Abstract
Objectives
The major clinical side-effect of the ERBB2 targeted breast cancer therapy, trastuzumab, is a decline in left ventricular ejection fraction (LVEF). Improved markers are needed to better identify patients susceptible to cardiotoxicity.
Methods
The NCCTG N9831 trial compared adjuvant doxorubicin and cyclophosphamide followed by either weekly paclitaxel (Arm A); paclitaxel then trastuzumab (Arm B); or concurrent paclitaxel and trastuzumab (Arm C) in patients with HER2-positive breast cancer. A GWAS was performed on all patients with available DNA (N=1,446). We used linear regression to identify SNPs associated with decline in LVEF, adjusting for age, baseline LVEF, anti-hypertensive medications and the first two principle components.
Results
618,863 SNPs passed quality control (QC) and DNA from 1,191 patients passed genotyping QC and were identified as whites of non-Hispanic origin. SNPs at six loci were associated with a decline in LVEF (p=7.73×10−6 to 8.93×10−8), LDB2, BRINP1, chr6 intergenic, RAB22A, TRPC6 and LINC01060, in patients who received chemotherapy plus trastuzumab (Arms BC, N=800). None of these loci were significant in patients who received chemotherapy only (Arm A, N=391) and did not increase in significance in analysis of all patients combined. We did not observe association, p<0.05 with SNPs previously associated with trastuzumab induced cardiotoxicity at ERBB2, I655V and P1170A. We replicated association, p<0.05, with SNPs previously associated with anthracycline cardiotoxicity at CBR3 and ABCB1.
Conclusions
Our study identified six putative novel cardiotoxicity loci in patients treated with combination chemotherapy and trastuzumab that require further investigation and confirmed known associations of anthracycline-induced cardiotoxicity.
Keywords: trastuzumab, doxorubicin, breast cancer, GWAS, cardiotoxicity, cardio-oncology, ERBB2, CBR3, ABCB1
Introduction
The targeted ERBB2 therapy, trastuzumab, has had a tremendous impact on the management of patients with HER2+ advanced and early breast cancer. The major adverse event for this drug is cardiotoxicity, but symptoms are not uniform across patients and differ from the well-known dose-dependent effect of anthracyclines1.
There are no clinically proven markers to predict which patients will experience cardiotoxicity following trastuzumab treatment. Left ventricular ejection fraction (LVEF) <55%, age >60 years, and antihypertensive medication prior to treatment are known risk factors2. The observed variability of cardiac symptoms in humans and animal models suggest a genetic influence. ErbB2-deficient conditional mutant adult mice develop a phenotype consistent with dilated cardiomyopathy (DCM)3,4. Some forms of heart disease presenting with decreased LVEF in humans, such as familial dilated and hypertrophic cardiomyopathy, are known to have a strong heterogeneous genetic basis5. 197 published rare variants in more than 30 causative genes have been reported in the literature6. Common variants in these genes are over-represented in sporadic cases7–9 suggesting the existence of modifying or risk variants that could be exacerbated by environmental stress factors such as trastuzumab and/or chemotherapy. To date, two common genetic polymorphisms within ERBB2, Ile655Val and Pro1170Ala, have been associated with an increased risk of trastuzumab-related cardiotoxicity, but sample sizes were small (N ranging 61–140 patients)10–14. Polymorphisms in several genes including ABCB1, CBR315,16, RAC2, NCF417,18 and SLC28A319,20 have been associated with anthracycline-related cardiotoxicity.
Trastuzumab-related cardiotoxicity commonly manifests as an asymptomatic decline in LVEF that is mostly reversible when treatment is stopped. However, it remains unclear whether trastuzumab therapy could have long-lasting effects on cardiac function such as progressive cardiac remodeling and DCM. This adverse effect of trastuzumab has resulted in strict eligibility criteria in clinical trials and/or discontinuation prior to completion of treatment; 5% of almost 3,500 eligible patients in the NSABP-B31 and N9831 trials were not allowed to initiate trastuzumab therapy and a further 5% of initially eligible women discontinued treatment due to cardiac side effects21,22. Data from multiple randomized adjuvant trials and observational studies suggest that the rate of discontinuation of trastuzumab treatment ranges between 6–31%, mainly due to cardiotoxicity. In patients aged ≥65 years old, this figure increases to a notable and clinically relevant rate of 41%23. Identification of genetic variants that predict which patients are predisposed to cardiotoxicity could improve patient treatment and management.
Observations of trastuzumab related cardiotoxicity are further complicated by the use of anthracyclines prior to trastuzumab treatment, particularly as anthracyclines can result in late-onset cardiac dysfunction24. All patients in the N9831 study received anthracyclines and paclitaxel prior to trastuzumab. The patients who had LVEF dysfunction at 6 years follow-up were the same patients who had LVEF dysfunction during trastuzumab-paclitaxel therapy25. In addition, LVEF dysfunction at 6 years was not statistically different among the chemotherapy only and chemotherapy plus trastuzumab treatment arms, so long-term LVEF dysfunction may not be related to trastuzumab but, possibly, to exposure to anthracyclines and/or increasing age. This is an important point because identification of genetic variants that predict which patients are predisposed to anthracycline cardiotoxicity could allow these patients to use a different chemotherapy regimen, allowing them to be eligible for and to complete trastuzumab therapy. The large size, design and detailed cardiac data collected in N9831 allowed us to examine the genetic influence of trastuzumab on cardiotoxicity in the context of both anthracycline chemotherapy and combination chemotherapy and trastuzumab.
Methods
Detailed information about the methods and study population are available in supplemental material. Briefly, genome-wide genotyping was performed by Affymetrix Axiom array from which 618,793 SNPs passed genotyping quality control. Primary analysis consisted of patients from N9831, Arms BC (N=800 individuals) who received doxorubicin, paclitaxel and trastuzumab (Figure 1). We performed statistical analyses using R version 3.1.1, PLINK version 1.07 and Locus Zoom26. Linear regression was used for change in LVEF (lowest recorded LVEF - baseline LVEF), adjusting for age, baseline LVEF, anti-hypertensive medications and the first two principle components in the 800 patients in Arms BC who received combination chemotherapy and trastuzumab.
Figure 1. Treatment Arms and cardiac monitoring of N9831.
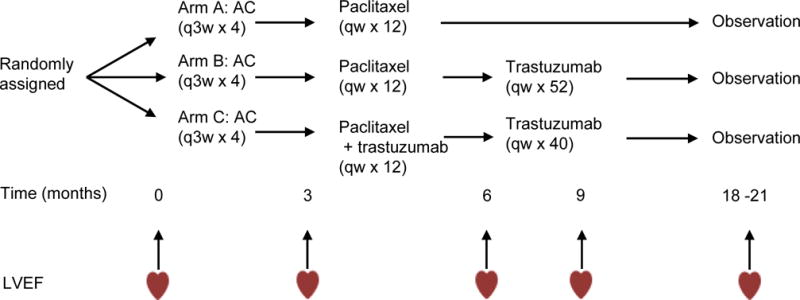
A, Doxorubicin 60 mg/m2; C, Cyclophosphamide 600mg/m2; paclitaxel 80 mg/m2; trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly (qw); LVEF, left ventricular ejection fraction.
We report novel associations at p<1×10−5 in these patients. We also report on published associations of trastuzumab-related cardiotoxicity of the variants ERBB2 Ile655Val and Pro1170Ala10,12–14 from patients in Arms BC and on published associations of anthracycline-related cardiotoxicity at the ABCB1, CBR3, RAC2, NCF4, SLC28A3, RARG and UGT1A6 loci27 in patients from Arms A, B and C combined, who all received doxorubicin and paclitaxel.
Results
Suggestive loci associated with combination chemotherapy and trastuzumab induced decline in LVEF
Seeking modifying/susceptibility genes for cardiotoxicity in patients undergoing treatment for HER2-positive breast cancer, we used a genome-wide association approach. Our primary analysis consisted of DNA samples from 800 patients (Arms BC) who received doxorubicin, paclitaxel and trastuzumab and were determined to be of White/non-Hispanic origin with complete LVEF data from baseline (prior to doxorubicin) and up to two years post treatment.
This analysis yielded six regions at p<1×10−5, with associated variants within or close to LDB2, BRINP1, an intergenic region on chromosome 6, RAB22A, TRPC6 and LINC01060 (Table 1), (QQ and Manhattan plots, Supplementary Figure 2). Locus zoom plots of each region are shown in Supplementary Figure 3. The most significant variant, rs55756123, p=8.93×10−8 mapped within 500bp of the 3′UTR of LDB2. Imputation did not identify any additional variants at this level of significance or any additional coding variants at p<0.05. In the subset of patients with congestive heart failure (CHF) (N=10) versus those without (N=789), a Fisher’s exact test of rs55756123 genotype counts was significant, p=0.007, suggesting enrichment of the associated allele in individuals with the worst cardiac outcome.
Table 1. Linear regression analysis of maximum decline in LVEF.
SNP ID, Chr, chromosome and Hg19 position. MAF, minor allele frequency, L95, lower and U95 upper 95% confidence intervals and P, p-value, is shown for Arms BC and Arm A additive linear regression analysis. Genotype count for each SNP is shown in the subset of patients with CHF, congestive heart failure, separated for Arms BC and Arm A, with genotype counts of allele 1 homozygote/heterozygote/allele 2 homozygote and minor allele frequency in parentheses.
| ID | Chr | Position Hg19 | Locus | Arms BC- Max Drop in LVEF (N=800) | Arm A- Max Drop in LVEF (N=391) | CHF (n=17) genotype count and (MAF) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MAF | Beta (L95−U95) | P (2−tail) | MAF | Beta (L95−U95) | P (2−tail) | Arms BC | Arm A | ||||
| rs55756123 | 4 | 16,502,767 | LDB2 | 0.02 | −6.11 (−8.32 to −3.89) | 8.93E-08 | 0.01 | 1.61 (−3.82 to 7.04) | 0.56 | 0/3/7 (0.15) | 0/0/7 (0.00) |
| rs10117876 | 9 | 121,859,107 | BRINP1 | 0.01 | −7.79 (−10.82 to −4.76) | 5.86E-07 | 0.01 | 2.12 (−3.35 to 7.59) | 0.44 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs7038023 | 9 | 121,859,198 | BRINP1 | 0.01 | −7.79 (−10.82 to −4.76) | 5.86E-07 | 0.01 | 1.83 (−3.26 to 6.92) | 0.48 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs7041012 | 9 | 121,859,463 | BRINP1 | 0.01 | −7.18 (−10.06 to −4.29) | 1.34E-06 | 0.01 | 1.16 (−3.92 to 6.24) | 0.66 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs1160584 | 9 | 121,861,009 | BRINP1 | 0.01 | −7.91 (−11.12 to −4.70) | 1.62E-06 | 0.01 | 2.85 (−3.64 to 9.35) | 0.38 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs230145 | 9 | 121,879,897 | BRINP1 | 0.01 | −7.91 (−11.12 to −4.70) | 1.62E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs230144 | 9 | 121,882,710 | BRINP1 | 0.01 | −7.90 (−11.12 to −4.69) | 1.70E-06 | 0.01 | −1.76 (−7.70 to 4.19) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs230142 | 9 | 121,883,736 | BRINP1 | 0.01 | −7.91 (−11.12 to −4.70) | 1.62E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs62573809 | 9 | 121,892,448 | BRINP1 | 0.01 | −6.88 (−9.84 to −3.93) | 5.70E-06 | 0.01 | −2.25 (−7.69 to 3.20) | 0.42 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs16908078 | 9 | 121,893,380 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.38) | 2.95E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs7851490 | 9 | 121,894,217 | BRINP1 | 0.01 | −7.48 (−10.60 to −4.36) | 3.12E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs7854066 | 9 | 121,899,889 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.37) | 3.04E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs62573837 | 9 | 121,900,834 | BRINP1 | 0.01 | −7.50 (−10.62 to −4.38) | 2.88E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs76586195 | 9 | 121,902,640 | BRINP1 | 0.01 | −7.73 (−10.95 to −4.52) | 2.90E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs7027658 | 9 | 121,904,365 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.38) | 2.95E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs75912020 | 9 | 121,909,304 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.38) | 2.95E-06 | 0.01 | −1.77 (−7.71 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs76890184 | 9 | 121,910,252 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.38) | 2.95E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs58944852 | 9 | 121,912,974 | BRINP1 | 0.01 | −7.49 (−10.61 to −4.38) | 2.87E-06 | 0.01 | −1.76 (−7.70 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs62568637 | 9 | 121,917,144 | BRINP1 | 0.01 | −8.50 (−11.81 to −5.19) | 6.01E-07 | 0.01 | −1.77 (−7.72 to 4.18) | 0.56 | 0/1/9 (0.05) | 0/0/7 (0.00) |
| rs4305714 | 6 | 22,957,737 | intergenic | 0.24 | −1.87 (−2.63 to −1.12) | 1.39E-06 | 0.24 | −0.51 (−1.64 to 0.63) | 0.38 | 0/5/5 (0.25) | 1/5/1 (0.50) |
| rs707557 | 20 | 56,942,312 | RAB22A | 0.40 | 1.46 (0.83 to 2.08) | 5.62E-06 | 0.38 | −0.31 (−1.33 to 0.71) | 0.55 | 1/3/6 (0.25) | 1/6/0 (0.57) |
| rs77679196 | 11 | 101,453,508 | TRPC6 | 0.01 | −7.00 (−10.05 to −3.96) | 7.72E-06 | 0.01 | −5.29 (−11.95 to 1.38) | 0.12 | 0/2/8 (0.10) | 0/0/7 (0.00) |
| rs7698718 | 4 | 189,555,947 | LINC01060 | 0.22 | 1.78 (1.01 to 2.56) | 7.73E-06 | 0.22 | 0.11 (−1.02 to 1.23) | 0.86 | 1/2/7 (0.20) | 0/5/2 (0.36) |
We observed a total of 18 SNPs, p<1×10−5 at the BRINP1 locus; the most significant an intronic variant, rs62568637, p=6.01×10−7 and variants in the 3′ flanking region, rs10117876 and rs7038023, p=5.86×10−7 in high LD, r2=0.815. Of note, rs62568637 and flanking SNPs on either side showed positive GERP scores (ranging 1.46–4.51) suggestive of sequence conservation (Supplementary Table 4). Imputation at this region identified a more significant 3′ flanking variant, rs7037449, p=1.87×10−7 in high LD with the other 3′ flanking variants.
rs4305714, p=1.39×10−6 maps to an intergenic region on chromosome 6p22.3. Imputation across the region was supportive, yielding an additional 18 variants at p<1×10−5 and two additional variants in high LD, rs72836853 and rs10485148 at p=9.85×10−7.
A common variant (MAF=0.40) within the 3′UTR of RAB22A, rs707557, was associated at p=5.62×10−6. The positive GERP score for this variant (2.47) may suggest some level of sequence conservation at this locus. Imputation across this region yielded two additional SNPs at the same level of significance in very high LD, rs1045408 and rs6128327, p=3.48 and 3.38×10−6, respectively, both of which map to sequences with very high GERP scores (ranging 4.32–5.40).
An intronic variant, rs77679196, within TRPC6 was associated at p=7.72×10−6. Imputation across the region yielded one additional SNP in the 5′ flanking region, with a slightly stronger association, rs75865789, beta= −8.62 (95% CI −12.07 to −5.178), p=1.14×10−6. In the subset of patients with CHF (N=10) versus those without (N=789), a Fisher’s exact test of rs77679196 genotype counts was significant (p=0.019) suggesting enrichment of the associated allele in individuals with the worst cardiac outcome.
Associated variants in the final significant region were entirely intergenic, the closest gene being LINC01060. Imputation supported the most significant SNP, rs7698718, with an additional variant at p<1×10−5 all in high LD, but no variants of greater association were identified.
We reasoned that variants associated with cardiotoxicity specifically from doxorubicin would be significant or show a trend in the chemotherapy Arm only and report these data separately in Table 1. None of the six regions from our discovery analyses of patients who received chemotherapy in combination with trastuzumab (N=800) were significant at p<0.05 (1 tailed) in the patients who received chemotherapy only (N=391). LDB2 and RAB22A showed an effect in the opposite direction in patients who received chemotherapy only, and combined analyses of all patients (N=1,191) became less significant, p=1.81×10−6 and p=0.0015, respectively. The most significant SNPs at BRINP1 and the intergenic region close to LINC01060 showed effects in the same direction and became slightly more significant in combined analyses of all patients (N=1,191), BRINP1, rs62568637, p=4.73×10−6; LINC01060, p=1.63×10−6. The intergenic SNP, rs4305714 became less significant in the combined analysis of all patients, p=1.20×10−5. rs7769196 in intron 1 of TRPC6 did show a similar effect size in the same direction and a trend for association (p=0.06,1-tailed) in patients who received chemotherapy only, increasing in significance in combined analysis of all patients, p=1.63×10−6.
Replication of variants associated with ERBB2-induced cardiotoxicity
Our study failed to find association of decline in LVEF with either of the published associated ERBB2 variants, rs1136201 (I655V) and rs1058808 (P1170A)10–14. To be consistent with published associations of the I655V variant, we also performed genotypic analyses of the AG+GG genotypes versus the AA genotype on a case control basis, where cases were defined as a decrease of at least 10% points from baseline with a resulting LVEF of less than 50%, a decrease of 15% with respect to baseline, or any decrease resulting in LVEF less than 45% at least once during the treatment or clinical manifestation of CHF14. We did not observe any association of the I655V or P1170A variants with either linear or logistic models (Table 2).
Table 2. SNPs previously associated with ERBB2 cardiotoxicity (Arms BC).
Association analyses were performed assuming an additive effect in both linear and logistic regression models. For logistic regression, we report genotype counts, odds ratio (OR) and P-values (P) using the more stringent definition of cases as per Gomez Pena et al, 2015: Decrease of at least 10% points from baseline with a resulting LVEF of less than 50% or decrease of 15% with respect to baseline, or any decrease resulting in LVEF less than 45% at least once during the treatment or clinical manifestation of CHF.
| Linear Max drop in LVEF | Logistic LVEF literature | ||||||
|---|---|---|---|---|---|---|---|
| SNP ID | AA change | Beta, L95-U95 | P | genotype case | genotype con | OR, L95-U95 | P |
| rs1136201 | I655V | −0.33 (−1.07 to 0.41) | 0.39 | GG=11 | GG=39 | 0.941 (0.71 to 1.25) | 0.67 |
| GA=59 | GA=228 | ||||||
| AA=103 | AA=360 | ||||||
| rs1058808 | P1170A | −0.45 (−1.14 to 0.24) | 0.20 | CC=21 | CC=65 | 1.034 (0.80 to 1.34) | 0.80 |
| CG=78 | CG=293 | ||||||
| GG=73 | GG=268 | ||||||
We also assessed association at ERBB2 by imputation including 200kb flanking region and linear regression of decline in LVEF, plotted in Locus Zoom (Supplementary Figure 4) in those patients who received combination chemotherapy and trastuzumab (Arms BC). This analysis identified 95/943 SNPs at p<0.05, although none of these remained significant following correction for multiple testing.
Variants associated with anthracycline-induced cardiotoxicity
Several SNPs have been associated with anthracycline-induced cardiotoxicity16,19,20,27,28. Under our linear model of decline in LVEF, we observed a significant association of the CBR3 V244M variant in combined analyses of all patients (N=1,191), p=0.004, Beta=0.84 when A (Met) allele is reference15,16. We also observed association with the published ABCB1 variant rs2235047 (p=0.018) and a trend for association of rs17863783 variant in UGT1A6 (p=0.078). We also looked for enrichment of the associated allele in the subset of individuals with CHF (N=17). There was no evidence of enrichment at either the CBR3 or ABCB1 loci. However, we did observe enrichment of reported associated alleles at the NCF4 and RARG loci in patients with CHF. Allele A of the NCF4 5′ flanking SNP, rs1883112, presented with a frequency of 0.44 in all patients who received chemotherapy (N=1,191) but only 0.24 (8/34 alleles) in patients with CHF, OR 0.35, p=0.011. The leucine allele of rs2229774 at the RARG locus was enriched in CHF patients relative to all patients who received chemotherapy (0.15 versus 0.07) and showed a trend for association, p=0.076. Locus zoom plots are shown in Supplementary Figure 5.
Discussion
Cardiotoxicity is a major clinical side-effect of both anthracycline and trastuzumab therapy, with a higher incidence in patients receiving combination treatment compared to those receiving only anthracycline25,29. The extent and frequency of cardiotoxicity is not uniform across patients, leading us to hypothesize a genetic influence. We performed a genome-wide association of decline in LVEF in patients treated with trastuzumab from the N9831 trial. This trial led to the use of trastuzumab as a standard of care in early onset HER2+ breast cancer. Our first approach used a quantitative analysis of the maximum observed decline in LVEF and DNA variants in patients from Arms BC (N=800) who received doxorubicin, paclitaxel and trastuzumab. We corrected for multiple testing with Benjamini-Hochberg and report those variants at q<0.2 for a total of 23 variants (p<1×10−5), from six novel loci: limb domain binding 2, (LDB2), an adapter molecule of transcriptional regulatory complexes30,31; BMP/retinoic acid-inducible neural-specific protein 1, (BRINP1), a suppressor of cell cycle progression32; transient receptor potential cation channel, subfamily C, member 6, (TRPC6), a central mediator of hypertrophic cardiomyopathy, cardiac remodeling33–36; a member of the RAS oncogene family, (RAB22A) involved in endocytic trafficking37–39; a LINC RNA, (LINC10160); and an intergenic region on chromosome 6.
Patients in Arms BC received both doxorubicin and trastuzumab. Therefore in an effort to discriminate between doxorubicin and trastuzumab-mediated cardiotoxicity, we reasoned that loci associated with decline in LVEF induced specifically by doxorubicin would be replicated in Arm A alone. However, none of the top variants were significant, p<0.05, in the patients in Arm A (N=391) (Table 1). We did observe a trend for association of decline in LVEF for the intronic variant in TRPC6 in patients who received chemotherapy only. This association was in the same direction and similar effect size as observed in patients receiving chemotherapy and trastuzumab, p=0.06 (1-tailed), suggesting that the TRPC6 variant could be a result of chemotherapy. Evidence from the literature could support this hypothesis, because TRPC6 expression which is increased in cardiac tissue from patients with dilated cardiomyopathy35 is also up-regulated by doxorubicin40. The failure to replicate other variants in patients in Arm A could infer that the novel associations in Arms BC are induced by combination chemotherapy and trastuzumab, although we acknowledge that Arm A (N=391) is smaller than Arms BC (N=800) and may lack statistical power to replicate. We do not believe associations in Table 1 are a statistical artifact of different cumulative doses of doxorubicin as all patients in this analysis received the same dose (4 × 60 mg/m2) regimen completing chemotherapy with post-anthracycline LVEF remaining above 50% to continue with trastuzumab treatment.
To further discriminate between chemotherapy and trastuzumab-mediated cardiotoxicity, we also performed an exploratory analysis of each novel region. We hypothesized that true risk alleles of cardiotoxicity might be enriched in the subset of patients with CHF. At LDB2, BRINP1 and TRPC6 (low frequency variants, MAF<0.02), we observed ≥ five-fold enrichment in the subset of patients with CHF who received trastuzumab (N=10). Interestingly, in those patients with CHF who received chemotherapy only (N=7) we did not observe any of the putative risk alleles at these loci, suggesting these associations may be trastuzumab-mediated with the caveat that our subset of patients with CHF is underpowered. In contrast, for the most common associated variants in Table 1, chr6 intergenic region, RAB22A and LINC01060, we observed enrichment in those patients with CHF who received chemotherapy only (MAF 0.50, 0.57 and 0.36, respectively). We did not observe the same trend in the patients receiving trastuzumab who also developed CHF. If these variants are truly associated with doxorubicin and/or trastuzumab-mediated cardiotoxicity, one could hypothesize the existence of large effect size, low frequency variants that increase susceptibility to cardiotoxicity with combination chemotherapy and trastuzumab, and the existence of common cardiac risk variants that increase susceptibility to anthracycline-related cardiotoxicity.
Our GWAS data also included variants from the current literature that were reported to be associated with cardiotoxicity from either, trastuzumab, anthracycline or combination therapy. We failed to observe genome-wide significant association with any variant ERBB2, the target of trastuzumab. We did not observe any association at p<0.05 for SNPs reported to be associated with trastuzumab-related cardiotoxicity in the current literature (i.e., rs1136201, I655V and rs1058808, P1170A). There are several possible reasons for this failure to replicate previous findings. We ruled out genotyping error based on concordant genotypes from multiple probes at rs1136201, genotypic data in Hardy-Weinberg equilibrium, observed allele frequencies in line with other reported studies and adequate cluster plots (Supplementary Figure 7).
We next considered our definition of cardiotoxicity as a reason for lack of replication with ERBB2 I655V and P1170A. Our primary analysis used a quantitative model of maximum decline in LVEF. Published associations used a binary definition of cardiotoxicity or no cardiotoxicity, but with different definitions in each case, including decrease in LVEF of at least 20% points10; decrease in LVEF of at least 10% points to less than 50% or any decrease to <45%11; decrease >10% points to <50% or any decrease >15% or any decrease resulting in <45% or diagnosis of CHF14. Hence we also looked for association with the same binary definition as Gomez Pena14. Again, we did not observe any associations, p<0.05. Acknowledging the caveat of the arbitrary definition of cardiotoxicity and the models we used, it is possible that our lack of replication was simply due to a smaller sample size in previous studies. Our data set from N9831 patients treated with trastuzumab (N=800) was over 2-fold greater compared to the four published studies combined (N=344, ranging 61–132 patients14), which showed large odds ratios ranging from 3.80 to 5.80. We note that there are no published negative studies of these variants with cardiotoxicity, which may suggest a publication bias towards positive associations in when using small sample sizes.
Our analysis of published SNPs associated with anthracycline cardiotoxicity in all patients who received doxorubicin (Arms A, B and C combined, N=1,191) was more successful in that we did observe association of the CBR3 V244M variant in our quantitative analysis of maximum decline in LVEF (p=0.004) with supporting evidence from multiple SNPs at this locus. Carbonyl reductases (CBRs) catalyze reduction of anthracyclines to cardiotoxic alcohol metabolites and polymorphisms in CBR1 and CBR3 influence the synthesis of these metabolites. CBR3 V244M was previously associated with a dose-dependent risk of anthracycline-related cardiomyopathy in childhood cancer survivors15, and with cardiotoxicity in a cohort of breast cancer patients16. In a literature review entitled, ‘Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity’ it was deemed that CBR3 required further validation. Our study confirms association of this variant in patients with breast cancer.
Similarly, we observed association of a variant in ABCB1, rs2235047, also previously associated with anthracycline-induced cardiotoxicity in children, where 266 were controls (defined as systolic function >30% during and after therapy, with follow-up of more than five years after completion of anthracycline therapy) and 78 cases (defined as systolic function ≤26% at any time during or after anthracycline therapy)19. This variant was not confirmed in a further study of children, consisting of 46 cases and 131 controls, analysed with the same methodology from the same group20, perhaps due to lack of power. However, in this study we observed an association of similar magnitiude (Beta = −2.34, p=0.018) in our linear model of decline in LVEF in 1,191 patients receiving doxorubicin, adding further evidence for this association. Certainly, this gene is a reasonable candidate due to its involvement in drug excretion in the kidney, liver, and heart41,42. Specifically, changes in ABCB1 expression or function prolong the presence of both doxorubicin in cardiac tissue and its cardiotoxic metabolite, doxorubicinol, within cardiac tissue43. Furthermore, the ERBB2 inhibitor, lapatinib, has been shown to inhibit the function of ABCB144, suggesting a possible mechanism for increased cardiotoxicity as a result of doxorubicin in combination with HER2 targeted therapy.
We did not observe an association of published SNPs associated with doxorubicin-induced cardiotoxicity in SLC28A3, NCF4, RAC2, RARG and UGT1A6 in our linear model of decline in LVEF in 1,191 patients receiving doxorubicin (Table 3). Given these were associations with CHF rather than decline in LVEF, we also tested for these variants in the limited subset of patients (N=17) with CHF. Aminkeng et al28 reported a highly signficant association of the 427Leu variant within retinoic acid receptor γ, RARG (rs2229774, Ser427Leu) with CHF in three studies of children (cases N=32, N=22 and N=19, respectively) treated with anthracyclines (either doxorubicin, daunorubicin, idarubicin, epirubicin or mitoxantrone). In our smaller subset of CHF patients from the N9831 trial treated with doxorubicin, we observed a weak association of the 427Leu variant, OR=2.39, p=0.038 (1-tailed). We also observed association of the NCF4 rs1883112 A allele, OR 0.35, p=0.01 (2-tailed) (A allele frequency in CHF patients = 0.24 vs 0.44 in patients who were not confirmed with CHF). However, our finding is in the opposite direction to other reported associations. The AA genotype of this variant was previously associated with CHF in adult non-Hodgkin lymphoma patients treated with doxorubicin, OR 2.00, p=0.01818 and the A allele was associated with CHF in cancer patients treated with anthracyclines, OR 5.11, p=0.01645.
Table 3. SNPs previously associated with anthracycline cardiotoxicity (Arms ABC).
Additive effect linear regression analyses in all Arms combined. Logistic regression was performed in those cases (N=17) with congestive heart failure (CHF) versus all other patients. P-values <0.05 are highlighted in bold.
| Linear Max drop in LVEF | Logistic CHF (N=17) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | rs ID | Function | Freq A1 (N=1191) | Beta, L95-U95 | P | Freq A1 (N=17) | OR, L95-U95 | P |
| ABCB1 | rs2235047 | intronic | 0.02 | −2.34 (−4.28 to −0.40) | 0.018 | 0.00 | NA | 0.996 |
| SLC28A3 | rs7853758 | L464L | 0.16 | −0.11 (−0.84 to 0.62) | 0.766 | 0.12 | 0.71 (0.25 to 2.02) | 0.523 |
| CBR3 | rs1056892 | V244M | 0.35 | 0.84 (0.26 to 1.41) | 0.004 | 0.35 | 1.01 (0.49 to 2.08) | 0.972 |
| NCF4 | rs1883112 | 5′ flanking | 0.44 | 0.19 (−0.36 to 0.72) | 0.492 | 0.24 | 0.35 (0.15 to 0.78) | 0.011 |
| RAC2 | rs13058338 | intronic | 0.26 | 0.05 (−0.55 to 0.66) | 0.861 | 0.29 | 1.21 (0.58 to 2.57) | 0.611 |
| RARG | rs2229774 | S427L | 0.07 | 0.44 (0.61 to 1.48) | 0.412 | 0.15 | 2.39 (0.91 to 6.23) | 0.076 |
| UGT1A6 | rs17863783 | V209V | 0.02 | −1.67 (−3.52 to 0.18) | 0.078 | 0.00 | NA | 0.996 |
A weakness of our study is the inability to validate our finidngs using other methods. Recognizing this weakness, we scanned the literature for reported cardiac modifying genes in animal models. This led us to examine four genes (SORBS2, RXRA, DNAJB6 and ANO5) reported as cardiac modifiers of doxorubicin-induced cardiotoxicity in zebrafish and/or mouse models46,47. Suggesting that our data can be replicated we also identified variants in our study of N9831 in SORBS2 and RXRA and p<0.01 in DNAJB6, p<0.001 (Supplementary Table 3). These finidngs suggest that these genes require further study as genes modified by chemotherapy treatments that could increase the risk for cardiomyopathy in patients.
In summary, our study confirmed previously reported associations of chemotherapy-induced cardiotoxicity with variants in the ABCB1 and CBR3 genes. We failed to replicate previous findings at ERBB2 with any model of cardiotoxicity (linear or logistic); however, our sample size was significantly larger than other published studies suggesting that previous associations may have occurred due to small sample size. In an effort to identify novel loci relevant to patients receiving combination chemotherapy and trastuzumab, we identified six potential susceptibility loci. Further study is needed to confirm the role of these loci in trastuzumab-induced cardiomyopathy.
Supplementary Material
Acknowledgments
Funding Sources. This work was supported by The Mackenzie Foundation, Breast Cancer Research Foundation, and a Gerstner Career Development Award to NN and National Institutes of Health (R01 HL111938, R21 ES024414) and American Heart Association (16GRNT30950007) grants to DF.
Footnotes
Conflicts of Interest: None declared
References
- 1.Narahara KA, Singer JW, Ritchie JL, Williams DL, Hamilton GW, Kennedy JW. Time- and dose-dependent changes in ejection fraction after anthracycline therapy. J Cardiovasc Pharmacol. 1979 Jul-Aug;1(4):395–401. doi: 10.1097/00005344-197907000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Advani P, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG (Alliance) N9831 adjuvant trastuzumab (H) trial. Journal of Clinical Oncology. 2014;32(15S):30s. doi: 10.1200/JCO.2015.61.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crone SA, Zhao YY, Fan L, Gu Y, Minamisawa S, Liu Y, Peterson KL, Chen J, Kahn R, Condorelli G, Ross J, Jr, Chien KR, Lee KF. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002 May;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 4.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013 Sep;10(9):531–547. doi: 10.1038/nrcardio.2013.105. [DOI] [PubMed] [Google Scholar]
- 6.Norton N, Robertson PD, Rieder MJ, Zuchner S, Rampersaud E, Martin E, Li D, Nickerson DA, Hershberger RE. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ Cardiovasc Genet. 2012 Apr 1;5(2):167–174. doi: 10.1161/CIRCGENETICS.111.961805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, Conner L, DePalma SR, McDonough B, Sparks E, Teodorescu DL, Cirino AL, Banner NR, Pennell DJ, Graw S, Merlo M, Di Lenarda A, Sinagra G, Bos JM, Ackerman MJ, Mitchell RN, Murry CE, Lakdawala NK, Ho CY, Barton PJ, Cook SA, Mestroni L, Seidman JG, Seidman CE. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. 2012 Feb 16;366(7):619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rampersaud E, Kinnamon DD, Hamilton K, Khuri S, Hershberger RE, Martin ER. Common susceptibility variants examined for association with dilated cardiomyopathy. Ann Hum Genet. 2010 Mar;74(2):110–116. doi: 10.1111/j.1469-1809.2010.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villard E, Perret C, Gary F, Proust C, Dilanian G, Hengstenberg C, Ruppert V, Arbustini E, Wichter T, Germain M, Dubourg O, Tavazzi L, Aumont MC, DeGroote P, Fauchier L, Trochu JN, Gibelin P, Aupetit JF, Stark K, Erdmann J, Hetzer R, Roberts AM, Barton PJ, Regitz-Zagrosek V, Aslam U, Duboscq-Bidot L, Meyborg M, Maisch B, Madeira H, Waldenstrom A, Galve E, Cleland JG, Dorent R, Roizes G, Zeller T, Blankenberg S, Goodall AH, Cook S, Tregouet DA, Tiret L, Isnard R, Komajda M, Charron P, Cambien F. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur Heart J. 2011 May;32(9):1065–1076. doi: 10.1093/eurheartj/ehr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, Hofman P, Ferrero JM, Pages G, Milano G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab-related cardiotoxicity. Ann Oncol. 2007 Aug;18(8):1335–1341. doi: 10.1093/annonc/mdm181. [DOI] [PubMed] [Google Scholar]
- 11.Lemieux J, Diorio C, Cote MA, Provencher L, Barabe F, Jacob S, St-Pierre C, Demers E, Tremblay-Lemay R, Nadeau-Larochelle C, Michaud A, Laflamme C. Alcohol and HER2 polymorphisms as risk factor for cardiotoxicity in breast cancer treated with trastuzumab. Anticancer Res. 2013 Jun;33(6):2569–2576. [PubMed] [Google Scholar]
- 12.Roca L, Dieras V, Roche H, Lappartient E, Kerbrat P, Cany L, Chieze S, Canon JL, Spielmann M, Penault-Llorca F, Martin AL, Mesleard C, Lemonnier J, de Cremoux P. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER-PACS 04 trial. Breast Cancer Res Treat. 2013 Jun;139(3):789–800. doi: 10.1007/s10549-013-2587-x. [DOI] [PubMed] [Google Scholar]
- 13.Stanton SE, Ward MM, Christos P, Sanford R, Lam C, Cobham MV, Donovan D, Scheff RJ, Cigler T, Moore A, Vahdat LT, Lane ME, Chuang E. Pro1170 Ala polymorphism in HER2-neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer. 2015;15:267. doi: 10.1186/s12885-015-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez Pena C, Davila-Fajardo CL, Martinez-Gonzalez LJ, Carmona-Saez P, Soto Pino MJ, Sanchez Ramos J, Moreno Escobar E, Blancas I, Fernandez JJ, Fernandez D, Correa C, Cabeza Barrera J. Influence of the HER2 Ile655Val polymorphism on trastuzumab-induced cardiotoxicity in HER2-positive breast cancer patients: a meta-analysis. Pharmacogenet Genomics. 2015 Aug;25(8):388–393. doi: 10.1097/FPC.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 15.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, Mays A, Friedman DL, Ginsberg JP, Hudson MM, Neglia JP, Oeffinger KC, Ritchey AK, Villaluna D, Relling MV, Bhatia S. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes–a report from the Children’s Oncology Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012 May 1;30(13):1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hertz DL, Caram MV, Kidwell KM, Thibert JN, Gersch C, Seewald NJ, Smerage J, Rubenfire M, Henry NL, Cooney KA, Leja M, Griggs JJ, Rae JM. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics. 2016 Feb;17(3):231–240. doi: 10.2217/pgs.15.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi D, Rasi S, Franceschetti S, Capello D, Castelli A, De Paoli L, Ramponi A, Chiappella A, Pogliani EM, Vitolo U, Kwee I, Bertoni F, Conconi A, Gaidano G. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B-cell lymphoma treated with R-CHOP21. Leukemia. 2009 Jun;23(6):1118–1126. doi: 10.1038/leu.2008.398. [DOI] [PubMed] [Google Scholar]
- 18.Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, Vonhof S, Bickeboller H, Toliat MR, Suk EK, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nurnberg P, Pfreundschuh M, Trumper L, Brockmoller J, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005 Dec 13;112(24):3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 19.Visscher H, Ross CJ, Rassekh SR, Barhdadi A, Dube MP, Al-Saloos H, Sandor GS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Brown AM, Rogers PC, Phillips MS, Rieder MJ, Carleton BC, Hayden MR. Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012 May 1;30(13):1422–1428. doi: 10.1200/JCO.2010.34.3467. [DOI] [PubMed] [Google Scholar]
- 20.Visscher H, Ross CJ, Rassekh SR, Sandor GS, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Rogers PC, Rieder MJ, Carleton BC, Hayden MR. Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer. 2013 Aug;60(8):1375–1381. doi: 10.1002/pbc.24505. [DOI] [PubMed] [Google Scholar]
- 21.Perez EA. Cardiac toxicity of ErbB2-targeted therapies: What do we know? Clin Breast Cancer. 2008 Mar;8:S114–S120. doi: 10.3816/cbc.2008.s.007. [DOI] [PubMed] [Google Scholar]
- 22.Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M, Coombes RC, Dodwell D, Pagani O, Madrid J, Hall M, Chen SC, Focan C, Muschol M, van Veldhuisen DJ, Piccart-Gebhart MJ. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010 Jul 20;28(21):3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 23.Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, Chen J. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. 2014 Jun 21; doi: 10.1007/s10549-014-3029-0. [DOI] [PubMed] [Google Scholar]
- 24.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. 2010 Sep-Oct;19(5):308–311. doi: 10.1016/j.carpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016 Feb 20;34(6):581–587. doi: 10.1200/JCO.2015.61.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010 Sep 15;26(18):2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aminkeng F, Ross CJ, Rassekh SR, Hwang S, Rieder MJ, Bhavsar AP, Smith A, Sanatani S, Gelmon KA, Bernstein D, Hayden MR, Amstutz U, Carleton BC. Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol. 2016 Sep;82(3):683–695. doi: 10.1111/bcp.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, Brunham LR, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Amstutz U, Rieder MJ, Bernstein D, Carleton BC, Hayden MR, Ross CJ. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nature genetics. 2015 Sep;47(9):1079–1084. doi: 10.1038/ng.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001 Mar 15;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 30.Agulnick AD, Taira M, Breen JJ, Tanaka T, Dawid IB, Westphal H. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature. 1996 Nov 21;384(6606):270–272. doi: 10.1038/384270a0. [DOI] [PubMed] [Google Scholar]
- 31.Jurata LW, Gill GN. Functional analysis of the nuclear LIM domain interactor NLI. Molecular and cellular biology. 1997 Oct;17(10):5688–5698. doi: 10.1128/mcb.17.10.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawano H, Nakatani T, Mori T, Ueno S, Fukaya M, Abe A, Kobayashi M, Toda F, Watanabe M, Matsuoka I. Identification and characterization of novel developmentally regulated neural-specific proteins, BRINP family. Brain research Molecular brain research. 2004 Jun 18;125(1–2):60–75. doi: 10.1016/j.molbrainres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Eder P, Molkentin JD. TRPC channels as effectors of cardiac hypertrophy. Circulation research. 2011 Jan 21;108(2):265–272. doi: 10.1161/CIRCRESAHA.110.225888. [DOI] [PubMed] [Google Scholar]
- 34.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. Journal of molecular and cellular cardiology. 2010 Apr;48(4):713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. The Journal of clinical investigation. 2006 Dec;116(12):3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honore E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010 Aug;460(3):571–581. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 37.Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. Journal of cell science. 2002 Mar 1;115(Pt 5):899–911. doi: 10.1242/jcs.115.5.899. [DOI] [PubMed] [Google Scholar]
- 38.Mesa R, Magadan J, Barbieri A, Lopez C, Stahl PD, Mayorga LS. Overexpression of Rab22a hampers the transport between endosomes and the Golgi apparatus. Exp Cell Res. 2005 Apr 1;304(2):339–353. doi: 10.1016/j.yexcr.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Mesa R, Salomon C, Roggero M, Stahl PD, Mayorga LS. Rab22a affects the morphology and function of the endocytic pathway. Journal of cell science. 2001 Nov;114(Pt 22):4041–4049. doi: 10.1242/jcs.114.22.4041. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HT, Wang WW, Ren LH, Zhao XX, Wang ZH, Zhuang DL, Bai YN. The mTORC2/Akt/NFkappaB Pathway-Mediated Activation of TRPC6 Participates in Adriamycin-Induced Podocyte Apoptosis. Cell Physiol Biochem. 2016;40(5):1079–1093. doi: 10.1159/000453163. [DOI] [PubMed] [Google Scholar]
- 41.Huls M, Russel FG, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration. J Pharmacol Exp Ther. 2009 Jan;328(1):3–9. doi: 10.1124/jpet.107.132225. [DOI] [PubMed] [Google Scholar]
- 42.Solbach TF, Paulus B, Weyand M, Eschenhagen T, Zolk O, Fromm MF. ATP-binding cassette transporters in human heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2008 May;377(3):231–243. doi: 10.1007/s00210-008-0279-6. [DOI] [PubMed] [Google Scholar]
- 43.Zhou ZY, Wan LL, Yang QJ, Han YL, Li D, Lu J, Guo C. Nilotinib reverses ABCB1/P-glycoprotein-mediated multidrug resistance but increases cardiotoxicity of doxorubicin in a MDR xenograft model. Toxicol Lett. 2016 Sep 30;259:124–132. doi: 10.1016/j.toxlet.2016.07.710. [DOI] [PubMed] [Google Scholar]
- 44.Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR, Jr, Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Research. 2008 Oct 1;68(19):7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cascales A, Pastor-Quirante F, Sanchez-Vega B, Luengo-Gil G, Corral J, Ortuno-Pacheco G, Vicente V, de la Pena FA. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. The oncologist. 2013;18(4):446–453. doi: 10.1634/theoncologist.2012-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding Y, Liu W, Deng Y, Jomok B, Yang J, Huang W, Clark KJ, Zhong TP, Lin X, Ekker SC, Xu X. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circ Res. 2013 Feb 15;112(4):606–617. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding Y, Long PA, Bos JM, Shih YH, Ma X, Sundsbak RS, Chen J, Jiang Y, Zhao L, Hu X, Wang J, Shi Y, Ackerman MJ, Lin X, Ekker SC, Redfield MM, Olson TM, Xu X. A modifier screen identifies DNAJB6 as a cardiomyopathy susceptibility gene. JCI Insight. 2016 Sep 8;1(14) doi: 10.1172/jci.insight.88797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


