Abstract
Purpose
This report describes initiation of apheresis capability in Liberia, Africa to support a clinical trial of convalescent plasma therapy for Ebola Virus Disease.
Methods
A bloodmobile was outfitted in the United States as a four-bed apheresis unit with capabilities including pathogen reduction, electronic blood establishment computer system, designated areas for donor counseling and laboratory testing, and onboard electrical power generation. After air transport to Liberia, the bloodmobile was positioned at ELWA Hospital, Monrovia, and connected to the hospital’s power grid. Liberian staff were trained to conduct donor screening, which included questionnaire and onsite blood typing and transfusion transmitted infection (TTI) testing, and plasma collection and processing.
Results
The bloodmobile was operational within three weeks after arrival of the advance team. Of 101 donors who passed the pre-screening questionnaire, 32 were deferred. Twenty-eight of 99 tested survivors were deferred for positive transfusion transmitted infection (TTI) tests; 21 were positive for hepatitis B, hepatitis C, or human immunodeficiency virus. The majority of donors had type O blood; all but one were Rh positive. Forty-three survivors donated at least once; 89 apheresis attempts resulted in 81 successful collections.
Conclusions
Apheresis capability was emergently established in Liberia to support an efficacy trial of Ebola Convalescent Plasma. Extensive cooperation among multinational team members, engineers, logisticians and blood safety technical personnel at the operational site was required to surmount challenges to execution posed by logistical factors. The high proportion of positive TTI tests supported the use of a pathogen reduction system to enhance product safety.
Keywords: survivor, blood groups, pathogen reduction, bloodmobile
INTRODUCTION
The Ebola Virus Disease (EVD) outbreak that ravaged West Africa in 2014–2015 caught the world unprepared for an effective clinical and research response. Although EVD had been recognized since 1976 and there had been at least 25 naturally occurring outbreaks since then,[1] there were no known effective treatments. In 2014, the health services of the severely affected countries of Guinea, Liberia and Sierra Leone were overwhelmed and medical intervention was limited. Typically, treatment consisted of fluid and electrolyte replacement, usually administered orally, with no clinical laboratory testing for guidance [2–5]. Oral administration was frequently preferred due to high caseloads and limited resources, including personnel and limited time to work in personal protective equipment[2].
Few candidate therapies were available. Repurposed and novel small-molecule antiviral drugs were urgently tested in in-vitro systems and progressively in rodent and non-human primate (NHP) models, but none had a convincingly positive therapeutic profile in the latter model [6]. Monoclonal antibody cocktails, for which efficacy in NHP models had been established up to two years previously, were in extremely short supply and had not undergone early phase testing[7].
A report from the 1995 EVD outbreak in Kikwit, Zaire (Democratic Republic of Congo since 1997),[8] suggested that administration of whole blood from EVD survivors might improve survival, but the uncontrolled nature of the data and limited number of treated patients (N = 8), precluded firm conclusions. If effective, convalescent blood would presumably confer benefit via anti-EVD immunoglobulins. Passive immunization with Ebola-immune globulin was demonstrated to improve outcome in NHP in 2012[9]. Although no conclusive evidence of efficacy of Ebola convalescent plasma (ECP) for treatment of EVD in humans was available, several patients repatriated to the US received ECP in addition to fluid management and intensive supportive care at a resource level not available in the severely affected countries in West Africa.[10, 11]. Additional treatments, including Zmapp, brincidofovir, siRNA, and favapiravir, were deployed with uncertain effectiveness as rapidly as feasible in affected countries in the hope of curbing the spread and decreasing mortality[11]. In September 2014, the World Health Organization (WHO) issued guidelines for collection and administration of ECP as an experimental therapy[12].
The use of ECP faced multiple challenges: development and ethical implementation of clinical trial designs to assess efficacy; rapid deployment of ECP collection in countries with limited blood collection or blood safety capacity; consistent production of safe blood components under suitable cold chain conditions; and prevention of harm to or exploitation of donors or clinical trial participants. The most significant was defining a statistically realistic but humane ‘control’ group. Despite evident challenges, three clinical trials of ECP were initiated in response to the WHO call, one each in Liberia, Guinea and Sierra Leone[13, 14]. This report describes establishment of capability for collection of apheresis plasma in Liberia to support implementation of the first ECP trial in West Africa during the 2014–2015 outbreak and results of donor screening and collection from the first 100 survivors who consented to donate plasma.
METHODS
The blood banking and plasma collection community; academic, governmental and non-governmental organizations and health care institutions; and philanthropic organizations provided crucial support for the trial. ClinicalRM, a contract research organization headquartered in Hinckley, OH, sponsored the trial and initiated protocol development in August 2014. These collaborative efforts enabled rapid design of one protocol for ECP collection from survivors and another for a therapeutic trial in patients with acute EVD. Protocols were approved by ethics committees of the participating US academic institutions (University of North Carolina and Duke University), the University of Liberia Pacific Institute for Research and Evaluation (PIRE) and the Liberia Medicines and Health Products Regulatory Authority. The clinical trial and plasma collection were conducted according to Good Clinical Practice (GCP) using the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines and applicable laws and regulations. Informed, written consent was obtained from all participating subjects.
ECP was collected at the Eternal Love Winning Africa (ELWA) Hospital near Monrovia, Liberia, to support a clinical trial of convalescent plasma efficacy and safety (clinical trials.gov NCT02333578), designed in accordance with WHO recommendations[12]. Dr. Jerry Brown, the ELWA Medical Director, was Principal Investigator. This report describes the implementation of apheresis capability and results of screening and ECP collection from December 2014 to May 2015.
Objectives and Eligibility
The primary study objective was to pre-qualify potential donors for ECP donation and collect ECP by apheresis for clinical trials or compassionate use. The secondary objective was to assess quantitative and qualitative changes in anti-EBOV antibody in EVD survivors over a period of one year. The eligible population comprised adult EVD survivors (diagnosis established by RT-PCR) who had onset of disease ≤ 2 years and ≥ 60 days prior to donation, and had been discharged ≥ 28 days previously from an Ebola Treatment Unit (ETU). Additional eligibility requirements included good general health, two negative RT-PCR blood tests for EVD at ≥ 48 hours apart; weight ≥ 50 kg; hemoglobin ≥ 12.0 g/dl; temperature ≤ 37.5°C; and negative test results for HIV-1 and HIV-2, hepatitis B, hepatitis C, malaria and syphilis.
Donor recruitment and screening
Donor recruitment, consent, initial screening and qualifying blood specimen collection were conducted at ELWA. Potential donors were identified by hospital staff from ELWA-2 discharge lists and contacted directly with study information. Safeguards to prevent coercion were included in the recruitment and consenting process. Prior to conduct of any study activities, individual counseling using an ICH-compliant, IRB-approved research consent process and written consent form were completed to minimize the risk of survivor coercion for participation. Subjects were screened for suitability using a modified detailed blood donation questionnaire[15]. Subjects underwent a standard focused physical examination. Blood specimens were collected for Ebola virus (EBOV) assays conducted at the Liberian Institute for Biomedical Research (LIBR) by RT-PCR under the direction of the U.S. Army Medical Research Institute of Infectious Disease (USAMRIID). Blood specimens were also collected for testing for transfusion-transmitted infections (TTIs) using rapid diagnostic tests (RDT) with a portion stored for future quantitative determination of binding and neutralizing anti-EBOV antibodies. Hemoglobin was measured from finger prick specimens using the CompoLab TS Hemoglobin Screening Device (Fenwal, a Fresenius Kabi company, Lake Zurich, IL). Urine beta-human chorionic gonadotropin was tested in women to ensure they were not pregnant. Blood Group was determined using GrifolsMD® cards. Subjects found to be TTI positive on initial screening were deferred, counselled in person by the PI, treated if appropriate, and referred for medical follow-up. All subjects were provided with a care package to include multivitamins, iron and folate, and with compensation sufficient to cover time and travel expenses.
ECP collection and donor care
Screening results from eligible donors were reviewed by the PI on the first return visit, (median 6 days, range 1 to 64 days). Prior to apheresis, potential donors were provided a meal, typically fish and rice. Donors were re-screened using the same questionnaire and procedures. In addition to a physical examination, potential donors were evaluated to exclude significant orthostatic drop in blood pressure. During the donation process, donors were encouraged to drink 500 ml Ca++ enriched water. Following plasma collection (650 ml), ECP was treated by pathogen reduction and split into six 100 ml aliquots, which were frozen within six hours of collection and managed as fresh frozen plasma (FFP)[16]. Fresh frozen plasma was not characterized for coagulation or protein factors, as these assays were not available in Liberia. Donors were eligible to return for repeat donation every 14 days, provided they continued to meet eligibility criteria and passed the pre- screening protocol. Compensation for time and travel was provided whether the donation was successful or not.
ECP collection equipment
Donation activities were conducted on the ELWA campus in a four-bed bloodmobile (Matthews Specialty Vehicles, Greensboro, NC) outfitted with apheresis machines (PCS2, Haemonetics Corporation, Braintree, MA), amotosalen/UV light pathogen reduction system (INTERCEPT, Cerus Corporation, Concord, CA), Fresenius sterile connection device (Compodock, Fresenius Kabi, Bad Homburg, Germany), blood establishment computer system (BECS, provided by BBCS, Auburn, Washington), and interview and laboratory space. Bloodmobiles were outfitted in the United States as apheresis units and airlifted to Liberia. Power was supplied by the primary hospital generators, with two back-up generators located on board the vehicle.
Data analysis
Data in this report were entered at the collection site and later retrieved from the BECS and exported to Microsoft Excel. Descriptive statistics were calculated using Microsoft Excel; confidence intervals for proportions were calculated at http://vassarstats.net/prop1.html.
RESULTS
The ECP efficacy trial was initiated in Liberia in December, 2014. ECP collection for purposes of this trial continued until May 2015, when enough ECP had been collected to meet potential requirements of the trial if the EVD outbreak should resume. Collection of ECP for production of immune globulin and follow up of donors in a longitudinal study continued beyond the dates included in this report.
Survivor characteristics
From 5 December 2014 to 30 April 2015, a total of 100 survivors were pre-qualified by history and consented to donate ECP. At first visit, survivors ranged in age from 19 to 67 years (34 ± 9 years) and weighed 69 ± 11 kg. Fifty-nine were males. Hemoglobin was tested in all 100 participants; 99 were tested for TTI and blood type. Hemoglobin was 13.8 ± 1.3 g/dl (14.2 ± 1.1 males, 13.2 ± 1.2 females). The majority of survivors were blood type O (N = 55, Figure 1). One donor was A negative; all the others were Rh positive.
Figure 1.
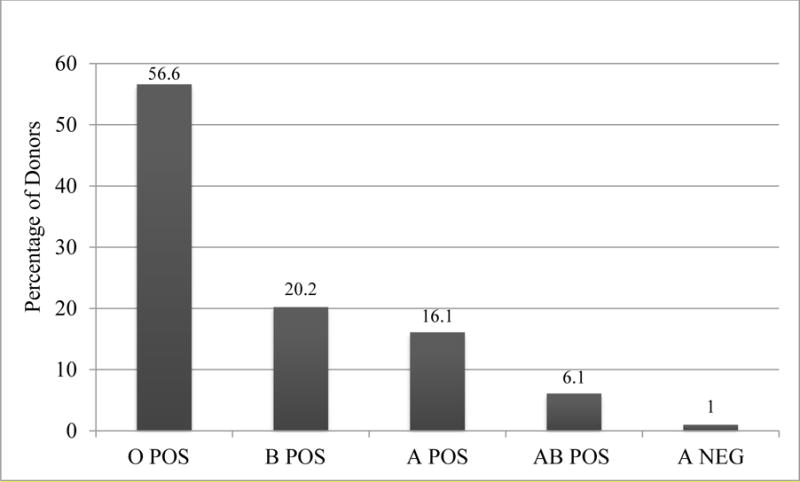
Distribution of ABO Groups among Screened Survivors
Deferrals
Disposition of the 100 enrolled participants (Figure 2) shows 99 provided specimens for TTI tests. Thirty-three were deferred prior to first donation. Twenty-eight were deferred for positive results of one or more TTI (Figures 2 and 3). Twenty-one (21.2%) of those tested were permanently deferred for positive HBV, HCV, or HIV tests. Two of the three survivors testing positive for syphilis were also positive for one of the permanently deferring tests; the other was positive for malaria. Six of the seven survivors positive for malaria were not positive for any other TTI.
Figure 2. Disposition of Screened Survivors and Collection Metrics1.
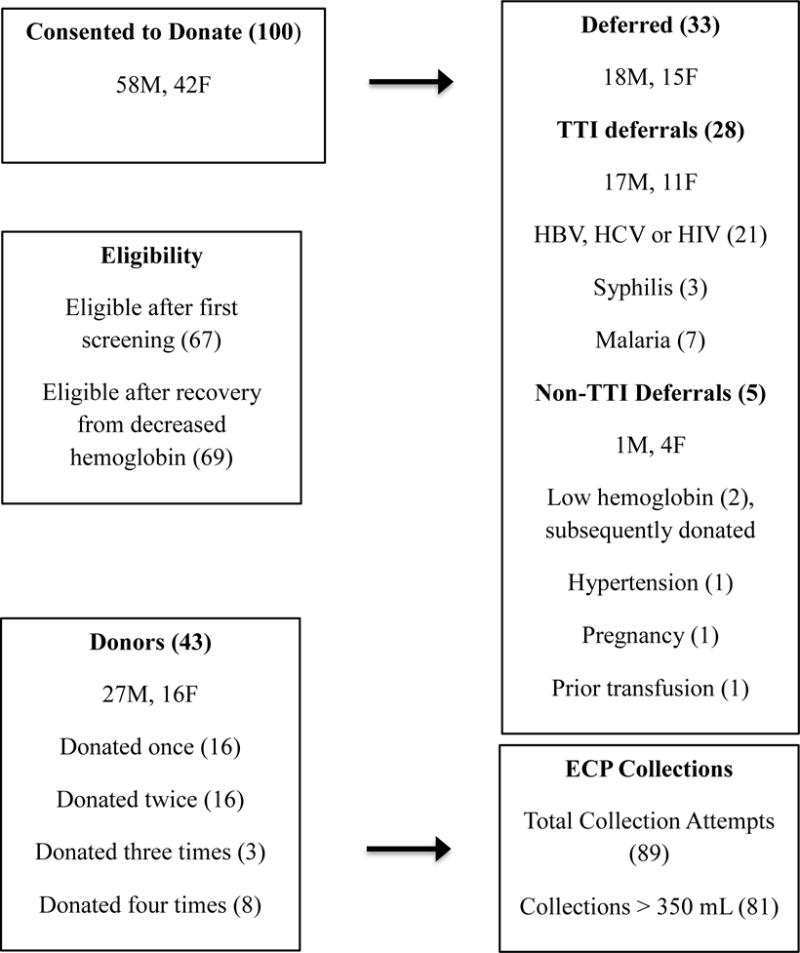
1Numbers in parentheses refer to total number of indicated participants or collection attempts. M = Male, F = Female
Figure 3.
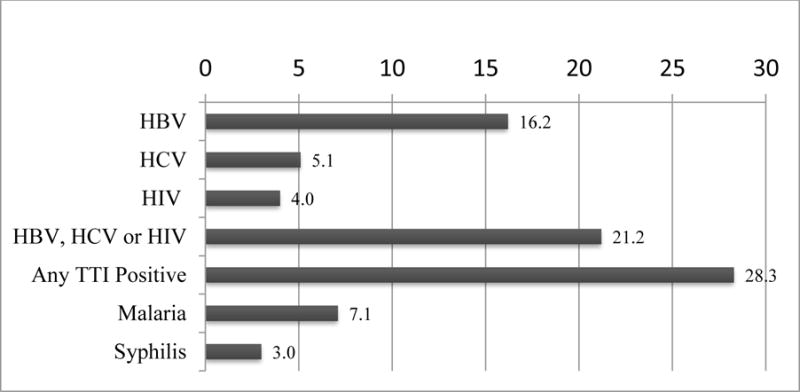
Percentage of Survivors with Positive Tests for TTI
Collections
Sixty-seven participants remained eligible after initial deferrals. Two of these, who were deferred for decreased hemoglobin, subsequently recovered and returned to donate (Figure 2). A total of 89 ECP collection attempts were made from 43 of these 69 eligible donors. Although donors were permitted to donate as often as every 14 days, the actual interval between donations ranged from 28 to 118 days, with a mean of 47 ± 20 days. A minimum of 350 ml of plasma, the volume required for subsequent processing in the pathogen reduction system, was collected and processed successfully in 81 (91.0%) attempts. Insufficient volume was collected in six attempts; two collections were not successfully processed.
Adverse effects
No adverse effects, including hypotensive episodes, were reported during the plasma collections. Analysis of hemoglobin levels of the 11 donors who donated at least three times showed no significant decline (Figure 4).
Figure 4.
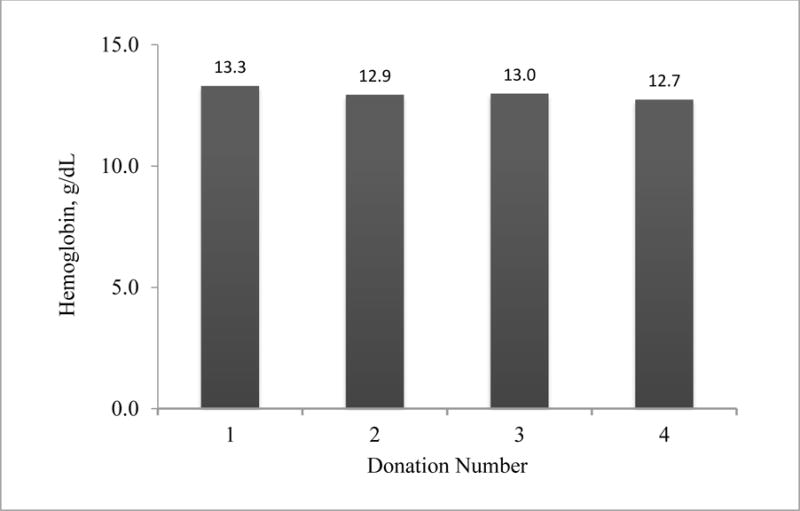
Hemoglobin Levels Prior to Repeated Donations
DISCUSSION
This report describes successful establishment of an emergency apheresis capability in Liberia during the 2014–2015 Ebola outbreak. The urgency of the Ebola crisis, limited functioning infrastructure and variable experience of local health care personnel informed our decisions on site selection, staffing, and logistics. The ELWA campus included ELWA Hospital, which was operational with limited capability throughout the outbreak, and ELWA-2, its associated ETU. The campus had independent primary and backup electrical generator power. Staff were well-trained in use of personal protective equipment (PPE) and had cared for numerous EVD patients in ELWA-2. Despite these important advantages of the ELWA site, there were some challenges. Initially, no suitable space with climate controlled work areas was available for the plasma collection process. Generator capability was marginal. Although provision of bloodmobiles outfitted with apheresis equipment mitigated many of these challenges, bloodmobiles alone did not provide a turnkey solution.
An advance, multi-national group of subject matter experts collaborated with onsite hospital technical staff to commission the apheresis unit and provide training to three ELWA Hospital laboratory technicians. Prior experience for blood bank operations consisted of occasional whole blood collection, blood typing and TTI screening by RDT kits. New training and competencies included the use of apheresis collection equipment, sterile connection, pathogen inactivation, equipment and vehicle maintenance, process and equipment validation, quality systems, donor management practices and use of the BECS system. Liberian staff were also trained in GCP, including principles of informed consent and appropriate methods to counsel, recruit and qualify donors for this purpose. Three weeks after the arrival of the multi-national team, initial visits for donor screening commenced.
Ongoing mentoring and monitoring of newly trained staff were essential to identify, solve and teach resolution strategies as new challenges arose. Donor screening, qualification and product management were designed around a 510(k) cleared BECS to assure quality and traceability of each process step. Electronic data management was relatively novel for local staff, and internet connectivity in Liberia was often unstable. As a result, data were frequently collected manually and subsequently uploaded to the US-hosted system during the period of this report. However, feasibility of intermittent uploading was demonstrated in this study.
Although power surges and outages challenged the project from the start, there were no equipment failures. A broadly competent on-site logistical and engineering team, robust supply chain, and back up for uninterrupted power supply were essential for successful operations.
We chose to use a pathogen reduction system to treat ECP immediately after collection to reduce potential risk of transmission of EBOV, known TTIs and undetected infectious agents. Disease has recurred in EVD survivors who have had repeated negative blood tests for EBOV [17, 18]. Moreover, prevalence rates of HCV and HBV are higher in West Africa than in other, better-resourced regions [19]. Automated donor screening tests were not available in the region, necessitating the use of less-sensitive rapid tests for donor qualification. The positive TTI test prevalence in Liberian survivors reported here may be greater than that previously reported for the general Liberian population. In 2014, the prevalence of HIV in Liberia was 1.2% for population aged 15 to 49 years [20], compared to 4.0% reported here. The 2014 rates of HBV and HCV positivity in blood donations in Liberia of 7.4% and 2.3%, respectively [19], are slightly less than half the rates, 16.2% and 5.1%, respectively, in individual donors reported here. Whether survivor donors have an increased rate of TTI compared to the general population will require more extensive investigation. These high rates of positive TTI tests appear to support the use of pathogen inactivation of blood products to reduce transfusion risk where possible.
The absence of adverse donor events in this series of 89 collection attempts is noteworthy. Direct donor observation by training staff did not indicate physiologically important adverse effects. A critical skill emphasized and role-played in training involved donor interaction and providing a positive donor experience. Many donors expressed apprehension and fear of pain caused by venipuncture. Implementation of lidocaine pre-treatment at the venipuncture site improved donor satisfaction and reinforced the concept of donor care. Other measures to provide for the safety and comfort of the donors, e.g., a light meal and pre-loading with calcium-enriched oral fluid, may in part account for the low reported adverse donor events.
Although the nutritional and metabolic status of the donors was an initial concern, no significant changes were observed in weight or hemoglobin levels with repeat donations, perhaps attributable in part to the relatively long mean interval of 47 days between donations. Assay of serum albumin or total protein, which was not available during the period covered by this report, and confirmatory research to assess potential negative consequences of repeated plasma donation in this population would be advisable.
Donors were compensated for travel and time spent for donations. The issue of compensation was controversial. Voluntary, non-remunerated blood donation is a pillar of blood safety in Africa and in many other regions (to discourage high risk blood donors) whereas source plasma donors in developed countries are routinely compensated. Concern over exploitation of Ebola survivors by providing compensation (as a possible incentive to donate) was balanced against a pressing need to support survivors, who faced issues of prolonged convalescence, stigmatization, isolation, loss of livelihoods, difficult travel logistics and bereavement. Local ethics committees and survivor groups concurred that compensation for time and travel was appropriate for the purpose of the clinical trial. There is no suggestion that this should be translated to the practice of national blood services for whole blood or routine donations of any sort, outside of a period of crisis.
CONCLUSIONS
This report describes the setup and operation of apheresis plasma collection activities in West Africa during the 2014–2015 Ebola outbreak. Eighty-one units of apheresis plasma were collected by Liberian staff from 43 Liberian EVD survivors to support clinical trials of ECP safety and efficacy in EVD. Provision of a fully-equipped apheresis unit, intensive training of local staff by an international team of blood safety and apheresis experts, and ready participation by the EVD survivor community led to rapid collection of sufficient ECP to fulfill projected trial requirements. A high rate of deferral for TTIs in prospective donors supported the use of an amotosalen/UV illumination pathogen reduction system to reduce transfusion risk. There were no reported adverse effects from the apheresis procedures; repeat donors maintained stable weights and hemoglobin levels. After an extended period of training and remote support dogged by erratic internet connectivity, implementation of a BECS ultimately provided satisfactory management of donor and product data but paper-based backup was essential.
Planning for collection of apheresis plasma in low-resource settings should include provisions for overcoming issues of limited and erratic electrical power supply, maintaining frozen storage capacity, climate control, maintenance of equipment and internet connectivity. Despite these challenges, the processes described in this report can be successfully implemented on relatively short notice in low-resource settings to provide product for further trials of convalescent plasma for EVD or other infectious diseases. They may also serve as a resource to support efforts at strengthening blood safety systems in West Africa.
Acknowledgments
We gratefully acknowledge the intense individual effort and support from many sources to make this study possible. Major funding was provided by the Bill and Melinda Gates Foundation. The U.S. Defense Threat Reduction Agency (CB10166) supported laboratory analyses. The Paul Allen and Jim Greenbaum Foundations donated bloodmobiles; the World Food Programme airlifted them. Cerus Corporation, Haemonetics, Fresenius, Digitrax, Grifols and BBCS donated equipment and supplies. Elan Weiner and Vernon Mailer coordinated apheresis equipment requirements. Daniel Arnold, Technical Services Engineer, Cerus Corporation provided 24/7 on demand remote support to calibrate the Intercept equipment. Beth Simon, Janel Hermsmeyer and Jeff Kriozere assisted with implementation of the BECS. Annie Winkler, Department of Pathology & Laboratory Medicine, Emory University, made her laboratories available at short notice so the advance team could be trained to commission the apheresis and pathogen reduction systems. Richard Benjamin, Obi Greenman, Larry Korash, John Fankhauser, Timothy Uyeki, Sridhar Basavaraju, and Matthew Kuehnert provided helpful advice on protocol design. Stephen Kennedy also advised on informed consent and protocol submission activities. ClinicalRM’s senior management and staff devoted untold effort and commitment to launching and organizing the project. Among these, Joseph Sgherza, Ned Taylor, Melissa Reyes and Charles Henry travelled to Liberia in the height of the outbreak and provided creative logistical and engineering support. The advance team efforts were augmented by the engineering staff of ELWA Hospital. Galakpai Gorvego, Darlington Komosee, and Uriah Glaybo conducted superb apheresis operations with expert advice from Ngoy Numbi and Claude Tayou, both of Safe Blood for Africa Foundation™ (SBFA). Sam Tozay and Gertrude Jeh-Mulbah and the staff of ELWA Hospital enthusiastically supported recruitment and donor management. David Norwood and the LIBR staff absorbed the additional workload of EBOV testing to provide rapid turnaround of results. Opinions, conclusions, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the US. Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense.
References
- 1.Centers for Disease Control and Prevention. Outbreaks Chronology: Ebola Virus Disease. 2016 [cited 2016 2/22/16]; Available from: http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html.
- 2.Chertow DS, et al. Ebola virus disease in West Africa–clinical manifestations and management. N Engl J Med. 2014;371(22):2054–7. doi: 10.1056/NEJMp1413084. [DOI] [PubMed] [Google Scholar]
- 3.Fowler RA, et al. Caring for critically ill patients with ebola virus disease. Perspectives from West Africa. Am J Respir Crit Care Med. 2014;190(7):733–7. doi: 10.1164/rccm.201408-1514CP. [DOI] [PubMed] [Google Scholar]
- 4.Hunt L, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15(11):1292–9. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 5.Qin E, et al. Clinical Features of Patients With Ebola Virus Disease in Sierra Leone. Clin Infect Dis. 2015;61(4):491–5. doi: 10.1093/cid/civ319. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza EJ, Qiu X, Kobinger GP. Progression of Ebola Therapeutics During the 2014–2015 Outbreak. Trends Mol Med. 2016;22(2):164–73. doi: 10.1016/j.molmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Plummer FA, Wong G, Kobinger GP. Experimental countermeasures against Ebola virus: current progress and an ethical conundrum. CMAJ. 2014;186(15):1129–30. doi: 10.1503/cmaj.141061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mupapa K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis. 1999;179(Suppl 1):S18–23. doi: 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 9.Dye JM, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A. 2012;109(13):5034–9. doi: 10.1073/pnas.1200409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uyeki TM, et al. Clinical Management of Ebola Virus Disease in the United States and Europe. N Engl J Med. 2016;374(7):636–46. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler AM, Koepsell SA. The use of convalescent plasma to treat emerging infectious diseases: focus on Ebola virus disease. Curr Opin Hematol. 2015;22(6):521–6. doi: 10.1097/MOH.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 12.Blood Regulators Network, W.H.O. Position Paper on Collection and Use of Convalescent Plasma or Serum as an Element in Filovirus Outbreak Response. 2014 [Google Scholar]
- 13.van Griensven J, et al. The Use of Ebola Convalescent Plasma to Treat Ebola Virus Disease in Resource-Constrained Settings: A Perspective From the Field. Clin Infect Dis. 2016;62(1):69–74. doi: 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Griensven J, et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Biologics Evaluation and Research, F.D.A. Guidance for Industry: Implementation of an Acceptable Abbreviated Donor History Questionnaire and Accompanying Materials for Use in Screening Frequent Donors of Blood and Blood Components. FDA CBER; Maryland: 2013. [Google Scholar]
- 16.AABB. Circular of Information for the Use of Human Blood and Blood Components. AABB; Bethesda, MD: 2013. [Google Scholar]
- 17.Varkey JB, et al. Persistence of Ebola Virus in Ocular Fluid during Convalescence. N Engl J Med. 2015;372(25):2423–7. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIntyre CR, Chughtai AA. Recurrence and reinfection-a new paradigm for the management of Ebola virus disease. Int J Infect Dis. 2015;43:58–61. doi: 10.1016/j.ijid.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Apata IW, et al. Progress toward prevention of transfusion-transmitted hepatitis B and hepatitis C infection–sub-Saharan Africa, 2000–2011. MMWR Morb Mortal Wkly Rep. 2014;63(29):613–9. [PMC free article] [PubMed] [Google Scholar]
- 20.UN Aids. HIV and Aids Estimates LIberia. 2014 2/9/16 [cited 2016; Available from: http://www.unaids.org/en/regionscountries/countries/liberia.


