Abstract
Rice is an important monocotyledonous crop worldwide; it differs from the dicotyledonous plant Arabidopsis in many aspects. In Arabidopsis, ethylene and auxin act synergistically to regulate root growth and development. However, their interaction in rice is still unclear. Here, we report that the transcriptional activation of OsEIL1 on the expression of YUC8/REIN7 and indole-3-pyruvic acid (IPA)-dependent auxin biosynthesis is required for ethylene-inhibited root elongation. Using an inhibitor of YUC activity, which regulates auxin biosynthesis via the conversion of IPA to indole-3-acetic acid (IAA), we showed that ethylene-inhibited primary root elongation is dependent on YUC-based auxin biosynthesis. By screening phenotypes of seedling primary root from mutagenesis libraries following ethylene treatment, we identified a rice ethylene-insensitive mutant, rein7-1, in which YUC8/REIN7 is truncated at its C-terminus. Mutation in YUC8/REIN7 reduced auxin biosynthesis in rice, while YUC8/REIN7 overexpression enhanced ethylene sensitivity in the roots. Moreover, YUC8/REIN7 catalyzed the conversion of IPA to IAA, truncated version at C-terminal end of the YUC8/REIN7 resulted in significant reduction of enzymatic activity, indicating that YUC8/REIN7 is required for IPA-dependent auxin biosynthesis and ethylene-inhibited root elongation in rice early seedlings. Further investigations indicated that ethylene induced YUC8/REIN7 expression and promoted auxin accumulation in roots. Addition of low concentrations of IAA rescued the ethylene response in the rein7-1, strongly demonstrating that ethylene-inhibited root elongation depends on IPA-dependent auxin biosynthesis. Genetic studies revealed that YUC8/REIN7-mediated auxin biosynthesis functioned downstream of OsEIL1, which directly activated the expression of YUC8/REIN7. Thus, our findings reveal a model of interaction between ethylene and auxin in rice seedling primary root elongation, enhancing our understanding of ethylene signaling in rice.
Author summary
Rice is an important crop worldwide and is grown in water-saturated environments during its life cycle. This unique feature confers that rice might have different aspects from Arabidopsis in ethylene signaling. Although the crosstalk between ethylene and auxin is well understood in Arabidopsis, however, the interaction in rice is largely unclear. Here, we show that YUC8/REIN7, a member of the YUC gene family, catalyzing the conversion of IPA to IAA in auxin biosynthesis, is transcriptionally modulated by ethylene signaling component OsEIL1, and mainly participates in auxin biosynthesis and ethylene-inhibited root growth. We first identified that ethylene-inhibited root elongation is suppressed by the inhibitor of YUC activity, and YUC8/REIN7 is required for IPA-dependent auxin biosynthesis, indicating that YUC8/REIN7 is involved in ethylene-inhibited root elongation in rice early seedlings. Moreover, ethylene induced YUC8/REIN7 transcription and promoted auxin accumulation in roots. Addition of low concentrations of IAA rescued the ethylene response in the rein7-1, demonstrating that ethylene stimulates auxin biosynthesis dependent on YUC8/REIN7 function. Further evidence revealed that OsEIL1 transcriptionally activates the expression of YUC8/REIN7, and YUC8/REIN7-mediated auxin biosynthesis genetically acts downstream of OsEIL1. Our data in the present report identified an interaction between ethylene and auxin in rice seedling primary root elongation, increasing our understanding of ethylene signaling in rice root growth.
Introduction
Root systems of higher plants play essential roles in absorbing water and nutrients and supporting the plant body. Improved root architecture is crucial for productivity and a very important contributor to extract water under water-limited stress [1]. The dicotyledonous plant Arabidopsis has a primary root and lateral roots, whereas monocotyledonous crops, including rice (Oryza sativa L.), have fibrous root systems composed of a primary root, lateral roots and adventitious roots. The primary root, initiated during embryo development, develops shortly after germination and is a fundamental part of the root system that absorbs mineral nutrients, and provides mechanical support for shoot growth [2–4]. Root development is affected by diverse endogenous and exogenous factors, such as ethylene and auxin, which are central regulators of this process [5–9].
Previous studies have shown that auxin biosynthesis, transport and auxin-dependent signaling affect root development [10–12]. Indole-3-acetic acid (IAA), the major form of auxin in plants, can be biosynthesized in tryptophan (Trp) -dependent and -independent pathways [13,14]. There are four pathways for IAA biosynthesis from Trp in plants: the YUCCA (YUC) pathway or the indole-3-pyruvic acid (IPA) pathway, the tryptamine (TAM) pathway, the indole-3-acetamide pathway, and the indole-3-acetaldoxime pathway [13]. The YUC pathway has been proposed as the most important pathway to produce auxin in plant [15–17], and the YUC family of flavin-containing monooxygenases and the TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS (TAA) family of aminotransferases are key enzymes in this pathway [13,18–20]. YUC catalyzes the conversion of IPA to IAA, a rate-limiting step in the IPA pathway [15,16]. The diversity of auxin biosynthesis indicates that different pathways may have distinctive roles in plant tissue growth and development.
Ethylene is a simple and very important gaseous phytohormone that modulates multiple plant growth and developmental processes [21]. In Arabidopsis, ethylene signaling is started from the perception by a family of endoplasmic reticulum-located receptors [22,23]. In the absence of ethylene, the active receptors recruit a Raf-like protein kinase, CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1), to phosphorylate the C-terminal domain of ETHYLENE INSENSITIVE 2 (EIN2), which represses the downstream ethylene response [24–27]. In the presence of ethylene, ethylene binding to the receptors inhibits the interaction with CTR1, resulting in that CTR1 cannot phosphorylate EIN2. Unphosphorylated EIN2 is cleaved by an unknown kinase, and the EIN2 C-terminus translocates into the nucleus [26,28,29], to stabilize the transcription factors EIN3 and EIN3-LIKE1 (EIL1), which are sufficient and necessary for activation of many ethylene-response genes. These changes ultimately cause different physiological responses [30,31].
Ethylene is known to inhibit root elongation [5,7]. This regulation is revealed to mediate the interaction with auxin [7,19,32–35]. For example, auxin increases 1-aminocyclopropane-1-carboxylic acid synthase (ACS) gene transcription and ethylene biosynthesis [36]. Similarly, ethylene application promotes the expression of IAA biosynthetic genes and IAA levels [19,33,34]. Furthermore, mutants related to auxin biosynthesis, distribution, or signaling display abnormal responses to ethylene [34,37–39], and the genes involved in local auxin biosynthesis, such as ANTHRANILATE SYNTHASE α1 (ASA1), ANTHRANILATE SYNTHASE β1 (ASB1), TAA1 and TRYPTOPHAN AMINOTRANSFERASE-RELATED PROTEIN 1 (TAR1), which are regulated by ethylene, exhibit ethylene-insensitive root growth [19,34]. YUC genes also play an important role in root responses to ethylene [16]. Recent studies have shown that several factors, such as EIN3, ETHYLENE RESPONSE FACTOR 1 (ERF1) and PHYTOCHROME INTERACTING FACTOR 4 (PIF4), function as crosstalk nodes between ethylene and auxin in primary root elongation [9,40,41], suggesting that ethylene-inhibited primary root growth in Arabidopsis requires auxin biosynthesis, transport, or signaling.
Rice is one of the most common crops worldwide and grown in water-saturated environments during its life cycle. Ethylene plays important roles in rice adaption to hypoxic conditions [42]. In the dark, ethylene promotes coleoptile growth of rice seedlings but inhibits root elongation [43,44]. The double response in rice is different from the triple response of dark-grown Arabidopsis seedlings, which have inhibited hypocotyls and roots, with an exaggerated apical hook [21]. Although rice has five receptors, including two members [ETHYLENE RESPONSE SENSOR 1 (OsERS1) and OsERS2] of subfamily I and three members [ETHYLENE RESPONSE 2 (OsETR2), OsETR3 and OsETR4] of subfamily II [45,46], an ETR1-type receptor is absent compared to Arabidopsis [44]. Moreover, a single loss-of-function mutation of ethylene receptors in rice can lead to phenotypic changes, whereas multiple receptor loss-of-function mutations in Arabidopsis can cause major phenotypic changes [23,47,48]. As discussed above, CTR1 is a key negative regulator of ethylene signaling [24]. Arabidopsis contains a single CTR1, whereas there are three CTR-like genes in rice, and ethylene receptor signal output is mediated in part by OsCTR2 [49]. Rice has six EIN3-like homologues, and only OsEIL1 and OsEIL2 are involved in the ethylene signaling [50]. OsEIL1 and OsEIL2 spatially regulate the ethylene response of roots and coleoptiles of etiolated seedlings, which differs from the incomplete ethylene insensitivity of ein3 and eil1 in Arabidopsis [50,51]. In rice, ethylene triggers root-specific accumulation of abscisic acid (ABA), which is required for root inhibition [48,52]. This ethylene-ABA interaction mode is different from previous reports, in which ABA negatively regulates ethylene production and then inhibits root growth in Arabidopsis [53–55]. These findings suggest that different features are present in rice ethylene signaling. Although the crosstalk between ethylene and auxin has been well studied in Arabidopsis, their interaction in rice is largely unclear. In this study, we report that YUC8/REIN7, a member of the YUC gene family, located genetically downstream of OsEIL1, is mainly involved in the conversion of IPA to IAA in auxin biosynthesis and root growth. Thus, the results provide a new insight into understanding of ethylene and auxin interaction in regulating rice root growth.
Results
YUC-based auxin biosynthesis is required for ethylene-inhibited root elongation
Ethylene promotes coleoptile growth of etiolated rice seedlings but inhibits root elongation [43,44,48,50,52], which is known as the double response. To determine whether the double response of ethylene in rice is conserved in different genetic backgrounds, we treated three japonica cultivars and three indica cultivars with ethylene. All genotypes exhibited increased coleoptile growth and inhibited root elongation (S1A–S1C Fig), suggesting that the ethylene double response is common in etiolated rice seedlings. Next, we investigated whether ethylene biosynthesis or signaling is involved in this process. We assessed the effect of the ethylene biosynthesis inhibitor 1-aminoethoxyvinyl-glycine (AVG) and ethylene competitive inhibitor 1-methylcyclopropene (1-MCP) on coleoptile and root growth in the presence of ethylene. Our data showed that the 1-MCP, but not AVG, suppressed the ethylene response of etiolated japonica and indica seedlings (S1A–S1C Fig). To further examine the requirements for the ethylene signaling pathway in this process, we assessed the ethylene double response in ethylene signaling mutants. Our results revealed that the osein2 mutant was insensitive to ethylene in both root elongation and coleoptile promotion, while oseil1 was insensitive to ethylene in root elongation, but showed normal coleoptile promotion S2A–S2C Fig). Correspondingly, overexpression of OsEIN2/OsEIL1 (EIN2-OX and EIL1-OX, respectively) resulted in an enhanced ethylene response following ethylene treatment (S2A–S2C Fig). These data indicate that ethylene-promoted coleoptile growth and ethylene-inhibited root elongation are primarily mediated by the ethylene signaling pathway.
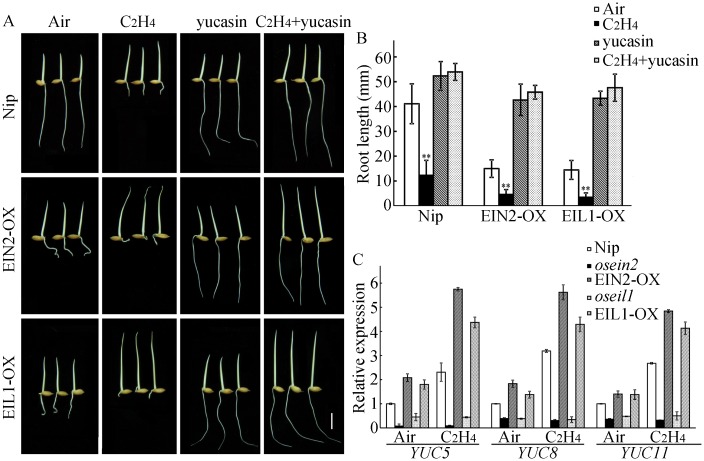
Multiple reports have demonstrated that ethylene-inhibited Arabidopsis root growth is mediated through the effects on auxin biosynthesis [9,19,33,34]. And YUC controls the rate-limiting step of auxin biosynthesis via the conversion of IPA to IAA [15,16]. There are 14 and 11 YUC genes in rice and Arabidopsis, respectively. To determine whether YUC-based auxin biosynthesis is required for ethylene-inhibited root growth in rice, we inhibited YUC activity with chemical inhibitor. Our observations showed that ethylene-inhibited root growth in the wild-type seedlings was suppressed by yucasin [5-(4-chlorophenyl)-4H-1,2,4-triazole-3-thiol], an inhibitor of YUC activity, in the presence of ethylene (Fig 1A and 1B). This suppression of ethylene-inhibited root elongation by yucasin was further confirmed using the overexpression transgenic lines EIN2-OX and EIL1-OX (Fig 1A and 1B). We then detected the expression of YUC genes in seedling roots. Our results showed that ethylene induced the expression of most YUC genes (Figs 1C and S3). Especially, the induction of YUC5, YUC8, and YUC11 was dependent on OsEIN2 and OsEIL1 (Fig 1C). These differential inducible characters might be due to the temporal and spatial expression patterns of YUC genes, some of them may be not involved in ethylene-inhibited root elongation in early seedlings, thus some YUC genes are not regulated by OsEIN2 and OsEIL1 in roots. Alternatively, the expression of YUC3 was obviously induced by ethylene, even in the absence of osein2 (S3 Fig), implying that there might have an OsEIN2-independent ethylene response pathway in rice. These findings indicate that ethylene-inhibited root elongation is mainly dependent on YUC activity-based auxin biosynthesis, and YUC5, YUC8, and YUC11 may be involved in ethylene-inhibited root elongation.
Fig 1. YUC-dependent auxin production is required for ethylene-inhibited root growth.
(A) Primary root phenotypes of Nipponbare (Nip), overexpressing OsEIN2 (EIN2-OX) and overexpressing OsEIL1 (EIL1-OX) transgenic lines treated with or without 10 ppm ethylene, in the absence or presence of 10 μM yucasin. Rice seedlings were grown in the dark for 3 d. Bar = 10 mm. (B) Root length of the plants shown in (A). Values are shown as the mean ± SD of 20–30 seedlings. The experiment was repeated at least three times with similar results. ** indicates significant difference compared to air at P < 0.01. (C) Expression of YUC genes in 3-d-old etiolated seedling roots. 3-d-old etiolated seedlings were treated with or without 10 ppm ethylene for 3 h. The RNAs from roots were isolated for qPCR. The experiment was repeated at least five times with similar results. Bars indicate ± SD.
YUC8, a flavin-containing monooxygenase, is required for IPA-dependent auxin biosynthesis
To identify the factors involved in ethylene-inhibited root elongation, we conducted a screen for ethylene-insensitive mutants from our mutagenesis (generated with fast neutron bombardment and chemical induction) and T-DNA insertion libraries in rice [56,57] in the dark. At least 7 rice ethylene-insensitive (rein) mutants were selected based on the phenotypes of the roots and coleoptile of etiolated seedlings (S4A Fig). Compared to the wild type, all the mutants, such as rein7-1, showed insensitivity or reduced sensitivity to ethylene in root elongation but exhibited normal coleoptile growth (S4B and S4C Fig), indicating that these mutation genes may be involved in ethylene-inhibited root elongation.
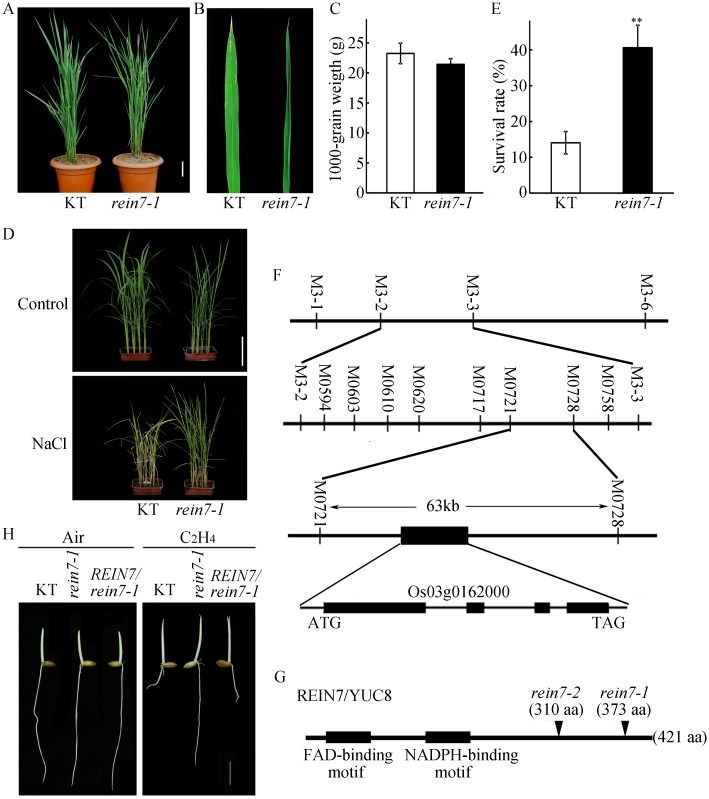
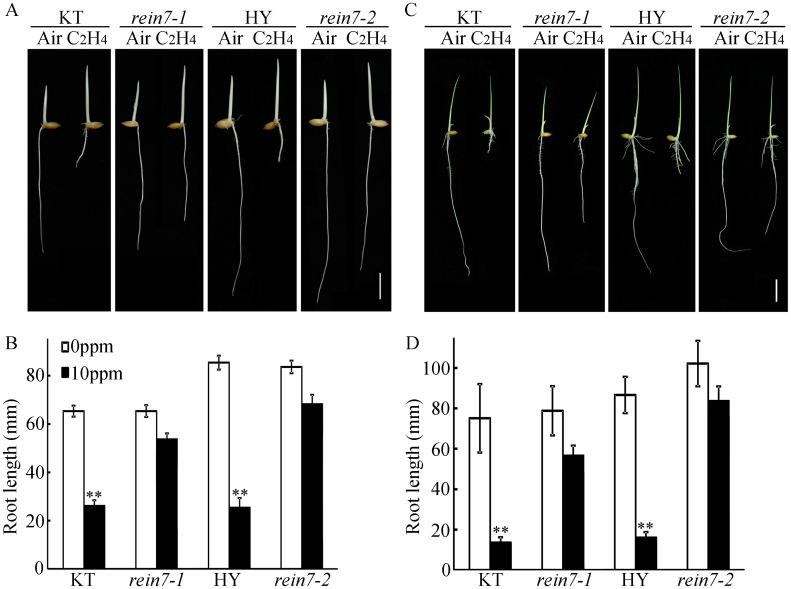
The field-grown rein7-1 mutant exhibited narrow, curled leaves, and enhanced salt tolerance but showed no significant difference in the 1000-grain weight compared with that of the wild-type plants (Fig 2A–2E), indicating that the rein7-1 confers salt adaptation without compromising yield. Analysis with map-based cloning using the F2 population of rein7-1 crossed with Dular (an indica cultivar) revealed that there is a single base pair substitution (G-A) in the fourth exon at nucleotide 2360 in Os03g0162000, resulting in a stop codon and loss of 47 amino acid residues from the C-terminal end of the REIN7 protein (Fig 2F–2G). The rein7-2 mutant was previously reported with a phenotype of curled leaves [58]. Sequence analysis suggests that REIN7 encodes a 421 amino acid protein containing two conserved sequence motifs, the FAD-binding motif and NADPH-binding motif (Fig 2G), and is a flavin-containing monooxygenase [59]. This protein shows significant sequence identity with YUC from Arabidopsis, which is required for the biosynthesis of auxin [18]. REIN7 corresponds to YUC8, belonging to the same group as rice YUC1 and Arabidopsis YUC1 and YUC4 (S5 Fig). To confirm that the mutation of YUC8/REIN7 locus is responsible for the mutant phenotype of rein7-1, we cloned a 5433 bp DNA fragment, including the complete Os03g0162000 genomic sequence, from Kitaake (a japonica cultivar) and transformed the construct into the rein7-1 plants. Ethylene response assays showed that the altered ethylene responsiveness of rein7-1 was rescued in the transgenic plants (Fig 2H), indicating that YUC8/REIN7 is located at Os03g0162000 locus. To further analyze the ethylene response of the rein7 mutants, we treated the two allelic mutants rein7-1 and rein7-2 with 10 ppm ethylene in dark or normal conditions; all of them exhibited reduced ethylene responses in roots in either dark or normal growth conditions (Fig 3A–3D). Thus our data showed that the C-terminal region of YUC8/REIN7 is required for ethylene-inhibited root elongation.
Fig 2. The rein7-1, a truncation of YUC8 at the C-terminus, displays rolled leaves and salt tolerance.
(A) Plant morphology of Kitaake (KT) and rein7-1 at the heading stage. Bar = 10 cm. (B) Typical image of rolled leaf. (C) The 1000-grain weight. Each value is the average of 30–50 plants. (D) Phenotypes of KT and rein7-1 under salt stress. Control indicates that rice seedlings were grown under normal conditions, and NaCl indicates that seedlings were treated with 150 mM NaCl aqueous solution. Bar = 10 cm. (E) Survival rate after salt treatment in (D). Approximately 50–60 seedlings were used in each experiment. Bars indicate ± SD of three independent assays. ** indicates a significant difference compared to KT at P < 0.01. (F) Map-based cloning of the YUC8/REIN7 gene. The locus was mapped to chromosome 3 within a 63 kb region between M0721 and M0728. ‘n’ indicates the number of samples used for map-based cloning, ‘M’ represents marker. (G) Mutation sites of two allelic mutants are indicated in the schematic diagram of the YUC8/REIN7 protein. (H) Functional complementation of the rein7 mutant. The KT, rein7-1 and complementary lines were treated with 10 ppm ethylene for 3 d under dark conditions. Bar = 10 mm.
Fig 3. Mutation of YUC8/REIN7 confers an insensitive phenotype of primary root growth in response to ethylene.
(A) and (C) Ethylene-response phenotypes of rein7 in dark or in normal growth conditions. KT and Hwayoung (HY) are the wild types. Seedlings were grown in the dark or in normal growth conditions for 3 d in the absence (air) or presence of 10 ppm of ethylene. Bar = 10 mm. (B) and (D) Root length in (A) and (C), correspondingly. Each column is the average of 20–30 seedlings, and bars indicate ± SD. The experiment was repeated at least three times with similar results. ** indicates significant difference compared to air at P < 0.01.
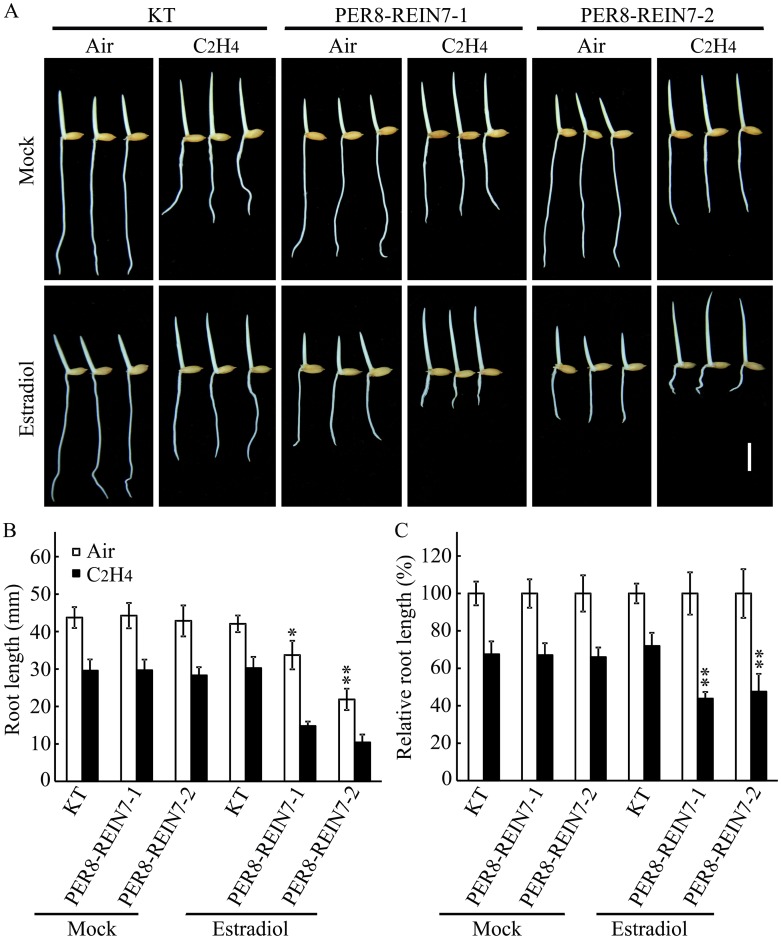
To further study the function of YUC8/REIN7 in rice ethylene response, we transformed the gene into the wild-type rice plants under the control of the CaMV 35S promoter. Due to alteration of the auxin content in the derived transgenic plants, most of the transformed calli exhibited overgrowth of the roots, and the rate of plant regeneration was quite low, and only two independent transgenic lines were obtained with a low transcription of YUC8/REIN7 (S6B Fig). To more directly observe the gene function, we used an estradiol-inducible system for YUC8/REIN7 expression. In the absence of estradiol treatment, the transgenic seedlings exhibited normal phenotypes and ethylene responses similar to those in the wild type (Fig 4A–4C). In contrast, when the plants were treated with estradiol, the primary root of transgenic seedlings was significantly shorter than that of the wild type. Following ethylene treatment, the transgenic seedlings showed enhanced ethylene response phenotypes in roots compared with those of the wild-type seedlings (Fig 4A–4C), further demonstrating that YUC8/REIN7 is required for ethylene-inhibited root elongation.
Fig 4. YUC8/REIN7 overexpression enhances ethylene response in roots.
(A) Ethylene response phenotypes of KT and inducible transgenic line (PER8-REIN7) seedlings grown in the dark for 3 d in the presence or absence of 2.5 μM estradiol. Bar = 10 mm. (B) Root length and (C) relative root length (ethylene-treated versus untreated in each genotype, respectively) in (A). Each column is the average of 20–30 seedlings, and bars indicate ± SD. The experiment was repeated at least three times with similar results. * and ** indicate significant difference compared to KT at P < 0.05 and P < 0.01.
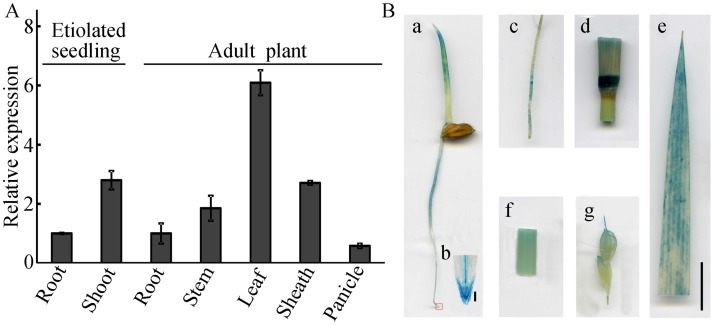
To study the function of YUC8/REIN7 in rice growth and development, we investigated its expression patterns by qPCR. The transcripts were detected in all organs from vegetative to reproductive stages and were preferentially expressed in young leaves (Fig 5A). GUS staining driven by the YUC8/REIN7 promoter in transgenic rice showed that the expression of YUC8/REIN7 was located in roots and coleoptiles of etiolated seedlings (Fig 5Ba and 5Bb). In field-grown plants, YUC8/REIN7 was expressed in the root tip, leaf, stem, young stem nodes and developing grains (Fig 5Bc–5Bg).
Fig 5. The transcripts of YUC8/REIN7 are expressed in developmental tissues.
(A) YUC8/REIN7 expression in different rice tissues detected by qPCR. Bars indicate ± SD from five independent experiments. (B) Tissue-specific expression of YUC8/REIN7 revealed by transgenic line (YUC8 promoter-GUS) analysis. (a) 3-d-old etiolated seedlings. (b) Root tip. Bar = 100 μm. (c) Root. (d) Young stem nodes. (e) Leaf. (f) Stem. (g) Developing grains. Bar = 10 mm.
Next, we investigated whether YUC8/REIN7 was involved in auxin biosynthesis in vivo. We generated transgenic lines containing 35S:REIN7 in the Arabidopsis yuc1-1 mutant, which has defective auxin biosynthesis [60]. The REIN7-OX/yuc1-1 plants exhibited longer hypocotyls, shorter primary roots and more root hairs than those of the control plants (S7A, S7D and S7E Fig). Mature leaves of REIN7-OX/yuc1-1 were longer, narrower and curled downward compared with those of the wild type (S7B and S7C Fig). These phenotypes are similar to those caused by elevated auxin levels as previously reported [18], while REIN7m-OX/yuc1-1 plants exhibited much longer petiole and slightly longer hypocotyl than the wild-type seedlings, but no obvious differences in roots and mature leaves compared with yuc1-1 (S7 Fig), demonstrating that truncated YUC8/REIN7 is partial active. Considering that FAD and NADPH binding sites of the truncated YUC8/REIN7 are remained, it should be possible that the truncated protein still has partial activity to bind to FAD and NADPH, consistence with the incompletely insensitive phenotype in rein7 mutants (Fig 3). These results suggest that YUC8/REIN7 is an auxin biosynthesis gene, and the C-terminus is important for its function.
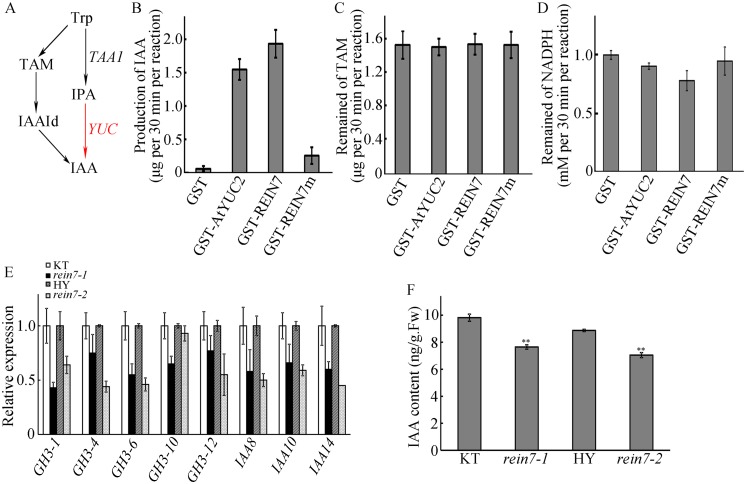
A previous study showed that Arabidopsis YUC1 converts tryptamine to N-hydroxylated tryptamine [18], but the mass spectrometry spectra were inconsistent with those for synthetic N-hydroxylated tryptamine [61]. Recent data showed that yuc mutants accumulate IPA, and in vitro data indicated that the YUC proteins convert IPA to IAA (Fig 6A) [15,16]. To determine whether YUC8/REIN7 functions in the conversion of IPA to IAA in the IPA pathway, we used kynurenine (Kyn), a potent inhibitor of in vivo TAA1/TAR activity [6], to treat the YUC8/REIN7 transgenic lines. Our data showed that the short root phenotype of YUC8/REIN7 overexpression lines was greatly suppressed by Kyn (S6A and S6C Fig). And this decrease of root growth suppressed by Kyn was further confirmed in estradiol-inducible transgenic lines (S6D and S6E Fig), suggesting that YUC8/REIN7 and TAA1/TAR work in a linear pathway to produce IAA. Next, we performed an enzymatic assay using purified GST-fused YUC8/REIN7 (S8A and S8B Fig). Our data showed that the GST-REIN7 fusion catalyzed the conversion of IPA to IAA. Loss of 47 amino acid residues from the C-terminal end of YUC8/REIN7 (GST-REIN7m) resulted in a significant decrease of enzymatic activity (Figs 6B, S9A and S9B), further supporting that the truncated protein of REIN7 is partially active. We did not detect the conversion of TAM in our assay conditions (Figs 6C, S9A and S9C), and the NADPH content in the reaction was slightly decreased when TAM was used as a substrate, indicating that there only have a few TAM turnovers (Fig 6D). These results suggest that YUC8/REIN7 mainly catalyzes conversion of IPA to IAA in the IPA pathway, and its C-terminus is important for enzymatic activity. Furthermore, the expression of auxin-responsive genes detected were decreased in rein7 mutants (Fig 6E), consistent with the decreased IAA content in the lines in 7-d-old seedlings (Fig 6F). These results suggest that YUC8/REIN7 is an important factor required for auxin biosynthesis.
Fig 6. YUC8/REIN7 is mainly required for IPA-dependent auxin biosynthesis.
(A) The simplified liner pathway of IAA biosynthesis. The red arrows indicate the function of YUC. Enzymatic assays with GST-AtYUC2, GST- REIN7, GST-REIN7m and the product IAA (B), the substrate TAM (C) were analyzed by LC-ESI-MS/MS. The bars represent ± SD from three independent experiments. (D) The content of remained NADPH in (C). The bars represent ± SD from three independent experiments. (E) Expression of auxin-inducible genes in 7-d-old normal grown seedlings. The bars represent ± SD from five independent experiments. (F) IAA content of 7-d-old normal grown seedlings. The bars represent ± SD from three independent experiments. ** indicates a significant difference compared to KT or HY at P < 0.01.
Ethylene enhances the YUC8/REIN7-dependent auxin biosynthesis
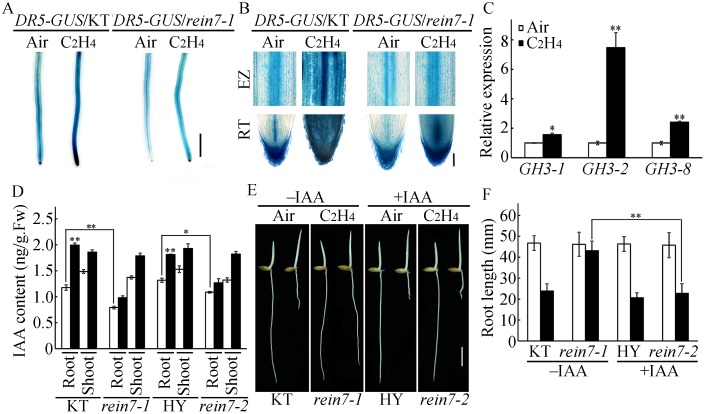
YUC8/REIN7 participates in auxin biosynthesis, and multiple studies have demonstrated that ethylene enhances auxin biosynthesis to inhibit root elongation [9,32,33]. We then investigated whether YUC8/REIN7 is involved in ethylene-enhanced auxin biosynthesis in rice root. Thus, we introduced a DR5:GUS reporter, an auxin reporter responding to endogenous auxin [62], into the wild type and the rein7 mutant. The expression of DR5:GUS in primary roots was significantly increased in the wild type after ethylene treatment, but this tendency was weakened in the rein7 roots (Fig 7A and 7B). The expression of auxin-responsive genes was dramatically induced by ethylene in roots of the wild-type seedlings (Fig 7C). In addition, the endogenous IAA content in rein7 was about 70% and 90% of that in the wild-type roots and shoots, respectively, indicating that YUC8/REIN7 mainly affects root auxin production. After ethylene treatment, endogenous IAA content did not significantly increased in rein7 roots, although obviously enhanced in the wild-type roots (Fig 7D), demonstrating that ethylene-enhanced IAA production is predominantly inhibited in rein7 roots. Thus, our data indicate that YUC8/REIN7 is essential for ethylene-enhanced auxin accumulation in rice roots.
Fig 7. YUC8/REIN7-mediated auxin biosynthesis is required for ethylene-inhibited root growth in etiolated seedlings.
(A) DR5-GUS expression in root. Seedlings of 3-d-old etiolated transgenic lines containing DR5-GUS in the wild type or the rein7-1 background were treated with or without 10 ppm ethylene for 8 h before GUS activity was assayed. Bar = 10mm. (B) Root tip and elongation zone in (A). ‘RT’ represents root tip, ‘EZ’ represents elongation zone. Bar = 100 μm. (C) qPCR analysis of auxin-response gene expression in response to ethylene. Dark-grown 3-d-old wild-type seedlings were treated with 10 ppm ethylene for 3 h. The RNAs from roots were isolated for qPCR. (D) IAA levels in 3-d-old wild-type and rein7 etiolated seedlings in the absence or presence of 10 ppm ethylene. (E) Rescue of the reduced ethylene sensitivity of rein7-1 root by IAA. The wild-type and rein7-1 seedlings were grown in the dark for 3 d in the absence or presence of 10 ppm ethylene, with or without supplementation of 10 nM IAA. Bar = 10 mm. (F) Quantification of root inhibition in (D). Each column is the average of 20–30 seedlings. The data are shown as the mean ± SD of three biological replicates. * and ** indicate significant differences between the compared two samples at P < 0.05 and P < 0.01, respectively.
Because the YUC8/REIN7 mutation leads to the ethylene insensitivity in rein7 roots, we next investigated whether addition of IAA could rescue the ethylene response of the mutant. We used 10 nM IAA in the complementation assay because this concentration of IAA had no obvious inhibitory effects on root growth in the wild-type seedlings (S10 Fig). In the presence of 10 nM IAA and 10 ppm ethylene, the defective response of rein7-1 roots to ethylene was largely rescued (Fig 7E and 7F), suggesting that reduced ethylene sensitivity of rein7 roots is most likely caused by the decrease of auxin biosynthesis.
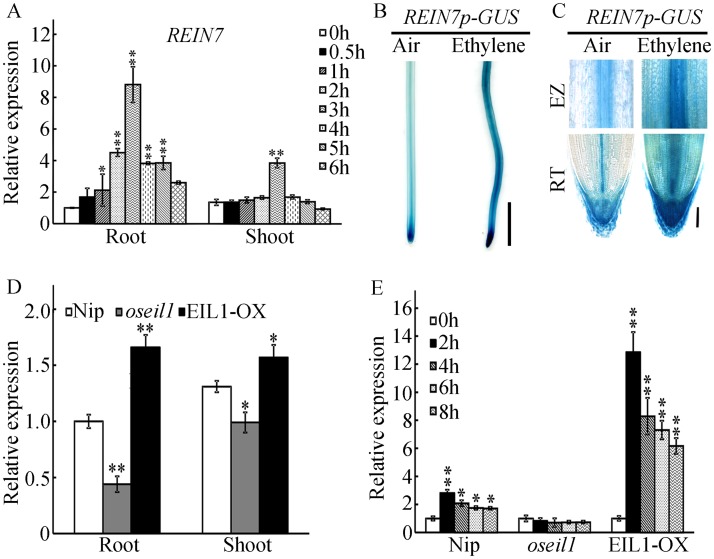
We then questioned whether ethylene activates YUC8/REIN7 transcripts. Our qPCR analyses showed that YUC8/REIN7 transcripts were significantly induced within 3 h with ethylene application in the wild-type roots and shoots (Fig 8A). Analysis with YUC8/REIN7 promoter-GUS transgenic line also showed that ethylene treatment stimulated YUC8/REIN7 promoter activity in roots (Fig 8B and 8C), suggesting a role for YUC8/REIN7 in root growth. Taken together, these findings reveal that ethylene transcriptionally activates the expression of YUC8/REIN7, resulting in auxin accumulation and inhibition of root elongation.
Fig 8. Mutation of OsEIL1 abolishes ethylene-induced YUC8/REIN7 transcription.
(A) Expression of YUC8/REIN7 in response to ethylene. The wild-type seedlings were grown in the dark for 3 d and then treated with 10 ppm ethylene. The RNAs from roots and shoots were isolated and used for qPCR. (B) Ethylene-induced GUS activity in roots of transgenic plants harboring REIN7p-GUS. Etiolated seedlings of 3-d-old plants were treated with or without 10 ppm ethylene for 8 h before GUS activity was assayed. Bar = 10mm. (C) Root tip and elongation zone in (B). ‘RT’ represents root tip, ‘EZ’ represents elongation zone. Bar = 100 μm. (D) qPCR analysis of YUC8/REIN7 expression in primary roots and shoots of Nip, oseil1 and EIL1-OX seedlings grown in the dark for 3 d. (E) Expression of YUC8/REIN7 in primary roots of Nip, oseil1 and EIL1-OX seedlings grown in the dark for 3 d and then treated with 10 ppm ethylene. The data are shown as the mean ± SD of five biological replicates. * and ** indicate significant differences compared to 0 h at P < 0.05 and P < 0.01, respectively.
YUC8/REIN7 functions downstream of OsEIL1 in ethylene-inhibited root elongation
Because ethylene-inhibited root elongation primarily mediates the ethylene signaling pathway, and ethylene transcriptionally activates the expression of YUC8/REIN7, we then examined whether the YUC8/REIN7 transcripts were controlled by OsEIL1. Our qPCR analyses showed that the expression of YUC8/REIN7 was significantly increased in OsEIL1 overexpression seedlings but decreased in oseil1 in both roots and shoots (Fig 8D), and ethylene-induced YUC8/REIN7 expression was completely abolished in oseil1 (Fig 8E). These results suggest that ethylene-inhibited root elongation via auxin biosynthesis is enhanced by OsEIL1 through regulation of YUC8/REIN7 expression.
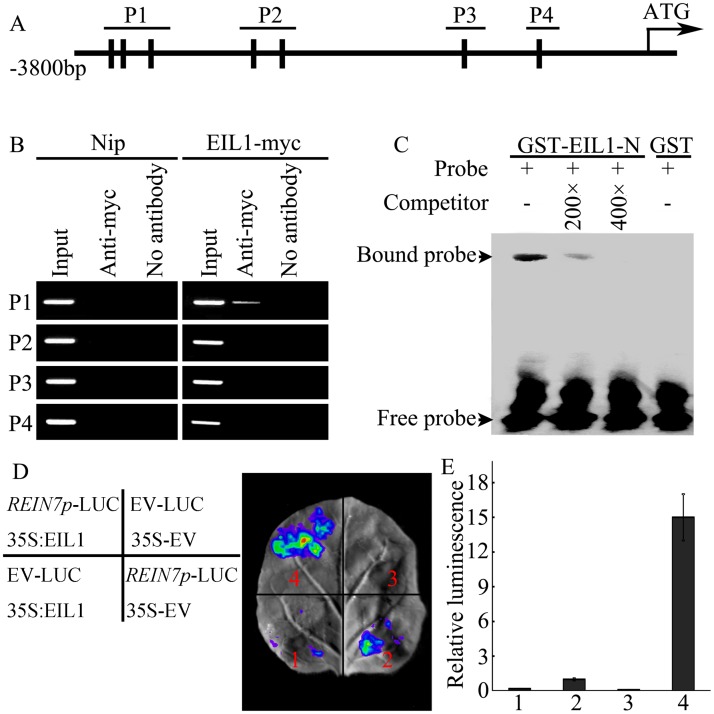
To determine whether OsEIL1 functions as a direct regulator of YUC8/REIN7, we first analyzed the promoter sequence of YUC8/REIN7 and found that there are seven EIN3-binding sites (EBS: ATGTA) (Fig 9A). Subsequently, we performed chromatin immunoprecipitation (ChIP) assay using the myc-tagged OsEIL1 (EIL1-myc) transgenic line. As shown in Fig 9B, the anti-myc antibodies precipitated the P1 fragment of the YUC8/REIN7 promoter. Next, we conducted electrophoretic mobility shift assay (EMSA) with GST-EIL1-N fusion protein expressed in E. coli. As shown in Fig 9C, the GST-EIL1-N fusion protein was able to directly bind to the DNA probes containing the EBS motif as in the P1 fragment of YUC8/REIN7 promoter, the binding was specific as demonstrated by competition assay using unlabeled competitor. These results indicate that OsEIL1 directly binds to YUC8/REIN7 promoter in vitro and in vivo.
Fig 9. OsEIL1 directly binds to YUC8/REIN7 promoter region.
(A) Schematic diagrams of putative EIN3 binding site (EBS) in the promoter of YCU8/REIN7. Black boxes indicate the positions of the EBS. P1-P4 are fragments of the YUC8/REIN7 promoter. (B) Anti-myc ChIP assays with DNA from 3-d-old etiolated seedling roots of Nip and overexpressing OsEIL1 with myc-tag (EIL1-myc) transgenic plants. (C) EMSA assay for binding to EBS sequence in the promoter of YUC8/REIN7 by OsEIL1 protein in vitro. Glutathione S-transferase (GST)-tagged OsEIL1 N-terminal fusion protein was incubated with biotin-labeled DNA fragments (Probe). Competition for the biotin-labeled promoter region was done by adding an excess of unlabeled probe (Competitor). Three biological replicates were performed with similar results. (D) The activation of OsEIL1 on the promoter activity of YUC8/REIN7 by transient expression assay in tobacco leaves. ‘EV’ represents empty vector. Three biological replicates were performed with similar results. (E) Quantitative analysis of luminescence intensity for each comparison in (D).
To test whether OsEIL1 could activate the expression of YUC8/REIN7, we used a tobacco transient expression assay system, the 3800 bp promoter sequence upstream from the ATG codon of YUC8/REIN7 was fused to the LUCIFERASE (LUC) reporter gene and cotransfected with the effector plasmid harboring 35S:EIL1 into the tobacco leaves, the cotransfected effector significantly increased the LUC activity driven by YUC8/REIN7 promoter, compared to control vector (Fig 9D and 9E). These results indicate that OsEIL1 could activate the expression of YUC8/REIN7.
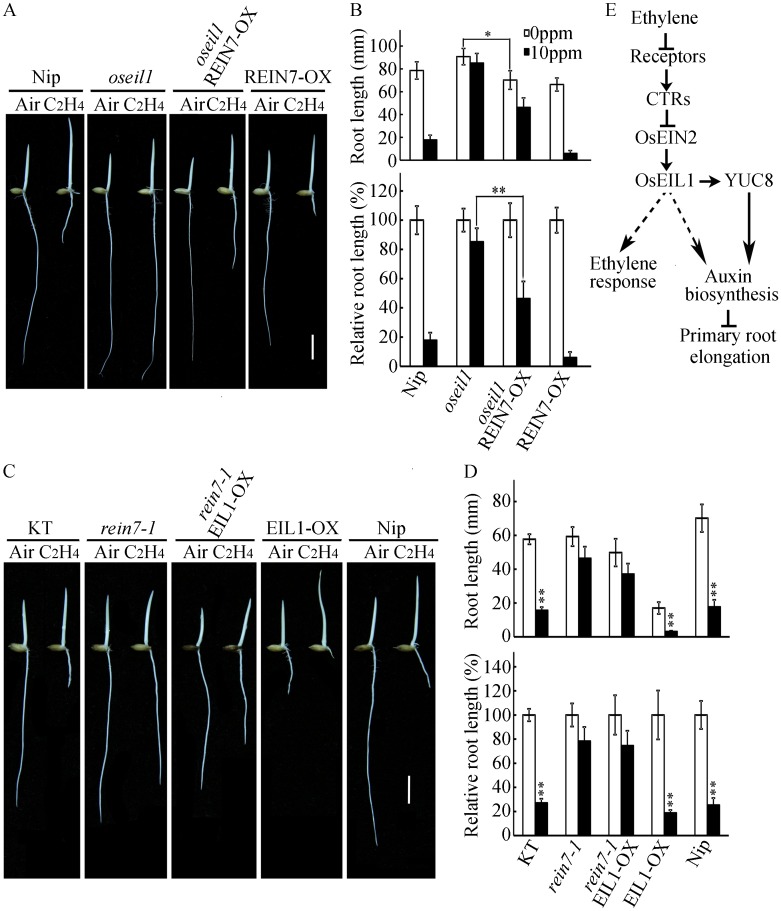
To examine the genetic relationship between YUC8/REIN7 and the ethylene signaling component OsEIL1, we first analyzed the ethylene response of the oseil1 REIN7-OX plants that were obtained by overexpression of YUC8/REIN7 on an oseil1 background. Our observations revealed that the root length of oseil1 REIN7-OX was shorter than that of oseil1 but similar to that of REIN7-OX in the absent of ethylene. Upon exposure to ethylene, the root length of the oseil1 REIN7-OX plants was significantly reduced compared with that of oseil1 but was longer than that of REIN7-OX (Fig 10A and 10B). To test whether IAA could rescue the response of root elongation of oseil1 to ethylene, we treated oseil1 seedlings in the presence of 10 nM IAA and 10 ppm ethylene, the result showed that IAA partially rescued the response of root elongation of oseil1 to ethylene (S11A and S11B Fig). These results suggest that there may have an OsEIL1-independent ethylene responsive pathway that regulates root elongation together with auxin, and YUC8/REIN7 can partially suppress ethylene insensitivity of roots in oseil1.
Fig 10. YUC8/REIN7 genetically functions downstream of OsEIL1.
(A) Phenotypes of Nip, oseil1, oseil1 REIN7-OX and REIN7-OX dark-grown seedlings in the presence or absence of 10 ppm ethylene for 3 d. Bars = 10 mm. (B) Root length and relative root length (ethylene-treated versus untreated in each genotype, respectively) in (A). (C) Phenotypes of KT, rein7-1, rein7-1 EIL1-OX, EIL1-OX and Nip dark-grown seedlings in the presence or absence of 10 ppm ethylene for 3 d. Bars = 10 mm. (D) Root length and relative root length (ethylene-treated versus untreated in each genotype, respectively) in (C). In (B) and (D), each column is the average of 20–30 seedlings, and bars indicate ± SD. * and ** indicates a significant difference compared to air at P < 0.05 and P < 0.01. (E) A proposed model of ethylene-inhibited primary root elongation in rice. Ethylene signaling acts upstream of the auxin biosynthesis to regulate primary root elongation. YUC8/REIN7 is a key regulator required for ethylene-inhibited primary root elongation.
To confirm the above genetic relationship, we further generated rein7-1 EIL1-OX plants by crossing the rein7-1 mutant with EIL1-OX plants. Without ethylene treatment, the root length of rein7-1 EIL1-OX seedlings was significantly longer than that of EIL1-OX seedlings. With ethylene treatment, the inhibition of root growth of EIL1-OX seedlings was partially alleviated in the rein7-1 EIL1-OX seedlings (Fig 10C and 10D). Because YUC8/REIN7 plays an important role in ethylene-inhibited root elongation, and ethylene treatment increases the expression of DR5:GUS and IAA content in the rein7-1 roots, these data are consistent with the detection of YUC gene expression in response to ethylene, indicating that YUC8/REIN7 is one of YUC members involved in ethylene-induced auxin accumulation in roots. Thus our data suggest that YUC8/REIN7 acts downstream of ethylene signaling pathway and the YUC8/REIN7-mediated pathway is partially required by OsEIL1 signaling for the regulation of the ethylene-inhibited root elongation.
Discussion
Previous studies have shown that the interaction of ethylene and auxin affects multiple physiological processes, including root growth [7,19,33,34]. Rice roots grow in water-saturated environments during their life cycle, and this unique habit might confer different features of ethylene signaling between water-grown rice and dry-land-cultured Arabidopsis [44]. Although the crosstalk between ethylene and auxin has been extensively investigated in Arabidopsis, the roles of ethylene and auxin in rice root elongation are still unclear. In the present report, we showed that YUC8/REIN7 via IPA-dependent auxin biosynthesis is essential for ethylene-inhibited root elongation. This process could be enhanced by ethylene and was dependent on transcriptional modulation of OsEIL1. Thus, our findings reveal a mode of interplay between ethylene and auxin in rice root elongation, enhancing our understanding of ethylene signaling in rice.
Ethylene plays important roles in root development [5,7]. In Arabidopsis, ethylene inhibits root growth through the interaction with auxin. For example, auxin biosynthetic genes, such as ASA1 [9,34] and TAA1 [6,19], have been identified in this process. YUC family proteins are pivotal for auxin biosynthesis [13,18] and catalyze the conversion of IPA to IAA, a rate-limiting step in the IPA pathway [15,16]. However, the function of the YUC family in this process is not well understood. Considering the different ethylene response between Arabidopsis and rice [44], we investigated whether YUC family members are necessary for rice ethylene response. In this report, we showed that YUC family members, at least YUC5, YUC8 and YUC11, are important for ethylene-inhibited root elongation, implying that there is functional redundancy of YUC genes in this process. Furthermore, we found that the YUC8/REIN7 mutation confers insensitivity to ethylene in root growth with reduced auxin levels, while YUC8/REIN7 overexpression enhanced ethylene responses in roots, revealing that YUC8/REIN7 is an important factor in ethylene-inhibited root elongation.
Previous genetic studies demonstrated that YUC catalyzes the N-oxygenation of TAM [18], and recent reports showed that YUC has a role in the conversion of IPA to IAA in Arabidopsis [15,16]. Considering the distinct aspects between Arabidopsis and rice, we first biochemically showed that YUC8/REIN7 functions mainly through IPA-dependent IAA biosynthesis in rice. Moreover, transgenic Arabidopsis lines overexpressing YUC8/REIN7 in the Arabidopsis mutant yuc1-1 showed auxin overproduction phenotypes, which are consistent with the AtYUC1 overexpression lines [18], suggesting that YUC8/REIN7 has similar functions to those of AtYUC1. In addition, the C-terminal region is important for YUC8/REIN7 enzymatic activity based on enzyme assay. Moreover, both of the mutants rein7-1 and rein7-2 exhibited reduced ethylene-insensitive in primary root growth, which FAD and NADPH binding sites are remained, implying that the truncated protein still has partial activity to bind to FAD and NADPH, supporting the observation of the incompletely insensitive phenotype in rein7 mutants. Thus our results indicate that the YUC pathway is conserved in Arabidopsis and rice, YUC8/REIN7 regulates IAA production through IPA-dependent IAA biosynthesis in rice, and the C-terminus is important for YUC8/REIN7 function.
YUC proteins play an important role in IAA biosynthesis; however, recent studies in Arabidopsis indicated that AtYUC6 confers drought tolerance independently of auxin biosynthesis [63], indicating the diversity of YUC function. Consistent with the results, we found that mutation of rein7-1 increased salt tolerance in this study. Most importantly, we discovered that YUC8/REIN7 mediates the ethylene-inhibited root growth as determined by analyses of root response to ethylene with rein7 alleles. Because the YUC8/REIN7 mutation decreases the auxin content, possibly through disruption of auxin biosynthesis, we propose that auxin may be a factor participating in ethylene-inhibited root growth. This conclusion is strongly supported by the following evidence: (1) ethylene treatment enhanced the DR5-GUS signal in roots; (2) ethylene induced YUC8/REIN7 expression and auxin accumulation specifically in roots; (3) exogenous application of IAA largely recovered the defective response of rein7 roots to ethylene; (4) ethylene induced expression of various auxin-responsive genes; (5) YUC8/REIN7 overexpression resulted in enhanced ethylene response in roots; and (6) YUC8/REIN7 function is a key factor required for the ethylene signaling component OsEIL1-mediated root ethylene response. Thus, ethylene-inhibited root growth requires YUC8/REIN7-dependent auxin biosynthesis.
EIN3 acts as a positive regulator at the most downstream position of the ethylene signaling transduction pathway and constitutes a major fraction of the ethylene response [30]. In rice, there are six members in the small family of the EIN3-like homologues (OsEIL1 to OsEIL6). Among these, OsEIL1 and OsEIL2 show the highest similarity to Arabidopsis EIN3 and spatially regulate ethylene response of the roots and coleoptiles in etiolated seedlings [44,50]. Because of the rein7 ethylene response in roots, the relationship between YUC8/REIN7 and the ethylene signaling component OsEIL1 was genetically identified, i.e., ethylene induces YUC8/REIN7 expression depending on OsEIL1, and OsEIL1 directly binds to YUC8/REIN7 promoter and positively regulates its expression, revealing that YUC8/REIN7 is key factor required for ethylene-inhibited root growth and acts downstream of ethylene signaling. Moreover, overexpression of YUC8/REIN7 in an oseil1 background partially rescued the ethylene response in oseil1 mutant, consistent with the detection that YUC8/REIN7 is one of YUC members involved in ethylene-induced auxin accumulation in roots, indicating that the YUC8/REIN7-mediated pathway is partially required by OsEIL1 signaling for the regulation of the ethylene-inhibited root elongation. Furthermore, the observation that supplementation of IAA partially rescues the response of root elongation of oseil1 to ethylene implies that there might have an OsEIL1-independent ethylene responsive pathway that regulates root elongation together with auxin.
In Arabidopsis, ABA-inhibited root growth is dependent on ethylene biosynthesis [53]. Our previous studies have shown that ABA represses ethylene biosynthesis through ABSCISIC ACID INSENSITIVE 4 (ABI4)-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis [54,55]. Moreover, studies in Arabidopsis have established that ethylene inhibits root growth through auxin action by modulating its biosynthesis, transport and signaling [19,32–35,37,38]. Additionally, various auxin biosynthesis genes were directly regulated by ethylene signaling components [9,40], strongly suggesting that ABA may exert its effect on root inhibition through regulating ethylene biosynthesis to stimulate accumulation of auxin in roots. These findings are different from previous reports, indicating that ethylene inhibits root growth by regulating accumulation of ABA [48,52]. All these findings suggest that different mechanisms are present in rice. Our present results demonstrated that ethylene inhibits root growth largely through auxin function, and YUC play an important role in this process. However, the crosstalk between auxin and ABA in rice is still unclear. ABA may regulate root elongation through the auxin pathway or vice versa, or the two hormones might act independently to mediate ethylene response. Our previous study indicated that ERF2 is required for the control of rice root architecture, ABA and ethylene response by fine-tuning the expression of genes involved in hormone signaling pathways [64]. Further investigations of the relationships among these hormones should elucidate their interaction in the control of rice roots.
Taken together, our results in the present investigation support a model (Fig 10E) that ethylene stimulates auxin biosynthesis in roots through the ethylene signaling component OsEIL1. YUC8/REIN7 is one of the factors that modulate auxin biosynthesis. And this regulation of YUC8/REIN7 is directly activated by OsEIL1. As a consequence of activating YUC8/REIN7 expression, ethylene increases the accumulation of auxin, which in turn decreases root elongation.
Materials and methods
Plant materials and grown conditions
The Arabidopsis yuc1-1 mutant used in this study was the SALK-106293 line as previously reported [60]. The rice knockout mutants osein2, oseil1, and overexpressing OsEIN2 (EIN2-OX) or OsEIL1 (EIL1-OX) transgenic lines were previously identified [43,50]. The isolation of ethylene-response rein mutants and ethylene treatment were performed as previously described [43]. The YUC8 (Os03g0162000) T-DNA knockout mutant rein7-2 (PFG_1C-07050.R) is on a Hwayoung (HY) background, identified by PCR using the T-DNA right border primer RB (5'-CCACAGTTTTCGCGATCCAGACTG-3') and gene-specific primers flanking the insertion site (RP, 5'-ATTCTGGCATGGAAGTGAGC-3'), was obtained from the POSTECH Biotech Center [65].
For the salt-tolerance assays in rice seedlings, the germinated rice seeds were cultured in soil for 2 weeks under normal growth conditions, watered with a 150 mM NaCl solution, and cultured for another 10–15 days before the phenotypes were observed. For material propagation, crossing, and investigating agronomic traits, rice plants were cultivated at the Experimental Station of the Chinese Academy of Agricultural Sciences in Beijing during the natural growing seasons.
Treatments and analysis of root growth
IAA, yucasin, and Kyn treatments were performed as previously described [52]. Briefly, germinated rice seeds were placed on cheesecloth on a stainless steel sieve that was placed in an air-tight plastic box of 10 L volume and incubated at 28°C. The seeds were subjected to the treatment with 4 L of water containing either 10 nM IAA, 10 μM yucasin/Kyn, or 10 ppm ethylene gas. The ethylene treatment was performed as previously described [43]. IAA was dissolved in ethanol, yucasin and Kyn were in DMSO. The controls were conducted with treatments containing equivalent volumes of air, ethanol or DMSO. At the end of the period, the roots were scanned and their length was measured from digitized images using Image J software.
Map-based cloning of the REIN7 gene
F2 mapping populations were generated from crosses between the rein7-1 mutant and indica variety Dular. Genomic DNA was isolated from seedlings with mutant phenotypes. A total of 246 mutant individuals selected from the F2 populations were used for fine mapping. PCR-based markers were developed based on the sequence difference between the japonica variety Nipponbare and indica variety 9311 (http://www.gramene.org/resources/). The primer sequences of the molecular markers used are listed in S1 Table. The REIN7 locus was mapped to chromosome 3 between M0721 and M0728 in a 63 kb region that contains 7 genes. The candidate gene was finally determined by DNA sequencing of all the candidate genes within this region.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from an approximately 0.5 g sample from 3-d-old seedlings or young leaves with an Ultrapure RNA Kit (CWBIO, CW0581S) according to the manufacturer’s instructions. Total RNA (approximately 2 μg) from each sample was reverse transcribed to cDNA with HiScript II Q RT SuperMix for the qPCR reaction, according to the manufacturer’s instructions (Vazyme, R223-01). qPCR was performed according to the manufacturer’s instructions (Bio-Rad iQ5), as previously described [66]. The rice Actin1 gene was used as the internal standard to normalize gene expression. The qPCR primers are listed in S1 Table.
Generation of transgenic rice
For overexpression of OsEIL1 in rice, the full coding sequence of OsEIL1 was cloned into the plant expression vector pCAMBIA1307 using the Xba I and BamH I sites. For overexpression of YUC8/REIN7 in rice, the full coding sequence of YUC8/REIN7 was cloned into the plant expression vector pCAMBIA1307 using the Sal I and BamH I sites. To generate YUC8/REIN7 inducible transgenic plants with an estradiol-inducible promoter, the full coding sequence of YUC8/REIN7 was cloned into the vector pER8 using the Csp451 and Spe I sites. For complementation, the YUC8/REIN7 genomic sequence (2504 bp), the upstream 2395 bp sequence of the YUC8/REIN7 ATG, and the downstream 534 bp sequence of the YUC8/REIN7 TGA were used. The full sequence (5433 bp) was cloned into the pCAMBIA1300 vector using the BamH I and Kpn I sites through In-Fusion cloning technology. For generation of the YUC8/REIN7p-GUS construct, the 4365 bp promoter region upstream of the start codon of YUC8/REIN7 was cloned into the pCAMBIA1381Z vector using the Xma I and Sal I sites. All vectors were introduced into Agrobacterium tumefaciens strain EHA105 through electroporation, and the resulting strains were introduced into the rice variety Nipponbare or Kitaake. The primers used for the constructs are listed in S1 Table.
Histochemical staining of GUS
For the histochemical staining of GUS in transgenic rice, the samples were incubated in sodium phosphate buffer (pH 7.0) containing 0.1% vol/vol Triton X-100 and 2 mM X-Gluc at 37°C for 12 h. After the samples were rinsed with 70% ethanol until the tissue cleared, they were photographed. To produce transverse section of roots, root segments were embedded in 3% agar. Transverse sections (25 μm) of root were produced using a vibratome (Leica VT 1000 S). The images of rice root autofluorescence were taken under a microscope (Nikon ECLIPSE Ni).
The expression and purification of YUC8 protein in vitro
The coding sequences of YUC8/REIN7 and truncated YUC8/REIN7 (loss of 47 amino acid residues, YUC8/REIN7m) were amplified by PCR using the primers described in S1 Table and then linked to the T-vector for sequencing. The correct YUC8/REIN7 and YUC8/REIN7m coding sequences were cloned into the expression vector pGEX-6p-1 using the BamH I and Sal I sites and fused with a GST-tag. Next, the constructed vectors were transformed into the E. coli BL21 (DE3) strain, and the transformed strains were cultured in Luria–Bertani (LB) medium and harvested after induction with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 16°C for 8 h. The fusion proteins were extracted with the lysis buffer containing 50 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 1% (vol/vol) Tween 20, and 20% (wt/vol) glycerol. The recombinant proteins were purified using a ProteinIso GST Resin according to the manufacturer’s instructions (Transgen, DP201-01). The concentration of the purified recombinant proteins was determined using the bicinchoninic acid protein assay (CWBIO, CW0014). The components of the purified proteins were analyzed by SDS-PAGE electrophoresis and Western blotting. The purified protein was immediately divided into aliquots and frozen in liquid nitrogen, and stored at -80°C for the further experiments.
Enzyme assay
The enzyme assays of GST-YUC8/REIN7 and GST-YUC8/REIN7m were performed as described [15]. Briefly, 20 μg of purified YUC8/REIN7, YUC8/REIN7m or AtYUC2 protein were added to a 100 μL reaction system containing 100 μM IPA or TAM, 40 μM FAD, and 1 mM NADPH in PBS buffer (pH 7.4) and incubated at 30°C for 30 min. IPA/TAM was added to the reaction just before the incubation at 30°C. In order to reduce the non-enzymatic conversion of IPA, the enzyme reactions were stopped by addition of acetonitrile and snap freezing in liquid nitrogen. And then, 20 μL of the mixture was injected into the HPLC instrument (Shimadzu LC-10A), and chromatographic separation was achieved on an YMC-Triart Diol-HILIC column (4.6 × 250 mm, 5 μm; YMC) and detected at 254 nm with an SPD M10A detector. The samples were eluted at a flow rate of 0.8 mL/min with 0.8% acetic acid (solvent A) and 100% acetonitrile (solvent B). Fractions eluting TAM (4.2 min)/IAA (7.2 min) were collected and further analyzed by LC-ESI-MS/MS in National Centre for Plant Gene Research (Beijing). The measurement meathod of TAM was similar to IAA. In parallel, 20 μg GST were used as a control, and only small amounts of IAA were produced non-enzymatically from IPA in a control reaction. The NADPH content was determined by measuring the optical density at 340 nm using a spectrophotometer.
IAA content measurement
IAA was quantified as previously described [6]. Briefly, 200 mg (fresh weight) of whole root or shoot for each treatment was quickly frozen in liquid nitrogen and ground into a fine powder, and then, tissues were homogenized and extracted for 24 h in methanol containing 2H-IAA (CDN isotopes) as an internal standard. Purification was performed using an Oasis Max solid phase extract cartridge (Waters) after centrifugation. IAA measurement was carried out with a liquid chromatography–tandem mass spectrometry system consisting of Acquity Ultra Performance Liquid Chromatography (Acquity UPLC; Waters) and a triple quadruple tandem mass spectrometer (QTRAP 5500; AB SCIEX).
ChIP-PCR assay
ChIP was conducted as described [67]. Briefly, approximately 2 g sample from 3-d-old overexpressing OsEIL1 with myc-tag (EIL1-myc) and Nipponbare etiolated seedling root were harvested and fixed with 1% formaldehyde in PBS under vacuum for 30 min at room temperature. After three washes with sterile deionized water, the samples were ground to a fine powder to extract the proteins and DNAs. The chromatin solution was then sonicated to shear the DNA into fragments. After centrifuging, the chromatin pellet was re-suspended in 300 μL buffer containing 50 mM Tris-HCl (pH 8.0), 10 mM EDTA, 1% SDS, 1 mM PMSF (phenylmethanesulfonyl fluoride) and protease inhibitors. The above solution was divided into three portions and then 900 mL buffer [1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl (pH 8.0) and 167 mM NaCl] was added. After adding 40 μL of salmon sperm DNA to each chromatin sample with gentle rotation and overnight incubation at 4°C, the pellet was rinsed three times and re-suspended in a buffer containing 50 mM Na3PO4 (pH 8.0), 167 mM NaCl, 10 mM imidazole and protease inhibitors. The input solution was then used for performing immunoprecipitation using anti-myc antibodies. The DNA fragments were cleaned up using a PCR DNA purification kit (Tiangen, DP214). Promoter fragments were amplified using 1 μL of purified DNA as a template in each PCR reaction. Primer sequences for ChIP PCR experiments are provided in S1 Table.
Electrophoretic mobility shift assay (EMSA)
To construct plasmid for the expression of N-terminal OsEIL1 (amino acids 1–350) region in E. coli BL21 (DE3), DNA fragment corresponding to the region was obtained and inserted into the pGEX-6p-1 vector using the BamH I and Not I sites.
EMSA was performed as previously described [50]. Briefly, single-stranded complementary oligonucleotide fragments corresponding to region of YUC8/REIN7 promoter (S1 Table) harboring the EBS elements were synthesized and biotinylated using the Biotin 3’ End DNA Labeling Kit (Thermo Fisher Scientific, 89818). Biotinylated and unlabeled complementary oligonucleotide pairs were annealed to make double-stranded biotin-labeled probes and competitors by mixing together equal amounts, denatured at 90°C for 1 min, then slowly cooled. EMSA reaction solutions were prepared according to the manufacturer’s protocol (LightShift Chemiluminescent EMSA Kit; Thermo Fisher Scientific, 20148). Reaction solutions were incubated for 20 min at room temperature. The protein-probe mixture was separated on a 5% polyacrylamide native gel and transferred to a nylon membrane (GE). After UV light cross-linking, the DNA on the membrane was detected using the Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific, 89880).
Transactivation assay in tobacco leaves
Transactivation assay was performed as previously described [50]. Briefly, the 3.8 kb sequence upstream from the ATG codons of YUC8/REIN7 was inserted into pGWB35 to generate promoter:LUC reporter construct using Gateway technology (Invitrogen). The reporter plasmid and the construct containing 35S:EIL1 were transformed into A. tumefaciens strain GV3101. The strains were incubated in Luria-Bertani medium and finally resuspended in infiltration buffer (10 mM MES, 0.2 mM acetosyringone, and 10 mM MgCl2) to an ultimate concentration of optical density at OD600 = 1. Equal amounts of different combined bacterial suspensions were infiltrated into the young leaves of 5-week-old tobacco (Nicotiana tabacum) plants using a needleless syringe. After infiltration, the plants were grown first in the dark for 12 h and then kept with 16 h of light/8 h of dark for 48 h at 24°C before CCD imaging. The leaves were sprayed with 100 mM luciferin (Promega) and placed in the dark for 5 min. LUC activity was observed with a low-light cooled CCD imaging apparatus (iXon; Andor Technology). Experiments were performed with three independent biological replicates.
Accession numbers
Sequence data from this article can be found in the MSU7.0 database (http://rice.plantbiology.msu.edu/) under the following accession numbers: OsActin1, Os03g50885; GH3-1, Os01g57610; GH3-2, Os01g55940; GH3-4, Os05g42150; GH3-6, Os05g05180; GH3-8, Os07g40290; GH3-10, Os07g38860; GH3-12, Os11g08340; IAA8, Os02g49160; IAA10, Os02g57250; IAA14, Os03g58350.
Supporting information
(A) Root and coleoptile phenotypes of japonica (Nip, HY, KT) and indica (IR29, YD6-Yangdao #6, ZS97-Zhenshan 97) cultivars treated with air, 10 ppm ethylene, 1 ppm 1-MCP, 1 ppm 1-MCP plus 10 ppm ethylene, 0.2 μM AVG, and 0.2 μM AVG plus 10 ppm ethylene. Rice seedlings were grown in the dark for 3 d in the presence of various reagents. Bar = 10 mm. (B) Root length for the plants shown in (A). (C) Coleoptile length for the plants shown in (A). Values are shown as the mean ± SD of 20–30 seedlings per genotype. The experiment was repeated at least three times with similar results.
(TIF)
(A) Ethylene-response phenotypes of Nip, osein2, EIN2-OX, oseil1 and EIL1-OX seedlings. The etiolated seedlings were grown in air or 10 ppm ethylene for 3 d. Bar = 10 mm. (B) Root length for the plants shown in (A). (C) Coleoptile length for the plants shown in (A). Values are shown as the mean ± SD of 20–30 seedlings per genotype. The experiment was repeated at least three times with similar results.
(TIF)
Nip, osein2, EIN2-OX, oseil1 and EIL1-OX seedlings grown in the dark for 3 d and then treated with or without 10 ppm ethylene for 3 h. The RNAs from roots were isolated and used for qPCR. The experiment was repeated at least five times with similar results. ‘ND’ represents not detected. Bars indicate ± SD.
(TIF)
(A) Ethylene-response phenotypes of various rein mutants. The etiolated seedlings were grown in air or 10 ppm ethylene for 3 d. Bar = 10 mm. (B) Root length of the wild type and rein mutants in response to ethylene. (C) Coleoptile length of the wild type and rein mutants in response to ethylene. Each column is the average of 20–30 seedlings, and bars indicate ± SD.
(TIF)
The phylogenetic tree of 14 rice and 11 Arabidopsis YUC genes was constructed using DNAMAN. Bootstrap analysis values are shown at the nodal branches.
(TIF)
(A) Root phenotype of Nip, constitutive overexpressing YUC8 transgenic (OX-1 and OX-2) etiolated seedlings. The seedlings were grown in the dark for 3 d in the presence or absence of 10 μM Kyn. Bar = 10 mm. (B) YUC8/REIN7 expression in 3-d-old etiolated seedlings. The experiment was repeated at least five times with similar results. Bars indicate ± SD. (C) Root length in (A). (D) Root phenotype of KT and inducible transgenic (PER8-REIN7) etiolated seedlings. The seedlings were grown in the dark for 3 d in the presence or absence of 2.5 μM estradiol, with or without supplementation of 10 μM Kyn. Bar = 10 mm. (E) Root length in (D). In C and E, each column is the average of 20–30 seedlings, and bars indicate ± SD. * and ** indicates a significant difference compared to mock at P < 0.05 and P < 0.01.
(TIF)
(A) The seedling phenotypes of Col-0, yuc1-1, transgenic lines overexpressing truncated (REIN7m-OX/yuc1-1) or full-length YUC8/REIN7 (REIN7-OX/yuc1-1) in Arabidopsis yuc1-1 mutant grown on MS medium for 7 d. (B) The phenotypes of adult Col-0, yuc1-1, REIN7m-OX/yuc1-1 and REIN7-OX/yuc1-1 lines. (C) Mature leaves of the Col-0, yuc1-1, REIN7m-OX/yuc1-1 and REIN7-OX/yuc1-1 lines. (D) Root length in (A). (E) Hypocotyl length in (A). Each column is the average of 20–30 seedlings and bars indicate ± SD. * and ** indicate significant differences compared to yuc1-1 at P <0.05 and P < 0.01, respectively.
(TIF)
(A) The purified proteins of GST-REIN7 and GST-REIN7m expressed in E. coli were identified by SDS-PAGE electrophoresis.–IPTG and +IPTG: the total proteins from E. coli that were induced or not by IPTG, respectively; Purified: the purified recombined protein. (B) The purified proteins of GST-REIN7 and GST-REIN7m expressed in E. coli were analyzed by an anti-GST antibody.
(TIF)
(A) The HPLC profile for authentic TAM, IPA and IAA with UV detection (254 nm). (B) The HPLC chromatogram for IAA that was produced from authentic IPA in GST, GST-AtYUC2, GST-REIN7 and GST-REIN7m reaction mixture. (C) The HPLC chromatogram for TAM that was remained in GST, GST-AtYUC2, GST-REIN7 and GST-REIN7m reaction mixture.
(TIF)
(A) Root phenotypes of the wild type Kitaake treated with various concentrations of IAA. The germinated seed was transferred to MS medium containing various concentrations of IAA and grown in the dark for 3 d. (B) Root length in (A). Each column is the average of 20–30 seedlings and bars indicate ± SD. ** indicates a significant difference compared to 0 μM IAA at P < 0.01.
(TIF)
(A) Partial recovery of the ethylene response of oseil1 root by IAA. The wild-type and oseil1 seedlings were grown in the dark for 3 d in the absence or presence of 10 ppm ethylene, with or without supplementation of 10 nM IAA. Bar = 10 mm. (B) Quantification of root inhibition in (A). Each column is the average of 20–30 seedlings. The data are shown as the mean ± SD of three biological replicates. * indicates significant differences between the compared two samples at P < 0.05.
(TIF)
(XLS)
Acknowledgments
We would like to thank Xiaohong Sun and Jinfang Chu [National Centre for Plant Gene Research (Beijing), Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, China] for determining the auxin contents. We are grateful to Dr. Jin-Song Zhang from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for kindly sharing the osein2 and oseil1 mutants and the EIN2-OX and EIL1-OX seeds. We thank Dr. Ying Sun from Hebei Normal University for her kind gift of the DR5-GUS vector.
Data Availability
The sequence data from this article can be found in the MSU7.0 database (http://rice.plantbiology.msu.edu/) under the following accession numbers: OsActin1, Os03g50885; GH3-1, Os01g57610; GH3-2, Os01g55940; GH3-4, Os05g42150; GH3-6, Os05g05180; GH3-8, Os07g40290; GH3-10, Os07g38860; GH3-12, Os11g08340; IAA8, Os02g49160; IAA10, Os02g57250; IAA14, Os03g58350.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2016YFD0100604), the National Science Foundation of China (31670304), and the National Key Program of Transgenic Biology (2016ZX08001-002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102. doi: 10.1038/ng.2725 [DOI] [PubMed] [Google Scholar]
- 2.Zheng HY, Pan XY, Deng YX, Wu HM, Liu P, et al. (2016) AtOPR3 specifically inhibits primary root growth in Arabidopsis under phosphate deficiency. Sci Rep 6: 24778 doi: 10.1038/srep24778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, Hu YF, Dai MQ, Huang LM, Zhou DX (2009) The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 21: 736–748. doi: 10.1105/tpc.108.061655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Cheng SF, Song YL, Huang YL, Zhou SL, et al. (2015) The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell 27: 2469–2483. doi: 10.1105/tpc.15.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stepanova AN, Alonso JM (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12: 548–555. doi: 10.1016/j.pbi.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 6.He WR, Brumos J, Li HJ, Ji YS, Ke M, et al. (2011) A small-molecule screen identifies L-Kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960. doi: 10.1105/tpc.111.089029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195. doi: 10.1016/j.tplants.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 8.Li J, Xu HH, Liu WC, Zhang XW, Lu YT (2015) Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol 168: 1777–1791. doi: 10.1104/pp.15.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mao JL, Miao ZQ, Wang Z, Yu LH, Cai XT, et al. (2016) Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet 12: e1005760 doi: 10.1371/journal.pgen.1005760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol, 63: 563–590. doi: 10.1146/annurev-arplant-042811-105501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smet I, White PJ, Bengough AG, Dupuy L, Parizot B, et al. (2012) Analyzing lateral root development: how to move forward. Plant Cell 24: 15–20. doi: 10.1105/tpc.111.094292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marhavy P, Vanstraelen M, De Rybel B, Ding ZJ, Bennett MJ, et al. (2013) Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J 32: 149–158. doi: 10.1038/emboj.2012.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao YD (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol, 61: 49–64. doi: 10.1146/annurev-arplant-042809-112308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljung K (2013) Auxin metabolism and homeostasis during plant development. Development 140: 943–950. doi: 10.1242/dev.086363 [DOI] [PubMed] [Google Scholar]
- 15.Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, et al. (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A 108: 18512–18517. doi: 10.1073/pnas.1108434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won C, Shen XL, Mashiguchi K, Zheng ZY, Dai XH, et al. (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A 108: 18518–18523. doi: 10.1073/pnas.1108436108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshikawa T, Ito M, Sumikura T, Nakayama A, Nishimura T, et al. (2014) The rice FISH BONE gene encodes a tryptophan aminotransferase, which affects pleiotropic auxin-related processes. Plant J 78: 927–936. doi: 10.1111/tpj.12517 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, et al. (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309. doi: 10.1126/science.291.5502.306 [DOI] [PubMed] [Google Scholar]
- 19.Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, et al. (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191. doi: 10.1016/j.cell.2008.01.047 [DOI] [PubMed] [Google Scholar]
- 20.Tao Y, Ferrer JL, Ljung K, Pojer F, Hong FX, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176. doi: 10.1016/j.cell.2008.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18. doi: 10.1146/annurev.cellbio.16.1.1 [DOI] [PubMed] [Google Scholar]
- 22.Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94: 261–271. [DOI] [PubMed] [Google Scholar]
- 23.Hua J, Sakai H, Nourizadeh S, Chen QHG, Bleecker AB, et al. (1998) EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell 10: 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441. [DOI] [PubMed] [Google Scholar]
- 25.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152. [DOI] [PubMed] [Google Scholar]
- 26.Ju C, Yoon GM, Shemansky JM, Lin DY, Ying ZI, et al. (2012) CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc Natl Acad Sci U S A 109: 19486–19491. doi: 10.1073/pnas.1214848109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salehin M, Estelle M (2015) Ethylene Prunes Translation. Cell 163: 543–544. doi: 10.1016/j.cell.2015.10.032 [DOI] [PubMed] [Google Scholar]
- 28.Qiao H, Shen ZX, Huang SSC, Schmitz RJ, Urich MA, et al. (2012) Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338: 390–393. doi: 10.1126/science.1225974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen X, Zhang CL, Ji YS, Zhao Q, He WR, et al. (2012) Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Res 22: 1613–1616. doi: 10.1038/cr.2012.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao QM, Rothenberg M, Solano R, Roman G, Terzaghi W, et al. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 31.Chang KN, Zhong S, Weirauch MT, Hon G, Pelizzola M, et al. (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. Elife 2: e00675 doi: 10.7554/eLife.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, et al. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196. doi: 10.1105/tpc.107.052100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, et al. (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212. doi: 10.1105/tpc.107.052126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242. doi: 10.1105/tpc.105.033365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185. doi: 10.1105/tpc.107.052068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136: 2982–3000. doi: 10.1104/pp.104.049999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman A, Amakawa T, Goto N, Tsurumi S (2001) Auxin is a positive regulator for ethylene-mediated response in the growth of arabidopsis roots. Plant Cell Physiol 42: 301–307. [DOI] [PubMed] [Google Scholar]
- 40.Franklin KA, Lee SH, Patel D, Kumar SV, Spartz AK, et al. (2011) PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) regulates auxin biosynthesis at high temperature. Proc Natl Acad Sci U S A 108: 20231–20235. doi: 10.1073/pnas.1110682108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu GC, Gao S, Tian HY, Wu WW, Robert HS, et al. (2016) Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis. PLoS Genet 12: e1006360 doi: 10.1371/journal.pgen.1006360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukao T, Bailey-Serres J (2008) Ethylene-A key regulator of submergence responses in rice. Plant Sci 175: 43–51. [Google Scholar]
- 43.Ma B, He SJ, Duan KX, Yin CC, Chen H, et al. (2013) Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant 6: 1830–1848. doi: 10.1093/mp/sst087 [DOI] [PubMed] [Google Scholar]
- 44.Yang C, Lu X, Ma B, Chen SY, Zhang JS (2015) Ethylene signaling in rice and Arabidopsis: conserved and diverged aspects. Mol Plant 8: 495–505. doi: 10.1016/j.molp.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 45.Watanabe H, Saigusa M, Hase S, Hayakawa T, Satoh S (2004) Cloning of a cDNA encoding an ETR2-like protein (Os-ERL1) from deep water rice (Oryza sativa L.) and increase in its mRNA level by submergence, ethylene, and gibberellin treatments. J Exp Bot 55: 1145–1148. doi: 10.1093/jxb/erh110 [DOI] [PubMed] [Google Scholar]
- 46.Yau CP, Wang LJ, Yu MD, Zee SY, Yip WK (2004) Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J Exp Bot 55: 547–556. doi: 10.1093/jxb/erh055 [DOI] [PubMed] [Google Scholar]
- 47.Wuriyanghan H, Zhang B, Cao WH, Ma BA, Lei G, et al. (2009) The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell 21: 1473–1494. doi: 10.1105/tpc.108.065391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma B, Yin CC, He SJ, Lu X, Zhang WK, et al. (2014) Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet 10: e1004701 doi: 10.1371/journal.pgen.1004701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Zhang W, Yin ZM, Wen CK (2013) Rice CONSTITUTIVE TRIPLE-RESPONSE2 is involved in the ethylene-receptor signalling and regulation of various aspects of rice growth and development. J Exp Bot 64: 4863–4875. doi: 10.1093/jxb/ert272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang C, Ma B, He SJ, Xiong Q, Duan KX, et al. (2015) MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol 169: 148–165. doi: 10.1104/pp.15.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, et al. (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci U S A 100: 2992–2997. doi: 10.1073/pnas.0438070100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin CC, Ma BA, Collinge DP, Pogson BJ, He SJ, et al. (2015) Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 27: 1061–1081. doi: 10.1105/tpc.15.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo XJ, Chen ZZ, Gao JP, Gong ZZ (2014) Abscisic acid inhibits root growth in Arabidopsis through ethylene biosynthesis. Plant J 79: 44–55. doi: 10.1111/tpj.12534 [DOI] [PubMed] [Google Scholar]
- 54.Li ZF, Zhang LX, Yu YW, Quan RD, Zhang ZJ, et al. (2011) The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J 68: 88–99. doi: 10.1111/j.1365-313X.2011.04670.x [DOI] [PubMed] [Google Scholar]
- 55.Dong ZJ, Yu YW, Li SH, Wang J, Tang SJ, et al. (2016) Abscisic acid antagonizes ethylene production through the ABI4-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis. Mol Plant 9: 126–135. doi: 10.1016/j.molp.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 56.Jeon JS, Lee S, Jung KH, Jun SH, Jeong DH, et al. (2000) T-DNA insertional mutagenesis for functional genomics in rice. Plant J 22: 561–570. [DOI] [PubMed] [Google Scholar]
- 57.Jeong DH, An S, Park S, Kang HG, Park GG, et al. (2006) Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. Plant J 45: 123–132. doi: 10.1111/j.1365-313X.2005.02610.x [DOI] [PubMed] [Google Scholar]
- 58.Woo YM, Park HJ, Su'udi M, Yang JI, Park JJ, et al. (2007) Constitutively wilted 1, a member of the rice YUCCA gene family, is required for maintaining water homeostasis and an appropriate root to shoot ratio. Plant Mol Biol 65: 125–136. doi: 10.1007/s11103-007-9203-6 [DOI] [PubMed] [Google Scholar]
- 59.Schlaich NL (2007) Flavin-containing monooxygenases in plants: looking beyond detox. Trends Plant Sci 12: 412–418. doi: 10.1016/j.tplants.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 60.Cheng Y, Dai X, Zhao Y (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799. doi: 10.1101/gad.1415106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tivendale ND, Davies NW, Molesworth PP, Davidson SE, Smith JA, et al. (2010) Reassessing the role of N-Hydroxytryptamine in auxin biosynthesis. Plant Physiol 154: 1957–1965. doi: 10.1104/pp.110.165803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472. [DOI] [PubMed] [Google Scholar]
- 63.Cha JY, Kim WY, Kang SB, Kim JI, Baek D, et al. (2015) A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat Commun 6: 8041 doi: 10.1038/ncomms9041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao GQ, Qin H, Zhou JH, Quan RD, Lu XY, et al. (2016) OsERF2 controls rice root growth and hormone responses through tuning expression of key genes involved in hormone signaling and sucrose metabolism. Plant Mol Biol 90: 293–302. doi: 10.1007/s11103-015-0416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yi J, An G (2013) Utilization of T-DNA tagging lines in rice. J Plant Biol 56: 85–90. [Google Scholar]
- 66.Zhang ZJ, Wang J, Zhang RX, Huang RF (2012) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71: 273–287. doi: 10.1111/j.1365-313X.2012.04996.x [DOI] [PubMed] [Google Scholar]
- 67.Wan LY, Zhang JF, Zhang HW, Zhang ZJ, Quan RD, et al. (2011) Transcriptional activation of OsDERF1 in OsERF3 and OsAP2-39 negatively modulates ethylene synthesis and drought tolerance in rice. PLoS One 6: e25216 doi: 10.1371/journal.pone.0025216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Root and coleoptile phenotypes of japonica (Nip, HY, KT) and indica (IR29, YD6-Yangdao #6, ZS97-Zhenshan 97) cultivars treated with air, 10 ppm ethylene, 1 ppm 1-MCP, 1 ppm 1-MCP plus 10 ppm ethylene, 0.2 μM AVG, and 0.2 μM AVG plus 10 ppm ethylene. Rice seedlings were grown in the dark for 3 d in the presence of various reagents. Bar = 10 mm. (B) Root length for the plants shown in (A). (C) Coleoptile length for the plants shown in (A). Values are shown as the mean ± SD of 20–30 seedlings per genotype. The experiment was repeated at least three times with similar results.
(TIF)
(A) Ethylene-response phenotypes of Nip, osein2, EIN2-OX, oseil1 and EIL1-OX seedlings. The etiolated seedlings were grown in air or 10 ppm ethylene for 3 d. Bar = 10 mm. (B) Root length for the plants shown in (A). (C) Coleoptile length for the plants shown in (A). Values are shown as the mean ± SD of 20–30 seedlings per genotype. The experiment was repeated at least three times with similar results.
(TIF)
Nip, osein2, EIN2-OX, oseil1 and EIL1-OX seedlings grown in the dark for 3 d and then treated with or without 10 ppm ethylene for 3 h. The RNAs from roots were isolated and used for qPCR. The experiment was repeated at least five times with similar results. ‘ND’ represents not detected. Bars indicate ± SD.
(TIF)
(A) Ethylene-response phenotypes of various rein mutants. The etiolated seedlings were grown in air or 10 ppm ethylene for 3 d. Bar = 10 mm. (B) Root length of the wild type and rein mutants in response to ethylene. (C) Coleoptile length of the wild type and rein mutants in response to ethylene. Each column is the average of 20–30 seedlings, and bars indicate ± SD.
(TIF)
The phylogenetic tree of 14 rice and 11 Arabidopsis YUC genes was constructed using DNAMAN. Bootstrap analysis values are shown at the nodal branches.
(TIF)
(A) Root phenotype of Nip, constitutive overexpressing YUC8 transgenic (OX-1 and OX-2) etiolated seedlings. The seedlings were grown in the dark for 3 d in the presence or absence of 10 μM Kyn. Bar = 10 mm. (B) YUC8/REIN7 expression in 3-d-old etiolated seedlings. The experiment was repeated at least five times with similar results. Bars indicate ± SD. (C) Root length in (A). (D) Root phenotype of KT and inducible transgenic (PER8-REIN7) etiolated seedlings. The seedlings were grown in the dark for 3 d in the presence or absence of 2.5 μM estradiol, with or without supplementation of 10 μM Kyn. Bar = 10 mm. (E) Root length in (D). In C and E, each column is the average of 20–30 seedlings, and bars indicate ± SD. * and ** indicates a significant difference compared to mock at P < 0.05 and P < 0.01.
(TIF)
(A) The seedling phenotypes of Col-0, yuc1-1, transgenic lines overexpressing truncated (REIN7m-OX/yuc1-1) or full-length YUC8/REIN7 (REIN7-OX/yuc1-1) in Arabidopsis yuc1-1 mutant grown on MS medium for 7 d. (B) The phenotypes of adult Col-0, yuc1-1, REIN7m-OX/yuc1-1 and REIN7-OX/yuc1-1 lines. (C) Mature leaves of the Col-0, yuc1-1, REIN7m-OX/yuc1-1 and REIN7-OX/yuc1-1 lines. (D) Root length in (A). (E) Hypocotyl length in (A). Each column is the average of 20–30 seedlings and bars indicate ± SD. * and ** indicate significant differences compared to yuc1-1 at P <0.05 and P < 0.01, respectively.
(TIF)
(A) The purified proteins of GST-REIN7 and GST-REIN7m expressed in E. coli were identified by SDS-PAGE electrophoresis.–IPTG and +IPTG: the total proteins from E. coli that were induced or not by IPTG, respectively; Purified: the purified recombined protein. (B) The purified proteins of GST-REIN7 and GST-REIN7m expressed in E. coli were analyzed by an anti-GST antibody.
(TIF)
(A) The HPLC profile for authentic TAM, IPA and IAA with UV detection (254 nm). (B) The HPLC chromatogram for IAA that was produced from authentic IPA in GST, GST-AtYUC2, GST-REIN7 and GST-REIN7m reaction mixture. (C) The HPLC chromatogram for TAM that was remained in GST, GST-AtYUC2, GST-REIN7 and GST-REIN7m reaction mixture.
(TIF)
(A) Root phenotypes of the wild type Kitaake treated with various concentrations of IAA. The germinated seed was transferred to MS medium containing various concentrations of IAA and grown in the dark for 3 d. (B) Root length in (A). Each column is the average of 20–30 seedlings and bars indicate ± SD. ** indicates a significant difference compared to 0 μM IAA at P < 0.01.
(TIF)
(A) Partial recovery of the ethylene response of oseil1 root by IAA. The wild-type and oseil1 seedlings were grown in the dark for 3 d in the absence or presence of 10 ppm ethylene, with or without supplementation of 10 nM IAA. Bar = 10 mm. (B) Quantification of root inhibition in (A). Each column is the average of 20–30 seedlings. The data are shown as the mean ± SD of three biological replicates. * indicates significant differences between the compared two samples at P < 0.05.
(TIF)
(XLS)
Data Availability Statement
The sequence data from this article can be found in the MSU7.0 database (http://rice.plantbiology.msu.edu/) under the following accession numbers: OsActin1, Os03g50885; GH3-1, Os01g57610; GH3-2, Os01g55940; GH3-4, Os05g42150; GH3-6, Os05g05180; GH3-8, Os07g40290; GH3-10, Os07g38860; GH3-12, Os11g08340; IAA8, Os02g49160; IAA10, Os02g57250; IAA14, Os03g58350.