Abstract
Only through concerted and well-executed research endeavors can we gain the requisite knowledge to advance pregnancy care and positively impact maternal and newborn health. Yet the heterogeneity inherent in individual studies limits our ability to compare and synthesize study results, thus impeding the capacity to draw meaningful conclusions that can be trusted to inform clinical care. The PhenX Toolkit (http://www.phenxtoolkit.org), supported since 2007 by the National Institutes of Health, is a web-based catalog of standardized protocols for measuring phenotypes and exposures relevant for clinical research. In 2016, a working group of pregnancy experts recommended 15 measures for the PhenX Toolkit that are highly relevant to pregnancy research. The working group followed the established PhenX consensus process to recommend protocols that are broadly validated, well-established, nonproprietary, and have a relatively low burden for investigators and participants. The working group considered input from the pregnancy experts and the broader research community and included measures addressing mode of conception, gestational age, fetal growth assessment, prenatal care, mode of delivery, gestational diabetes, behavioral and mental health, and environmental exposure biomarkers. These pregnancy measures complement the existing measures for other established domains in the PhenX Toolkit, including reproductive health, anthropometrics, demographic characteristics, and alcohol, tobacco, and other substances. The preceding domains influence a woman's health during pregnancy. For each measure, the PhenX Toolkit includes data dictionaries and data collection worksheets that facilitate incorporation of the protocol into new or existing studies. The measures within the pregnancy domain offer a valuable resource to investigators and clinicians and are well poised to facilitate collaborative pregnancy research with the goal to improve patient care. To achieve this aim, investigators whose work includes the perinatal population are encouraged to utilize the PhenX Toolkit in the design and implementation of their studies, thus potentially reducing heterogeneity in data measures across studies. Such an effort will enhance the overall impact of individual studies and increasing the ability to draw more meaningful conclusions that can then be translated into clinical practice.
Keywords: Conception, cross-study analyses, data harmonization, delivery, depression, environmental exposure biomarkers, epidemiologic surveys, fetal growth assessment, gestational age, gestational diabetes, mental health, phenotypes, PhenX, PhenX Toolkit, pregnancy, prenatal care, standard measures
Introduction
The principal goals of the practice of medicine are to promote health: forestall disease, offer a cure when feasible, and ease suffering when a cure is not an option.1 Historically, attempts to reach these goals have included unproven treatments based on traditional mores, convention, or sometimes solely relying on the practitioner's instinct.2 This changed dramatically with the introduction of the concept of evidence-based medicine (EBM), defined as the scrupulous and astute incorporation of the best available evidence into clinical decision-making for individual patients.3 Clinicians practicing EBM rely on a combination of their expertise and the best available evidence;4 for the process to be effective, this evidence must be valid, clear, and consistent.
The first step in achieving this aim is to recognize the existence of heterogeneity inherent in individual studies and the limits that this imposes on between-study comparisons and meta-analysis of data;5 these limits are further compounded by inexact or absent definitions of measures and outcomes, which are inherent in many reports of original research.6 Curtailing such barriers at the origin of a study is apt to be the most successful strategy leveraged to enable harmonization of research endeavors. The creation of an easily accessible, common set of measures and outcomes for use by researchers worldwide provides an opportunity to do so.
This is the intention of an effort known as the consensus measures for Phenotypes and eXposures (PhenX) project,7 funded as a genomic resource by the National Institutes of Health (NIH) and led by RTI International. PhenX is driven by the scientific community, with overall guidance provided by the PhenX Steering Committee (SC). Measures for the Toolkit are selected using an established consensus process.8 Launched in 2007, the goal of PhenX is to deliver high-quality, low-burden, well-established measures to facilitate cross-study analysis of genome-wide association studies, where even optimally designed individual investigations often lack sufficient sample sizes to address statistical power stipulations.9 The original scope of the PhenX project included 20 research domains, with a domain defined as an area of investigation with common features and clearly itemized measures.7 Some of the original domains included demographics, anthropometrics, complex medical conditions (e.g., diabetes, cancer), body systems (e.g., ocular, respiratory), and exposures (e.g., nutrition and dietary supplements, physical activity and physical fitness).7 For each domain, a Working Group (WG) of from six to nine experts, chosen to balance domain expertise with proficiency in epidemiology, biostatistics, and genomics research, was convened and tasked with selecting (via consensus) 15 measures for inclusion in the PhenX Toolkit.7 Available at no cost, the PhenX Toolkit (https://www.phenxtoolkit.org) is a web-based catalog that provides ready access to the standard measures of each domain, along with protocols for their use. In 2013, the scope of the PhenX project was expanded to embrace rare genetic conditions and a variety of study designs, as well as four new domains, and the April 2017 version 21.0 of the PhenX Toolkit contains 523 measures in 24 domains;10 one of these domains is pregnancy.
Pregnancy is a critical time during which exposures have the potential to influence the fetal phenotype significantly, as demonstrated through evolving areas of study, such as the exposome (the sum of all environmental exposures from conception through the lifespan),11 and investigation into the developmental origins of health and disease (the hypothesis that in utero exposures at critical times induce certain changes in the fetus, some of which are at the epigenetic level, for instance, in preparation for the ex utero environment).12 Data collected about lifestyle and biomarkers that represent environmental exposures are essential to fully understand the factors that influence maternal and fetal/neonatal health.13 Thus, the vital need for utilization of standard measures in studies focused on the perinatal period is readily apparent, and the importance of including pregnancy as one of the PhenX Toolkit domains easily follows, supporting the American Congress of Obstetricians and Gynecologists (ACOG) endorsement of standardization of practice to improve clinical care and health outcomes.14
Recognizing this critical need, the PhenX Pregnancy Working Group (PWG) was prioritized by the PhenX-SC. The aim of this report is to describe the consensus process and results of the PWG's deliberations and to introduce the Pregnancy domain measures included in the PhenX Toolkit.
Material and Methods
The PhenX SC, with input from the NIH PhenX liaisons, established the PWG, identifying individuals on the basis of their expertise, making a deliberate effort to include senior and junior investigators, clinician scientists, academic researchers, and at least one geneticist.8 The nine PWG members represented obstetrics, maternal-fetal medicine, pediatrics, reproductive genetics, perinatal and reproductive epidemiology, biostatistics, and toxicology. Also involved were personnel from RTI International and a representative from the PhenX-SC. The PWG roster is available on the PhenX website: https://www.phenx.org/Default.aspx?tabid=1066.
The PhenX SC developed the initial scope of the Pregnancy domain, elements of which were refined by the PWG via a review of the PhenX Toolkit to ascertain currently existing measures, thereby allowing for cross-linkage with the new measures and avoiding duplication. Following this review, the PWG selected 15 high-priority measures reflective of the domain, recommending an established and readily available protocol for each measure according to the selection criteria previously developed by the SC (Table 1). Consensus on preliminary measures was reached following deliberations in the form of a 1-day in-person meeting where proposals for measures and protocols were presented by the PWG members for discussion as well as subsequent conference calls, e-mail exchanges, and portal discussions. The PWG then sought input on these preliminary measures from the scientific research community, reviewed data gathered from the outreach, and selected the final measures for inclusion in the PhenX Toolkit (Figure 1). These measures were approved by the SC for release in the PhenX Toolkit. As part of the release, data collection worksheets and dictionaries were created, the measures were linked to “essential” and “related” measures of relevance (terms described below), and keywords were associated with the Smart Query Tool.
Table 1. PhenX Toolkit measure criteria.
| Standard criteria | Additional criteria |
|---|---|
|
|
Figure 1. Standard approach to the PhenX consensus process.
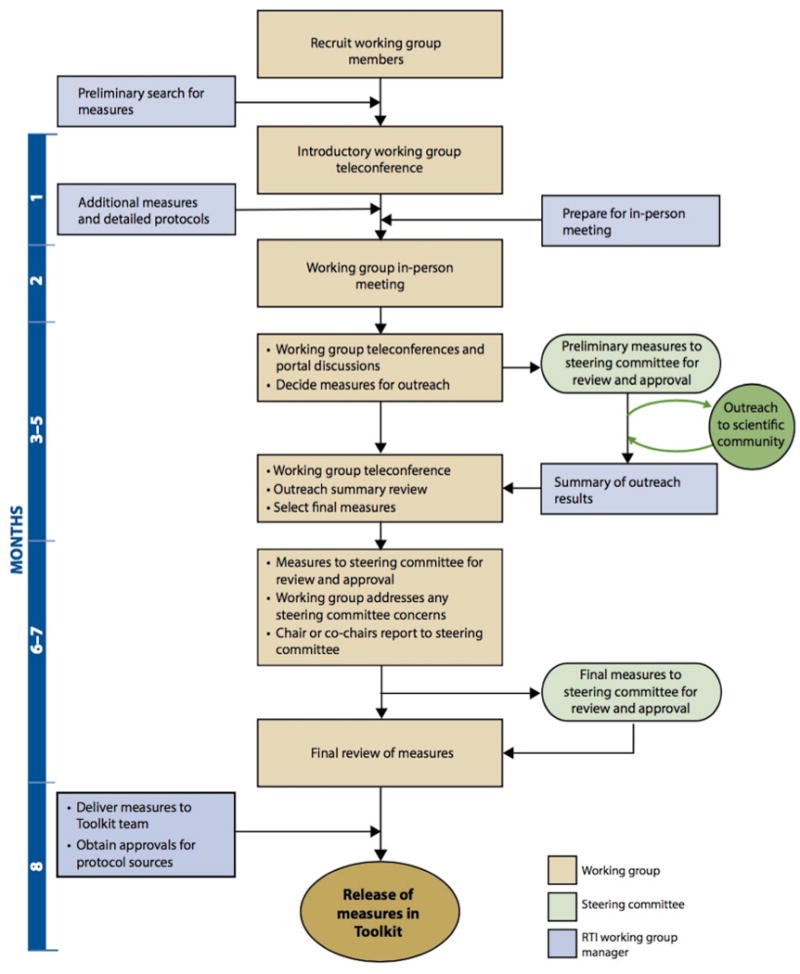
Originally published by Maiese et al.8 PhenX—establishing a consensus process to select common measures for collaborative research. 2013. RTI Press and Dr. Maiese have granted permission to the authors to publish this figure.
An essential measure is defined as one that is critical or integral to the collection of the chosen measure; without it, data would be incomplete or interpretation of results compromised.7 A related measure is defined as a suggested, additional PhenX measure, which may be of value to allow a researcher to use the chosen measure to its full capacity. The related measure may, for instance, permit calculation of a derived variable (e.g., height and weight for the calculation of Body Mass Index), or it may be conceptually linked to the chosen measure (e.g., Annual Family Income and Current Educational Attainment).
Results
The Pregnancy domain is unique, yet it is closely related to specific elements already addressed by existing PhenX domains, especially the Reproductive Health domain. To refine the initial scope of the Pregnancy domain, a review of the PhenX Toolkit identified 44 existing measures that are deemed relevant and applicable to research focusing on the perinatal period. These are listed in Table 2, and detailed protocols can be found at https://www.phenxtoolkit.org/index.php?pageLink=browse.relevantmeasures&id=3045.
Table 2. Additional relevant measures existing in the PhenX Toolkit and applicable to the pregnancy domain.
| Existing measure | Domain | |
|---|---|---|
| Birthplace | Subject | Demographics |
| Parents | ||
| Grandparents | ||
| Cigarette Smoking | Age of first use | Alcohol, Tobacco and Other Substances |
| Age of cessation | ||
| Smoking status | ||
| Dependence on cigarettes/nicotine | ||
| Pregnancy status and tobacco use | Tobacco Regulatory Research | |
| Environmental tobacco smoke exposure | Environmental Exposures | |
| Tobacco[non-cigarette] | Product type | Oral Health |
| Use | Tobacco Regulatory Research: Vector | |
| 30-day quantity and frequency | Alcohol, Tobacco and Other Substances | |
| Alcohol | Age of first use | Alcohol, Tobacco and Other Substances |
| 30-day quantity and frequency | ||
| Maximum drinks in 24 hours | ||
| Lifetime use | ||
| Lifetime abuse and dependence | ||
| Substances | Age of first use | Alcohol, Tobacco and Other Substances |
| 30-day frequency | ||
| Lifetime use | ||
| Lifetime abuse and dependence | ||
| Patterns of use | Substance Abuse and Addiction | |
| Screening and severity of substance use problems | Assessment of Substance Use and Substance Use Disorders | |
| Reproductive Health | Ovulation history | Cancer |
| History of pre-pubertal development | Reproductive Health | |
| Assessment of pubertal development | ||
| Human papilloma virus vaccine use | ||
| Contraceptive methods | ||
| Sexual history | ||
| Menstrual history | ||
| Reproductive history | ||
| Female reproductive organ surgical procedures | ||
| Testes development | ||
| Male sexual function | ||
| Prostate health | ||
| Difficulty conceiving | ||
| Causes and treatments of known infertility | ||
| Hormonal therapy | ||
| Male reproductive tract birth defects, | ||
| Pregnancy | Pregnancy weight gain | Anthropometrics |
| Serial pregnancy weight gain | ||
| Mother and baby health | Tobacco Regulatory Research | |
| High blood pressure during pregnancy | Cardiovascular | |
| Medical History | Personal history of type I and type II Diabetes | Diabetes |
Following this review, the process of selecting the new measures unfolded over 7 months of deliberations, with an in-person meeting taking place on May 23, 2016, in Bethesda, MD. This meeting culminated in the PWG's recommendation to add 15 new measures to the PhenX Toolkit, reflecting the Pregnancy domain that has yet to be explored by other WGs (Table 3). These were then connected with pertinent essential and related measures (Table 4, providing easy access to measures that relate to one another, thereby allowing researchers to contemplate the inclusion of other measures they may not have otherwise considered.
Table 3. PhenX measures and protocols in the pregnancy domain.
| Measure name* | Protocol sources | Protocol description and rationale | |
|---|---|---|---|
| Adequacy of Prenatal Care | Kotelchuck Index, also called the Adequacy of Prenatal Care Utilization (APNCU) | Adequacy of prenatal care is assessed by the month of the initial visit in the pregnancy and total number of prenatal visits until delivery, with that number being calculated as a percentage of recommended visits. Compared to other utilization of care indexes, the Kotelchuck Index is preferred, as it includes a category for women who receive more than the recommended amount of care (adequate plus, or intensive utilization). | |
| Concentrations of Flame Retardants | NCS, Biospecimen Adult Blood Procedures: Standard Operating Procedures NHANES, CDC Laboratory Procedure Manual, PBDEs | Blood samples are collected and analyzed to determine the concentrations of several PBDEs, which are chemicals used as flame retardants. High concentrations of PBDEs in the blood of a pregnant woman or young child may lead to neurobehavioral problems. The NCS was planned as the largest pregnancy cohort studies in the United States, and the NHANES is a major cross-sectional study in the United States. The collection methods used in both been validated previously. | |
| Concentrations of PCBs and Persistent Pesticides | NCS, Biospecimen Adult Blood Procedures: Standard Operating Procedures NHANES, CDC Laboratory Procedure Manual, PCBs and Persistent Pesticides | Blood samples are collected and analyzed to determine the concentrations of several PCBs and persistent pesticides. Prenatal exposure PCBs may lead to poor cognitive function and attention deficit/hyperactivity disorder (ADHD). Prenatal exposures to persistent pesticides (e.g., DDT) may have adverse neurodevelopmental effects on children. The NCS was planned as the largest pregnancy cohort studies in the United States, and the NHANES is a major cross-sectional study in the United States. The collection methods used in both been validated previously. | |
| Concentrations of Phenols and Parabens | NCS Biospecimen Adult Urine Procedures: Standard Operating Procedures NHANES Laboratory Procedure Manual, Bisphenol A, Other Environmental Phenols, and Parabens in Urine | Urine samples are collected and analyzed to determine the concentrations of several phenolic compounds such as bisphenol A (BPA) used to make plastic water bottles, baby bottles, and children's toys. This bioassay also permits analysis of parabens, which are chemicals used as preservatives in foods and beverages, cosmetics, and pharmaceuticals. Some phenols and parabens are endocrine disruptors and some have been associated with adverse health effects. The NCS was planned as the largest pregnancy cohort studies in the United States, and the NHANES is a major cross-sectional study in the United States. The collection methods used in both been validated previously. | |
| Concentration of Trace Metals | NCS Biospecimen Adult Blood Procedures: Standard Operating Procedures NHANES, CDC Laboratory Procedure Manual, Cadmium, Lead, Manganese, Mercury, and Selenium | Blood is collected and analyzed to determine
the concentrations of trace metals, which may include cadmium, lead,
manganese, selenium, and mercury. High concentrations of metals in the
blood may cause neurodevelopmental problems, particularly in a
developing fetus or in young children. The NCS was planned as one the largest pregnancy cohort studies in the United States, and the NHANES is a major cross-sectional study in the United States. The collection methods used in both been validated previously. |
|
| Current Pregnancy Status | (Self-report) NHANES Reproductive Health Module (Assay) NCS, Biospecimen Adult Urine Procedures: Standard Operating Procedures NHANES Mobile Examination Center Laboratory Procedures Manual | This measure is used to determine whether a woman is currently pregnant. It may be needed to determine suitability for participation in a research study and because pregnancy may influence the results of several physical and health measures such as weight and blood pressure. Depending on specific needs and implications, researchers may accept self-report of pregnancy or require a biological sample (urine or blood) for confirmation. The NHANES question on current pregnancy status was chosen for self-report. However, it was acknowledged that a biological assay is the most accurate pregnancy test and should be used for confirmation if pregnancy determination is critical to the study (i.e., prior to certain investigations such computed tomography, or with the use of pharmaceutical agents). The NCS was planned as the largest pregnancy cohort studies in the United States, and the NHANES is a major cross-sectional study in the United States. The collection methods used in both been validated previously. | |
| Difficulties in Pregnancy | nuMoM2b47 - Difficulties in Pregnancy Visit 1, Form V1E | A measure in the form of a single-item (with 13 subparts) used to capture worries, concerns, and difficulties a woman has experienced related to her pregnancy. Likert-style, self-report questionnaire, administered at 6–13 weeks, and again at 22–29 weeks. The nuMoM2b is a major prospective cohort study collecting data throughout pregnancy on approximately 10,000 nulliparous women. The data collection instruments were developed by the nuMoM2b investigators in collaboration with Project Scientists from the NICHD. | |
| Family History of Pregnancy Complications | nuMoM2b Difficulties in Pregnancy, Maternal Interview Visit 2 | Questions to assess a woman's family history of pregnancy complications. Of relevance, as researchers should be knowledgeable about a woman's family history of pregnancy complications, given some complications may be passed from one generation to the next. The nuMoM2b is a major prospective cohort study collecting data throughout pregnancy on approximately 10,000 nulliparous women. The data collection instruments were developed by the nuMoM2b investigators in collaboration with project scientists from NICHD. | |
| Fetal Growth Assessment | Fetal Growth Standards based on NICHD Fetal Growth Studies | This measure includes abstraction of fetal growth and ultrasound information from a medical record. Fetal growth is a gestational age–dependent measure of fetal size, in relation to a defined standard growth curve. Fetal growth at both extremes of pathology (SGA and LGA) affects fetal and neonatal outcomes, and has been linked with a variety of co-morbidities encountered in later life. The Fetal Growth Standards based on NICHD Fetal Growth Studies offer robust methodology and account for ethnic differences in fetal growth. The protocol is accessible and easy to use. The formula chosen to calculate the EFW, which was then used to develop the fetal growth centiles, is well-known and broadly used. Researchers searching for a protocol for fetal growth assessment can apply this formula with ease to calculate the EFW centile for their study, using ultrasound-derived biometry measures, which are then plotted on the available NICHD growth curves. | |
| Gestational Age (GA) | (Maternal Interview) ELGAN Study Maternal Interview (Medical Record Abstraction) GA | For maternal interview, the protocol originates from the well-established ELGAN Project, a large-scale, multi-center, epidemiologic study. Maternal Record Abstraction is the preferred option for establishing GA, with maternal interview provided as an alternate when medical record abstraction is untenable. GA is established using criteria for determination of EDD adapted from ACOG, AIUM, SMFM, and SOGC. Determination of EDD is based on review and correlation of menstrual dating with ultrasound parameters in natural conception and on ART-derived EDD when ART is used. | |
| Gestational Diabetes | nuMoM2b: CLA Prenatal Labs | Abstraction of prenatal laboratory data to
determine the presence of gestational diabetes. Pregnant women are
commonly screened for gestational diabetes because untreated or poorly
controlled gestational diabetes can cause negative health outcomes for
the mother and baby, including macrosomia, hypoglycemia, and increased
risk for cesarean section delivery. The nuMoM2b is a major prospective cohort study collecting data throughout pregnancy on approximately 10,000 nulliparous women. The data collection instruments were developed by the nuMoM2b investigators in collaboration with the NICHD. |
|
| Health and Wellness in Pregnancy | Early Life Exposure Assessment Tool (ELEAT) | The measure is used to determine a woman's general physical and mental health in the time period surrounding her pregnancy. The ELEAT tool has been used in previous studies. | |
| Mode of Conception | (Maternal Interview) PRAMS Phase 7 Core Questions and Standard Questions (Medical Record Abstraction) ELGAN Chart Abstraction Form | Questions assessing whether medical intervention of any kind was needed to achieve pregnancy. Information about natural or assisted conception (i.e., infertility treatments or reproductive technologies) is essential to determine fertility status. Questions for the maternal interview protocol have been derived from PRAMS, a major ongoing survey. Questions for the medical record abstraction protocol have been derived from the ELGAN project, a large, multicenter epidemiologic study. | |
| Mode of Delivery | (Maternal Interview) PRAMS Phase 7 Standard Questions (Medical Record Abstraction) A Randomized Trial of Induction Versus Expectant Management (ARRIVE) | Information about the initiating event of a woman's delivery, the mode of delivery, and if it was an assisted delivery. The mode of delivery may influence the health of both the mother and the neonate. Questions for the maternal interview protocol have been derived from PRAMS, a major ongoing survey. | |
| Prenatal and Postpartum Depression | Edinburgh Postnatal Depression Scale© (EPDS) | This measure is a questionnaire that can be
used to screen for recent symptoms of depression, including perinatal
and postnatal depression. There are 10 questions to assess a
mother's postnatal depression in the previous 7 days. A depression screening tool helps health care providers assess women for symptoms of depression before and after their pregnancy. The EPDS is one of the most widely-used, validated self-report instruments to screen for depression during and after pregnancy. |
|
| Supplemental information | |||
| Placental Assessment | Amsterdam Placental Workshop Group Consensus Statement & College of American Pathologists Practice Guideline for Examination of the Placenta | A protocol for the histopathologic examination of gross and microscopic placental features. The protocol provides explicit instructions on specimen collection and sampling of placental tissues and guidance on definitions, diagnostic criteria, and classification of placental lesions to assist in international comparability of research studies and continued evolution of knowledge regarding the significance of placental lesions associated with adverse pregnancy and later health outcomes. | |
| International Fetal Growth Standards | Stirnemann J, et al. (2016). International Estimated Fetal Weight Standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol. doi: 10.1002/uog.17347 Hadlock FP, et al. (1985). Estimation of fetal weight using head, body and femur measurements - a prospective study. Am J Obstet Gynecol, 151:333-337. | This measure includes abstraction of fetal growth and ultrasound information from a medical record. | |
All measures and protocols are available at: https://www.phenxtoolkit.org/index.php?pageLink=browse.measures&id=240000 ACOG – American Congress of Obstetricians and Gynecologists; AIUM – American Institute of Ultrasound in Medicine; ART – Assisted Reproductive Technology; BPA – bisphenol A; CDC – Centers for Disease Control; EDD – Estimated Date of Delivery; EFW – Estimated Fetal Weight; ELEAT – Early Life Exposure Assessment Tool; ELGAN – Extremely Low Gestational Age Newborns; GA – Gestational Age; LGA – Large for Gestational Age; NCS – National Children's Study; NHANES – National Health and Nutrition Examination Survey; NICHD – Eunice Kennedy Shriver National Institute of Child Health and Human Development; nuMoM2b – Nulliparous Pregnancy Outcomes Study Monitoring Mothers-to-Be; PBDEs – Polybrominated Diphenyl Ethers; PCBs – polychlorinated biphenyls; PRAMS – Pregnancy Risk Assessment Monitoring System; SGA – Small for Gestational Age; SMFM – Society for Maternal-Fetal Medicine; SOGC – Society of Obstetricians and Gynecologists of Canada
Table 4. Essential and related measures in the PhenX Toolkit, which are relevant to the use of the new Pregnancy domain measures.
| Essential measures | Related measures | |||
|---|---|---|---|---|
| Pregnancy domain measure | Measure | Domain | Measure | Domain |
| Adequacy of Prenatal Care | Gestational Age | Pregnancy | Current Age | Demographics |
| Current Pregnancy Status | Pregnancy | |||
| Reproductive History | Reproductive Health | |||
| Concentrations of Flame Retardants | Current Age | Demographics | CBC | Rare Genetic Conditions |
| Current Pregnancy Status | Pregnancy | |||
| Gestational Age | Pregnancy | |||
| Concentrations of Phenols and Parabens | Current Age | Demographics | Home and Workplace Exposures to Floor and Wall Materials | Environmental Exposures |
| Current Pregnancy Status | Pregnancy | Personal Care Products | Environmental Exposures | |
| Gestational Age | Pregnancy | |||
| Concentrations of PCBs and Persistent Pesticides | Current Age | Demographics | CBC | Rare Genetic Conditions |
| Current Pregnancy Status | Pregnancy | |||
| Gestational Age | Pregnancy | |||
| Concentrations of Trace Metals | Current Age | Demographics | CBC | Rare Genetic Conditions |
| Current Pregnancy Status | Pregnancy | Selenium | Nutritional and Dietary Supplements | |
| Gestational Age | Pregnancy | |||
| Current Pregnancy Status | Current Age | Demographics | Reproductive History | Reproductive Health |
| Gender | Demographics | |||
| Difficulties in Pregnancy | Gender | Demographics | Prenatal and Postpartum Depression | Pregnancy |
| Reproductive History | Reproductive Health | |||
| Family History of Pregnancy Complications | Current Age | Demographics | Causes and Treatments of Known Infertility | Reproductive Health |
| Gender | Demographics | Contraceptive Methods | Reproductive Health | |
| Difficulty in Conceiving | Reproductive Health | |||
| Family Health History | Rare Genetic Conditions | |||
| Female Reproductive Organ Surgical Procedure | Reproductive Health | |||
| Reproductive History | Reproductive Health | |||
| Gestational Age | Current Age | Demographics | Current Age | Demographics |
| Pregnancy Status—Mother and Baby Health | Tobacco Regulatory Research | |||
| Reproductive History | Reproductive Health | |||
| Serial Pregnancy Weight Gain | Anthropometrics | |||
| Total Pregnancy Weight Gain | Anthropometrics | |||
| Birth Weight | Anthropometrics | |||
| Fetal Growth | Gestational Age | Demographics | Birth Weight | Anthropometrics |
| Height | Anthropometrics | Pregnancy Status—Mother and Baby Health | Tobacco Regulatory Research | |
| Serial Pregnancy Weight Gain | Anthropometrics | Current Age | Demographics | |
| Race | Demographics | Total Pregnancy Weight Gain | Anthropometrics | |
| Ethnicity | Demographics | |||
| Reproductive History | Reproductive Health | |||
| Gestational Diabetes | Current Pregnancy Status | Pregnancy | Family History of Diabetes | Diabetes |
| Gender | Demographics | Fasting Plasma Glucose | Diabetes | |
| Fasting Serum Insulin | Diabetes | |||
| Oral Glucose Tolerance Test | Diabetes | |||
| Personal History of Type I and Type II Diabetes | Diabetes | |||
| Reproductive History | Reproductive Health | |||
| Serial Pregnancy Weight Gain | Anthropometrics | |||
| Total Pregnancy Weight Gain | Anthropometrics | |||
| Health and Wellness Before, During, and After Pregnancy | Gender | Demographics | Body Mass Index | Anthropometrics/Substance Abuse and Addiction Collection |
| Cigarette Smoking Status | Alcohol, Tobacco, and Other Substances | |||
| Current Age | Demographics | |||
| Current Quality of Life | Psychosocial | |||
| Depression | Psychiatric | |||
| Ethnicity | Demographics | |||
| Gestational Diabetes | Pregnancy | |||
| Height | Anthropometrics | |||
| Weight | Anthropometrics | |||
| Reproductive History | Reproductive Health | |||
| Mode of Conception | Gender | Demographics | Causes and Treatments of Known Infertility | Reproductive Health |
| Contraceptive Methods | Reproductive Health | |||
| Difficulty in Conceiving | Reproductive Health | |||
| Reproductive History | Reproductive Health | |||
| Mode of Delivery | Gender | Demographics | Reproductive History | Reproductive Health |
| Prenatal and Postpartum Depression | Current Age | Demographics | Aggression and Hostility | Mental Health Research: Suicide Specialty Collection |
| Current Marital Status | Demographics | Classification of Suicidal Ideation and Suicidal Behaviour | Mental Health Research: Suicide Specialty Collection | |
| Ethnicity | Demographics | Depression | Psychiatric | |
| Gender | Demographics | Hopelessness | Mental Health Research: Suicide Specialty Collection | |
| Race | Demographics | Impairment | Mental Health Research Core: Tier 1 | |
| Insomnia | Mental Health Research: Suicide Specialty Collection | |||
| Life Events | Social Environments | |||
| Reproductive History | Reproductive Health | |||
CBC – complete blood count; PCB – polychlorinated biphenyl
To highlight the structure of the Toolkit, three of the included Pregnancy measures are characterized in more detail and several other measures that were considered are discussed.
Select PhenX Measures
Gestational Age
Gestational age is an essential component of research on pregnancy outcomes. It is often used for stratification purposes, influences interpretation of many related measures, and is inversely associated with many neonatal complications. Preterm birth closely correlates with many adverse neonatal outcomes and its estimates are higher when best obstetric estimates of gestational age (as outlined below) are used.15 The PWG recommends that gestational age be established using criteria for determination of estimated due date (EDD) adapted from ACOG,16 the American Institute of Ultrasound in Medicine,16 the Society for Maternal-Fetal Medicine,16 and the Society of Obstetricians and Gynaecologists of Canada.17 With natural conception, these criteria are based on correlation of menstrual dating and ultrasound parameters, whereas with assisted reproductive technology (ART), an ART-derived EDD is used. If gestational age cannot be established based on medical record abstraction, a maternal interview can be used as an alternative, although this is the less preferred approach. The protocol chosen to address gestational age via maternal interview originates from the well-established Extremely Low Gestational Age Newborns (ELGAN) Project,18,19 a large-scale, multicenter, epidemiologic study. Finally, the PWG advocated consideration of the following definitions developed by a work group organized by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)20 for infants born at particular gestational age time frames: early preterm (<34 weeks, 0 days), late preterm (34 weeks, 0 days to 36 weeks, 6 days), early term (37 weeks, 0 days to 38 weeks, 6 days), full term (39 weeks, 0 days to 40 weeks, 6 days), late term (41 weeks, 0 days to 41 weeks, 6 days), and postterm (≥42 weeks, 0 days).
Fetal Growth Assessment
Fetal growth is a gestational age-dependent measure of fetal size in relation to a defined standard growth curve. Fetal growth at both extremes of pathology (small and large for dates) can negatively impact fetal and neonatal outcomes and has been linked to various comorbidities in later life.21,22For the measure to be accurate, precise determination of gestational age is required. The fetal growth assessment is a three-step process: (i) ascertainment of estimated fetal weight (EFW); (ii) verification of gestational age at which the EFW was recorded; and (iii) determination of the weight's centile by plotting the EFW at the appropriate gestational age on a predefined fetal growth curve. The National Standards for Fetal Growth (NSFG), a NICHD-funded multicenter project, is based on a robust methodology that accounts for racial and ethnic differences in fetal growth in US populations. The PhenX work group proposed the NSFG as the preferred protocol.23The protocol is accessible and easy to use, and it does not require major equipment or specialized training. A second protocol, the International Fetal and Newborn Consortium for the 21st Century (INTERGROWTH-21st) fetal growth standards was also considered,24 partly because the standards were developed utilizing methods of the World Health Organization Multicenter Growth Reference Study to complete the Fetal Growth and Longitudinal Study. The PWG recommended these standards as Supplemental Information because debate still exists as to whether pooling of data across the eight geographically diverse study sites was the optimal approach and also because the EFW used to calculate the fetal growth percentiles has not yet been tested outside of the original study.25,26
Prenatal and Postpartum Depression
One in eight new mothers suffer major postpartum depression, and a large majority experience the “baby blues”.27 The Edinburgh Postnatal Depression Scale© (EPDS) was developed to screen for depression and has been validated in pregnancy.28 The 10-question self-reported instrument screens for depression in the previous 7 days, with a score above 9 identifying candidates who would benefit from a complete clinical assessment.27 It is one of the most widely used, validated instruments available, has been translated into numerous languages, and has been used with diverse cultural groups, providing an excellent objective screening measure for studies on the topic.29-32 Feasibility of the use of EPDS as an initial universal screen for depression in the antenatal and postnatal period to identify those who may benefit from further diagnostic evaluation has also been demonstrated.33
Other Measures Considered
When the PhenX SC developed the original scope for this domain, very broad coverage of pregnancy-related research was proposed. In its initial deliberation, the PWG determined that some components of the scope were sufficiently covered elsewhere in the Toolkit, such as dietary intake, nutrients and supplements, and medication use. Other components failed to meet selection criteria, such as inflammatory markers associated with preterm birth (absence of a well-established protocol), biobank samples (no phenotype correlation), preeclampsia (available protocols too complicated and long for widespread use in research that may have a different overall focus), and social media (absence of an established protocol).
One further measure, which was deliberated upon for consideration for inclusion, was “placental assessment”. The placenta provides significant insight into environmental exposures over the course of the gestation, and offers indispensable information about pregnancy outcome, as emphasized by the current NIH Human Placenta Project.34-37 While considered an asset to the domain, the placental assessment protocol lacked the prerequisite that measures in the PhenX Toolkit must be characterized by an actual phenotype and was added to the Supplemental Information section of the Pregnancy domain as an additional resource for users.
Comment
The PhenX PWG was successful in adding 15 new measures uniquely suited to address outcomes pertinent to the state of pregnancy, which complement previously established measures in the PhenX Toolkit. These well-established, low-burden, high-quality measures offer a valuable resource to investigators and clinicians for whom pregnancy is a primary research focus but are perhaps even more indispensable for those who hold expertise in other areas and wish to include pregnancy-related measures in their studies. With this in mind, the PhenX Toolkit provides not only detailed protocols for collecting the data but also all the information necessary to implement the measure properly.
The work of the PWG expands on the earlier efforts of the Reproductive Health WG. The Reproductive Health domain was recently reviewed by an Expert Review Panel (ERP). New measures and updated protocols for this domain were added in version 21.0 of the Toolkit in April 2017. To ensure that the PhenX Toolkit remains scientifically relevant, all domains are systematically reexamined by ERPs on a rotating basis.
Relevant pregnancy-related measures will also be included in two Pregnancy and Birth Collections:38 (1) the Pregnancy and Birth Health Conditions Collection (http://phenxtoolkitstage.rti.org/index.php?pageLink=browse.conceptualgroups&id=3385&bread crumbs=3374,3385), and (2) the Pregnancy and Birth Adverse Pregnancy Outcome Risk Factors Collection(http://phenxtoolkitstage.rti.org/index.php?pageLink=browse.conceptualgroups&id=3293&bread crumbs=3293). Collections in the Toolkit do not provide new measures; instead, they offer an alternate path for accessing the measures of interest, characterized by grouping measures linked by a particular theme.
Several other features within the Toolkit also contribute to a convenient user experience. Measures can be browsed by domain, by collection (e.g., risk factors or life stages),7 or by a specific measure whereby interrelated measures will be highlighted. The search utility supports keyword and full-text searches to locate relevant measures that can be added to “My Toolkit,” which can be individually set up and saved for download. Each measure is complemented by a downloadable data collection worksheet and data dictionary options. Users who register have the added option of saving their work, sharing their Toolkit with their collaborators, and receiving Toolkit updates. Research Electronic Data Capture (REDCap) data dictionary zip files are also now available for all PhenX protocols through the Shared Library website (https://redcap.vanderbilt.edu/consortium/library/search.php), enabling easy inclusion of PhenX measures in clinical and translational research projects that use REDCap.39
Beyond delivering an effortless platform for access and use of common outcome measures, the PhenX Toolkit encourages the use of standard protocols across research studies and establishes common data elements. In addition to the advantages of standard measures as they apply to genome-wide association studies,7 these benefits also readily extend to biomedical and epidemiologic investigations. Advantages of standard protocols for collecting data include the ability to combine data (i) from prevailing phenotypic and environmental exposure measures, which are historically collected in individual studies using disparate methods; (ii) from studies on diverse populations, allowing for validation of original findings; (iii) from similar studies to increase statistical power, enabling confirmation of weak associations and exploration of rare diseases.7
Despite the numerous benefits of the use of standard measures and protocols, the propensity for each researcher to incorporate a set of uniquely defined variables into their study remains,40 limiting the ability to meta-analyze data across studies. The PhenX Toolkit stands well poised to champion progress in this regard, and there is evidence that PhenX measures are increasingly recognized across the scientific community. PhenX has been recommended by several NIH institutes in 205 Funding Opportunity Announcements as of March 2017.41 Furthermore, concerted efforts are underway to align PhenX with other standardization platforms. PhenX collaborates with the National Library of Medicine as well as the database of Genotypes and Phenotypes (dbGaP; https://www.ncbi.nlm.nih.gov/gap), and the data elements are posted in the NIH Common Data Elements Repository (https://cde.nlm.nih.gov/home). This approach enables ready identification of investigations using common variables, which will allow for cross-study analysis.40
Expanding on the virtues of data harmonization, Fortier et al. describe two approaches: stringent, and flexible.9 The stringent approach calls for collection of data across studies with identical tools and protocols. Its advantages lie in ease of data synthesis, and its disadvantages include (i) the challenge of imposing rigid measures and protocols across studies that are thematically, methodologically, and geographically diverse, and have variable resources and funding; and (ii) restriction of data synthesis solely to studies using the prescribed measures, potentially limiting generalizability and risking bias. Conversely, the flexible approach calls for collection of data across studies with distinct tools and protocols. Its advantages include the capacity for synthesis of existing data and the ability to synthesize data across a wide breadth of studies. Its disadvantages include the challenge of safeguarding sufficient compatibility across studies to allow for valid comparisons, and the challenge of synthesis across a sufficiently large number of investigations to allow for valid conclusions.
Reminiscent of the stringent approach, the PhenX Toolkit utilizes standardized protocols to ensure collected data are directly comparable, which facilitates cross-study analysis.7 At the same time, PhenX reflects a flexible approach by providing investigators with protocols for specific measures particular to different areas of interest (e.g., demographics, physical activity and physical fitness), different patient populations (e.g., age, gender), and different modes of administration (e.g., interview, medical record abstraction, bioassay) to be used, as needed, in addressing specific research questions in a uniform, easily replicable manner.42 The PhenX Toolkit also supports flexibility by providing custom data dictionaries that are compatible with REDCap and dbGaP.39,40
Another data harmonization effort, the Core Outcome Measures in Effectiveness Trials (COMET) Initiative is an example of the flexible approach. The COMET Initiative catalogs Core Outcome Sets (COS) that were developed using prescribed methodology and pertaining to trials investigating specific clinical conditions.43-46 It has been endorsed by the CoRe Outcomes in WomeN's health (CROWN) Initiative, which includes editors from more than 50 journals pertaining to women's health,43 and is encouraged by funding bodies in the United Kingdom,44 where it originated. COS represents a standardized set of outcomes for a particular health condition, forming the minimum expectation for trial reporting,44 with additional outcome measures of relevance to the specific study hypothesis to be included at the investigator's discretion.
The stringent and flexible approaches are both valuable in striving for harmonization,9 and thus the PhenX Toolkit and the COMET Initiative should be viewed as highly complementary entities, with investigators drawing on the strengths of each to achieve the goal of standardization so as to strengthen global research initiatives, with the ultimate goal of improving patient care.
The key strengths of the PhenX Toolkit measures, in general, and of the measures in the Pregnancy domain, in particular, lie in that they are well-established, easily and freely accessible, and chosen with the intent of minimizing the burden on study participants and personnel.7 The next challenge is to promote these measures' use among investigators worldwide so that the measures are adopted by a critical mass of investigators and that the aim of data synthesis across multiple studies can be realized.9
Some of the limitations encountered by the PWG were in part imposed by the stipulations of PhenX, which in fact make the Toolkit robust. Specifically, selecting only 15 high-priority measures and also selecting only protocols meeting criteria for easy accessibility, free availability, and a low-burden nature precluded consideration of certain protocols (e.g. for a multigeneration family history of reproductive issues). Similarly, certain measures had to be abandoned because no suitable protocol could be identified (e.g. pre-eclampsia), decreasing the comprehensiveness of the Pregnancy domain.
Collaborative efforts aimed at standardizing data collection methodologies will improve the quality and consistency of study data. This improvement has the potential to accelerate research progress by allowing data synthesis across studies, contributing to enhanced statistical power and the ability to draw more meaningful conclusions. The creation of the Pregnancy domain with its 15 new measures is well poised to facilitate collaborative pregnancy research. To achieve this aim, investigators whose work includes the perinatal population are encouraged to utilize the PhenX Toolkit in the design and implementation of their studies. Only through concerted and well-executed research endeavors can we gain the requisite knowledge to advance pregnancy care and positively impact maternal and newborn health.
Acknowledgments
Role of Funding Source: RTI International's work on PhenX is funded by the National Human Genome Research Institute (cooperative agreement number U41 HG007050) with co-funding from the National Institute on Drug Abuse.
The authors would like to acknowledge the contributions of the PhenX liaisons from the National Institutes of Health.
Footnotes
Conflict of Interest/Disclosure Statement: The authors report no conflicts of interest.
Disclosures: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the U.S. Environmental Protection Agency and National Institutes of Health.
Disclaimer: This work represents the opinions of the authors and does not represent the official policy of the U.S. Environmental Protection Agency or the National Institutes of Health.
Paper Presentation Information: Presented at the 66th Annual Meeting of the American Society for Human Genetics Vancouver, Canada, October 16–22, 2016; the National Institute of Environmental Health Sciences, NIEHS Environmental Health Sciences Fest, Research Triangle Park, NC, December 5–8, 2016; and 56th Annual Meeting of the Society of Toxicology, Baltimore, MD, March 12–16, 2017.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Callahan D. Managed care and the goals of medicine. J Am Geriatr Soc. 1998;46:385–8. doi: 10.1111/j.1532-5415.1998.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 2.Whitley R, Rousseau C, Carpenter-Song E, Kirmayer LJ. Evidence-based medicine: opportunities and challenges in a diverse society. Can J Psychiatry. 2011;56:514–22. doi: 10.1177/070674371105600902. [DOI] [PubMed] [Google Scholar]
- 3.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–2. doi: 10.1136/bmj.312.7023.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sackett DL. Evidence-based medicine. Seminars in Perinatology. 1997;21:3–5. doi: 10.1016/s0146-0005(97)80013-4. [DOI] [PubMed] [Google Scholar]
- 5.Chess LE, Gagnier JJ. Applicable or non-applicable: investigations of clinical heterogeneity in systematic reviews. BMC Med Res Methodol. 2016;16:19. doi: 10.1186/s12874-016-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials. 2007;8:39. doi: 10.1186/1745-6215-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–60. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese DR, Hendershot T, Strader L, et al. RTI Methods Report series. Research Triangle Park, NC: RTI Press publication; 2013. PhenX - establishing a consensus process to select common measures for collaborative research. [PubMed] [Google Scholar]
- 9.Fortier I, Doiron D, Burton P, Raina P. Invited commentary: consolidating data harmonization--how to obtain quality and applicability? Am J Epidemiol. 2011;174:261–4. doi: 10.1093/aje/kwr194. author reply 65-6. [DOI] [PubMed] [Google Scholar]
- 10.PhenX toolkit. Version 21.0. Released April 11, 2017. Available at: http://www.phenxtoolkit.org/
- 11.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–50. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 12.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27:391–402. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buck Louis GM, Sundaram R. Exposome: time for transformative research. Stat Med. 2012;31:2569–75. doi: 10.1002/sim.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG Committee on Patient Safety and Quality Improvement. ACOG Committee Opinion No. 526: Standardization of practice to improve outcomes. Obstet Gynecol. 2012;119:1081–2. doi: 10.1097/AOG.0b013e31825649ad. [DOI] [PubMed] [Google Scholar]
- 15.Duryea EL, McIntire DD, Leveno KJ. The rate of preterm birth in the United States is affected by the method of gestational age assignment. Am J Obstet Gynecol. 2015;213:231 e1–5. doi: 10.1016/j.ajog.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obsetricians and Gynecologists, American Institute of Ultrasound in Medicine, Society for Maternal-Fetal Medicine. Committee opinion no 611: method for estimating due date. Obstet Gynecol. 2014;124:863–6. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- 17.Butt K, Lim K, Society of O, Gynaecologists of C Determination of gestational age by ultrasound. J Obstet Gynaecol Can. 2014;36:171–83. doi: 10.1016/S1701-2163(15)30664-2. [DOI] [PubMed] [Google Scholar]
- 18.O'Shea TM, Allred EN, Dammann O, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joseph RM, Korzeniewski SJ, Allred EN, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a largecohort study of 10-year-old children born at 23-27 weeks' gestation. Am J Obstet Gynecol. 2017;216:304 e1–04 e16. doi: 10.1016/j.ajog.2016.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309:2445–6. doi: 10.1001/jama.2013.6235. [DOI] [PubMed] [Google Scholar]
- 21.Chiavaroli V, Marcovecchio ML, de Giorgis T, Diesse L, Chiarelli F, Mohn A. Progression of cardio-metabolic risk factors in subjects born small and large for gestational age. PLoS One. 2014;9:e104278. doi: 10.1371/journal.pone.0104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wennerstrom EC, Simonsen J, Melbye M. Long-Term Survival of Individuals Born Small and Large for Gestational Age. PLoS One. 2015;10:e0138594. doi: 10.1371/journal.pone.0138594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449 e1–49 e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH-21st Project. Ultrasound Obstet Gynecol. 2017;49:478–86. doi: 10.1002/uog.17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanson M, Kiserud T, Visser GH, Brocklehurst P, Schneider EB. Optimal fetal growth: a misconception? Am J Obstet Gynecol. 2015;213:332 e1–4. doi: 10.1016/j.ajog.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Villar J, Papageorghiou AT, Pang R, et al. Monitoring human growth and development: a continuum from the womb to the classroom. Am J Obstet Gynecol. 2015;213:494–9. doi: 10.1016/j.ajog.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Wisner KL, Parry BL, Piontek CM. Clinical practice. Postpartum depression. N Engl J Med. 2002;347:194–9. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- 28.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 29.Husain N, Rahman A, Husain M, et al. Detecting depression in pregnancy: validation of EPDS in British Pakistani mothers. J Immigr Minor Health. 2014;16:1085–92. doi: 10.1007/s10903-014-9981-2. [DOI] [PubMed] [Google Scholar]
- 30.Eberhard-Gran M, Eskild A, Tambs K, Opjordsmoen S, Samuelsen SO. Review of validation studies of the Edinburgh Postnatal Depression Scale. Acta Psychiatr Scand. 2001;104:243–9. doi: 10.1034/j.1600-0447.2001.00187.x. [DOI] [PubMed] [Google Scholar]
- 31.Adouard F, Glangeaud-Freudenthal NM, Golse B. Validation of the Edinburgh postnatal depression scale (EPDS) in a sample of women with high-risk pregnancies in France. Arch Womens Ment Health. 2005;8:89–95. doi: 10.1007/s00737-005-0077-9. [DOI] [PubMed] [Google Scholar]
- 32.Adewuya AO, Ola BA, Dada AO, Fasoto OO. Validation of the Edinburgh Postnatal Depression Scale as a screening tool for depression in late pregnancy among Nigerian women. J Psychosom Obstet Gynaecol. 2006;27:267–72. doi: 10.1080/01674820600915478. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh KK, Nadel H, Blewett D, Freeman MP, Kaimal AJ, Riley LE. Implementation of universal screening for depression during pregnancy: feasibility and impact on obstetric care. Am J Obstet Gynecol. 2016;215:517 e1–8. doi: 10.1016/j.ajog.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Sadovsky Y, Clifton VL, Burton GJ. Invigorating placental research through the “Human Placenta Project”. Placenta. 2014;35:527. doi: 10.1016/j.placenta.2014.06.367. [DOI] [PubMed] [Google Scholar]
- 35.Guttmacher AE, Spong CY. The human placenta project: it's time for real time. Am J Obstet Gynecol. 2015;213:S3–5. doi: 10.1016/j.ajog.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 36.Nelson DM. How the placenta affects your life, from womb to tomb. Am J Obstet Gynecol. 2015;213:S12–3. doi: 10.1016/j.ajog.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. Am J Obstet Gynecol. 2015;213:S14–20. doi: 10.1016/j.ajog.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead NS, Hammond JA, Williams MA, et al. The PhenX Toolkit pregnancy and birth collections. Ann Epidemiol. 2012;22:753–8. doi: 10.1016/j.annepidem.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips M, Grant T, Giampietro P, et al. PhenX measures for phenotyping rare genetic conditions. Genet Med. 2017 doi: 10.1038/gim.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan H, Tryka KA, Vreeman DJ, et al. Using PhenX measures to identify opportunities for cross-study analysis. Hum Mutat. 2012;33:849–57. doi: 10.1002/humu.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.PhenX Toolkit Funding Opportunity Annoucements (FOAs) - 205. Updated March 16, 2017. Available at: https://www.phenxtoolkit.org/index.php?pageLink=news.funding.
- 42.Hendershot T, Pan H, Haines J, et al. Using the PhenX Toolkit to Add Standard Measures to a Study. Curr Protoc Hum Genet. 2015;86:1 21 1–17. doi: 10.1002/0471142905.hg0121s86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women's health. BJOG. 2014;121:1181–2. doi: 10.1111/1471-0528.12929. [DOI] [PubMed] [Google Scholar]
- 44.Clarke M, Williamson P. Core outcome sets and trial registries. Trials. 2015;16:216. doi: 10.1186/s13063-015-0738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khan KS, Romero R. Chief Editors of Journals participating in CI. The CROWN initiative: journal editors invite researchers to develop core outcomes in women's health. Am J Obstet Gynecol. 2014;211:575–6. doi: 10.1016/j.ajog.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Khan KS. The CROWN initiative: journal editors invite researchers to develop core outcomes in women's health. Paediatr Perinat Epidemiol. 2014;28:356–8. doi: 10.1111/ppe.12148. [DOI] [PubMed] [Google Scholar]
- 47.Haas DM, Parker CB, Wing DA, et al. A description of the methods of the Nulliparous Pregnancy Outcomes Study: monitoring mothers-to-be (nuMoM2b) Am J Obstet Gynecol. 2015;212:539 e1–39 e24. doi: 10.1016/j.ajog.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


