Abstract
Aim:
Symphytum officinale (comfrey) is a medicinal plant commonly used in decoction and to treat ailments. It protects the skin against ultraviolet (UV)-irradiation. UV irradiation may induce variable effects on the constituents of herbal extracts and thereby may limit or improve the advantages of using these extracts as medicinal supplements. This study aimed to assess the effect of UV radiations including UV-A, UV-B, and UV-C on the constituents of S. officinale aqueous and alcoholic extracts.
Materials and Methods:
Comfrey extracts (1% w/v) were prepared using distilled water, ethanol, and methanol. They were exposed to wavelengths of UV-A, UV-B, and UV-C for 10 min. The principal peak on the UV-spectroscopy scanning, the flavonoids, reducing power, and the allantoin levels were determined before and after irradiation.
Results:
UV irradiation reduces the magnitude of the principle peak at 355 nm wavelength of the aqueous infusion and methanol extracts. It improves the levels of flavonoids and reducing power of the aqueous extracts and increases the levels of allanotoin in aqueous and methanol extracts.
Conclusions:
UV-radiation enhances the yields of active ingredient of comfrey extracted with methanol, whereas improves the flavonoids, reducing power, and allantoin levels of comfrey extracted by the aqueous infusion method. UV-radiation reduces the levels of flavonoids, reducing power and allantoin when the comfrey extracted by alcohols.
Keywords: Allantoin, comfrey, flavonoids, reducing power, ultraviolet irradiation
INTRODUCTION
Symphytum officinale (comfrey) is a medicinal plant commonly used in decoction and to treat ailments. It contains therapeutic bioactive compounds such as allantoin, rosmarimic, and hepatotoxic pyrrolizidine alkaloids such as lycopsamine [1,2]. Comfrey has anti-inflammatory and wound healing properties [3-5]. Aqueous extract of comfrey root contained high quantities of allantoin and its inclusion in the topical applications reduced skin irritation [6]. Alkan et al. reported that comfrey has antioxidant and free radical scavenging properties when it extracted in aqueous and alcoholic solvents [7].
Exposure of supplemental ultraviolet-B (UV-B) leads to alterations of the metabolism of reactive oxygen species in plants [8]. The extracts of certain plants are either reduced the toxic effect of UV-A radiation as with green tea or they showed photoprotection against UV-A exposure as with rosmarimic acid extract [9,10]. The comfrey contained many pyrrolizidine alkaloid related substances, which have the affinity to chelate the cellular DNA molecule and thereby they responsible for the genotoxic effect of comfrey [11].
The rationale of this study was the UV radiation in respect to their wavelengths induces variable effects on the constituents of herbal extracts and thereby may limit or improve the advantages of using these extracts as medicinal supplements. Therefore, the aim of the study is to assess the effect of the wide spectral range of the UV radiations including UV-A, UV-B, and UV-C on the constituents of S. officinale aqueous and alcoholic extracts. These constituents including the flavonoids, reducing power and allantoin levels as the measurements of antioxidant, scavenging property and antiaging, respectively.
MATERIALS AND METHODS
This study was conducted in the Department of Pharmacology and the Department of Physiology, College of Medicine at Al-Mustansiriya University in Baghdad, Iraq. S. officinale seeds (comfrey) obtained from local markets, which imported from Saudi Arabia and identified by the Department of Biology, College of Science. The seeds grinded mechanically and sieved to get a fine powder before their extraction with distilled water (aqueous) and alcohols (ethanol and methanol). 1 g of fine powder of comfrey extracted with 100 ml of distilled water, ethanol and methanol (1% w/v) for 24 h in a dark room at a temperature of 25°C followed by filtration the extracts. Another aqueous extract of 1% (w/v) prepared by infusion method by adding a boiling distilled water to the herbal powder, left for 15 min then followed by filtrating the extract.
Each extract irradiated with UV light; 4 ml of each extract in quartz cell was exposed to UV radiation (UV-A; at 320 and 360 nm; UV-B at 280; and 300 nm and UV-C at 220 and 260 nm) for 10 min. The UV-visible spectra of each aqueous extract were obtained by scanning the extract using UV-visible spectrophotometer (Aquarius, France, Cecil series with scanning utility) before and after each exposure of UV radiation. The mean of three readings was determined.
Quantification of Total Flavonoids
The method based on the quantification of the yellow color produced by the interaction of flavonoids with aluminum chloride (AlCl3) reagent [12]. Aliquots of 1.5 ml of each extract added to an equal volume of a solution of 2% AlCl3.6H2O (2 g in 100 ml methanol). The mixture vigorously shakes and the absorbance at 367 nm recorded after 10 min of incubation. The average of three readings of each sample was calculated. The flavonoids content was determined by applying the linear regression equation based on the calibration curve of rutin; therefore, the contents of flavonoids were determined as µg rutin equivalent per milligram dry weight of comfrey seeds. The results expressed as a mean of three readings.
Assessment of Reducing Power
To 1 ml of each extract was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% of potassium ferricyanide (K3Fe(CN)6, then the mixture was incubated at 50°C for 30 min. Afterward, 2.5 ml of trichloroacetic acid (1%) was added to the mixture, then centrifuged at 3000 rpm for 10 min. Finally, 2.5 ml of the upper layer solution was mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1%), and the absorbance was recorded at 700 nm. The increased absorbance of the reaction mixture indicates increased reducing power. The results expressed as a mean of three readings.
Determination of Allantoin
This determinant was carried out as described previously [13] using Ehrlich’s reagent, which consisted of 1 g p-dimethylaminobenzaldehyde in a mixture of 25 ml of concentrated hydrochloric acid and 75 ml of methanol. 1 ml of each extract mixed with Ehrlich’s reagent (1:2 v/v), incubated at room temperature, and read the absorbance at 440 nm. The allantoin contents calculated using the linear regression equation based on the standard allantoin calibration curve. The results expressed as a mean of three readings.
Statistical Analysis
The results are expressed as numbers and percentages. The mean and the standard deviation of three readings of each experiment were calculated. The accuracy of measurements was determined by calculating the coefficient variation which ranged in this study between 1% and 2.5% and indicating the precision of the methodology.
RESULTS
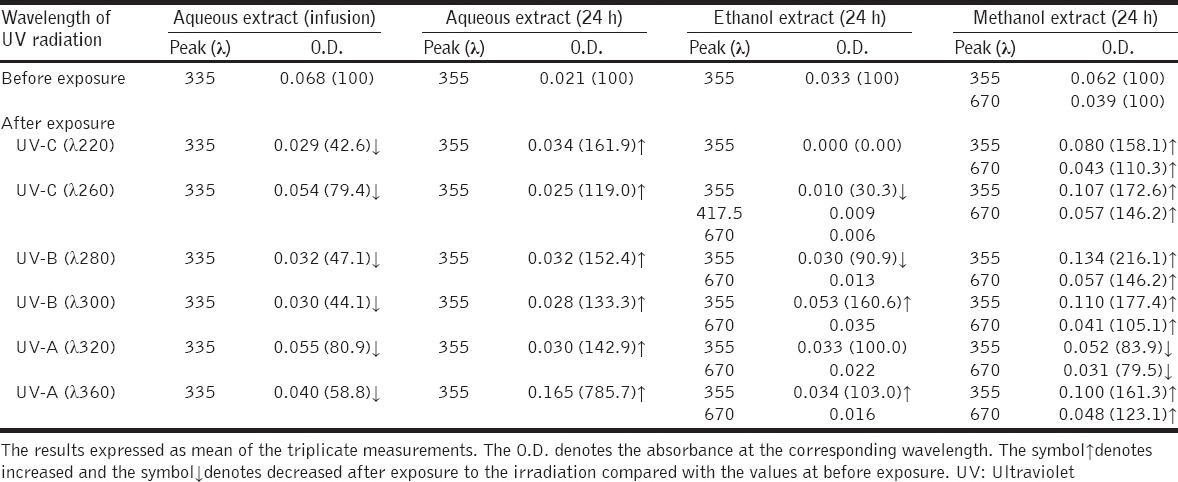
UV-visible spectra showed a principal peak of absorbance of comfrey extract at wavelength 355 nm [Table 1]. Another peak at wavelength 670 nm observed in methanol extract. UV radiation reduced the optic density (absorbance) of aqueous extract prepared by infusion but it produced reverse effects on the aqueous extract prepared by incubation for 24 h. The effect of UV radiation on ethanol and methanol extracts was variable for each extract and UV exposure [Table 1]. The UV-visible spectra showed an absorbance peak at 670 nm of ethanol and methanol extracts exposed to UV-irradiation.
Table 1.
Effect of UV radiation on the absorbance peak (O.D.) of comfrey extract diluted to the final concentration of 0.1% (w/v)
Determination of Flavonoids
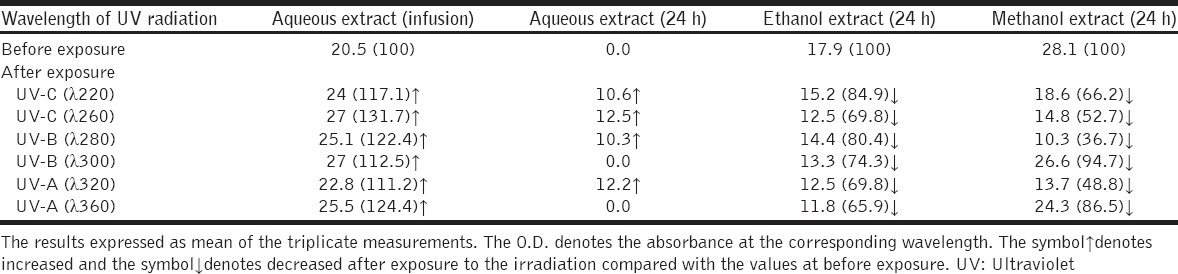
The yields of flavonoids in the methanol extract were higher than other extracts. The levels of flavonoids in the infusion aqueous extract were higher after irradiation with UV lights and it declines with ethanol and methanol extracts [Table 2].
Table 2.
Effect of UV radiation on the flavonoids levels (expressed as rutin µg/mg seeds weight) on the extracts of comfrey
Assessment of Reducing Power
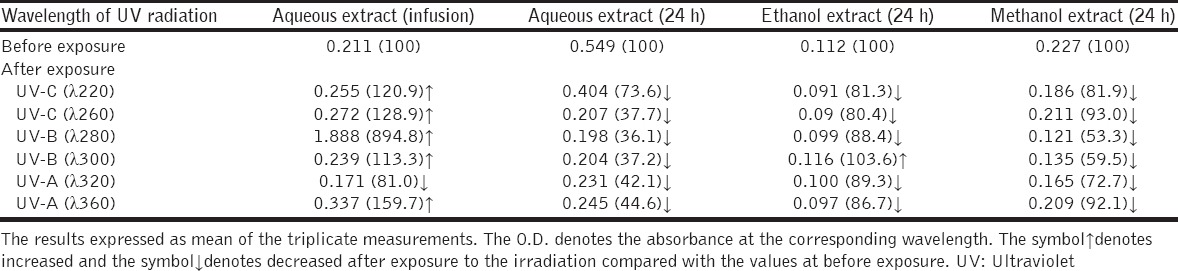
The highest value of reducing power was of the comfrey extracted with aqueous media over 24 h compared with other extraction media [Table 3]. The reducing power of UV-irradiated infusion aqueous extract of comfrey was increased compared with overnight aqueous, ethanol, and methanol extracts which were decreased after exposing to the UV-radiations of whatever their wavelength [Table 3].
Table 3.
Effect of UV radiation on the reducing power (expressed as absorbance percentage of the non-irradiated) of the extracts of comfrey
Determination of Allantoin
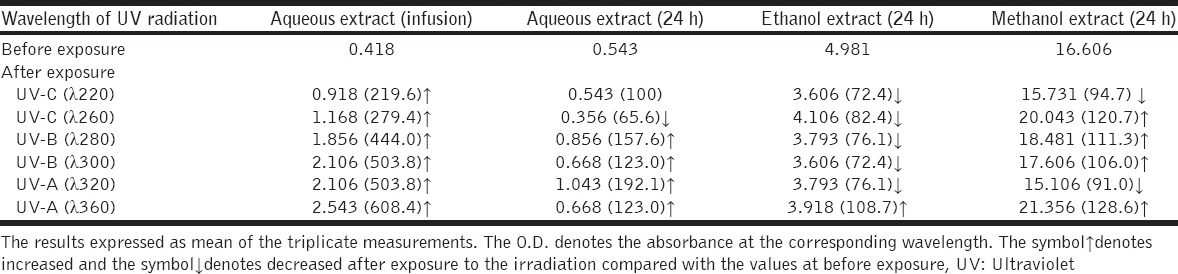
Extraction of comfrey with alcohols (ethanol or methanol) resulted in higher concentrations of allantoin. The effect of UV radiation on the allantoin levels on the medicinal plant extracts was related on the type of UV radiation, extracted solution as well as the nature of the plant. UV-C irradiation resulted in the increase of the allantoin levels in infusion aqueous extract and reduced in the overnight aqueous ethanol extracts for both medicinal plants [Table 4]. UV-C (l 220 nm) but not UV-C (l 260 nm) reduced the allantoin levels of the methanol extracts of comfrey [Table 4]. UV-B irradiation either increased the levels of allantoin of the comfrey extracts or did not show any effect except the ethanol extract by which the levels decreased. The results of UV-B (l 300 nm), UV-A (l 320 nm), and UV-A (l 360 nm) on the levels of allantoin are inconsistent [Table 4]. In general, irradiated infusion of comfrey or saffron aqueous extracts resulted in high levels of allantoin at any wavelength of UV irradiation.
Table 4.
Effect of UV radiation on the allantoin levels (µg/mg weight seeds) on the extracts of comfrey
DISCUSSION
The results of this study show that the yields of the active constituents of the comfrey are relating to the methods of the extraction. UV-radiation improves the extraction of the active ingredient of the comfrey in the overnight aqueous and methanol extracts. UV-radiation increases the levels of flavonoids of the aqueous extracts, but not of the alcoholic extracts whereas it increases the reducing power of the aqueous infusion extract. UV-radiation improves the extraction of the allantoin from the aqueous infusion and overnight extracts. Therefore, the results of this study point out the importance of using UV-radiation as an assisted method of extraction of certain substances, and on the other hand, it may induce damage to the other constituents taking in consideration the solvents and the methods of extraction. The principal peak at UV-spectra was 355 nm wavelength indicating that this peak is related to the lycopsamine (a substance related to the pyrrolizidine), which is available in high quantity in comfrey seeds [14]. UV-irradiation reduced the magnitude of the principal peak of the aqueous and methanol extracts, and this effect is in favor of the UV-radiation, as the pyrrolizidine can induce tumor [15].
The previous studies show that irradiation of the apple juice by UV-light did not reduce the activity of antioxidant polyphenols by inactivating the polyphenol oxidase enzyme as our results show that the flavonoids levels are increasing after exposure to UV-radiation [16]. Moreover, g-rays significantly increased the antioxidant activity of the polyphenols of the red and black maca extracts (Lepidium meyenii walp) and UV-C irradiation increase the total flavonoids and the reducing power of the fresh cut mango (Mangifera indica L. cv. Chokanan) [17,18]. Therefore, our encouraging results may utilize to use UV-irradiation to enhance the extraction of the antioxidants as an assisted method of extraction using aqueous media. As early as 1990, Inaba et al. found that rats exposed to the microwave irradiation increase the level of plasma allantoin as a byproduct of uric acid [19]. This study adds a new information that UV irradiation increases the levels of allantoin in aqueous extracts, and thereby it can be used this method in the preparation of the wound-healing creams that contained allantoin [20]. The net results of this study that UV-irradiation of the comfrey extracts leads to improve the antioxidant properties and reduced the tumorigenicity of the comfrey.
CONCLUSIONS
We conclude that UV-radiation enhances the yields of active ingredient of comfrey extracted with methanol whereas improves the flavonoids, reducing power and allantoin levels of comfrey extracted by the aqueous infusion method. UV-radiation reduces the levels of flavonoids, reducing power and allantoin when the comfrey extracted by alcohol.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pawar RS, Grundel E, Mazzola E, White KD, Krynitsky AJ, Rader JI. Chiral stationary phases for separation of internecine and lycopsamine enantiomers from Symphytum uplandicum. J Separation Sci. 2010;33:200–5. doi: 10.1002/jssc.200900611. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Wan SY, Jiang Z, Li SF, Ong ES, Osorio JC. Determination of pyrrolizidine alkaloids in comfrey by liquid chromatography-electrospray ionization mass spectrometry. Talanta. 2009;80:916–23. doi: 10.1016/j.talanta.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Bleakley CM, McDonough SM, MacAuley DC. Some conservative strategies are effective when added to controlled mobilization with external support after acute ankle sprain: A systematic review. Aust J Physiother. 2008;54:7–20. doi: 10.1016/s0004-9514(08)70061-8. [DOI] [PubMed] [Google Scholar]
- 4.D'Anchise R, Bulitta M, Giannetti B. Comfrey extract ointment in comparison to diclofenac gel in the treatment of acute unilateral ankle sprains (distortions) Arzneimittel Forschung. 2007;57:712–6. doi: 10.1055/s-0031-1296672. [DOI] [PubMed] [Google Scholar]
- 5.Araújo LU, Reis PG, Barbosa LC, Saúde-Guimarães DA, Grabe-Guimarães A, Mosqueira VC, et al. In vivo wound healing effects of Symphytum officinale L. Leaves extract in different topical formulations. Pharmazie. 2012;67:355–60. [PubMed] [Google Scholar]
- 6.Savić VL, Nikolić VD, Arsić IA, Stanojević LP, Najman SJ, Stojanović S, et al. Comparative study of the biological activity of allantois and aqueous extract of the comfrey root. Phytother Res. 2015;29:1117–22. doi: 10.1002/ptr.5356. [DOI] [PubMed] [Google Scholar]
- 7.Alkan FU, Anlas C, Ustuner O, Bakırel T, Sari AB. Antioxidant and proliferative effects of aqueous and ethanolic extracts of Symphytum officinale on 3T3 Swiss albino mouse fibroblast cell line. Asian J Plant Sci Res. 2014;4:62–8. [Google Scholar]
- 8.Singh SK, Kakani VG, Surabhi GK, Reddy KR. Cowpea (Vigna unguiculata[L.] Walp.) geno-types response to multiple abiotic stresses. J Photochem Photobiol B. 2010;100:135–46. doi: 10.1016/j.jphotobiol.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Malhomme de la Roche H, Seagrove S, Mehta A, Divekar P, Campbell S, Curnow A. Using natural dietary sources of antioxidants to protect against ultraviolet and visible radiation-induced DNA damage: An investigation of human green tea ingestion. J Photochem Photobiol B. 2010;101:169–73. doi: 10.1016/j.jphotobiol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Campillo M, Gabaldon JA, Castillo J, Benavente-García O, del Baño MJ, Alcaraz M, et al. Rosmarinic acid, a photo-protective agent against UV and other ionizing radiations. Food Chem Toxicol. 2009;47:386–92. doi: 10.1016/j.fct.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Mei N, Guo L, Fu PP, Fuscoe JC, Luan Y, Chen T. Metabolism, genotoxicity, and carcinogenicity of comfrey. J Toxicol Environ Health B Crit Rev. 2010;13:509–26. doi: 10.1080/10937404.2010.509013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamaison JL, Carnet A. The levels of the main flavonoids completing of the flower of Craaegeus monogyna Jawq and Crataegeus laevigata (poiret) as a function of the vegetation. Pharm Acta Helv. 1990;65:315–20. [Google Scholar]
- 13.Vrbaski MM, Grujić-Injac B, Gajić D. A new method for allantoin determination and its application in allantoin determination in Agrostemma githago L. Seed. Anal Biochem. 1978;91:304–8. doi: 10.1016/0003-2697(78)90844-8. [DOI] [PubMed] [Google Scholar]
- 14.Brown AW, Stegelmeier BL, Colegate SM, Gardner DR, Panter KE, Knoppel EL, et al. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and internecine in chicks (Gallus gallus domesticus) J Appl Toxicol. 2016;36:716–25. doi: 10.1002/jat.3205. [DOI] [PubMed] [Google Scholar]
- 15.Xia Q, Zhao Y, von Tungeln LS, Doerge DR, Lin G, Cai L, et al. Pyrrolizidine alkaloid-derived DNA adducts as a common biological biomarker of pyrrolizidine alkaloid-induced tumorigenicity. Chem Res Toxicol. 2013;26:1384–96. doi: 10.1021/tx400241c. [DOI] [PubMed] [Google Scholar]
- 16.Juarez-Enriquez E, Salmerón I, Gutierrez-Mendez N, Ortega-Rivas E. Ultraviolet irradiation effect on apple juice bioactive compounds during shelf storage. Foods. 2016;5:e10. doi: 10.3390/foods5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zevallos-Concha A, Nuñez D, Gasco M, Vasquez C, Quispe M, Gonzales GF. Effect of gamma irradiation on phenol content, antioxidant activity and biological activity of black maca and red maca extracts (Lepidium meyenii walp) Toxicol Mech Methods. 2016;26:67–73. doi: 10.3109/15376516.2015.1090512. [DOI] [PubMed] [Google Scholar]
- 18.George DS, Razali Z, Santhirasegaram V, Somasundram C. Effect of postharvest ultraviolet-C treatment on the proteome changes in fresh cut mango (Mangifera indica L. cv. Chokanan) J Sci Food Agric. 2016;96:2851–60. doi: 10.1002/jsfa.7454. [DOI] [PubMed] [Google Scholar]
- 19.Inaba R, Watanabe S, Okada A, Moroji T. Effects of whole-body microwave exposure on the plasma corticosterone, glucose, uric acid and allantois levels in rats. Nihon Eiseigaku Zasshi. 1990;45:904–8. doi: 10.1265/jjh.45.904. [DOI] [PubMed] [Google Scholar]
- 20.Pazyar N, Yaghoobi R, Rafiee E, Mehrabian A, Feily A. Skin wound healing and phytomedicine: A review. Skin Pharmacol Physiol. 2014;27:303–10. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]


