Abstract
Gestational diabetes mellitus (GDM) poses well-established risks to both the mother and infant. As over 50% of women with GDM will develop type 2 diabetes mellitus (T2DM) in their lifetime, performing postpartum oral glucose tolerance testing (OGTT) is paramount to initiation of appropriate lifestyle interventions and pharmacologic therapy. Nonetheless, test completion among women with GDM is estimated to be <50%, with particularly low rates in Latina patients, as well as patients with public insurance, low education levels, and low health literacy. Data suggest our current health services infrastructure loses patients in the postpartum gap between pregnancy-focused care and primary care. As previous studies have demonstrated strategies to promote OGTT completion for T2DM prevention, hereto is a proposal of best practices including 1) enhanced patient support for identifying long-term health care providers 2) patient-centered medical home utilization when possible 3) patient and provider test reminders, and 4) formalized obstetrician-primary care provider hand-offs using the “SBAR” (Situation Background Assessment Recommendation) mnemonic. These strategies deserve future investigation to solidify a multi-level approach for identifying and preventing the continuum of diabetes.
Keywords: gestational diabetes mellitus, glucose tolerance testing, patient hand-offs, preventive medicine, postpartum care, SBAR, transitions of care, type II Diabetes Mellitus
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance developed during pregnancy.1 As with type 2 diabetes mellitus (T2DM), the incidence of GDM is growing; GDM currently affects an estimated 5–10% of pregnancies in the United States, with approximately 250,000 new cases each year.2 Not only has there been a steady increase in the prevalence of GDM over the past 20 years, but the rising national trends of advanced maternal age, obesity, and decreased physical activity will contribute to a further increase in the prevalence of GDM in years to come.3
The diagnosis of GDM bears associated short-term and long-term risks for both the infant and mother. The correlation between GDM and macrosomia, neonatal hypoglycemia, birth trauma, and subsequent overweight in the offspring has been well-established.4 For the mother, GDM is associated with increased risk of hypertensive disorders, cesarean delivery, and other perinatal complications.5 Furthermore, 30% of women with GDM remain glucose intolerant after delivery, and over half ultimately receive the diagnosis of overt diabetes.6 The risks of T2DM in pregnancies subsequent to an index pregnancy with GDM are amplified beyond those incurred by GDM alone.7–9
The first step in long-term risk management of women with GDM is postpartum glucose tolerance testing. Groups such as the American College of Obstetrics and Gynecology (ACOG) and the American Diabetes Association (ADA) recommend that women with GDM receive care 6–12 weeks after delivery to assess blood pressure, body mass index (BMI), and metabolic profile, in addition to routine postpartum concerns (Table 1). Women are additionally recommended to visit their primary care provider (PCP) within a year of delivery; PCPs may perform further metabolic testing, recommend pharmacologic therapy, and utilize lifestyle modalities to promote weight loss, which has been shown to reduce the onset of diabetes, as demonstrated in the Diabetes Prevention Program.10–13
Table 1.
Current Guidelines for Postpartum Care of Women with GDM
| Recommended Testing Timeline and Method | Recommended Interventions | Offspring | |
|---|---|---|---|
| American Diabetes Association1 |
6–12 weeks postpartum Screen women with GDM for persistent diabetes using 75g OGTT and non-pregnancy criteria
|
|
|
| Endocrine Society56 |
24–72 hours after delivery Fasting plasma glucose OR fasting self-monitored blood glucose 6–12 weeks postpartum 2-hour, 75-g OGTT
|
|
|
| American College of Obstetricians and Gynecologists5 |
6–12 weeks postpartum. Fasting plasma glucose test OR a 75-g, two-hour oral glucose tolerance test
|
|
Despite these recommendations, data reveal that less than 50% of women with GDM partake in any form of postpartum glucose testing.14–18 Our goal is to use a public health and health services perspective to discuss barriers to optimal postpartum care for women with GDM, review evidence based interventions, and offer recommendations for a multi-level approach for serving this important and growing population.
Barriers to Diabetes Postpartum Care
Receipt of appropriate postpartum glucose testing is contingent upon returning for postpartum care. Others have reported extensively on barriers to receiving postpartum care, and we will highlight issues unique to women with GDM. Indeed the social, financial, and structural barriers to receiving postpartum care additionally serve as barriers to receiving diabetes-specific care. Such barriers include out-of-pocket costs, lack of health insurance, appointment wait times, childcare availability, transportation costs, demanding work schedules, and lack of supported parental leave.19–21 Additional barriers specific to completing the postpartum oral glucose tolerance test (OGTT) can be divided into patient characteristics and beliefs, inadequate provider training, and ineffective system-level practices, which are discussed below.
Several studies have sought to identify individual characteristics associated with postpartum appointment attendance and OGTT completion in women with GDM. Patients who are Asian, older, nulliparous, or with medication-controlled GDM are more likely to return for testing.14,15,22 Risk factors for poor follow up include Latina ethnicity, public insurance, less education, and lower health literacy.14,15,23 Low health literacy has a well-studied association with inadequate health service utilization, and in this particular population, limited literacy/numeracy could limit T2DM risk estimation or result in confusion regarding instructions.24 Among women who report to understand their future T2DM risk but do not complete their OGTT, many express anxiety about receiving a T2DM diagnosis, citing fears of diabetes complications and needing lifelong insulin.20,21,25
From a provider perspective, ACOG best practices include counseling women with GDM about their higher lifetime risk of cardiometabolic disease, as well as ensuring that these women undergo postpartum glucose screening.19 The ADA recommends women with a history of GDM receive education about lifestyle modification.1 While the recommendations are well established, the literature suggests room for improvement in provider knowledge and implementation. One study, for example, showed the minority of obstetricians/gynecologists knew that >40% of women with GDM will progress to T2DM within 10 years.26 This work also showed that exercise counseling and nutrition referrals were low for both obstetrician/gynecologists and certified nurse-midwives.26,27
Limited access to care and inadequate obstetric-primary care transitions pose system-level barriers to long-term diabetes prevention and management. In many states, Medicaid coverage for the mother extends to only 60 days postpartum.28 Subsequent care may require women to pay out of pocket, which poses a cost burden that likely deters mothers from pursuing long-term health care. While new mothers are encouraged to seek health care through systems such as the Affordable Care Act, enrollment can be challenging and may not occur in a timely manner. Thus, for women in underserved communities in particular, many of whom may not have physician contact prior to or between pregnancies, it can be especially difficult to focus on primary prevention.29
Care transition is also suboptimal. A 2014 study showed that among women with Medicaid, 65% of those with a pregnancy complicated by GDM or hypertensive disorders had a postpartum obstetric visit within three months of delivery, and only 56.6% visited a primary care doctor within a year.30 This clearly represents an opportunity for improving care coordination, especially since an estimated 44.9% of the nation’s births are covered by Medicaid each year.28 Furthermore, of women who do see a PCP after delivery, many fail to disclose a pregnancy complicated by GDM.31 While this gap could be ameliorated by better communication, transitional health care between obstetricians/gynecologists and PCPs remains inconsistent in both frequency and efficacy.
Bridging the Diabetes Postpartum Gap
There are multiple levels of barriers to optimal postpartum care for women with GDM, including systems-based and communications barriers. We seek to close the communication gap between obstetricians and PCPs by highlighting existing data and areas for future research on care transition. We propose a more streamlined transition between providers could provide more optimally identify and aid women following a pregnancy complicated by GDM. Thus we offer a four-pronged model for best practices including 1) enhanced support for identifying long-term care 2) reducing barriers via patient centered medical home utilization when possible 3) reminder systems, and 4) formalized obstetrician-PCP hand-offs using the “SBAR” (Situation Background Assessment Recommendation) mnemonic.
1) Establishing long-term care
“Transition of care” refers to the period in which a patient’s healthcare setting and team change based on her evolving needs. The postpartum period is a prototype for transition of care, in which women transition from intensive pregnancy-focused care to long-term primary health needs. We find that care transitions are typically initiated shortly before a provider is ready to transfer responsibility of a patient’s care to another provider. This sequence makes it far too easy for a patient to leave obstetrical care without a concrete or feasible follow-up plan.
Thus, our transition model begins upon establishment of prenatal care (Figure 1). We recommend that upon establishment of prenatal care, the obstetric team initiate dialogue with each patient regarding the importance of healthcare outside pregnancy. If necessary and feasible, the team can utilize support services (social work, nursing support) to secure this patient-PCP relationship. Simultaneously, transition preparation is benefitted by use of the electronic medical record (EMR). Documentation of the GDM diagnosis can enhance care by reminding the obstetrician of the diagnosis and need for postpartum transition, as well as serve as direct communication with a PCP when providers share EMR systems.
Figure 1.
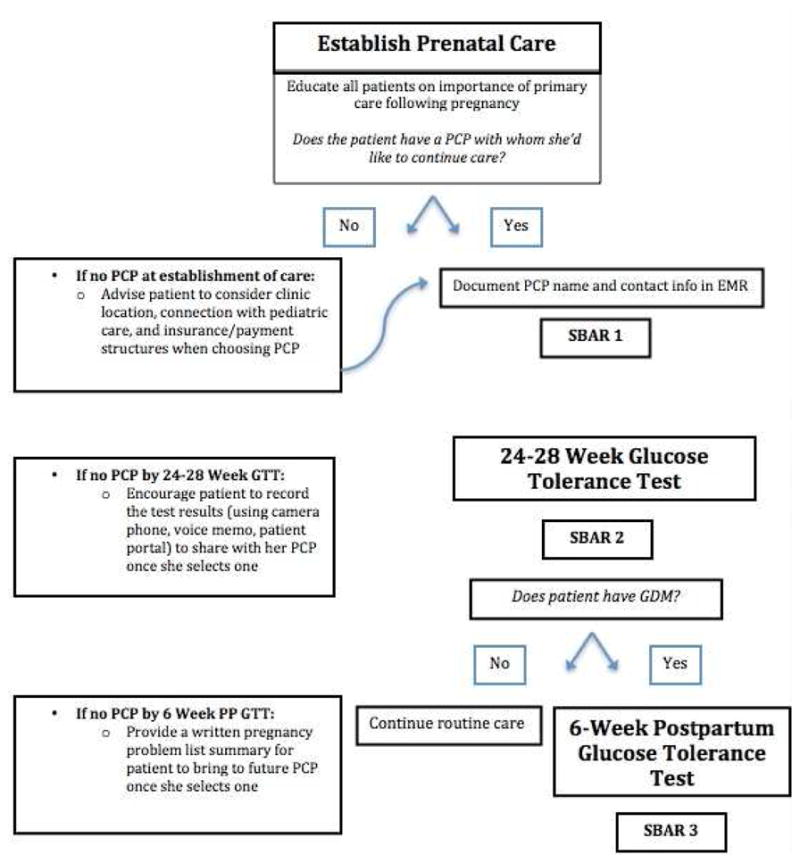
2) Reducing barriers via patient-centered medical home
It is paramount to identify women without an established PCP and assist them as they navigate the healthcare system on behalf of themselves and their newborns. Work by our group on patient navigation suggested that concerted efforts to decrease patient logistical barriers to making and attending postpartum appointments is associated with improved postpartum attendance.32 However, common patient feedback in this program included the desire for coordinated maternal-newborn care. To address these needs, one strategy is the patient-centered medical home (PCMH), a model which revolves around preventive care and coordination of care.33 This model’s emphasis on collaboration between team members (physicians, nutritionists, social workers) also makes it well-equipped to transition between OGTT completion and execution of an individualized, concrete action plan. As the PCMH model has only recently gained momentum, only 10% of PCPs are at National Committee for Quality Assurance recognized PCMHs, so this will not yet be accessible to many patients.34 Nonetheless, the prenatal care team can convey the importance of seeking a PCP and identifying one who will minimize burdens to engaging in long-term health care, which may include coordinating with pediatric care.
3) Reminder systems
An emerging branch of postpartum diabetes screening research has been dedicated to the use of reminder systems targeting the patient, doctor, or both using email, letter, text, phone call, and/or EMR. A 2013 survey found that obstetricians who were more likely to perform a postpartum OGTT were significantly more likely to call or mail reminders to patients as well as use EMR alerts.26 Several prospective studies have also addressed such reminder systems. In one example, women with GDM were randomized to receive reminders mailed 3 months after delivery to both physician and patient, physician only, patient only, or neither. The physician reminder included the Canadian Diabetes Association’s postpartum screening recommendations, and patient reminders included information about the importance of screening and a lab order to complete the OGTT. Each intervention group had a statistically significant increased rate of OGTT completion, with screening rates ranging from 51.6–60.5%, compared to 14.3% without reminders.35 Similarly, the 2013 DIAMIND trial randomized Australian women with GDM to a single text message reminder at 6 months postpartum versus text reminders at 6 weeks, 3 months and 6 months postpartum.36 While this study did not produce statistically significant results, such a protocol has been proposed in larger populations, and future work on reminder systems for both screening and uptake of prevention strategies has been recommended.37
Given the growing evidence base for reminder systems, we recommend that clinics institute reminder systems to promote patient and provider adherence to screening recommendations. Such reminder systems may vary in format, including electronic, nurse-initiated, or paper, depending on patient needs and clinical resources. In addition, we recommend communicating with patients via patient health care portals when possible. Many of these portals have associated smartphone applications that promote ease of communication. Notably, studies examining communication preferences in postpartum women show that the majority want information and reminders sent electronically or to their phones rather than through voicemail or mail.38–40
4) Using SBAR to execute effective hand-off communication
Once a PCP is chosen, transitions have been discussed, and reminder systems are in place, the remaining critical step requires the prenatal care provider to execute effective hand-off communication. ACOG defines “effective” as complete, clear, concise, and timely, and recommends emphasizing 1) interactive communications, 2) limited interruptions, 3) a process for verification, and 4) an opportunity to review any relevant historical data to maximize clarity.41
To achieve these goals, there is an array of proposed hand-off mnemonics in the literature. A 2009 systematic review of hand-off mnemonics highlighted 24 mnemonics, including the commonly cited “I PASS THE BATON” (Introduction Patient identifiers Assessment Situation Safety concerns THE Background Actions Timing Ownership Next) and SBAR (Situation Background Assessment Recommendations).42 Of the 24 mnemonics reviewed, the majority were designed for emergency department or inpatient settings; none were developed to convey long-term care goals. However, we believe use of a simple mnemonic may be as applicable to the outpatient setting as it is to higher acuity care. Therefore, to enhance our multi-level approach for obstetric-PCP hand-offs, we prioritized use of SBAR, a concise, widely recognizable mnemonic.
SBAR is an evidence-based, Joint Commission-endorsed mnemonic which has functioned in studies representing multiple healthcare settings, specialties, and levels of practitioners.43,44 SBAR is user-friendly across specialties and allows providers to include as much pertinent clinical information as needed. SBAR exchanges can be done via verbal, written, or EMR-based communication, and should preferably allow for questions and closed loop communication. As exemplified in Table 2, the obstetrician should use SBAR1 to connect with the PCP upon establishment of prenatal care and SBAR2 following the GDM diagnosis to update the PCP and communicate the management plan. Postpartum, the obstetrician should initiate SBAR3. If the patient did not attend her appointment, or the OGTT was not completed, SBAR3 should reflect the urgency of follow-up. We also propose that a patient-centered version of SBAR3 be delivered to the patient, as a reminder to her about the need for this next step of care. It should also clearly state whether the obstetrician intends to achieve that follow-up or if he/she is officially passing responsibility to the PCP.
Table 2.
Obstetrician to PCP Hand-Off Model
| SBAR Model57 | SBAR 1 Initiation of prenatal care | S AR 2 24–28 week OGTT: New diagnosis of GDM | SBAR 3 Postpartum |
|---|---|---|---|
|
Situation: State what is happening at the present time that has warranted the SBAR communication Background: Explain circumstances leading up to this situation. Put the situation into context for the reader/listener. Assessment: What do you think the problem is? Recommendation: What would you do to correct the problem? |
Situation:
|
Situation:
|
Situation:
|
PCP, primary care provider; OGTT, oral glucose tolerance test; EDD, estimated due date; BMI, body mass index; PCOS, polycystic ovary syndrome
Alternative Postpartum Testing Methods
In addition to the systems issues proposed above, there are multiple diabetes diagnostic methods available to postpartum providers. The 2014 ADA Standards of Care recommend administering an OGTT 6–12 weeks after delivery, requiring women to fast 8 hours prior to the test, then stay at the appointment for 2 hours for multiple blood draws. Additional costs include phlebotomy services, clinic time, and laboratory analysis. While the OGTT is the “gold standard”, the Endocrine Society and ACOG affirm the use of fasting plasma glucose (FPG) and/or self-monitored blood glucose for postpartum screening (Table 1). The ADA similarly acknowledges that in clinical settings, the FPG test is preferred because of “ease of administration, convenience, acceptability to patients, and lower cost.”45 Additionally, in 2009, an International Expert Committee added the hemoglobin A1c (HbA1c) as an option; the HbA1C does not require fasting and reflects a 3-month average of blood glucose levels, making the reading less prone to variation with stress or illness.46
Many investigators have explored whether these alternative methods could increase test completion rates without sacrificing predictive value. A recent study showed that performing the OGTT on postpartum day 2 resulted in 92% test completion, which is among the highest reported test completion rates in the literature.23 As this approach occurs during a critical transition period, the fasting occurs overnight, with the blood draw occurring first thing in the morning to minimize fasting while breastfeeding. This study demonstrated 100% sensitivity and 94% specificity for detecting T2DM, but the method was less sensitive and specific for lesser degrees of abnormal glucose tolerance.23
A 2016 study explored the potential role of third trimester HbA1c, and found that HbA1c >6.5% identified all women who ultimately received a T2DM diagnosis five years after pregnancy.47 A 2014 meta-analysis shared this study’s conclusion that HbA1c alone lacks the sensitivity to detect non-T2DM abnormal glucose tolerance, but it could be used to identify high-risk women who could benefit from early lifestyle interventions and close monitoring. 47,48 Similarly, studies combining HbA1c and FPG offered approximately 90% sensitivity and specificity for detecting abnormal glucose tolerance in the postpartum period, potentially decreasing the number of necessary OGTTs and providing a more convenient approach to risk stratification.49, 50
We do not believe that current literature supports an alternative testing method at this time; however, we support the ongoing evaluation of how changing testing method and/or test timing may improve test completion without sacrificing predictive value. Additionally, a metabolomics approach shows promise in providing another accurate, low-hassle testing method for predicting incident T2DM cases.51
Developing an Evidence Base for Multilevel Care
Existing data suggest any one approach, such as reminder systems alone, may be inadequate. For example, Zera et al performed a randomized trial of an EMR reminder system for the postpartum OGTT and found no improvement in the rate of screening.52 Similarly, our patient navigation program increased postpartum follow-up rates from 70.3% to 88.1% (p<0.001), but there was no statistically significant increase in OGTT completion.32 A 2012 Kaiser Permanente study, however, sought to combine appointment reminders with other systems processes.53 The nursing protocol was revised to allow nursing staff to order the tests, and nurse care managers were encouraged to make at least one postpartum reminder call. Second, the team altered the EMR to add a check box for the FPG within order entry sets. Clinical staff attended a training program and received a patient handout describing the importance of follow-up screening and risks associated with T2DM. Lastly, the nurse case manager called each woman up to three times if she had not completed her OGTT by 3 months postpartum, then sent an email or letter if necessary. While the rate of completed glucose screenings was not statistically significantly increased within 3 months of delivery, the second round of reminders yielded improvements in overall test completion (71.5% v. 59.5%).53
These emerging data suggest multilevel interventions may be an effective approach. We propose that important next steps in improving care for postpartum women with GDM will be to study approaches such as those proposed. Many individual health systems have likely instituted their own GDM quality improvement programs; we advocate for rigorous evaluation and dissemination of these programs to optimize care. Our group proposes implementing a step-by-step quality improvement initiative in which we begin the transition process early in prenatal care. We intend to incorporate an EMR-based reminder system such that all women with GDM on the “problem list” receive OGTT reminders via the online health portal and has her chart flagged with a provider reminder. We then propose to institute SBAR. Evaluation of this program will require understanding provider satisfaction, efficacy (long-term follow-up rates), and resource utilization, all of which will be critical to developing an effective transition program.
Next steps must also focus on the unique needs of disadvantaged populations, where postpartum follow up rates are lower and yet medical needs are often greater. Implementing system-level changes is a particular burden to low-resource care settings. However, our hope is that the mandatory meaningful use of EMRs by Medicaid-reimbursed providers may be leveraged into improved diagnosis documentation, provider-provider communication, and automated reminders. Additionally, patient-centered exploration of barriers and needs must inform effective implementation in less resourced communities. Finally, carrying out these proposed interventions is time-intensive and costly. Detailed cost analyses must be planned alongside future investigations of the clinical efficacy of these potential strategies.
Summary
GDM is a major public health and clinical concern.3 Up to 70% of women with GDM will develop T2DM within their lifetime, posing enormous medical, emotional, and financial burdens to this population.54 As pregnancy is considered a “window to future health,”55 we propose taking advantage of this time to provide women with GDM appropriate screening, counseling, and support to identify and prevent T2DM. We believe that systematic partnerships between obstetricians and PCPs could secure the transition of care necessary to achieve disease detection, prevention, and long term care.
To combat the multitude of patient, provider, and systems barriers to optimal care for women with GDM, we have examined literature-supported strategies and proposed additional strategies which warrant further research (Table 3). We hope these recommendations will encourage providers to build upon routine postpartum conversations, such as contraception counseling, by tailoring counseling based on a patient’s GDM history. We believe this proposal for collaborative care continuity will meet the unique needs of the postpartum patient with GDM, and in doing so reduce this population’s risk of developing diabetes and diabetes-related complications. We invite our peers to further this endeavor through quality improvement and research endeavors.
Table 3.
Recommendations for Best Practices for Care of Women with Gestational Diabetes Mellitus
| Time Point | Priority |
|---|---|
| Ongoing |
|
| Initiation of Prenatal Care |
|
| Gestational Age: 24–28 Weeks |
|
| Gestational Age: >28 Weeks |
|
| Delivery |
|
| Delivery – 6 Weeks Postpartum |
|
| Postpartum Appointment: 6–12 Weeks |
|
GDM, gestational diabetes mellitus; PCP, primary care provider; SBAR, situation-background assessment-recommendation; OGTT, oral glucose tolerance test
Acknowledgments
FUNDING: LMY is supported by the NICHD K12 HD050121-11.
Footnotes
DISCLOSURES: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Standards of Medical Care in Diabetes—2014. Diabetes care. 2014;37(Supplement 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. 2007;30:S141–146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 4.Garrison A. Screening, diagnosis, and management of gestational diabetes mellitus. 2015;91(7):460–467. [PubMed] [Google Scholar]
- 5.Committee on Practice B-O. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstetrics and gynecology. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 6.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. 2007;30(4):878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 7.Sheffield JS, Butler-Koster EL, Casey BM, McIntire DD, Leveno KJ. Maternal diabetes mellitus and infant malformations. Obstet Gynecol. 2002;100(5 Pt 1):925–930. doi: 10.1016/s0029-7844(02)02242-1. [DOI] [PubMed] [Google Scholar]
- 8.Corrigan N, Brazil DP, McAuliffe F. Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res A Clin Mol Teratol. 2009;85(6):523–530. doi: 10.1002/bdra.20567. [DOI] [PubMed] [Google Scholar]
- 9.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Absetz P, Valve R, Oldenburg B, et al. Type 2 diabetes prevention in the “real world”: one-year results of the GOAL Implementation Trial. Diabetes care. 2007;30(10):2465–2470. doi: 10.2337/dc07-0171. [DOI] [PubMed] [Google Scholar]
- 11.Absetz P, Oldenburg B, Hankonen N, et al. Type 2 diabetes prevention in the real world: three-year results of the GOAL lifestyle implementation trial. Diabetes care. 2009;32(8):1418–1420. doi: 10.2337/dc09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermunt PW, Milder IE, Wielaard F, de Vries JH, van Oers HA, Westert GP. Lifestyle counseling for type 2 diabetes risk reduction in Dutch primary care: results of the APHRODITE study after 0.5 and 1.5 years. Diabetes care. 2011;34(9):1919–1925. doi: 10.2337/dc10-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Annals of internal medicine. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 14.Stasenko M, Cheng YW, McLean T, Jelin AC, Rand L, Caughey AB. Postpartum follow-up for women with gestational diabetes mellitus. 2010;27(9):737–742. doi: 10.1055/s-0030-1253557. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenthal DB, Maiden K, Rogers S, Ball A. Postpartum healthcare after gestational diabetes and hypertension. 2014;23(9):760–764. doi: 10.1089/jwh.2013.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tovar A, Chasan-Taber L, Eggleston E, Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Preventing chronic disease. 2011;8(6):A124. [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara A, Peng T, Kim C. Trends in postpartum diabetes screening and subsequent diabetes and impaired fasting glucose among women with histories of gestational diabetes mellitus: A report from the Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes care. 2009;32(2):269–274. doi: 10.2337/dc08-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietz PM, Vesco KK, Callaghan WM, et al. Postpartum screening for diabetes after a gestational diabetes mellitus-affected pregnancy. Obstetrics and gynecology. 2008;112(4):868–874. doi: 10.1097/AOG.0b013e318184db63. [DOI] [PubMed] [Google Scholar]
- 19.Committee Opinion No. 666: Optimizing Postpartum Care. Obstetrics and gynecology. 2016;127(6):e187–192. doi: 10.1097/AOG.0000000000001487. [DOI] [PubMed] [Google Scholar]
- 20.Bennett WL, Ennen CS, Carrese JA, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. Journal of women's health. 2011;20(2):239–245. doi: 10.1089/jwh.2010.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stasenko M, Liddell J, Cheng YW, Sparks TN, Killion M, Caughey AB. Patient counseling increases postpartum follow-up in women with gestational diabetes mellitus. American journal of obstetrics and gynecology. 2011;204(6):522.e521–526. doi: 10.1016/j.ajog.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggleston EM, LeCates RF, Zhang F, Wharam JF, Ross-Degnan D, Oken E. Variation in Postpartum Glycemic Screening in Women With a History of Gestational Diabetes Mellitus. Obstet Gynecol. 2016;128(1):159–167. doi: 10.1097/AOG.0000000000001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werner EF, Has P, Tarabulsi G, Lee J, Satin A. Early Postpartum Glucose Testing in Women with Gestational Diabetes Mellitus. Am J Perinatol. 2016;33(10):966–971. doi: 10.1055/s-0036-1583193. [DOI] [PubMed] [Google Scholar]
- 24.Yee LM, Niznik CM, Simon MA. Examining the Role of Health Literacy in Optimizing the Care of Pregnant Women with Diabetes. American journal of perinatology. 2016;33(13):1242–1249. doi: 10.1055/s-0036-1584540. [DOI] [PubMed] [Google Scholar]
- 25.Van Ryswyk E, Middleton P, Shute E, Hague W, Crowther C. Women's views and knowledge regarding healthcare seeking for gestational diabetes in the postpartum period: A systematic review of qualitative/survey studies. Diabetes research and clinical practice. 2015;110(2):109–122. doi: 10.1016/j.diabres.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Ko JY, Dietz PM, Conrey EJ, et al. Strategies associated with higher postpartum glucose tolerance screening rates for gestational diabetes mellitus patients. Journal of women's health. 2013;22(8):681–686. doi: 10.1089/jwh.2012.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ko JY, Dietz PM, Conrey EJ, et al. Gestational diabetes mellitus and postpartum care practices of nurse-midwives. Journal of midwifery & women's health. 2013;58(1):33–40. doi: 10.1111/j.1542-2011.2012.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin SC, Osterman MJ, Uddin SF, Sutton SR, Reed PR. Source of payment for the delivery: births in a 33-state and District of Columbia reporting area, 2010. 2013;62(5):1–20. [PubMed] [Google Scholar]
- 29.Paez KA, Eggleston EM, Griffey SJ, et al. Understanding why some women with a history of gestational diabetes do not get tested for diabetes. 2014;24(4):e373–379. doi: 10.1016/j.whi.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Bennett WL, Chang HY, Levine DM, et al. Utilization of primary and obstetric care after medically complicated pregnancies: an analysis of medical claims data. 2014;29(4):636–645. doi: 10.1007/s11606-013-2744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah BR, Lipscombe LL, Feig DS, Lowe JM. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG : an international journal of obstetrics and gynaecology. 2011;118(12):1484–1490. doi: 10.1111/j.1471-0528.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 32.Yee L, Martinez N, Nguyen A, Hajjar N, Chen M, Simon M. Using a patient navigator to improve postpartum care in an urban women's health clinic. Obstetrics and Gynecology. 2017 doi: 10.1097/AOG.0000000000001977. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellerman R, Kirk L. Principles of the patient-centered medical home. 2007;76(6):774–775. [PubMed] [Google Scholar]
- 34.Assurance NCfQ. The Future of Patient-Centered Medical Homes: Foundation for a Better Health Care System. 2014. [Google Scholar]
- 35.Clark HD, Graham ID, Karovitch A, Keely EJ. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. 2009;200(6):634.e631–637. doi: 10.1016/j.ajog.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Heatley E, Middleton P, Hague W, Crowther C. The DIAMIND study: postpartum SMS reminders to women who have had gestational diabetes mellitus to test for type 2 diabetes: a randomised controlled trial - study protocol. 2013;13:92. doi: 10.1186/1471-2393-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. 2014;3:CD009578. doi: 10.1002/14651858.CD009578.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tezcan B, Von Rege I, Henkson H, Oteng-Ntim E. Unified communication to reach vulnerable mothers. J Obstet Gynaecol. 2011;31(2):122–124. doi: 10.3109/01443615.2010.542515. [DOI] [PubMed] [Google Scholar]
- 39.Welch RA, Thomas RL. Survey of Postpartum Patients Regarding Electronic Communication Between Obstetricians and Patients. Obstet Gynecol. 2014;123:178s–178s. [Google Scholar]
- 40.Van Ryswyk EM, Middleton PF, Hague WM, Crowther CA. Women's views on postpartum testing for type 2 diabetes after gestational diabetes: Six month follow-up to the DIAMIND randomised controlled trial. Prim Care Diabetes. 2016;10(2):91–102. doi: 10.1016/j.pcd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 41.ACOG Committee Opinion No. 517: Communication strategies for patient handoffs. Obstetrics and gynecology. 2012;119(2 Pt 1):408–411. doi: 10.1097/AOG.0b013e318249ff4f. [DOI] [PubMed] [Google Scholar]
- 42.Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. American journal of medical quality : the official journal of the American College of Medical Quality. 2009;24(3):196–204. doi: 10.1177/1062860609332512. [DOI] [PubMed] [Google Scholar]
- 43.Riehle A, Braun BI, Hafiz H. Improving patient and worker safety: exploring opportunities for synergy. Journal of nursing care quality. 2013;28(2):99–102. doi: 10.1097/NCQ.0b013e3182849f4a. [DOI] [PubMed] [Google Scholar]
- 44.Commission TJ. Transitions of Care: The need for a more effective approach to continuing patient care [Google Scholar]
- 45.American Diabetes A. Gestational diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S103–105. doi: 10.2337/diacare.26.2007.s103. [DOI] [PubMed] [Google Scholar]
- 46.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Claesson R. HbA1c as a predictor of diabetes after gestational diabetes mellitus. Prim Care Diabetes. 2016 doi: 10.1016/j.pcd.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Su X. Hemoglobin A1c for Diagnosis of Postpartum Abnormal Glucose Tolerance among Women with Gestational Diabetes Mellitus: Diagnostic Meta-Analysis. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noctor E, Crowe C, Carmody LA, et al. ATLANTIC DIP: simplifying the follow-up of women with previous gestational diabetes. 2013;169(5):681–687. doi: 10.1530/EJE-13-0491. [DOI] [PubMed] [Google Scholar]
- 50.Megia A, Naf S, Herranz L, et al. The usefulness of HbA1c in postpartum reclassification of gestational diabetes. BJOG : an international journal of obstetrics and gynaecology. 2012;119(7):891–894. doi: 10.1111/j.1471-0528.2012.03325.x. [DOI] [PubMed] [Google Scholar]
- 51.Allalou A. A Predictive Metabolic Signature for the Transition From Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes. 2016;65:2529–2539. doi: 10.2337/db15-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zera C, Bates D, Stuebe A, Ecker J, Seely E. Diabetes screening reminder for women with prior gestational diabetes: A randomized controlled trial. Obstetrics and Gynecology. 2015;126(1):109–114. doi: 10.1097/AOG.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vesco KK, Dietz PM, Bulkley J, et al. A system-based intervention to improve postpartum diabetes screening among women with gestational diabetes. 2012;207(4):283.e281–286. doi: 10.1016/j.ajog.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 54.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 55.Saade GR. Pregnancy as a window to future health. 2009;114(5):958–960. doi: 10.1097/AOG.0b013e3181bf5588. [DOI] [PubMed] [Google Scholar]
- 56.Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. 2013;98(11):4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haig KM, Sutton S, Whittington J. SBAR: a shared mental model for improving communication between clinicians. Jt Comm J Qual Patient Saf. 2006;32(3):167–175. doi: 10.1016/s1553-7250(06)32022-3. [DOI] [PubMed] [Google Scholar]


