomes assessed: 1, 2 weeks, 1, 3 months after surgery |
| Starting date | July 2013 |
| Contact information | Prof. Rudy MM Nuijts, MD, PhD, +31 43 387 1594, rudy.nuijts@mumc.nl Laura HP Wielders, MD, +31 43 387 1594, laura.wielders@mumc.nl |
| Notes | Study sponsor: Maastricht University Medical Center |
Pollack 2016.
| Trial name or title | Nepafenac Once Daily for Macular Edema (Study 1 and 2) |
| Methods |
Study design: parallel‐group RCT Number enrolled: 615 and 605 randomized in Study 1 and Study 2, respectively |
| Participants |
Country: United States Condition: non‐proliferative diabetic retinopathy Gender: both Age: 18 years and older Inclusion criteria: planned cataract extraction by phacoemulsification with implantation of a posterior chamber intraocular lens, history of type 1 or 2 diabetes and non‐proliferative diabetic retinopathy (mild, moderate, or severe) in the study eye, BCVA of 73 letters or worse in the study eye with expectation of improvement after surgery, understand and sign an informed consent document, other protocol‐defined inclusion criteria may apply Exclusion criteria: pre‐existing macular edema in the study eye, history in the study eye of retinal detachment, wet age‐related macular degeneration, chronic or recurrent inflammatory eye disease, or prior procedures, planned cataract surgery in the fellow eye after randomization and prior to the Day 90 postoperative study visit or through study exit, planned multiple procedures for the study eye during the cataract/intraocular lens implantation surgery, use of exclusionary medications including NSAIDs and steroids as specified in protocol, participation in any other clinical study within 30 days of the screening visit, females of childbearing potential who are breastfeeding, have a positive urine pregnancy test at screening, are not willing to undergo a urine pregnancy test upon entering or exiting the study, intend to become pregnant during the study, or do not agree to use adequate birth control methods for the duration of the study, other protocol‐defined exclusion criteria may apply |
| Interventions |
Comparison analyzed: NSAID plus corticosteroid vs corticosteroid alone NSAID + corticosteroid: nepafenac 0.3% 1 drop 1 day prior to surgery through 90 days following surgery plus prednisolone acetate Corticosteroid: prednisolone acetate Length of follow‐up: Planned: 90 days |
| Outcomes |
Outcomes: proportion of participants with BCVA improvement of ≥ 15 letters from preoperative baseline, proportion of participants who develop macular edema Intervals at which outcomes assessed: not reported |
| Starting date |
Study period: June 2013 to May 2015 Trial registry #: NCT01853072 and NCT01872611 |
| Contact information | Clinical Project Lead, Alcon Research |
| Notes | Study sponsor: Alcon Research |
anti‐VEGF: anti‐vascular endothelial growth factor CME: cystoid macular edema DR: diabetic retinopathy IOP: intraocular pressure NPDR: non‐proliferative diabetic retinopathy NSAID: non‐steroidal anti‐inflammatory drug RCT: randomized controlled trial
Differences between protocol and review
We included methods for the 'Summary of findings' tables and the GRADE assessment that were not included in the original protocol.
We did not do a sensitivity analysis to determine the implication of missing data because too few trials provided information on missing data and loss to follow‐up.
The protocol called for meta‐analysis on anterior chamber cell and flare as dichotomous outcomes, however we performed meta‐analyses on continuous cell and flare data as this was how this information was reported in many of the included studies.
Contributions of authors
VVJ, EC, and RSC coordinated and contributed significantly to the design and writing of the review. VVJ, EC, and RSC provided clinical, methodological, policy, and consumer perspectives to the review. RSC performed previous work that was the foundation of the current study. EC performed the data analysis and prepared the results. VVJ drafted the discussion and conclusions.
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Eyes and Vision US Project, supported by Grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
VVJ: None known. EC: None known. RSC: None known.
New
References
References to studies included in this review
Adam 2005 {published data only}
- Adam R, Rodallec T, Nordmann JP. Indometacine and macular thickness undergoing cataract surgery: a prospective randomized study. Investigative Ophthalmology & Visual Science 2005; Vol. 46:E‐Abstract 787.
Almeida 2008 {published data only}
- Almeida DR, Johnson D, Hollands H, Smallman D, Baxter S, Eng KT, et al. Effect of prophylactic nonsteroidal antiinflammatory drugs on cystoid macular edema assessed using optical coherence tomography quantification of total macular volume after cataract surgery. Journal of Cataract and Refractive Surgery 2008;34(1):64‐9. [DOI] [PubMed] [Google Scholar]
Almeida 2012 {published data only}
- Almeida DR, Khan Z, Xing L, Bakar SN, Rahim K, Urton T, et al. Prophylactic nepafenac and ketorolac versus placebo in preventing postoperative macular edema after uneventful phacoemulsification. Journal of Cataract and Refractive Surgery 2012;38(9):1537‐43. [DOI] [PubMed] [Google Scholar]
Asano 2008 {published data only}
- Asano S, Miyake K, Ota I, Sugita G, Kimura W, Sakka Y, et al. Reducing angiographic cystoid macular edema and blood‐aqueous barrier disruption after small‐incision phacoemulsification and foldable intraocular lens implantation. Multicenter prospective randomized comparison of topical diclofenac 0.1% and betamethasonone 0.1%. Journal of Cataract and Refractive Surgery 2008;31(1):57‐63. [DOI] [PubMed] [Google Scholar]
Bucci 2001 {published data only}
- Bucci FA, Matta AZ. An objective comparison of acular vs vexol to control inflammation following phacoemulsification. American Academy of Ophthalmology; 2001 Nov 11‐14; New Orleans (LA). 2001:117.
Cervantes‐Coste 2009 {published data only}
- Cervantes‐Coste G, Sánchez‐Castro Y, Orozco‐Carroll M, Mendoza‐Schuster E, Velasco‐Barona C. Inhibition of surgically induced miosis and prevention of postoperative macular edema with nepafenac. Clinical Ophthalmology 2009;3(1):219‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatziralli 2011 {published data only}
- Chatziralli IP, Papazisis L, Sergentanis TN. Ketorolac plus tobramycin/dexamethasone versus tobramycin/dexamethasone after uneventful phacoemulsification surgery: a randomized controlled trial. Ophthalmologica 2011;225(2):89‐94. [DOI] [PubMed] [Google Scholar]
- Chatziralli IP, Sergentanis TN, Parikakis EA, Papazisis LE, Mitropoulos P, Moschos MM. The impact of non‐steroidal anti‐inflammatory agents after phacoemulsification on quality of life: a randomized study. Ophthalmology and Therapy 2017;6(1):133‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen J, Wang GQ. Clinical observation of bromfenac sodium 0.1% eye drops on cataract surgery. International Eye Science 2015;15(12):2102‐4. [Google Scholar]
Dal 2014 {published data only}
- Dal D, Saraç O, Toklu Y, Cakmak HB, Gungor ED. Efficacy of topical ketoroloc on macular thickness in patients undergoing cataract surgery. Ophthalmologica 2014;232(Suppl 2):43. [Google Scholar]
Demco 1997 {published data only}
- Demco TA. Topical diclofenac versus prednisolone acetate in control of inflammation after phacoemulsification. American Academy of Ophthalmology 1995:150.
- Demco TA, Sutton H, Demco CJ, Raj PS. Topical diclofenac sodium compared with prednisolone acetate after phacoemulsification‐lens implant surgery. European Journal of Ophthalmology 1997;7(3):236‐40. [DOI] [PubMed] [Google Scholar]
Donnenfeld 2006 {published data only}
- Donnenfeld ED, Perry HD, Wittpenn JR, Solomon R, Nattis A, Chou T. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic‐response curve. Journal of Cataract and Refractive Surgery 2006;32(9):1474‐82. [DOI] [PubMed] [Google Scholar]
Duong 2014 {published data only}
- Duong HV, Westfield KC, Singleton IC. Treatment paradigm after uncomplicated cataract surgery: a prospective evaluation. Acta Ophthalmologica 2015;93(4):e314‐5. [DOI] [PubMed] [Google Scholar]
- Duong HV, Westfield KC, Singleton IC. Treatment paradigm after uncomplicated cataract surgery: a prospective evaluation. Asia‐Pacific Journal of Ophthalmology 2014;3(4):220‐5. [DOI] [PubMed] [Google Scholar]
el‐Harazi 1998 {published data only}
- el‐Harazi SM, Ruiz RS, Feldman RM, Villanueva G, Chuang AZ. A randomized double‐masked trial comparing ketorolac tromethamine 0.5%, diclofenac sodium 0.1%, and prednisolone acetate 1% in reducing post‐phacoemulsification flare and cells. Ophthalmic Surgery and Lasers 1998;29(7):539‐44. [PubMed] [Google Scholar]
Elsawy 2013 {published data only}
- Elsawy MF, Badawi N, Khairy HA. Prophylactic postoperative ketorolac improves outcomes in diabetic patients assigned for cataract surgery. Clinical Ophthalmology 2013;7:1245‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endo 2010 {published data only}
- Endo N, Kato S, Haruyama K, Shoji M, Kitano S. Efficacy of bromfenac sodium ophthalmic solution in preventing cystoid macular oedema after cataract surgery in patients with diabetes. Acta Ophthalmologica 2010;88(8):896‐900. [DOI] [PubMed] [Google Scholar]
- Kitano S, Endo N, Shoji M, Haruyama K, Kato S. Effect of bromfenac sodium ophthalmic solution on preventing cystoids macular edema and inflammation after phacoemulsification and implantation of intraocular lens in diabetic patients. Investigative Ophthalmology & Visual Science 2008; Vol. 49:ARVO E‐Abstract 3470.
Entezari 2016 {published data only}
- Entezari M, Ramezani A, Nikkhah H, Yaseri M. The effect of topical sodium diclofenac on macular thickness in diabetic eyes after phacoemulsification: a randomized controlled trial. International Ophthalmology 2017;37(1):13‐8. [DOI: 10.1007/s10792-016-0209-4] [DOI] [PubMed] [Google Scholar]
Guzey 2000 {published data only}
- Guzey M, Karadede Z, Dogan Z, Satici A. Ketorolac‐tobramycin combination vs fluorometholone‐tobramycin combination in reducing inflammation following phacoemulsification cataract extraction with scleral tunnel incision. Ophthalmic Surgery and Lasers 2000;31(6):451‐6. [PubMed] [Google Scholar]
Hessemer 1996 {published data only}
- Hessemer V, Schartner H. Low influence of anti‐inflammatory therapy on intraocular inflammation following minimally invasive phacoemulsification [Geringer Einfluß antiinflammatorischer Therapie auf den intraokularen Reizzustand nach minimal invasiver Phakoemulsifikation]. Der Ophthalmologe 1997;94(1):30‐2. [DOI] [PubMed] [Google Scholar]
- Hessemer V, Schmidt K, Schartner H. Minimally inflammatory cataract surgery [Minimal inflammatorische Kataraktchirurgie (MIK)]. Klinische Montasblätter für Augenheilkunde 1996;209(6):331‐9. [DOI] [PubMed] [Google Scholar]
Holzer 2002 {published data only}
- Holzer MP, Solomon KD, Sandoval HP, Vroman DT. Comparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification. Journal of Cataract and Refractive Surgery 2002;28(1):93‐9. [DOI] [PubMed] [Google Scholar]
Jung 2015 {published data only}
- Jung JW, Chung BH, Kim EK, Seo KY, Kim TI. The effects of two non‐steroidal anti‐inflammatory drugs, bromfenac 0.1% and ketorolac 0.45%, on cataract surgery. Yonsei Medical Journal 2015;56(6):1671‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kato 1998 {published data only}
- Kato S, Boku C, Onishi T, Koike M, Kageyama T, Nishihara H, et al. Comparison of postoperative inflammation with two types of intraocular lense and anti‐inflammatory agents. Folia Ophthalmologica Japonica 1998;49:286‐9. [Google Scholar]
Laurell 2002 {published data only}
- Laurell CG, Zetterström C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. British Journal of Ophthalmology 2002;86(12):1380‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2011 {published data only}
- Li MC, Yang XR, Liu F, Shao DP, Li YB. Effect of topical NSAIDs in preventing macular edema after phacoemulsification in diabetes. International Journal of Ophthalmology 2011;11(9):1614‐6. [Google Scholar]
Mathys 2010 {published data only}
- Aschbrenner MW, Mathys KC, Cohen KL. Do biological parameters and phaco metrics affect OCT measured CMT with and without nepafenac 0.1% treatment?. Investigative Ophthalmology & Visual Science 2008;49(13):389. [Google Scholar]
- Mathys KC, Cohen KL. Effect of nepafenac on macular thickness following uncomplicated cataract surgery. Investigative Ophthalmology & Visual Science 2008; Vol. 49:ARVO E‐Abstract 5674.
- Mathys KC, Cohen KL. Impact of nepafenac 0.1% on macular thickness and postoperative visual acuity after cataract surgery in patients at low risk for cystoid macular oedema. Eye 2010;24(1):90‐6. [DOI] [PubMed] [Google Scholar]
McColgin 1999 {published data only}
- McColgin AZ, Raizman MB. Efficacy of topical voltaren in reducing the incidence of postoperative cystoid macular edema. Investigative Ophthalmology & Visual Science 1999; Vol. 40:ARVO E‐Abstract 1529.
Miyake 2007 {published data only}
- Miyake K, Nishimura K, Harino S, Ota I, Asano S, Kondo N, et al. The effect of topical diclofenac on choroidal blood flow in early postoperative pseudophakias with regard to cystoid macular edema formation. Investigative Ophthalmology & Visual Science 2007;48(12):5647‐52. [DOI] [PubMed] [Google Scholar]
Miyake 2011 {published data only}
- Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. Journal of Cataract and Refractive Surgery 2011;37(9):1581‐8. [DOI] [PubMed] [Google Scholar]
Miyanaga 2009 {published data only}
- Miyanaga M, Miyai T, Nejima R, Maruyama Y, Miyata K, Kato S. Effect of bromfenac ophthalmic solution on ocular inflammation following cataract surgery. Acta Ophthalmologica 2009;87(3):300‐5. [DOI] [PubMed] [Google Scholar]
Moschos 2012 {published data only}
- Moschos MM, Chatziralli IP, Pantazis P, Rouvas AA, Sergentanis TN. Is topical diclofenac essential before and after uneventful phacoemulsification cataract surgery?. Journal of Ocular Pharmacology and Therapeutics 2012;28(4):335‐9. [DOI] [PubMed] [Google Scholar]
Mulet 2001 {published data only}
- Mulet ME, Alio JL, Ayala MJ, Hoz F, Beda JL. Efficacy of 0.5% ketorolac (Acular™) as an anti‐inflammatory treatment after cataract surgery. Investigative Ophthalmology & Visual Science 2001;42:ARVO E‐Abstract 66. [Google Scholar]
Ostrov 1997 {published data only}
- Ostrov CS, Sirkin SR, Deutsch WE, Masi RJ, Chandler JW, Lindquist TD. Ketorolac, prednisolone, and dexamethasone for postoperative inflammation. Clinical Therapeutics 1997;19(2):259‐72. [DOI] [PubMed] [Google Scholar]
Roberts 1995 {published data only}
- Brennan KM, Brown RM, Roberts CW. A comparison of topical non‐steroidal anti‐inflammatory drugs to steroids for control of post cataract inflammation. Journal of the American Society of Ophthalmic Registered Nurses 1993;18(1):8‐11. [PubMed] [Google Scholar]
- Brown RM, Roberts CW. Preoperative and postoperative use of nonsteroidal antiinflammatory drugs in cataract surgery. Journal of the American Society of Ophthalmic Registered Nurses 1996;21(1):13‐6. [DOI] [PubMed] [Google Scholar]
- Roberts CW. A comparison of non‐steroidal anti‐inflammatory drug to steroids for postoperative inflammation. American Academy of Ophthalmology 1992; Vol. 22.
- Roberts CW, Brennan KM. A comparison on topical diclofenac with prednisolone for postcataract inflammation. Archives of Ophthalmology 1995;113(6):725‐7. [DOI] [PubMed] [Google Scholar]
Ruiz Rodríguez 2011 {published data only}
- Rodríguez YR, PereraII YM, Hernández IM, ÁvilaII RI, Hernández Silva JR, Ochoa MJ. Diclofenac sodium eyedrops in cataract's surgery by phacoemulsification [Colirio de diclofenaco sódico en cirugía de catarata por facoemulsificación]. Revista Cubana de Oftalmología 2011;24(1):46‐54. [Google Scholar]
Sahu 2015 {published data only}
- Sahu S, Ram J, Bansal R, Pandav SS, Gupta A. Effect of topical ketorolac 0.4%, nepafenac 0.1%, and bromfenac 0.09% on postoperative inflammation using laser flare photometry in patients having phacoemulsification. Journal of Cataract and Refractive Surgery 2015;41(10):2043‐8. [DOI] [PubMed] [Google Scholar]
Schmitt 1995 {published data only}
- Schmitt TH. Anti‐inflammatory therapy after cataract surgery — a laser‐flare‐cell photometer controlled study [Zur antiinflammatorischen therapie nach kataraktchirurigie. Eine kontrollierte studie mittels Laser‐Flare‐Cell‐Photometrie]. Aktuelle Augenheilkunde 1995;20(5):263‐8. [Google Scholar]
Shimazaki 1996 {published data only}
- Shimazaki J, Fujishima H, Yagi Y, Tsubota K. Effects of diclofenac eye drops on corneal epithelial structure and function after small‐incision cataract surgery. Ophthalmology 1996;103(1):50‐7. [DOI] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh R, Alpern L, Jaffe GJ, Lehmann RP, Lim J, Reiser HJ, et al. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clinical Ophthalmology 2012;2012(6):1259‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2001 {published data only}
- Solomon KD, Vroman DT, Barker D, Gehlken J. Comparison of ketorolac tromethamine 0.5% and rimexolone 1% to control inflammation after cataract extraction. Journal of Cataract and Refractive Surgery 2001;27(8):1232‐7. [DOI] [PubMed] [Google Scholar]
Ticly 2014 {published data only}
- Ticly FG, Lira RP, Zanetti FR, Machado MC, Rodrigues GB, Arieta CE. Prophylactic use of ketorolac tromethamine in cataract surgery: a randomized trial. Journal of Ocular Pharmacology and Therapeutics 2014;30(6):495‐501. [DOI] [PubMed] [Google Scholar]
Trinavarat 2003 {published data only}
- Trinavarat A, Atchaneeyasakul L, Surachatkumtonekul T, Kosrirukvongs P. Comparison of topical prednisolone acetate, ketorolac tromethamine and fluorometholone acetate in reducing inflammation after phacoemulsification. Journal of the Medical Association of Thailand 2003;86(2):143‐50. [PubMed] [Google Scholar]
Tzelikis 2015 {published data only}
- Tzelikis PF, Vieira M, Hida WT, Motta AF, Nakano CT, Nakano EM, et al. Comparison of ketorolac 0.4% and nepafenac 0.1% for the prevention of cystoid macular oedema after phacoemulsification: prospective placebo‐controlled randomised study. British Journal of Ophthalmology 2015;99(5):654‐8. [DOI] [PubMed] [Google Scholar]
Voudouri 2002 {published data only}
- Voudouri A, Zafirakis P, Livir‐Rallatos G, Livir‐Rallatos C, Canakis C, Markomichelakis N. Diclofenac versus prednisolone post phacoemulsification treatment: a randomized clinical trial. Investigative Ophthalmology & Visual Science 2002; Vol. 43:ARVO E‐Abstract 373.
Wang 2013 {published data only}
- Wang QW, Yao K, Xu W, Chen PQ, Shentu XC, Xie X, et al. Bromfenac sodium 0.1%, fluorometholone 0.1% and dexamethasone 0.1% for control of ocular inflammation and prevention of cystoid macular edema after phacoemulsification. Ophthalmologica 2013;229(4):187‐94. [DOI] [PubMed] [Google Scholar]
Wittpenn 2008 {published data only}
- Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, et al. A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low‐risk cataract surgery patients. American Journal of Ophthalmology 2008;146(4):554‐60. [DOI] [PubMed] [Google Scholar]
Yavas 2007 {published data only}
- Yavas GF, Oztürk F, Küsbeci T. Preoperative topical indomethacin to prevent pseudophakic cystoid macular edema. Journal of Cataract and Refractive Surgery 2007;33(5):804‐7. [DOI] [PubMed] [Google Scholar]
Zaczek 2014 {published data only}
- Zaczek A, Artzen D, Laurell CG, Stenevi U, Montan P. Nepafenac 0.1% plus dexamethasone 0.1% versus dexamethasone alone: effect on macular swelling after cataract surgery. Journal of Cataract and Refractive Surgery 2014;40(9):1498‐505. [DOI] [PubMed] [Google Scholar]
Zanetti 2012 {published data only}
- Zanetti FR, Fulco EA, Chaves FR, Costa Pinto AP, Arieta CE, Lira RP. Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. Indian Journal of Ophthalmology 2012;60(4):277‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2008 {published data only}
- Zhang HY, Zhu SQ. Clinical study of pranoprofen eyedrops on prophylaxis of cystoids macular edema after cataract surgery. International Journal of Ophthalmology 2008;8(7):1370‐2. [Google Scholar]
References to studies excluded from this review
Abelson 1989 {published data only}
- Abelson MB, Smith LM, Ormerod LD. Prospective, randomized trial of oral piroxicam in the prophylaxis of postoperative cystoid macular edema. Journal of Ocular Pharmacology 1989;5(2):147‐53. [DOI] [PubMed] [Google Scholar]
Bahar 2007 {published data only}
- Bahar I, Rosenblat I, Erenberg M, Eldar I, Gaton D, Avisar R, et al. Effect of dexamethasone eyedrops on blood glucose profile. Current Eye Research 2007;32(9):739‐42. [DOI] [PubMed] [Google Scholar]
Barequet 2002 {published data only}
- Barequet IS, Wygnanski‐Jaffe T, Sachs D. Effect on posterior capsule opacification of topical diclofenac sodium vs dexamethasone phosphate after cataract surgery. Annals of Ophthalmology 2002;34(2):142‐7. [Google Scholar]
Barequet 2004 {published data only}
- Barequet IS. Posterior capsule opacification: The effect of postoperative topical therapy with Voltaren vs. Maxidex after cataract surgery. American Academy of Ophthalmology 1999, issue AAO abstract 244.
- Barequet IS, Wygnanski‐Jaffe T, Sachs D. Effect of diclofenac versus dexamethasone on posterior capsule opacification. Journal of Cataract and Refractive Surgery 2004;30(11):2253‐4. [DOI] [PubMed] [Google Scholar]
Burde 1972 {published data only}
- Burde RM, Waltman SR. Topical corticosteroids after cataract surgery. Annals of Ophthalmology 1972;4(4):290‐3. [PubMed] [Google Scholar]
Carenini 1993 {published data only}
- Carenini BB, Brancato R, Molfetta V, Frezzotti R, Balestrazzi E, Cardia L, et al. Indomethacin ophthalmic suspension in the prevention of cystoid macular oedema in cataract extraction patients [L'Indometacina, sospensione oftalmica, nella prevenzione dell'edema maculare cistoide in pazienti sottoposti ad estrazione di cataratta]. Annali di Ottalmologia e Clinica Oculistica 1993;119(9):681‐7. [Google Scholar]
Chang 2016 {published data only}
- Chang A, Arnold J, Davies P, Heriot W, Adewale A, Jaross N. Efficacy and safety of nepafenac 0.3% ophthalmic suspension administered once‐daily in patients with diabetic retinopathy following cataract surgery. Clinical and Experimental Ophthalmology 2016;44(Suppl 1):57‐8. [Google Scholar]
Corbett 1993 {published data only}
- Corbett MC, Hingorani M, Boulton JE, Shilling JS. Subconjunctival betamethasone is of benefit after cataract surgery. Eye 1993;7(6):744‐8. [DOI] [PubMed] [Google Scholar]
EUCTR2009‐017031‐18‐NL {unpublished data only}
- EUCTR2009‐017031‐18‐NL. Treatment of cystoid macular edema following cataract surgery. A randomized, double‐masked, placebo‐controlled, clinical trial — treatment of post‐cataract CME. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐017031‐18‐NL (accessed 5 October 2016).
EUCTR2015‐003296‐30‐FI {unpublished data only}
- EUCTR2015‐003296‐30‐FI. Efficacy of steroid and nonsteroidal anti‐inflammatory eye drops in management of post‐operative inflammation and prevention of pseudophakic cystic macular edema after uncomplicated cataract surgery. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015‐003296‐30‐FI (accessed 5 October 2016).
Farooq 2003 {published data only}
- Farooq Q, Ali SI. Comparison of anti‐inflammatory activity of ofloxacin + dexamethasone eye drops and flurbiprofen eye drops in post operative cases. Pakistan Journal of Ophthalmology 2003;19(4):122‐9. [Google Scholar]
Ferreira 2006 {published data only}
- Ferreira PC, Bauer Koerich LC, Lima GS, Ramalho F, Figueiredo AM. Influence of prednisolone acetate 1% and ketorolac 0.5% eyedrops in endothelial cell loss after cataract surgery. American Academy of Ophthalmology; 2006 Nov 11‐14; Las Vegas (NV). 2006.
Hessemer 1994 {published data only}
- Hessemer V, Schmitt K. Local treatment with steroids and/or non‐steroid antiphlogistic agents in cataract surgery? A lasertyndallometric answer [Lokaltherapie mit Steroiden und/oder nichtsteroidalen Antiphlogistika in der Kataraktchirurgie? Eine lasertyndallometrische Antword]. Klinische Monatsblatter Fur Augenheilkunde 1994;204:186. [Google Scholar]
Hossain 2010 {published data only}
- Hossain MM, Mohiuddin AA, Hossain MA, Aziz MA. Diclofenac sodium and prednisolone acetate ophthalmic solution in controlling inflammation after cataract surgery. Mymensingh Medical Journal 2010;19(3):343‐7. [PubMed] [Google Scholar]
Hosseini 2016 {published data only}
- Hosseini K, Walters T, DaVanzo R, Lindstrom RL. A randomized double‐masked study to compare the ocular safety, tolerability, and efficacy of bromfenac 0.075% compared with vehicle in cataract surgery subjects. Clinical Ophthalmology 2016;10:2311‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN02628492 {unpublished data only}
- ISRCTN02628492. Prevention of pseudophakic cystoid macula oedema with pre‐ and post‐operative ketorolac. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN02628492 (accessed 5 October 2016).
Knopf 1970 {published data only}
- Knopf MM. A double‐blind study of fluorometholone. American Journal of Ophthalmology 1970;70:739‐40. [DOI] [PubMed] [Google Scholar]
Kraff 1990 {published data only}
- Kraff MC, Sanders DF, McGuigan L, Raanan MG. Inhibition of blood‐aqueous humor barrier breakdown with diclofenac. Archives of Ophthalmology 1990;7(1):380‐3. [DOI] [PubMed] [Google Scholar]
Liou 1991 {published data only}
- Liou SW, Yen RJ. The effect of 0.1% indomethacin eyedrops on cataract surgery. Journal of Ocular Pharmacology 1991;7(1):77‐81. [DOI] [PubMed] [Google Scholar]
Luo 2013 {published data only}
- Luo Y, Feng J, Lu M, Cheng XK. Clinical assessment of diclofenac sodium eye drops in toric intraocular lens implantation. International Eye Science 2013;13(5):880‐2. [Google Scholar]
Maheshwari 1995 {published data only}
- Maheshwari R, Ather M, Bhandari V. A study on comparative efficacy of topical non‐steroidal anti inflammatory drug, flurbiprofen and corticosteroids in post operative ocular inflammation. Afro‐Asian Journal of Ophthalmology 1995;14(1):12‐4. [Google Scholar]
McDonald 1998 {published data only}
- McDonald MB, Schanzlin D. Diclofenac Sodium (DS) versus Prednisolone Acetate (PA) in the treatment of inflammation following cataract extraction. American Academy of Ophthalmology 1998:147.
Meconi 1998 {published data only}
- Meconi S, Scorolli L, Scalinci SZ, Massimi M, Meduri R. A comparison of topical diclofenac with indomethacin for post‐phacoemulsification CME (cystoid macular edema) [Comparazione dell'utilizzo del diclofenac e dell'indometacina nell'emc post‐facoemulsificazione]. Annali di Ottolmaologia e Clinica Oculistica 1998;124(11‐12):357‐65. [Google Scholar]
Missotten 2001 {published data only}
- Missotten L, Richard C, Trinquand C. Topical 0.1% indomethacin solution versus topical 0.1% dexamethasone solution in the prevention of inflammation after cataract surgery. Ophthalmologica 2001;215(1):43‐50. [DOI] [PubMed] [Google Scholar]
Miyake 1998 {published data only}
- Miyake K, Masuda K, Shirato S, Oshika T, Equchi K, Hoshi H, et al. Preventive effects of diclofenac ophthalmic solution on post‐cataract‐surgical cystoid macular edema. Nippon Ganka Gakkai Zasshi 1998;102(8):522‐30. [PubMed] [Google Scholar]
Miyake 2000 {published data only}
- Miyake K, Masuda K, Shirato S, Oshika T, Egucha K, Hoshi H, et al. Comparison of diclofenac and fluorometholone in preventing cystoid macular edema after small incision cataract surgery: a multicentered prospective trial. Japanese Journal of Ophthalmology 2000;44(1):58‐67. [DOI] [PubMed] [Google Scholar]
Mullins 2016 {published data only}
- Mullins A, Meijer P, Airey R, Gunda P, Banhazi J. Cost‐effectiveness of nepafenac 3 mg/ml for the reduction in risk of post‐operative macular oedema associated with cataract surgery: from UK NHS perspective. Value in Health 2016;19(7):A569‐70. [Google Scholar]
NCT00407017 {unpublished data only}
- NCT00407017. Therapeutic variables in cataract surgery. clinicaltrials.gov/ct2/show/NCT00407017 (accessed 5 October 2016).
NCT00433225 {unpublished data only}
- NCT00433225. Efficacy of topical ketorolac versus placebo for improving visual outcomes following multifocal IOL implantation. clinicaltrials.gov/ct2/show/NCT00433225 (accessed 5 October 2016).
NCT00698724 {unpublished data only}
- NCT00698724. Comparing optical coherence tomography (OCT) and visual acuity outcomes in subjects undergoing cataract surgery, who receive xibrom ophthalmic solution and standard presurgical care vs. xibrom ophthalmic solution plus prednisolone acetate 1% and standard presurgical care. clinicaltrials.gov/ct2/show/NCT00698724 (accessed 5 October 2016).
NCT00758199 {unpublished data only}
- NCT00758199. Determination of optimum duration of treatment with bromfenac (xibrom) eyedrops following cataract surgery. clinicaltrials.gov/ct2/show/NCT00758199 (accessed 5 October 2016).
NCT01193504 {unpublished data only}
- NCT01193504. Randomized, masked comparison of bromfenac and besifloxacin BID with either prednisolone BID or loteprednol 0.5% BID for prevention of retinal thickening and CME following phacoemulsification. clinicaltrials.gov/ct2/show/NCT01193504 (accessed 5 October 2016).
Nishino 2009 {published data only}
- Nishino M, Equchi H, Iwata A, Shiota H, Tanaka M, Tanaka T. Are topical steroids essential after an uneventful cataract surgery?. Journal of Medical Investigation 2009;56(1‐2):11‐5. [DOI] [PubMed] [Google Scholar]
Othenin‐Girard 1994 {published data only}
- Othenin‐Girard P, Tritten JJ, Pittet N, Herbort CP. Dexamethasone versus diclofenac sodium eyedrops to treat inflammation after cataract surgery. Journal of Cataract and Refractive Surgery 1994;20(1):9‐12. [DOI] [PubMed] [Google Scholar]
Ozkurt 2003 {published data only}
- Ozkurt Y, Oral Y. Comparison of postoperative anti‐inflammatory effects of ketorolac 0.5% and prednisolone 1% after phacoemulsification and intraocular lens implantation. SOE 2003: XIV Congress of the European Society of Ophthalmology 2003:305‐9. [Google Scholar]
Pollack 2017 {published data only}
- Pollack A, Staurenghi G, Sager D, Mukesh B, Reiser H, Singh RP. Prospective randomised clinical trial to evaluate the safety and efficacy of nepafenac 0.1% treatment for the prevention of macular oedema associated with cataract surgery in patients with diabetic retinopathy. British Journal of Ophthalmology 2017;101:423‐7. [DOI] [PubMed] [Google Scholar]
Raju 2005 {published data only}
- Raju AS, Raju NS, Rajendran B, Paju B. Prospective randomized controlled trial between diclofenac sodium and prednisolone acetate eye drops in cataract surgery. American Academy of Ophthalmology; 2005 Oct 15‐18; Chicago (IL). 2005.
Ramakrishnan 2015 {published data only}
- Ramakrishnan S, Baskaran P, Talwar B, Venkatesh R. Prospective, randomized study comparing the effect of 0.1% nepafenac and 0.4% ketorolac tromethamine on macular thickness in cataract surgery patients with low risk for cystoid macular edema. Asia‐Pacific Journal of Ophthalmology 2015;4(4):216‐20. [DOI] [PubMed] [Google Scholar]
Rocha 2009 {published data only}
- Rocha G. A randomized, masked comparison of topical ketorolac 0.4% plus steroid alone in low‐risk cataract surgery patients. Evidence‐Based Ophthalmology 2009;10(2):92‐3. [DOI] [PubMed] [Google Scholar]
Sabiston 1987 {published data only}
- Sabiston DW, Robinson IG. An evaluation of the anti‐inflammatory effect of flurbiprofen after cataract extraction. British Journal of Ophthalmology 1987;71(6):418‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smerdon 1986 {published data only}
- Smerdon DL, Hung SO, Akingbehin T. Double‐blind controlled trial to compare anti‐inflammatory effects of tolmetin 2%, prednisolone 0.5%, and placebo in post‐cataract extraction eyes. British Journal of Ophthalmology 1986;70(10):761‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sourdille 1993 {published data only}
- Sourdille P, Zanlonghi X, Bron V, Allaire C, Trinquand C. Comparison of prednisolone and topical indomethacin in post‐operative inflammation. European Journal of Implant and Refractive Surgery 1993;5(3):1959‐63. [Google Scholar]
Suharwardy 1994 {published data only}
- Suharwardy J, Ling C, Bell JA, Munton CG. A comparative trial between diclofenac‐gentamicin and bethamethasone‐neomycin drops in patients undergoing cataract extraction. Eye 1994;8(4):550‐4. [DOI] [PubMed] [Google Scholar]
Szymanski 1994 {published data only}
- Szymański A, Gierek‐Lapińska A, Koziak M. Influence of steroidal and nonsteroidal anti‐inflammatory drugs on post‐operative blood‐aqueous barrier in pseudophakia. Klinika Oczna 1993;96(4‐5):145‐7. [PubMed] [Google Scholar]
Tauber 2006 {published data only}
- Tauber S, Gessler J, Scott W, Peterson C, Hamlet P. The effect of topical ketorolac 0.4% on cystoid macular edema following routine cataract surgery. Investigative Ophthalmology & Visual Science 2006; Vol. 47:ARVO E‐Abstract 683.
Tunc 1999 {published data only}
- Tunc M, Saatci OA, Ergin MH, Ergin S. Cystoid macular edema early after uncomplicated cataract surgery: the efficacy of diclofenac in prophylaxis. Annals of Ophthalmology ‐ Glaucoma 1999;31(1):27‐32. [Google Scholar]
Turan‐Vural 2013 {published data only}
- Turan‐Vural E, Halili E, Serin D. Assessing the effect of ketorolac and acetazolamide on macular thickness by optical coherence tomography following cataract. International Ophthalmology 2013;34(3):525‐31. [DOI] [PubMed] [Google Scholar]
Waseem 2009 {published data only}
- Waseem M, Humayun S. Comparison of anti‐inflammatory activity of dexamethasone and diclofenac sodium eye drops in phacoemulsification. Journal of the College of Physicians and Surgeons Pakistan 2009;19(9):570‐4. [PubMed] [Google Scholar]
Yasuda 2016 {published data only}
- Yasuda K, Motohashi R, Kotake O, Nakagawa H, Noma H, Shimura M. Comparative effects of topical diclofenac and betamethasone on inflammation after vitrectomy and cataract surgery in various vitreoretinal diseases. Journal of Ocular Pharmacology and Therapeutics 2016;32(10):677‐84. [DOI] [PubMed] [Google Scholar]
Yung 2007 {published data only}
- Yung CW, Hui SL, Wang J, Gao H, Peracha MO, Pratt LM, et al. The effect of topical ketorolac tromethamine 0.5% on macular thickness in diabetic patients after cataract surgery. American Academy of Ophthalmology; 2007 Nov 10‐13; New Orleans (LA). 2007.
References to studies awaiting assessment
ChiCTR‐TRC‐12002600 {unpublished data only}
- ChiCTR‐TRC‐12002600. Bromfenac sodium 0.1%, fluorometholone 0.1% and dexamethasone 0.1% for control of ocular inflammation and prevention of cystoid macular edema after phacoemulsification. www.chictr.org.cn/showprojen.aspx?proj=6951 (accessed 5 October 2016).
Coassin 2016 {published data only}
- Coassin M, Iovieno A, Soldani A, Cavuto S, Cimino L, Sartori A, et al. Bromfenac ophthalmic solution 0.09% as an adjunctive therapy to topical steroids after cataract surgery in pseudoexfoliation syndrome. Journal of Cataract and Refractive Surgery 2016;42(8):1119‐25. [DOI] [PubMed] [Google Scholar]
Jirásková 2000 {published data only}
- Jirásková N, Rozsíval P, Liláková D, Klimešová J. Nonsteroidal anti‐inflammatory agents after cataract surgery [Nesteroidní antiflogistika po operaci katarakty]. Ceská a slovenská oftalmologie : casopis Ceské oftalmologické spolecnosti a Slovenské oftalmologické spolecnosti 2000;56(3):176‐9. [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu SC, Wang LQ. Study on the preventive effect of 1g/L bromfenac sodium hydrate ophthalmic solution on macular edema after cataract surgery. International Eye Science 2016;16(12):2250‐3. [Google Scholar]
Malik 2016 {published data only}
- Malik A, Sadafale A, Gupta YK, Gupta A. A comparative study of various topical nonsteroidal anti‐inflammatory drugs to steroid drops for control of post cataract surgery inflammation. Oman Journal of Ophthalmology 2016;9(3):150‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00366691 {unpublished data only}
- NCT00366691. Comparison of Acular LS with Lotemax to prevent inflammation after cataract surgery and intraocular lens implantation. clinicaltrials.gov/ct2/show/NCT00366691 (accessed 16 June 2017).
NCT00992355 {unpublished data only}
- NCT00992355. Tobramycin‐dexamethasone versus tobramycin‐dexamethasone plus ketorolac after phacoemulsification surgery. clinicaltrials.gov/ct2/show/NCT00992355 (accessed 16 June 2017).
Stringa 1996 {published data only}
- Stringa A, Iato MS, Pasinetti GM, Reposi S. Anti‐inflammatory non steroidal and steroidal drugs: their effects on postsurgical inflammation [Antiinfiammatori non steroidei e steroidei: effetti sull'infiammazione postchirurgica]. Annali Di Ottalmologia e Clinica Oculistica 1996;122:125‐31. [Google Scholar]
References to ongoing studies
NCT01774474 {unpublished data only}
- NCT01774474. PRevention of Macular EDema after cataract surgery (PREMED). clinicaltrials.gov/ct2/show/NCT01774474 (accessed 16 June 2017).
Pollack 2016 {published data only}
- Pollack A, Lehmann R, Staurenghi G, Narvekar A, Adewale A, Singh R. Clinical efficacy and safety of nepafenac 0.3% administered once‐daily in patients with diabetic retinopathy following cataract surgery: outcomes from two identical, randomised, phase 3 studies. Ophthalmologica 2016;236:11. [Google Scholar]
Additional references
Barnes 2006
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. British Journal of Pharmacology 2006;148(3):245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colin 2007
- Colin J. The role of NSAIDs in the management of postoperative ophthalmic inflammation. Drugs 2007;67(9):1291‐308. [DOI] [PubMed] [Google Scholar]
Congdon 2004
- Congdon N, Vingerling JR, Klein BE, West S, Friedman DS, Kempen J, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Archives of Ophthalmology 2004;122(4):487‐94. [DOI] [PubMed] [Google Scholar]
DeCroos 2008
- DeCroos FC, Afshari NA. Perioperative antibiotics and anti‐inflammatory agents in cataract surgery. Current Opinion in Ophthalmology 2008;19(1):22‐6. [DOI] [PubMed] [Google Scholar]
Donnenfeld 2007
- Donnenfeld ED, Holland EJ, Stewart RH, Gow JA, Grillone LR, Bromfenac Ophthalmic Solution 0.09% (Xibrom) Study Group. Bromfenac ophthalmic solution 0.09% (Xibrom) for postoperative ocular pain and inflammation. Ophthalmology 2007;114(9):1653‐62. [DOI] [PubMed] [Google Scholar]
Flach 1987
- Flach AJ, Dolan BJ, Irvine AR. Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution for chronic aphakic and pseudophakic cystoid macular edema. American Journal of Ophthalmology 1987;103(4):479‐86. [DOI] [PubMed] [Google Scholar]
Flach 1988
- Flach AJ, Lavelle CJ, Olander KW, Retzlaff JA, Sorenson LW. The effect of ketorolac tromethamine solution 0.5% in reducing postoperative inflammation after cataract extraction and intraocular lens implantation. Ophthalmology 1988;95(9):1279‐84. [DOI] [PubMed] [Google Scholar]
Flach 1991
- Flach AJ, Jampol LM, Weinberg D, Kraff MC, Yannuzzi LA, Campo RV, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. American Journal of Ophthalmology 1991;112(5):514‐9. [DOI] [PubMed] [Google Scholar]
Flach 2002
- Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. International Ophthalmology Clinics 2002;42(1):1‐11. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 10 January 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guidera 2001
- Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical non‐steroidal anti‐inflammatory drugs. Ophthalmology 2001;108(5):936‐44. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirneiss 2005
- Hirneiss C, Neubauer AS, Kampik A, Schönfeld CL. Comparison of prednisolone 1%, rimexolone 1% and ketorolac tromethamine 0.5% after cataract extraction: a prospective, randomized, double‐masked study. Graefe's Archives of Clinical and Experimental Ophthalmology 2005;243(8):768‐73. [DOI] [PubMed] [Google Scholar]
Jabs 2005
- Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American Journal of Ophthalmology 2005;140(3):509‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessel 2014
- Kessel L, Tendal B, Jorgensen KJ, Erngaard D, Flesner P, Andresen JL, et al. Post‐cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti‐inflammatory eye drops: a systematic review. Ophthalmology 2014;121(10):1915‐24. [DOI] [PubMed] [Google Scholar]
Kim 2015
- Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti‐inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2015;122(11):2159‐68. [DOI] [PubMed] [Google Scholar]
Lane 2007
- Lane SS, Modi SS, Lehmann RP, Holland EJ. Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. Journal of Cataract and Refractive Surgery 2007;33(1):53‐8. [DOI] [PubMed] [Google Scholar]
Lim 2016
- Lim BX, Lim CHL, Lim DK, Evans JR, Bunce C, Wormald R. Prophylactic non‐steroidal anti‐inflammatory drugs for the prevention of macular oedema after cataract surgery. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD006683.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McColgin 2000
- McColgin AZ, Heier JS. Control of intraocular inflammation associated with cataract surgery. Current Opinion in Ophthalmology 2000;11(1):3‐6. [DOI] [PubMed] [Google Scholar]
Needleman 1986
- Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annual Reviews in Biochemistry 1986;55(1):69‐102. [DOI] [PubMed] [Google Scholar]
Review Manager 5 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossetti 1996
- Rossetti L, Bujtar E, Castoldi D, Torrazza C, Orzalesi N. Effectiveness of diclofenac eyedrops in reducing inflammation and the incidence of cystoid macular edema after cataract surgery. Journal of Cataract and Refractive Surgery 1996;22(Suppl 1):794‐9. [DOI] [PubMed] [Google Scholar]
Sharir 1997
- Sharir M. Exacerbation of asthma by topical diclofenac. Archives of Ophthalmology 1997;115(2):294‐5. [DOI] [PubMed] [Google Scholar]
Sivaprasad 2012
- Sivaprasad S, Bunce C, Crosby‐Nwaobi R. Non‐steroidal anti‐inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004239.pub3] [DOI] [PubMed] [Google Scholar]
Snyder 2000
- Snyder RW, Siekert RW, Schwiegerling J, Donnenfeld E, Thompson P. Acular as a single agent for use as an antimiotic and anti‐inflammatory in cataract surgery. Cataract and Refractive Surgery 2000;26(8):1225‐7. [DOI] [PubMed] [Google Scholar]
Solomon 1995
- Solomon LD. Efficacy of topical flurbiprofen and indomethacin in preventing pseudophakic cystoid macular edema. Journal of Cataract and Refractive Surgery 1995;21(1):73‐81. [DOI] [PubMed] [Google Scholar]
Vane 1996
- Vane JR, Botting RM. Mechanism of action of anti‐inflammatory drugs. Scandinavian Journal of Rheumatology, Supplement 1996;25(Suppl 102):9‐21. [DOI] [PubMed] [Google Scholar]
Vane 1998
- Vane JR, Botting RM. Anti‐inflammatory drugs and their mechanism of action. Inflammation Research 1998;47(2):S78‐S87. [DOI] [PubMed] [Google Scholar]
Wielders 2015
- Wielders LH, Lambermont VA, Schouten JS, Biggelaar FJ, Worthy G, Simmons RW, et al. Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta‐analysis. American Journal of Ophthalmology 2016;160(5):968‐81. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gonzales 2013
- Gonzales JA, Gritz DC, Channa R, Quinto GG, Kim A, Chuck RS. Non‐steroidal anti‐inflammatory drugs versus corticosteroids for controlling inflammation after uncomplicated cataract surgery. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD010516] [DOI] [PMC free article] [PubMed] [Google Scholar]


