Abstract
Ocular complications occur after transplant in 60–90% of chronic graft versus host disease (GVHD) patients and significantly impair vision-related quality of life. Ocular surface inflammation and dry eye disease (DED) are the most common manifestations of ocular GVHD (oGVHD). oGVHD can be viewed as an excellent pre- clinical model that can be studied to understand the immune pathogenesis of this common and debilitating disease. A limitation of this is that only a few experimental models mimic the ocular complications following HSCT and have focused on the acute GVHD process. To address this issue, we used a pre-clinical animal model developed by our group where ocular involvement was preceded by systemic GVHD to gain insight regarding the contributing immune mechanisms. Employing this "MUD" model enabled the development of a clinical scoring criterion, which readily identified different degrees of ocular pathology at both the ocular surface and adnexa dependent on the level of conditioning prior to HSCT. As far as we are aware we report that for the first time these clinical and immune responses occur not only on the ocular surface, but also heavily involve the lid margin region. In total, the present study reports a pre-clinical scoring model that can be applied to animal models as investigators look to further explore GVHD's immunologic effects at the level of the ocular surface and eyelid adnexa compartments. We speculate that future studies will use this clinical scoring index in combination with what is recognized histologically and correlated with serum biomarkers being identified in chronic/ocular GVHD.
Keywords: Allogeneic Hematopoietic Stem Cell Transplant, Graft vs. Host Disease, Ocular GVHT, Ocular Adenexa, Ocular scoring, Lacrimal gland
INTRODUCTION
Graft versus host disease (GVHD) following allogeneic hematopoietic stem cell transplantation (HSCT) is a multi-organ disorder stemming from an immunological attack by donor allo-reactive T cells, resulting in damage to the liver, skin, GI tract, and hematopoietic tissues and additional compartments during chronic disease including the ocular surface of the eye[1]. Ocular complications occur after transplant in 60–90% of chronic GVHD patients and significantly impair vision-related quality of life [2–5]. (Ocular surface inflammation and dry eye disease (DED) are the most common manifestations of ocular GVHD, and are a hallmark finding in chronic GVHD[1, 2]. Similarly to other forms of dry eye syndrome related to inflammation, ocular GVHD can lead to loss of vision due to refractive changes as a result of the lacrimal film and in severe cases secondary to corneal ulceration/perforation [6]. Furthermore, it is known clinically that lid margin abnormalities contributes to ocular surface disease, often in the form of meibomian gland dysfunction[7], which is also a common manifestation of GVHD [8]). In contrast to other ocular surface disorders where there could be multiple pathways of disease, ocular GVHD disease is primarily immune mediated with a “time-zero” initiation (transplant) which allows more accurate monitoring to dissect the underlying immune pathogenesis. [9, 10]. Therefore, ocular GVHD provides a useful model to test novel therapies for the prevention and treatment of dry eye.
Interestingly, there have been reports of ocular involvement actually preceding the diagnosis of clinical chronic systemic GVHD, but regardless, early recognition of ocular pathology would enable more timely initiation of systemic and local therapies[11]. Although, specific criterion exists for the diagnosis and assessment of systemic and ocular GVHD in humans[12], precise scoring criteria to evaluate ocular involvement in animal studies has not been established. The standardization of ocular manifestations of GVHD in pre-clinical animal models would not only assist in the evaluation of patient manifestations of ocular GVHD but also provide a method to uniformly communicate progression of disease and impact of interventional therapies.
Presently, few experimental models mimic the ocular complications following HSCT, and those almost exclusively focus on the acute GVHD process [13, 14]. To address this issue, we generated a pre- clinical animal model where ocular involvement was preceded by systemic GVHD to gain insight regarding the contributing immune mechanisms [15]. Our results demonstrated that following experimental MHC-matched minor histocompatibility-mismatched HSCT, ocular GVHD involves the presence of donor T cells in the cornea, as well as conjunctiva and lacrimal gland involvement which lead to pathologic changes in the ocular compartment[15]. Notably, employing this MHC-matched, minor transplantation antigen mismatched allogeneic “MUD” model has allowed us to develop a clinical scoring criterion, which readily identified different degrees of ocular pathology at both the ocular surface and adnexa dependent on the level of conditioning prior to HSCT. In this study we exploit our ability to monitor in real time the association of these clinical changes with the development of immune responses around the ocular adnexa to validate the role of inflammation in our scoring scale. As far as we are aware we report that for the first time these clinical and immune responses occur not only on the ocular surface, but also heavily involve the lid margin region.
METHODS
Animals
All animal studies were conducted according to protocols approved by the University of Miami Animal Care and Use Committee and in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J (B6) (H2b), C3H.SW (H2b), B6.PL-Thy1a/CyJ (B6-Thy1.1) and Enhanced green fluorescent protein B6-EGFP transgenic (H2b) mice were initially obtained from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facilities at the University of Miami School of Medicine. All mice used in experiments were 8–10 weeks old, free from ocular surface and eyelid disease at baseline, and fed with a standard caloric diet for their age. The animals were routinely monitored prior to all procedures and until experiment end.
Hematopoietic stem cell transplantation (HSCT)
Mice were temporarily placed in a holding device to transport them for total body irradiation (TBI) with 7.5 or 10.5 Gy (n=8) using a Gamma Cell 40 device 3–4 hours prior to transplantation. All animals were provided antibiotic water from day -3 to day 14 post-transplant for prophylaxis against bacterial infection. Donor cells were obtained from un-manipulated mice differing from recipients at selected genetic loci. Donor B6 mice (H-2b, Thy1.1) were euthanized by cervical dislocation, and lymph node tissue were harvested and processed as previously described [16, 17]. Femurs and tibiae were removed from donor B6- eGFP+ mice and bone marrow cells (BMCs) flushed with cold RPMI. Donor marrow inoculum (TCD-BM) was prepared using anti-Thy-1.2 Miltenyi MACS magnetic beads and negative selection to remove T cells, washed, and adjusted before transplant to 5x106/ml. To prepare donor T cells, lymph node cells were incubated on anti-sIg-coated plastic dishes for 45 minutes at 4°C to remove B cells. Cell suspensions containing donor bone marrow and T cells were adjusted in serum-free RPMI to a concentration of 4.6 x106/ml for intravenous (0.5ml) injection of 2.3 x106 T cells/mouse.
Systemic GVHD Assessment
The immune phenotype of systemic GVHD was assessed by fluorescent conjugated mAbs to analyze CD4/CD8 ratio, and B cell levels in peripheral blood. Clinical scoring was performed on all mice at baseline, and recorded 2–7 weeks post-transplant
Animals were monitored for established signs of GVHD by clinical assessment using modified version of a standard scoring system previously described by Cook et al.[18]. This system incorporates 7 clinical traits measuring the degree of systemic GVHD: posture, activity, weight loss, fur texture, skin integrity, degree of alopecia, and presence of diarrhea. Each clinical parameter was scored from 0–2 resulting in a range from 0–14.
Clinical Evaluation of Ocular GVHD
Clinical photographs were obtained to evaluate clinical characteristics of ocular disease progression. Clinical components analogous to what is monitored in patients with ocular GVHD were used to develop the scoring system in mice following allogeneic HSCT. The rationale for why these scoring criteria were selected was based on clinical changes classically reported in the eyes of patients with chronic GVHD[3, 19, 20]. For the murine studies, at each time of analysis individual animals were evaluated and graded from 0–4 for the clinical parameters related to both clinical manifestations reflecting the spectrum from no involvement to severe manifestations in each anatomical domain (Table 1).
Table 1.
Total ocular GVHD Score incorporates all clinical, eGFP, and MGI scoring parameters encompassing both the lid margin scoring index and cornea scoring index to generate a total ocular GVHD score with a maximum value of 24. The level of scores obtained were compared with total systemic GVHD scores to monitor overall disease progression.
| Ocular criteria and scoring system used to assess pre-clinical model with ocular graft vs. host disease | |||||
|---|---|---|---|---|---|
| Total Ocular GVHD Score |
Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Clinical Lid Margin | Clear | Mild lid edema | Edema and partial lid closure | Edema, partial lid closure, skin swelling | Full lid closure |
| Lid Margin eGFP | Clear | Minimal eGFP infiltrate: upper/lower lid | eGFP infiltrate: upper/lower lid | eGFP infiltrate both lids & 0–1mm skin involvement | eGFP infiltrate both lids & 1mm skin involvement |
| MGI Lid Margin | 0–25 | 25–50 | 50–75 | 75–100 | >100 |
| Clinical Cornea | Clear | Epithelial haze | Diffuse keratopathy, pupil visualized | Confluent keratopathy, pupil not visualized | Ulceration |
| Cornea eGFP | Clear | eGFP infiltrate: 25%cornea / limbus | eGFP infiltrate: 50%cornea / limbus | Diffuse eGFP infiltrate: 100% cornea | Confluent eGFP infiltrate: 100% cornea |
| MGI Cornea | 0–25 | 25–50 | 50–75 | 75–100 | >100 |
In Vivo Evaluation of Immunological Evaluation of Ocular GVHD
In order to correlate the clinical ocular findings and scoring system to in-situ immunological responses, mouse recipients of eGFP+ expressing cell populations were assessed at weekly time points using intra-vital fluorescent microscopy, allowing precise measurement of eGFP in the cornea and eyelid, which was quantified as mean green intensity (MGI) as described previously[21]. Photographs of the cornea and eyelid adnexa were taken using an automated fluorescence microscope (Leica MZ16FA; Leica Microsystems, Wetzlar, Germany). The fluorescent images were analyzed using ImagePro software (Media Cybernetics, Rockville, MD, USA) and MGI calculated for the cornea and eyelid adnexa.
Statistical Analysis
Unpaired t-test was used to compare control versus experimental treatment groups for systemic and ocular GVHD scores at each weekly time points. Both eyes of each mouse used in the experiments were included in the statistical analysis. A p-value of .05 was used to determine statistical significance. All statistical calculations were performed with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA)
RESULTS
Novel clinical scoring grading of ocular involvement in a pre-clinical model of post-HSCT
Ocular GVHD represents a disease of the ocular surface that allows investigators to understand the natural history of disease and of inflammation in this compartment related to the development of dry eye in this disease. Thus, investigating how the ocular adnexa are involved in pre-clinical models of ocular GVHD should promote understanding of the mechanisms of action responsible for the development of the ocular disease. We used our previous MHC-matched, minor transplantation antigen mismatched allogeneic “MUD” to develop a novel ocular GVHD pre-clinical scoring system using criterion derived from clinical pathology typically observed in patients.
In this model, the two main ocular structures that were affected clinically were the cornea and lid margin, similar to patients undergoing ocular GVHD. To standardize usage to multiple pre-clinical allo-HSCT models, we developed the following criteria to quantitate target tissue damage. Mice were analyzed for corneal ulceration and lid margin inflammation. At each time of analysis mice were evaluated and graded from 0–4 for the clinical parameters related to both clinical manifestations reflecting the spectrum from no involvement to severe manifestations in each anatomical domain (Table 1). As demonstrated by the data, lid margin involvement was represented by the presence and degree of worsening levels of eyelid edema and closure. (Fig 1A). A clinical score of 1 was assigned to mild lid edema limited to the lid margin and a score of 3 – 4 was given when disease progression resulted in partial to complete lid closure (Fig. 1A left). Clinically, focal epithelial hazing of the ocular surface was scored a 1 (Fig. 1B left). As the severity of ocular GVHD increased, scores of 2 or 3 were given dependent on the surface area of the keratopathy (diffuse or confluent). Ulceration of the ocular surface would be scored a 4. Notably, our scoring system parallels the scoring systems previously employed to evaluate systemic GVHD in other tissues (see below).
Fig 1. Clinical and immunological criteria for pre-clinical ocular GVHD scoring.
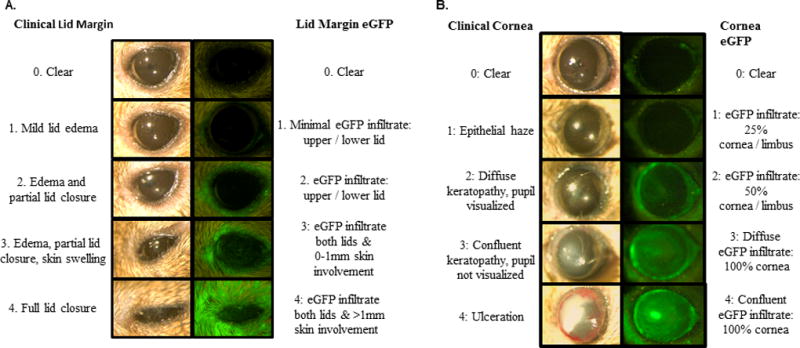
Lid margin and cornea involvement are the predominant clinical manifestations occurring in the ophthalmic compartment in the ocular adnexa following allogeneic HSCT. These clinical changes correlate with the fluorescence intensity of infiltrating cells labeled with eGFP. A) Individual mice received a score of 0 to 4 for grade of both clinical and eGFP degree of lid margin involvement. Clinical score was based on the severity of lid edema and to the degree of lid involvement causing local skin swelling and lid closure. EGFP parameters were based on the presence and intensity of eGFP cells in the upper or lower lid, and the degree of extension in the surrounding skin. B) Clinical and eGFP scoring criteria used to identify the degree of corneal involvement throughout GVHD progression. The clinical spectrum of corneal involvement ranged from clear to varying degrees of keratopathy to corneal ulceration. EGFP parameters were based on the percentage of cornea/limbus surface area infiltrated by eGFP cells.
Correlation of clinical score to donor related immune cell recruitment
To confirm that the clinical score correlated to an objective measurement of immunological mechanism, a transplant was performed utilizing eGFP+ donor cells which allows in vivo fluorescent imaging to monitor the degree of corneal and lid margin donor cell infiltration. Previously, we performed a transplant using eGFP+ donor T cells and non-fluorescent marrow in which we observed a modest level of fluorescence in the ocular compartment (Supplemental Fig. 1). Accordingly, in the present experiments we employed eGFP+ donor bone marrow. Lid margin eGFP score (Fig. 1A right) and cornea eGFP score (Fig. 1B right) were analyzed to assess the extent of eGFP+ immune cell presence in the lid and cornea. Mean green intensity (MGI) of the lid margin score (Table 1 top), and MGI of cornea score (Table 1 bottom) were recorded to analyze the intensity of the immune cell infiltrate. MGI scores were then combined with the eGFP and clinical scores to generate an index for both the lid margin and cornea, resulting in a maximum score of 12 per index (Table 1). The combined scores for the cornea and lid margin were summed to obtain a total ocular GVHD score, with a maximum value of 24. In the present transplant, mice began to express clinical disease by weeks 3–4 post-transplant as demonstrated by lid margin edema (Fig. 2A). Eyelid involvement correlated with the clinical findings of severe lid sickness and closure, as eGFP+ cells were present in the upper and lower lids, as well as the surrounding skin (Fig 2B). Clinical examination of the ocular surface identified mild corneal pathology between 6–7 weeks post-transplant. These findings indicate that using the new criteria correlates with the clinical changes and infiltration observed post-HSCT.
Fig 2. Tempo of clinical and immune infiltrative changes in pre-clinical model of GHVD.
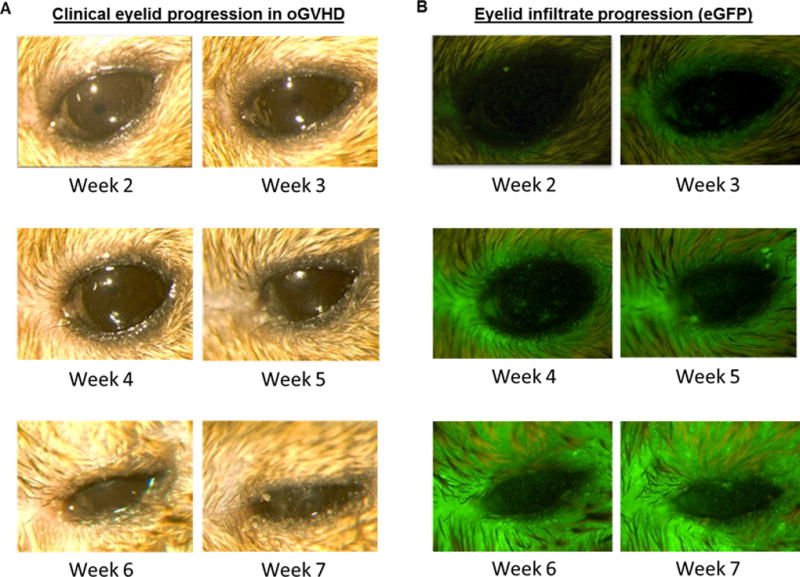
Animals were conditioned with a 10.5 Gy and transplanted with B6-eGFP bone marrow and CD90.1 T-cells. Clinical photographs and eGFP expression was captured by light and in vivo stereo fluorescent microscopy at different time-points post-allogeneic HSCT. A) external photographs demonstrating disease progression at the lid margin. At weeks 4–5, lid edema was present and by week 6, partial lid closure was evident. Severe lid closure occurred by week 7. Clinical examination revealed mild corneal pathology between weeks 6–7 post transplant. B) Fluorescent stereomicroscopy photographs demonstrating increasing eGFP cell infiltrate, with prominent lid margin involvement by week 6. Note the presence of eGFP infiltrate at week 3 correlating with worsening clinical lid edema and closure, with increased infiltrate by weeks 6 and 7. eGFP cell infiltrate localization to the ocular surface precedes development of mild clinical corneal pathology.
Clinical scoring scale reflects level of ocular involvement post-HSCT
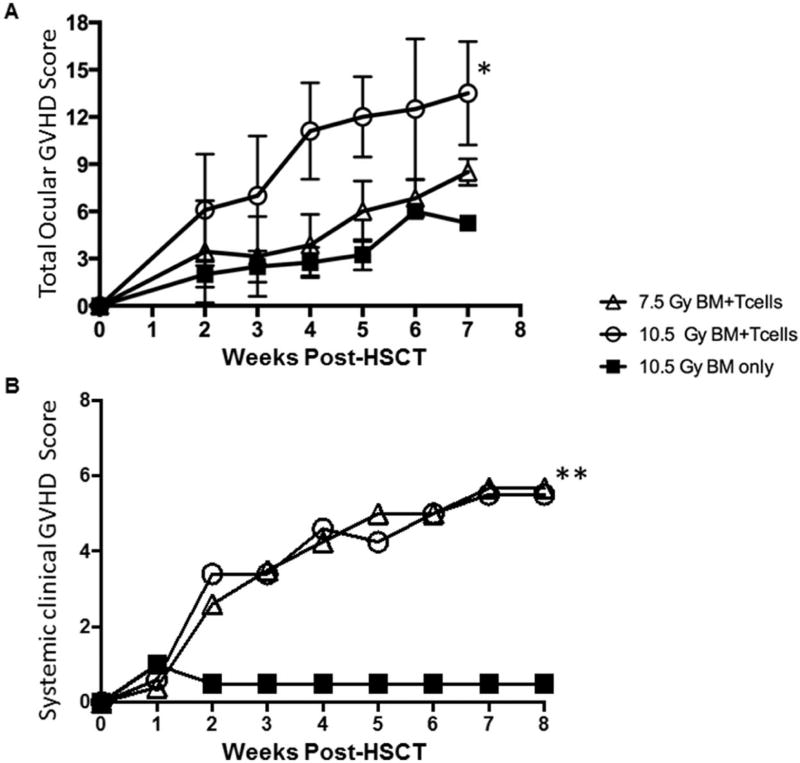
We next wanted to assess the ability of the new clinical scoring scale to detect differing degrees of ocular involvement following allogeneic HSCT. Therefore, mice were treated with different doses of TBI irradiation and administered bone marrow alone or together with donor T cells. Clinical analyses obtained during weeks 2–7 post-HSCT of both groups of mice receiving bone marrow plus T cells exhibited worsening clinical progression. Notably, involvement of the lid margin in mice conditioned with 10.5 Gy TBI and transplanted with allogeneic donor T cells and marrow versus marrow alone showed more severe ocular involvement vs. the 7.5 Gy conditioned mice as determined by clinical score (Fig. 3A). As noted above, at weeks 4–5, lid edema was present and by week 6, partial lid closure was evident (Fig. 2A). By week 7, the degree of involvement at the ocular surface and lid margin was greater in transplanted mice conditioned with 10.5 Gy vs. 7.5 Gy TBI (Fig. 3A). It should be noted that while donor T cells + TBI resulted in significant ocular involvement, high doses of TBI alone did induce detectable clinical changes. Importantly, our scoring system was able to capture this difference by the clinical appearance, EGFP expression and mean green fluorescence intensity (MGI). (Fig 3B,C). These observations were validated in mice from an independent experiment (Supplemental Fig. 2). Additionally, both groups of recipients transplanted with B6 T cells developed corneal involvement and the new scoring system also successfully captured changes and differences in this compartment (Fig 4). Of particular interest, donor eGFP+ cells correlated with these observations as they were visualized (Fig 3B) by fluorescent stereomicroscopy beginning 2 to 3 weeks post-transplant and further, confirmed by flow cytometric analyses (data not shown). These results were consistent with our prior study that identified donor T-cell populations in recipient corneas of mice with GVHD by week 3 (data not shown[15]. Recipient mice conditioned with 10.5 Gy TBI and transplanted with TCD-BM + B6 T cells demonstrated diffuse eGFP+ cell infiltrate of the cornea by week 6 and confluent infiltrate by week 7 (Fig. 4). The observed presence of eGFP infiltrate prior to clinical evidence of ocular surface disease was consistent in all mice.
Fig 3. Utilization of scoring system to quantitate disease progression and discriminate between severity of eyelid disease induced by level of total body irradiation (TBI) conditioning.
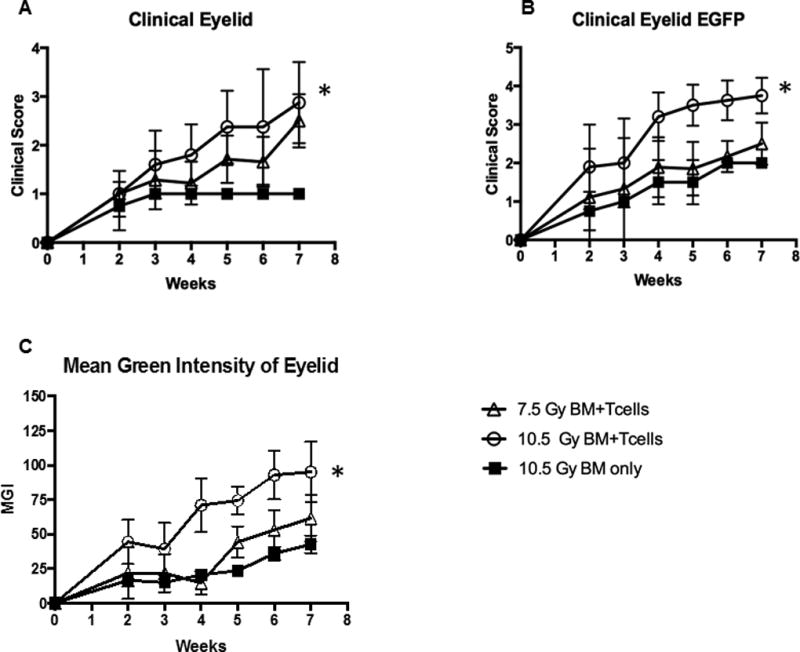
Animals were conditioned with low (7.5Gy) or high (10.5 Gy) TBI and transplanted as in Fig. 2. The scoring system (Table 1) was applied to monitor and score the development of oGVHD. A) Clinical lid margin score with 10.5Gy BM+T cells demonstrated significant eyelid involvement relative to control, with peak score at week 7. In contrast, mice with low conditioning exhibited marginal changes vs. control, B) eGFP lid margin score in mice receiving high conditioning exhibited significantly more eGFP cell infiltrate compared to control. 105Gy+ T cells reached near peak infiltrate by week 4. Similarly to clinical scoring, mice receiving low conditioning exhibited marginal changes. C) Quantification of eGFP by MGI also demonstrated significant changes in high but not low conditioned animals. Mean values are mean score +/− SEM of data from all mice in the injected T cell or control groups. *p < .05. **p < .0.01, paired t-test
Fig 4. Corneal scoring of disease and infiltration using scoring system.
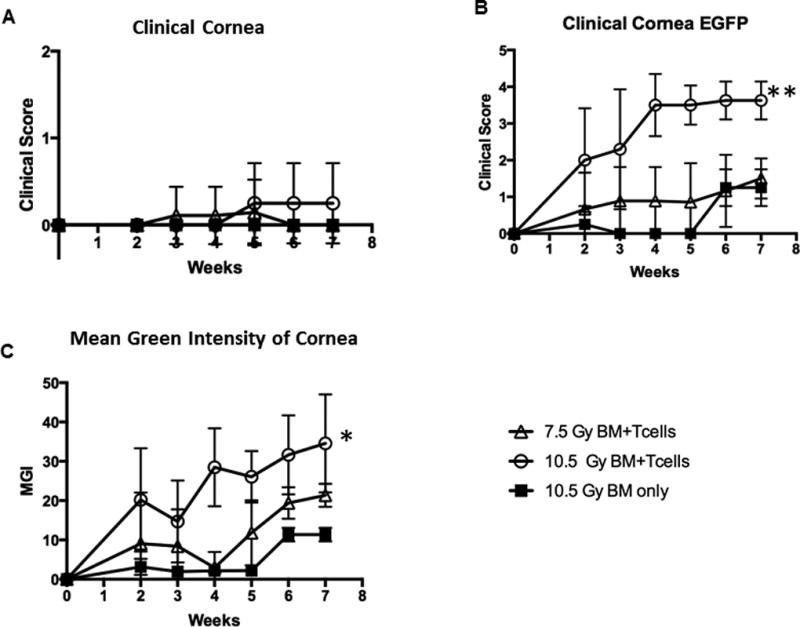
Animals from the same experiment described in Figure 3 scored (Table 1) for corneal changes and disease. A) Clinical cornea score did not detect any significant differences between all groups, B) eGFP cornea score in mice receiving high conditioning demonstrated significantly more eGFP labelled inflammatory cell infiltrate compared to controls. Similar to lid margin involvement, this reached near peak score by week 4. Marginal changes were observed in mice receiving low conditioning before transplant. C) Quantification of eGFP by MGI illustrated more intense eGFP than controls. Mean values are mean score +/− SEM of data from all mice in the injected T cell or control groups. *p < .05. **p < .0.01, paired t-test.
Correlation between the overall ocular and systemic GVHD score
Total systemic scoring combines assessment of multiple clinical signs including changes in the skin posture, weight loss and other scoring criteria (Ref = Cook) (Fig. 5A,B). Systemic involvement, evident by dramatic weight loss and hunching, significantly worsened between weeks 1 and 2 in all groups with injected B6 T-cells
Fig 5. Total ocular GVHD score identifies differences between animals expressing comparable systemic GVHD changes.
Animals as described in Figs. 3 and 4 were assessed for both overall systemic GVHD clinical changes and total ocular GVHD scoring. A) Total ocular GVHD score, combining clinical and eGFP scores for both anatomic domains, reflect overall ocular GVHD progression and illustrate significant differences between low and high conditioned groups. B) Total systemic GVHD score for all groups of conditioned mice did not identify differences between low and high conditioned animals. Mean values are mean score +/− SEM of data from all mice in the injected T cell or control groups. *p < .05. **p < .0.01, paired t-test
Mice transplanted with donor B6 TCD-BM and injected with B6 T cells developed the clinical signs of systemic and ocular GVHD (Fig. 5). The trends in degree of ocular involvement paralleled those occurring systemically in the periphery which involve assessment of the skin, weight loss and other scoring criteria [18]. To date this systemic score has not included ocular involvement commonly occurred in chronic [2–6]. To capture the overall pathological changes occurring in the ocular adnexa we developed a composite score described above (Table 1). Using this combined scoring system, changes were evident by the second to third week post-HSCT (Fig. 5A). Furthermore, a distinct difference (p < 0.5) in overall score was detected between groups receiving 10.5 Gy (higher ocular score) vs 7.5Gy (lower ocular score) level of TBI. Systemic involvement, evident by dramatic weight loss and hunching, significantly worsened between weeks 1 and 2 in all groups with injected B6 T-cells and in some experiments ocular manifestations appeared with similar kinetics (Fig. 5B). Overall, we have found the clinical corneal scores vary somewhat more than the clinical eyelid. Accordingly we propose that the total ocular GVHD scoring system correlates with systemic GVHD and therefore should be utilized to monitor ocular involvement in other pre-clinical models of graft versus host disease – particularly when non-fluorescent labeled cells are used for transplant.
DISCUSSION
Following allogeneic hematopoietic stem cell transplantation, monitoring and predicting ocular involvement in clinical transplant practice is difficult. Moreover, it remains unknown why certain patients develop more severe forms of ocular pathology than others, and the immunologic processes mediating systemic disease to predict ocular involvement are unclear [22]. Developing an assessment tool that enables visualization of varying degrees of ocular involvement would aid in further understanding onset of disease. Since laboratories continue to develop and study pre-clinical animal models towards advancing our understanding and treatment of GVHD, we wanted to create a standardized scoring system to capture clinical and pathological changes at the level of the ocular compartment. In the present study, we utilized our previously established pre-clinical animal model to develop a scoring system that can be used to assess the severity of damage in the ocular compartment [15]. Furthermore we have linked clinical evaluation with the immune process by first evaluating the changes clinically and then assessing changes by monitoring cell infiltration via imaging together with eGFP expression. Establishing a reproducible scoring system for evaluating the degree of ocular involvement from both a clinical and fluorescent imaging perspective provides a comprehensive tool for evaluation of the pathologic alterations typically accompanying pre-clinical development of ocular GVHD.
Lid margin abnormalities are a significant factor in the development of dry eye disease associated with ocular GVHD [23]. Lid margin infiltration with blepharitis and lid edema have been observed and in some cases meibomian gland dysfunction (MGD) has been reported as the second most common complication of chronic ocular GVHD[8, 24]. Our findings support this by demonstrating worsening lid margin involvement throughout GVHD progression, with increased presence of graft derived cell populations. This correlates with what has been found histologically in GVHD patients, where T-cell infiltration of the posterior lid margin causes significant meibomian gland destruction. In addition, severe inflammation leading to excessive fibrosis and obstruction of meibomian gland orifices, as well as infiltration of zeiss glands has been characterized[23]. While these clinical findings are not demonstrated histologically in this study, the model enables us to quantify and characterize immune cell trafficking to the immediate site of pathologic injury. Future studies will look to characterize the immune cell populations and pathologic alterations specifically at the lid margin in this pre-clinical model.
Consistent with heightened GVHD responses subsequent to aggressive/ablative conditioning regimens and the established correlation between systemic GVHD and ocular symptoms[25], the greatest degree of ocular infiltration in our pre-clinical transplant model using the scoring system was detected in 10.5 Gy TBI exposed mice that received donor T-cells. We wanted to develop a scoring system that would sensitively detect ocular changes during the development of systemic GVHD. Notably, imaging together with eGFP expression readily facilitated direct visualization of the immunologic responses associated with the infiltration of donor cells leading to lid margin damage and the onset of pathologic changes in the ocular compartment during onset and development of GVHD.
In total, the current study reports a pre-clinical scoring model that can be applied to animal models as investigators look to further explore GVHD’s immunologic effects at the level of the ocular surface and eyelid adnexa compartments. Using two independent MHC-matched minor antigen mismatched strain combinations (C3H→AKR/J - kindly provided by Drs. Judy Shizuru and Antonieta Mueller, Stanford University) and B10.D2→BALB/c), we have also observed similar clinical involvement in the ocular adnexa (personal observations and Supplementary Figure 4). We posit that future studies can use this clinical scoring index in combination with what is recognized histologically and correlated with novel serum biomarkers which are beginning to be identified in chronic GVHD [26, 27]. Notably, because clinical corneal scores vary somewhat more than the eyelid, we propose that the total ocular GVHD scoring system should be utilized to monitor ocular involvement.
This clinical scoring system that correlates with the immune phenotype will facilitate a standardized analysis of the immunologic and pathologic findings in the eye that occurs in animal models of ocular GVHD. Furthermore, uniform scoring of the ocular adnexa will be useful for future pre-clinical studies investigating mechanisms of ocular GVHD and the development of interventional strategies to prevent and treat ocular GVHD.
Supplementary Material
Supplemental Figure 1 Legend. Bone marrow transplant performed using 2.3x106 B6-eGFP+ T cells (CD4 + CD8) together with 5x106 B6-CD45.1 bone marrow cells transplanted into conditioned (10.5Gy) MHC-matched C3H.SW recipients (see Materials and Methods). In vivo fluorescent microscopy demonstrates the presence of eGFP+ donor T cells in the ocular ad nexa correlating with clinical ocular GVHD.
Supplemental Figure 2 Legend. Level of ocular lid involvement correlates with increased radiation conditioning doses. An independent transplant as described in Figure 3 was performed and lid margin involvement assessed during the first 5 weeks post-HSCT. Findings confirm those observations detected in a prior HSCT (Fig. 3).
Supplemental Figure 3 Legend. Level of cornea involvement also correlates with increased radiation conditioning doses. An independent transplant as described in Figure 3 was performed and lid margin involvement assessed during the first 5 weeks post-HSCT. Findings confirm those observations detected in a prior HSCT (Fig. 4).
Supplemental Figure 4 Legend. Clinical photos of the progression of ocular adnexa involvement in recipients of MUD HSCT between mice B10.D2 donors and BALB/c recipient mice. B10.D2 T cells (25x106 spleen cells) and bone marrow (8x106) were transplanted into 8.0Gy TBI treated BALB/c recipients. Animals were anaesthetized prior to photographic imaging. Progression of disease between 2 and 5 weeks is illustrated by lid margin edema, erythema and closure. Photographs of two additional BALB/c recipients illustrating ocular changes (weeks 5 and 8) post-HSCT.
Highlights.
Employing an MHC-matched, minor transplantation antigen mismatched allogeneic “MUD” model we developed clinical scoring criterion identifying degrees of ocular pathology at both the ocular surface and adnexa.
Monitoring in real time the association of these clinical changes with the development of immune responses around the ocular adnexa.
Report for the first time these clinical and immune responses occur not only on the ocular surface, but also heavily involve the lid margin region.
Studies facilitate a standardized analysis of the immunologic and pathologic findings in the eye that occurs in animal models of ocular GVHD.
Acknowledgments
The authors would like to acknowledge the Flow Cytometry Department of the Sylvester Cancer Center. The research reported in this publication was supported by grants EY024484-01 from National Eye Institute, AI055815 National Institute of Allergy and Infectious Disease, and internal support from the Sylvester Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: Author Robert Levy consults for Capricor Therapeutics and Allergan. The remaining authors have nothing to disclose.
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franklin RM, Kenyon KR, Tutschka PJ, Saral R, Green WR, Santos GW. Ocular manifestations of graft-vs-host disease. Ophthalmology. 1983;90(1):4–13. doi: 10.1016/s0161-6420(83)34604-2. [DOI] [PubMed] [Google Scholar]
- 3.Riemens A, te Boome L, Imhof S, Kuball J, Rothova A. Current insights into ocular graft-versus-host disease. Curr Opin Ophthalmol. 2010;21(6):485–94. doi: 10.1097/ICU.0b013e32833eab64. [DOI] [PubMed] [Google Scholar]
- 4.Hirst LW, Jabs DA, Tutschka PJ, Green WR, Santos GW. The eye in bone marrow transplantation. I. Clinical study. Arch Ophthalmol. 1983;101(4):580–4. doi: 10.1001/archopht.1983.01040010580010. [DOI] [PubMed] [Google Scholar]
- 5.Impact of Ocular Chronic Graft-versus-Host Disease on Quality of Life. Sun YC, Chai X, Inamoto Y, Pidala J, Martin PJ, Flowers ME, Shen TT, Lee SJ, Jagasia M. Biol Blood Marrow Transplant. 2015 Sep;21(9):1687–91. doi: 10.1016/j.bbmt.2015.05.020. Epub 2015 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sales CS, Johnston LJ, Ta CN. Long-term clinical course of dry eye in patients with chronic graft-versus-host disease referred for eye examination. Cornea. 2011;30(2):143–9. doi: 10.1097/ICO.0b013e3181e9b3bf. [DOI] [PubMed] [Google Scholar]
- 7.Knop E, Korb DR, Blackie CA, Knop N. The lid margin is an underestimated structure for preservation of ocular surface health and development of dry eye disease. Dev Ophthalmol. 2010;45:108–22. doi: 10.1159/000315024. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa Y, Okamoto S, Wakui M, et al. Dry eye after haematopoietic stem cell transplantation. British Journal of Ophthalmology. 1999;83(10):1125–1130. doi: 10.1136/bjo.83.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2(2):124–30. doi: 10.1016/s1542-0124(12)70148-9. [DOI] [PubMed] [Google Scholar]
- 10.Nassar A, Tabbara KF, Aljurf M. Ocular manifestations of graft-versus-host disease. Saudi J Ophthalmol. 2013;27(3):215–22. doi: 10.1016/j.sjopt.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balaram M, Rashid S, Dana R. Chronic ocular surface disease after allogeneic bone marrow transplantation. Ocul Surf. 2005;3(4):203–11. doi: 10.1016/s1542-0124(12)70207-0. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa Y, Kim SK, Dana R, et al. International Chronic Ocular Graft-vs-Host-Disease (GVHD) Consensus Group: proposed diagnostic criteria for chronic GVHD (Part I) Sci Rep. 2013;3:3419. doi: 10.1038/srep03419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez RL, Pérez-Simón JA, Caballero-Velazquez T, et al. Limbus damage in ocular graft-versus-host disease. Biol Blood Marrow Transplant. 2011;17(2):270–3. doi: 10.1016/j.bbmt.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Hassan AS, Clouthier SG, Ferrara JL, et al. Lacrimal gland involvement in graft-versus-host disease: a murine model. Invest Ophthalmol Vis Sci. 2005;46(8):2692–7. doi: 10.1167/iovs.05-0040. [DOI] [PubMed] [Google Scholar]
- 15.Herretes S, Ross DB, Duffort S, et al. Recruitment of Donor T Cells to the Eyes During Ocular GVHD in Recipients of MHC-Matched Allogeneic Hematopoietic Stem Cell Transplants. Invest Ophthalmol Vis Sci. 2015;56(4):2348–57. doi: 10.1167/iovs.14-15630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker MB, Altman NH, Podack ER, Levy RB. The role of cell-mediated cytotoxicity in acute GVHD after MHC-matched allogeneic bone marrow transplantation in mice. J Exp Med. 1996;183(6):2645–56. doi: 10.1084/jem.183.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker MB, Riley RL, Podack ER, Levy RB. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proc Natl Acad Sci U S A. 1997;94(4):1366–71. doi: 10.1073/pnas.94.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88(8):3230–9. [PubMed] [Google Scholar]
- 19.Ogawa Y, Yamazaki K, Kuwana M, et al. A significant role of stromal fibroblasts in rapidly progressive dry eye in patients with chronic GVHD. Invest Ophthalmol Vis Sci. 2001;42(1):111–9. [PubMed] [Google Scholar]
- 20.Espana EM, Shah S, Santhiago MR, Singh AD. Graft versus host disease: clinical evaluation, diagnosis and management. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1257–66. doi: 10.1007/s00417-013-2301-z. [DOI] [PubMed] [Google Scholar]
- 21.Carlson EC, Drazba J, Yang X, Perez VL. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47(1):241–8. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- 22.Jacobs R, Tran U, Chen H, et al. Prevalence and risk factors associated with development of ocular GVHD defined by NIH consensus criteria. Bone Marrow Transplant. 2012;47(11):1470–3. doi: 10.1038/bmt.2012.56. [DOI] [PubMed] [Google Scholar]
- 23.Ban Y, Ogawa Y, Ibrahim OM, et al. Morphologic evaluation of meibomian glands in chronic graft-versus-host disease using in vivo laser confocal microscopy. Mol Vis. 2011;17:2533–43. [PMC free article] [PubMed] [Google Scholar]
- 24.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea. 2012;31(3):299–310. doi: 10.1097/ICO.0b013e318226bf97. [DOI] [PubMed] [Google Scholar]
- 25.Balaram M, Dana MR. Phacoemulsification in patients after allogeneic bone marrow transplantation. Ophthalmology. 2001;108(9):1682–7. doi: 10.1016/s0161-6420(01)00675-3. [DOI] [PubMed] [Google Scholar]
- 26.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris AC, Ferrara JL, Braun TM, Holler E, Teshima T, Levine JE, et al. Plasma biomarkers of lower gastrointestinal and liver acute GVHD. Blood. 2012;119:2960–2963. doi: 10.1182/blood-2011-10-387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Legend. Bone marrow transplant performed using 2.3x106 B6-eGFP+ T cells (CD4 + CD8) together with 5x106 B6-CD45.1 bone marrow cells transplanted into conditioned (10.5Gy) MHC-matched C3H.SW recipients (see Materials and Methods). In vivo fluorescent microscopy demonstrates the presence of eGFP+ donor T cells in the ocular ad nexa correlating with clinical ocular GVHD.
Supplemental Figure 2 Legend. Level of ocular lid involvement correlates with increased radiation conditioning doses. An independent transplant as described in Figure 3 was performed and lid margin involvement assessed during the first 5 weeks post-HSCT. Findings confirm those observations detected in a prior HSCT (Fig. 3).
Supplemental Figure 3 Legend. Level of cornea involvement also correlates with increased radiation conditioning doses. An independent transplant as described in Figure 3 was performed and lid margin involvement assessed during the first 5 weeks post-HSCT. Findings confirm those observations detected in a prior HSCT (Fig. 4).
Supplemental Figure 4 Legend. Clinical photos of the progression of ocular adnexa involvement in recipients of MUD HSCT between mice B10.D2 donors and BALB/c recipient mice. B10.D2 T cells (25x106 spleen cells) and bone marrow (8x106) were transplanted into 8.0Gy TBI treated BALB/c recipients. Animals were anaesthetized prior to photographic imaging. Progression of disease between 2 and 5 weeks is illustrated by lid margin edema, erythema and closure. Photographs of two additional BALB/c recipients illustrating ocular changes (weeks 5 and 8) post-HSCT.