Abstract
Early-life environmental stressors, including sickness, have the potential to disrupt development in ways that could severely impact fitness. Despite what is known about the effects of sickness on reproduction, the precise physiological mechanisms have not yet been determined. The goal of this study was to investigate the effects of a neonatal immune challenge on adult reproductive physiology and opposite-sex social behavior. Male and female Siberian hamster (Phodopus sungorus) pups were administered lipopolysaccharide ([LPS]; a cell wall component of gram-negative bacteria) or saline injections on postnatal days 3 and 5 and body mass, food intake, and measures of reproductive maturity were taken throughout development. In adulthood, hamsters were placed in staged mating pairs with reproductively mature individuals of the opposite sex, during which a series of behaviors were scored. We found that although males and females showed no change in food intake, body mass, or reproductive behaviors, LPS-treated females had abnormal estrous cycles and smaller ovaries. Females also showed increased investigation of and increased aggression towards males in a reproductive context. In contrast, LPS-treated males showed no change in any physiological measures, nor did they show any changes in behavior. The present findings demonstrate that females may be more robustly affected by neonatal sickness than males and that these effects could have potential impacts on reproductive success. Collectively, the results of this study can be used to expand upon what is already known about sickness and reproduction, specifically the importance of social behaviors involved in pre-copulation and information necessary to choose the appropriate mate.
Keywords: development, immune system, lipopolysaccharide, social behavior
1. Introduction
Animals encounter environmental fluctuations throughout their lifetime, and in order to produce appropriate behavioral responses, neural circuits must integrate external stimuli with internal physiology. The hypothalamo-pituitary-gonadal (HPG) axis integrates environmental stimuli and regulates reproductive activity through hypothalamic release of gonadotropin-releasing hormone (GnRH), concomitant release of gonadotropic hormones from the pituitary, and subsequently, sex steroids from the gonads [1,2]. The HPG axis is strongly affected by physiological responses to external stressors, and the timing of environmental stressors is critical in determining the effect they will have on an organism's development. The neonatal period is an extremely sensitive time in the life of an individual. In particular, environmental stressors during this time may increase susceptibility to a range of nervous system disorders (e.g., anxiety, depression, autism, schizophrenia, and learning disabilities) and may result in subtle alterations in the physiological and behavioral response an individual has to subsequent stressors or to new experiences in adulthood [3–5].
Many social behaviors are crucial to successfully attracting a mate and successful reproduction. Reproductive behaviors are extremely diverse, and they are dependent not only species, but also on sex and gonadal steroid levels, as well as time of year [6]. In most female mammals, including Siberian hamsters (Phodopus sungorus), lordosis is used to determine the receptivity of a female, and is characterized by a fixed posture accompanied by a dorsiflexion of the back [7]. Male rodents mount shortly after being introduced to a receptive female, followed by intromission and ejaculation [8].
Prior to lordosis and mounting and when first introduced to a conspecific, both sexes will perform vigorous chemoinvestigatory behaviors, allowing them to retrieve the necessary cues for choosing an appropriate mate and copulating [9]. Animals perform nose-to-nose and nose-to-anogenital investigation to identify sex, age, and other characteristics of their conspecifics to determine whether or not the individual is a suitable mate [10,11]. Nose-to-nose sniffing involves the rubbing and passing of the animal's snout through the fur of the conspecific, which often occurs during the early phases of sexual behavior [11,12]. Nose-to-anogenital sniffing involves the rubbing and passing of the animal's snout through the fur of the conspecifics anus and genitalia [11,12].
While animals can adjust to environmental stressors during certain periods of their life, some stressors during the early stages of life can have lasting consequences on physiology and behavior. The precise cause of behavioral abnormalities is not completely understood, yet it appears that an inflammatory or sickness response during pregnancy or shortly after birth may play a role. Studies have shown that male mice exposed to prenatal stress have increased expression of cytokines in the hippocampus compared with non-stressed individuals, and they exhibit greater activation of microglia and astrocytes in response to an immune challenge in adulthood [13]. Treatment with lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, is commonly employed to induce a robust immune response in animals. When LPS is injected into the peritoneal cavity, it enters circulation within fifteen minutes and levels stay elevated for at least 2 hours following injection [14]. One way LPS administration mimics the actions of a live bacterial infection is by way of binding to toll-like receptor (TLR)-4, which stimulates the activation of transcription factors and pathways leading to the subsequent release of pro- and anti-inflammatory mediators in the body [15]. These molecules can then act downstream to contribute to the acute-phase response (APR) initiated primarily by hepatocytes, eliciting a sickness response, including but not limited to fever, lethargy, decreased food intake, and enhanced pain [3,16–18].
Although exogenous LPS has been shown to produce increases in mainly proinflammatory cytokines (interleukin-1 [IL-1], IL-6, and tumor necrosis factor-alpha [TNF-α]), one study has suggested that treatment with exogenous LPS not only causes an increase in IL-1 (a pro-inflammatory cytokine), but further, they determined that LPS can also stimulate release of the anti-inflammatory cytokine, IL-2, and these two cytokines can differentially influence behavioral responses [19]. For example, rats treated with IL-2 at three weeks of age exhibit enhanced locomotor activity and greater levels of exploration, whereas rats treated with IL-1 at eight weeks of age show an increase in startle response [20], suggesting that the release of these specific cytokines may be the trigger for changes in the amount and duration of particular social behaviors. Work in our lab suggests that maternal immune activation with LPS affects offspring physiological and behavioral development. Specifically, male offspring from LPS-treated dams show greater cortisol response following an intruder encounter and higher frequency of bites during that agonistic encounter when compared with offspring from control-treated dams. Further, these two measures are positively correlated, suggesting the hypothalamo–pituitary–adrenal (HPA) axis may play a role in these behavioral changes [21].
The goals of the present study were to assess the effects of postnatal immune activation on offspring reproductive development and behavior and to evaluate the potential sex differences in response to neonatal treatment. We hypothesized that early postnatal immune activation with LPS would produce changes in both reproductive development and behavior, as well as other social behaviors in both males and females. The results of this study will help to further our understanding of how the neuroendocrine and immune systems interact during early development in male and female Siberian hamsters. More importantly, they will shed light on an important aspect of reproductive behavior that is often missing from the literature; here we focus on the social behaviors particularly important in pre-copulation, which is crucial in determining the optimal mate.
2. Materials and Methods
2.1 Animal Housing and Immune Challenge
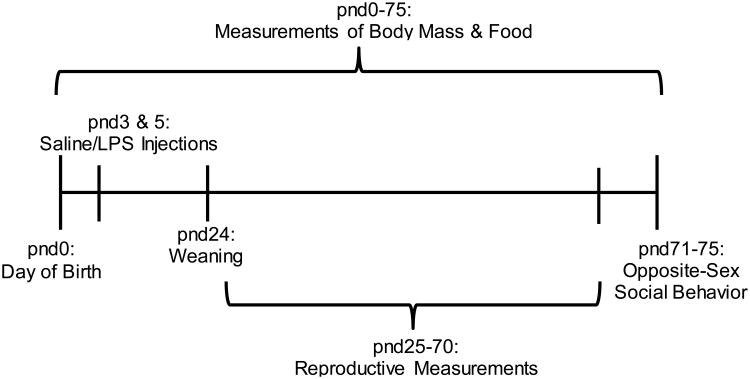
Male and female hamsters were paired (n=18 pairs) and housed in a 16:8 light:dark photoperiod, in polypropylene cages (28 × 17 × 12 cm). Ambient temperature was maintained at 20 ± 2C, and relative humidity was maintained at 55 ± 5%. Hamsters were given ad libitum access to tap water and standard laboratory rodent chow (Lab Diet 5001, PMI Nutrition) throughout the experiment. Males of each breeding pair were removed from the breeding cage 16 days after being paired to prevent post-partum pregnancy. Pups remained in their litter until weaning on postnatal day (pnd) 24. On pnd 3, half of the litters were given a single intraperitoneal (i.p.) injection (100μl) of 50 μg/kgof LPS (from Salmonella enterica serotype typhimurium, Sigma-Aldrich, St. Louis, MO,USA), suspended in 0.9% sterile saline and the other half of the litters received injections of 0.9% sterile saline. A second injection of LPS or saline was given on pnd5 according to a previously validated protocol, as there is heightened sensitivity of the GnRH pulse generator at these time points [22]. All pups in an individual litter received the same treatment (LPS or saline), and the time for mothers to return to nursing was monitored across all litters. Once injected, pups were monitored and weighed for the remainder of the experiment. Animals were weaned at pnd24 and housed individually for the remainder of the study. At the conclusion of the study, reproductive behavior assays were conducted to determine both pre-copulatory behaviors (e.g., investigation, scent-marking, and aggression) and copulatory behaviors (e.g., lordosis, mounting, and ejaculation). See Figure 1 for experimental timeline. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC) at Indiana University.
Figure 1.
Experimental timeline demonstrating when treatments were performed and when physiological and behavioral measures were collected. Postnatal day (pnd) 0 represents the time point at which pups were born, and pnd24 represents the time point at which each animal was individually housed for the remainder of the study.
2.2 Reproductive Physiology
Beginning at pnd25 and once per week thereafter until reproductive maturity (for no more than 2 weeks), males (n=20 saline-treated; n=26 LPS-treated) were lightly anesthetized with isoflurane, and the length and width of the left testis were measured externally (+/- 0.1 mm) with calipers following previously outlined methods [23–26]. Estimated testis volume (ETV) was calculated as the length × width2, which is directly correlated with testis mass and spermatogenesis [23,27]. An ETV of 400mm3 indicates a mass of approximately 200mg, which is correlated with the critical mass for production of viable spermatids [23,27]. Beginning at pnd25 and every day thereafter until reproductive maturity, all female offspring (n=24 saline-treated; n=29 LPS-treated) were monitored daily to determine the time of initial vaginal opening [28]. Female estrous cycles were monitored via vaginal cytology during the 5 consecutive days of behavioral testing (pnd71-75) [29,30]. Vaginal cell samples were obtained via vaginal lavage. Following lavage, samples were transferred to microscope slides, fixed with methanol, and stained with Giemsa. Samples were then evaluated for estrous stage (diestrus, proestrus, estrus, metestrus, and anestrus) under 100× magnification [29,31,32]. Estrous cycling provides a measure of reproductive functioning, specifically ovarian functioning, in Siberian hamsters [33]. Therefore, tracking estrous cycles via vaginal cytology provides us with important information about the functioning of the reproductive axis.
2.3 Behavioral Trials and Analyses
We recorded and analyzed reproductive behavior, aggression, investigation, and scent-marking using the resident-intruder paradigm with opposite-sex social partners per previously outlined methods for this species [34]. All experimental trials were performed under low illumination (25W), red light conditions, with a video camera (Sony Handycam HD R-SR7) positioned in front of the cage with mirrors on either side, to ensure visibility of all cage corners. To identify intruders, small patches of fur were shaved on the dorsal surface at least 24 hours before the start of the behavior assays.
Beginning on pnd71, a subset of the experimental animals was placed in staged mating pairs. Adult males (n=7 saline-treated; n=12 LPS-treated) and females (n=10 saline-treated; n=10 LPS-treated) were introduced to a novel individual of the opposite sex, and behavior was recorded within a 30-minute testing period. Each of the interactions took place on 5 consecutive nights with the same pairs in order to capture when females were receptive [34]. Although it is possible to identify changes in vaginal cytology, we are unable to determine a pattern between the estrous stage and the receptivity of the female during behavioral assessments in Siberian hamsters [35]. Thus receptivity was determined by the female's performance of behaviors showing readiness to copulate, including lordosis and the absence of attacks towards the male conspecific. Additionally, a receptive female has the tendency to approach a male, remain in close proximity to the male, and investigate the male more often than a non-receptive female [36]. If a female was not receptive, both animals were returned to their home cage and the procedure was performed again on the next night. On the fifth night, if a female was still not receptive, the pair was recorded and was placed in a non-receptive group for analysis and assessed as such. All behavioral trials were conducted 30 minutes following the onset of darkness until no more than 3 hours after darkness, since it has been found that females come into estrus starting at the beginning of darkness to 3 hours after dark [37]. Behavioral interactions were scored by a trained observer blind to the treatment group using ODlog™ software (Macropod).
We analyzed female reproductive behaviors, including the occurrence of receptivity, frequency of lordosis, and lordosis quotient (LQ = number of lordoses/10 mounts × 100) [34,38–40]. Their male intruders were monitored for the occurrence of ejaculation, as determined by the male thrusting for a period of time followed by the male falling on its side while still intromitting. This was usually followed by a short pause, temporary disinterest in the female, and vigorous grooming of the genital area [29,41–43]. Focal male reproductive behaviors included receptivity within the pair, frequency and duration (s) of vaginal mounting, (scored when the male mounted with an orientation that would permit intromission), frequency and duration (s) of mis-mounting (scored when the male mounted with an orientation that would not permit intromission), and occurrence of ejaculation [29,41,42,44]. We measured investigation in both males and females. For investigation, we quantified the frequency and duration (s) of nose-to-anogenital and nose-to-nose investigation. In all females, we also measured aggression towards the male conspecific. To do so, we quantified latency to first attack (s) and the frequency and duration (s) of attacks. In all males, we measured scent-marking by quantifying the frequency and duration (s) of scent depositing.
2.4 Tissue Collection
At the end of the experiment, all animals were euthanized via a lethal i.p. injection of a ketamine and xylazine cocktail in 0.9% saline. Following euthanasia, testes, ovaries, and uterine horns were dissected and weighed to determine the effects of treatment on gross anatomy.
3. Statistical Analyses
We performed all statistical analyses in R v. 3.3.1 (R Core Team 2015) and attributed statistical significance at p < 0.05. Data were log or square root transformed to attain normality and equal variances. For all behavioral and physiological data, we used generalized linear mixed models (GLMMs), including the fixed effects of the model (e.g., treatment) as well as the random effect of litter in a hierarchical experimental design. This enables us to take into account the fact that individual pups from the same litter may not truly be independent samples. Differences in repeated measures (i.e., body mass, food intake) were assessed via repeated-measures GLMMs. Only a randomized portion of the male and female pups was used for behavioral trials; therefore, sample sizes vary across different measures. All sample sizes are noted in the methods section. Spearman's rank correlations were conducted on female ovarian mass and behavioral measures.
4. Results
4.1 Early-life immune activation did not affect litter physiology
The total number of pups in each litter did not differ across treatment groups (t11=-1.444, p=0.176). Further, the time for mothers to return to nursing after the first injection (t16=-0.727, p=0.478) and after the second injection (t16=0.727, p=0.478) was not affected by treatment.
4.2 Early-life immune activation did not affect adult body mass or food intake
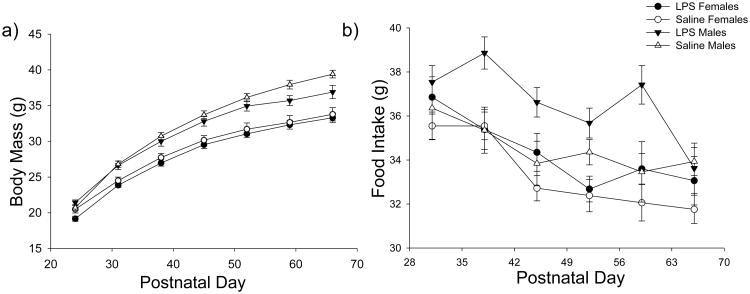
LPS treatment did not affect adult female body mass at pnd66 (t15=1.031, p=0.319), nor did it affect adult male body mass at pnd66 (t13=-1.353, p=0.199) (Figure 2). Food intake and body mass over the course of the entire study were also not affected by treatment (Figure 2) (p > 0.05 in all cases).
Figure 2.
Mean ± S.E.M. of a) body mass and b) food intake over time. There was no change in body mass or food intake in male (n=20 saline-treated; n=26 LPS-treated) and female (n=24 saline-treated; n=29 LPS-treated) hamsters treated with LPS when compared with hamsters treated with saline. White circles represent saline-treated females; white triangles represent saline-treated males; black circles represent LPS-treated females; and black triangles represent LPS-treated males;
4.3 Early-life immune activation affected reproductive physiology in females but not in males
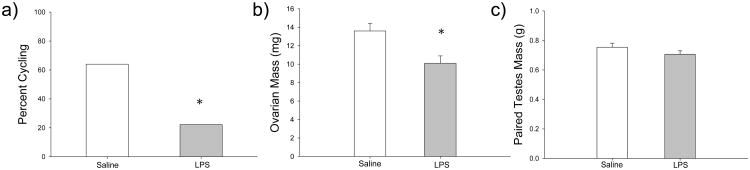
Postnatal LPS administration did not affect the timing of female vaginal opening (t14=-0.445, p=0.663) or reproductive maturity in males, as determined by estimated testis volume (t14=0.628, p=0.540). However, postnatal LPS treatment disrupted female estrous cycles. Fewer LPS-treated females exhibited normal estrous cycles when compared with saline-treated females (t15=3.142, p=0.007) (Figure 3a).
Figure 3.
Effects of LPS treatment on reproductive physiology in female (n=24 saline-treated; n=29 LPS-treated) and male (n=20 saline-treated; n=26 LPS-treated) hamsters. a) LPS-treated females (gray bars) exhibited a higher percentage of abnormal estrous cycles when compared to saline-treated females (white bars), b) LPS-treated females had smaller ovaries when compared with saline-treated females, and c) male paired testes mass was not affected by LPS treatment. Bar heights represent mean ± S.E.M. An asterisk (*) indicates statistically significant differences between group means (p < 0.05).
Additionally, postnatal LPS affected reproductive organ mass in adult females. LPS-treated females had significantly smaller ovaries in adulthood (t12=3.276, p=0.007) (Figure 3b), yet the difference between uterine horns in LPS-treated and saline-treated females was not significantly different (t12=0.303, p=0.767). In contrast, postnatal LPS treatment did not affect male reproductive organ mass in adulthood. Paired testes mass did not differ between LPS-treated males and saline-treated males (t11=1.300, p=0.220) (Figure 3c).
4.4 Early-life immune activation did not affect receptivity in males or females
There was no difference in receptivity in males or females across treatment groups. LPS-treated females showed no significant difference in the frequency of receptivity when compared to saline-treated females (t12=0.509, p=0.620). Similarly, females that were paired with LPS-treated males showed no difference in the frequency of receptivity (t11=1.603, p=0.137) when compared with saline-treated males.
4.5 Early-life immune activation did not affect reproductive behaviors in males or females
Postnatal LPS did not significantly affect female or male reproductive behaviors in receptive pairs. Lordosis quotients did not differ between LPS- and saline-treated females (t4=0.005, p=0.997), and the frequency (t4=-0.868, p=0.434) and duration (t4=-0.663, p=0.544) of lordosis did not differ between LPS- and saline-treated females (Table 1). Additionally, experimental males paired with a receptive female (receptive males) showed no significant difference in the frequency (t4=1.081, p=0.341) or duration (t4=0.998, p=0.375) of vaginal mounting or the frequency (t4=-0.962, p=0.391) or duration (t4=-0.959, p=0.392) of mis-mounting (Table 2). Similarly, there was no difference in frequency (t7=-0.788, p= 0.457) or duration (t7=-0.751, p=0.477) of mis-mounting in males paired with a non-receptive female (non-receptive males) as well (Table 2). Further, LPS-treated males paired with a receptive female displayed no difference in the occurrence of ejaculation when compared with saline-treated males (t4=1.633, p=0.178) (Table 2). Non-receptive females of both treatment groups showed no lordosis (Table 1), and males in non-receptive pairs displayed no vaginal mounting or ejaculations (Table 2). All reproductive behaviors performed by intruders did not significantly differ across treatment groups (p > 0.05 in all cases).
Table 1.
Mean ± S.E.M. of investigation, aggression, and reproductive behaviors in female hamsters (n=10 saline-treated; n=10 LPS-treated) across treatment groups. The frequency and duration of nose-to-nose investigation, the frequency and duration of total investigation, and the duration of attacks were significantly different between receptive treatment groups. An asterisk (*) indicates statistically significant differences between group means (p < 0.05).
| Female | Receptive Pairs | Non-Receptive Pairs | ||
|---|---|---|---|---|
| Control | LPS | Control | LPS | |
| Frequency of Nose-to-Nose Investigation | 8.75 ± 1.70 | 17.67 ± 2.60* | 42.00 ± 5.13 | 31.14 ± 7.24 |
| Duration of Nose-to-Nose Investigation | 6.73 ± 2.01 | 20.43 ± 4.33* | 47.67 ± 10.19 | 41.01 ± 17.37 |
| Frequency of Nose-to-Anogenital Investigation | 2.25 ± 1.11 | 12.67 ± 4.91 | 11.33 ± 1.31 | 14.43 ± 2.53 |
| Duration of Nose-to-Anogenital Investigation | 1.98 ± 1.15 | 15.17 ± 7.05 | 11.47 ± 2.49 | 19.34 ± 5.39 |
| Total Frequency of Investigation | 11.00 ± 2.48 | 30.33 ± 7.45* | 53.33 ± 5.23 | 45.57 ± 9.60 |
| Total Duration of Investigation | 8.70 ± 2.76 | 35.60 ± 11.32* | 59.13 ± 11.58 | 60.36 ± 22.05 |
| Frequency of Attacks | 4.75 ± 2.78 | 13.00 ± 2.08 | 21.00 ± 5.65 | 17.86 ± 2.34 |
| Duration of Attacks | 2.50 ± 1.28 | 11.40 ± 1.86* | 23.62 ± 5.77 | 17.01 ± 3.13 |
| Latency to First Attack | 138.75 ± 57.82 | 180.00 ± 70.77 | 94.17 ± 42.53 | 25.00 ± 7.40 |
| Frequency of Lordosis | 24.25 ± 5.71 | 31.33 ± 7.80 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Duration of Lordosis | 47.95 ± 13.74 | 62.47 ± 17.54 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Lordosis Quotient | 47.50 ± 11.81 | 46.67 ± 3.33 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Table 2.
Mean ± S.E.M. of investigation, scent-marking, and reproductive behavior in male hamsters (n=7 saline-treated; n=12 LPS-treated) across treatment groups. No values were significantly different across treatment groups.
| Male | Receptive Pairs | Non-Receptive Pairs | ||
|---|---|---|---|---|
| Control | LPS | Control | LPS | |
| Frequency of Nose-to-Nose Investigation | 92.75 ± 12.13 | 106.50 ± 13.50 | 38.00 ± 7.37 | 40.75 ± 10.56 |
| Duration of Nose-to-Nose Investigation | 185.95 ± 51.63 | 234.15 ± 59.45 | 60.90 ± 24.66 | 54.30 ± 16.85 |
| Frequency of Nose-to-Anogenital Investigation | 53.75 ± 6.69 | 55.50 ± 4.50 | 36.33 ± 6.94 | 65.38 ± 12.87 |
| Duration of Nose-to-Anogenital Investigation | 104.63 ± 18.37 | 106.25 ± 12.55 | 65.57 ± 12.47 | 114.60 ± 25.73 |
| Total Frequency of Investigation | 146.50 ± 9.99 | 162.00 ± 18.00 | 74.33 ± 11.79 | 106.13 ± 20.32 |
| Total Duration of Investigation | 290.58 ± 43.83 | 340.40 ± 72.00 | 126.47 ± 36.37 | 168.90 ± 33.04 |
| Frequency of Scent-marking | 14.25 ± 6.86 | 7.00 ± 9.90 | 36.33 ± 6.94 | 8.63 ± 2.83 |
| Duration of Scent-marking | 16.98 ± 8.99 | 5.7 ± 8.06 | 6.1 ± 2.95 | 7.59 ± 2.34 |
| Frequency of Vaginal Mounting | 30.00 ± 11.37 | 11.50 ± 2.50 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Duration of Vaginal Mounting | 72.35 ± 30.10 | 27.25 ± 3.25 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Frequency of Mis-Mounting | 15.50 ± 5.58 | 30.50 ± 21.50 | 0.00 ± 0.00 | 6.87 ± 5.64 |
| Duration of Mis-Mounting | 17.70 ± 8.57 | 38.85 ± 29.25 | 0.00 ± 0.00 | 9.70 ± 8.44 |
| Frequency of Ejaculations | 1.00 ± 0.41 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
4.6 Early-life immune activation affected investigative behaviors in females but not in males
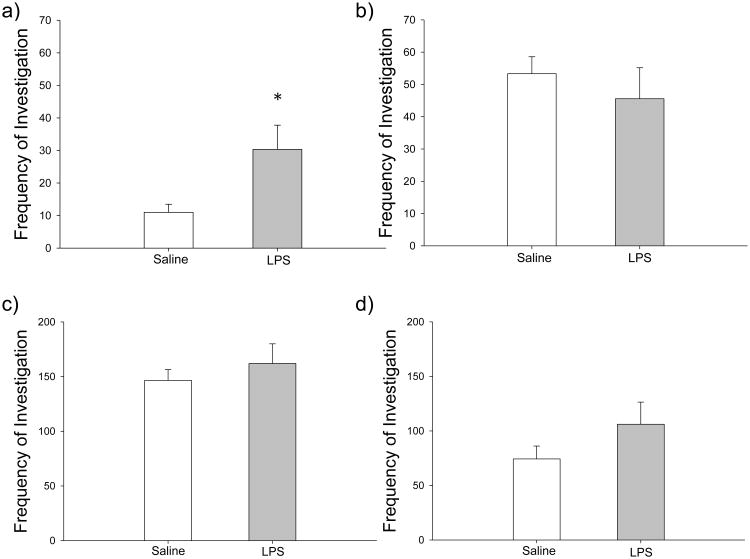
Postnatal LPS treatment significantly affected female investigation with a male conspecific, but only in receptive pairs. The total frequency (t4=-3.006, p=0.040) and duration (t4=-3.162, p= 0.034) of nose-to-nose investigation was significantly increased in receptive LPS-treated females, and the total frequency (t4=-2.415, p=0.073) and duration (t4=-2.178, p=0.095) of nose-to-anogenital investigation was slightly increased, showing a trend towards significance in receptive females (Table 1). Additionally, in receptive LPS-treated females, the frequency (t4=-2.807, p=0.049) and duration (t4=-2.685, p=0.055) of total investigation was greater than in saline-treated females (Figure 4a, Table 1).
Figure 4.
Effects of LPS treatment on total investigation in male and female hamsters. a) LPS-treated (gray bars) receptive females exhibited a higher total frequency of investigative behavior when compared to saline-treated (white bars) receptive females (n=4 saline; n=3 LPS); however, there was no difference in total frequency of investigation in b) non-receptive females (n=6 saline; n=7 LPS), c) receptive males (n=4 saline; n=3 LPS), or d) non-receptive males (n=3 saline; n=8 LPS). Bar heights represent mean ± S.E.M. An asterisk (*) indicates statistically significant differences between group means (p < 0.05).
In contrast, in non-receptive females, treatment did not affect the frequency (t9=1.184, p=0.267) or duration (t9=0.415, p=0.688) of nose-to-nose investigation or the frequency (t9=-0.708, p=0.497) or duration (t9=-1.251, p=0.242) of nose-to-anogenital investigation (Table 1). Additionally, the frequency (t9=0.675, p=0.516) and duration (t9=0.073, p=0.944) of total investigation in non-receptive females was not affected by treatment (Figure 4b, Table 1).
In males, treatment did not affect any investigative behaviors. LPS treatment did not affect the frequency (t4=-0.688, p=0.529) or duration (t4=-0.563, p=0.603) of nose-to-nose investigation or the frequency (t4=-0.168, p=0.875) or duration (t4=-0.057, p=0.958) of nose-to-anogenital investigation in receptive pairs (Table 2). Similarly, in non-receptive pairs, treatment did not affect the frequency (t7=-0.150, p=0.885) or duration (t7=0.228, p=0.826) of nose-to-nose investigation or the frequency (t7=-1.572, p=0.160) or duration (t7=-1.277, p=0.242) of nose-to-anogenital investigation in experimental males (Table 2). Further, in males paired with receptive females, there was no change in the frequency (t4=-0.629, p=0.563) or duration (t4=1.081, p=0.341) of total investigation (Table 2). Likewise, males paired with non-receptive females showed no change in the frequency (t7=-1.050, p=0.329) or duration (t7=-0.867, p=0.415) of total investigation (Figure 4c&d, Table 2). All investigative behaviors performed by intruders did not significantly differ across treatment groups (p > 0.05 in all cases).
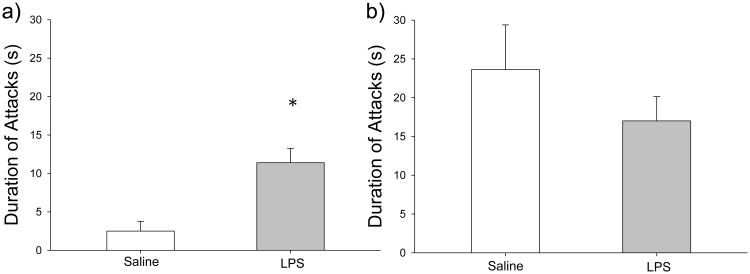
4.7 Early-life immune activation affected aggression in females
LPS treatment significantly affected the duration of attacks in receptive females (t4=-4.100, p=0.015) (Figure 5a and Table 1), but the difference in the frequency of the attacks did not reach significance (t4=-2.086, p=0.105). In contrast, the frequency (t9=0.544, p=0.600) and duration (t9=1.049, p=0.322) of attacks were not significantly affected in non-receptive females (Figure 5b and Table 1). The latency to first attack was not different across treatment groups (Table 1). Receptive females that were LPS-treated displayed no difference in the latency to first attack (t4=-0.571, p=0.599), and similarly, non-receptive females displayed no difference in latency to first attack across treatment groups (t9=1.734, p=0.117) (Table 1). All aggressive behaviors performed by female intruders did not significantly differ across treatment groups (p > 0.05 in all cases).
Figure 5.
Effects of LPS treatment on aggressive behavior in female hamsters. a) Receptive LPS-treated females (gray bars) exhibited significantly longer attacks on male conspecifics when compared to saline-treated receptive females (white bars) (n=4 saline; n=3 LPS), and b) there was no difference in aggressive behavior in non-receptive females (n=6 saline; n=7 LPS). Bar heights represent mean ± S.E.M. An asterisk (*) indicates statistically significant differences between group means (p < 0.05).
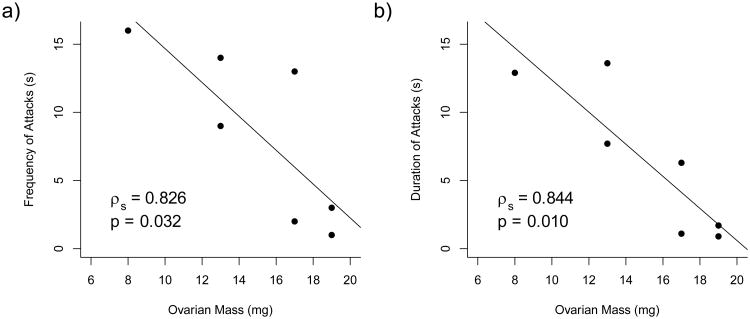
4.8 Receptive female behavior is associated with ovarian mass
The frequency and duration of attacks in receptive female hamsters were significantly associated with ovarian mass in adulthood (ρs=0.826, n=7, p=0.032; ρs=0.844, n=7, p=0.010) (Table 3, Figure 6). Although the frequency and duration of nose-to-nose investigation and the frequency and duration of total investigation in receptive females did not reach significance, there were clear associations between these behaviors and ovarian mass in adulthood (Table 3).
Table 3.
Relationships between ovarian mass and social behavior in receptive females (n=4 saline; n=3 LPS). An asterisk (*) indicates statistically significant correlations between ovarian mass and behavior (p < 0.05).
| Behavior | ρs | n | p |
|---|---|---|---|
| Frequency of Nose-to-Nose Investigation | 0.771 | 7 | 0.055 |
| Duration of Nose-to-Nose Investigation | 0.771 | 7 | 0.058 |
| Total Frequency of Investigation | 0.624 | 7 | 0.052 |
| Total Duration of Investigation | 0.624 | 7 | 0.070 |
| Frequency of Attacks | 0.826 | 7 | 0.032* |
| Duration of Attacks | 0.844 | 7 | 0.010* |
Figure 6.
Associations between aggression and ovarian mass in receptive females. There were associations between the frequency (a) and duration (b) of attacks and ovarian mass in receptive females in adulthood.
4.9 Early-life immune activation did not affect scent-marking behavior in males
LPS-treated males paired with receptive females exhibited no change in the frequency (t4=0.650, p=0.551) or duration (t4=0.809, p=0.464) of scent-marking, and similarly, LPS-treated males paired with non-receptive females showed no difference in the frequency (t7=-0.254, p=0.807) or duration (t7=-0.348, p=0.738) of scent-marking as well (Table 2). Scent-marking performed by intruders was not significantly different across treatment groups (p > 0.05 in all cases).
5. Discussion
It is becoming increasingly clear that early-life infection can have profound impacts on adult behavior [reviewed in 5]. The precise mechanisms by which early-life infection influences subsequent adult physiology and behavior, as well as sex differences seen in response to these stressors, however, remain unclear despite potential consequences for fitness and reproductive success [3,4,22]. Here, we tested the effects of early-life immune challenge on reproductive development and social behavior. We paired hamsters and mimicked a bacterial infection in early life, by administering LPS to experimental pups or saline to control pups of both sexes at pnd3 and pnd5. In adulthood, hamsters were placed in staged mating pairs with reproductively mature individuals of the opposite sex, and we scored an array of social behaviors. Although males and females showed no change in food intake, body mass, or reproductive behaviors, LPS-treated females showed impaired measures of reproductive physiology and function (i.e., abnormal estrous cycles, smaller ovaries) in adulthood. Additionally, LPS-treated females displayed increased investigation of male conspecifics and increased aggression towards males in a reproductive context. We found significant associations between aggression and ovarian mass in those receptive females as well. In contrast, LPS-treated males showed no change in any of the physiological measures that we investigated, nor did they show any changes in behavior. Here we provide evidence that early-life sickness can have profound sex-specific effects on physiology and behavior. More specifically, we suggest that females may be more strongly affected by immune challenge than males, as determined by the specific measures investigated here, and that these changes may have the potential to produce long-term consequences on fitness. Although small sample sizes in some behavioral measures restrict our ability to control for multiple comparisons, we feel confident that the changes we see in behavior are specific in nature, and they help us to better understand how early-life infection may modulate social behaviors particularly important in opposite-sex social interactions across rodent species. Further, the sex-specific physiological effects of early-life LPS on males and females within the same litter that we measure here (e.g., ovaries, testes), which have larger samples sizes, enable us to make meaningful contributions to the possible role of sex in response to early-life inflammation.
5.1 Sex Differences in Response to Immune Challenge
A wide range of studies has investigated the effects of immune challenge on physiology and behavior; however, many of these studies focus solely on male rodents or on varying physiological measures. For example, LPS treatment attenuates testicular development in pre-pubertal male hamsters, though the same investigation has not been done in females [24]. In another study, male rats that received neonatal E. coli had attenuated corticosterone responses following tail shock when compared with vehicle-injected males [45]. Further, studies examining the effects of immune challenge on physiology and behavior in females are often inconsistent. For example, in one study in female rats, neonatal LPS resulted in delayed vaginal opening and first estrus [22]. In contrast, in another study, female rats showed compensatory growth, and consequently, earlier onset of puberty and first estrus, as well as smaller ovaries and fewer primordial follicles in adulthood [46].
Although the focus of recent work has shifted away from the near-exclusive study of the effects of treatment on males, with increasing experimental attention being given to females, the literature is still lacking in comparisons across sexes within the same study [47]. Here, we have focused on not only the role that immune challenge plays in social development, but further, we looked at both males and females within the same litters and across litters, with the goal of identifying how sex may affect the response to the same treatment. Similar to some previous studies, we found that females were robustly affected by neonatal LPS treatment. Unlike other studies, however, we did not see a change in timing of vaginal opening; instead we found that LPS-treated females showed higher rates of abnormal estrous cycling and smaller ovaries in adulthood. Though the reproductive measures that we investigated here are not equivalent, and therefore cannot be compared directly to each other (e.g., ETV cannot be compared directly to vaginal patency), some of the sex differences we see in response to immune challenge may be due to gonadal steroids. Many studies suggest that there are complex interactions between sex steroids and the immune system, and that testosterone is an immune suppressor, whereas, estrogens are immune enhancers [48]. Thus, it is possible that females may have a more intense cellular and humoral immune response following LPS exposure and that they are more strongly affected by treatment than males [49]. In our study, however, we investigated only a subset of the potential measures to determine effects of LPS on physiology; therefore, it is possible that not all aspects of physiology are affected in the same manner.
Further, it is well known that maternal behavior itself is an extremely important facet in the development of offspring behavior and that in some rodent models (e.g., domestic rats and mice), cross-fostering is often a preferred approach to studying these types of effects [50]. In Siberian hamsters, however, dams will often cannibalize their own pups and/or unfamiliar pups [3, personal observation]. These hamster mothers are notoriously sensitive to environmental perturbations that can trigger cannibalism. Because of this, we maintained all pups in the litter and provided the same treatment to all pups within the litter. We do recognize, however, that dams may have provided differential maternal care (e.g., to females) within the same litter providing the opportunity for maternal behavior to have contributed to the effects of LPS on development of physiology and behavior [reviewed in 52]. Therefore, we cannot be sure that the effects of LPS on social behavioral development here are direct. Future studies will be needed to further investigate the potential effects of LPS on additional aspects of physiology.
5.2 The importance of Social Behavior in Successful Reproduction
Many studies have focused on the effects of sickness on a number of different non-social behaviors, as well as same-sex social interactions [reviewed in 5]. For example, adult male rats show increased anxiety-like behavior following neonatal LPS administration, as evidenced by decreased exploration of the open arm of the elevated plus maze [53]. Further, male rats postnatally injected with E. coli show reduced exploration following an adult stressor, but they are able to recover more quickly than males injected with phosphate buffered saline (PBS) [45]. While it is important to understand how animals behave in a non-social context, animals must often interact with others in a social setting in order to successfully reproduce and survive. Immune challenge has been shown to affect social behaviors as well, but studies are inconsistent and often investigate a single sex. For example, in an aggressive line of male mice, neonatal LPS exposure decreases aggression but increases social reactivity in adulthood [54]. In a separate study, both male and female rats exposed to neonatal sickness show decreased defensive behavior in adulthood, but they show no change in social behavior or olfactory discrimination [55]. The species used in studies throughout the literature may play a role in the varying findings, yet, this has not yet been investigated thoroughly.
Although we found that male and female reproductive behaviors were not disrupted, we demonstrated marked differences in pre-copulatory behaviors in female hamsters. Because animals are faced with many different potential mates in the wild, choosing the optimal mate requires the appropriate interpretation of cues to determine the best chance for successful copulation. To do so, many rodents, including Siberian hamsters, rely on chemoinvestigation to identify information about potential mates. In addition to the effects of postnatal sickness on reproductive physiology in females that we demonstrate here, we also show that neonatal sickness affects female chemoinvestigation and aggression with a male conspecific, behaviors important for choosing an appropriate mate. Outside of the laboratory, it is only likely for an animal to be successful in finding a mate when it appropriately integrates information, including chemosensory cues from its conspecifics.
5.3 Potential Mechanisms Mediating the Influence of Immunity on Behavior
While the physiological mechanisms underlying the influence of immunity on behavior were not examined in the present study, several likely candidates exist. One potential mechanism mediating the physiological and behavioral response to early-life immune activation is activation of the hypothalamo-pituitary-adrenal (HPA) axis. For example, rats exposed to LPS as neonates exhibit greater adrenocorticotrophic hormone (ACTH) and corticosterone release to restraint stress than control-treated animals in adulthood, and they exhibit a more rapid corticosterone response to immune stress later in life [5,56]. Additionally, cytokines are released after exposure to LPS, which may cross the blood brain barrier (BBB) due to increased permeability during the developmental period. Exposure to exogenous LPS increases cytokine levels in pubertal and adult mice as well. More specifically, pro-inflammatory cytokines are higher in adult mice than in pubertal mice, but pubertal mice administered LPS exhibit greater levels of anti-inflammatory cytokines than LPS-treated adult mice [49].
Further, the complex interactions between the HPA axis and cytokines may mediate the response to immune challenge. For example, the release of cytokines following LPS administration may alter glucocorticoid receptor function [56,57]. It has been suggested that exposure to LPS during critical stages of development may decrease glucocorticoid receptor density, thereby reducing inhibition of ACTH synthesis and increasing HPA axis sensitivity [58]. Conversely, because glucocorticoids block transcription of some cytokines, including IL-1β, IL-2, and IL-6, it is possible that the presence of particular cytokines may be altered via glucocorticoid action following an LPS injection, but these effects are not completely understood [59,60]. In addition, structural lesions have been found in brain tissue of patients with psychological disease, which may be correlated with common, brain-specific antibodies that penetrate the BBB during fetal development, when the barrier is vulnerable to a number of different molecules [61]. Therefore, antibodies may be capable of inducing alterations in brain function in otherwise healthy individuals, and the effects of these antibodies may not transpire until the individual is exposed to a stressor or a new experience in adulthood [62].
Damage to neural circuits induced by antibodies or toxins may affect the integration of sensory stimuli and the response produced. For example, it is possible that the molecules crossing the BBB during development may induce changes in the emotion-regulating limbic system, therefore creating abnormal reactions to stressors. Further, there are various locations in the brain that can modulate reproductive behavior, including appetitive and precopulatory behaviors (e.g., investigation, locomotion, aggression) and consummatory behaviors (e.g., lordosis, ejaculation) [63]. Areas of the brain involved in providing the motivation to approach a potential mate and investigate odors produced by an individual (e.g., bed nucleus of the stria terminalis, medial preoptic area) may also play an important role in modulating the behavioral response to immune challenge [64]. Changes in these brain areas may influence how individuals find potential mates and even when and how they initiate sexual behaviors [64]. Immune activation may also have effects on dopaminergic pathways. For example, LPS administration reduces dopamine levels in the brain, particularly in the nucleus accumbens, which could influence appetitive and aversive behaviors, suggesting that specific brain regions could influence investigative behaviors, particularly in a reproductive context [65].
Conclusion
Early-life experience, such as resource availability, social environment, and sickness can greatly influence the development of adult physiology and behavior. In order to produce appropriate responses, animals must appropriately integrate stimuli with internal physiology. Here, we provide evidence that early-life immune activation can have profound sex-specific effects on physiology and behavior, and specifically, we suggest that females may be more strongly affected by immune challenge than males, as indicated by the measures we investigated here. Additionally, the effects we demonstrate here may have important consequences on fitness. The results of this study will help to further our understanding of how the neuroendocrine and immune systems interact during early development. More importantly, these results will contribute to a greater understanding of the importance of studying social behavior across sexes, and in particular, studying social behaviors that are crucial in determining optimal mates inside and outside of a laboratory setting.
Highlights.
LPS-treated females showed impaired reproductive physiology and function in adulthood.
Male reproductive physiology and function was not affected by early-life sickness.
Male and female reproductive behavior was not affected by early-life sickness.
Female pre-copulatory investigation was increased following early-life sickness.
Females were more aggressive towards male conspecifics following early-life sickness.
Acknowledgments
The authors thank Dr. Jeff Alberts for help in study design, and L. Achiry, A.C. Amez, J. Bazan, E.D. Carlton, P. Guardado, K.J. O'Malley, E.A. St. John, and E. Weigel for assistance in behavioral filming, necropsies, and general animal procedures. This work was supported by a Sigma Xi Grant in Aid of Research (K.E.S.), a Kinsey Institute Graduate Student Research Award (K.E.S.), the National Institute of Child Health and Human Development (T32HD49336; K.E.S.), and Indiana University.
Footnotes
Author contributions: K.E.S. and G.E.D. designed the research; K.E.S. performed the research and analyzed the data; and K.E.S. and G.E.D. wrote the paper.
Conflict of interest: The authors declare no conflict of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Greives TJ, Kriegsfeld LJ, Bentley GE, Tsutsui K, Demas GE. Recent advances in reproductive neuroendocrinology: a role for RFamide peptides in seasonal reproduction? Proc Biol Sci. 2008;275:1943–51. doi: 10.1098/rspb.2008.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henson JR, Carter SN, Freeman DA. Exogenous T(3) elicits long day-like alterations in testis size and the RFamides Kisspeptin and gonadotropin-inhibitory hormone in short-day Siberian hamsters. J Biol Rhythm. 2013;28:193–200. doi: 10.1177/0748730413487974. [DOI] [PubMed] [Google Scholar]
- 3.Harvey L, Boksa P. Prenatal and postnatal animal models of immune activation: Relevance to a range of neurodevelopmental disorders. Dev Neurobiol. 2012;72:1335–1348. doi: 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- 4.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shanks N, Windle RJ, Perks Pa, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould L, Zeigler T. Variation in Fecal Testosterone Levels, Inter-Male Aggression, Dominance Rank and Age During Mating and Post-Mating Periods in Wild Adult Male Ring Tailed Lemurs (Lemuar catta) Am J Primatol. 2007;69:1325–1339. doi: 10.1002/ajp. [DOI] [PubMed] [Google Scholar]
- 7.Joppa MA, Meisel RL, Garber MA. c-Fos Expression in Female Hamster Brain Following Sexual and Aggressive Behaviors. Neuroscience. 1995;68:783–792. doi: 10.1016/0306-4522(95)00179-m. [DOI] [PubMed] [Google Scholar]
- 8.Kriegsfeld LJ, Demas GE, Huang PL, Burnett AL, Nelson RJ. Ejaculatory Abnormalities in Mice Lacking the Gene for Endothelial Nitric OxideSynthase (eNOS -/-) Physiol Behav. 1999;67:561–566. doi: 10.1016/s0031-9384(99)00100-6. [DOI] [PubMed] [Google Scholar]
- 9.Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23:181–95. doi: 10.1016/0166-4328(87)90019-2. http://www.ncbi.nlm.nih.gov/pubmed/3555537. [DOI] [PubMed] [Google Scholar]
- 10.Alberts JR, Galef BG. Olfactory cues and movement: stimuli mediating intraspecific aggression in the wild Norway rat. J Comp Physiol Psychol. 1973;85:233–42. doi: 10.1037/h0035050. http://www.ncbi.nlm.nih.gov/pubmed/4796493. [DOI] [PubMed] [Google Scholar]
- 11.Pellis SM, Pellis VC. Identification of the Possible Origin of the Body Target That Differentiates Play Fighting From Serious Fighting in Syrian Golden Hamsters (Mesocricetus auratus) Aggress Behav. 1988;14:437–449. doi: 10.1002/1098-2337(198814:6<437∷AID-AB2480140605>3.0.CO;2-Q. [DOI] [Google Scholar]
- 12.Bunnell BN, Boland BD, Dewsbury Da. Copulatory behavior of golden hamsters (Mesocricetus auratus) Behaviour. 1977;61:180–206. [Google Scholar]
- 13.Diz-Chaves Y, Astiz M, Bellini MJ, Garcia-Segura LM. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Hansen MK, Nguyen KT, Fleshner M, Goehler LE, Gaykema RP, Maier SF, Watkins LR. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2000;278:R331–336. doi: 10.1152/ajpregu.2000.278.2.R331. http://www.ncbi.nlm.nih.gov/pubmed/10666132. [DOI] [PubMed] [Google Scholar]
- 15.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan N, Banks Wa. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Cray C, Zaias J, Altman N. Acute phase response in animals: a review. Comp Med. 2009;59:517–526. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2798837/ [PMC free article] [PubMed] [Google Scholar]
- 19.Costalonga M, Zell T. Lipopolysaccharide enhances in vivo interleukin-2 production and proliferation by naive antigen-specific CD4 T cells via a Toll-like receptor 4-dependent mechanism. Immunology. 2007;122:124–130. doi: 10.1111/j.1365-2567.2007.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tohmi M, Tsuda N, Watanabe Y, Kakita A, Nawa H. Perinatal inflammatory cytokine challenge results in distinct neurobehavioral alterations in rats: implication in psychiatric disorders of developmental origin. Neurosci Res. 2004;50:67–75. doi: 10.1016/j.neures.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 21.French SS, Chester EM, Demas GE. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol Behav. 2013;119:175–184. doi: 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Knox AM, Li XF, Kinsey-Jones JS, Wilkinson ES, Wu XQ, Cheng YS, Milligan SR, Lightman SL, O'Byrne KT. Neonatal lipopolysaccharide exposure delays puberty and alters hypothalamic Kiss1 and Kiss1r mRNA expression in the female rat. J Neuroendocr. 2009;21:683–689. doi: 10.1111/j.1365-2826.2009.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorman MR. Seasonal adaptations of Siberian hamsters. I. Accelerated gonadal and somatic development in increasing versus static long day lengths. Biol Reprod. 1995;53:110–5. doi: 10.1095/biolreprod53.1.110. http://www.ncbi.nlm.nih.gov/pubmed/7669841. [DOI] [PubMed] [Google Scholar]
- 24.Prendergast BJ, Hotchkiss AK, Bilbo SD, Nelson RJ. Peripubertal immune challenges attenuate reproductive development in male Siberian hamsters (Phodopus sungorus) Biol Reprod. 2004;70:813–820. doi: 10.1095/biolreprod.103.023408. [DOI] [PubMed] [Google Scholar]
- 25.Paul MJ, Pyter LM, Freeman Da, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. J Neuroendocrinol. 2009;21:1007–14. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey AM, Legan SJ, Demas GE. Exogenous Kisspeptin Enhances Seasonal Reproductive Function in Male Siberian Hamsters. Funct Ecol. 2017:1–11. doi: 10.1111/1365-2435.12846. [DOI] [Google Scholar]
- 27.Schlatt S, De Geyter M, Kliesch S, Nieschlag E, Bergmann M. Spontaneous recrudescence of spermatogenesis in the photoinhibited male Djungarian hamster, Phodopus sungorus. Biol Reprod. 1995;53:1169–77. doi: 10.1095/biolreprod53.5.1169. http://www.ncbi.nlm.nih.gov/pubmed/8527522. [DOI] [PubMed] [Google Scholar]
- 28.Adam CL, Moar KM, Logie TJ, Ross aW, Barrett P, Morgan PJ, Mercer JG. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology. 2000;141:4349–56. doi: 10.1210/endo.141.12.7807. [DOI] [PubMed] [Google Scholar]
- 29.Scotti MAL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;52:183–90. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Rendon NM, Rudolph LM, Sengelaub DR, Demas GE. The agonistic adrenal : melatonin elicits female aggression via regulation of adrenal androgens. Proc R Soc B Biol Sci. 2015 doi: 10.1098/rspb.2015.2080. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffatt-Blue CS, Sury JJ, Young Ka. Short photoperiod-induced ovarian regression is mediated by apoptosis in Siberian hamsters (Phodopus sungorus) Reproduction. 2006;131:771–82. doi: 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlton ED, Cooper CL, Demas GE. Metabolic stressors and signals differentially affect energy allocation between reproduction and immune function. Gen Comp Endocrinol. 2014;208:21–29. doi: 10.1016/j.ygcen.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shahed A, Young KA. Differential Ovarian Expression of KiSS-1 and GPR-54 During the Estrous Cycle and Photoperiod Induced Recrudescence in Siberian Hamsters (Phodopus sungorus) Mol Reprod Dev. 2009;76:444–452. doi: 10.1124/dmd.107.016501.CYP3A4-Mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey AM, Rendon NM, O'Malley KJ, Demas GE. Food as a supplementary cue triggers seasonal changes in aggression, but not reproduction, in Siberian hamsters. Physiol Behav. 2016;167:298–308. doi: 10.1016/j.physbeh.2016.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wynne-Edwards KE, Lisk RD. Behavioral interactions differentiate Djungarian (Phodopus campbelli) and Siberian (Phodopus sungorus) hamsters. Can J Zool. 1987;65:2229–2235. doi: 10.1139/z87-337. [DOI] [Google Scholar]
- 36.Beach FA, Stern B, Carmichael M. Comparisons of Sexual Receptivity and Proceptivity in Female Hamsters 1. Vol. 487. Department of Psychology, University of California; Berkeley, California 94720: 1976. pp. 473–487. [DOI] [PubMed] [Google Scholar]
- 37.Harvey E, Yanagimachi R, Chang MC. Onset of Estrus and Ovulation in the Golden Hamster'. J Exp Zool. 1961 doi: 10.1002/jez.1401460303. [DOI] [PubMed] [Google Scholar]
- 38.Mani SK, Allen JM, Rettori V, McCann SM, O'Malley BW, Clark JH. Nitric oxide mediates sexual behavior in female rats. Proc Natl Acad Sci U S A. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erskine MS. Mating-induced increases in FOS protein in preoptic area and medial amygdala of cycling female rats. Brain Res Bull. 1993;32:447–451. doi: 10.1016/0361-9230(93)90289-N. [DOI] [PubMed] [Google Scholar]
- 40.Wynne-Edwards KE, Lisk RD. Differences in behavioral responses to a competitive mating situation in two species of dwarf hamster (Phodopus campbelli and P. sungorus) J Comp Psychol. 1988;102:49–55. doi: 10.1037/0735-7036.102.1.49. [DOI] [Google Scholar]
- 41.Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- 42.Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240:27–41. doi: 10.1016/0006-8993(82)90641-2. http://www.ncbi.nlm.nih.gov/pubmed/7093718. [DOI] [PubMed] [Google Scholar]
- 43.Etgen AM, Shamamian P. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Horm Behav. 1986;20:166–180. doi: 10.1016/0018-506X(86)90015-2. [DOI] [PubMed] [Google Scholar]
- 44.Powers JB, Steel Ea, Hutchison JB, Hastings MH, Herbert J, Walker aP. Photoperiodic Influences on Sexual Behavior in Male Syrian Hamsters. J Biol Rhythms. 1989;4:61–78. doi: 10.1177/074873048900400105. [DOI] [PubMed] [Google Scholar]
- 45.Bilbo SD, Yirmiya R, Amat J, Paul ED, Watkins LR, Maier SF. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology. 2008;33:261–9. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sominsky L, Meehan CL, Walker AK, Bobrovskaya L, McLaughlin Ea, Hodgson DM. Neonatal immune challenge alters reproductive development in the female rat. Horm Behav. 2012;62:345–55. doi: 10.1016/j.yhbeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: A review of the evidence. Anim Behav. 2004;68:227–239. doi: 10.1016/j.anbehav.2004.05.001. [DOI] [Google Scholar]
- 49.Cai KC, van Mil S, Murray E, Mallet JF, Matar C, Ismail N. Age and sex differences in immune response following LPS treatment in mice. Brain Behav Immun. 2016;58:327–337. doi: 10.1016/j.bbi.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9:128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 51.Day CS, Galef BG. Pup cannibalism: One aspect of maternal behavior in golden hamsters. J Comp Physiol Psychol. 1977;91:1179–1189. doi: 10.1037/h0077386. [DOI] [Google Scholar]
- 52.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Granger Da, Hood KE, Dreschel Na, Sergeant E, Likos a. Developmental effects of early immune stress on aggressive, socially reactive, and inhibited behaviors. Dev Psychopathol. 2001;13:599–610. doi: 10.1017/S0954579401003108. [DOI] [PubMed] [Google Scholar]
- 55.Foley Ka, Macfabe DF, Vaz A, Ossenkopp KP, Kavaliers M. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: Implications for autism spectrum disorders. Int J Dev Neurosci. 2014;39:1–11. doi: 10.1016/j.ijdevneu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Shanks N, Meaney J. Neonatal Endotoxin Exposure Responsivity to Stress Alters the Development of the Axis : Early Illness and Later. 1995;15 doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84. doi: 10.1523/JNEUROSCI.15-01-00376.1995. http://www.ncbi.nlm.nih.gov/pubmed/7823142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franchimont D, Kino T, Galon J, Meduri GU, Chrousos G. Glucocorticoids and inflammation revisited: The state of the art - NIH Clinical Staff Conference. Neuroimmunomodulation. 2002;10:247–260. doi: 10.1159/000069969. [DOI] [PubMed] [Google Scholar]
- 60.Smoak K, Cidlowski Ja. Glucocorticoids Regulate Tristetraprolin Synthesis and Posttranscriptionally Regulate Tumor Necrosis Factor Alpha Inflammatory Signaling. Mol Cell Biol. 2006;26:9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diamond J, Bond A. A Comparative Analysis of Social Play in Birds. Behaviour. 2003;140:1091–1115. doi: 10.1163/156853903322589650. [DOI] [Google Scholar]
- 62.Adde-Michel C, Hennebert O, Laudenbach V, Marret S, Leroux P. Effect of perinatal alcohol exposure on ibotenic acid-induced excitotoxic cortical lesions in newborn hamsters. Pediatr Res. 2005;57:287–293. doi: 10.1203/01.PDR.0000148712.30716.9D. [DOI] [PubMed] [Google Scholar]
- 63.Pfaus JG, Kippin TE, Centeno S. Conditioning and sexual behavior: a review. Horm Behav. 2001;40:291–321. doi: 10.1006/hbeh.2001.1686. doi:10.1006/hbeh.2001.1686\nS0018-506X(01)91686-1 [pii] [DOI] [PubMed] [Google Scholar]
- 64.Been LE, Petrulis A. Dissociated functional pathways for appetitive and consummatory reproductive behaviors in male Syrian hamsters. Horm Behav. 2012;61:204–211. doi: 10.1016/j.yhbeh.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann N Y Acad Sci. 2004;1018:281–287. doi: 10.1196/annals.1296.033. [DOI] [PubMed] [Google Scholar]