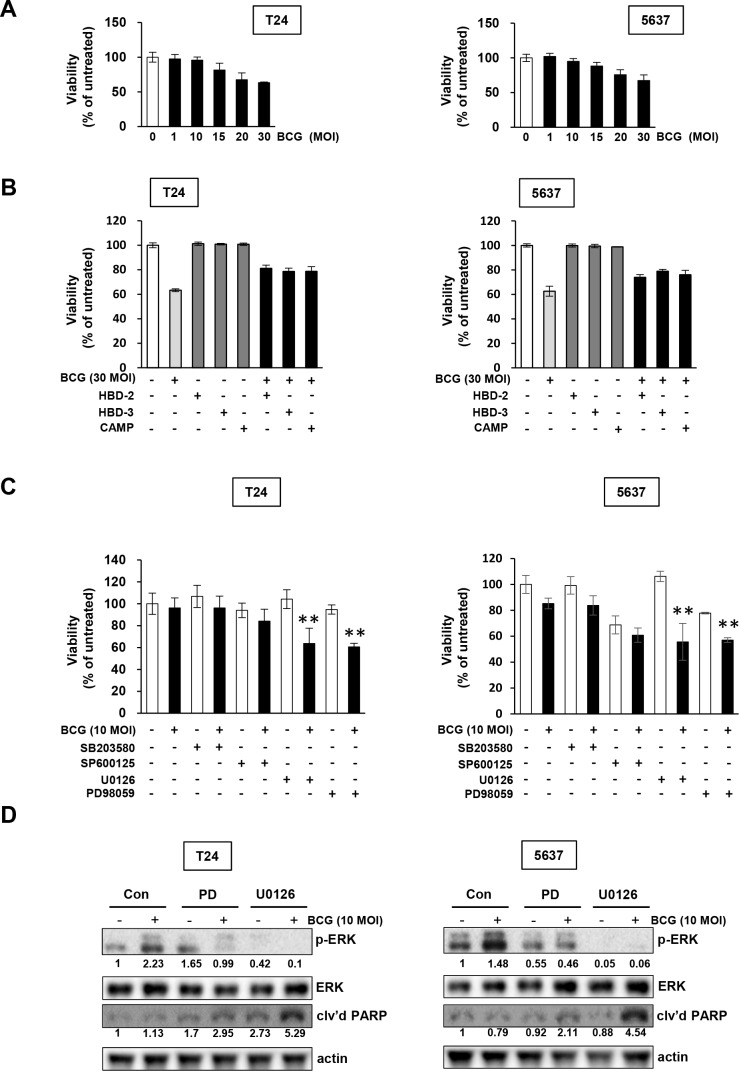
Figure 2. Pharmacological inhibition of MEK pathways increases the anti-proliferative effects of BCG in bladder cancer cells.
(A) Cells were plated in 96-well plates 24 h before adding BCG. Viable cells were quantified by MTT assays. (B) Cells were pretreated with antimicrobial peptides (HBD-2, HBD-3, and CAMP), and subsequently infected with 30 MOI of BCG (i.e. a dosage determined by data in panel A to induce greater than 30 % growth inhibition). After 48 hours, viable cells were quantified by MTT assays. (C) Cells were pretreated with specific inhibitors of p38 (SB203580; 10 μM), JNK (SP600125; 5 μM) and/or ERK (U0126; 10 μM or PD98059; 1 μM). After 2 hours, the cells were infected with 10 MOI of BCG (i.e. sub-optimal dosage to suppress cell proliferation as shown in the panel A) for 48 hours, followed by MTT cell viability assays. Data are mean ± SD (n=6 per group). **P<0.01, U0126/ BCG+U0126, BCG/BCG+U0126, PD98059/ BCG+PD98059, or BCG/BCG+PD98059. Student's T-test. (D) Western blotting analyses showing apoptosis by combined treatment with MEK inhibitors and BCG in T24 (left panels) and 5637 (right panels) cells pre-treated with PD98059 (a specific MEK1 inhibitor; 1 μM) or U0126 (a specific MEK1/2 inhibitor; 10 μM) for 2 h, followed by BCG infection (10 MOI for 48 hours). DMSO was used as empty vector controls (Con). The expressions of phosphorylated ERK1/2 was calculated as the ratio of phosphorylated ERK/2 to total ERK1/2 protein expression using densitometric analyses. The ratio of cleaved PARP product to actin protein expression were calculated similarly. Expression ratios were normalized to the untreated group.