Abstract
Linoleic acid (LA) and alpha-linolenic acid (ALA) in plant or algae oils are precursors to oxidized fatty acid metabolites known as oxylipins. Liquid chromatography tandem mass spectrometry was used to quantify oxylipins in soybean, corn, olive, canola and four high-oleic acid algae oils at room temperature or after heating for 10 minutes at 100°C. Flaxseed oil oxylipin concentrations were determined in a follow-up experiment that compared it to soybean, canola, corn and olive oil. Published economic disappearance data for soybean, canola, corn and olive oil were used to estimate daily oxylipin intake. The LA and ALA fatty acid composition of the oils was generally related to their respective oxylipin metabolites, except for olive and flaxseed oil which had higher LA-derived mono-hydroxy and ketone oxylipins than other oils, despite their low LA content. Algae oils had the least amount of oxylipins. The change in oxylipin concentrations was not significantly different amongst the oils after short-term heating. Estimated oxylipin intake from non-heated soybean, canola, corn and olive oil was 1.1 mg per person per day. These findings suggest that oils represent a dietary source of LA- and ALA- derived oxylipins and that the response of oils to short-term heating does not differ amongst the various oils.
Keywords: Linoleic acid, alpha-linolenic acid, oxidized fatty acids, oxylipins, lipidomics, plant oils, soybean, corn, canola, flaxseed, olive, algae oils, mass spectrometry
Graphical Abstract

Introduction
Plant oils are a major dietary source of the essential polyunsaturated fatty acids, linoleic (LA, 18:2n-6) and alpha-linolenic acid (ALA, 18:3n-3). LA and ALA are required for infant development and supporting optimal nutrition. The estimated requirements are 0.5–1% of calories for ALA and 2% for LA 1, 2. Currently, however, LA accounts for approximately 7% of caloric intake in the US due to the widespread consumption of high-LA oils such as soybean and corn 3. Estimated US intake of ALA is 0.72% energy 3, provided mainly through soybean oil and to a lesser extent, canola oil 3.
LA and ALA are precursors to bioactive oxidized fatty acid metabolites known as oxylipins. LA-derived oxylipins are involved in inflammatory cascades, pain perception and skin barrier integrity 4–7. Little is known about the role of ALA-derived oxylipins in mammals, although plants produce them enzymatically to regulate root development and defense against pathogens 8, 9.
Oxylipins can be synthesized in vivo from their precursor fatty acids via oxygenase enzymes 10–12, or obtained through food or dietary oils 13–16. Ingested hydroxyl, epoxy and dihydroxy oxylipins are bioavailable 17–21. LA-derived hydroperoxides have been shown to degrade in the stomach into aldehydes, which accumulate in liver 22, 23.
The oxidation of fatty acids in oils is non-enzymatic 24, although enzymatic oxidation via lipoxygenase enzymes occurs during the extraction of oils from seed sources 25. Studies reported the formation of LA-derived hydroxy, hydroperoxy, ketone dienes or epoxy monoenes in oils heated at 40, 100 or 180°C for 10–264 hours or up to 156 days 13–15. Oils with higher LA content, such as safflower oil, produced more LA-derived oxylipins as compared to low-LA oils 13–15. In these studies, oxylipins were detected after prolonged heating of the oils but not at room temperature, likely because the analytical methods involving high performance liquid chromatography with an ultraviolet detection, gas chromatography coupled to a flame ionization detection (GC-FID) or nuclear magnetic resonance, lacked sensitivity 13–15. Fankhauser-Noti et al., however, detected the presence of LA-derived mono-epoxy and di-epoxy fatty acids with GC-FID in fresh (non-heated) and 4-year old olive and sunflower oil 26. We are not aware of studies that reported on ALA-derived oxylipins in oils.
The advent of lipidomic analysis with ultra-high pressure liquid chromatography tandem mass spectrometry (UPLC-MS/MS) has enabled the separation, detection and quantitation of many oxylipin species at picomolar concentrations 27. To gain detailed analytical insight into the type and quantity of oxylipins in oils, the aim of the present study was to measure LA and ALA derived oxylipins in plant and algae oils containing varying amounts of LA and ALA at room temperature or after heating for 10 minutes with UPLC-MS/MS 27. Algae oils were tested because they are an emerging and potentially sustainable source of dietary fat 28. Thus, like plants, algae synthesize unsaturated fatty acids and contain oxygenase enzymes that may contribute to lipid oxidation during the extraction 29. Oxylipins were measured in oils at room temperature or after heating for 10 minutes at 100°C to simulate low-heat cooking or simmering conditions. This allowed us to test whether highly unsaturated oils were more vulnerable to oxidation compared to less saturated oils. Daily intake of LA and ALA derived oxylipins was also estimated based on published consumption data of commonly consumed plant oils in the US3.
We hypothesized that concentrations of LA and ALA derived oxylipins at room temperature will be proportional to their precursor fatty acid concentrations in oils, and that high LA or ALA oils will produce more oxylipins following short-term heating for 10 minutes compared to low LA or ALA oils. Understanding the dietary contribution of oils to potentially bioactive oxylipins is likely to have significant implications for human health and disease.
Materials and Methods
Chemicals and Reagents
Methanol, ethyl acetate, chloroform, toluene, and hexane were obtained from Fisher Scientific (Hampton, NH). Methanol was LC-MS grade, whereas all other solvents were HPLC grade. Acetic acid, butylated hydroxytoluene, sodium carbonate, glycerol, triphenylphosphine, ethylenediaminetetraacetic acid (EDTA), and hydrochloric acid were purchased from Sigma-Aldrich (St. Lous, MO). Oxylipin standards were purchased from Cayman Chemicals (Cayman Chemical, Ann Arbor, MI) or synthesized by Dr. Bruce Hammock’s lab at UC Davis. The synthetic standards included the following ALA-derived oxylipins: 15(16)-EpODE, 12(13)-EpODE, 9(10)-EpODE, 15,16-DiHODE, 12,13-DiHODE and 9,10-DiHODE. Fatty acid standards were purchased from NuCheck Prep (Elysian, MN). Oils were purchased from local stores.
General Study Design
Two experiments were carried out to test the aforementioned hypotheses. In the first experiment, oxylipins and fatty acids were measured in soybean, corn, canola and olive oil as well as 4 algae oils provided by TerraVia Holdings, Inc. that were high in oleic acid, low in LA and ALA, and either lacked or contained 1000 ppm of mixed tocopherols (FORTIUM® MT70 IP Liquid from Kemin IA, USA) as an antioxidant. The algae oils tested were a High Stability Algae Oil (HSAO), HSAO without added antioxidants (w/AO), Ultra Omega-9 Algae oil (“Thrive”), and “Thrive” w/AO. Oxylipins were measured at room temperature or after heating for 10 minutes at 100°C. Heat was applied at 100°C (212°F), because it is below the smoke point range of the oils used in the present study (140–244°C; 280–471°F) 30, 31.
In the second experiment, we confirmed oxylipin concentrations in off-the-shelf oils tested in Experiment 1 (corn, canola, soybean and olive oil), and compared them to flaxseed (linseed) oil, which may be prone to oxidation due to its high ALA (54% of total fatty acids) content 32. Estimated oxylipin intake levels in the US diet were then calculated from published consumption data for soybean, corn, canola and olive oil 3 and measured oxylipins in these oils. The fatty acid composition of the oils in both experiments was confirmed with GC-FID.
The same soybean, canola, and corn brands were used in both experiments. They were purchased from different markets, however. Experiment 1 oils were purchased from local supermarkets in San Francisco. Experiment 2 oils were purchased from local supermarkets in Davis. Spectrum and STAR extra virgin olive oil brands were used in Experiments 1 and 2, respectively.
Experiment 1
Five mL of each of the soybean, corn, canola, olive and 4 algae oils were added to 8 mL glass vials (17 mm diameter, 64 mm height) and heated, uncapped at 100°C on a heating block for 10 minutes. Temperature was measured each minute. Samples were staggered 1 minute apart to allow time for temperature recording (Supplementary Figure 2). Two 10 μL samples were taken from each oil at baseline (room temperature) and after 10 minutes of heating for fatty acid and oxylipin analysis (methodological details below). There were three replicates per oil in total, and each was performed on a separate day (i.e. all 8 oils heated and sampled on day 1 and then on days 2 and 3). Oils were stored in a 4°C fridge and thawed prior to aliquoting samples each day.
Experiment 2
Soybean, corn, canola, olive and flaxseed oils were purchased from various supermarket outlets in Davis (CA, USA). Supplementary Table 1 shows the oil brand, store they were purchased from, date they were purchased and expiry date. For each oil, 5 bottles from the same brand but with different lot numbers were purchased from the various stores, except for flaxseed oil, because we were not able to find the same brand in different stores. Therefore, 3 different brands of flaxseed oil were purchased, with one brand being purchased in duplicate from the same store to increase the sample size to 4 (Supplementary Table 1). The reason we aimed to keep the oil company source consistent was to confirm the validity of our methods on the same oil brand. Oxylipins were extracted and measured within a week after purchasing the oils. Fatty acid composition of the oils was confirmed a few weeks later.
Oxylipin Analysis with UPLC-MS/MS
A total of 8 LA and 8 ALA oxylipins were measured by targeted UPLC-MS/MS (Supplementary Table 2). Ten μL of oil sample were mixed with 200 μL of methanol containing 0.1% acetic acid and 0.1% butylated hydroxytoluene (BHT) after adding 10 μL surrogate standard solution (purity ≥ 95%) containing 5 pmol (per sample) of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-6-keto-PGF1α, d4-9- HODE, d4-LTB4, d4-PGE2, d4-TXB2, d6-20-HETE and d8-5-HETE in methanol27 and 10μL of antioxidant solution (0.2 mg/mL BHT, ethylenediaminetetraacetic acid (EDTA), and triphenylphosphine (TPP) in water/methanol (1:1 v/v)). While the test oils did not contain arachidonic and docosahexaenoic acid, d4-6-keto-PGF1α, d4-LTB4, d4-PGE2 and d4-TXB2, which are standards for arachidonate or docosahexaenoate derived oxylipins, constituted part of our routinely used surrogate standard mix 10, 11. They were included in the assay but were not used in any of the LA or ALA oxylipin calculations.
Oils were hydrolyzed by adding 200 μL of 0.25 M sodium carbonate solution (1.13 g in 21.3 mL water and 21.3 mL methanol) to 10 μL oil aliquot containing antioxidant and surrogate standard 10, 33, 34. The samples were vortexed and heated for 30 minutes at 60°C under constant shaking. The samples were allowed to cool to room temperature and 25 μL acetic acid and 1575 μL of Millipore water were added to each sample. Litmus paper was used to confirm that the pH was below 7 after adding the acetic acid.
Oxylipins were extracted using solid phase extraction (SPE) Waters Oasis HLB columns (60 mg, 3cc cartridges; Waters, Milford, MA). The SPE columns were washed with 4 mL ethyl acetate and twice with 4 mL of methanol and pre-conditioned twice with 4 mL of SPE wash buffer containing 95/5/0.1 v/v/v Millipore water/methanol/acetic acid. The hydrolyzed oil samples were loaded onto the column and washed with 4 mL SPE buffer twice. The SPE filter was dried under high vacuum for 20 minutes. Oxylipins were then eluted from the filter with 0.5 mL methanol and 1.5 mL ethyl acetate into collection tubes containing 6 μL of 30% glycerol (in methanol). Samples were dried with vacuum centrifugation and reconstituted in 50 μL methanol containing 200 nM 1-cyclohexyl ureido, 3-dodecanoic acid (CUDA) as a surrogate recovery standard. The reconstituted samples were filtered using Ultrafree-MC VV Centrifugal Filter (0.1 μm;EMD Millipore, Bedford, MA, USA) tubes and transferred to LC-MS/MS vials.
A surrogate standard mix containing 10, 50 or 200 nM of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-6-keto-PGF1α, d4-9-HODE, d4-LTB4, d4-PGE2, d4-TXB2, d6-20-HETE and d8-5-HETE was spiked with 200 nM CUDA and used to calculate accuracy and sample percent recovery. The accuracy was determined by dividing the observed concentrations of the surrogate standards using CUDA, by the expected concentrations and multiplying by 100 (n=3). The observed concentration of the surrogate standards was calculated as follows:
The percent recovery in our samples was calculated as follows:
The slope of surrogate standard curve was derived from a plot of the standard surrogate concentration / CUDA concentration versus surrogate area / CUDA area,
Oxylipins were analyzed on an Agilent 1200 SL LC series UPLC system (Agilent Corporation, Palo Alto, CA, USA) connected to a 4000 QTRAP tandem mass spectrometer (Applied Biosystems Instrument Corporation, Foster City, CA, USA) equipped with an electrospray source (Turbo V). The system was operated in negative electrospray ionization mode and used optimized multiple reaction monitoring (MRM) conditions 27. Oxylipins were separated on an Agilent Eclipse Plus C-18 reverse-phase column (2.1 × 150 mm, 1.8 μm particle size). The auto-sampler temperature was kept constant at 4°C and the column at 50°C. Mobile phase A contained Millipore water containing 0.1% glacial acetic acid, and mobile phase B contained acetonitrile/methanol (80/15 v/v) with 0.1% glacial acetic acid. The flow rate was 250 μL/min. Solvent B was held at 35% for 0.25 min, and then increased to 45% between 0.25 and 1 min, 55% from 1 to 3 min, 66% from 3 to 8.5 min, 72% from 8.5 to 12.5 min, 82% from 12.5 to 15 min and 95% from 15 to 16.5 min. It was maintained at 95% to 18 min, lowered to 35% from 18 to 18.1 min and held at 35% between 18.1 and 21 min. The retention time, MRM conditions, collision energy, limits of quantitation and surrogate standard used for each oxylipin are presented in Supplementary Table 3. The limits of detection were set at 3 times the signal to noise ratio, whereas the limits of quantitation were set at 10 times the signal to noise ratio.
Oil hydrolysis under air or nitrogen
To ensure that heat applied during the hydrolysis process described above does not cause oxylipin artefacts, we performed the hydrolysis reaction described above with soybean oil samples (10 uL per sample) capped under air (n=3) or flushed with nitrogen prior to capping (n=3) at 60°C for 30 minutes. The hydrolyzed oxylipins were subjected to SPE and measured on an Agilent 1290 Infinity ultrahigh-pressure liquid chromatography system interfaced to a 6460 triple-quadrupole mass spectrometer with electrospray ionization LC-MS/MS. The same oxylipin method was adapted from the 4000 QTRAP tandem mass spectrometer system (Applied Biosystems) to the Agilent 6460 tandem mass spectrometer. The data shown in Supplementary Table 4 confirm no differences between oxylipins hydrolyzed under air or nitrogen.
Fatty Acid Analysis with gas chromatography
Ten μL of oil sample was nonadecanoic acid (19:0) ethyl ester in chloroform/methanol (2:1 v/v; Experiment 1) or free heptadecanoic acid in methanol (Experiment 2) as internal standards. TFatty acids were analyzed according to the method of Ichihara et al. 35. Four hundred μL of toluene, 3 mL methanol and 600 μL of 8% hydrochloric acid in methanol solution were added to each sample before placing vials on a dry heating block at 90°C for 60 minutes. The samples were allowed to cool at room temperature for approximately 10 minutes. One mL of hexane and 1 mL of water were added to each sample. The samples were vortexed and the hexane and water layer were allowed to separate by leaving the sample undisturbed for 15 minutes. Six hundred μL of the upper hexane layer were transferred to a micro-centrifuge tube containing sodium sulfate as a drying agent. The hexane layer containing fatty acid methyl esters (FAMEs) was transferred into new micro-centrifuge tubes and stored at −80°C until analysis.
FAMEs were analyzed on a Varian 3800 gas-chromatography system equipped with a DB-23 fused silica capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). The injector and detector temperature were set at 250°C and 300°C, respectively. The initial oven temperature was held at 50°C for 2 minutes, and was increased by 10°C/min to 180°C, held at 180°C for 5 min, increased to 240°C at 5°C/min and held at 240°C for 5 min. The total run time was 37 min. The carrier gas was helium, which was maintained at a flow rate of 1.3 mL/min. A custom mix of 31 fatty acid methyl ester standards (NuChek Prep, Elysian, MN, USA) was used to identify the individual fatty acids. Retention times of the main fatty acids in oils are presented in Supplementary Table 5. Fatty acid concentrations were determined by comparison of the GC peak areas to the internal standard area. Data were expressed as percent of total identified fatty acid peaks or absolute concentrations.
Estimation of dietary LA and ALA-derived oxylipin intake levels
US oil intake data reported by Blasbalg et al. (2011)3 was used to derive the mean amount of oxylipins consumed in the US diet. The amount of soybean, corn, canola and olive oil consumed in grams per person per day based on the Blasbalg et al study is 31.8, 2.2, 2.2 and 1.9, respectively (Supplementary Table 6). This amount was multiplied by the measured mean of oxylipin concentrations in each oil, to estimate LA and ALA oxylipin intake levels, after correcting for oil density.
Statistical Analysis
Statistical analysis was performed on GraphPad Prism v. 6.05 (La Jolla, CA, USA). For Experiment 1, the effect of heating on the rise in temperature of the 8 oils over time was determined using a two-way repeated measures analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests. The differences in fatty acid and oxylipin composition and in change of oxylipin concentration after heating between the different oils were assessed using a Kruskall-Wallis one-way ANOVA. No post-hoc tests were performed in Experiment 1 because the sample size was too small (n = 3 per oil) to provide accurate comparisons without risking a type I or II statistical error. Data from Experiment 1 were presented as median and range of the lowest and highest points.
Data for Experiment 2 were analyzed by Kruskall-Wallis test, followed by Dunn’s multiple comparison test. The sample size (n= 4–5 per group) was sufficient to allow meaningful comparisons. Fatty acid and oxylipin data for Experiment 2 were presented as median and interquartile ranges (25th and 75th percentiles). Spearman’s correlation analysis was used to correlate LA and ALA concentrations (μM) to LA- and ALA-derived oxylipins (nM).
Oxylipin intake data was expressed as mean without standard deviation, because it is a calculated value so true variability cannot be established. Hence, no statistical comparisons were done for calculated oxylipin intake data.
Statistical significance was set at p<0.05.
Results
Experiment 1
Standard recovery
Mean accuracy for d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-9-HODE and d8-5-HETE, the surrogates used to quantify LA- and ALA-derived oxylipins was 82%, 81%, 92% and 82%, respectively. The correlation coefficient reflecting linearity of the standard curve was above 0.99 for all measured oxylipins. Mean percent recovery of d11-11(12)-EpETrE, d11-14,15-DiHETrE, d4-9-HODE and d8-5-HETE, the surrogates used to quantify LA- and ALA-derived oxylipins was 39%, 70%, 55% and 48%, respectively. The low percent recovery of the surrogate standards in the oils is likely due to their partial degradation during base hydrolysis and matrix effects caused by ion suppression 36, 37. However, the use of the surrogate standards added prior to base hydrolysis and SPE corrects for these losses 36. Although a reduced percent recovery may decrease sensitivity, only peaks with a signal to noise ratio above 10 were included in the analysis to minimize the risk of quantitating noise. A representative LCMS/MS chromatogram of peaks detected in soybean oil before and after 10 minutes of heating is shown in Supplementary Figure 1.
Temperature
Two-way repeated measures ANOVA showed a significant effect of time on oil temperature (F(10,160)=3204; P<0.0001) during the 10-minute heating period. Oil temperature increased significantly within the first minute compared to baseline and reached a steady-state level of 100°C within 7 minutes (Supplementary Figure 2).
Baseline oil fatty acid composition
Oil fatty acid percent composition is shown in Figure 1. Concentration data are presented in Supplementary Figure 3. Differences in fatty acid percent composition amongst the oils were reflected in concentration data. Therefore, only statistical analysis of the percent composition data will be discussed in this section.
Figure 1. Oil fatty acid percent composition.
Fatty acid composition of the different oils at room temperature. Data are presented as median ± range. Significant differences between oils were assessed using a nonparametric Kruskall-Wallis test. Significant P values (<0.05) are reported on the figure. Only the main fatty acids present in the oils are represented in the figure, namely, myristic acid (14:0), palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1 n-9), vaccenic acid (18:1 n-7), linoleic acid (18:2 n-6) and α-linolenic acid (18:3 n-3).
Kruskall-Wallis test showed significant differences amongst the groups for myristic acid (14:0; p=0.014), palmitic acid (16:0; p=0.003), stearic acid (18:0; p=0.007), oleic acid (18:1n-9; p=0.003), vaccenic acid (18:1n-7; p=0.013), LA (p=0.002) and ALA (p=0.01). Myristate, stearate and vaccinate were generally low in composition (<5%) or not detected in some oils. Palmitate was highest in olive (12%), corn (11.5%) and soybean oils (11%), followed by canola oil (4%), HSAO (neat and w/AO; ~3.5%) and “Thrive” (neat and w/AO; ~1.5%). Oleate was highest in the 4 algae oils (~91%), followed by olive (74%), canola (63.5%), soybean (21%) and corn (29%) oil.
LA composition was highest in corn and soybean oil (55%) followed by canola (19%), olive (7%), “Thrive” (neat and w/AOs; ~3.9%) and HSAO (neat and w/AO; ~1.5%). ALA was highest in canola oil (7.2%), followed by soybean oil (6.5%), corn oil (0.8%) and olive oil (0.4%) It was negligible or not detected in the 4 algae oils.
Baseline oxylipin concentrations
The LA and ALA oxylipin data are presented in Figures 2-A and 2-B, respectively. Overall, high LA or ALA oils had high concentrations of their respective LA or ALA metabolites, except for olive oil, which had comparable levels of LA and ALA monohydroxylated products compared to corn oil despite being low in LA (7%) and ALA (0.4%). The presence or absence of α-tocopherol in the algae oils did not appear to affect oxylipin concentrations.
Figure 2. Oil LA (A) or ALA (B) derived oxylipin concentrations at room temperature.
Oxidized linoleic acid (A.) and α-linolenic acid (B.) metabolite concentrations (nM) in the different oils at room temperature. Data are presented as median ± range. Significant differences between oils were assessed using a nonparametric Kruskall-Wallis test. Significant P values (<0.05) are reported on the figure. HODE, hydroxyoctadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; DiHOME, dihydroxyoctadecamonoenoic acid; HOTrE, hydroxyoctadecatrienoic acid; EpODE, epoxyoctadecadienoic acid; DiHODE, dihydroxyoctadecadienoic acid.
Results from one-way ANOVA indicated that LA-derived oxylipin median values differed significantly among the groups for 9-HODE (p = 0.006), 13-HODE (p = 0.006), 9-oxo-ODE (p = 0.003), 13-oxo-ODE (p = 0.007), 9,10-DiHOME (p = 0.006) and 12,13-DiHOME (p = 0.006) (Figure 2-A). Monohydroxylated metabolites (9-HODE and 13-HODE) were 19 to 24 times higher in olive and corn oil than soybean and canola oil, which were both 3 to 4 times higher than the 4 algae oils. LA dihydroxylated metabolites (9,10-DiHOME and 12,13-DiHOME) were at least 11 times higher in corn and soybean oil as compared to other oils. No significant differences were observed for epoxy-metabolites of LA (9(10)-EpOME and 12(13)-EpOME).
Kruskall-Wallis test also showed statistically significant differences amongst the groups in ALA-derived monohydroxy (9-HOTrE, p = 0.019; 13-HOTrE, p = 0.005), epoxy (9(10)-EpODE, p = 0.04; 15(16)-EpODE, p = 0.035) and dihydroxy metabolites (9,10-DiHODE, p = 0.008; 12,13-DiHODE, p = 0.004; 15,16-DiHODE, p = 0.004) (Figure 2-B). Monohydroxylated ALA metabolites (9- and 13-HOTrE) were highest in olive oil, followed sequentially by corn, soybean and canola oil, compared to algae oils. Epoxidized ALA metabolites (9(10)-EpODE, 15(16)-EpODE), and the dihydroxylated ALA metabolite 15,16-DiHODE, were 5- to 22- fold higher in canola and soybean oil compared to the other oils. Dihydroxylated ALA-derived 9,10-DiHODE was 4-fold higher in soybean oil compared to corn oil, which was 4- to 19- fold higher compared to other oils. 12,13-DiHODE was detected in corn, canola and soybean oils, but was negligible or undetected in the remaining oils.
Effect of oil type on the change in oxylipin and fatty acid concentrations after short-term heating
Heat was applied at 100°C for 10 minutes to test whether oxylipins increased more in oils with higher levels of LA and ALA compared to less unsaturated oils such as algae. Kruskall-Wallis test found no significant differences amongst the oils in the change (from baseline) in LA or ALA oxidized metabolites (Supplementary Figure 4-A and 4-B). There were no significant differences amongst the oils in the change in fatty acid concentrations or percent composition from baseline (data not shown).
Experiment 2
Fatty acid composition of various off-the-shelf oils
The fatty acid percent composition data for olive, corn, canola, soybean and flaxseed oils obtained from 4 to 5 stores (one oil per store) are presented in Table 1. Fatty acid analysis confirmed the composition of the oils13, 38. Flaxseed oil contained the highest amount of ALA (56%), followed by canola (9%), soybean (7%), corn (1%) and olive (0.8%) oil. Corn and soybean oil contained the highest amount of LA (55–57%) followed by canola (20%), flaxseed (15%) and olive (9%) oil. Significant differences among the various oils are shown in Table 1.
Table 1.
Fatty acid composition of the different oils (soybean, corn, canola, olive and flaxseed)
| Soybean Oil | Corn Oil | Canola Oil | Olive Oil | Flaxseed Oil | |
|---|---|---|---|---|---|
| 16:0 | 10.2 (10.0 – 10.5)ab | 11.9 (11.8 – 11.9)ac | 4.0 (4.0 – 4.2)b | 13.7 (12.5 – 14.3)a | 5.3 (5.2 – 5.5)bc |
| 18:0 | 3.8 (3.7 – 4.0)a | 1.5 (1.5 – 1.6)b | 1.6 (0.0 – 1.7)ab | 2.6 (2.6 – 3.1)ab | 3.7 (3.4 – 4.1)ab |
| 18:1 n-9 | 22.5 (22.4 – 22.8)ac | 28.5 (28.1 – 28.7)bc | 61.5 (60.9 – 63.1)ab | 71.3 (67.4 – 73.2)b | 20.2 (18.9 – 21.4)c |
| 18:1 n-7 | 1.2 (1.1 – 1.2)ab | 0.0 (0.0 – 0.0)a | 1.6 (1.5 – 2.2)b | 1.6 (1.3 – 1.8)b | 0.0 (0.0 – 0.0)a |
| 18:2 n-6 | 55.0 (54.7 – 55.0)ac | 56.9 (56.8 – 57.3)a | 19.7 (19.6 – 19.8)ab | 8.6 (7.2 – 11.2)b | 15.0 (14.5 – 15.5)bc |
| 18:3 n-3 | 7.1 (7.0 – 7.1)ab | 1.0 (1.0 – 1.1)ac | 8.9 (8.7 – 8.9)bc | 0.8 (0.8 – 0.9)a | 55.7 (54.2 – 57.0)b |
| 18:2 n-6/18:3 n-3 | 7.8 (7.6 – 7.9)abc | 54.3 (52.3 – 61.6)a | 2.2 (2.2 – 2.3)bc | 12.0 (8.6 – 12.5)ac | 0.3 (0.3 – 0.3)b |
Data (% of total detected fatty acids) are expressed as median and interquartile range (25th and 75th percentiles). Data were analyzed by Kruskall-Wallis test followed by Dunn’s multiple comparison post-hoc test. For each row, different alphabetical superscripts mean that the oils differed significantly (P<0.05) from each other. Only main fatty acids present in the oils are presented, namely, palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1 n-9), vaccenic acid (18:1 n-7), linoleic acid (18:2 n-6) and α-linolenic acid (18:3 n-3).
Oxylipin concentrations of various off-the-shelf oils
Oxylipin concentrations of five off-the-shelf oils are shown in Table 2. Kruskall-Wallis test followed by Dunn’s multiple comparison test revealed that LA- and ALA- oxidized metabolite concentrations differed significantly amongst the different oils.
Table 2.
Concentrations of linoleic acid (LA) and α-linolenic acid (ALA)-derived metabolites in nM in the different oils (olive, corn, canola, soybean and flaxseed).
| Soybean Oil | Corn Oil | Canola Oil | Olive Oil | Flaxseed Oil | |
|---|---|---|---|---|---|
| 13-HODE | 1570 (1025–2095)ab | 8400 (6085–10205)bc | 147 (101–232)b | 14300 (6235–17600)ac | 24100 (16900–45925)c |
| 9-HODE | 333 (260–795.5)ab | 5450 (4270–6065)bc | 215 (165.5–303)b | 14000 (4915–16000)ac | 68050 (40900–78100)c |
| 13-oxo-ODE | 277 (147.5–352.5)ab | 446 (330.5–545)bc | 120 (64.4–181)b | 4420 (2980–5240)ac | 25800 (20275–50000)c |
| 9-oxo-ODE | 892 (722–1590)a | 2320 (1470–3485)ab | 679 (444.5–963)a | 10300 (4850–12250)b | 2880 (1640–3805)ab |
| 12(13)-EpOME | 5100 (3510–6140)ab | 13000 (11050–16600)a | 2880 (2445–3210)ab | 1210 (609–1445)b | 12970 (7905–18575)a |
| 9(10)-EpOME | 4430 (3135–5345)ab | 17300 (16250–21200)a | 2550 (2025–2975)b | 250 (939–2685)b | 9200 (6368–11950)ab |
| 12,13-DiHOME | 18800 (10870–22700)a | 9030 (5230–13565)a | 982 (906–1065)ab | 56.9 (40.85–78.1)b | 352.5 (159–868.5)ab |
| 9,10-DiHOME | 23900 (14200–28800)a | 15400 (9130–21050)a | 357 (314.5–414)ab | 75.5 (59.8–99.5)b | 444 (158–934)ab |
| Total LA-metabolites | 56590 (37475–63568)ab | 72802 (60932–84871)a | 7922 (6850–8963)b | 46244 (20954–55257)ab | 143157 (111198–193904)a |
|
| |||||
| 9-HOTrE | 99 (59–140)ab | 231 (208–283)bc | 42 (26–63)b | 2090 (1915–2955)ac | 50750 (28275–77800)c |
| 13-HOTrE | 160 (113–243)ab | 42 (28–53)a | 95 (77–114)ac | 908 (604–1260)bc | 19300 (11890–34975)b |
| 15(16)-EpODE | 3590 (2525–4095)ab | 2430 (2155–3055)ab | 3840 (3210–4075)a | 224 (194–235)b | 248500 (141500–384750)a |
| 12(13)-EpODE | 574 (353–628)ab | 213 (206–260)ac | 851 (710.5–1024)bc | 85 (64–116)a | 15050 (9165–21325)b |
| 9(10)-EpODE | 742 (457–791)ab | 1010 (809–1370)ac | 1030 (899–1375)bc | 178 (122–231)a | 20650 (13950–27500)c |
| 15,16-DiHODE | 28000 (16250–32900)a | 1530 (935–2630)b | 11200 (9710–12600)ab | 15 (11–27)b | 7990 (2600–14700)ab |
| 12,13-DiHODE | 720 (415–914)a | 187 (126–277)ab | 103 (85–114)bc | 14 (10–17)b | 381 (225–519)ac |
| 9,10-DiHODE | 9160 (5000–11150)a | 1720 (939–2775)ab | 146 (132–173)bc | 7 (6–13)c | 306 (180–507)ac |
| Total ALA-metabolites | 43127 (26067–49923)ab | 7073 (6362–9890)ac | 17192 (16166–18277)bc | 3570 (2942–4810)c | 355205(279990–497593)b |
Data are expressed as median and interquartile range (25th and 75th percentiles). HODE, hydroxyoctadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; DiHOME, dihydroxyoctadecamonoenoic acid; OxLAM, oxidized linoleic acid metabolites; HOTrE, hydroxyoctadecatrienoic acid; EpODE, epoxyoctadecadienoic acid; DiHODE, dihydroxyoctadecadienoic acid; ALA, α-linolenic acid. Data were analyzed by Kruskall-Wallis test followed by Dunn’s multiple comparison tests. For each row, different alphabetical superscripts mean that the oils differed significantly (P<0.05) from each other.
With regard to LA metabolites, concentrations of LA-derived monohydroxy (9- and 13- HODE) and ketone metabolites (9- and 13-oxo-ODE) were highest in flaxseed and olive oil relative to corn, soybean and canola oil. 9- and 13-HODE and 13-oxo-ODE were significantly higher by 15–316 fold in flaxseed oil as compared to canola (p<0.001) and soybean oil (p<0.05). These metabolites were also significantly higher by 37–97 fold in olive oil as compared to canola oil (p<0.05). 9-oxo-ODE was significantly higher in olive oil as compared to both canola (p<0.001) and soybean oils (p<0.05) by 15 and 12 fold, respectively.
Flaxseed and corn oil had the highest concentration of LA-derived epoxides (9(10)- and 12(13)- EpOME). Both of these oils had an 11-fold significantly higher concentration of 12(13)-EpOME relative to olive oil (p<0.01). 9(10)-EpOME concentration was significantly higher than canola (p<0.01) and olive oil (p<0.001) by 7 and 69 fold, respectively.
Dihydroxy products of LA-derived epoxides (9,10-DiHOME and 12,13-DiHOME) were significantly higher in soybean (p<0.001) and corn oil (p<0.01) by 159–330 and 200–317 fold, respectively, as compared to olive oil.
With regard to ALA metabolites, flaxseed oil had the highest concentration of monohydroxylated ALA-derived metabolites, followed by olive oil (Table 2). 9- and 13- HOTrE were significantly higher in flaxseed oil than soybean, corn and canola oil by 121–1208 fold. 9-HOTrE was significantly higher in olive oil than canola oil by 510-fold, whereas 13- HOTrE was significantly higher than corn oil by 22-fold.
Epoxy-ALA metabolites (15(16)-, 12(13)- and 9(10)-EpODE) were highest in flaxseed oil, followed by canola oil. All three epoxidized ALA metabolites were 116–1109 and 6–10 times higher in flaxseed and canola oil (p<0.05), respectively, than olive oil, which had the lowest concentration of epoxidized ALA metabolites amongst the five oils. Soybean, corn and canola oil had similar concentrations of epoxidized ALA metabolites; however, 12(13)-EpODE was 98% lower in corn oil relative to flaxseed oil (p<0.05) and 9(10)-EpODE was 83% lower in soybean oil than flaxseed oil (p<0.05).
Dihydroxy ALA metabolites (15,16-, 12,13-, and 9,10-diHODEs) were highest in soybean oil and lowest in olive oil. The differences were statistically significant between soybean and olive oil for all three diHODEs, which were lower in olive oil by 98–99.9% (p<0.001). Soybean oil also had a higher concentration of 12,13-DiHODE (+85%; p<0.05) and 9,10-DiHODE (+98%; p<0.05) compared to canola oil.
Reproducibility between experiments
Supplementary Table 7 summarizes mean oil oxylipin concentrations from Experiments 1 and 2, and the experiment comparing air to nitrogen capping during hydrolysis (“Experiment 3”). As shown, oxylipin concentrations were similar between Experiments. Epoxides in canola oil, however, differed by 4–7 folds between Experiments 1 and 2, suggesting they are unstable in this particular oil. The oils used came from different outlets (San Francisco or Davis) and likely had different expiration dates. Fankhauser-Noti et al. reported similar fluctuations in epoxides between fresh and old olive and sunflower oil26.
Estimated daily oxidized fatty acid intake
Mean daily intake of oxidized LA and ALA metabolites from soybean, corn, canola and olive oil was estimated, based on published mean consumption (kg/p/year) data on these oils 3. Consumption data for flaxseed oil and the four algae oils were not available.
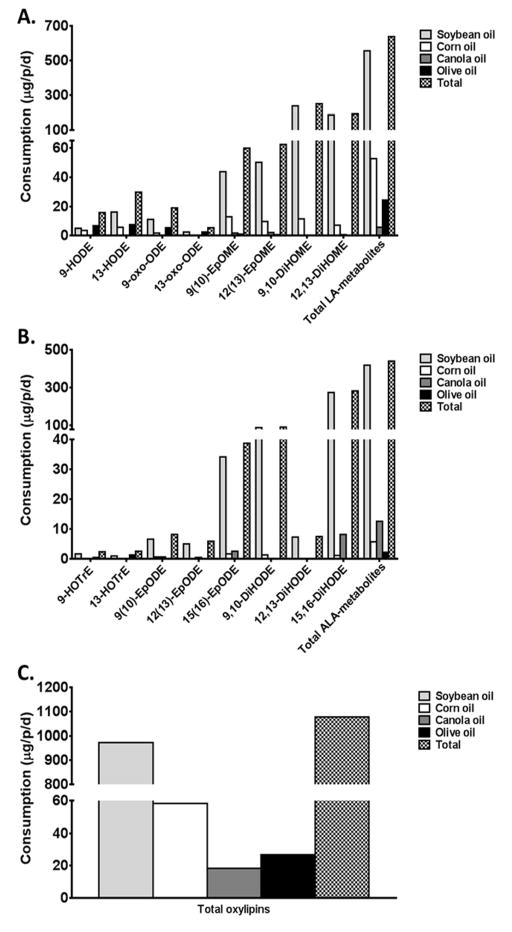
As shown in Figures 3-A and 3-B, of the four oils, soybean oil contributed the most of LA and ALA oxylipins in the diet, because it is the most consumed plant oil in the US (Supplementary Table 6) 3. The majority of oxylipins in the diet were in the form of epoxy and dihydroxy metabolites of LA and ALA.
Figure 3. Estimated US consumption of LA (A) and ALA (B) derived oxylipins.
Mean intake of the linoleic acid (LA)-derived (A.), α-linolenic acid (ALA)-derived (B.) and total oxylipins (C.) through the consumption of the four commonly consumed oils in US. Values are expressed as μg of oxylipins per person per day (μg/p/d). HODE, hydroxyoctadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; DiHOME, dihydroxyoctadecamonoenoic acid; LA, linoleic acid; HOTrE, hydroxyoctadecatrienoic acid; EpODE, epoxyoctadecadienoic acid; DiHODE, dihydroxyoctadecadienoic acid; ALA, alpha-linolenic acid.
Estimated daily intake of total oxylipins from olive, corn, canola and soybean oils averaged 1.1 mg/day (Figure 3-C). Of the measured oils, soybean oil contributed most oxylipins in the diet, followed by corn, olive and canola oil (Figure 3-C).
Correlations
Oil LA and ALA concentrations positively correlated with concentrations of their respective metabolites as shown in Table 4. LA correlated positively with LA-derived 9(10)- and 12(13)-EpOME and 9,10- and 12,13-DiHOME (P<0.05). It also correlated with ALA-derived 9-HOTrE and DiHODEs (P<0.05).
Table 4.
Spearman’s correlation between fatty acid and oxylipin concentrations from Experiment 2
| Correlation Between Fatty Acid and Oxylipin Concentration | ||||
|---|---|---|---|---|
|
| ||||
| LA concentration | ALA concentration | |||
|
| ||||
| r-value | p-value | r-value | p-value | |
| LA-oxylipins | ||||
|
| ||||
| 9-HODE | −0.3191 | 0.1285 | −0.00956 | 0.9646 |
| 13-HODE | −0.2643 | 0.2119 | −0.01565 | 0.9421 |
| 9-oxo-ODE | −0.3148 | 0.1341 | −0.3696 | 0.0755 |
| 13-oxo-ODE | −0.3765 | 0.0698 | 0.04174 | 0.8465 |
| 9(10)-EpOME | 0.4991 | 0.0130 | 0.06174 | 0.7744 |
| 12(13)-EpOME | 0.4652 | 0.0220 | 0.2565 | 0.2263 |
| 9,10-DiHOME | 0.8652 | <0.0001 | 0.1104 | 0.6075 |
| 12,13-DiHOME | 0.86 | <0.0001 | 0.06174 | 0.7744 |
|
| ||||
| ALA-oxylipins | ||||
|
| ||||
| 9-HOTrE | −0.4904 | 0.0150 | 0.4304 | 0.0358 |
| 13-HOTrE | −0.3852 | 0.0630 | −0.0087 | 0.9678 |
| 9(10)-EpODE | 0.01566 | 0.9421 | 0.5214 | 0.0090 |
| 12(13)-EpODE | 0.04001 | 0.8527 | 0.855 | <0.0001 |
| 15(16)-EpODE | 0.1339 | 0.5327 | 0.7896 | <0.0001 |
| 9,10-DiHODE | 0.8339 | <0.0001 | 0.2009 | 0.3466 |
| 12,13-DiHODE | 0.6551 | 0.0005 | 0.492 | 0.0146 |
| 15,16-DiHODE | 0.5466 | 0.0057 | 0.6458 | 0.0007 |
Table depicts correlations between total LA and ALA concentration and total LA- and ALA-oxylipin concentrations in all oils. Data was analyzed using Spearman Correlation Analysis. Significant p-values were determined to be <0.05. HODE, hydroxyoctadecadienoic acid; oxo-ODE, oxo-octadecadienoic acid; EpOME, epoxyoctadecamonoenoic acid; DiHOME, dihydroxyoctadecamonoenoic acid; OxLAM, oxidized linoleic acid metabolites; HOTrE, hydroxyoctadecatrienoic acid; EpODE, epoxyoctadecadienoic acid; DiHODE, dihydroxyoctadecadienoic acid; ALA, α-linolenic acid.
ALA did not correlate with LA-derived oxylipins, but positively correlated with ALA-derived hydroxy, epoxy and dihydroxy metabolites (P<0.05).
Discussion
The present study reported the presence of LA- and ALA- derived oxylipin species in plant and algae oils. Concentrations of LA- and ALA- derived oxylipins were generally proportional to the concentrations of their precursor fatty acids except for flaxseed and olive oil, which had higher oxylipin concentrations than other oils. Estimated oxylipin intake levels based on available consumption data on soybean, corn, canola and olive oils averaged 1.1 mg per person per day. Oxylipin concentrations were comparable between experiments and to one study which reported epoxy-LA metabolites in olive oil26, thus confirming the validity and reproducibility of our measurements.
The oxylipins detected in non-heated oils were likely formed by non-enzymatic or enzymatic pathways during the seed extraction process. Non-enzymatic auto-oxidation is known to be influenced by storage or processing conditions 39. Enzymatic oxidation is mediated in part by lipoxygenase, which is activated when barrier integrity of the seed, fruit or algae is compromised by homogenization during the oil extraction process 25, 29, 40. Other plant or algae enzymes involved in oxylipin formation include soluble epoxide hydrolase, cytochrome P450 or pathogen-inducible oxygenases 41–44, although their activation during the oil extraction process is not known.
There were no significant differences amongst the oils in the change in oxylipin concentrations following 10 minutes of heat relative to baseline (Experiment 1, Supplementary Figure 4). Previous studies reported the formation of LA-derived oxylipins after heating high LA oils at 40, 100 or 180°C for 10–264 hours 13–15. We predicted, however, that using UPLC-MS/MS, we might detect changes in oxylipins in high LA or ALA oils within 10 minutes of heating at 100°C. The lack of difference between the oils could be due to the short heating duration as previous work has demonstrated that prolonged heating is required to oxidize oils 13, 15. It is also possible that non-enzymatic oxylipin products of heat-induced oxidation (HODEs, oxoODEs) increased in the high LA or ALA oils during heating, but were rapidly degraded into secondary volatile compounds within the 10 minute heating period 45, 46.
The LA and ALA content of the oils from Experiment 2 were related to the concentration of their respective oxylipin metabolites (Table 4). LA also correlated highly with ALA-derived oxylipins. This association was likely driven by olive and flaxseed oil which unexpectedly contained a high amount of LA-derived oxylipins potentially caused by processing, storage or handling conditions that need to be further investigated. Overall, however, the findings suggest that precursor fatty acid pool in oils is an important determinant of oxylipin concentrations, consistent with the observation that the low LA and ALA algae oils had the least concentration of LA- and ALA- derived oxylipins compared to other plant oils, irrespective of vitamin E content (Experiment 1, Figure 2). The presence of vitamin E may not be critical for low polyunsaturated fatty acid oils when heated for a short period of time (10 minutes) at 100°C.
In both experiments, olive oil had higher concentrations of LA-derived ketones (9- and 13-oxo-ODE), and monohydroxylated LA (9- and 13-HODE) and ALA (9- and 13-HOTrE) metabolites, than soybean, canola and corn oil, despite being low in LA (9%) and ALA (0.8%). This could be due to enhanced lipoxygenase activity upon homogenizing the olive fruit to extract the oil compared to seeds (soybean, canola or corn)40. However, Jarén-Galán et al. reported higher lipoxygenase activity from soybean compared to olives 47. It is possible that other oxygenase enzymes that differ in activity between soybeans and olives account for the unexpected high oxylipin concentrations in olive oil. Other factors such as processing or storage conditions may also explain the high mono-hydroxylated oxylipin metabolites detected in olive oil relative to other higher-LA or ALA oils.
LA-derived monohydroxy and ketone metabolites, and ALA-derived monohydroxy and epoxy metabolites were highest in flaxseed oil as compared to other oils (Experiment 2). The high concentration of ALA-derived oxylipins is expected because flaxseed oil contained the highest concentration of ALA (54%) compared to the other oils tested in this study (2–6%). Flaxseed oil has less LA relative to soybean oil (14% versus 50%), yet LA-metabolites were 3-fold higher in flaxseed oil compared to soybean oil. It is not known whether these metabolites were formed during processing, storage or flaxseed crushing. It is possible that the high concentrations of ALA epoxides may have catalyzed the oxidation of LA via electrophilic attack of the allylic carbon next to the double bonds, although this remains to be determined.
The consumption of LA has increased from 2% to 7% of energy over the past century, due to increased consumption of high LA plant oils, such as soybean oil 3. Soybean oil is the most commonly consumed plant oil in the US and the fourth major contributor of total calories 3. It is likely that the consumption of oxidized LA metabolites concomitantly increased over the past few decades with increased soybean oil intake. Approximating the levels of dietary oxylipins will allow future studies evaluating the bioavailability and effect of oxylipins on health and disease to utilize relevant doses.
Oxylipins are bioavailable, and circulating LA-derived oxylipins in particular have been associated with atherosclerosis, pain syndromes and hypertension, consistent with their role in mediating pro-inflammatory signaling in tissues or vasculature 4, 48, 49. A recent meta-analysis reported that fried food consumption was associated with hypertension and weight gain 50. In vivo, the concentration of circulating oxylipins depends on the availability of their precursor fatty acid 10, 11, 51. However, studies have demonstrated that dietary oxylipins are absorbed 17–20 and incorporated into blood chylomicrons 52. The relative plasma contribution of dietary oxylipins compared to endogenously produced oxylipins is not known and merits future evaluation.
Estimated US daily intake of LA and ALA oxylipins was 0.64 and 0.44 mg, respectively, and amounted to a total of 1.1 mg per person per day. These values are likely underestimated, however, because 1) oxylipins in this study were quantified in off-the-shelf oils maintained at room temperature, 2) oxylipin content of commonly consumed foods such as peanut butter and French Fries was not measured 53, 54, and 3) a targeted UPLC-MS/MS approach was used to quantify oxylipins, which means that other oxylipin species such as hydroperoxides of LA or ALA or oleic acid-derived compounds were not accounted for in our estimates. Accounting for the amount and type of oxylipins produced during food processing, cooking or prolonged storage, in relation to water, metal and antioxidant content will provide a better estimate of daily oxylipin consumption levels 39, 55, 56. The use of non-targeted mass-spectrometry methods may identify other oxylipin species in commonly consumed oils that can be quantified with targeted UPLC-MS/MS as performed in the present study 57.
Limitations of this study include the low sample size and lack of information on the processing methods used to produce the oils and storage conditions and duration since date of production. The risk of statistical errors associated with the low sample size or number of replicates in Experiment 1 was mitigated by reproducing the measurements of some oils (corn, soybean, olive and canola) in Experiment 2. Information on processing and storage would require coordination with each of the oil manufacturers in future studies.
In summary, this study quantified LA and ALA oxylipins in various oils and found them to be related to LA and ALA fatty acid composition with a notable exception being olive and flaxseed oils. The amount of oxylipins consumed through commonly consumed plant oils in the US was estimated to be 1.1 mg per person per day, but this value is underestimated because it does not account for oxylipins in commonly consumed oils not measured in this study, or processing, frying or storage effects. Knowing the amount and type of oxylipins chronically consumed through dietary oils is important for understanding their health implications.
Supplementary Material
Acknowledgments
This work was supported by TerraVia Holdings, Inc., USDA National Institute of Food and Agriculture, Hatch/Taha (project #1008787), NIEHS R01 ES002710, NIEHS/Superfund Research Program P42 ES004699 and NIH/NIDDK U24 DK097154. Mark S. Horowitz is thanked for statistical programming expertise.
Abbreviations
- ALA
alpha-linolenic acid
- AO
antioxidants
- CUDA
1-cyclohexyl-dodecanoic acid urea
- DiHODE
dihydroxyoctadecadienoic acid
- DiHOME
dihydroxyoctadecamonoenoic acid
- EDTA
triphenylphosphine, ethylenediaminetetraacetic acid
- EpODE
epoxyoctadecadienoic acid
- EpOME
epoxyoctadecamonoenoic acid
- FID
flame ionization detector
- GC
gas-chromatography
- GC-FID
gas chromatography coupled to a flame ionization detection
- HODE
hydroxyoctadecadienoic acid
- HOTrE
hydroxyoctadecatrienoic acid
- HSAO
High Stability Algae Oil
- LA
Linoleic acid
- LC-MS/MS
liquid chromatography tandem mass spectroscopy
- MRM
multiple reaction monitoring
- NMR
nuclear magnetic resonance
- oxo-ODE
oxo-octadecadienoic acid
- PUFAs
polyunsaturated fatty acids
- SPE
solid-phase extraction
- UV
ultraviolet
- w/AO
without added antioxidants
Footnotes
Conflicts of interest
The authors have no conflicts of interests to declare.
References
- 1.Hansen AE, Haggard ME, Boelsche AN, Adam DJ, Wiese HF. Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency. The Journal of nutrition. 1958;66:565–76. doi: 10.1093/jn/66.4.565. [DOI] [PubMed] [Google Scholar]
- 2.Holman RT, Johnson SB, Hatch TF. A case of human linolenic acid deficiency involving neurological abnormalities. The American journal of clinical nutrition. 1982;35:617–23. doi: 10.1093/ajcn/35.3.617. [DOI] [PubMed] [Google Scholar]
- 3.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–62. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–26. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiba T, Thomas CP, Calcutt MW, Boeglin WE, O’Donnell VB, Brash AR. The precise structures and stereochemistry of trihydroxy-linoleates esterified in human and porcine epidermis and their significance in skin barrier function: IMPLICATION OF AN EPOXIDE HYDROLASE IN THE TRANSFORMATIONS OF LINOLEATE. The Journal of biological chemistry. 2016 doi: 10.1074/jbc.M115.711267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nature medicine. 1997;3:562–6. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51:3046–54. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcos R, Izquierdo Y, Vellosillo T, Kulasekaran S, Cascon T, Hamberg M, Castresana C. 9-Lipoxygenase-Derived Oxylipins Activate Brassinosteroid Signaling to Promote Cell Wall-Based Defense and Limit Pathogen Infection. Plant physiology. 2015;169:2324–34. doi: 10.1104/pp.15.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamberg M, Sanz A, Rodriguez MJ, Calvo AP, Castresana C. Activation of the fatty acid alpha-dioxygenase pathway during bacterial infection of tobacco leaves. Formation of oxylipins protecting against cell death. The Journal of biological chemistry. 2003;278:51796–805. doi: 10.1074/jbc.M310514200. [DOI] [PubMed] [Google Scholar]
- 10.Taha AY, Hennebelle M, Yang J, Zamora D, Rapoport SI, Hammock BD, Ramsden CE. Regulation of rat plasma and cerebral cortex oxylipin concentrations with increasing levels of dietary linoleic acid. Prostaglandins Leukot Essent Fatty Acids. 2016 doi: 10.1016/j.plefa.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsden CE, Ringel A, Majchrzak-Hong SF, Yang J, Blanchard H, Zamora D, Loewke JD, Rapoport SI, Hibbeln JR, Davis JM, Hammock BD, Taha AY. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: Implications for idiopathic pain syndromes? Molecular pain. 2016;12 doi: 10.1177/1744806916636386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinaud O, Delaforge M, Boucher JL, Rocchiccioli F, Mansuy D. Oxidative metabolism of linoleic acid by human leukocytes. Biochem Biophys Res Commun. 1989;161:883–91. doi: 10.1016/0006-291x(89)92682-x. [DOI] [PubMed] [Google Scholar]
- 13.Marmesat S, Velasco J, Dobarganes MC. Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J Chromatogr A. 2008;1211:129–34. doi: 10.1016/j.chroma.2008.09.077. [DOI] [PubMed] [Google Scholar]
- 14.Arturo Moralesa SM, Carmen Dobarganesa M, Márquez-Ruizb Gloria, Velascoa Joaquín. Quantitative analysis of hydroperoxy- keto- and hydroxy-dienes in refined vegetable oils. Journal of Chromatography A. 2012;1229:190–197. doi: 10.1016/j.chroma.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Goicoechea E, Guillen MD. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100 degrees C. J Agric Food Chem. 2010;58:6234–45. doi: 10.1021/jf1005337. [DOI] [PubMed] [Google Scholar]
- 16.Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Development and validation of a gas chromatography-flame ionization detection method for the determination of epoxy fatty acids in food matrices. J Agric Food Chem. 2014;62:2982–8. doi: 10.1021/jf405664c. [DOI] [PubMed] [Google Scholar]
- 17.Ferreiro-Vera C, Priego-Capotea F, Mata-Granadosa JM, Luque de Castroa MD. Short-term comparative study of the influence of fried edible oils intake on the metabolism of essential fatty acids in obese individuals. Food Chem. 2013;136:576–84. doi: 10.1016/j.foodchem.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 18.Wilson R, Lyall K, Smyth L, Fernie CE, Riemersma RA. Dietary hydroxy fatty acids are absorbed in humans: implications for the measurement of ‘oxidative stress’ in vivo. Free radical biology & medicine. 2002;32:162–8. doi: 10.1016/s0891-5849(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 19.Wilson R, Smith R, Wilson P, Shepherd MJ, Riemersma RA. Quantitative gas chromatography-mass spectrometry isomer-specific measurement of hydroxy fatty acids in biological samples and food as a marker of lipid peroxidation. Analytical biochemistry. 1997;248:76–85. doi: 10.1006/abio.1997.2084. [DOI] [PubMed] [Google Scholar]
- 20.Goicoechea E, Brandon EF, Blokland MH, Guillen MD. Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011;49:115–24. doi: 10.1016/j.fct.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Wilson R, Fernie CE, Scrimgeour CM, Lyall K, Smyth L, Riemersma RA. Dietary epoxy fatty acids are absorbed in healthy women. European journal of clinical investigation. 2002;32:79–83. doi: 10.1046/j.1365-2362.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- 22.Glavind J, Sylven C. Intestinal absorption and lymphatic transport of methyl linoleate hydroperoxide and hydroxyoctadecadienoate in the rat. Acta chemica Scandinavica. 1970;24:3723–8. doi: 10.3891/acta.chem.scand.24-3723. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa K, Ashida H. Dietary hydroperoxides of linoleic acid decompose to aldehydes in stomach before being absorbed into the body. Biochimica et biophysica acta. 1998;1393:349–61. doi: 10.1016/s0005-2760(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Fan YW, Li J, Tang L, Hu JN, Deng ZY. Evaluating and predicting the oxidative stability of vegetable oils with different fatty acid compositions. J Food Sci. 2013;78:H633–41. doi: 10.1111/1750-3841.12089. [DOI] [PubMed] [Google Scholar]
- 25.Pulvera ZM, Kitamura K, Hajika M, Shimada K, Matsui K. Oxylipin metabolism in soybean seeds containing different sets of lipoxygenase isozymes after homogenization. Bioscience, biotechnology, and biochemistry. 2006;70:2598–603. doi: 10.1271/bbb.60121. [DOI] [PubMed] [Google Scholar]
- 26.Anja Fankhauser-Noti KF, Biedermann-Brem Sandra, Grob Koni. Assessment of epoxidized soy bean oil (ESBO) migrating into foods: Comparison with ESBO-like epoxy fatty acids in our normal diet. Food and Chemical Toxicology. 2006;44:1279–1286. doi: 10.1016/j.fct.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81:8085–93. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul Abishek M, Patel J, Prem Rajan A. Algae oil: a sustainable renewable fuel of future. Biotechnol Res Int. 2014;2014:272814. doi: 10.1155/2014/272814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquemoud D, Pohnert G. Extraction and Analysis of Oxylipins from Macroalgae Illustrated on the Example Gracilaria vermiculophylla. Methods Mol Biol. 2015;1308:159–72. doi: 10.1007/978-1-4939-2684-8_10. [DOI] [PubMed] [Google Scholar]
- 30.Morgan DA. Smoke, fire, and flash points of cottonseed, peanut, and other vegetable oils. JAOCS. 1942;19:193–98. [Google Scholar]
- 31.Choi H, Lee E, Lee KG. Quality evaluation of noble mixed oil blended with palm and canola oil. Journal of oleo science. 2014;63:653–60. doi: 10.5650/jos.ess14023. [DOI] [PubMed] [Google Scholar]
- 32.Douny C, Razanakolona R, Ribonnet L, Milet J, Baeten V, Rogez H, Scippo ML, Larondelle Y. Linseed oil presents different patterns of oxidation in real-time and accelerated aging assays. Food Chem. 2016;208:111–5. doi: 10.1016/j.foodchem.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Arnold C, Markovic M, Blossey K, Wallukat G, Fischer R, Dechend R, Konkel A, von Schacky C, Luft FC, Muller DN, Rothe M, Schunck WH. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. The Journal of biological chemistry. 2010;285:32720–33. doi: 10.1074/jbc.M110.118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat. 2014;113–115:21–9. doi: 10.1016/j.prostaglandins.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichihara K, Fukubayashi Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res. 2010;51:635–40. doi: 10.1194/jlr.D001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panuwet P, Hunter RE, Jr, D’Souza PE, Chen X, Radford SA, Cohen JR, Marder ME, Kartavenka K, Ryan PB, Barr DB. Biological Matrix Effects in Quantitative Tandem Mass Spectrometry-Based Analytical Methods: Advancing Biomonitoring. Crit Rev Anal Chem. 2016;46:93–105. doi: 10.1080/10408347.2014.980775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willenberg I, Ostermann AI, Schebb NH. Targeted metabolomics of the arachidonic acid cascade: current state and challenges of LC-MS analysis of oxylipins. Anal Bioanal Chem. 2015;407:2675–83. doi: 10.1007/s00216-014-8369-4. [DOI] [PubMed] [Google Scholar]
- 38.Jana Orsavova LM, Ambrozova Jarmila Vavra, Vicha Robert, Mlcek J. Fatty Acid composition of vegetable oils and its contribution to dietary energy intake dependence of cardiovascular mortality on dietary intake of fatty acids. Int J Mol Sci. 2015;16:12871–12890. doi: 10.3390/ijms160612871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claxson AW, Hawkes GE, Richardson DP, Naughton DP, Haywood RM, Chander CL, Atherton M, Lynch EJ, Grootveld MC. Generation of lipid peroxidation products in culinary oils and fats during episodes of thermal stressing: a high field 1H NMR study. FEBS Lett. 1994;355:81–90. doi: 10.1016/0014-5793(94)01147-8. [DOI] [PubMed] [Google Scholar]
- 40.Soldo B, Sprung M, Musac G, Pavela-Vrancic M, Ljubenkov I. Evaluation of Olive Fruit Lipoxygenase Extraction Protocols on 9- and 13-Z,E-HPODE Formation. Molecules. 2016;21 doi: 10.3390/molecules21040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morisseau C, Beetham JK, Pinot F, Debernard S, Newman JW, Hammock BD. Cress and potato soluble epoxide hydrolases: purification, biochemical characterization, and comparison to mammalian enzymes. Arch Biochem Biophys. 2000;378:321–32. doi: 10.1006/abbi.2000.1810. [DOI] [PubMed] [Google Scholar]
- 42.Sanz A, Moreno JI, Castresana C. PIOX, a new pathogen-induced oxygenase with homology to animal cyclooxygenase. Plant Cell. 1998;10:1523–37. doi: 10.1105/tpc.10.9.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grausem B, Widemann E, Verdier G, Nosbusch D, Aubert Y, Beisson F, Schreiber L, Franke R, Pinot F. CYP77A19 and CYP77A20 characterized from Solanum tuberosum oxidize fatty acids in vitro and partially restore the wild phenotype in an Arabidopsis thaliana cutin mutant. Plant Cell Environ. 2014;37:2102–15. doi: 10.1111/pce.12298. [DOI] [PubMed] [Google Scholar]
- 44.Petkova-Andonova M, Imaishi H, Ohkawa H. CYP92B1, A cytochrome P450, expressed in petunia flower buds, that catalyzes monooxidation of long-chain fatty acids. Bioscience, biotechnology, and biochemistry. 2002;66:1819–28. doi: 10.1271/bbb.66.1819. [DOI] [PubMed] [Google Scholar]
- 45.MDGEaG. Volatile compounds generated in corn oil stored at room temperature. Presence of toxic compounds. European Journal of Lipid Science and Technology. 2014;116:395–406. [Google Scholar]
- 46.Katragadda HRFA, Sidhu S, Carbonell-Barrachina AA. Emissions of volatile aldehydes from heated cooking oils. Journal of Food Chemistry. 2010;120:59–65. [Google Scholar]
- 47.Jaren-Galan M, Carmona-Ramon C, Minguez-Mosquera MI. Interaction between chloroplast pigments and lipoxygenase enzymatic extract of olives. J Agric Food Chem. 1999;47:2671–7. doi: 10.1021/jf980900n. [DOI] [PubMed] [Google Scholar]
- 48.Leong XF, Salimon J, Mustafa MR, Jaarin K. Effect of repeatedly heated palm olein on blood pressure-regulating enzymes activity and lipid peroxidation in rats. Malays J Med Sci. 2012;19:20–9. [PMC free article] [PubMed] [Google Scholar]
- 49.Jaarin K, Mustafa MR, Leong XF. The effects of heated vegetable oils on blood pressure in rats. Clinics (Sao Paulo) 2011;66:2125–32. doi: 10.1590/S1807-59322011001200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sayon-Orea C, Carlos S, Martinez-Gonzalez MA. Does cooking with vegetable oils increase the risk of chronic diseases?: a systematic review. Br J Nutr. 2015;113(Suppl 2):S36–48. doi: 10.1017/S0007114514002931. [DOI] [PubMed] [Google Scholar]
- 51.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87:135–41. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staprans I, Rapp JH, Pan XM, Kim KY, Feingold KR. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler Thromb. 1994;14:1900–5. doi: 10.1161/01.atv.14.12.1900. [DOI] [PubMed] [Google Scholar]
- 53.Storey ML, Anderson PA. Contributions of white vegetables to nutrient intake: NHANES 2009–2010. Adv Nutr. 2013;4:335S–44S. doi: 10.3945/an.112.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stroehla BC, Malcoe LH, Velie EM. Dietary sources of nutrients among rural Native American and white children. Journal of the American Dietetic Association. 2005;105:1908–16. doi: 10.1016/j.jada.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Aladedunye FA, Przybylski R. Degradation and Nutritional Quality Changes of Oil During Frying. Journal of the American Oil Chemists’ Society. 2008;86:149–156. [Google Scholar]
- 56.Spiteller P, Spiteller G. Strong dependence of the lipid peroxidation product spectrum whether Fe2+/O2 or Fe3+/O2 is used as oxidant. Biochimica et biophysica acta. 1998;1392:23–40. doi: 10.1016/s0005-2760(97)00209-9. [DOI] [PubMed] [Google Scholar]
- 57.Cajka T, Fiehn O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal Chem. 2016;88:524–45. doi: 10.1021/acs.analchem.5b04491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.