Abstract
Background
Pathogenesis of severe fever with thrombocytopenia syndrome (SFTS) has not been well described yet. Recent studies indicate that SFTSV could replicate in endothelial cells. Here we performed a case-control study to determine whether endothelial activation/dysfunction occurred in SFTSV infection and to identify the biomarkers reflecting endothelial dysfunction.
Methodology/Principal findings
In a case-control study of 134 SFTS patients and 68 healthy controls, serum levels of plasminogen activator inhibitor 1, tissue plasminogen activator, P-selectin, platelet endothelial cell adhesion molecular, CD40 ligand, E-selectin, vascular endothelial growth factor A, serum amyloid antigen 1 (SAA-1) and vascular cell adhesion molecular 1 were significantly enhanced in the patients than the controls (all P<0.05), indicating the occurrence of endothelial activation/dysfunction in SFTS. The intercellular adhesion molecular 1 (ICAM-1) and SAA-1 at the convalescent phase were also significantly associated with severe patients, after adjusting for the potential confounders. The odds ratio was estimated to be 3.364 (95% CI 1.074–10.534) for ICAM-1, and 1.881 (95% CI 1.166–3.034) for SAA-1, respectively. Cutoff value of 1.1×107 pg/mL SAA-1 or 1.2×106 pg/mL ICAM-1 were found to have moderate power of predicting fatal cases.
Conclusions
The endothelial dysfunction may be one of the pathogenic mechanism of SFTS. The serum levels of ICAM-1 and SAA-1 might be used to predict adverse outcome.
Author summary
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne viral disease and first reported in the rural areas of China. Pathogenesis of the disease has not been well described yet. Recent studies indicated that SFTSV replicated in endothelial cells. So, we performed a case-control study to explore whether endothelial activation/dysfunction occurred in SFTSV infection and to identify biomarkers reflecting endothelial dysfunction. We found that the occurrence of endothelial activation/dysfunction in severe fever with thrombocytopenia syndrome and the serum levels of ICAM-1 and SAA-1 might be used to predict adverse outcome.
Introduction
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne viral disease that is caused by a novel bunyavirus, SFTSV, which was first reported in the rural areas of China [1]. A genetically similar virus, heartland virus was also demonstrated to cause mortality in USA [2,3]. The disease is clinically characterized by fever, thrombocytopenia and leucopenia, with the mortality rate varying between 10% and 30% in different studies. The severe cases could present with hemorrhagic, neurologic, multiple organs dysfunction, and even developing fatal outcome [1,4]. Pathogenesis of the disease has not been well described yet. Recent studies indicate that SFTSV could replicate in endothelial cells [4] and it’s hypothesized that SFTSV-infected endothelial cells may directly contribute to viremia, vascular permeability. On the other hand, it’s suggested that SFTSV might also mediate endothelial cell activation via an indirect route, considering the significant elevation of circulating TNF-α in SFTS cases [5,6], a strong activator of vascular endothelium. Endothelial dysfunction usually includes several proinflammatory and procoagulant changes as well as endothelial activation [7]. Here in order to determine whether endothelial activation/dysfunction occurred in SFTSV infection and to identify biomarkers reflecting endothelial dysfunction that can be used to predict disease outcome in SFTSV infection, we performed a case-control study in PLA 154 hospital in Xinyang City, Henan Province, China. The serum levels of endothelial function makers were evaluated and compared regarding their clinical progression and disease severity.
Methods
Subjects
The SFTS patients who were treated in PLA154 hospital during 2015–2016 were included in this study. SFTSV infection was confirmed by real-time reverse transcription-polymerase chain reaction (RT-PCR) tests or serological tests as guided by the Ministry of Health, China [8]. The serum samples that were collected on admission were used as acute phase samples, and all were within seven days after the onset of disease. The second samples were collected when the patients had their clinical manifestations resolved and the main laboratory abnormalities (decreased white blood cell and platelet counts, increased transaminase) restored to normal. For the fatal patients, the second samples were collected at the time of disease deterioration. Severe patients were defined by the presence of hemorrhagic manifestations (melena, hematemesis, hemoptysis, ophthalmorrhagia and gingival bleeding), any one or more organ failure or encephalitis development [9].Healthy blood donors with comparable age and gender who were determined to be SFTSV negative by both real-time RT-PCR test and serological test were enrolled as controls from the department of physical examination in the same hospital. After the participants were enrolled, their underlying disease was further checked and those with hypertension or cardiovascular diseases were excluded from the study, due to the known endothelial activation/dysfunction that occurred in these diseases.
Ethics statement
The study was approved by the ethics committee of PLA 154 hospital. All participants gave written informed consent.
Detection of cytokines and adhesion molecules reflecting endothelial dysfunction
Serum levels of vascular endothelial growth factor A (VEGF-A), P-selectin, E-selectin, platelet endothelial cell adhesion molecular (PECAM-1), CD40 ligand (CD40L), tissue plasminogen activator (tPA), plasminogen activator inhibitor 1 (PAI-1), serum amyloid antigen 1 (SAA-1), vascular cell adhesion molecular 1 (VCAM-1) and intercellular adhesion molecular 1 (ICAM-1) were determined by the ProcartaPlex multiplex immunoassays panels (Affymetrix, USA) according to the manufacturer instructions. Each serum sample was tested in duplicates and the concentration was further determined according to the dilution curve of standard materials.
Statistical analysis
Serum levels of the above-mentioned molecules reflecting endothelial dysfunction were compared between groups after performing base 10 logarithmic transformations. For variables that were not normally distributed, comparisons were made with the Mann-Whitney U test. The logistic regression model was performed to explore the correlations introduced by estimating the odds ratios (ORs) and 95% confidence intervals (CIs) for levels of biomarkers predicting severe and fatal SFTS patients. Receiver operator characteristic (ROC) curves were constructed and the area under the ROC curve (AUC) was calculated as a measure of discriminative ability. P-value of <0.05 was considered statistically significant. All analyses were performed using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
The subjects
Among all 219 patients with clinical suspicion of SFTS, 134 patients were confirmed to be infected with SFTSV and included into the study, their mean age was 61 years (Standard deviation, 11 years) and 45 (33.6%) were male. The demographic characteristics were comparable with those of the 68 controls (age 58±17 years, male gender 42.7%, P = 0.132 and 0.206, respectively). Fifty-seven (42.5%) patients had severe outcome and 12 (9.0%) patients died. The median duration between the disease onset and the day in which acute serum samples were collected was 6 days (interquartile range 5–7 days). The severe and mild patients had their convalescence samples collected at 16 (interquartile range 14–18) days and 14 (interquartile range 12–16) after disease onset, respectively. For the fatal cases, the median duration between the disease onset and the day in which the second sample was collected was 12 days (interquartile range 10–14 days). The clinical information of the SFTS patients were listed in Table 1.
Table 1. The characteristics and clinical manifestations of SFTS patients.
| Characteristic | SFTS patients (n = 134) |
|---|---|
| Demographic characteristics | |
| Male gender/ No. (%) | 45 (33.6) |
| Age, y, mean±SD | 61±11 |
| Days delay, median (IQR) | 6 (5–7) |
| Hospital duration, median (IQR) | 16 (14–17) |
| Clinical manifestations | |
| Fever >38°C | 134 (100) |
| Gastrointestinal syndromes | 121 (90.3) |
| Nausea | 118 (88.1) |
| Vomiting | 49 (36.6) |
| Diarrhea | 38 (28.4) |
| Neurological symptoms | 37 (27.6) |
| Dysphoria | 15 (11.2) |
| Convulsion | 27 (20.2) |
| Clouding of consciousness | 22 (16.4) |
| Lethargy | 5 (3.7) |
| Coma | 7 (5.3) |
| Hemorrhagic manifestation | 34 (25.4) |
| Melena | 11 (8.2) |
| Hematemesis | 1 (0.8) |
| Hemoptysis, | 7 (5.2) |
| Gingival bleeding | 21 (15.7) |
| Malaise | 134 (100) |
| Lymphadenectasis | 57 (42.5) |
| Cough | 95 (70.9) |
| Sputum production | 74 (55.2) |
| Dizzy | 24 (17.9) |
| Headache | 18 (13.4) |
| Dyspnea | 4 (3.0) |
| Severe | 57 (42.5) |
| Fatal | 12 (9.0) |
| Laboratory features on admission | |
| White blood cell count < 4×109/L | 115 (85.8) |
| Platelet count < 100 ×109/L | 110 (82.1) |
| Neutrophils > 70% | 68 (50.8) |
| Lymphocytes < 20% | 57 (42.5) |
| Hemoglobin < 110 g/L | 22 (16.4) |
| Aspartic transaminase> 40 U/L | 114 (85.1) |
| Alanine aminotransferase > 40 U/L | 72 (53.7) |
| Total protein <60 g/L | 15 (11.2) |
| Albumin < 35 g/L | 11 (8.2) |
| Alkaline phosphatase > 150 U/L | 3 (2.2) |
| Gamma-glutamyl transpeptidase >50 U/L | 23 (17.2) |
| Lactate dehydrogenase > 245 U/L | 118 (88.1) |
| Creatine kinase >200 U/L | 85 (63.4) |
| Urea nitrogen >7.14 mmol/L | 50 (37.3) |
| Total bilirubin >17.1 μmol/L | 4 (3.0) |
| Creatinine > 97 μmol/L | 22 (16.4) |
| Amylase > 115 U/L | 32 (23.9) |
Comparison between case and control groups
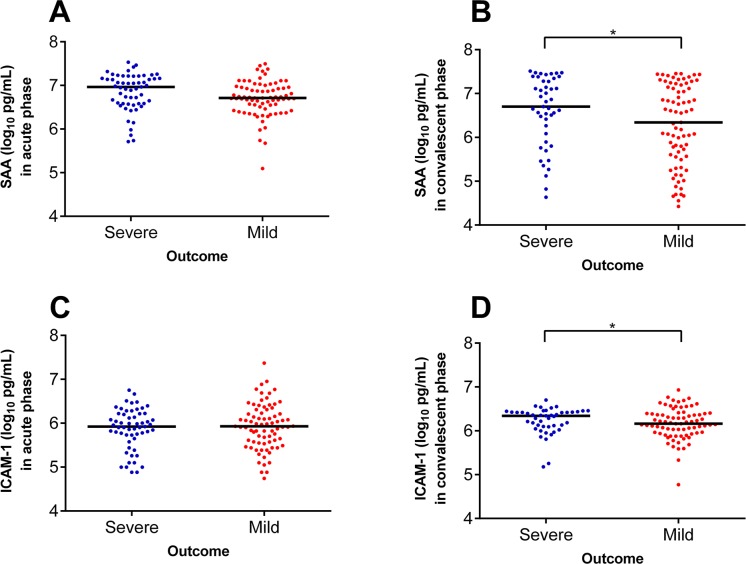
Nine of the 10 parameters that were evaluated at acute phase of SFTSV infection, i.e., PAI-1, tPA, P-selectin, PECAM-1, CD40L, E-selectin, VEGFA, SAA-1 and VCAM-1 were increased to significantly higher levels in the cases than in the controls. Only ICAM-1 was comparable between two groups (Table 2). At convalescence, all the 10 parameters were found to be significantly different between cases and controls. When the evaluations were compared between severe and mild patients, only the ICAM-1 and SAA-1 at the convalescent phase showed significantly higher levels in severe patients than in the mild patients after multivariate analysis (P = 0.004 and 0.010 respectively, Table 3 and Fig 1). The ORs of severe outcome was 3.364 (95% CI 1.074–10.534) for ICAM-1 (log10-transformed, pg/mL), and 1.881 (95% CI 1.166–3.034) for SAA-1 (log10-transformed, pg/mL) (Table 4). All the other evaluations were comparable between two groups, by applying multivariate analysis to adjust the effect from the age, sex the interval from disease onset to admission and underlying diseases (Table 3).
Table 2. The levels of the cytokines and adhesion molecules reflecting endothelial dysfunction in cases and controls.
| Biomarkers (pg/mL) | Case group | Control group (n = 68) |
P value | |||
|---|---|---|---|---|---|---|
| Acute sampling (n = 134) | Second sampling (n = 122) | Acute vs. Second | Acute vs. Control | Second vs. Control | ||
| VEGFA | 2.74 (2.52, 3.01) | 2.92 (2.55, 3.11) | 1.14 (0.21, 2.65) | 0.033 | <0.001 | <0.001 |
| ICAM-1 | 5.92 (5.57, 6.22) | 6.24 (6.03, 6.44) | 6.06 (5.84, 6.23) | <0.001 | 0.073 | <0.001 |
| CD40L | 0.93 (0.13, 1.72) | 1.69 (0.75, 2.07) | -0.22 (-0.27, 1.53) | <0.001 | <0.001 | <0.001 |
| PAI-1 | 4.61 (4.42, 4.77) | 4.37 (4.10, 4.66) | 3.38 (1.01, 4.07) | <0.001 | <0.001 | <0.001 |
| tPA | 3.76 (3.26, 4.04) | 3.19 (2.78, 3.76) | 2.22 (1.97, 3.12) | <0.001 | <0.001 | <0.001 |
| VCAM-1 | 5.94 (5.83, 6.1) | 5.87 (5.78, 5.94) | 5.82 (5.68, 5.89) | <0.001 | <0.001 | 0.013 |
| P-selectin | 4.12 (3.87, 4.31) | 4.19 (3.93, 4.4) | 3.9 (3.43, 4.36) | 0.068 | 0.011 | <0.001 |
| PECAM-1 | 4.53 (4.3, 4.68) | 4.46 (4.17, 4.71) | 4.07 (3.34, 4.42) | 0.081 | <0.001 | <0.001 |
| E-selectin | 3.99 (3.7, 4.19) | 3.99 (3.63, 4.25) | 3.36 (3.01, 3.95) | 0.480 | <0.001 | <0.001 |
| SAA-1 | 6.74 (6.55, 7.07) | 6.66 (5.8, 7.28) | 5.05 (4.71, 5.32) | 0.064 | <0.001 | <0.001 |
Data are given as log-transformed median (interquartile range); The comparison was made by applying Mann-Whitney U test.
Table 3. The levels of the cytokines and adhesion molecules reflecting endothelial dysfunction in the severe patients and mild patients.
| Biomarkers (pg/mL) | Acute phase of SFTS | Convalescent phase of SFTS | ||||
|---|---|---|---|---|---|---|
| Severe (n = 57) | Mild (n = 77) | P | Severe (n = 45) | Mild (n = 77) | P | |
| tPA | 3.71 (3.31, 3.99) | 3.79 (3.26, 4.07) | 0.697 | 3.08 (2.78, 3.65) | 3.42 (2.78, 3.77) | 0.160 |
| PAI-1 | 4.61 (4.43, 4.77) | 4.61 (4.41, 4.76) | 0.822 | 4.5 (4.06, 4.66) | 4.33 (4.11, 4.67) | 0.737 |
| P-selectin | 4.12 (3.86, 4.28) | 4.12 (3.91, 4.33) | 0.381 | 4.2 (3.9, 4.42) | 4.19 (3.97, 4.39) | 0.754 |
| PECAM-1 | 4.43 (4.27, 4.65) | 4.56 (4.37, 4.7) | 0.090 | 4.45 (4.17, 4.85) | 4.47 (4.21, 4.67) | 0.518 |
| CD40L | 0.42 (0.13, 1.59) | 1.21 (0.13, 1.72) | 0.051 | 1.43 (0.31, 1.96) | 1.8 (1.23, 2.18) | 0.022 |
| E-selectin | 3.98 (3.63, 4.19) | 4 (3.73, 4.18) | 0.729 | 3.98 (3.58, 4.26) | 4.02 (3.73, 4.23) | 0.792 |
| VEGFA | 2.71 (2.51, 2.92) | 2.75 (2.58, 3.02) | 0.283 | 2.89 (2.22, 3.11) | 2.93 (2.69, 3.12) | 0.357 |
| SAA-1 | 6.96 (6.58, 7.16) | 6.71 (6.46, 6.95) | 0.098 | 7.01 (6.42, 7.34) | 6.34 (5.57, 7.14) | 0.004 |
| ICAM-1 | 5.92 (5.73, 6.22) | 5.93 (5.57, 6.23) | 0.724 | 6.36 (6.13, 6.46) | 6.16 (5.95, 6.39) | 0.010 |
| VCAM-1 | 5.92 (5.82, 6.05) | 5.95 (5.85, 6.13) | 0.230 | 5.87 (5.76, 5.95) | 5.87 (5.79, 5.94) | 0.632 |
Data are given as log-transformed median (interquartile range); The comparison was made by applying Mann-Whitney U test.
Fig 1. The distribution of serum amyloid A proteins 1 (SAA-1) and intercellular adhesion molecular 1 (ICAM-1) between severe and mild patients at acute phase.
The comparison was performed by adjusting the effect from age, sex, the interval from disease onset to admission and underlying diseases. A, SAA-1 in acute phase; B, SAA-1 in convalescent phase; C, ICAM-1 in acute phase; D, ICAM-1 in convalescent phase.
Table 4. The association between the cytokines and adhesion molecules reflecting endothelial dysfunction and severe outcome.
| Biomarkers (pg/mL) | Severe | Mild | Crude | Adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |||
| Acute phase | ||||||||
| SAA-1 | 6.96 (6.58, 7.16) | 6.71 (6.46, 6.95) | 2.584 | 1.061–6.339 | 0.037 | 2.168 | 0.868–5.419 | 0.098 |
| Convalescent phase | ||||||||
| CD40L | 1.43 (0.31, 1.96) | 1.80 (1.23, 2.18) | 0.648 | 0.433–0.970 | 0.035 | 0.666 | 0.438–1.011 | 0.056 |
| ICAM-1 | 6.36 (6.13, 6.46) | 6.16 (5.95, 6.39) | 3.386 | 1.123–10.209 | 0.030 | 3.364 | 1.074–10.534 | 0.037 |
| SAA-1 | 7.01 (6.42, 7.34) | 6.34 (5.57, 7.14) | 1.861 | 1.211–2.859 | 0.005 | 1.881 | 1.166–3.034 | 0.010 |
Note: The ORs (95% CI) were calculated using the log10-transformed data of the biomarkers.
*adjusted for the variables of age, sex the interval from disease onset to admission and underlying diseases.
The levels of PAI-1, tPA and VCAM-1 were significantly decreased in convalescence compared with the acute samples, however, remained significantly different from those of the controls (Table 2). In contrast, the level of CD40L and VEGFA were further increased, with the convalescence level were significantly higher, even than the acute phase (Table 2).
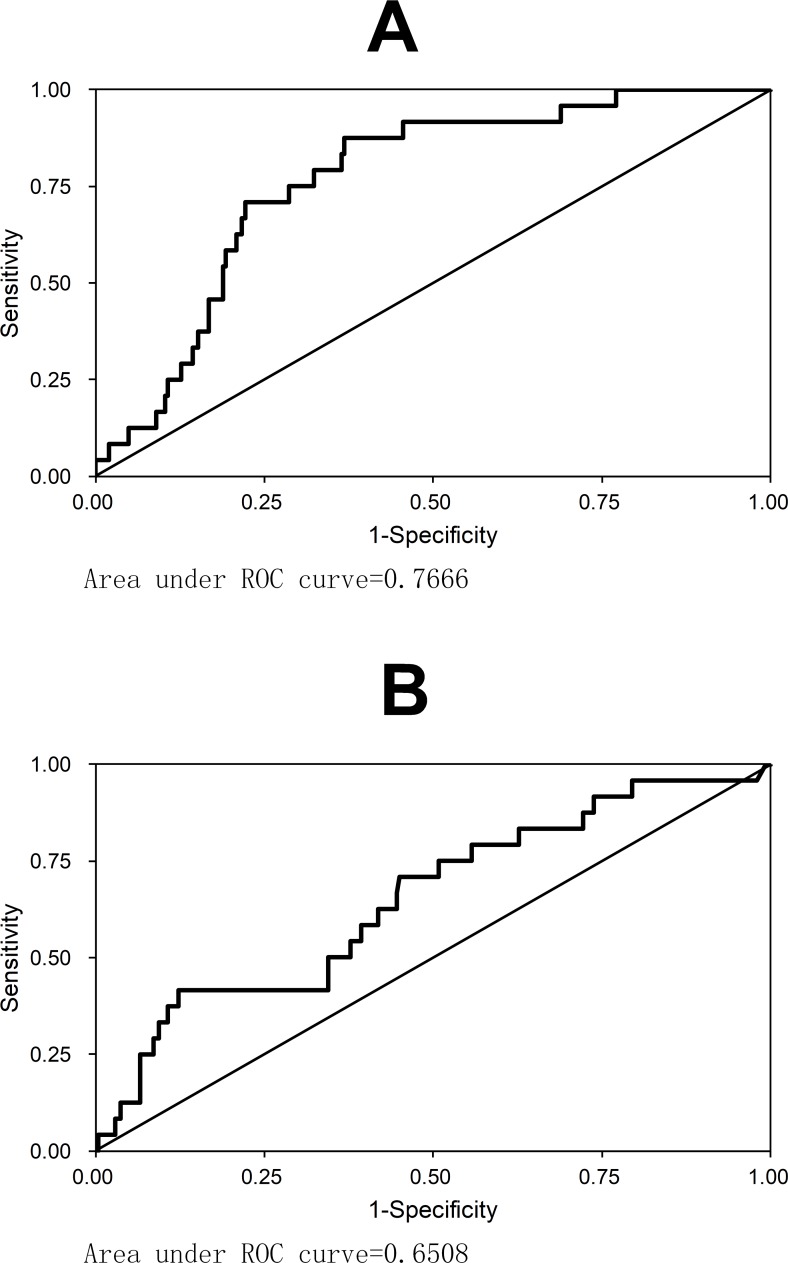
It’s interesting that the ICAM-1, although comparable between cases and controls, turned to be increased to higher than the controls (Table 1), and even higher in severe patients than in mild patients (Fig 1). When the fatal cases were analyzed separately, the levels of ICAM-1 and SAA-1 in second samples of fatal cases were found to be even more significantly increased compared to the non-fatal patients in the convalescent phase (Table 5). ROC analysis demonstrated the good diagnostic accuracy for SAA-1 (AUC = 0.767) and ICAM-1 (AUC = 0.651) to the prediction of mortality (Fig 2). The SAA-1 had 75.0% sensitivity and 71.3% specificity for prediction of mortality at a cutoff of 1.1×107 pg/mL; and the ICAM-1 had 75.0% sensitivity and 49.2% specificity for prediction of mortality at a cutoff of 1.2×106 pg/mL. These results indicated these two parameters could be used as consistent markers as to differentiate the cases from controls, and predicting the severe patients and even fatal patients.
Table 5. The association between the cytokines and adhesion molecules reflecting endothelial dysfunction and fatal outcome of SFTS patients.
| Biomarkers (pg/mL) | Fatal | Non-Fatal | Crude | Adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |||
| Acute phase | ||||||||
| CD40L | -.027 (-0.27, 0.48) | 1.05 (0.13, 1.74) | 0.223 | 0.071–0.697 | 0.010 | 0.218 | 0.067–0.713 | 0.012 |
| PECAM-1 | 4.18 (3.13, 4.26) | 4.56 (4.36, 4.69) | 0.001 | <0.001–0.026 | <0.001 | <0.001 | <0.001–0.006 | <0.001 |
| E-selectin | 3.60 (3.33, 3.80) | 4.01 (3.73, 4.21) | 0.041 | 0.007–0.240 | <0.001 | 0.032 | 0.005–0.239 | 0.001 |
| VEGFA | 2.41 (2.07, 2.55) | 2.78 (2.58, 3.02) | 0.032 | 0.005–0.191 | <0.001 | 0.017 | 0.002–0.138 | <0.001 |
| SAA-1 | 7.13 (6.95, 7.22) | 6.72 (6.54, 7.05) | 13.277 | 1.754–100.472 | 0.012 | 11.409 | 1.493–87.183 | 0.019 |
| Fatal cases at deterioration vs. non-fatal cases in convalescent phase | ||||||||
| CD40L | -0.27 (-0.27, 1.32) | 1.74 (1.09, 2.12) | 0.185 | 0.075–0.456 | <0.001 | 0.181 | 0.068–0.483 | 0.001 |
| ICAM-1 | 6.61 (6.34, 6.69) | 6.21 (6.02, 6.42) | 129.446 | 7.402–2263.522 | 0.001 | 122.669 | 6.609–2276.791 | 0.001 |
| VEGFA | 1.98 (1.64, 2.56) | 2.93 (2.65, 3.12) | 0.117 | 0.038–0.357 | <0.001 | 0.120 | 0.040–0.365 | <0.001 |
| SAA-1 | 7.30 (7.22, 7.37) | 6.60 (5.73. 7.22) | 8.306 | 1.566–44.045 | 0.013 | 8.166 | 1.356–49.189 | 0.022 |
Note: The ORs (95% CI) were calculated using the log10-transformed data of the biomarkers.
*adjusted for the variables of age, sex the interval from disease onset to admission and underlying diseases.
Fig 2.
The ROC curves of SAA-1 (A) and ICAM-1 (B) for predicting mortality in SFTS patients.
Discussion
In this case control study, we identified that elevated levels of adhesion molecule were associated with SFTS disease. All these elevated plasma measurements could be considered as the marker of endothelial activation/dysfunction; therefore, we suggest that the endothelial activation/dysfunction might act as one of the pathogenesis of SFTSV infection. We also found that serum levels of ICAM-1 and SAA-1 might be an indicator of adverse prognosis in SFTS, independent of other impact factors. Using a discrimination analysis, we are able to infer a cutoff value that might predict the occurrence of fatal SFTS.
Like several previous reports, the serum level of ICAM-1 alone, but not E-selectin or VCAM-1 was found to be an independent predictor of severity or mortality in the cases [10,11]. ICAM-1 is the member of immunoglobulin superfamily, which is expressed by leucocytes and the endothelial cells of vessels. ICAM-1 is present at very low levels in healthy humans. Their concentration might be increased markedly in various inflammatory conditions [12,13]. Levels of ICAM-1 were even higher in severe and fatal cases in late disease, which may related to the adverse outcome.
Consistent with this alteration is the increased SAA-1 levels in patient, also seen in those severe patients. SAA-1 has also been proven for its potent impact on the dysfunction of endothelial, through the suppression of endothelial nitric oxide synthase function and endothelial-derived nitric oxide bioviability [14]. The higher expression of ICAM-1 has been linked to the systematic injuries of vascular endothelial cells in the LPS stimulated disseminated intravascular coagulation (DIC) mice model [15], and SAA-1 was found to disturb the balance of endothelial tissue factor pathway, which acts as a component of initiation of coagulation cascade [16]. Similarly, these two indicators, through mediating the endothelial damage and activation, contribute to hemostatic failure by stimulating platelet aggregation and degranulation, resulting in consequent activation of the intrinsic coagulation cascade [7]. This is consistent with the late development of DIC in most of the severe and fatal SFTS cases [17].
VEGFA is known to be the most specific mitogenic factor of endothelial cells. It is originally found as a vascular permeability factor that causes vascular leakage. In the Dengue virus study, an increased level of VEGFA associated with a decrease in its soluble receptor, VEGFR2 were observed in patients with Dengue hemorrhagic fever [18], and the significant elevation of VEGFA in the acute phase may directly indicate the generalized capillary damage and vascular leakage [19]. In the current SFTS patients, a prolonged high level of VEGFA in the recovery period may indicate the sustained vascular angiogenesis and repairmen, similar with hantavirus infection, also from the Bunyaviridae [20].
The role of vascular endothelium in the pathogenesis of vascular disease has been better known in the last 30 years, and also been increasingly related to the infectious disease, especially bacterial sepsis and viral hemorrhagic fever [21]. As displayed in previous findings, endothelial activation/dysfunction has an important role in the pathogenesis of known members of viral hemorrhagic fever virus, such as CCHF, Ebola virus. In case of vascular dysregulation and hyperpermeability, patients might exhibit thrombocytopenia, leukopenia, proteinuria, and fluid distribution problems. Consistent with previous findings, all these symptoms have occurred in SFTSV infection and clinically related to more severe disease.
Several investigations have performed in the SFTS patients or animal models to determine whether circulating cytokines and chemokines have their diagnostic or prognostic value, but only yielding conflicting results [5,22,23]. These cytokines and chemokines displayed rapid alteration and recovery in the viral hemorrhagic fever conditions, especially in severe cases, therefore their levels are remarkably influenced by the sampling time. In contrast, the measurement of adhesion molecules, like SAA-1 and ICAM-1 would have a better reproducibility in the correlation with the disease, due to their persistent alteration after disease [14].
Our study has limitation that we assessed endothelial dysfunction solely by means of serum biomarkers and do not have other measures of vascular physiology, which needs further investigation. Despite of this limitation, through a relatively large study population, we found that elevated plasma levels of biomarkers reflecting endothelial activation/dysfunction were observed in SFTSV infection, and independently associated with severe disease outcome after SFTSV infection. As the significant differences were only seen at later phase of infection, the predictive use of these biomarkers was restricted. More studies were needed to further demonstrate the dynamic profile of the biomarkers.
The occurrence of endothelial dysregulation, which is a common feature of viral hemorrhagic fever, also developed in SFTS. Thus, similar with other known viral hemorrhagic fever, endothelial dysfunction might be one of the pathogenic mechanism of SFTS, and the therapies that control endothelial dysfunction might be applied in the treatment of SFTS.
Acknowledgments
We thank all the tested individuals for their participation.
Data Availability
Data are available from the PLA 154 hospital Ethics Committee for researchers who meet the criteria for access to confidential data (pla154yy@sina.com).
Funding Statement
This work was supported by grants from the National Natural Science Foundation (No. 81473023 and 81703274), the State Key Laboratory of Pathogen and Biosecurity (Academy of Military Medical Science, SKLPBS1442) and the Youth Talent Support Program by the School of Public Health, Peking University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, et al. (2011) Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364: 1523–1532. doi: 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fill MA, Compton ML, McDonald EC, Moncayo AC, Dunn JR, et al. (2016) Novel Clinical and Pathologic Findings in a Heartland Virus-Associated Death. Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, et al. (2012) A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 367: 834–841. doi: 10.1056/NEJMoa1203378 [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Qi Y, Liu C, Gao W, Chen P, et al. (2014) Nonmuscle myosin heavy chain IIA is a critical factor contributing to the efficiency of early infection of severe fever with thrombocytopenia syndrome virus. J Virol 88: 237–248. doi: 10.1128/JVI.02141-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng B, Zhang S, Geng Y, Zhang Y, Wang Y, et al. (2012) Cytokine and chemokine levels in patients with severe fever with thrombocytopenia syndrome virus. PLoS One 7: e41365 doi: 10.1371/journal.pone.0041365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding YP, Liang MF, Ye JB, Liu QH, Xiong CH, et al. (2014) Prognostic value of clinical and immunological markers in acute phase of SFTS virus infection. Clin Microbiol Infect 20: O870–878. doi: 10.1111/1469-0691.12636 [DOI] [PubMed] [Google Scholar]
- 7.Ozturk B, Kuscu F, Tutuncu E, Sencan I, Gurbuz Y, et al. (2010) Evaluation of the association of serum levels of hyaluronic acid, sICAM-1, sVCAM-1, and VEGF-A with mortality and prognosis in patients with Crimean-Congo hemorrhagic fever. J Clin Virol 47: 115–119. doi: 10.1016/j.jcv.2009.10.015 [DOI] [PubMed] [Google Scholar]
- 8.(2011) Ministry of Health China. Guideline for prevention and treatment of severe fever with thrombocytopenia syndrome (2010 version). Chin J Clin Infect Dis 4: 193–194. [Google Scholar]
- 9.Lu QB, Cui N, Hu JG, Chen WW, Xu W, et al. (2015) Characterization of immunological responses in patients with severe fever with thrombocytopenia syndrome: a cohort study in China. Vaccine 33: 1250–1255. doi: 10.1016/j.vaccine.2015.01.051 [DOI] [PubMed] [Google Scholar]
- 10.Kayal S, Jais JP, Aguini N, Chaudiere J, Labrousse J (1998) Elevated circulating E-selectin, intercellular adhesion molecule 1, and von Willebrand factor in patients with severe infection. Am J Respir Crit Care Med 157: 776–784. doi: 10.1164/ajrccm.157.3.9705034 [DOI] [PubMed] [Google Scholar]
- 11.Knapp S, Thalhammer F, Locker GJ, Laczika K, Hollenstein U, et al. (1998) Prognostic value of MIP-1 alpha, TGF-beta 2, sELAM-1, and sVCAM-1 in patients with gram-positive sepsis. Clin Immunol Immunopathol 87: 139–144. [DOI] [PubMed] [Google Scholar]
- 12.Boldt J, Wollbrück M, Kuhn D, Linke LC, Hempelmann G (1995) Do plasma levels of circulating soluble adhesion molecules differ between surviving and nonsurviving critically ill patients? Chest 107: 787–792. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, et al. (1995) Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med 151: 1420–1427. doi: 10.1164/ajrccm.151.5.7735595 [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Chai H, Wang Z, Lin PH, Yao Q, et al. (2008) Serum amyloid A induces endothelial dysfunction in porcine coronary arteries and human coronary artery endothelial cells. Am J Physiol Heart Circ Physiol 295: H2399–2408. doi: 10.1152/ajpheart.00238.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koide N, Abe K, Narita K, Kato Y, Sugiyama T, et al. (1997) Expression of intercellular adhesion molecule-1 (ICAM-1) on vascular endothelial cells and renal tubular cells in the generalized Shwartzman reaction as an experimental disseminated intravascular coagulation model. FEMS Immunol Med Microbiol 18: 67–74. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Zhou S, Heng CK (2007) Impact of serum amyloid A on tissue factor and tissue factor pathway inhibitor expression and activity in endothelial cells. Arterioscler Thromb Vasc Biol 27: 1645 doi: 10.1161/ATVBAHA.106.137455 [DOI] [PubMed] [Google Scholar]
- 17.Gai ZT, Zhang Y, Liang MF, Jin C, Zhang S, et al. (2012) Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206: 1095–1102. doi: 10.1093/infdis/jis472 [DOI] [PubMed] [Google Scholar]
- 18.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, et al. (2007) Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol 81: 1592–1600. doi: 10.1128/JVI.01642-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG (2005) Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol 43: 99–102. doi: 10.1016/j.femsim.2004.10.004 [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Liu B, Yuan B, Wang J, Yu H, et al. (2012) Sustained High Level of Serum VEGF at Convalescent Stage Contributes to the Renal Recovery after HTNV Infection in Patients with Hemorrhagic Fever with Renal Syndrome. Clin Dev Immunol 2012: 812386 doi: 10.1155/2012/812386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiropoulou CF, Srikiatkhachorn A (2013) The role of endothelial activation in dengue hemorrhagic fever and hantavirus pulmonary syndrome. Virulence 4: 525–536. doi: 10.4161/viru.25569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin C, Jiang H, Liang M, Han Y, Gu W, et al. (2015) SFTS virus infection in non-human primates. J Infect Dis 211: 915–925. doi: 10.1093/infdis/jiu564 [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Jin C, Zhan F, Wang X, Liang M, et al. (2012) Host cytokine storm is associated with disease severity of severe fever with thrombocytopenia syndrome. J Infect Dis 206: 1085–1094. doi: 10.1093/infdis/jis452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the PLA 154 hospital Ethics Committee for researchers who meet the criteria for access to confidential data (pla154yy@sina.com).