Abstract
Some human diseases, including obesity, Type II diabetes, and numerous cancers, are thought to be influenced by environments experienced in early life, including in utero. Maternal diet during the perinatal period may be especially important for adult offspring energy balance, potentially affecting both body composition and physical activity. This effect may be mediated by the genetic background of individuals, including, for example, potential “protective” mechanisms for individuals with inherently high levels of physical activity or high basal metabolic rates. To examine some of the genetic and environmental factors that influence adult activity levels, we used an ongoing selection experiment with 4 replicate lines of mice bred for high voluntary wheel running (HR) and 4 replicate, non-selected control lines (C). Dams (half HR and half C) were fed a “Western” diet (WD, high in fat and sucrose) or a standard diet (SD) from 2 weeks prior to mating until their pups could feed on solid food (14 days of age). We analyzed dam and litter characteristics from birth to weaning, and offspring mass and physical activity. One male offspring from each litter received additional metabolic and behavioral tests. Maternal WD caused pups to eat solid food significantly earlier for C litters, but not for HR litters (interaction of maternal environment and genotype). With dam mass as a covariate, mean pup mass was increased by maternal WD but litter size was unaffected. HR dams had larger litters and tended to have smaller pups than C dams. Home-cage activity of juvenile focal males was increased by maternal WD. Juvenile lean mass, fat mass, and fat percent were also increased by maternal WD, but food consumption (with body mass as a covariate) was unaffected (measured only for focal males). Behavior in an elevated plus maze, often used to indicate anxiety, was unaffected by maternal WD. Maximal aerobic capacity (VO2max) was also unaffected by maternal WD, but HR had higher VO2max than C mice. Adult lean, fat, and total body masses were significantly increased by maternal WD, with greater increase for fat than for lean mass. Overall, no aspect of adult wheel running (total distance, duration, average running speed, maximum speed) or home-cage activity was statistically affected by maternal WD. However, analysis of the 8 individual lines revealed that maternal WD significantly increased wheel running in one of the 4 HR lines. On average, all groups lost fat mass after 6 days of voluntary wheel running, but the absolute amount lost was greater for mice with maternal WD resulting in no effect of maternal WD on absolute or % body fat after wheel access. All groups gained lean and total body mass during wheel access, regardless of maternal WD or linetype. Measured after wheel access, circulating leptin, adiponectin, and corticosterone concentrations were unaffected by maternal WD and did not differ between HR and C mice. With body mass as a covariate, heart ventricle mass was increased by maternal WD in both HR and C mice, but fat pads, liver, spleen, and brain masses were unaffected. As found previously, HR mice had larger brains than C mice. Body mass of grand-offspring was unaffected by grand-maternal WD, but grand-offspring wheel running was significantly increased for one HR line and decreased for another HR line by grand-maternal WD. In summary, maternal Western diet had long-lasting and general effects on offspring adult morphology, but effects on adult behavior were limited and contingent on sex and genetic background.
Keywords: Exercise, Genotype-by-environment interaction, Maternal diet, Selection experiment, Spontaneous Physical Activity, Wheel running
1. Introduction
The obesity epidemic has been expanding at an alarming rate. Poor diet and lack of physical activity are generally viewed as key components contributing to the increase, but recent research highlights the importance of several other factors, including environmental experiences in early life [1]. Specifically, maternal overnutrition during the perinatal stage (gestation + lactation) has been associated with increased risk for childhood obesity, cardiovascular diseases, and other ailments, with effects often lasting into adulthood. Changes to epigenetic regulation of these traits may be passed on to further generations, compounding the epidemic.
Rodent models have reported that high-fat diet induces offspring leptin insensitivity and altered hypothalamic and hippocampal function through direct and epigenetic effects [2–4, review in 5]. Maternal high-fat diets have been reported to decrease offspring birth mass in mice [6] and rats [7,8], but increase offspring mass (with increase in fat and lean mass) from weaning into adulthood [7,9,10]. Studies of laboratory rats found that dams fed a high-fat diet have greater fat content in their milk after the first week of lactation (no difference in the first week) and increased milk production [11,12].
Maternal high-fat diet alters the glucocorticoid pathway of the dam, affecting offspring in utero and in later development in mice [13]. Offspring of dams receiving a high-fat diet may have higher corticosterone levels as adults [14]. Maternal high-fat diets may also lead to increased adult leptin [9,15,16] and decreased adiponectin [15]. Maternal overnutrition during this stage has also been reported to alter neurobiological processes, including dietary preferences, reward signaling, learning, and memory [e.g., 17–20]. Changes in maternal care have also been reported to impact stress responses of offspring as juveniles to adults. Maternal high-fat diet increased anxiety-related behaviors of offspring in an elevated plus maze in mice [2] and rats [21]. In another rat study, maternal high-fat diet decreased adolescent offspring anxiety, but increased adult offspring anxiety [13], suggesting potential differences in the effect of maternal diet at various offspring ages.
High levels of physical activity reduce risks for various diseases and promote mental health and physical fitness. Levels of physical activity are likely influenced by maternal diet. In mice, a cross-fostering study found decreased spontaneous physical activity (SPA) and increased obesity in female pups of genetically obese (Avy/a) dams, even when they were fostered from birth to lean (a/a) dams [22]. Similarly, cross-fostering mice to smaller litters at birth increased body mass and adiposity and decreased physical activity and energy expenditure, apparently related to sex-specific alterations in hypothalamic DNA methylation and gene expression [23]. Another study of mice found that maternal Western diet decreased adult offspring activity in their home cage [24], though a similar study of rats observed no effect [25]. Maternal diet with added sunflower oil actually elevated SPA of offspring at 20 and >65 days of age for one rat study [26]. Another study found that adult offspring of mice given high carbohydrate diets were hyperactive in their home cage [24,27]. Voluntary exercise was decreased by maternal WD in a study of mice [28]. Clearly, maternal diet affects offspring physical activity and related traits.
Effects of maternal diet are likely dependent on the genetic background and associated behavioral and physiological traits of both the mother and her offspring. For example, individuals with inherently high levels of physical activity or high basal metabolic rates might experience some degree of “protection” from the adverse effects of maternal Western diet. This possibility could be addressed in various ways, such as through comparisons of strains of rodents that vary in activity levels [e.g., 29]. Here, we used the High Runner (HR) mouse lines that have been bred for increased voluntary wheel running for 70+ generations and compared them with non-selected control lines. HR lines run 2.5-3× times more revolutions per night when given wheel access [review in 30] and are more active in their home-cage without wheels [31]. The HR lines have also been reported to increase adult wheel running when given Western diet from weaning through adulthood, indicating changes in energy balance from the C lines [32]. HR lines show changes in other relevant “lower-level” traits, including increased heart mass, increased VO2max, increased circulating corticosterone and adiponectin concentrations, but reduced leptin levels, and an altered brain reward system [33–36].
We tested the overarching hypothesis that the early-life environment can affect both spontaneous physical activity and voluntary exercise of adults, but that these effects would be different for HR and control lines of mice. We hypothesized that the genetic predisposition for high voluntary wheel running in HR mice would be protective against negative consequences of a maternal high-fat, high-sucrose diet. We further predicted that these effects could be mediated by changes in epigenetic regulation of genes as described above, in which case we should see the effects in the grand-offspring of dams fed WD. The replication of selected HR lines (N = 4) and non-selected C lines (N = 4) in this selection experiment helps to mimic the polygenic nature of human population differences in complex traits such as physical activity.
2. Materials and Methods
All experiments and methods were approved by the Institutional Animal Use and Care Committee of the University of California, Riverside.
2.1.1. Experimental animals
Mice used for this experiment were from generation 73 from an ongoing, long-term artificial selection experiment that breeds for high voluntary wheel running [30, for reviews, see 37]. A base population of 224 outbred individuals from the Hsd:ICR strain of house mice was bred randomly for 2 generations, then separated into 8 closed lines, each starting with 10 breeding pairs. The 8 lines were randomly designated into 4 lines bred for high voluntary wheel running (HR: lab designated as lines 3, 6, 7, 8) and 4 control lines bred without regard to wheel running (C: lab designated as 1, 2, 4, 5). Each generation, 6-8 week old mice from HR and C lines are individually housed for 6 days in cages attached to a Wahman-type activity wheel (1.12 m circumference, 35.7 cm diameter, 10 cm wide wire mesh running surface) with a recording device to count revolutions of the wheel in 1-minute intervals for the duration of the experiment. In the HR lines, the highest running female and male from each family are chosen as breeders for the next generation based on their wheel running on days 5 and 6. Breeders are chosen randomly for C lines. All families are represented in the breeders to the next generation (termed “within-family selection”) and sibling pairs are disallowed. Room temperatures are maintained at approximately 22°C, with lights on at 0700 for a 12:12 photoperiod, and water and standard diet (SD: Teklad Rodent Diet W-8604, 14% kJ from fat, 54% kJ from carbohydrates, and 32% kJ from protein, no added sugars [less than ∼9% naturally-occurring sugars by weight, mostly from grains]) are available ad libitum.
2.1.2. Litter characteristics
In the present study, we manipulated the diet of dams (from generation 72 of the selection experiment) from 2 weeks before mating until pups were observed attempting to feed on solid food (∼14 days after birth). All dams were given wheel access as young adults prior to diet manipulation as a normal selection generation. Dams were 14-16 weeks old when they birthed pups. 100 dams (50 HR, 50 C) were fed a “Western” diet (WD: Harlan Teklad TD.88137, 42% kJ from fat, 42.7% kJ from carbohydrates, 15.2% kJ from protein, 34.1% added sucrose by weight) and another 100 dams (50 HR, 50 C) stayed on SD. The source of fat in WD was anhydrous milk fat, the source of protein was casein, and the sources of carbohydrates were sucrose and cornstarch (34.1 and 15.0 g/100 g, respectively). In addition, the high-fat diet contained 0.15% cholesterol.
Starting from birth and continuing until the pups were weaned at 3 weeks of age, 100 families (50 WD, 50 SD) were observed twice daily for developmental markers of the pups (i.e., first day for eye opening, moving, and feeding on solid food). Each cage was observed with a quick look (“spot check”). When pups were 15 days old, dams and litters were all switched to SD. Pups were weaned at 21 days of age and weighed; 2 males and 2 females were saved for C litters and up to 5 males and 5 females were saved for HR litters. One additional male pup from 50 WD dams and 50 SD dams (half C and HR in each group) were considered focal mice and received various additional tests (Fig. 1).
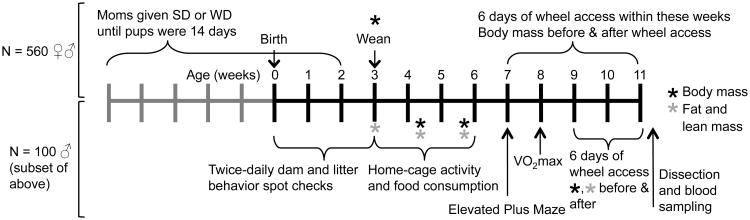
Fig. 1.
Timeline of experimental design. Above the line are procedures given to all mice (N = 560), which include maternal diet manipulation (SD = standard diet, WD = Western diet), weaning at 3 weeks and 6 days of wheel access in adulthood. Below the line are procedures for 100 “focal” families and one male offspring from each of those families, which includes twice-daily behavior checks during the first 3 weeks, monitoring of home-cage activity and food consumption, behavioral testing on an elevated plus maze, and maximal aerobic capacity (VO2max). Each tick mark represents one week and asterisks indicate when mice were weighed (in black) and measured for fat and lean composition (in grey).
2.2. Body mass and body composition
All mice were weighed at weaning (3 weeks old) and before and after 6 days of wheel access (7-11 weeks old).
Focal males were additionally weighed at ∼4.5 weeks and ∼5.5 weeks of age. Focal male body composition was also measured by non-invasive quantitative magnetic resonance (EchoMRI-100; Echo Medical Systems LLC, Houston, Texas, USA). The body composition scanner independently calculated fat mass, lean mass, and water mass in grams. Fat mass was analyzed as a percentage of total body mass.
Change in body mass after wheel access was calculated as an absolute change (post-exercise minus pre-exercise mass) and as percent change:
Similarly, change in lean mass was calculated as an absolute change and using the equation above. Change in fat mass was calculated as an absolute change in grams, change in percent fat mass, and using the equation above with fat mass in grams and percent fat mass.
2.3. Food Consumption
For focal males, juvenile food consumption of SD (grams/day) was measured from 3-6 weeks of age. Food hoppers were weighed and any obvious shredding or wasting of food was noted. Food consumption was calculated as the absolute change in grams from 3 to 4.5 weeks of age and again from 4.5 to 6 weeks of age.
2.4. Maximal aerobic capacity (VO2max)
VO2max was measured during forced exercise in a 900 ml enclosed wheel metabolic chamber as described previously [38,39]. Each trial lasted 5 minutes and each mouse was tested twice, with a rest day in between. Trials started by placing a mouse in the wheel chamber and manually spinning the wheel, slowly increasing the spinning speed over the trial. Air was pumped into the wheel at 2000ml per minute. A subsample of air (150ml) was pumped out, ran through Drierite and soda lime to remove moisture and CO2, and then the volume of O2 was measured in an oxygen analyzer (S-3A Applied Electrochemistry INC. Sunnyvale, CA). Outputs from the instruments were digitized by an analog-to-digital converter (ADAM-4017 data Acquisition Module) and recorded every second on a computer using LabHelper software (Warthog Systems, www.warthog.ucr.edu). The highest 1-minute interval of oxygen consumption in either trial was used to measure VO2max per mouse.
Trials were graded for quality according to how cooperative the mouse was in running on the wheel (1 through 5) and how tired it looked after the trial (1, 2 or 3). Highly cooperative trials indicated that the mouse kept up with the wheel speed until a clear limit in O2 consumption had been reached. Tiredness was graded by how many seconds (<1, 1-5, or >5s) elapsed after the trial before the mouse was able to walk, groom, or engage in exploratory activities. The wheel apparatus for measuring VO2max was chosen over the more traditional treadmill-based test because they obtain equivalent values and the wheel apparatus closely mimics the behavior for which HR mice have been bred [40].
2.5. Behavior in the elevated plus maze
As adults, focal males were tested for their behavior in an elevated plus maze. The maze consists of a plus-sign shaped platform, 1 meter off the ground, with two exposed and two enclosed arms (length: 100 cm, width: 9 cm) joined to a central square platform (9 cm × 9 cm). Each trial lasted 5 minutes and the percentage of time a mouse spends in the enclosed arms is often used as a measure of anxiety [41]. Behavior in the maze was obtained at 1000h-1400h (3-7 hours after lights on) in a lit room, recorded with HD Webcam C525 (Logitech International S.A, Lausanne, Switzerland) and analyzed using TopScan LITE software (Clever Sys, Inc., Reston, Virginia, USA). The surface of the maze was cleaned before each trial and mice were placed in the center square at the beginning of the trial.
2.6. Spontaneous physical activity
From weaning to 6 weeks of age, focal males were housed individually and monitored daily for home-cage activity using passive infrared motion-detector sensors [42,43]. A computer with custom Activity Recording Software (developed by Dr. Mark A. Chappell, UC Riverside) measured activity per 1-minute intervals for 23 hours [36]. Activity in home cages was also measured during wheel testing (described in 2.1.1.).
2.7. Organ masses and plasma hormone concentrations
Focal males were sacrificed by decapitation 6-16 days post wheel access (counterbalanced by diet and linetype). Analyses of organs and hormones used as a covariate the number of days between the end of wheel access and sacrifice. Blood samples and various organ tissues were dissected and weighed: posterior subcutaneous fat pads, caudal portions of the abdominal pelvic fat pad [44], heart ventricles, livers, spleens, and brains. Heparinized blood samples were spun at 13,000 RPM for 12 minutes and collected plasma was stored at -20°C.
Plasma leptin was measured using a Crystal Chem Enzyme-linked Immunosorbent Assay (ELISA) kit (Mouse Leptin Assay Catalog #90030), without dilution and measured in duplicate in 96-well plates. Absorbances were read at 450 nm in an EPOCH2 microplate reader, using GEN5 2.07 reading software (microplate and reading software: BioTek Instruments, Inc., Winooski, VT, USA) and compared with a standard curve generated individually for each plate. Plasma adiponectin was measured similarly with an AssayPro ELISA kit (Mouse Adiponectin ACRP30 Catalog #EMA 2500-1), diluted 400-fold and measured in duplicate. Plasma corticosterone was measured similarly with an Arbor Assays ELISA kit (Corticosterone EIA kit Catalog #K014-H1), diluted 150-fold and measured in duplicate.
Leptin and adiponectin hormone concentrations were analyzed with covariates of fat percent of body mass.
2.8. Grand-offspring characterist ics
In order to measure the effects of grand-maternal diet, males and females whose dams had the same diet (SD or WD) were paired to breed following the same protocol as the normal selection experiment. That is, for each HR line, we chose the highest running male and female from each family and paired them to the highest runners from other families, but in this case only to mice whose mothers had the same diet. In each C line, we chose breeders without regard to their wheel running, but also paired based on maternal diet and disallowed mating between siblings. Then, the offspring of these pairings (i.e., grand-offspring of dams fed SD or WD) were given 6 days of wheel access as adults and weighed before and after.
2.9. Statistical analyses
All analyses were performed using the Mixed Procedure in SAS 9.1.3 (SAS Institute, Cary, NC, USA) to apply analysis of covariance models with Type III tests of fixed effects and REML estimation. Linetype (HR or C) and maternal diet were fixed effects; replicate lines were nested within linetype as a random affect. Effects of linetype, maternal diet, and their interaction were tested relative to the variance among replicate lines, and degrees of freedom were always 1 and 6 for these analyses. The foregoing description applies to both the 100 focal males and the ∼560 male and female offspring for which we obtained data on body mass at weaning, before and after adult wheel access, as well as adult wheel running for 6 days (Fig. 1). For the latter set of mice, we also used dam as a random effect nested within linetype to allow for possible litter effects.
Covariates depended on the trait being analyzed and included age, body mass, wheel freeness (inverse measure of rotational resistance), home cage sensor sensitivity [43], total wheel running (revolutions), and/or time from end of wheel access to sacrifice. In the results when we refer to traits being “adjusted for” by various variables, we mean that these variables were used as covariates in ANCOVA. Dependent variables were transformed as necessary to improve normality of residuals. All P values are 2-tailed unless otherwise indicated.
Body mass and voluntary wheel running were further analyzed by line to measure differences among replicate populations. Although HR lines experienced the same directional selection, each replicate line (and sexes within lines) differed in response time to the selection pressure and mean voluntary wheel running [45], as well as other phenotypes [30, e.g., 37, 46]. Thus, the replicate HR lines have had “multiple solutions” [47] and may be expected to differ in response to manipulation of maternal diet. When the interaction between diet and line was statistically significant, we checked the P value for differences of least-squares means for effect of diet on each line (SAS Procedure Mixed).
Mini-muscle status was also included as a cofactor in some analyses for focal males. Mini-muscle is a simple Mendelian genetic trait [48,49] that causes ∼50% reduced hindlimb muscle mass in two HR lines (all mice in line 3 and a subset in line 6). Mini-muscle status was determined for focal males at the end of the study by inspection of triceps surae muscle mass regressed on body mass. All 10 focal males in line 3 (4 SD and 6 WD) and 3 of the 15 focal males in line 6 (1 SD and 2 WD) were mini- muscle individuals. Among several other phenotypic effects observed in adult mice, mini-muscle individuals were reported previously to have enlarged internal organs [e.g., 33,50,51] and an elevated cost of transport during voluntary wheel running [52].
3. Results
Western diet had a variety of effects on the behavior and body mass of both dams and their offspring, and some of these effects were specific to mice from replicate lines (genotype-by-environment interaction).
3.1. Litter characteristics
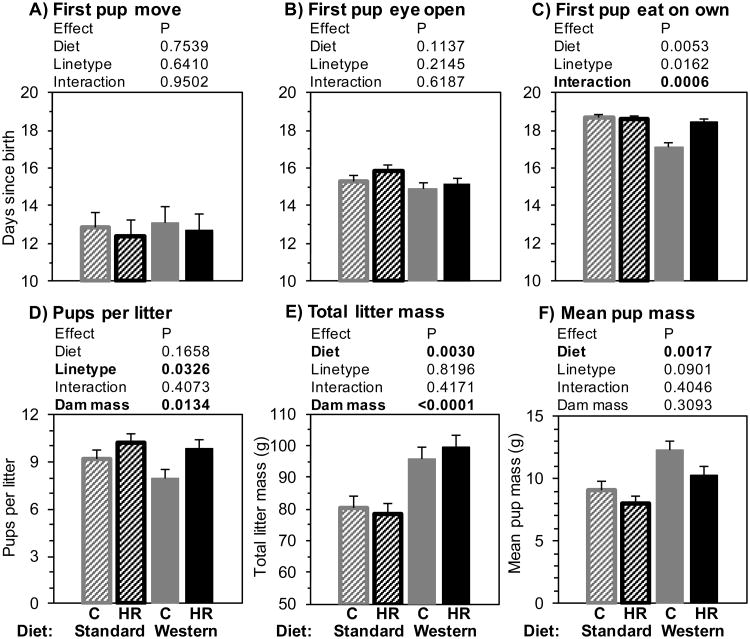
All analyses of litter characteristics used age of the dam as a covariate. Analyses of pup behaviors were performed with and without litter size as a covariate. Litter size was not significant (P > 0.05) when used as a covariate, so the results without litter size are presented here (Fig. 2 A, B, and C; see Online Supplemental Table 1 for results when litter size was included). The timing of the first occurrences for any pup of a litter to move on their own or open their eyes were not significantly affected by maternal WD and did not differ between HR and C lines (Fig. 2A and 2B, respectively). The first sighting for a pup to eat solid food was earlier for C litters with maternal WD (Diet-by-linetype interaction; Fig. 2C).
Fig. 2.
Litter characteristics. A-C: the first day that developmental markers were observed for at least one pup in any litter. D-F: number of pups per litter and total and mean pup mass at weaning (3 weeks after birth), adjusted for dam mass (grand mean = 29.34g, standard deviation = 3.38g). N = 25 per group. Bars represent least-squares means (LSM) + standard error (SE). Striped bars indicate maternal standard diet, solid bars indicate maternal Western diet, grey bars are control mice, and black bars are high-runner (HR) mice. Results of statistical analyses (fixed effects) are shown above each graph. All analyses included covariates of dam age (not shown).
Adjusted for dam age, dam mass was not significantly affected by maternal WD and did not differ between HR and C (Online Supplemental Table 1). Analyses of litter size, total litter mass, and mean litter mass are shown in Fig. 2 (D, E, and F). Adjusted for dam mass and age, the number of pups per litter at weaning was not significantly affected by maternal WD but was larger for HR than C dams (Fig. 2D). Total litter mass was increased by maternal WD but did not differ between HR and C families (Fig. 2E). Mean pup mass was increased by maternal WD and tended to be lower for HR than C (Fig. 2F). Adjusting for dam age, time from pairing to birthing pups was not affected by maternal WD and did not differ between HR and C mice (Online Supplemental Table 1). Sex ratio of pups (measured as number of female pups divided by total number of pups) was also not affected by maternal WD and did not differ between HR and C mice (Online Supplemental Table 1).
3.2.1. Body mass
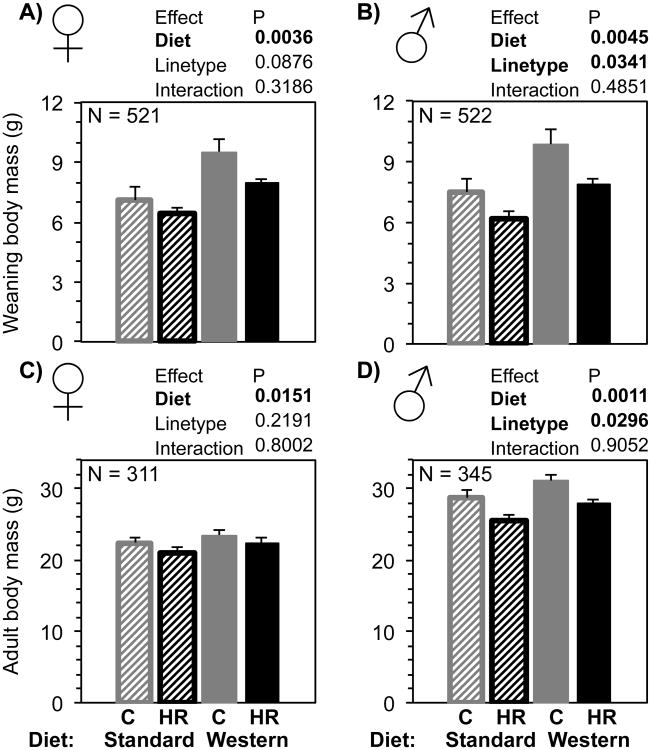
Body mass at weaning was increased by maternal Western diet for both female and male offspring (Fig. 3A and B), and HR males were smaller than C males (Fig. 3B). Juvenile male mass was also increased by maternal WD at ∼5 weeks of age and ∼6 weeks of age (Fig. 4). Females were not weighed as juveniles.
Fig. 3.
Body mass at weaning (3 weeks of age) and as adults (just prior to wheel testing, 7-11 weeks of age) of C and HR mice given maternal SD or WD, with separate analyses for females and males. Bars are age-adjusted LSM + SE and N were approximately equal for each group. Striped bars indicate maternal standard diet, solid bars indicate maternal Western diet, grey bars are control mice, and black bars are high-runner (HR) mice. Results of statistical analyses (fixed effects) are shown above each graph. All analyses included covariates of age (not shown).
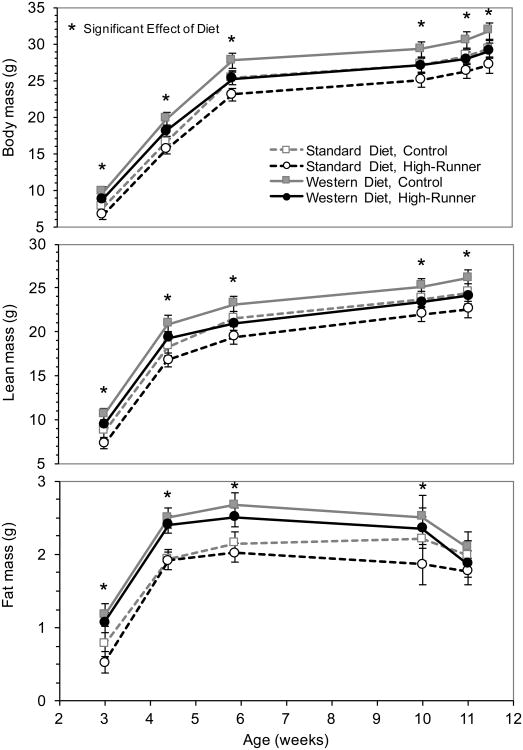
Fig. 4.
Focal male body composition (total body mass, lean mass, and fat mass), adjusted for age. 3 weeks of age corresponds to weaning and 10 weeks of age represents measurements when mice were first granted adult wheel access (though this ranged from 9-10 weeks old), and 11 weeks represents measurements after 6 days of wheel access. Age at approximately 11.5 weeks represents measurements at sacrifice. Each point represents a LSM for ∼25 males and error bars are standard errors. Open points and dashed lines indicate maternal standard diet, filled in points and solid bars indicate maternal Western diet, control mice are in grey, and high-runner mice are in black. 2-tailed P < 0.05 for effect of maternal diet at the time points indicated by an asterisk (*).
Body mass at the start of wheel testing (∼10 weeks of age) was increased for both females and males by maternal WD (Fig. 3C and D), and HR males were significantly smaller than C males (Fig. 3D). All groups significantly gained body mass during wheel access, regardless of maternal WD or linetype. However, the amount of increase in body mass after 6 days of wheel access was not affected by maternal WD or linetype (Online Supplemental Table 1). Analyses of body mass in relation to replicate lines are presented in (Supplemental Figure 4).
3.2.2. Focal male body composition
Both lean and fat masses were increased by maternal WD at all measurements from weaning to adulthood (Fig. 4). The increase for fat mass was greater than for lean mass in terms of both absolute and relative (% increase) values (results not shown). All groups gained lean mass during wheel access (all P < 0.05 except HR whose mothers had SD, for which P = 0.0841). However, the amount of increase in lean mass after 6 days of wheel access was not affected by maternal WD or linetype (Online Supplemental Table 1). Absolute and percent change in lean mass after 6 days of wheel access was not affected by maternal WD and did not differ between HR and C mice (Online Supplemental Table 1).
Regardless of maternal diet or linetype, mice lost fat after 6 days of wheel access. The decrease in absolute fat mass was significantly greater with maternal WD (P = 0.0340), though percent change in fat percentage was not affected by maternal WD and did not differ between HR and C mice (Online Supplemental Table).
3.3. Food consumption
Food consumption of SD was measured for juvenile focal males from 3-6 weeks of age. Using body mass as a covariate, food consumption was not affected by maternal WD or linetype, with no interaction (Online Supplemental Table 1).
3.4. Maximal aerobic capacity (VO2max)
For focal male mice, mass- and age-adjusted VO2max was unaffected by maternal WD, but HR had higher VO2max than C mice (Fig. 5). Mini-muscle individuals had elevated VO2max.
Fig. 5.
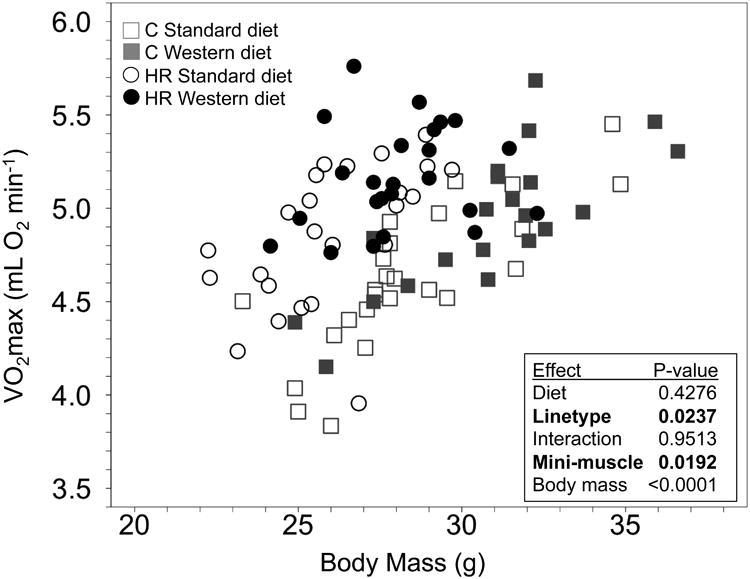
Maximal aerobic capacity (VO2max) measured at ∼8 weeks of age for focal males. N = 91, approximately equal number in each group. Open points indicate maternal standard diet, filled in points indicate maternal Western diet, control mice are in grey, and high-runner (HR) mice are in black. Results of statistical analyses (fixed effects) are shown on the right. All analyses included covariates of age (not shown).
3.5. Behavior in the elevated plus maze
Total duration in the maze varied slightly (range = 293 - 314 seconds), so trial duration was used as a covariate in all analyses. Mice spent 54.4% ± 13.6% (mean ± standard deviations) of the 5-minute test in the closed arms of the maze, compared with 23.7% ± 12.5% in the open arms and 21.7% ± 9.2% in the center. They moved on average 15.9 meters total (range: 4.00 – 20.66 m). Distance moved in each section was 11.2 ± 3.2 meters in the closed arms, 2.6 ± 2.1 meters in the open arms, and 2.1 ± 0.9 meters in the center square.
We analyzed measures putatively related to anxiety such as percent entries into open arms and percent of time spent in open arms [41], as well as number of fecal pellets and urine pools at the end of the trial. We also analyzed, for each zone of the elevated plus maze (closed arms, open arms, and the center): latency to enter from the start of the test, number of entries, time spent, distance moved, and velocity. None of the above measurements of behavior were significantly affected by maternal WD or linetype, with no interaction and no effect of mini-muscle status (Online Supplemental Table 1).
3.6. Spontaneous physical activity
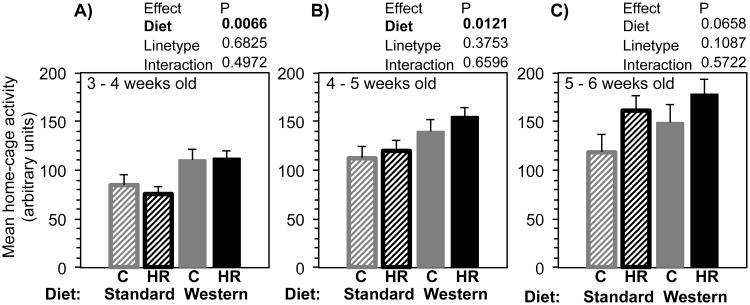
Maternal WD significantly increased home-cage activity of focal HR and C males at 3-4 and at 4-5 weeks of age, but by 5-6 weeks of age, the effect was no longer statistically significant (Fig. 6). At 5-6 weeks of age, HR mice tended to be somewhat more active than C mice, but this effect was not statistically significant. Adding body mass to the models did not change statistical results in any important way (results not shown).
Fig. 6.
Juvenile home-cage activity (measure of spontaneous physical activity, in arbitrary units, averaged across days in the week) for focal males. Panels show 3 consecutive weeks. Bars are age-adjusted LSM + SE, N = 25 in each group. Striped bars indicate maternal standard diet, solid bars indicate maternal Western diet, grey bars are control mice, and black bars are high-runner (HR) mice. Results of statistical analyses (fixed effects) are shown above each graph. All analyses included covariates of age (not shown).
Adult focal male home-cage activity (measured during the 6 days of wheel access) was statistically unaffected by maternal WD, linetype, or their interaction. Additional analyses with amount of wheel running on days 5 and 6 as covariates did not change results (Online Supplemental Table 1).
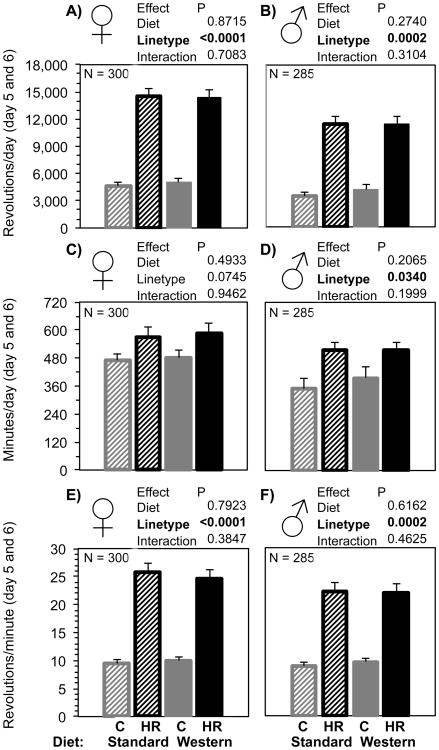
3.7. Voluntary exercise
Wheel running of adult females was 3-fold higher in HR than C and 3.1-fold higher in adult male HR than C (Fig. 7 A and B). Average number of minutes spent running per day was not different between HR and C females, but HR males ran for more minutes than C males (Fig. 7 C and D). Average speed of wheel running was higher in HR females than C females and higher in HR males than C males (Fig. 7 E and F). No aspect of adult wheel running (total distance, duration, average running speed, maximum speed) was statistically affected by maternal WD, with no interaction between linetype and WD. Analysis of wheel running by replicate lines are presented in section 3.10.
Fig. 7.
Total voluntary wheel running revolutions, duration, and average speed of days 5+6 of 6 days of wheel access of adult C and HR mice given maternal SD or WD, with separate analyses for females and males. Bars are age- and wheel-freeness-adjusted LSM + SE and N were approximately equal for each group. Striped bars indicate maternal standard diet, solid bars indicate maternal Western diet, grey bars are control mice, and black bars are high-runner (HR) mice. Results of statistical analyses (fixed effects) are shown above each graph. All analyses included covariates of age (not shown).
3.8. Organ masses
Adjusting for body mass and age, heart ventricle mass was increased by maternal WD, with no effect of linetype and no interaction (Table 1). Posterior subcutaneous fat pad, abdominal pelvic fat pad, liver, spleen, and brain masses were unaffected by maternal WD (Table 1). As reported previously [53], HR mice tended to have larger brains than C mice. Mice with the mini-muscle phenotype had larger hearts, livers, spleens, and both fat pads (Table 1), with several of these effects being reported previously (see Discussion).
Table 1.
Significance levels (P values) from statistical analyses of adult internal organ masses of mice after 6 days of wheel access. Log10 body mass, age, and days from the end of wheel access to sacrifice were used as covariates (results not shown). The grand mean of body mass was 29.90 g with standard deviation = 3.16 g (N = 92). Groups were approximately equal HR and C, with maternal SD and WD. Signs following P values indicate direction of effect: for diet, “+” indicates Western diet > standard diet; for linetype, “+” indicates high-runner lines > control lines.
| Organ mass (log10 transformed) | P values of effects | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Body mass | Diet | Linetype | Interaction | Mini | |
| Abdominal pelvic fat pad | 92 | <0.0001+ | 0.8054- | 0.7513- | 0.4243 | 0.0380+ |
| Posterior subcutaneous fat pad | 91 | <0.0001+ | 0.6195+ | 0.9624- | 0.4475 | 0.0371+ |
| Heart ventricle | 92 | <0.0001+ | 0.0050+ | 0.2327+ | 0.4932 | 0.0005+ |
| Liver | 92 | <0.0001+ | 0.1638- | 0.9518- | 0.9902 | 0.0070+ |
| Spleen | 89 | <0.0001+ | 0.0578- | 0.6383- | 0.0932 | 0.0014+ |
| Total brain | 91 | <0.0001+ | 0.3560+ | 0.0680+ | 0.3159 | 0.1455+ |
| Cerebellum | 91 | 0.0101+ | 0.6049- | 0.2192+ | 0.2955 | 0.9785+ |
| Non-cerebellar brain | 91 | 0.0001+ | 0.2636+ | 0.1516+ | 0.7370 | 0.0825+ |
3.9. Plasma hormone concentrations
With age and days from the end of wheel access to sacrifice as covariates, plasma leptin, adiponectin, and corticosterone concentrations were unaffected by maternal WD and did not differ between HR and C lines, with no interaction (Online Supplemental Table 2).
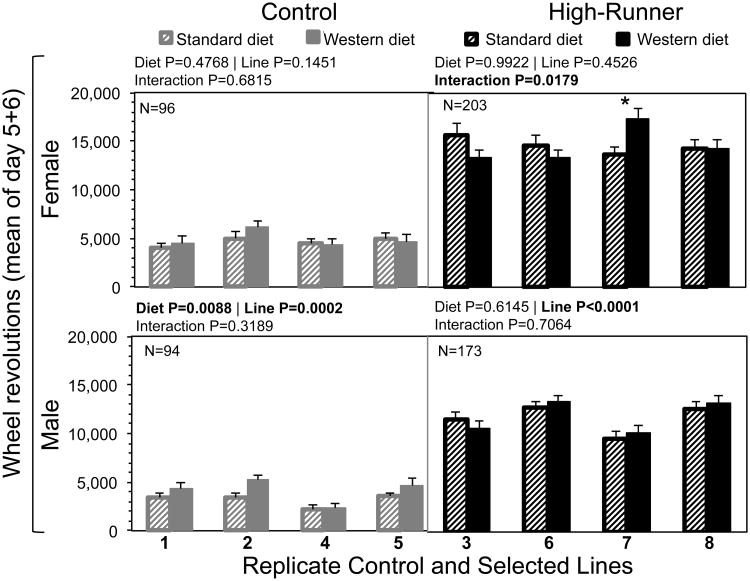
3.10. Line analyses of wheel running
Analyses of variation among the replicate lines were performed separately by sex and linetype (Fig. 8). These analyses revealed that replicate lines of C (N=4) and HR (N=4) mice responded differently to maternal WD, and that these responses were influenced by sex.
Fig. 8.
Total wheel revolutions of adult mice given maternal standard diet (striped bars) or Western diet (solid bars), with separate analyses for sex and replicate control (in grey) and high-runner (in black) lines. Bars are LSM + SE (age and wheel freeness used as covariates). Total N is shown in the upper left-hand corner, and Ns were approximately equal for each line within linetype and diet within line. Results of statistical analyses (fixed effects) are shown above each graph. Asterisks indicate differences of LSM within each line between diets (P < 0.05) from SAS Procedure Mixed. All analyses included covariates of age (not shown).
For wheel running (mean revolutions/day on days 5 & 6) by C females, maternal WD had no statistical effect and replicate lines did not differ significantly (Fig. 8). In HR females, however, maternal WD significantly increased wheel running in one line (HR line 7). In C males, maternal WD increased wheel running, and lines differed significantly. In HR males, maternal WD did not significantly affect wheel running, but lines differed significantly.
We analyzed the number of intervals run per day (1-minute intervals containing at least one revolution), the mean wheel-running speed (revolutions/intervals), and the maximum speed (single highest 1-minute interval), as averages of days 5 & 6 (Online Supplemental Figures 1-3). We also analyzed body mass by replicate line (Online Supplemental Figure 4).
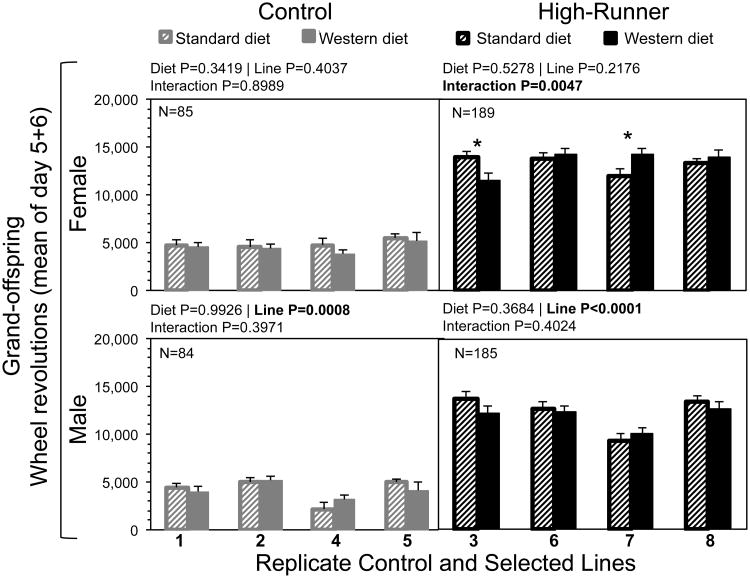
3.11. Effects on grand-offspring
In order to measure the effects of grand-maternal diet, males and females whose dams had the same diet (SD or WD) were paired to breed. Grand-offspring body mass and wheel running as adults were unaffected by grand-maternal WD when analyzed by linetype (Online Supplemental Table 3).
Line analyses of grand-offspring wheel running revealed that replicate lines of HR mice were affected by grand-maternal WD, but only in female mice (Fig. 9). Specifically, maternal WD significantly increased wheel running for females in HR line 7 and significantly decreased it for females in HR line 3 (differences of least squares means from SAS Procedure Mixed). C females were not affected by grand-maternal WD and replicate lines did not differ significantly. In C and HR males, maternal WD did not affect wheel running but replicate lines differed significantly. We also analyzed grand-offspring body mass by sex and replicate line, but found no differences due to grand-maternal diet (Online Supplemental Figure 5).
Fig. 9.
Total wheel revolutions of grand-offspring of dams given standard diet (striped bars) or Western diet (solid bars), with separate analyses for sex and replicate control (in grey) and high-runner (in black) lines. Grand-offspring were produced by mating males and females within each line whose dams had the same diet. Bars are LSM + SE (age and wheel freeness used as covariates). Total N is shown in the upper left-hand corner, and Ns were approximately equal for each group. Results of statistical analyses (fixed effects) are shown above each graph. Asterisks indicate differences of LSM within each line between diets (P < 0.05) from SAS Procedure Mixed. All analyses included covariates of age (not shown).
4. Discussion
We tested the general hypothesis that early-life exposure to maternal Western diet (beginning prior to conception and lasting through ∼2/3 of the lactation period) would affect adult offspring body composition, levels of physical activity, and associated lower-level traits (e.g., organ masses and hormone levels). We expected that any such effects would be mediated by genetic background; in particular, we predicted that the four selectively bred High Runner lines of mice would be somewhat protected against negative consequences of maternal overnutrition as compared with the four non-selected Control lines. For most measures, we obtained data on ∼100 focal adult males, but for adult wheel running and body mass we also obtained data for ∼200 additional males and ∼300 total females.
4.1. Maternal diet changes offspring body composition
Maternal WD had long-lasting effects on the morphology of offspring, increasing fat mass, lean mass, and percent fat of body mass well into adulthood (Fig. 4). Fat % has been found to be increased by maternal high-fat diets in rats [7,10], but to our knowledge, ours study is the first to report increased lean mass. As illustrated in Figure 4 and in Online Supplemental Table 1, maternal WD significantly increased absolute fat mass at weaning, at 4.5 weeks of age, at 5.5 weeks of age, and at ∼10 weeks of age (all P < 0.01), which was immediately prior to the start of wheel access. However, the effect on fat mass was no longer statistically significant after wheel access (P = 0.28). Moreover, the absolute amount of fat lost during wheel access was significantly greater for mice whose dams had experienced WD (Online Supplemental Table 1). Thus, even acute exercise can reverse the negative effect of maternal WD on fat mass, and this occurred for both HR and C mice. In future studies, it would be of considerable interest to determine if the beneficial effect of adult exercise persists.
Maternal WD increased adult heart ventricle mass, even after adjusting for variation in body mass by ANCOVA. Although we have not tested whether this increase was beneficial (physiological) or detrimental (pathological), other studies of mice have reported that maternal high-fat, high-sugar diet caused pathological increases in ventricle mass [54,55]. In any case, maternal WD did not increase offspring maxima l aerobic capacity, which should generally correlate positively with heart size [e.g., 34]. Interestingly, we found that individuals with the mini-muscle phenotype (found only in two of the HR lines in the present sample of mice) had statistically higher VO2max. Previous studies have generally not found this to be the case [40,50], although mini-muscle individuals did have higher VO2max when tested in hypoxia [34] and in a comparison of only one control and two HR lines [51].
4.2. Maternal diet apparently changes offspring energy balance
Maternal WD increased juvenile (3-6 week old) home-cage activity. All mice became more active with age, and at 5-6 weeks, HR tended to be more active than C, as expected from previous studies when these mice are housed without wheels [31,42,43,56]. However, consumption of standard chow, measured only during the juvenile stage for focal males and analyzed using body mass as a covariate, was not affected by maternal WD and did not differ between HR and C mice (Online Supplemental Table 1). This result is consistent with a previous study of mice which reported that maternal over-nutrition increased offspring preference for high-fat food but did not change intake of control food [57]. Given that offspring spontaneous physical activity was increased by maternal WD, coincident with increased offspring body mass (Fig. 4, weeks 3-6) but no change in juvenile food consumption (adjusted for variation in body mass), it is possible that basal or resting metabolic rate was reduced by maternal WD.
In general, maternal Western diet did not affect voluntary exercise on wheels by adult offspring. However, we did find line- and sex-specific effects. Specifically, maternal WD increased wheel running for female offspring of HR line 7, which emphasizes our previous findings that the replicate HR lines have undergone somewhat different evolutionary paths under the same selection regime [46–48]. A sex-specific effect was also found in a study of rats with maternal high-fat diet, which reported less active male and more active female offspring when given free wheel access for one week [8]. Furthermore, grand-offspring of the dams fed WD had significantly altered wheel running in two HR lines (Fig. 9). Maternal WD significantly increased wheel running in HR line 7 offspring and grand-offspring. In HR line 3, maternal WD only tended to decrease running in the offspring, but significantly decreased running in the grand-offspring, showing an “amplifying” effect through two generations. Transgenerational amplification of obesity in mice can be mediated by epigenetic alterations via DNA methylation [58], which could be the case for activity levels as well.
A couple previous studies on these lines of mice have reported that cage activity is reduced when rodents are housed with wheels [42,43]. In the present study, we only measured home-cage activity of adults with wheel access and found no statistical effect of maternal WD and no difference between HR and C mice (Online Supplemental Table 1), the latter result consistent with previous reports [42,43]. Another study of mice, housed without wheels, reported that a maternal high-fat, high-sugar diet decreased spontaneous physical activity of adult offspring measured over one week via telemetry [24].
4.3. No apparent effects of maternal diet on offspring hormone levels
Hormone concentrations of offspring were measured 6-16 days after 6 days of adult wheel access, which may have influenced results. As stated in section 4.1., after 6 days of wheel access, fat mass no longer differed between groups. Additionally, we found no effect of maternal WD on fat pad masses measured at dissection (Table 1). In general, body fat is a positive predictor of leptin levels in mice, including in previous studies of the lines we studied [42,59]. As expected, we found that leptin levels were positively associated with the masses of both posterior subcutaneous fat pads and abdominal pelvic fat pads (Online Supplemental Table 2). Therefore, leptin levels may have been affected by maternal WD before adult wheel access, but been returned to “baseline” values due to exercise-related fat loss. Alternatively, the lack of effect of maternal WD might be a common outcome, as a review of rat studies with maternal high-fat diet reported that 4 of 8 studies did not find significant effects on plasma leptin levels [60].
One previous study of ICR mice found that maternal WD decreased circulating adiponectin concentrations in adult offspring [15], but we did not observe such an effect. Circulating adiponectin concentration is expected to be strongly negatively related to body fat [e.g., 61,62], which, as noted in the previous paragraph, was not affected by maternal WD after 6 days of adult wheel access. Furthermore, we did not replicate the finding of a previous study that showed higher plasma adiponectin in HR versus C males [63].
Maternal high-fat diet has been reported to lower offspring circulating corticosterone levels in mice [13,14], but we did not observe this effect. Previous studies have reported that HR mice have higher circulating corticosterone levels than C mice [e.g., 31,64], but we did not obtain this result (Online Supplemental Table 2), possibly because mice were measured after 6 days of wheel access, followed by several days of sedentary housing.
Physical activity is frequently a confounding factor in measurements of circulating hormone levels. Ideally, we would have taken blood samples prior to adult wheel testing, but we chose not take any blood samples at that time because we did not want to risk affecting the wheel-running phenotype that was a crucial outcome variable for this study. Therefore, we cannot definitively conclude the lack of endocrine effects due to maternal WD. (Similar cautions would apply to organ masses, although we did find an effect of maternal WD on heart size.) Future studies should examine hormone levels at different stages of development for offspring of mothers with high-fat, high-sugar diets while including treatments of sedentary vs. active. Another approach would be to examine the density of central leptin receptors in the hypothalamus, given the differences in body fat mass resulting from maternal diet (Fig. 4, Online Supplemental Table 1).
4.4. Concluding remarks
In summary, we found that maternal Western diet can have long-lasting effects on offspring adult morphology and behavior, although these effects can be mediated by sex and genetic background. However, some effects, such as that observed for body fat (Fig. 4), can be reversed by as little as 6 days of wheel access. Use of the polygenic High Runner mouse lines and their control counterparts allows for a wide scope of exploration of variation in the complex phenotypes related to activity levels, including the importance of population differences and effects of genetic background in interacting with environmental factors. To our knowledge, ours is the first study to characterize effects of maternal WD on mouse strains with inherent propensity for high levels of physical activity.
Supplementary Material
Highlights.
4 High Runner (HR) lines bred for wheel running and 4 non-selected control lines.
Females fed standard/Western diet (WD) before mating until pups were 2 weeks old.
WD increased fat & lean mass; 6 days of adult wheel access reversed effect on fat.
WD increased home-cage activity of juvenile male offspring for both HR and C lines.
WD increased wheel running of offspring and grand-offspring in 1 HR line.
Acknowledgments
This work was supported in part by US NSF grant IOS-1121273 to T.G. and US NIH grant R21HD084856 to T.G. and Wendy Saltzman. We thank Mikkal Blick and William Agnew-Svoboda for assistance with experimental design and data collection.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, Allison DB. Ten Putative Contributors to the Obesity Epidemic. Crit Rev Food Sci Nutr. 2009;49:868–913. doi: 10.1080/10408390903372599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guidotti S, Meyer N, Przybyt E, Scheurink AJW, Harmsen MC, Garland T, Jr, van Dijk G. Diet-induced obesity resistance of adult female mice selectively bred for increased wheel-running behavior is reversed by single perinatal exposure to a high-energy diet. Physiol Behav. 2016;157:246–257. doi: 10.1016/j.physbeh.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Moody L, Chen H, Pan YX. Early-Life Nutritional Programming of Cognition—The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process. Adv Nutr Int Rev J. 2017;8:337–350. doi: 10.3945/an.116.014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalueff AV, Tuohimaa P. Mouse grooming microstructure is a reliable anxiety marker bidirectionally sensitive to GABAergic drugs. Eur J Pharmacol. 2005;508:147–153. doi: 10.1016/j.ejphar.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki A, de Vega W, Sivanathan S, St-Cyr S, McGowan PO. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience. 2014;272:92–101. doi: 10.1016/j.neuroscience.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Bellisario V, Panetta P, Balsevich G, Baumann V, Noble J, Raggi C, Nathan O, Berry A, Seckl J, Schmidt M, Holmes M, Cirulli F. Maternal high-fat diet acts as a stressor increasing maternal glucocorticoids' signaling to the fetus and disrupting maternal behavior and brain activation in C57BL/6J mice. Psychoneuroendocrinology. 2015;60:138–150. doi: 10.1016/j.psyneuen.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KLK. Maternal High-Fat Diet During Gestation or Suckling Differentially Affects Offspring Leptin Sensitivity and Obesity. Diabetes. 2012;61:2833–2841. doi: 10.2337/db11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: A review. Biochim Biophys Acta BBA - Mol Basis Dis. 2014;1842:507–519. doi: 10.1016/j.bbadis.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehmer F, Bertino M, Jen KLC. The effects of high fat diet on reproduction in female rats. Behav Neural Biol. 1979;27:120–124. doi: 10.1016/S0163-1047(79)92828-0. [DOI] [PubMed] [Google Scholar]
- 11.Rolls BJ, Rowe EA. Pregnancy and lactation in the obese rat: Effects on maternal and pup weights. Physiol Behav. 1982;28:393–400. doi: 10.1016/0031-9384(82)90130-5. [DOI] [PubMed] [Google Scholar]
- 12.Sasson IE, Vitins AP, Mainigi MA, Moley KH, Simmons RA. Pre-gestational vs gestational exposure to maternal obesity differentially programs the offspring in mice, Diabetologia. 2015;58:615–624. doi: 10.1007/s00125-014-3466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howie GJ, Sloboda DM, Kamal T, Vickers MH. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J Physiol. 2009;587:905–915. doi: 10.1113/jphysiol.2008.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.F da S Cunha, Molle RD, Portella AK, C da S Benetti, Noschang C, Goldani MZ, Silveira PP. Both food restriction and high-fat diet during gestation induce low birth weight and altered physical activity in adult rat offspring: the “similarities in the inequalities” model. PLOS ONE. 2015;10:e0118586. doi: 10.1371/journal.pone.0118586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers TJG, Morgan MD, Heger AH, Sharpe RM, Drake AJ. High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci Rep. 2016;6:31857. doi: 10.1038/srep31857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolls BA, Gurr MI, Van Duijvenvoorde PM, Rolls BJ, Rowe EA. Lactation in lean and obese rats: Effect of cafeteria feeding and of dietary obesity on milk composition. Physiol Behav. 1986;38:185–190. doi: 10.1016/0031-9384(86)90153-8. [DOI] [PubMed] [Google Scholar]
- 17.Purcell RH, Sun B, Pass LL, Power ML, Moran TH, Tamashiro KLK. Maternal stress and high-fat diet effect on maternal behavior, milk composition, and pup ingestive behavior. Physiol Behav. 2011;104:474–479. doi: 10.1016/j.physbeh.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 19.Grissom NM, George R, Reyes TM. The hypothalamic transcriptional response to stress is severely impaired in offspring exposed to adverse nutrition during gestation. Neuroscience. 2017;342:200–211. doi: 10.1016/j.neuroscience.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-Like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology. 2012;153:2823–2830. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- 21.Taylor PD. The influence of maternal obesity on offspring cardiovascular control and insights from rodent models. In: Green LR, Hester RL, editors. Parent Obes Intergenerational Program Consequences. Springer; New York: 2016. pp. 307–334. [DOI] [Google Scholar]
- 22.Frazier CRM, Mason P, Zhuang X, Beeler JA. Sucrose exposure in early life alters adult motivation and weight gain. PLOS ONE. 2008;3:e3221. doi: 10.1371/journal.pone.0003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162:924–932. doi: 10.1016/j.neuroscience.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozanne SE, Siddle K. Metab Syndr. 2nd. Blackwell: 2011. Developmental origins of insulin resistance and Type 2 diabetes; pp. 60–80. [Google Scholar]
- 26.Baker MS, Li G, Kohorst JJ, Waterland RA. Fetal growth restriction promotes physical inactivity and obesity in female mice. Int J Obes. 2015;39:98–104. doi: 10.1038/ijo.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Kohorst JJ, Zhang W, Laritsky E, Kunde-Ramamoorthy G, Baker MS, Fiorotto ML, Waterland RA. Early postnatal nutrition determines adult physical activity and energy expenditure in female mice. Diabetes. 2013 doi: 10.2337/db12-1306. DB_121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EHJM, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension and insulin resistance. Hypertension. 2008;51:383–392. doi: 10.1161/HYPERTENSIONAHA.107.101477. [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JMC, Coen CW, Poston L, Taylor PD. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55:76–82. doi: 10.1161/HYPERTENSIONAHA.109.139402. [DOI] [PubMed] [Google Scholar]
- 30.Brenneman DE, Rutledge CO. Effect of dietary lipid on locomotor activity and response to psychomotor stimulants. Psychopharmacology (Berl) 1982;76:260–264. doi: 10.1007/BF00432557. [DOI] [PubMed] [Google Scholar]
- 31.Roghair RD, Segar JL, Volk KA, Chapleau MW, Dallas LM, Sorenson AR, Scholz TD, Lamb FS. Vascular nitric oxide and superoxide anion contribute to sex-specific programmed cardiovascular physiology in mice. Am J Physiol - Regul Integr Comp Physiol. 2009;296:R651–R662. doi: 10.1152/ajpregu.90756.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SA, Javurek AB, Painter MS, Murphy CR, Conard CM, Gant KL, Howald EC, Ellersieck MR, Wiedmeyer CE, Vieira-Potter VJ, Rosenfeld CS. Effects of a maternal high-fat diet on offspring behavioral and metabolic parameters in a rodent model. J Dev Orig Health Dis. 2016:1–14. doi: 10.1017/S2040174416000490. [DOI] [PubMed] [Google Scholar]
- 33.Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- 34.Swallow JG, Hayes JP, Koteja P, Garland T., Jr Selection experiments and experimental evolution of performance and physiology. Exp Evol Concepts Methods Appl Sel Exp. 2009:301–351. [Google Scholar]
- 35.Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, Middleton KM, Garland T., Jr Behavioral despair and home-cage activity in mice with chronically elevated baseline corticosterone concentrations. Behav Genet. 2009;39:192–201. doi: 10.1007/s10519-008-9246-8. [DOI] [PubMed] [Google Scholar]
- 36.Meek TH, Eisenmann JC, Garland T., Jr Western diet increases wheel running in mice selectively bred for high voluntary wheel running. Int J Obes. 2010;34:960–969. doi: 10.1038/ijo.2010.25. [DOI] [PubMed] [Google Scholar]
- 37.Swallow JG, Rhodes JS, Garland T., Jr Phenotypic and evolutionary plasticity of organ masses in response to voluntary exercise in house mice. Integr Comp Biol. 2005;45:426–437. doi: 10.1093/icb/45.3.426. [DOI] [PubMed] [Google Scholar]
- 38.Rezende EL, Garland T, Jr, Chappell MA, Malisch JL, Gomes FR. Maximum aerobic performance in lines of Mus selected for high wheel-running activity: effects of selection, oxygen availability and the mini-muscle phenotype. J Exp Biol. 2006;209:115–127. doi: 10.1242/jeb.01883. [DOI] [PubMed] [Google Scholar]
- 39.Garland T, Jr, Zhao M, Saltzman W. Hormones and the evolution of complex traits: insights from artificial selection on behavior. Integr Comp Biol. 2016;56:207–224. doi: 10.1093/icb/icw040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson Z, Argueta D, Garland T, Jr, DiPatrizio N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol Behav. 2017;170:141–150. doi: 10.1016/j.physbeh.2016.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of mice selected for high voluntary wheel-running activity. Integr Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- 42.Claghorn GC, Thompson Z, Wi K, Van L, Garland T., Jr Caffeine stimulates voluntary wheel running in mice without increasing aerobic capacity. Physiol Behav. 2017;170:133–140. doi: 10.1016/j.physbeh.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 43.Dlugosz EM, Harris BN, Saltzman W, Chappell MA. Glucocorticoids aerobic physiology and locomotor behavior in California mice. Physiol Biochem Zool Ecol Evol Approaches. 2012;85:671–683. doi: 10.1086/667809. [DOI] [PubMed] [Google Scholar]
- 44.Dlugosz EM, Schutz H, Meek TH, Acosta W, Downs CJ, Platzer EG, Chappell MA, Garland T., Jr Immune response to a Trichinella spiralis infection in house mice from lines selectively bred for high voluntary wheel running. J Exp Biol. 2013;216:4212–4221. doi: 10.1242/jeb.087361. [DOI] [PubMed] [Google Scholar]
- 45.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acosta W, Meek TH, Schutz H, Dlugosz EM, Vu KT, Garland T., Jr Effects of early-onset voluntary exercise on adult physical activity and associated phenotypes in mice. Physiol Behav. 2015;149:279–286. doi: 10.1016/j.physbeh.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Copes LE, Schutz H, Dlugosz EM, Acosta W, Chappell MA, Garland T., Jr Effects of voluntary exercise on spontaneous physical activity and food consumption in mice: Results from an artificial selection experiment. Physiol Behav. 2015;149:86–94. doi: 10.1016/j.physbeh.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 48.Cinti S. Adipose Tissue Adipokines Health Dis. Springer; 2007. [accessed October 18, 2016]. The adipose organ; pp. 3–19. http://link.springer.com/10.1007/978-1-59745-370-7_1. [Google Scholar]
- 49.Careau V, Wolak ME, Carter PA, Garland T., Jr Limits to behavioral evolution: the quantitative genetics of a complex trait under directional selection. Evolution. 2013;67:3102–3119. doi: 10.1111/evo.12200. [DOI] [PubMed] [Google Scholar]
- 50.Wallace IJ, Garland T., Jr Mobility as an emergent property of biological organization: Insights from experimental evolution: Mobility and biological organization. Evol Anthropol Issues News Rev. 2016;25:98–104. doi: 10.1002/evan.21481. [DOI] [PubMed] [Google Scholar]
- 51.Garland T, Jr, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, Van Cleave SL, Middleton KM. How to run far: multiple solutions and sex-specific responses to selective breeding for high voluntary activity levels. Proc R Soc B Biol Sci. 2011;278:574–581. doi: 10.1098/rspb.2010.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garland T, Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA. Evolution of a small-muscle polymorphism in lines of house mice selected for high activity levels. Evolution. 2002;56:1267–1275. doi: 10.1111/j.0014-3820.2002.tb01437.x. [DOI] [PubMed] [Google Scholar]
- 53.Kelly SA, Bell TA, Selitsky SR, Buus RJ, Hua K, Weinstock GM, Garland T, Jr, Pardo-Manuel de Villena F, Pomp D. A novel intronic single nucleotide polymorphism in the myosin heavy polypeptide 4 gene is responsible for the mini- muscle phenotype characterized by major reduction in hind-limb muscle mass in mice. Genetics. 2013;195:1385–1395. doi: 10.1534/genetics.113.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, Garland T., Jr Erythropoietin elevates VO2max but not voluntary wheel running in mice. J Exp Biol. 2010;213:510–519. doi: 10.1242/jeb.029074. [DOI] [PubMed] [Google Scholar]
- 55.Templeman NM, Schutz H, Garland T, Jr, McClelland GB. Do mice bred selectively for high locomotor activity have a greater reliance on lipids to power submaximal aerobic exercise? Am J Physiol - Regul Integr Comp Physiol. 2012;303:R101–R111. doi: 10.1152/ajpregu.00511.2011. [DOI] [PubMed] [Google Scholar]
- 56.Dlugosz EM, Chappell MA, McGillivray DG, Syme DA, Garland T., Jr Locomotor trade-offs in mice selectively bred for high voluntary wheel running. J Exp Biol. 2009;212:2612–2618. doi: 10.1242/jeb.029058. [DOI] [PubMed] [Google Scholar]
- 57.Kolb EM, Rezende EL, Holness L, Radtke A, Lee SK, Obenaus A, Garland T., Jr Mice selectively bred for high voluntary wheel running have larger midbrains: support for the mosaic model of brain evolution. J Exp Biol. 2013;216:515–523. doi: 10.1242/jeb.076000. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez-Twinn DS, Blackmore HL, Siggens L, Giussani DA, Cross CM, Foo R, Ozanne SE. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology. 2012;153:5961–5971. doi: 10.1210/en.2012-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blackmore HL, Niu Y, Fernandez-Twinn DS, Tarry-Adkins JL, Giussani DA, Ozanne SE. Maternal diet-induced obesity programs cardiovascular dysfunction in adult male mouse offspring independent of current body weight. Endocrinology. 2014;155:3970–3980. doi: 10.1210/en.2014-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland T., Jr Differential sensitivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selectively bred for hyperactive wheel-running behavior. Psychopharmacology (Berl) 2001;158:120–131. doi: 10.1007/s002130100857. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki A, Erb S, McGowan PO. The effect of maternal avernutrition on reward and anxiety in offspring. In: Green LR, Hester RL, editors. Parent Obes Intergenerational Program Consequences. Springer New York; New York, NY: 2016. [accessed December 13, 2016]. pp. 187–200. http://link.springer.com/10.1007/978-1-4939-6386-7_9. [Google Scholar]
- 62.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes. 2008;32:1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girard I, Rezende EL, Garland T., Jr Leptin levels and body composition of mice selectively bred for high voluntary locomotor activity. Physiol Biochem Zool. 2007;80:568–579. doi: 10.1086/521086. [DOI] [PubMed] [Google Scholar]
- 64.Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes. 2011;35:325–335. doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- 65.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 66.Stefan N, Vozarova B, Funahashi T, Matsuzawa Y, Weyer C, Lindsay RS, Youngren JF, Havel PJ, Pratley RE, Bogardus C, Tataranni PA. Plasma Adiponectin Concentration Is Associated With Skeletal Muscle Insulin Receptor Tyrosine Phosphorylation, and Low Plasma Concentration Precedes a Decrease in Whole-Body Insulin Sensitivity in Humans. Diabetes. 2002;51:1884–1888. doi: 10.2337/diabetes.51.6.1884. [DOI] [PubMed] [Google Scholar]
- 67.Vaanholt L, Meerlo P, Garland T, Jr, Visser G, van Dijk G. Plasma adiponectin is increased in mice selectively bred for high wheel-running activity but not by wheel running per sé. Horm Metab Res. 2007;39:377–383. doi: 10.1055/s-2007-976542. [DOI] [PubMed] [Google Scholar]
- 68.Malisch JL, deWolski K, Meek TH, Acosta W, Middleton KM, Crino OL, Garland T., Jr Acute restraint stress alters wheel-running behavior immediately following stress and up to 20 hours later in house mice. Physiol Biochem Zool. 2016;89:546–552. doi: 10.1086/688660. [DOI] [PubMed] [Google Scholar]
- 69.Eclarinal JD, Zhu S, Baker MS, Piyarathna DB, Coarfa C, Fiorotto ML, Waterland RA. Maternal exercise during pregnancy promotes physical activity in adult offspring. FASEB J. 2016 doi: 10.1096/fj.201500018R. fj.201500018R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly SA, Hua K, Wallace JN, Wells SE, Nehrenberg DL, Pomp D. Maternal exercise before and during pregnancy does not impact offspring exercise or body composition in mice. J Negat Results Biomed. 2015;14:13. doi: 10.1186/s12952-015-0032-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, Roseboom TJ. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88:1648–1652. doi: 10.3945/ajcn.2008.26140. [DOI] [PubMed] [Google Scholar]
- 72.Stein AD, Rundle A, Wada N, Goldbohm RA, Lumey LH. Associations of gestational exposure to famine with energy balance and macronutrient density of the diet at age 58 years differ according to the reference population used. J Nutr. 2009;139:1555–1561. doi: 10.3945/jn109.105536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Almeida SS, Garcia RA, de Oliveira LM. Effects of early protein malnutrition and repeated testing upon locomotor and exploratory behaviors in the elevated plus-maze. Physiol Behav. 1993;54:749–752. doi: 10.1016/0031-9384(93)90086-U. [DOI] [PubMed] [Google Scholar]
- 74.Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats, Hypertension. 2003;41:168–175. doi: 10.1161/01.HYP.0000047511.97879.FC. [DOI] [PubMed] [Google Scholar]
- 75.Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes. 2006;30:729–738. doi: 10.1038/sj.ijo.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wahlqvist ML, Krawetz SA, Rizzo NS, Dominguez-Bello MG, Szymanski LM, Barkin S, Yatkine A, Waterland RA, Mennella JA, Desai M, Ross MG, Krebs NF, Young BE, Wardle J, Wrann CD, Kral JG. Early-life influences on obesity: from preconception to adolescence. Ann N Y Acad Sci. 2015;1347:1–28. doi: 10.1111/nyas.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutton EF, Gilmore LA, Dunger DB, Heijmans BT, Hivert MF, Ling C, Martinez JA, Ozanne SE, Simmons RA, Szyf M, Waterland RA, Redman LM, Ravussin E. Developmental programming: State-of-the-science and future directions-Summary from a Pennington Biomedical symposium. Obesity. 2016;24:1018–1026. doi: 10.1002/oby.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meek TH, Dlugosz EM, Vu KT, Garland T., Jr Effects of leptin treatment and Western diet on wheel running in selectively bred high runner mice. Physiol Behav. 2012;106:252–258. doi: 10.1016/j.physbeh.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Donovan EL, Hernandez CE, Matthews LR, Oliver MH, Jaquiery AL, Bloomfield FH, Harding JE. Periconceptional undernutrition in sheep leads to decreased locomotor activity in a natural environment. J Dev Orig Health Dis. 2013;4:296–299. doi: 10.1017/S2040174413000214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.