Despite dramatic improvements in treatment and survival, acute lymphoid malignancies remain the leading cause of death in children with cancer and a highly lethal malignancy in adults [1]. Acute lymphoblastic leukemia (ALL) is the most common lymphoid tumor in children, and includes B-lineage acute lymphocytic leukemia, (B-ALL), which comprises 25% of all cancers occurring in children under 15 years of age [1]. T-lineage ALL accounts for 15% of all childhood ALL and carries a worse prognosis due higher relapse rates. Burkitts lymphoma/leukemia is an aggressive lymphoid malignancy of more mature B cells that presents in childhood or adults. Although most children with ALL or Burkitts are cured with current chemotherapeutic regimens, approximately 15% will relapse and 5–8% will ultimately succumb to their disease [1]. Even patients who are cured of their disease are at risk for long-germ sequelae, including obesity, diabetes, and heart disease. Adults with these lymphoid tumors fare even worse, particularly older adults who are frequently unable to tolerate intensive cytotoxic therapies. Thus, there is an urgent need for studies to elucidate molecular mechanisms that could be targeted in therapy, and in particular, to identify therapies that are well-tolerated and without significant toxicities.
Increasing evidence underscores a central role for chromatin remodeling proteins in malignant hematopoiesis. In fact, nuclear chromatin structure is the most important morphologic feature that distinguishes a leukemic blast from a normal white blood cell. The high mobility group A1 (HMGA1) chromatin remodeling proteins have emerged as master regulators of tumor progression, refractory disease, and cancer stem cell properties in diverse malignancies [2–14]. These proteins include the HMGA1a/HMGA1b isoforms, which result from alternatively spliced mRNA [2]. The HMGA1 gene is highly expressed during embryogenesis, with low or undetectable levels in adult, differentiated tissues. HMGA1 gene expression and proteins are enriched in all high-grade, poorly differentiated or refractory tumors studied to date [2–14], and high expression portends a poor prognosis in childhood B-ALL [13], and other diverse tumor types. Because HMGA1 regulates gene expression by remodeling chromatin and recruiting transcription factor complexes to AT-rich regions in DNA, targeting downstream pathways induced by HMGA1 provides an approach to disrupt its function. We previously discovered that HMGA1 induces expression of diverse genes that drive tumor progression, including signal transducer and activator of transcription 3 (STAT3) [6,14]. Similar to HMGA1, STAT3 is also linked to refractory status in diverse tumors and cancer stem cell properties [15].
To determine if STAT3 cold be targeted in aggressive lymphoid malignancies overexpressing HMGA1, we tested BP-1-102, a salicylic acid-based inhibitor, in preclinical models of ALL and Burkitts lymphoma [15]. BP-1-102 prevents STAT3 phosphorylation at tyrosine 705 (Tyr705), thereby inhibiting STAT3 dimerization, DNA binding, and activation of downstream genes, with little or no effect on phosphorylation of Shc, Src, Jak-1/2, Erk1/2 or Akt [15]. BP-1-102 also decreases nuclear and cytoplasmic phosphorylated STAT3 (pSTAT3). Importantly, cytotoxicity was not observed in non-transformed cell lines or transformed cell lines lacking STAT3 activation treated with BP-1-102 [15]. We therefore treated cultured cells derived from B-lymphoid tumors previously shown to express high levels of HMGA1 and STAT3, including B-ALL (REH cells) and Burkitts leukemia (Ramos) at concentrations ranging from 5–40 µM. We discovered a striking arrest in proliferation at concentrations as low as 5 µM (Fig. 1A). Next, we assessed T-ALL cells (Jurkat; 5 µM) and observed decreased proliferation, albeit less dramatic as compared to the B-lineage cell lines (Fig. 1A). After exposure to BP-1-102 for 96 hours, proliferation in Jurkat cells increased, which could reflect emerging resistance to BP-1-102 or decreased drug stability in these cells.
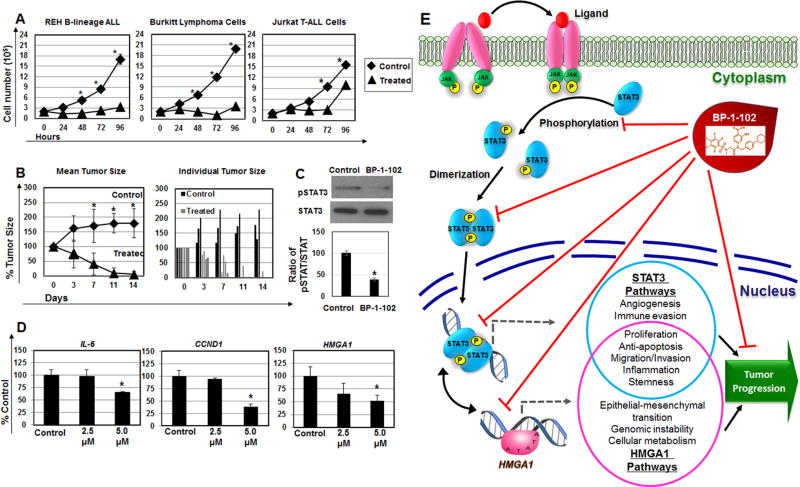
Figure 1. BP-1-102 inhibits proliferation, tumor growth, STAT3 phosphorylation, STAT3 transcriptional targets, and HMGA1 gene expression in REH B-lineage ALL cells.
(A) BP-1-102 arrests cell proliferation in REH B-ALL, Burktte (Ramos) and Jurkat T-ALL cells grown in vitro. Cells were cultured for 96 hours in the presence of 5 uM BP-1-102. Cell number was assessed every 24 hours. Note that this concentration (5 µM) is readily achieved in plasma of murine models after oral administration. (* denotes P <0.05; student’s t-test).
(B) Mice bearing REH tumors were treated orally by gavage with 3.0 mg/kg of inhibitor dissolved in dimethyl sulfoxide (DMSO; 100 mg/mL) as vehicle daily for 14 days; the control arm received the same volume of vehicle alone by gavage daily. Tumor sizes were measured every 2–3 days. All treated mice responded, and three tumors disappeared. Tumors were 100–200 mm3 at the start of treatment, and considered 100%. The difference between the control and inhibitor treatment arms was significant by day 7 and remained statistically significant throughout the remainder of the treatment as assessed by student’s t-test (P = 0.04 on day 7; P = 0.004 on day 11; P = 0.02 on day 14). The bar graph shows individual tumors. As above, tumors were assigned a value of 100% at the start of therapy. As shown, 3 treated tumors completely disappeared, and 2 regressed dramatically. (* denotes P <0.05).
(C) Western blot shows a decrease in relative pSTAT:STAT3 in REH cells treated with BP-102 at 5.0 µM at 6 hours. The bar graph shows densitometry of the ratio of pSTAT3/STAT3 from control cells (assigned a value of 100%) compared to BP-1-102 treated cells (100% ± 5.4% for controla arm versus 38.7% ± 3.2% for BR-1-102 treatment arm; P < 0.001 by student’s t-test).
(D) The STAT3 transcriptional target genes, CCND1 and IL-6, are repressed in REH B-ALL cells following 6 hours of treatment with BP-1-102. HMGA1 is also repressed in REH B-ALL cells treated with BP-1-102 (at 6 hours). Gene expression was normalized to the PO gene. (*P <0.05 by student’s t-test).
(E) Model for BP-1-102 anti-tumor activity: BP-1-102 blocks STAT3 phosphorylation, dimerization, localization to the nucleus, and activation of STAT3 tumor promoting pathways, including HMGA1. HMGA1 also induces the STAT3 gene, and other tumor progression pathways [6].
Next, we sought to determine whether BP-1-102 has anti-tumor effects in vivo. We therefore treated nude mice with B-ALL (REH) xenograft tumors (≥100 mm3) following subcutaneous implantation (107 cells). After 2 weeks of BP-1-102 therapy (3 mg/kg by oral gavage), there was a marked regression in tumors in the treatment arm with rapid tumor enlargement in the control arm (vehicle alone; Fig. 1B). There was no evidence for toxicity based on similar weight gain in treated and control mice nor was there evident toxicity on gross necropsy of the liver, kidney, heart, gastrointestinal tract, and lungs (not shown). In contrast to the dramatic tumor regression in REH B-ALL cells, we observed no effect on tumor growth in vivo in xenografts generated from Burkitts or Jurkat cells (not shown). We also tested our Hmga1 transgenic model crossed onto the Cdkn2a null background, which develop aggressive T-lymphoid tumors with complete penetrance by 20 weeks of age [8]. In prior studies, we demonstrated that spleen weight is a reliable surrogate for tumor burden in this model [14]. After 2 weeks of gavage therapy with BP-1-102, however, there was no effect on tumor growth from therapy (not shown). The basis for the treatment failures is unclear, but could reflect lower levels of pSTAT3 or less dependence upon pSTAT3 pathways. Alternatively, resistant tumors could evolve to maintain exceptionally high levels of pSTAT3 that cannot be depleted by the inhibitor.
Because BP-1-102 had significant anti-proliferative effects in vitro and anti-tumor effects in vivo in B-ALL cells, we reasoned that BP-1-102 effectively blocks STAT3 phosphorylation and represses downstream genes in this setting. To test this, we performed Western analysis of cell extracts from REH cells before and after BP-1-102. There was a decrease in phosphorylated STAT3 at Tyr705 (pSTAT2; Fig. 1E). To determine whether BP-1-102 represses STAT3 transcriptional target genes, we performed quantitative RT-PCR for mRNA corresponding to CCND1, IL-6, STAT3, and MCL-1 in REH cells. Both CCND1 and IL-6 were repressed (Fig. 1F), although neither STAT3 nor MCL1 expression changed (Fig. 1G). These findings indicate that BP-1-102 blocks STAT3 phosphorylation in REH B-ALL cells and represses a subset of STAT3 transcriptional targets, namely CCND1 and IL-6. Because there was a dramatic anti-proliferative response to BP-1-102 in lymphoid tumor cells which paralleled what we reported in Ramos Burkitt cells and other cancer cells after silencing HMGA1 [3–4,10], we hypothesized that BP-1-102 also represses HMGA1 (Fig. 1H). We found a significant decrease in HMGA1 mRNA in REH cells following treatment with BP-1-102, suggesting that STAT3 directly or indirectly induces HMGA1 expression. Interestingly, we identified a conserved STAT3 consensus DNA binding site (TTN5AA) at position −1250 in humans (−1073 in mice) that may mediate STAT3 binding and promoter transactivation.
Here, we show for the first time dramatic anti-proliferative and anti-tumor efficacy with BP-1-102 in REH B-ALL cells, using both in vitro and in vivo preclinical models. The STAT3 transcriptional target genes, IL-6 and CCND1, were also repressed. Although BP-1-102 disrupted proliferation of Burkitts lymphoma and Jurkat T-cell ALL tumor cells in vitro, there was no anti-tumor efficacy in murine xenograft models. While the basis for this is unclear, the microenvironment of resistant tumor cells could provide a protective niche, either by limiting exposure to the drug, or by inducing alternative oncogenic pathways that bypass the inhibitory effects of BP-1-102. In the studies reported here, we focused on the response of aggressive tumor cell lines to BP-1-102. In published work, there was no demonstrable toxicity to cells derived from normal thymus stromal epithelium (TE-71), Stat3-null mouse embryonic fibroblasts, or transformed cells that lack aberrantly activated Stat3 (NIH 3T3/v-Ras, A2789 human ovarian carcinoma cells) [15]. The efficacy of oral administration in the REH B-ALL tumor model presented here along with prior studies demonstrating anti-tumor effects in solid tumors models suggest that BP-1-102, or similar agents, will be effective drugs in diverse malignancies. Further preclinical studies are warranted to better define the bioavailability of this drug in human plasma, downstream metabolites, and to determine whether any off-target effects contribute to the anti-tumor efficacy.
Unexpectedly, we also discovered that BP-1-102 represses HMGA1, thus providing evidence for an HMGA1-STAT3 “feed forward loop” (Fig. 1H). We showed previously that HMGA1 induces STAT3 expression [6], and our data here suggests that STAT3 also feeds forward to up-regulate HMGA1, leading to enhanced expression of both genes during tumor progression. This feed forward loop could drive a stem-like state in tumor cells. Consistent with this model, we also found a putative STAT3 consensus DNA binding site (TTN5AA) in the HMGA1 promoter region that is conserved in humans and mice and could mediate STAT3 dimer binding and transactivation. The contribution of the putative STAT3 binding site to HMGA1 induction is not yet known, however. Alternatively, or in conjunction with direct transactivation, STAT3 could induce downstream factors that up-regulate HMGA1 expression. STAT3 regulates many genes encoding inflammatory cytokines and signals, and HMGA1 is also induced in the setting of inflammation or viral infection [2]. Thus, STAT3 inflammatory mediators could amplify both HMGA1 expression and STAT3 signaling. Further work will be needed to elucidate the relevant pathways affected by BP-1-102 or similar agents in responsive tumors. Nonetheless, this brief report highlights the potent anti-proliferative and anti-tumor effects of STAT3 inhibition in B-ALL cells in preclinical models, and provides compelling data that targeting STAT3 and HMGA1 could be effective adjuvant therapy in a subset of lymphoid malignancies.
References
- 1.Hunger SP, Loh ML, Whitlock JA, et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:957–63. doi: 10.1002/pbc.24420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resar LM. The high mobility group A1 gene: Transforming inflammatory signals into cancer? Cancer Res. 2010;70:436–9. doi: 10.1158/0008-5472.CAN-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood LJ, Mukherjee M, Dolde CE, et al. HMG-I/Y, a new c-myc target gene and potential oncogene. Mol Cell Biol. 2000;20:5490–502. doi: 10.1128/mcb.20.15.5490-5502.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Sumter TF, Bhattacharya R, et al. The HMG-I oncogene causes highly penetrant, aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res. 2004;64:3371–5. doi: 10.1158/0008-5472.CAN-04-0044. [DOI] [PubMed] [Google Scholar]
- 5.Pedulla ML, Treff NR, Resar LMS, Reeves R. Sequence and analysis of the murine Hmgiy (Hmga1) gene. Gene. 2001;271:51–8. doi: 10.1016/s0378-1119(01)00500-5. [DOI] [PubMed] [Google Scholar]
- 6.Hillion J, Dhara S, Sumter TF, et al. The high-mobility group A1a/signal transducer and activator of transcription-3 axis: An achilles heel for hematopoietic malignancies? Cancer Res. 2008;68:10121–7. doi: 10.1158/0008-5472.CAN-08-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuldenfrei A, Belton A, Kowalski J, et al. HMGA1 drives stem cell, inflammatory pathway, and cell cycle progression genes during lymphoid tumorigenesis. BMC Genomics. 2011;12:549. doi: 10.1186/1471-2164-12-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Cello F, Dhara S, Hristov AC, et al. Inactivation of the Cdkn2a locus cooperates with HMGA1 to drive T-cell leukemogenesis. Leuk Lymphoma. 2013;54:1762–8. doi: 10.3109/10428194.2013.764422. [DOI] [PubMed] [Google Scholar]
- 9.Nelson DM, Joseph B, Hillion J, Segal J, Karp JE, Resar LM. Flavopiridol induces BCL-2 expression and represses oncogenic transcription factors in leukemic blasts from adults with refractory acute myeloid leukemia. Leuk Lymphoma. 2011;52:1999–2006. doi: 10.3109/10428194.2011.591012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belton A, Gabrovsky A, Bae YK, et al. HMGA1 induces intestinal polyposis in transgenic mice and drives tumor progression and stem cell properties in colon cancer cells. PLoS One. 2012;7:e30034. doi: 10.1371/journal.pone.0030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SN, Kerr C, Cope L, et al. HMGA1 reprograms somatic cells into pluripotent stem cells by inducing stem cell transcriptional networks. PLoS One. 2012;7:e48533. doi: 10.1371/journal.pone.0048533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somervaille TC, Matheny CJ, Spencer GJ, et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–40. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, Di Cello F, Kowalski J, Hristov AC, Tsai H-L, Bhojwana D, Meyer JA, Carroll WL, Belton A, Resar LMS. HMGA1 overexpression correlates with relapse in childhood B-lineage acute lymphoblastic leukemia. Leuk Lymphoma. 2013;54:2565–7. doi: 10.3109/10428194.2013.782610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillion J, Di Cello F, Belton A, Shah SN, Turkson J, Ning J, Tweardy D, Huso, Resar LMS. Nanoparticle delivery of inhibitory STAT3 GQ-oligonucleotides blocks tumor growth in an HMGA1 transgenic model of T-cell leukemia. Leuk Lymphoma. 2014;55:1194–7. doi: 10.3109/10428194.2013.821202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Yue P, Page BD, et al. Orally bioavailable small-molecule inhibitor of transcription factor Stat3 regresses human breast and lung cancer xenografts. Proc Natl Acad Sci U S A. 2012;109:9623–9628. doi: 10.1073/pnas.1121606109. [DOI] [PMC free article] [PubMed] [Google Scholar]