Abstract
Background:
This study was planned to investigate the effectiveness of the whey protein isolate (WPI) of high purity and a galactooligosaccharides (GOS) preparation on glucose homeostasis and insulin resistance in high fat diet (HFD) (45.47% energy from fat) fed conditions in C57BL/6J mice.
Methods:
Fasting blood glucose level, serum insulin, and glucagon-like peptide-1 (enzyme-linked immunosorbent assay) were measured; also, homeostasis model assessment of insulin resistance (HOMA-IR) was determined in different treatment groups. mRNA expression of gluconeogenesis genes in liver and small intestine tissues was analyzed by quantitative real time-polymerase chain reaction.
Results:
Dietary incorporation of WPI and GOS was observed to significantly resist (P < 0.001) the HFD-induced increase in blood glucose levels indicating a mitigating effect on glycemic load. It is important to note that no additive effects of administration of WPI and GOS could be observed. The administration of WPI and GOS exhibited maximum resistance (37.8%) to the rise in insulin level. Thus, the resistance to the increase in HOMA-IR was also noticed on the dietary incorporation of two functional ingredients . The positive effects on mRNA expression of phosphoenolpyruvate carboxykinase and glucose 6-phosphatase could be detected in liver only.
Conclusion:
Both types of functional components exhibit potential to improve glucose homeostasis under HFD fed conditions. Resistance to HFD-induced hyperinsulinemia and HOMA-IR is also recorded .
Keywords: Galactooligosaccharides, glucose homeostasis, high fat diet, insulin resistance, whey protein isolate
INTRODUCTION
Western diets, which are high in fat content as well as refined sugars, combined with sedentary lifestyles, are recognized to be the causes of major health threats such as increasing obesity and associated metabolic disorders. The toxicological concerns regarding pharmacological intervention to control obesity have prompted researchers to try dietary interventions, e.g., changes in proportion/type of fat, carbohydrate, protein, and fiber. Whey protein isolate (WPI) is a rich source of branched-chain amino acids (BCAAs): Leucine, isoleucine, and valine. The BCAAs are thought to play an important role in the maintenance of lean body mass [1]. Protein-induced satiety appears to be of vital importance for weight loss and weight maintenance. After a breakfast with whey, increase in insulin and active glucagon-like peptide-1 (GLP-1) has been reported to be larger than a breakfast with casein and soy [2]. Maurer et al. [3] reported that weaning diets high in protein or fiber caused a rapid increase in the secretion of satiety hormones and the expression of genes involved in glucose and lipid metabolism that reflect the response to a high-energy diet in adulthood. Protein feeding markedly increases the expression of the regulatory genes of gluconeogenesis (glucose 6-phosphatase [G6Pase] and phosphoenolpyruvate carboxykinase [PEPCK]) in rat small intestines. This promotes endogenous glucose synthesis and its release into the portal blood, a phenomenon lasting during the postabsorptive time. This portal glucose flux, potentiated by the portal glucose sensor, has been suggested to activate the hypothalamic nuclei involved in the regulation of food intake and to cause a decrease in subsequent food consumption [4].
Dietary fiber may decrease a diet’s metabolizable energy . Prebiotic fibers (both insoluble and soluble) are among the functional food ingredients which are gaining a lot of popularity. A prebiotic is a selectively fermentable ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora, that confer benefits on host well-being and health [5]. Administration of prebiotics leads to an improvement of fasting and/or post-oral glucose load glycemia [6]. Nondigestible carbohydrates which are largely fermented in the colon, such as oligofructose (OFS), when added in the diet, improve glucose tolerance, insulin secretion, and lower food intake in animals and humans alike. These effects are often associated with higher plasma GLP-1 content [7].
Galactooligosaccharides (GOS) and fructooligosaccharides (FOS) are currently used in a wide range of commercial commodities, including infant formulas, dairy products, sauces, soups, breakfast cereals, and beverages [8]. Although lot of information is available signifying the role of GOS in the improvement of gut environment through modulation of gut microflora [9-11], very little information is available on efficacy of GOS in glucose homeostasis and insulin resistance under high fat diet (HFD) fed conditions. The GOS (prebiotic fiber) and WPI are expected to act through different mechanisms resulting in various physiological effects. The aim of this study was to investigate the effectiveness of WPI and GOS individually, and in combination on glucose homeostasis and insulin resistance developed under HFD fed conditions in C57BL/6 mice.
MATERIALS AND METHODS
Animals and Experimentation
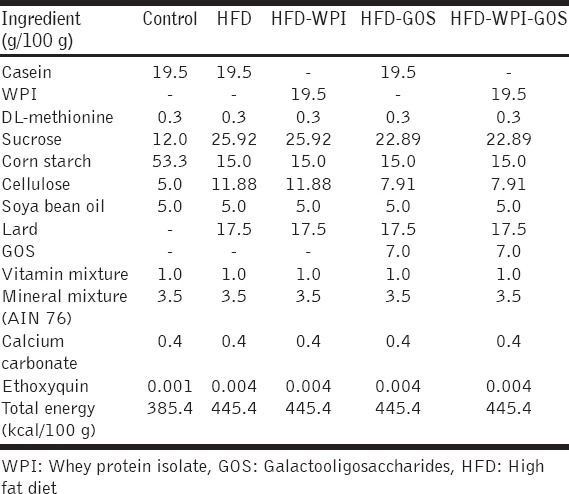
Male C57BL/6J mice were purchased from National Institute of Nutrition, Hyderabad (India). The experimental animals (19-24 g) were housed in ventilated plastic cages under 12 h light/12 h dark conditions. The experiment was carried out in accordance with the guidelines of Institutional Animal Ethics Committee. After 2 weeks of acclimatization, the mice were divided into five groups (n = 10 mice/group), viz., control, HFD, HFD with WPI, HFD with GOS, and HFD containing WPI and GOS. The mice in different groups were fed on respective experimental diets . The HFD (4.45kcal/g) contained 45.47% energy from fat (lard) and 17.51% energy from protein. The low-fat control diet (3.85 kcal/g) contained 11.68% energy from fat and 20.24% energy from protein, and detailed composition of all diets was given in Table 1. The prebiotic fiber (GOS 70-75% pure, gifted by Tata Chem., Ltd.) was incorporated at 7% (w/w) in HFD while WPI (80-90% pure, Glanbia Cheese, USA) was incorporated at 20% as a substitute of casein . All experimental diets contained ethoxyquin as an antioxidant. The animals were fed ad-libitum, had free access to water, and the feeding schedule was followed for 18 weeks.
Table 1.
Composition of diets
The weekly body weight of different treatment groups were recorded for up to 18 weeks. After 14 weeks, a significant reduction in the body weight gain was observed in WPI, GOS and in combination as compared to HFD group (Supplementary Figure 1).
Supplementary Figure 1.

Effects of dietary supplementation of whey protein isolate (WPI), galactooligosaccharides (GOS) and WPI + GOS on body weight of mice fed high fat diet. Values are expressed as mean ± standard error of mean (*P < 0.05 and **P < 0.01)
Collection of Blood and Tissue Samples
Five animals from each group were sacrificed after 6 weeks of dietary intervention, and the remaining animals were continued on their respective diets up to 18 weeks and sacrificed by cervical dislocation under anesthesia using diethyl ether . Blood was collected by cardiac puncture using a sterile syringe and stored in sterile 1.5 ml Eppendorf Tubes. After clot formation, the blood samples were centrifuged at 2000 ×g (HERMLE Labortechnik) for 15 min at 4°C. Upper layer of serum was transferred to 1.5 ml of Eppendorf Tube and stored at −20°C. This was used for determining the serum GLP-1 and insulin level . Portions of liver and small intestine tissues were stored in RNAlater® (Sigma-Aldrich, Bengaluru, India) at −20°C for gene expression analysis.
Fasting Blood Glucose, Serum Insulin, Homeostasis Model Assessment of Insulin Resistance (HOMA-IR), and GLP-1
Tail vein was punctured to collect a drop of blood, and the fasting glucose levels were measured from overnight fasting animals using glucometer (ACCU-CHEK® Active, Roche Diagnostics). Serum insulin levels were measured by sandwich enzyme-linked immunosorbent assay (Crystal chem. Inc, USA). IR was assessed by HOMA. It was calculated by the formula: HOMA-IR = [Fasting serum insulin (mU/ml) × Fasting blood glucose (mmol/l)]/22.5 [12]. Active form of serum GLP-1 was measured by human/mouse/Rat enzyme immunoassay (EIA) kit based on the principle of competitive EIA (RayBiotech Inc Norcross, Georgia).
Isolation of Total RNA and Gene Expression Analysis by Quantitative Real Time-Polymerase Chain reaction (qRT-PCR)
Total RNA was isolated from liver and the entire portion of small intestine tissues using TRIzol (Sigma-Aldrich, USA). RNA was determined by measuring absorbance at 260 nm using nanodrop (NanoQuant M200 pro), and the purity was assessed by measuring the ratio of absorbance at 260 and 280 nm. The total RNA with A260/ A280 between 1.7 and 1.9 was acceptable for subsequent use. The integrity of RNA was determined by subjecting it to agarose gel electrophoresis. The cDNA template was synthesized by reverse transcription of 500 ng of total RNA using first strand cDNA synthesis kit (Thermo Scientific). The primers used for qRT-PCR are listed in Table 2. SYBR green was used for real-time PCR detection. The qPCR data were analyzed by the method of Livak and Schmittgen [13].
Table 2.
Primer sequences used for qRT-PCR

Statistical Analysis
Data were expressed as mean ± standard error of mean (SEM). The statistical analysis was performed with one-way ANOVA followed by Tukey’s tests (GraphPad Software, version 5.01) for fasting blood glucose, serum insulin, HOMA-IR, GLP-1, and gene expression.
RESULTS
Fasting Blood Glucose
As shown in Figure 1, the fasting blood glucose level was found to increase significantly on feeding HFD and was measured to be 151.6 ± 3.14 mg/dl as compared to a low level of 91.80 ± 3.89 mg/dl (mean ± SEM) in the case of the control group at 6 weeks. The dietary incorporation of WPI/GOS was observed to significantly resist (P < 0.001) the HFD-induced increase in blood glucose levels indicating a mitigating effect on glycemic load. Meanwhile, no additive effect of administration of WPI and GOS could be observed. A similar trend was observed after 18 weeks of feeding also.
Figure 1.

Effect of dietary supplementation of whey protein isolate (WPI), galactooligosaccharides (GOS) and WPI + GOS on fasting blood glucose levels in mice fed high fat diet. Values are expressed as mean ± standard error of mean (**P < 0.01 and ***P < 0.001)
Serum Insulin and HOMA-IR
The data on serum insulin level and HOMA-IR at 6 and 18 weeks of feeding period are presented in Figure 2. Serum insulin level was observed to rise as a consequence of HFD feeding (HFD group), however, it did not reach to a statistically significant level as compared to control group at 6 weeks. Feeding of HFD for a longer duration of 18 weeks resulted in a significant rise in the serum insulin concentration in HFD group which reached to the level of 1.72 ± 0.15 ng/ml, and was found to be significantly higher (P < 0.01) as compared to a low level (0.90 ± 0.18 ng/ml) in the control group. Dietary incorporation of WPI or GOS in HFD was observed to exhibit protective effect against the rise in insulin level caused by HFD feeding. Although the insulin level in HFD-WPI (1.12 ± 0.06 ng/ml) was lower than that in the case of HFD-GOS (1.21 ± 0.12 ng/ml), but these values were not statistically different. The administration of WPI and GOS exhibited maximum resistance (37.8%) to the rise in insulin level. The insulin concentration in HFD-WPI-GOS was measured to be 1.07 ± 0.07 ng/ml.
Figure 2.

Effects of whey protein isolate (WPI), galactooligosaccharides (GOS) and WPI + GOS on serum insulin, homeostasis model assessment of insulin resistance score, and glucagon-like peptide-1 levels in mice fed high fat diet. Values are expressed as mean ± standard error of mean. a-bMean values with different superscripts differ significantly (**P < 0.01 and *P < 0.5)
Both at 6 and 18 weeks, the HOMA-IR score was found to be significantly higher in HFD group as compared to the control group. After 6 weeks of feeding, HOMA-IR score in HFD group was calculated to be 7.35 ± 0.92 as compared to a significantly lower (P < 0.01) value of 3.1 ± 0.79 in the control group. Different dietary treatments involving incorporation of WPI/GOS resulted in protection from increase in HOMA-IR, almost to the same extent . After 18 weeks of HFD administration, HOMA-IR further increased and reached to a significantly higher level of 14.01 ± 1.52 (P < 0.001) compared to the level in the control group. Resistance to the increase in HOMA-IR was recorded on dietary incorporation of WPI as well as GOS. Apparently, there seemed to be a slight additive effect of two types of functional components, however, it did not differ significantly in comparison to the individual effects.
Serum GLP-1
Results of serum GLP-1 measured after 18 weeks are also depicted in Figure 2. It was found to decrease significantly as a consequence of HFD feeding, and the levels in HFD versus control group were measured to be 8.71 ± 2.29 versus 29.48 ± 3.08 pg/ml (mean ± SEM). The dietary incorporation of WPI/GOS exhibited significant protective effect against the decrease in serum GLP-1 levels. The GLP-1 levels in HFD-WPI, HFD-GOS, and HFD-WPI-GOS were measured to be 36.39 ± 5.47, 39.31 ± 1.73, and 42.34 ± 3.34 pg/ml, which were significantly higher (P < 0.05) when compared to the HFD group and also indicated that maximum effect was reached in the case of HFD-WPI+GOS group (>4-fold vs. HFD level).
mRNA Expression Analysis of Genes Related to Gluconeogenesis
Quantification of mRNA expression levels of genes related to gluconeogenesis in liver and small intestine as affected by different dietary treatments was performed by qRT-PCR [Table 3]. The expression of PEPCK in liver tissue of animals fed HF-calorie rich diet was observed to be significantly increased (P < 0.05) and reached to the level of 2.02 ± 0.23 fold in HFD group as compared to the control group (1.02 ± 0.10). The dietary incorporation of WPI/GOS was found to be significantly effective in resisting the upregulation of PEPCK [Figure 3]. The results showed that WPI as well as GOS were almost equally and significantly effective individually, and did not exhibit any additive effect. A similar trend could be seen in small intestinal tissue in different groups. Although the PEPCK expression was higher in HFD group as compared to control group, the levels were not found to differ significantly among different treatment groups.
Table 3.
Effects of WPI, GOS, and WPI+GOS on gluconeogenesis genes in liver and small intestinal tissues

Figure 3.

Effects of dietary supplementation of whey protein isolate (WPI), galactooligosaccharides (GOS) and WPI + GOS on the expression of phosphoenolpyruvate carboxykinase in liver and small intestine of mice fed high fat diet. Values are expressed as mean ± standard error of mean. a-bMean values with different superscripts differ significantly
The mRNA expression of G6Pase (important enzyme of gluconeogenesis) in liver was significantly increased in HFD fed animals (HFD group) and reached to the level of 1.79 ± 0.21 fold. The incorporation of either of the functional ingredient in HFD was significantly effective in resisting the upregulation of G6Pase, indicating 53% (P < 0.01) and 69.5% (P < 0.001) reduction in HFD-WPI and HFD-GOS group, respectively. The coadministration of WPI and GOS did not reveal any additive effect. As far as the expression of G6Pase in the small intestine is concerned, a similar trend, as observed in the case of liver, could be seen. However, the differences among different treatment groups were not found to be statistically significant.
The relative mRNA expression of glucose transporter 2 (glut2), (an important GLUT) as affected by different dietary treatments was also determined in liver as well as the small intestine. Glut2 expression in both tissues appeared to increase as a consequence of HFD feeding (HFD group), though it did not differ significantly in comparison to the control group. There seemed to be a positive effect in animals fed either WPI or GOS. However, these values were not found to be statistically different as compared to the HFD group.
DISCUSSION
Increased intake of calorie-rich food (HF and refined sugar) is known to induce obesity leading to insulin resistance and type 2 diabetes. The health benefits associated with increased dairy food intake may be attributed to the whey components of dairy proteins [14]. In this study, we could demonstrate the ameliorative effect of both functional components, viz., WPI as well as GOS. Fasting blood glucose levels were significantly increased as a consequence of HFD feeding for 6/18 weeks. Our results are in agreement with the findings of Tranberg et al. [15] who demonstrated that replacement of casein with WPI in the HFD (62% energy from fat) resulted in significantly lowered fasting blood glucose in the HF Whey group compared to HF Casein on the basis of fasting blood glucose levels measured before oral glucose tolerance test after 12 weeks of dietary intervention. To the best of our knowledge, no studies are available about how rodents are affected by GOS treatment on diet-induced hyperglycemia. We could demonstrate a significant effect of GOS in normalizing blood glucose level under HFD fed conditions.
Our results indicate a significant rise in serum insulin concentration in HFD group and protective effect of WPI as well as GOS against the rise in serum insulin levels which may be correlated with the significantly lowered blood glucose level in HFD-WPI and HFD-GOS groups. Zhang et al. [16] reported a reduced weight gain and improved glucose homeostasis when HFD was used along with leucine supplementation with drinking water. Other research workers have also reported beneficial effects of leucine supplementation on insulin resistance without affecting body fat gain on a HFD [17]. In our study, the significantly higher HOMA-IR level could be seen in HFD group, and a distinct protection could also be seen as a consequence of dietary incorporation of WPI/GOS, and the maximal effect on the administration of the two functional components.
Whey protein is rich in BCAAs, e.g., leucine. Petersen et al. [18] explored the glycemic effect of adding escalating doses of a glycemic index lowering peptide fraction (rich in BCAAs) from whey to a glucose drink and reported decreased postprandial blood glucose levels.
Improved insulin sensitivity, mediated through leucine supplementation or administration of a high amount of whey protein diet for 20 weeks in male C57BL/6 mice, which was fed a HFD (20% w/w of fat), has been presented by the other research group also [19]. Shertzer et al. [20] evaluated the influence of WPI on systemic energy balance and metabolic changes in female C57BL/6J mice fed a HFD (40% energy derived from fat) for 11 weeks with or without 100 g WPI/L drinking water. Mice administered WPI had improved glucose tolerance and insulin sensitivity. They also showed that HOMA-IR values in mice receiving WPI were one-third the value observed with HFD alone.
Our findings also indicated greater insulin sensitivity in the HFD-WPI group as the HOMA-IR in this group was 42.3% of the value in HFD group at 18 weeks. Improvement in insulin sensitivity could be observed as a consequence of dietary incorporation of GOS also. The maximum positive effect on insulin sensitivity was recorded in HFD-WPI-GOS group as the HOMA-IR was 37.5% of the value in HFD group. In a recent study, Tranberg et al. [15] replaced casein with whey in HFD of C57BL/6NT ac mice and found that whey significantly alleviated certain parameters of the metabolic syndrome compared to casein along with attenuated glucose intolerance and insulin resistance in 14 weeks. The effectiveness of different prebiotic fibers in improvement of insulin sensitivity has been reported in different animal studies and human trials; as significant blunting of hyperglycemia and hyperinsulinism on administration of 10% (w/v) gum acacia dissolved in tap water to mice [21] and the lowering effects on of insulin concentration by administration of FOS [22] and by Bi2muno (B-GOS), a galactooligosaccharide mixture [23] in humans have been reported [21-23].
In this study, we could also demonstrate a significant decrease in serum GLP-1 level as a consequence of HFD feeding, and protective effect of dietary incorporation of WPI/GOS against the decrease in serum GLP-1 level . Akhavan et al. [24] in their attempt to identify the mechanism of action of whey protein on area under curve of insulin on the reduction of post-meal glycemia in healthy young men, reported that compared with glucose, whey protein resulted in higher post-meal GLP-1 and PYY, and lower insulin concentrations without altering the insulin secretion. They concluded that premeal consumption of whey protein lowered postmeal glycemia by both insulin-dependent and insulin-independent mechanisms.
However, McAllan et al. [25] did not observe any impact of WPI when compared to casein on GLP-1 level in C57BL/6J mice fed HFD for 8 weeks. The significant increase in GLP-1 due to GOS administration, observed in this study, is in conformity with similar effects of other prebiotic fibers, viz., higher level of GLP-1 and PYY in HF-arabinoxylan oligosaccharides than in HF group mice [26], higher GLP-1 (7-36) amide concentration in portal vein serum of OFS and synergy-1 (consisting of Raftilose P95-Raftiline HP 1:1) fed Wistar rats [7].
Insulin resistance is the hallmark of metabolic syndrome. Increased endogenous glucose production has been recognized to be a crucial step during the development of illness from insulin resistance toward impaired glucose tolerance and further to diabetes [27]. Pillot et al. 2009 [27] have demonstrated that small intestine is the third gluconeogenic organ (after liver and kidney) expressing glucose 6-phosphase and other genes required for gluconeogenesis, and is able to contribute to endogenous glucose production in fasting situation and insulinopenic diabetes. In our study, significant increase in mRNA expression of PEPCK, as well as glucose 6-phosphase in liver of HFD, fed mice could be observed. However, only a tendency was observed in small intestine. The two functional components, WPI and GOS, were found to be effective in resisting the upregulation of PEPCK, as well as glucose 6-phosphase. Mithieux et al. [4] have reported in that the protein diet-induced glucose release by the intestine, initiating satiety signals to the brains [28,29]. Reports are also available suggesting that the presence of glucose and insulin in the portal blood is necessary and sufficient to suppress hepatic glucose release and promote glycogen storage. The results can be correlated with the decreased expression of two important gluconeogenesis genes (PEPCK and glucose 6-phosphase) and decreased blood glucose levels on administration of WPI and/or GOS. Pillot et al [27] have strongly suggested that a redistribution of glucose production among gluconeogenic organs might occur on protein feeding. Contrary to expectations, we could not observe significant differences in the expression of PEPCK and glucose 6-phosphase in the small intestine of different treatment groups.
CONCLUSION
In this study, we have been able to demonstrate the effectiveness of WPI of high purity (80-90%) and a GOS preparation (70-75% purity) in amelioration of blood glucose levels under HFD fed conditions in C57BL/6 mice. To the best of our knowledge, this is the first report delineating the potential of GOS in improvement of glucose homeostasis under HFD fed conditions. Both types of functional components exhibited resistance to HFD-induced hyperinsulinemia and HOMA-IR . There seemed to be some additive effect in terms of resistance to the increase in insulin as well as HOMA-IR score, however, it was not significantly higher than the effects due to individual ingredients. Certainly, more studies are warranted to link the influences of these functional components of different chemical architecture on gut physiology and glucose metabolism.
ACKNOWLEDGMENTS
PKK expresses sincere thanks the ICAR-National Dairy Research Institute for providing the Institute’s fellowship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136(1 Suppl):319S–23. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 2.Veldhorst M, Nieuwenhuizen A, Hochstenbach-Waelen A, van Vught A, Westerterp K, Engelen M, et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–82. doi: 10.1016/j.physbeh.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Maurer AD, Chen Q, McPherson C, Reimer RA. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. J Physiol. 2009;587:679–91. doi: 10.1113/jphysiol.2008.161844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mithieux G, Misery P, Magnan C, Pillot B, Gautier-Stein A, Bernard C, et al. Portal sensing of intestinal gluconeogenesis is a mechanistic link in the diminution of food intake induced by diet protein. Cell Metab. 2005;2:321–9. doi: 10.1016/j.cmet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 6.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, et al. Prebiotic effects: Metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–6. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- 8.Yang ST, Silva EM. Novel products and new technologies for use of a familiar carbohydrate, milk lactose. J Dairy Sci. 1995;78:2541–62. doi: 10.3168/jds.S0022-0302(95)76884-9. [DOI] [PubMed] [Google Scholar]
- 9.Arora T, Sharma R. Prebiotic effectiveness of galactooligosaccharides and b-glucan in stimulation of growth of Lactobacillus acidophilus NCDC 13 in vitro. Curr Top Nutraceutical Res. 2011;9:67–70. [Google Scholar]
- 10.Hernandez-Hernandez O, Marin-Manzano M, Rubio L, Moreno F, Sanz M, Clemente A. Monomer and linkage type of galacto-oligosaccharides affect their resistance to ileal digestion and prebiotic properties in rats. J Nutr. 2012;142:1232–9. doi: 10.3945/jn.111.155762. [DOI] [PubMed] [Google Scholar]
- 11.Foolad N, Armstrong AW. Prebiotics and probiotics: The prevention and reduction in severity of atopic dermatitis in children. Benef Microbes. 2014;5:151–60. doi: 10.3920/BM2013.0034. [DOI] [PubMed] [Google Scholar]
- 12.Haffner S, Miettinen H, Stern M. The homeostasis model in the San Antonio heart study. Diabetes Care. 1997;20:1087–92. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 13.Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Pal S, Ellis V, Dhaliwal S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br J Nutr. 2010;104:716–23. doi: 10.1017/S0007114510000991. [DOI] [PubMed] [Google Scholar]
- 15.Tranberg B, Hellgren LI, Lykkesfeldt J, Sejrsen K, Jeamet A, Rune I, et al. Whey protein reduces early life weight gain in mice fed a high-fat diet. PLoS One. 2013;8:e71439. doi: 10.1371/journal.pone.0071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–54. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 17.Macortela Y, Emanuelli B, Bang AM, Espinoza D, Boucher J, Beebe K, et al. Dietary leucine - An environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen BL, Ward LS, Bastian ED, Jenkins AL, Campbell J, Vuksan V. A whey protein supplement decreases post-prandial glycemia. Nutr J. 2009;8:47. doi: 10.1186/1475-2891-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freudenberg A, Petzke KJ, Klaus S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J Nutr Biochem. 2012;23:1524–30. doi: 10.1016/j.jnutbio.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Shertzer HG, Woods SE, Krishan M, Genter MB, Pearson KJ. Dietary whey protein lowers the risk for metabolic disease in mice fed a high-fat diet. J Nutr. 2011;141:582–7. doi: 10.3945/jn.110.133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasir O, Artunc F, Wang K, Rexhepaj R, Foller M, Ebrahim A, et al. Downregulation of mouse intestinal Na+-coupled glucose transporter SGLT1 by gum Arabic (Acacia senegal) Cell Physiol Biochem. 2010;25:203–10. doi: 10.1159/000276554. [DOI] [PubMed] [Google Scholar]
- 22.Genta S, Cabrera W, Habib N, Pons J, Carillo IM, Grau A, et al. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin Nutr. 2009;28:182–7. doi: 10.1016/j.clnu.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Vulevic J, Juric A, Tzortzis G, Gibson GR. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J Nutr. 2013;143:324–31. doi: 10.3945/jn.112.166132. [DOI] [PubMed] [Google Scholar]
- 24.Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr. 2010;91:966–75. doi: 10.3945/ajcn.2009.28406. [DOI] [PubMed] [Google Scholar]
- 25.McAllan L, Keane D, Schellekens H, Roche HM, Korpela R, Cryan JF, et al. Whey protein isolate counteracts the effects of a high-fat diet on energy intake and hypothalamic and adipose tissue expression of energy balance-related genes. Br J Nutr. 2013;110:2114–26. doi: 10.1017/S0007114513001396. [DOI] [PubMed] [Google Scholar]
- 26.Neyrinck AM, Van Hée VF, Piront N, De Backer F, Toussaint O, Cani PD, et al. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr Diabetes. 2012;2:e28. doi: 10.1038/nutd.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillot B, Soty M, Gautier-Stein A, Zitoun C, Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology. 2009;150:616–24. doi: 10.1210/en.2008-0601. [DOI] [PubMed] [Google Scholar]
- 28.Sindelar DK, Chu CA, Neal DW, Cherrington AD. Interaction of equal increments in arterial and portal vein insulin on hepatic glucose production in the dog. Am J Physiol. 1997;273:E972–80. doi: 10.1152/ajpendo.1997.273.5.E972. [DOI] [PubMed] [Google Scholar]
- 29.Guignot L, Mithieux G. Mechanisms by which insulin, associated or not with glucose, may inhibit hepatic glucose production in the rat. Am J Physiol. 1999;277:E984–9. doi: 10.1152/ajpendo.1999.277.6.E984. [DOI] [PubMed] [Google Scholar]


