Abstract
BACKGROUND
In the United States, prices for therapeutically similar drugs vary widely, which has prompted efforts by public and private insurers to steer patients toward the lower-priced options. Under reference pricing, the insurer or employer establishes a maximum contribution it will make toward the price of a drug or procedure, and the patient pays the remainder.
METHODS
We used difference-in-differences multivariable regression methods to analyze changes in prescriptions and pricing for 1302 drugs in 78 therapeutic classes in the United States, before and after implementation of reference pricing by an alliance of private employers. We assessed trends for the study group relative to those for an employee group that was not subject to reference pricing. The study included 1,122,741 prescriptions that were reimbursed during the period from 2010 through 2014.
RESULTS
Implementation of reference pricing was associated with a higher percentage of prescriptions that were filled for the lowest-priced reference drug within its therapeutic class (difference in probability, 7.0 percentage points; 95% confidence interval [CI], 4.0 to 9.9), a lower average price paid per prescription (−13.9%; 95% CI, −23.8 to −2.7), and a higher rate of copayment by patients (5.2%; 95% CI, 0.2 to 10.4) than in the comparison group. During the first 18 months after implementation, spending for employers was $1.34 million lower and the amount of copayments for employees was $0.12 million higher than in the comparison group.
CONCLUSIONS
Implementation of reference pricing was associated with significant changes in drug selection and spending for a population of patients covered by employment-based insurance in the United States. (Funded by the Agency for Healthcare Research and Quality and the Genentech Foundation.)
In the United States, the pharmaceutical market has an increasing potential for cost-reducing competition, as manufacturers launch branded, generic, and biosimilar products that have safety and efficacy profiles equivalent to existing treatments. However, potential competition will translate into actual competition only to the extent that physicians and patients select drugs on the basis of price as well as clinical performance.
The majority of insurers, employers, and pharmacy-benefit managers negotiate price discounts and rebates from pharmaceutical manufacturers by implementing tiered formularies, which link the patients’ cost-sharing obligation (copayment) to the price of each drug.1 In tiered formularies, generic drugs often have a low copayment, branded drugs offering price discounts have a moderate copayment, and nondiscounted and specialty drugs have higher copayments and coinsurance. In response, many pharmaceutical manufacturers offer copayment-assistance programs that attenuate the patient’s incentive to prefer low-priced drugs over high-priced drugs.2,3
Tiered formularies have been successful in attenuating the growth in pharmaceutical spending. Overall drug spending remained flat for a decade beginning in 2005, as price decreases for products that faced competition compensated for price increases for products without competition. 4,5 In recent years, however, drug spending has again increased. New products are being launched at ever-higher prices, and manufacturers have substantially raised the prices of some well-established drugs.6–9
Many nations use reference pricing as one strategy for attenuating increases in pharmaceutical spending.10,11 In reference-pricing programs, individual drugs are grouped according to therapeutic class and payment is limited to the price of the cheapest, or one of the cheapest, drugs in each class. Patients who use a more expensive drug must pay the difference between the payer’s contribution limit and the price charged by the manufacturer, unless they obtain an exemption on clinical grounds. In this study, we assessed the effect of a reference-pricing initiative for outpatient drugs implemented by an alliance of private employers in the United States.
METHODS
REFERENCE-PRICING INITIATIVE
The RETA Trust is a national association of 55 Catholic organizations that purchases health care for clergy, school teachers, and other lay and religious employees.12 The trust is self-insured but contracts with private health plans and pharmacy-benefit managers to pay claims and negotiate prices with pharmaceutical manufacturers and medical providers.
During the period before the implementation of reference pricing, the RETA Trust employers maintained tiered pharmaceutical formularies for covered employees and required a $10 consumer copayment for generic drugs and a range of copayment and coinsurance levels for branded drugs. The effectiveness of the formulary had begun to weaken, however, because the copayment levels did not account for the price variation and increases within each tier. Enrollees faced an incentive to choose a drug from a low-copayment tier but not to select a low-priced drug from within the tier or to switch selection after an increase in drug price.
In July 2013, the RETA Trust instituted a reference-pricing program for 1302 outpatient drugs, which were grouped into 78 therapeutic classes on the basis of a model developed by the consulting firm RxTE Health.13 In the year before implementation, these drug classes constituted 56.1% of the $15.9 million in the RETA Trust spending under its pharmacy benefit. Therapeutic classes were defined according to the criteria of the American Hospital Formulary Service Pharmacologic–Therapeutic Classification, which is used in classifying drugs for Medicaid and Medicare Part D formularies. Drug classes were included in the reference-price initiative if there was extensive price variation among therapeutically equivalent products. Therapeutic classes that include complex and expensive specialty drugs were not included in the program but continued to be subject to the tiered formulary, in order to allow the RETA Trust to gain experience with the application of reference pricing to less complex and costly medications.
Under the reference-pricing initiative, the payment from the RETA Trust was limited to the price of the least-costly drug in each therapeutic category. Patients who used drugs for which the manufacturers charged prices that were higher than the price of the reference drug were notified that lower-priced alternatives were available. They were also advised that they should discuss the alternatives with their physicians. If physicians thought that patients would have unacceptable side effects or not have a response to the reference product or if the drug was contraindicated owing to other clinical factors, they would submit an exemption request to be reviewed by the clinical staff at the pharmacy-benefit manager contracted by the RETA Trust. The group’s policy was to accept all physician exemption requests that contained a clinical justification for continued use of an expensive drug and, in such cases, to pay for the more expensive drug. Absent a physician’s intervention, however, patients who continued to use the highest-priced drugs were obligated to pay the price difference themselves.
PHARMACY CLAIMS AND COMPARISON-GROUP DATA
We obtained pharmacy claims incurred from July 1, 2010, through December 31, 2014, from the RETA Trust. Claims included a drug identifier (National Drug Code, formulation, dose, and days of treatment), price paid (allowed charge and copayments), and patients’ demographic characteristics (sex, employee or dependent status, and ZIP Code of residence). Prescription prices and copayments by patients were calculated on a uniform monthly basis. The RETA Trust drug-data file included 573,456 prescriptions over the 5-year period. We also obtained a file containing the therapeutic class to which the drug was assigned and the reference (lowest-priced) drug for the class.
Trends in pharmaceutical prices reflect the launch of new drugs, price increases for older drugs, patent expirations, new generic competition, changes in direct-to-consumer advertising, changes in manufacturer-funded patient-support programs, and other factors. In order to assess the effect of reference pricing on drug selection and pricing, it is necessary to control for changing characteristics of markets as well as the characteristics of the patients. As a comparison group against which to evaluate the experience at the RETA Trust, we obtained 549,285 pharmaceutical claims from the health-benefits trust of a labor union that maintained a drug formulary with copayments similar to those for the RETA Trust but that did not implement reference pricing. These comparison-group claims were incurred during the same period as those incurred by RETA Trust employees. We obtained these claims from EnvisionRx, the pharmacy-benefit manager currently overseeing the RETA Trust pharmacy benefit. The total number of employees who were covered by the RETA Trust and the labor union trust fluctuated with trends in employment and employer participation in the alliances. At the time of the implementation of reference pricing in July 2013, a total of 17,500 employees were covered by the RETA Trust and 30,000 by the labor union trust.
STATISTICAL ANALYSIS
We used pharmacy claims from 2012 to illustrate the variation in prices paid by the RETA Trust for therapeutically similar drugs before the implementation of reference pricing. Within each therapeutic category, we calculated the percentage of prescriptions for the reference drug (including drugs with a price within $5 per month of the lowest-priced drug, which also were exempted from reference-price copayments), the price of the lowest-priced drug, the maximum price charged for any drug within the class, and the difference between the highest-priced and lowest-priced drugs in the class.
For each quarter between 2010 and 2014, we calculated the percentage of prescriptions that were written for the reference drug within each therapeutic class for employees at both the RETA Trust and the union trust to illustrate trends in drug selection. For each quarter, we computed the average price paid (allowed charge) for selected drugs. We calculated separately the copayment amount paid per prescription by the patients.
We used multivariable difference-in-differences regressions to measure the association between the implementation of reference pricing and three end points: the probability that a prescription would be written for the lowest-priced reference drug within its therapeutic class, the price paid, and the copayment. Difference-in-differences analysis compares changes in the treatment group with changes in the comparison group, thereby removing the effect of market-level factors that affect both groups.14 Difference-in-differences analysis interprets the effect of reference pricing as the residual change in each RETA Trust end-point measure after subtracting the trends observed in the comparison group.
The regressions included binary variables for the insurance sponsor of the patient (RETA Trust vs. union trust), the year of the claim, the interaction between the sponsor (RETA Trust vs. union trust) and indicators for the post-implementation periods, and binary variables for each therapeutic class, the patient’s sex, and the calendar month of the year. Drug prices and copayments were measured in logarithmic units to account for skewed data and to permit the interpretation of trends as the percentage rate of change. Measurements for the statistical model were estimated with the use of ordinary least squares and include robust standard errors that have been clustered according to therapeutic drug class. A variety of alternative statistical models and methods were used as checks on the robustness of the core model. Details regarding the statistical analysis are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
RESULTS
BEFORE IMPLEMENTATION OF REFERENCE PRICING
Table 1 provides data for 2012 on the minimum and maximum prices paid by the RETA Trust within each of the 30 therapeutic drug classes with the highest rates of prescription. Names of the individual drugs are provided in Table S2 in the Supplementary Appendix. This variation is what motivated the RETA Trust to adopt reference pricing in the following year. There is a substantial difference between the price of the lowest-priced drug and the highest-priced drug in almost every therapeutic class. The median variation in the monthly price of the reference drug and the price of the most costly drug within a class was $222. Across the 30 therapeutic classes, the percentage of prescriptions that were written for the lowest-priced drug ranged from 0.1% to 60.8%, with a median of 6.8%. The low rate of prescription for the lowest-priced drugs within therapeutic classes highlights the limitations of the group’s traditional tiered-formulary strategy to motivate price-conscious choice and was another factor in generating interest in reference pricing as an alternative. The percentage of prescriptions that were written for the highest-priced drug within each class ranged from 0.02 to 37.1%.
Table 1.
Price Variation and Market Shares in 2012, According to Therapeutic Class.*
| Drug Class | Prescriptions Filled | Price of Drug in Class | Difference between Highest and Lowest Price | Share of Drug in Class | ||
|---|---|---|---|---|---|---|
| Lowest-Priced | Highest-Priced | Lowest-Priced | Highest-Priced | |||
| no. | dollars | % | ||||
| Statins | 11,701 | 12.3 | 447.2 | 434.9 | 0.3 | <0.1 |
|
| ||||||
| Thyroid hormones | 8,386 | 5.3 | 33.4 | 28.1 | 0.3 | 0.1 |
|
| ||||||
| Selective serotonin-reuptake inhibitors | 7,287 | 10.3 | 201.0 | 190.7 | 10.2 | 0.1 |
|
| ||||||
| ACE inhibitors | 6,601 | 5.9 | 50.4 | 44.5 | 2.0 | 0.1 |
|
| ||||||
| Beta-blockers | 5,490 | 6.1 | 78.0 | 71.9 | 6.1 | 3.9 |
|
| ||||||
| Proton-pump inhibitors | 5,345 | 25.7 | 296.1 | 270.4 | 28.7 | 0.5 |
|
| ||||||
| Biguanides | 4,185 | 11.8 | 525.2 | 513.4 | 41.0 | 0.8 |
|
| ||||||
| Hydrocodone combinations | 4,073 | 27.8 | 297.4 | 269.6 | 7.7 | 1.4 |
|
| ||||||
| Nonsteroidal antiinflammatory drugs | 4,021 | 9.9 | 521.0 | 511.1 | 12.3 | 0.1 |
|
| ||||||
| Calcium-channel blockers | 3,864 | 14.6 | 221.8 | 207.2 | 3.2 | 0.4 |
|
| ||||||
| Angiotensin II receptor antagonists | 3,497 | 11.5 | 166.6 | 155.1 | 8.6 | 0.4 |
|
| ||||||
| Benzodiazepines | 3,286 | 3.0 | 15.1 | 12.1 | 0.1 | 7.8 |
|
| ||||||
| Anticonvulsants | 3,224 | 17.9 | 292.2 | 274.3 | 0.2 | 0.5 |
|
| ||||||
| Nasal glucocorticoids | 2,952 | 34.0 | 422.1 | 388.1 | 60.8 | 0.3 |
|
| ||||||
| Thiazides and thiazide-like diuretics | 2,647 | 4.1 | 69.4 | 65.3 | 0.3 | 0.2 |
|
| ||||||
| Serotonin–norepinephrine reuptake inhibitors | 2,644 | 41.5 | 299.7 | 258.2 | 17.7 | 2.6 |
|
| ||||||
| Beta agonists | 2,379 | 8.0 | 489.4 | 481.3 | 0.2 | 0 |
|
| ||||||
| Nonbenzodiazepine GABA-receptor modulators | 2,233 | 34.3 | 221.4 | 187.1 | 12.6 | 0.1 |
|
| ||||||
| Human insulin | 2,070 | 108.9 | 323.2 | 214.3 | 2.8 | 16.0 |
|
| ||||||
| Angiotensin II receptor antagonists and thiazide and thiazide-like diuretics | 1,987 | 16.0 | 139.5 | 123.5 | 14.0 | 6.2 |
|
| ||||||
| Other antidepressants | 1,896 | 28.0 | 97.4 | 69.4 | 2.5 | 37.1 |
|
| ||||||
| Estrogens | 1,777 | 7.3 | 146.6 | 139.3 | 7.5 | 1.1 |
|
| ||||||
| Central muscle relaxants | 1,746 | 15.4 | 781.9 | 766.5 | 8.6 | 0.1 |
|
| ||||||
| Sulfonylureas | 1,715 | 7.4 | 24.3 | 17.0 | 16.3 | 8.9 |
|
| ||||||
| Opioid agonists | 1,688 | 7.5 | 2,827.6 | 2,820.1 | 0.1 | 0.4 |
|
| ||||||
| Fibric acid derivatives | 1,568 | 18.2 | 173.0 | 154.9 | 2.0 | 2.0 |
|
| ||||||
| Leukotriene receptor antagonists | 1,544 | 57.8 | 147.8 | 90.0 | 1.1 | 0.2 |
|
| ||||||
| ACE inhibitors and thiazide and thiazide-like diuretics | 1,462 | 11.4 | 40.5 | 29.1 | 16.9 | 1.4 |
|
| ||||||
| Adrenergic combinations | 1,163 | 197.5 | 624.3 | 426.8 | 2.3 | 0.7 |
|
| ||||||
| Selective serotonin 5-HT1B/1D receptor agonists | 1,093 | 178.9 | 2,296.9 | 2,118.1 | 35.3 | 1.6 |
Shown are drug prices and the share of prescriptions in drug classes for a list of 30 commonly prescribed drug classes in 2012, before the RETA Trust, a national association of 55 Catholic organizations that purchases health care for the organizations, instituted a reference-pricing program. Certain combinations of drugs are listed under a single category because the drugs are frequently prescribed together. ACE denotes angiotensin-converting enzyme, 5-HT 5-hydroxytryptamine, and GABA gamma-aminobutyric acid.
AFTER IMPLEMENTATION OF REFERENCE PRICING
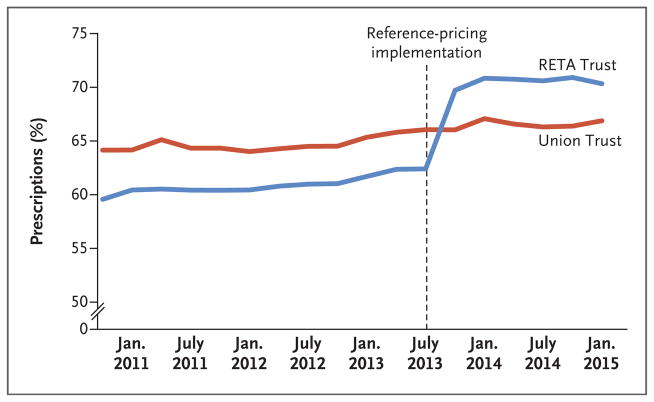
Figure 1 shows the percentage of RETA Trust and union trust prescriptions that were written for lowest-priced drugs within their therapeutic classes, from the third quarter of 2010 to the fourth quarter of 2014. The use of lowest-priced drugs was rising before the implementation of reference pricing because of the incentive created by the tiered formulary for patients to select drugs with the lowest copayment. From July 2010 through July 2013 (the date of implementation of reference pricing), the share of prescriptions that were written for the lowest-priced drugs rose from 59.5% to 62.4% for the RETA Trust and from 64.1% to 66.1% for the labor union trust. In the first quarter after implementation of reference pricing, the share of the lowest-priced reference drug in each class increased to 69.7% for the RETA Trust and then stabilized, whereas the share did not change significantly for the union trust. Approximately 1% of prescriptions were exempted from reference pricing because a physician had provided a clinical justification for continued use of a high-priced drug within the therapeutic class.
Figure 1. Percentage of Prescriptions Written for Lowest-Priced Drugs within Therapeutic Classes (2010–2014).
Shown are the percentages of prescriptions that were written for the lowest-priced drugs for the employees of the RETA Trust, a national association of 55 Catholic organizations that purchases health care for the organizations, and for those of a labor union trust before and after July 2013 (vertical dashed line), when the RETA Trust instituted a reference-pricing program for 1302 outpatient drugs, which were grouped into 78 therapeutic classes. In the first quarter after implementation of reference pricing, the use of the lowest-priced reference drug in each class increased to 69.7% for the RETA Trust and then stabilized, while the use did not change for the union trust.
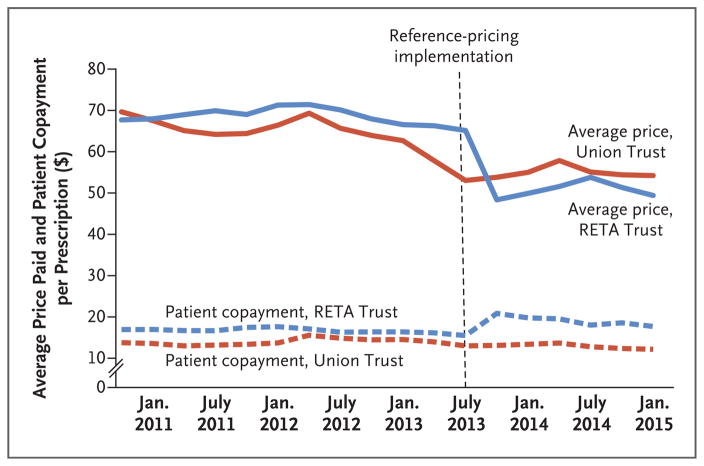
Figure 2 shows trends in the average price and the copayment per prescription. The average price that was paid declined slightly in 2012 because of patent expirations for several major drugs. Average prices then dropped substantially lower for the RETA Trust after implementation of reference pricing than for the union trust. The amount of the copayment per prescription was stable for 2 years and then increased for RETA Trust employees after the implementation of reference pricing.
Figure 2. Monthly Drug Prices and Copayments per Prescription (2010–2014).
Shown are the dollar amounts of the average price of drugs and copayments by patients per prescription before and after the implementation of reference pricing. After a slight decrease in the average drug price in 2012 because of patent expirations for several major drugs, average prices then dropped substantially lower for the RETA Trust than for the union trust after implementation of reference pricing. During the same period, the average copayment per prescription was stable for 2 years and then increased for RETA Trust employees.
THREE KEY MEASUREMENTS OF DIFFERENCES
Table 2 provides key measurements from the difference-in-differences regression analyses of the association between the implementation of reference pricing and the three end points: the probability that the lowest-priced drug in each therapeutic class was prescribed, the price of the drug, and the copayment. (The full set of measurements is presented in Tables S3, S4, and S5 in the Supplementary Appendix.)
Table 2.
Difference between RETA Trust and Union Trust in the Share of Prescriptions for Lowest-Priced Drugs within a Therapeutic Class, in Average Drug Prices, and in Patients’ Copayments (2010–2014).*
| Variable | Share of Prescriptions for Lowest-Priced Drugs† | Average Drug Price per Prescription‡ | Copayment per Prescription§ |
|---|---|---|---|
| percentage points (95% CI) | % (95% CI) | ||
| Difference between RETA Trust and union trust after implementation of reference pricing | 7.0 (4.0 to 9.9)¶ | −13.9 (−23.8 to −2.7)|| | 5.2 (0.2 to 10.4)|| |
|
| |||
| Difference between RETA Trust and union trust before implementation of reference pricing | −2.6 (−4.6 to −0.6)|| | 10.6 (−2.3 to 25.2) | 30.9 (15.1 to 48.9)¶ |
|
| |||
| Difference between RETA Trust and union trust in average market trends in first 12 mo after implementation of reference pricing | 0.8 (0.1 to 1.5)|| | −12.2 (−17.9 to −6.1)¶ | −4.9 (−8.1 to −1.5)¶ |
|
| |||
| Difference between RETA Trust and union trust in average market trends in period from 13 to 18 mo after implementation of reference pricing | 1.3 (0.1 to 2.5)|| | −18.7 (−26.0 to −10.7)¶ | −12.2 (−16.6 to −7.7)¶ |
Shown are estimates from the difference-in-differences regression analyses of the association between the implementation of reference pricing by the RETA Trust in July 2013 and three key end points, as compared with the union trust, for 1,122,741 prescriptions. Coefficients for the drug price and copayment have been log-transformed so that the values can be directly interpreted as percentages. CI denotes confidence interval.
This column shows the between-group difference in the percentage of prescriptions that were filled for the reference drugs within each therapeutic class after adjustment for changes that occurred at the union trust and for the patient’s sex and for the drug therapeutic class.
This column shows the relative percent difference between the RETA Trust and the union trust in drug prices, after adjustment for market trends that affected the two groups equally.
This column shows the relative percent difference between the RETA Trust and the union trust in patients’ copayments, after adjustment for the initial difference and the overall trend in copayments.
P<0.01.
P<0.05.
The frequency with which prescriptions were filled for the reference drugs within each therapeutic class was higher for the RETA Trust by 7.0 percentage points (95% confidence interval [CI], 4.0 to 9.9) after adjustment for changes that occurred at the union trust and for patients’ sex and for the drug therapeutic class. On the basis of the baseline rate of 62%, this difference of 7.0 percentage points translated into a rate of use of lowest-priced reference drugs that was 11.3% higher among RETA Trust employees than among union trust employees. Before the implementation of reference pricing, RETA Trust employees were 2.6% less likely than the union trust employees to use reference drugs. The rate of use of reference drugs within each therapeutic class increased for both RETA Trust and union trust employees over the course of the study period for reasons aside from implementation of reference pricing by the RETA Trust. These reasons include market-wide price declines that were due to the introduction of generic drugs into several major therapeutic classes during this period.
After the implementation of reference pricing, the RETA Trust paid prices that were 13.9% lower (95% CI, −23.8 to −2.7) than prices paid by the union trust. On the basis of the baseline mean price of $66.48, this percentage change translated into an average price that was $9.24 lower per monthly prescription for the RETA Trust than for the union trust. Multiplying the lower price per prescription by the 144,520 RETA Trust prescriptions that were filled during the 18-month period after implementation results in a savings of $1.34 million for the RETA Trust. Before the implementation of reference pricing, the RETA Trust paid an average of 10.6% more per prescription than did the union trust. Both payers benefited from an overall market trend toward price decline, with the RETA Trust paying 12.2% less during the first year after implementation of reference pricing and 18.7% less during the second year (from 13 to 18 months).
The implementation of reference pricing was associated with out-of-pocket spending that was 5.2% (95% CI, 0.2 to 10.4) higher for RETA Trust employees than for union trust employees, after adjustment for the initial difference and the overall trend in copayments. Applying this percentage change to the baseline RETA Trust copayment of $16.15 per prescription results in a $0.84 increase in copayments per prescription. This sums to $0.12 million (for 144,520 prescriptions) in higher copayments for RETA Trust employees during the 18-month period after implementation of reference pricing. RETA Trust employees paid an average of 30.9% more in copayments per prescription than did the union employees before the implementation of reference pricing, a difference that reflects the generosity of the benefits negotiated by the labor union for its members.
DISCUSSION
Under reference pricing, the payer sets a maximum payment for drugs within each therapeutic class that is equal to the lowest price, or one of the lowest prices, charged for any drug in the category. Patients who select drugs with prices above the reference level must pay the full difference themselves, unless they obtain an exemption on clinical grounds.
In this study, we evaluated the association between reference pricing, drug prescriptions, prices, and out-of-pocket copayments by patients for an association of private employers in the United States. Before July 2013, the RETA Trust paid widely different prices for drugs within therapeutic classes (Table 1). In July 2013, the RETA Trust limited its payment to the price of the least costly drugs in each class, which resulted in an increase of 7.0 percentage points in the probability that patients would select the lowest-priced drug in the therapeutic class, a 13.9% reduction in the average price paid per prescription, and a 5.2% increase in the average copayment, as compared with the union trust. In the first 18 months after implementation, reference pricing was associated with a spending reduction for the employer alliance of $1.34 million but an out-of-pocket spending increase for RETA Trust employees of $0.12 million versus the comparison group.
Pharmaceutical reference pricing has been associated with a decrease of 10 to 12% in drug prices in European and other nations, a reduction that is very similar to that reported here for a privately insured U.S. population.10,11 In the United States, reference pricing has been used primarily for surgical and diagnostic procedures and has resulted in spending reductions of 20% for joint replacement,15 18% for cataract removal,16 21% for colonoscopy,17 17% for arthroscopy,18 12% for computed tomography,19 and 32% for laboratory assays.20
The results of this study should be interpreted in light of its limitations. We evaluated a reference-pricing initiative from one alliance of private employers, and its generalizability to other employers and to public health insurance programs is unknown. The scale of the association was similar, however, to that experienced by other private U.S. employers for other tests and treatments. 21 We could not assess whether the changes in drug selection exerted any effect on adherence to medication therapy for individual employees or their health outcomes.22,23 Since we did not have data on the use of nonpharmaceutical services, we could not assess any indirect effects of reference drug pricing on the use of these services. The RETA Trust program included an exemption policy that mandated that the trust would continue to pay for high-priced drugs if the patient’s physician indicated a clinical justification. Future evaluations of reference pricing will need to assess health outcomes, especially if reference pricing is extended to complex specialty drug classes.
The recent spikes in drug prices have increased the attention that policymakers are paying to pharmaceutical spending. Reference pricing may be one instrument for influencing drug choices by patients and drug prices paid by employers and insurers. In the future, pharmaceutical manufacturers who wish to charge premium prices may need to supply evidence of commensurately premium performance.
Supplementary Material
Acknowledgments
Supported by the Agency for Healthcare Research and Quality and the Genentech Foundation.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288:1733–9. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 2.Ubel PA, Bach PB. Copay assistance for expensive drugs: a helping hand that raises costs. Ann Intern Med. 2016;165:878–9. doi: 10.7326/M16-1334. [DOI] [PubMed] [Google Scholar]
- 3.Dafny LS, Ody CJ, Schmitt MA. Undermining value-based purchasing — lessons from the pharmaceutical industry. N Engl J Med. 2016;375:2013–5. doi: 10.1056/NEJMp1607378. [DOI] [PubMed] [Google Scholar]
- 4.Aitken M, Berndt ER, Cutler D, Kleinrock M, Maini L. Has the era of slow growth for prescription drug spending ended? Health Aff (Millwood) 2016;35:1595–603. doi: 10.1377/hlthaff.2015.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estimate of Medicare Part D costs after accounting for manufacturer rebates. IMSHealth; 2016. ( http://www.imshealth.com/en/thought-leadership/quintilesims-institute/reports/estimate-of-medicare-part-d-costs-after-accounting-for-manufacturer-rebates) [Google Scholar]
- 6.Howard DH, Bach PB, Berndt ER, Conti RM. Pricing in the market for anticancer drugs. J Econ Perspect. 2015;29:139–62. doi: 10.1257/jep.29.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Bennette CS, Richards C, Sullivan SD, Ramsey SD. Steady increase in prices for oral anticancer drugs after market launch suggests a lack of competitive pressure. Health Aff (Millwood) 2016;35:805–12. doi: 10.1377/hlthaff.2015.1145. [DOI] [PubMed] [Google Scholar]
- 8.Government Accountability Office. Generic drugs under Medicare: Part D generic drug prices declined overall, but some had extraordinary price increases. 2016 Sep 12; ( http://www.gao.gov/products/GAO-16-706)
- 9.Hauptman PJ, Goff ZD, Vidic A, Chibnall JT, Bleske BE. Variability in retail pricing of generic drugs for heart failure. JAMA Intern Med. 2017;177:126–8. doi: 10.1001/jamainternmed.2016.6955. [DOI] [PubMed] [Google Scholar]
- 10.Lee JL-Y, Fischer MA, Shrank WH, Polinski JM, Choudhry NK. A systematic review of reference pricing: implications for US prescription drug spending. Am J Manag Care. 2012;18(11):e429–e437. [PubMed] [Google Scholar]
- 11.Acosta A, Ciapponi A, Aaserud M, et al. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev. 2014;10:CD005979. doi: 10.1002/14651858.CD005979.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reta — a Catholic healthcare trust. ( https://www.retatrust.org/)
- 13.RxTE health home page. ( http://www.rxtehealth.com/)
- 14.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: the difference-in-differences approach. JAMA. 2014;312:2401–2. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JC, Brown TT. Increases in consumer cost sharing redirect patient volumes and reduce hospital prices for orthopedic surgery. Health Aff (Millwood) 2013;32:1392–7. doi: 10.1377/hlthaff.2013.0188. [DOI] [PubMed] [Google Scholar]
- 16.Robinson JC, Brown T, Whaley C. Reference-based benefit design changes consumers’ choices and employers’ payments for ambulatory surgery. Health Aff (Millwood) 2015;34:415–22. doi: 10.1377/hlthaff.2014.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson JC, Brown TT, Whaley C, Finlayson E. Association of reference payment for colonoscopy with consumer choices, insurer spending, and procedural complications. JAMA Intern Med. 2015;175:1783–9. doi: 10.1001/jamainternmed.2015.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson JC, Brown TT, Whaley C, Bozic KJ. Consumer choice between hospital-based and freestanding facilities for arthroscopy: impact on prices, spending, and surgical complications. J Bone Joint Surg Am. 2015;97:1473–81. doi: 10.2106/JBJS.O.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson JC, Whaley C, Brown TT. Reference pricing, consumer cost-sharing, and insurer spending for advanced imaging tests. Med Care. 2016;54:1050–5. doi: 10.1097/MLR.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JC, Whaley C, Brown TT. Association of reference pricing for diagnostic laboratory testing with changes in patient choices, prices, and total spending for diagnostic tests. JAMA Intern Med. 2016;176:1353–9. doi: 10.1001/jamainternmed.2016.2492. [DOI] [PubMed] [Google Scholar]
- 21.Robinson JC, Brown TT, Whaley C. Reference pricing changes the “choice architecture” of health care for consumers. Health Aff (Millwood) 2017;36:524–30. doi: 10.1377/hlthaff.2016.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–9. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra A, Gruber J, McKnight R. Patient cost-sharing and hospitalization offsets in the elderly. Am Econ Rev. 2010;100:193–213. doi: 10.1257/aer.100.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.