Abstract
Objective
The objectives of this study were to determine: 1) the longitudinal profile of plasma-soluble ST2 (sST2) concentrations in patients with preeclampsia and those with uncomplicated pregnancies; 2) whether the changes in sST2 occur prior to the diagnosis of preeclampsia; and 3) the longitudinal sST2 profile of women with early or late preeclampsia.
Materials and Methods
This longitudinal nested case-control study included singleton pregnancies in the following groups: 1) uncomplicated pregnancies (n=160); and 2) those complicated by early (<34 weeks, n=9) and late (≥34 weeks, n=31) preeclampsia. sST2 concentrations were determined by enzyme-linked immunosorbent assays. Mixed-effects models were used for the longitudinal analysis.
Results
1) Plasma sST2 concentration profiles across gestation differed significantly among cases and controls (p<0.0001); 2) women with early preeclampsia had higher mean sST2 concentrations than controls >22 weeks of gestation; cases with late preeclampsia had higher mean concentrations >33 weeks of gestation (both p<0.05); and 3) these changes started approximately six weeks prior to clinical diagnosis.
Conclusions
Maternal plasma sST2 concentrations are elevated six weeks prior to the clinical diagnosis of preeclampsia. An increase in maternal plasma concentration of sST2 may contribute to an exaggerated intravascular inflammatory response and/or the Th1/Th2 imbalance in some cases.
Keywords: interleukin-1, interleukin-33, intravascular inflammation, prediction of preeclampsia, Th1/Th2 immune response
Introduction
Preeclampsia, a syndrome largely evident during the third trimester, remains a major concern due to its association with maternal (1–6) and perinatal morbidity and mortality worldwide (7–17). The early (<34 weeks of gestation) and late (≥ 34 weeks of gestation) features of preeclampsia share clinical presentations of hypertension and proteinuria, but differ substantially in the severity of the disease, the rate of maternal and fetal/neonatal complications, and the severity of the placental lesions (18–27). Moreover, there is a difference in the underlying mechanisms implicated in the pathogenesis of early-onset versus late-onset preeclampsia (28) (i.e., maternal transcriptome (29), balance of angiogenic and antiangiogenic factors (30–54), oxidative stress (55–58), activation of coagulation and thrombic generation (59–61), and inflammation (22,60,62–64).
Preeclampsia is associated with a shift from T helper 2 (Th2)-associated anti-inflammatory cytokines indicating normal pregnancy to a predominant increase of T helper 1 (Th1)-associated pro-inflammatory cytokines (65–70). Cytokines produced by Th1 cells, such as interleukin (IL)-2 (68,71,72), interferon (IFN)-γ (67,71–73), and tumor necrosis factor (TNF)-α (74–77), have each been found to be higher in women with preeclampsia than in those with normal pregnancies. However, the information available regarding their role in early-onset and late-onset preeclampsia is scarce.
Suppressor of tumorigenicity 2 (ST2) is a member of the interleukin-1 receptor (IL-1R) family (78) and, together with its ligand IL-33, it is associated with a Th2 immune response via the production of anti-inflammatory cytokines, such as IL-4, IL-5, and IL-13 (79). The ST2 receptors comprise a membrane-anchored receptor (ST2L) and a soluble ST2 (sST2) receptor that act as decoys for IL-33 (80). This receptor is secreted by endothelial cells during inflammation (81–83). The binding of sST2 to IL-33 shifts the pro-inflammatory phenotype toward a Th1 response. Soluble ST2 concentrations are increased in the third trimester of normal pregnancy and, together with IL-33 and ST2L, might play an important role in maintaining the immunoregulation of normal pregnancy (83–86). Changes in sST2 concentrations were reported at the onset of disease in several pregnancy complications, including miscarriage (87), fetal inflammatory response syndrome (FIRS) (88), and preterm labor (86). Our group (85) and other investigators (84) showed that maternal plasma sST2 concentrations were significantly higher in women with preeclampsia than in those with normal pregnancies. Plasma concentrations of sST2 were higher in early-onset preeclampsia compared to the late onset of the disease and also in severe preeclampsia compared to mild preeclampsia (85). However, due to the lack of information regarding the changes of sST2 concentrations throughout gestation in normal and preeclamptic pregnancies, we conducted a longitudinal study to determine 1) whether patients with preeclampsia have a different longitudinal profile of plasma sST2 concentration than those with uncomplicated pregnancies; 2) if the changes in sST2 occur prior to the diagnosis of preeclampsia; and 3) the longitudinal profile of sST2 between women who subsequently develop early-onset or late-onset preeclampsia.
Materials and Methods
Study design and participants
A longitudinal nested case-control study was performed that included 200 women with singleton pregnancies in the following groups: 1) normal pregnant women (n =160) and 2) patients who developed preeclampsia (n=40). Patients with preeclampsia were subdivided into early (<34 weeks, n=9) and late (≥ 34 weeks, n=31) groups according to the gestational age at delivery. Exclusion criteria included 1) patients with chronic hypertension, 2) known major fetal or chromosomal anomalies, and 3) multiple gestations. All women were enrolled in the prenatal clinic at the Sótero del Rió Hospital, Santiago, Chile, and followed until delivery.
A minimum of three samples were collected from all patients during pregnancy (ranging from 3–7 samples). Subjects were included only if their plasma samples were available at least once prior to 24 weeks of gestation. Plasma samples from each patient were selected once during the following seven intervals: 1) 6–14.9 weeks; 2) 15–19.9 weeks; 3) 20–24.9 weeks; 4) 25–27.9 weeks; 5) 28–31.9 weeks; 6) 32–36.9 weeks; and 7) 37 weeks of gestation or more. The earliest sample from each interval was used for sST2 profiling. Samples collected after the clinical diagnosis of preeclampsia were excluded.
Clinical definitions
Preeclampsia was defined as hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) and as proteinuria (≥ 300 mg in a 24-hour urine collection or one dipstick measurement ≥2+) (89). Severe preeclampsia was defined as previously described (39). Patients with preeclampsia diagnosed before 34 weeks of gestation were defined as having early-onset preeclampsia, while late-onset preeclampsia was diagnosed after 34 weeks of gestation (90). Pregnant women were considered normal if they had no medical, obstetrical, or surgical complications and if they delivered a normal term (≥ 37 weeks) infant whose birthweight was appropriate for gestational age (10th–90th percentile) (91).
The collection and utilization of the samples were approved by the Human Investigation Committee of the Sótero del Rió Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago), and by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). Many of these samples were used in previous studies.
Sample collection and sST2 immunoassay
Blood samples were collected into tubes containing EDTA. The samples were centrifuged for 10 min at 4°C and stored at −70°C. Laboratory personnel were blinded to the clinical diagnoses. Maternal plasma concentrations of sST2 were determined using sensitive and specific immunoassays (R&D Systems, Minneapolis, MN). All immunoassays utilized a sandwich enzyme-based technique and had been validated for plasma determinations of the analytes. The inter- and intra-assay coefficients of variation were 4.6% and 3.9%, respectively. The sensitivity of the assays was 17.5 pg/mL.
Statistical analysis
Cross-sectional analysis of the demographic and clinical characteristics data
The Kolmogorov-Smirnov test was used to assess the distribution of the data. Since the data were not normally distributed, we used the Mann-Whitney U test to compare continuous variables between the groups. A Chi-squared or a Fisher’s exact test was used for comparisons of categorical variables. Statistical analysis was performed using SPSS 19 (IBM Corp, Armonk, NY) and SAS 9.3 (SAS Inc., Cary, NC). A p value < 0.05 was considered statistically significant.
Longitudinal analysis of plasma sST2 concentration
The data collected in this study contain longitudinal measurements from the same individuals belonging to the two groups (preeclampsia and controls). An appropriate statistical method for longitudinal data analysis is the linear mixed-effects model, in which both fixed and random effects are allowed (92, 93). The inherent correlation (similarity) in the data for samples taken from the same individual is accounted for by a subject-specific coefficient in the model called random effect. Therefore, the baseline response (sST2 concentration), treated as a random effect, is allowed to differ from one subject to the next, yet the concentration profile (rate of change over time, i.e., gestational age) is assumed to be similar and, hence, modeled via polynomial terms of gestational age treated as fixed effects. The fixed effects included the group indicator variable (preeclampsia vs. controls), polynomial terms of the gestational age up to the third degree, nulliparity, and smoking. Body mass index (BMI), maternal age, and storage time were tested but did not improve the model fit as determined by a likelihood ratio test and, therefore, were not retained in the model. In addition, to allow the concentration profiles over time to be different between groups, the fixed effects in the model included interaction terms between polynomial components of gestational age and the group variable. With such an interaction model, the effect of the group variable is dependent on the gestational age; hence, we estimated the magnitude and significance of between-group differences in sST2 concentrations at every week of gestation.
The Imer function from the Ime4 package under the R statistical environment was used for mixed-effects model fitting. Significance of the coefficients was determined using the ANOVA method in the Ime4 package, which performs a likelihood ratio test between the model fit with and without the coefficient of interest. All analyses were performed under the R statistical environment (www.r-project.org).
Backward longitudinal analysis
Backward longitudinal analysis was conducted to determine how many weeks prior to the diagnosis that the sST2 concentration in women with preeclampsia differed from the controls. Plasma samples from healthy pregnant women were matched for gestational age to those of patients with preeclampsia (in a 4:1 ratio). The samples from the matched controls of patients with preeclampsia, taken after the gestational age indicated at the time of diagnosis, were removed so that the distribution of gestational age values was similar between the disease and control groups. To test for the differences between groups as a function of time to the disease diagnosis, the same mixed-effects model used in the forward longitudinal analysis was applied.
Results
Demographic and clinical characteristics of the study groups
This study included a total of 1328 samples; of these samples, 1101 were obtained from women with uncomplicated pregnancies and 227 from those with preeclampsia. The demographic and clinical characteristics of the study groups are displayed in Table 1. Patients who developed preeclampsia had a significantly lower median maternal age, a higher median pre-pregnancy BMI, and a higher proportion of nulliparous women compared to those with normal pregnancies. There was no significant difference in the median gestational age at enrollment between patients who subsequently developed preeclampsia and those with normal pregnancies. The median gestational age at delivery and birthweight were lower in patients with preeclampsia than in those with normal pregnancies (p < 0.001 both; Table 1). Among women with preeclampsia, 27.5% (11/40) delivered neonates whose birthweights were less than the 10th percentile, 35% (14/40) delivered preterm before 37 weeks of gestation, 22% (9/40) delivered before 34 weeks of gestation, and 65% (26/40) had a severe preeclampsia.
Table 1.
Demographic and clinical characteristics of the study groups
| Normal pregnancy (n=160) | Preeclampsia (n=40) | p | |
|---|---|---|---|
| Maternal age (years) | 24 (20–30) | 20.5 (19–25) | 0.02 |
| Pre-pregnancy body mass index (BMI) (kg/m2) | 23.9 (21.6–26.8) | 25.7 (23.5–29.2) | 0.005 |
| Smoking | 23 (14.4) | 2 (5) | 0.55 |
| Nulliparity | 71 (44.4) | 26 (65) | 0.02 |
| Previous preeclampsia | 2 (1.3) | 5 (12.5) | 0.04 |
| Gestational age at enrollment (weeks) | 9.4 (8–11.4) | 10 (8.7–11.4) | 0.3 |
| Gestational age at delivery (weeks) | 40 (39.2–40.7) | 38 (34.8–39.3) | <0.001 |
| Birthweight (g) | 3415 (3190–3560) | 2980 (1990–3537) | <0.001 |
Data expressed as median (interquartile range) and number (percentage)
Plasma sST2 concentrations are increased prior to the clinical manifestation of preeclampsia: a longitudinal analysis
Plasma concentrations of sST2 were modeled using a linear mixed-effects model as described in the Methods section. Actual sST2 concentrations were in agreement with the prediction of the model (Figure S1).
Patients who subsequently developed preeclampsia presented a different profile of maternal plasma sST2 (concentration over time) compared to patients with normal pregnancies after adjusting for potential confounders (p<0.0001).
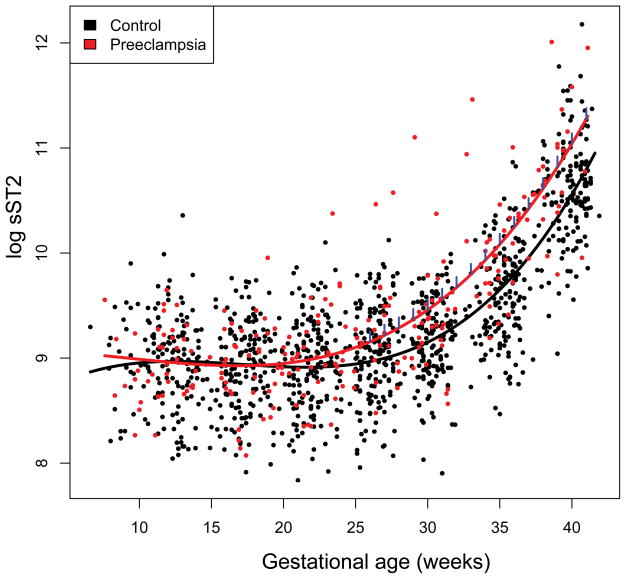
The mean maternal plasma sST2 concentrations were significantly higher in women who subsequently developed preeclampsia from 26 weeks of gestation onward than in women with normal pregnancies (p<0.05), and the magnitude of differences increased as term approached (Table 2, Figure 1).
Table 2.
Magnitude and significance of differences in sST2 concentration between patients with preeclampsia and those with normal pregnancies according to gestational age. The magnitude of differences is expressed as percentage changes [(preeclampsia-control)/control * 100] and significance p-values was obtained from linear mixed-effects models.
| GA | sST2 (pg/mL) | Percentage difference | p-value | |
|---|---|---|---|---|
| Preeclampsia | Control | |||
| 10 | 7294.5 | 7368.0 | −1.0 | 0.91 |
| 11 | 7192.7 | 7436.7 | −3.3 | 0.67 |
| 12 | 7098.0 | 7466.2 | −4.9 | 0.48 |
| 13 | 7013.9 | 7462.6 | −6.0 | 0.37 |
| 14 | 6943.6 | 7432.7 | −6.6 | 0.32 |
| 15 | 6890.4 | 7383.4 | −6.7 | 0.31 |
| 16 | 6857.5 | 7321.7 | −6.3 | 0.33 |
| 17 | 6848.0 | 7254.4 | −5.6 | 0.40 |
| 18 | 6865.6 | 7188.0 | −4.5 | 0.50 |
| 19 | 6914.0 | 7129.0 | −3.0 | 0.65 |
| 20 | 6997.5 | 7083.5 | −1.2 | 0.86 |
| 21 | 7121.1 | 7057.6 | 0.9 | 0.89 |
| 22 | 7290.6 | 7057.5 | 3.3 | 0.62 |
| 23 | 7513.3 | 7089.4 | 6.0 | 0.37 |
| 24 | 7797.6 | 7160.3 | 8.9 | 0.19 |
| 25 | 8154.4 | 7277.8 | 12.0 | 0.079 |
| 26 | 8596.9 | 7450.8 | 15.4 | 0.028 |
| 27 | 9142.0 | 7690.2 | 18.9 | 0.0087 |
| 28 | 9811.1 | 8009.2 | 22.5 | 0.0024 |
| 29 | 10631.5 | 8424.5 | 26.2 | 0.0006 |
| 30 | 11638.5 | 8957.7 | 29.9 | 0.0001 |
| 31 | 12878.3 | 9636.8 | 33.6 | <0.0001 |
| 32 | 14411.2 | 10498.9 | 37.3 | <0.0001 |
| 33 | 16317.3 | 11593.7 | 40.7 | <0.0001 |
| 34 | 18703.8 | 12988.2 | 44.0 | <0.0001 |
| 35 | 21715.6 | 14774.8 | 47.0 | <0.0001 |
| 36 | 25550.7 | 17081.5 | 49.6 | <0.0001 |
| 37 | 30482.1 | 20088.8 | 51.7 | <0.0001 |
| 38 | 36891.6 | 24054.3 | 53.4 | <0.0001 |
| 39 | 45318.7 | 29351.6 | 54.4 | <0.0001 |
| 40 | 56535.2 | 36531.0 | 54.8 | <0.0001 |
| 41 | 71660.5 | 46416.4 | 54.4 | 0.0001 |
Figure 1.
Longitudinal profiles of plasma sST2 concentration. Maternal plasma concentration of sST2 (log, base e, thereof) in women with normal pregnancy (black dots) and those who subsequently developed preeclampsia (red dots). The gestational age dependence of the sST2 concentration in women with normal pregnancy (black line) and those with preeclampsia (red line) was estimated using a linear mixed-effect model and a third degree polynomial function. The blue vertical lines on the preeclampsia curve denote statistical significance of the difference between the two groups at the corresponding gestational age according to a linear mixed-effects model adjusting for covariates (gestational age at venipuncture, nulliparity, and smoking).
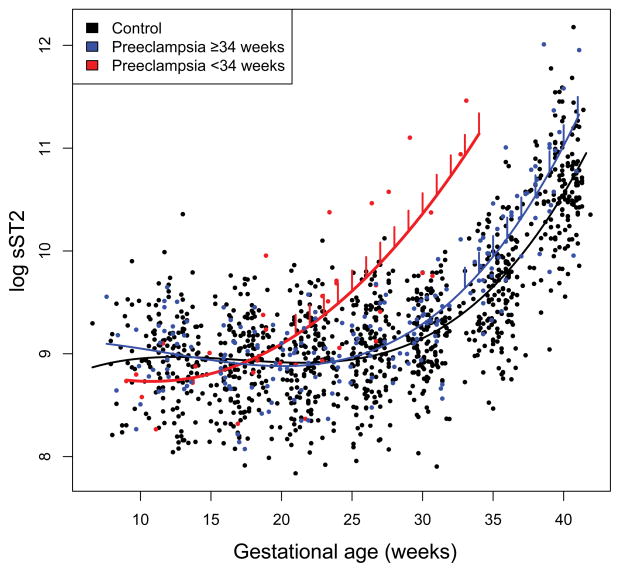
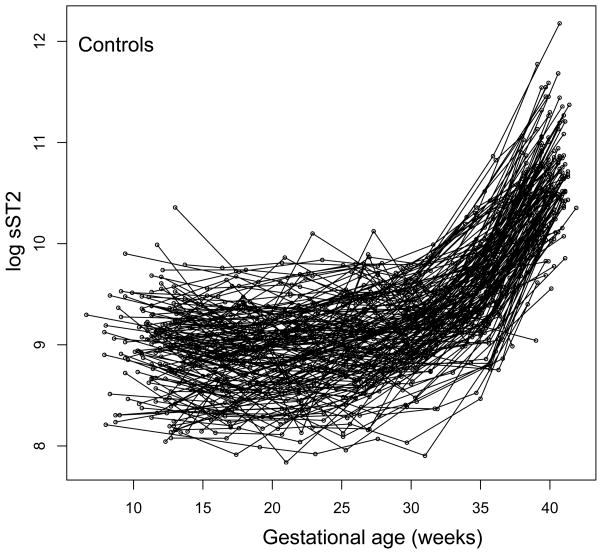
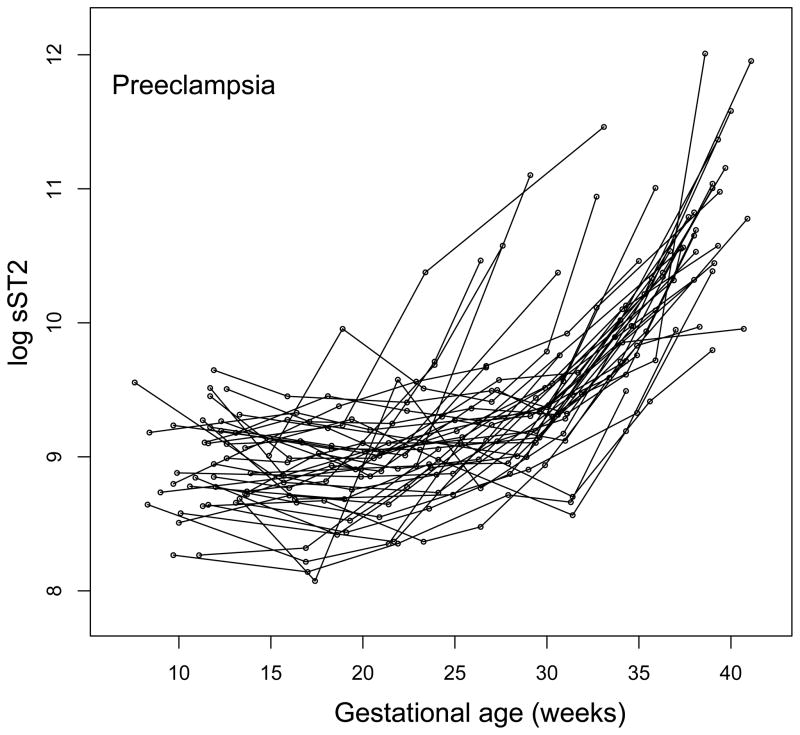
The mean plasma sST2 concentrations were significantly increased (p<0.05) at an earlier stage of gestation (from 22 weeks onward) in patients who subsequently developed early-onset preeclampsia as opposed to those who developed late-onset preeclampsia (from 33 weeks onward) (Figure 2). Individual plasma sST2 concentration profiles of women with normal pregnancies and those who subsequently developed preeclampsia are shown in Figure 3.
Figure 2.
Longitudinal profiles of plasma sST2 concentration in early-onset and late-onset preeclampsia. Maternal plasma concentration of sST2 (log, base e, thereof) in women with normal pregnancy (black dots), early-onset preeclampsia (delivered < 34 weeks of gestation; red dots), and late-onset preeclampsia (blue dots). The gestational-age dependence of the sST2 concentration in women with normal pregnancy (black line), the early-onset preeclampsia group (red line), and the late-onset preeclampsia group (blue line) was estimated using a linear mixed-effect model and a third degree polynomial function. The vertical lines on the preeclampsia curve denote statistical significance of the difference between the preeclampsia and normal pregnancy at the corresponding gestational age according to a linear mixed-effects model, adjusting for covariates (gestational age at venipuncture, nulliparity, and smoking).
Figure 3.
Individual longitudinal plasma sST2 concentration profiles. sST2 plasma concentration (log, base e, thereof) in women with normal pregnancy (n=160) and those who subsequently developed preeclampsia (n=40) is shown as a function of gestational age. Each line corresponds to one patient.
Plasma sST2 concentration is increased 6 weeks prior to the clinical manifestation of preeclampsia (backward analysis)
We conducted a backward analysis to determine when the sST2 concentrations varied at any given time before diagnosis. The mean plasma sST2 concentration was significantly higher in patients who subsequently developed preeclampsia than in those with normal pregnancies at approximately 6 weeks before clinical diagnosis, after adjusting for covariates (all p<0.05; Table 3).
Table 3.
The differences in plasma sST2 concentration between normal pregnancy and preeclampsia as a function of the number of weeks before clinical diagnosis. The magnitude of differences (expressed as fold change between cases and controls) and corresponding significance p-values were estimated using linear mixed-effect models.
| Weeks before clinical diagnosis | Preeclampsia vs Controls | |
|---|---|---|
| Fold change | P value | |
| 0 | 1.65 | <0.0001 |
| 1 | 1.54 | <0.0001 |
| 2 | 1.44 | <0.0001 |
| 3 | 1.36 | <0.0001 |
| 4 | 1.29 | 0.0007 |
| 5 | 1.23 | 0.007 |
| 6 | 1.17 | 0.03 |
| 7 | 1.13 | 0.12 |
| 8 | 1.09 | 0.27 |
| 9 | 1.05 | 0.50 |
| 10 | 1.02 | 0.76 |
| 11 | −1.00 | 0.97 |
| 12 | −1.02 | 0.75 |
| 13 | −1.04 | 0.58 |
| 14 | −1.06 | 0.45 |
| 15 | −1.07 | 0.38 |
| 16 | −1.07 | 0.33 |
| 17 | −1.08 | 0.31 |
| 18 | −1.08 | 0.31 |
| 19 | −1.08 | 0.33 |
| 20 | −1.07 | 0.37 |
Discussion
Principal findings
1) Maternal plasma sST2 concentration differs between patients destined to develop preeclampsia and those with a normal pregnancy from 27 weeks of gestation onward; 2) the change in the maternal plasma sST2 concentration of women with early-onset preeclampsia was observed at 22 weeks of gestation; and 3) in comparison to normal pregnant women, mean maternal plasma sST2 concentration was significantly higher approximately six weeks before the clinical onset of preeclampsia.
Biology of ST2
ST2 is a member of the IL-1 receptor family; it was originally identified as being induced by serum or oncogene expression in mouse fibroblasts (94,95), and it does not bind IL-1α, IL-1β, or IL-1R antagonist (96–98). The two best-characterized isoforms are ST2L and sST2. The cellular activity of ST2 promotes a Th2 inflammatory response through ligation with IL-33 (99,100). The binding of IL-33 to ST2L initiates the production of Th2-associated cytokines (IL-4, IL-5, and IL-13) (101–109). IL-33 is considered an alarmin, as it can be expressed and released by the endothelial cells exposed to an insult (110,111). In addition to its conjoined activity with IL-33, ST2L has an anti-inflammatory property through the inhibition of Toll-like receptor (TLR) signaling (112–114). Macrophages from ST2 knockout mice produce more pro-inflammatory cytokines in response to lipopolysaccharide (LPS) than wild-type mice (115).
Soluble ST2 is identical to the extracellular region of ST2L, except for additional nine amino acids present at the C terminus of the molecule (116, 117). This molecule is expressed in the embryonic tissues, mammary tumors and fibroblasts (94,95,117), alveolar epithelial cells (118), brain, small bowel cells, placenta (84,119), endothelial cells in various tissues (81,83,120,121), and cardiomyocytes (118). Soluble ST2 acts as a decoy that binds IL-33 and prevents its intracellular signaling, shifting the inflammatory response to a Th1 type.
Elevated circulating sST2 concentration has been observed in various conditions characterized by 1) intravascular inflammation, such as LPS-induced lung acute inflammation (119,122–124), dengue fever (125–127), myocardial infarction (128–131), heart failure (132,133), atherosclerosis (134), sepsis (124,135), trauma (124); and in 2) disorders associated with an abnormal Th2 immune response (105,136,137), such as systemic lupus erythematous (138), atopic dermatitis (139,140), idiopathic pulmonary fibrosis (141), acute eosinophilic pneumonia (142), and asthma (143–146).
Changes in soluble ST2 during pregnancy
This longitudinal study describes for the first time the changes observed in maternal plasma sST2 concentrations during normal pregnancy. The concentrations of sST2 are relatively constant until 30 weeks of gestation, from which they increase steadily until delivery (Figure 2). This pattern of increased concentration toward the end of pregnancy is similar to previous reports regarding the changes of cytokine concentrations, e.g., IL-6, IL-8, IL-12, and TNF-α during gestation (147–150). It has been proposed that the increase in the pro-inflammatory cytokines during the third trimester may be associated with physiological changes toward the onset of parturition (151–158). An additional etiology for the elevation of sST2 during the third trimester, after 30 weeks of gestation, is a change in the volume of maternal circulation. It may be that a sense of volume overload promotes the release of sST2 endothelial cells (81,128,132). This may be inferred from studies by Bartunek et al. that demonstrated increased endothelial cell production of sST2 in cases of volume overload in patients with diastolic cardiac dysfunction(81).
The inflammatory phenotype of patients with preeclampsia
Preeclampsia is associated with a systemic maternal inflammatory response (159,160) almost akin to sepsis (161). Indeed, we and other investigators have described changes in the metabolic activity of leukocytes (161,164–166), oxidative stress (55–58), and cytokine profiles (22,60,62,64). These changes were described mainly during the clinical presentation of the disease. Preeclampsia is characterized by an improper inflammatory response initiated and maintained by cytokines such as IL-1β, TNF-α, IL-6, IL-8, and IL-17, which are secreted from Th1 and Th17 cells (162,163). Previous flow cytometry studies have demonstrated that women with preeclampsia exhibit a change in the phenotype and in the metabolic activity of immune cells consistent with leukocyte activation, which is higher than that of normal pregnancy (161,164–166). In addition, the changes in the inflammatory response differ between early-onset and late-onset preeclampsia (167,168). Indeed, higher cytokine concentrations of IL-12, TNF-α, and IL-1β were reported in patients with early-onset versus late-onset preeclampsia (167).
Changes in sST2 in preeclampsia
Our group and other investigators reported an increase of circulating sST2 in the maternal plasma of patients with preeclampsia in comparison to those with normal pregnancies (84,85,169). In addition, women with early-onset preeclampsia had higher sST2 concentrations than those with late onset of this syndrome. The sources for such elevation in sST2 in the maternal circulation among women with preeclampsia were either placental or of the vascular endothelium in response to the exaggerated maternal inflammatory state associated with this syndrome (161,165,170–172). Indeed, the maternal concentration of pro-inflammatory cytokines are higher in patients with early-onset preeclampsia (173–175) and severe preeclampsia (174,176,177) than in women with normal pregnancies. Pro-inflammatory cytokines such as TNF-α and IL-1β, activate endothelial cells; for in vitro studies, investigators were able to stimulate vascular endothelial cells to secrete sST2 into the supernatant (81,83). Since sST2 has been associated with heart failure and volume overload in the vasculature, one cannot but wonder whether the maternal hemodynamic changes of the cardiovascular system in women with preeclampsia may also contribute to the elevation of sST2 concentration observed in this syndrome.
The longitudal profile of sST2 and other biomarkers in preeclampsia
Plasma concentration of sST2 in preeclampsia was strongly correlated with plasma concentrations of anti-angiogenic factors (sVEGFR-1 and sEng) and inversely correlated with the angiogenic placental growth factor (PlGF) (85). The performance of sST2 in identifying patients with preterm preeclampsia was equivalent to that of angiogenic (PlGF) and anti-angiogenic (sVEGFR-1, sEng) factors and their ratios (PlGF/sVEGFR-1, PlGF/sEng) (85). Although the mean plasma sST2 concentration was reported to be significantly increased prior to the onset of preeclampsia (84), the current study extends these observations by demonstrating the profile of plasma sST2 concentration changes throughout pregnancy. Soluble ST2 concentrations were higher toward the end of pregnancy for women with normal pregnancies and for preeclamptic women, although a higher magnitude was seen in early-onset preeclampsia. We also showed that women with early-onset preeclampsia had a higher, earlier increase of plasma sST2 concentration than those with late-onset preeclampsia. The finding that plasma sST2 concentration increased six weeks prior to the diagnosis of preeclampsia is similar to other biomarkers (178). An increase in sVEGFR-1 concentration was demonstrated 6–10 weeks before clinical manifestations (31,178–184). A lower plasma PlGF/sVEGFR-1 ratio was detected in women who developed preterm and term preeclampsia at 20 weeks and at 14 weeks before the clinical diagnosis, respectively (182). A plasma ratio of PlGF/sVEGFR-1 below the 2.5th percentile at 20–23 weeks of gestation was also strongly predictive of delivery before 34 weeks with an increasing number of histological lesions consistent with maternal underperfusion (185), a major placental lesion found in women with preeclampsia (160). Similarly, a lower plasma PlGF/sEng ratio was observed in women with preeclampsia at 20 weeks and at 10 weeks before the clinical diagnosis for preterm and term preeclampsia (182). We were able to identify that sST2 increases significantly in the maternal plasma about six weeks prior to the onset of the syndrome, similar to other biomarkers of this disease. It is not clear whether this observation reflects the systemic maternal changes (hemodynamic, immune system, and endothelial dysfunction) prior to the onset of preeclampsia, or whether this elevation in sST2 concentration has a role in shifting the maternal immune response to the Th1 type (by inhibiting IL-33) associated with preeclampsia (65,67,69).
Strengths and limitations
This study is the first to demonstrate the different profiles of plasma sST2 concentrations in relation to gestational age in women with a normal pregnancy and in those with preeclampsia as well as between early-onset and late-onset preeclampsia. Its observational nature precludes us from any conclusions regarding causality and specific mechanisms that may derive from this study.
Conclusions
Our study demonstrates the change in plasma sST2 concentration throughout gestation. Women who subsequently developed preeclampsia have a higher sST2 concentration when compared to healthy pregnant women. This change is observed approximately six weeks prior to the clinical manifestation of preeclampsia. Patients with early-onset preeclampsia showed a substantial change in the maternal plasma sST2 concentration beginning at 22 weeks of gestation.
Supplementary Material
Evaluation of a linear mixed-effects model fit of sST2 concentration. Estimated values are plotted against observed plasma sST2 concentration (R2=0.83)
Backward analysis of longitudinal profiles of plasma sST2 concentration. Maternal plasma concentration of sST2 in women with normal pregnancy (black lines) and patients who subsequently developed preeclampsia (red lines) are shown as a function of the number of weeks prior to the disease diagnosis. The gestational-age dependence of the sST2 concentration in women with normal pregnancy (black thick line) and those in the preeclampsia group (red thick line) was estimated using a linear mixed-effect model and a third degree polynomial function. The blue vertical lines on the preeclampsia curve denote statistical significance of the difference between the two groups at the corresponding number of weeks prior to diagnosis.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD/NIH under Contract No. HSN275201300006C.
Footnotes
Recipient of the Frederick P. Zuspan Award for the oral presentation at the XIX World Congress of the International Society for the Study of Hypertension in Pregnancy, October 28, 2014, New Orleans, Louisiana.
References
- 1.von Dadelszen P, Menzies J, Magee LA. The complications of hypertension in pregnancy. Minerva Medica. 2005;96:287–302. [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 3.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21:521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 4.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 5.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. doi: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Alsnes IV, Janszky I, Forman MR, Vatten LJ, Okland I. A population-based study of associations between preeclampsia and later cardiovascular risk factors. Am J Obstet Gynecol. 2014;211:657.e1–7. doi: 10.1016/j.ajog.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12:302–23. [PubMed] [Google Scholar]
- 8.Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks’ gestation. Am J Obstet Gynecol. 2000;182:607–11. doi: 10.1067/mob.2000.104224. [DOI] [PubMed] [Google Scholar]
- 9.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–9. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 11.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 12.Ganzevoort W, Rep A, de Vries JI, Bonsel GJ, Wolf H. Prediction of maternal complications and adverse infant outcome at admission for temporizing management of early-onset severe hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2006;195:495–503. doi: 10.1016/j.ajog.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol. 2006;30:16–19. doi: 10.1053/j.semperi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 16.Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta. 2012;33:S48–S54. doi: 10.1016/j.placenta.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Seeho SK, Algert CS, Roberts CL, Ford JB. Early-onset preeclampsia appears to discourage subsequent pregnancy but the risks may be overestimated. Am J Obstet Gynecol. 2016;215:785.e1–e8. doi: 10.1016/j.ajog.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 18.Burton GJ, Jauniaux E. What is the placenta? Am J Obstet Gynecol. 2015;213:S6.e1–S6-e8. doi: 10.1016/j.ajog.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213:S53–S69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim JS, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28:2001–9. doi: 10.3109/14767058.2014.976198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213:S21–S28. doi: 10.1016/j.ajog.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 22.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. Am J Obstet Gynecol. 2015;213:S9.e1, S9–11. doi: 10.1016/j.ajog.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Vahanian SA, Lavery JA, Ananth CV, Vintzileos A. Placental implantation abnormalities and risk of preterm delivery: a systematic review and metaanalysis. Am J Obstet Gynecol. 2015;213:S78–S90. doi: 10.1016/j.ajog.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 24.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215:S1–S46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MY, Buyon JP, Guerra MM, Rana S, Zhang D, Laskin CA, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol. 2016;214:108.e1–e14. doi: 10.1016/j.ajog.2015.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maulik D, De A, Ragolia L, Evans J, Grigoryev D, Lankachandra K, et al. Down-regulation of placental neuropilin-1 in fetal growth restriction. Am J Obstet Gynecol. 2016;214:279.e1–e9. doi: 10.1016/j.ajog.2015.09.068. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui MF, Nandi P, Girish GV, Nygard K, Eastabrook G, de Vrijer B, et al. Decorin over-expression by decidual cells in preeclampsia: a potential blood biomarker. Am J Obstet Gynecol. 2016;215:361.e1–e15. doi: 10.1016/j.ajog.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–80. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaiworapongsa T, Romero R, Whitten A, Tarca AL, Bhatti G, Draghici S, et al. Differences and similarities in the transcriptional profile of peripheral whole blood in early and late-onset preeclampsia: insights into the molecular basis of the phenotype of preeclampsiaa. J Perinat Med. 2013;41:485–504. doi: 10.1515/jpm-2013-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–7. doi: 10.1016/j.ajog.2004.03.043. discussion 7–50. [DOI] [PubMed] [Google Scholar]
- 31.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 32.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 33.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early-versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–9. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 34.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirashima C, Ohkuchi A, Matsubara S, Suzuki H, Takahashi K, Usui R, et al. Alteration of serum soluble endoglin levels after the onset of preeclampsia is more pronounced in women with early-onset. Hypertens Res. 2008;31:1541–8. doi: 10.1291/hypres.31.1541. [DOI] [PubMed] [Google Scholar]
- 36.Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat Diagn. 2009;29:464–70. doi: 10.1002/pd.2203. [DOI] [PubMed] [Google Scholar]
- 37.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wikstrom AK, Larsson A, Akerud H, Olovsson M. Increased circulating levels of the antiangiogenic factor endostatin in early-onset but not late-onset preeclampsia. Reprod Sci. 2009;16:995–1000. doi: 10.1177/1933719109339348. [DOI] [PubMed] [Google Scholar]
- 39.Chaiworapongsa T, Romero R, Kusanovic JP, Mittal P, Kim SK, Gotsch F, et al. Plasma soluble endoglin concentration in pre-eclampsia is associated with an increased impedance to flow in the maternal and fetal circulations. Ultrasound Obstet Gynecol. 2010;35:155–62. doi: 10.1002/uog.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25:498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alahakoon TI, Zhang W, Trudinger BJ, Lee VW. Discordant clinical presentations of preeclampsia and intrauterine fetal growth restriction with similar pro- and anti-angiogenic profiles. J Matern Fetal Neonatal Med. 2014;27:1854–9. doi: 10.3109/14767058.2014.880882. [DOI] [PubMed] [Google Scholar]
- 42.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nature Rev Nephrol. 2014;10:531–40. doi: 10.1038/nrneph.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Major HD, Campbell RA, Silver RM, Branch DW, Weyrich AS. Synthesis of sFlt-1 by platelet-monocyte aggregates contributes to the pathogenesis of preeclampsia. Am J Obstet Gynecol. 2014;210:547.e1–e7. doi: 10.1016/j.ajog.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore Simas TA, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, et al. Angiogenic biomarkers for prediction of early preeclampsia onset in high-risk women. J Matern Fetal Neonatal Med. 2014;27:1038–48. doi: 10.3109/14767058.2013.847415. [DOI] [PubMed] [Google Scholar]
- 45.Lim R, Acharya R, Delpachitra P, Hobson S, Sobey CG, Drummond GR, et al. Activin and NADPH-oxidase in preeclampsia: insights from in vitro and murine studies. Am J Obstet Gynecol. 2015;212:86.e1–e12. doi: 10.1016/j.ajog.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Aggarwal S, Sunderland N, Thornton C, Xu B, Hennessy A, Makris A. A longitudinal analysis of angiotensin II type 1 receptor antibody and angiogenic markers in pregnancy. Am J Obstet Gynecol. 2017;216:170.e1–170.e8. doi: 10.1016/j.ajog.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 47.Biberoglu E, Kirbas A, Daglar K, Biberoglu K, Timur H, Demirtas C, et al. Serum angiogenic profile in abnormal placentation. J Matern Fetal Neonatal Med. 2016;29:3193–7. doi: 10.3109/14767058.2015.1118044. [DOI] [PubMed] [Google Scholar]
- 48.Chaiworapongsa T, Romero R, Whitten AE, Korzeniewski SJ, Chaemsaithong P, Hernandez-Andrade E, et al. The use of angiogenic biomarkers in maternal blood to identify which SGA fetuses will require a preterm delivery and mothers who will develop pre-eclampsia. J Matern Fetal Neonatal Med. 2016;29:1214–28. doi: 10.3109/14767058.2015.1048431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diguisto C, Piver E, Le Gouge A, Eboue F, Le Vaillant C, Marechaud M, et al. First trimester uterine artery Doppler, sFlt-1 and PlGF to predict preeclampsia in a high-risk population. J Matern Fetal Neonatal Med. 2017;30:1514–19. doi: 10.1080/14767058.2016.1183631. [DOI] [PubMed] [Google Scholar]
- 50.Gallo DM, Wright D, Casanova C, Campanero M, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19–24 weeks’ gestation. Am J Obstet Gynecol. 2016;214:619.e1–e17. doi: 10.1016/j.ajog.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Kurtoglu E, Avci B, Kokcu A, Celik H, Cengiz Dura M, Malatyalioglu E, et al. Serum VEGF and PGF may be significant markers in prediction of severity of preeclampsia. J Matern Fetal Neonatal Med. 2016;29:1987–92. doi: 10.3109/14767058.2015.1072157. [DOI] [PubMed] [Google Scholar]
- 52.Lagana AS, Favilli A, Triolo O, Granese R, Gerli S. Early serum markers of pre-eclampsia: are we stepping forward? J Matern Fetal Neonatal Med. 2016;29:3019–23. doi: 10.3109/14767058.2015.1113522. [DOI] [PubMed] [Google Scholar]
- 53.O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–e12. doi: 10.1016/j.ajog.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 54.Yang J, Pearl M, DeLorenze GN, Romero R, Dong Z, Jelliffe-Pawlowski L, et al. Racial-ethnic differences in midtrimester maternal serum levels of angiogenic and antiangiogenic factors. Am J Obstet Gynecol. 2016;215:359.e1–e9. doi: 10.1016/j.ajog.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raijmakers MT, Peters WH, Steegers EA, Poston L. NAD(P)H oxidase associated superoxide production in human placenta from normotensive and pre-eclamptic women. Placenta. 2004;25(Suppl A):S85–S89. doi: 10.1016/j.placenta.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Roy S, Dhobale M, Dangat K, Mehendale S, Lalwani S, Joshi S. Differential oxidative stress levels in mothers with preeclampsia delivering male and female babies. J Matern Fetal Neonatal Med. 2015;28:1973–80. doi: 10.3109/14767058.2014.974537. [DOI] [PubMed] [Google Scholar]
- 57.Daglar K, Kirbas A, Timur H, Ozturk Inal Z, Danisman N. Placental levels of total oxidative and anti-oxidative status, ADAMTS-12 and decorin in early- and late-onset severe preeclampsia. J Matern Fetal Neonatal Med. 2016;29:4059–64. doi: 10.3109/14767058.2016.1154942. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Deng Q, Luo X, Chen Y, Shan N, Qi H. Oxidative stress-induced Gadd45alpha inhibits trophoblast invasion and increases sFlt1/sEng secretions via p38 MAPK involving in the pathology of pre-eclampsia. J Matern Fetal Neonatal Med. 2016;29:3776–85. doi: 10.3109/14767058.2016.1144744. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Zhang Y, Yang F, Li L, Liu S, Xu Z, et al. Complement component C4A and apolipoprotein A-I in plasmas as biomarkers of the severe, early-onset preeclampsia. Mol Biosyst. 2011;7:2470–9. doi: 10.1039/c1mb05142c. [DOI] [PubMed] [Google Scholar]
- 60.Boij R, Svensson J, Nilsson-Ekdahl K, Sandholm K, Lindahl TL, Palonek E, et al. Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol. 2012;68:258–70. doi: 10.1111/j.1600-0897.2012.01158.x. [DOI] [PubMed] [Google Scholar]
- 61.He Y, Xu B, Song D, Yu F, Chen Q, Zhao M. Expression of the complement system’s activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am J Reprod Immunol. 2016;76:205–11. doi: 10.1111/aji.12541. [DOI] [PubMed] [Google Scholar]
- 62.Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK, Allen J, Knudsen UB. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90:791–6. doi: 10.1111/j.1600-0412.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 63.Fisher SJ. Why is placentation abnormal in preeclampsia? Am J Obstet Gynecol. 2015;213:S115–S122. doi: 10.1016/j.ajog.2015.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuzcu ZB, Asicioglu E, Sunbul M, Ozben B, Arikan H, Koc M. Circulating endothelial cell number and markers of endothelial dysfunction in previously preeclamptic women. Am J Obstet Gynecol. 2015;213:533.e1–e7. doi: 10.1016/j.ajog.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 65.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 67.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1:Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y, et al. Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol. 1999;41:297–306. doi: 10.1111/j.1600-0897.1999.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 69.Borzychowski AM, Croy BA, Chan WL, Redman CW, Sargent IL. Changes in systemic type 1 and type 2 immunity in normal pregnancy and pre-eclampsia may be mediated by natural killer cells. Eur J Immunol. 2005;35:3054–63. doi: 10.1002/eji.200425929. [DOI] [PubMed] [Google Scholar]
- 70.Peixoto AB, Araujo E, Junior, Ribeiro JU, Rodrigues DB, Castro EC, Caldas TM, et al. Evaluation of inflammatory mediators in the deciduas of pregnant women with pre-eclampsia/eclampsia. J Matern Fetal Neonatal Med. 2016;29:75–9. doi: 10.3109/14767058.2014.987117. [DOI] [PubMed] [Google Scholar]
- 71.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J, Oleszczuk J. T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 1999;86:165–70. doi: 10.1016/s0301-2115(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 72.Rein DT, Schondorf T, Gohring UJ, Kurbacher CM, Pinto I, Breidenbach M, et al. Cytokine expression in peripheral blood lymphocytes indicates a switch to T(HELPER) cells in patients with preeclampsia. J Reprod Immunol. 2002;54:133–42. doi: 10.1016/s0165-0378(01)00128-0. [DOI] [PubMed] [Google Scholar]
- 73.Ozkan ZS, Simsek M, Ilhan F, Deveci D, Godekmerdan A, Sapmaz E. Plasma IL-17, IL-35, interferon-gamma, SOCS3 and TGF-beta levels in pregnant women with preeclampsia, and their relation with severity of disease. J Matern Fetal Neonatal Med. 2014;27:1513–7. doi: 10.3109/14767058.2013.861415. [DOI] [PubMed] [Google Scholar]
- 74.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–7. discussion 7–9. [PubMed] [Google Scholar]
- 75.Meekins JW, McLaughlin PJ, West DC, McFadyen IR, Johnson PM. Endothelial cell activation by tumour necrosis factor-alpha (TNF-alpha) and the development of pre-eclampsia. Clin Exp Immunol. 1994;98:110–114. doi: 10.1111/j.1365-2249.1994.tb06615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102:20–5. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 77.Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. 2013;70:412–427. doi: 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]
- 78.Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–4. doi: 10.1016/0014-5793(89)81679-5. [DOI] [PubMed] [Google Scholar]
- 79.Peine M, Marek RM, Lohning M. IL-33 in T cell differentiation, function, and immune homeostasis. Trends Immunol. 2016;37:321–33. doi: 10.1016/j.it.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–9. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 81.Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardio. 2008;52:2166–74. doi: 10.1016/j.jacc.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aoki S, Hayakawa M, Ozaki H, Takezako N, Obata H, Ibaraki N, et al. ST2 gene expression is proliferation-dependent and its ligand, IL-33, induces inflammatory reaction in endothelial cells. Mol Cell Biochem. 2010;335:75–81. doi: 10.1007/s11010-009-0244-9. [DOI] [PubMed] [Google Scholar]
- 83.Topping V, Romero R, Than NG, Tarca AL, Xu Z, Kim SY, et al. Interleukin-33 in the human placenta. J Matern Fetal Neonatal Med. 2013;26:327–38. doi: 10.3109/14767058.2012.735724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Granne I, Southcombe JH, Snider JV, Tannetta DS, Child T, Redman CW, et al. ST2 and IL-33 in pregnancy and pre-eclampsia. PloS One. 2011;6:e24463. doi: 10.1371/journal.pone.0024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stampalija T, Chaiworapongsa T, Romero R, Chaemsaithong P, Korzeniewski SJ, Schwartz AG, et al. Maternal plasma concentrations of sST2 and angiogenic/anti-angiogenic factors in preeclampsia. J Matern Fetal Neonatal Med. 2013;26:1359–70. doi: 10.3109/14767058.2013.784256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stampalija T, Chaiworapongsa T, Romero R, Tarca AL, Bhatti G, Chiang PJ, et al. Soluble ST2, a modulator of the inflammatory response, in preterm and term labor. J Matern Fetal Neonatal Med. 2014;27:111–121. doi: 10.3109/14767058.2013.806894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaitu’u-Lino TJ, Tuohey L, Tong S. Maternal serum interleukin-33 and soluble ST2 across early pregnancy, and their association with miscarriage. J Reprod Immunol. 2012;95:46–9. doi: 10.1016/j.jri.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Stampalija T, Romero R, Korzeniewski SJ, Chaemsaithong P, Miranda J, Yeo L, et al. Soluble ST2 in the fetal inflammatory response syndrome: in vivo evidence of activation of the anti-inflammatory limb of the immune response. J Matern Fetal Neonatal Med. 2013;26:1384–93. doi: 10.3109/14767058.2013.784258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 90.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 91.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 92.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 93.Douglas B, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999999-0. 2012 Available from: http://CRAN.R-project.org/package=Ime4.
- 94.Haslett C, Savill JS, Meagher L. The neutrophil. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 95.Klemenz R, Hoffmann S, Werenskiold AK. Serum- and oncoprotein-mediated induction of a gene with sequence similarity to the gene encoding carcinoembryonic antigen. Proc Natl Acad Sci USA. 1989;86:5708–12. doi: 10.1073/pnas.86.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104.e1–e11. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 97.Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–54. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Southcombe JH, Redman CW, Sargent IL, Granne I. Interleukin-1 family cytokines and their regulatory proteins in normal pregnancy and pre-eclampsia. Clin Exp Immunol. 2015;181:480–90. doi: 10.1111/cei.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naccasha N, Gervasi MT, Chaiworapongsa T, Berman S, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol. 2001;185:1118–23. doi: 10.1067/mob.2001.117682. [DOI] [PubMed] [Google Scholar]
- 100.Fock V, Mairhofer M, Otti GR, Hiden U, Spittler A, Zeisler H, et al. Macrophage-derived IL-33 is a critical factor for placental growth. J Immunol. 2013;191:3734–3743. doi: 10.4049/jimmunol.1300490. [DOI] [PubMed] [Google Scholar]
- 101.Pakandl M, Pecka Z. A domestic duck as a new host for Blastocystis sp. Folia Parasitol. 1992;39:59–60. [PubMed] [Google Scholar]
- 102.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci USA. 1998;95:6930–5. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iwahana H, Yanagisawa K, Ito-Kosaka A, Kuroiwa K, Tago K, Komatsu N, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem/FEBS. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 104.Tominaga S, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Komatsu N. Presence and expression of a novel variant form of ST2 gene product in human leukemic cell line UT-7/GM. Biochem Biophys Res Commun. 1999;264:14–18. doi: 10.1006/bbrc.1999.1469. [DOI] [PubMed] [Google Scholar]
- 105.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 106.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–4. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 107.Komai-Koma M, Xu D, Li Y, McKenzie AN, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 108.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 109.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gajardo Carrasco T, Morales RA, Perez F, Terraza C, Yanez L, Campos-Mora M, et al. Alarmin’ Immunologists: IL-33 as a putative target for modulating T cell-dependent responses. Front Immunol. 2015;6:232. doi: 10.3389/fimmu.2015.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eda Gokdemir I, Ozdegirmenci O, Elmas B, Sarikaya E, Tokmak A, Kazanci FH, et al. Evaluation of ADAMTS12, ADAMTS16, ADAMTS18 and IL-33 serum levels in pre-eclampsia. J Matern Fetal Neonatal Med. 2016;29:2451–2456. doi: 10.3109/14767058.2015.1087497. [DOI] [PubMed] [Google Scholar]
- 112.Yanagisawa K, Naito Y, Kuroiwa K, Arai T, Furukawa Y, Tomizuka H, et al. The expression of ST2 gene in helper T cells and the binding of ST2 protein to myeloma-derived RPMI8226 cells. J Biochem. 1997;121:95–103. doi: 10.1093/oxfordjournals.jbchem.a021577. [DOI] [PubMed] [Google Scholar]
- 113.Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–6639. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 114.Takezako N, Hayakawa M, Hayakawa H, Aoki S, Yanagisawa K, Endo H, et al. ST2 suppresses IL-6 production via the inhibition of IkappaB degradation induced by the LPS signal in THP-1 cells. Biochem Biophys Res Comm. 2006;341:425–32. doi: 10.1016/j.bbrc.2005.12.206. [DOI] [PubMed] [Google Scholar]
- 115.Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O’Neill LA, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 116.Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994;13:1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGill SN, Ahmed NA, Hu F, Michel RP, Christou NV. Shedding of L-selectin as a mechanism for reduced polymorphonuclear neutrophil exudation in patients with the systemic inflammatory response syndrome. Arch Surg. 1996;131:1141–1146. doi: 10.1001/archsurg.1996.01430230023005. discussion 7. [DOI] [PubMed] [Google Scholar]
- 118.Mildner M, Storka A, Lichtenauer M, Mlitz V, Ghannadan M, Hoetzenecker K, et al. Primary sources and immunological prerequisites for sST2 secretion in humans. Cardiovasc Res. 2010;87:769–777. doi: 10.1093/cvr/cvq104. [DOI] [PubMed] [Google Scholar]
- 119.Kumar S, Tzimas MN, Griswold DE, Young PR. Expression of ST2, an interleukin-1 receptor homologue, is induced by proinflammatory stimuli. Biochem Biophys Res Commun. 1997;235:474–478. doi: 10.1006/bbrc.1997.6810. [DOI] [PubMed] [Google Scholar]
- 120.Demyanets S, Kaun C, Pentz R, Krychtiuk KA, Rauscher S, Pfaffenberger S, et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. doi: 10.1016/j.yjmcc.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zeyda M, Wernly B, Demyanets S, Kaun C, Hammerle M, Hantusch B, et al. Severe obesity increases adipose tissue expression of interleukin-33 and its receptor ST2, both predominantly detectable in endothelial cells of human adipose tissue. Int J Obes (Lond) 2013;37:658–665. doi: 10.1038/ijo.2012.118. [DOI] [PubMed] [Google Scholar]
- 122.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. ST2 protein induced by inflammatory stimuli can modulate acute lung inflammation. Biochem Biophys Res Comm. 2002;299:18–24. doi: 10.1016/s0006-291x(02)02578-0. [DOI] [PubMed] [Google Scholar]
- 123.Oshikawa K, Yanagisawa K, Tominaga S, Sugiyama Y. Expression and function of the ST2 gene in a murine model of allergic airway inflammation. Clin Exp Allergy. 2002;32:1520–1526. doi: 10.1046/j.1365-2745.2002.01494.x. [DOI] [PubMed] [Google Scholar]
- 124.Brunner M, Krenn C, Roth G, Moser B, Dworschak M, Jensen-Jarolim E, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intensive Care Med. 2004;30:1468–1473. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 125.Becerra A, Warke RV, de Bosch N, Rothman AL, Bosch I. Elevated levels of soluble ST2 protein in dengue virus infected patients. Cytokine. 2008;41:114–120. doi: 10.1016/j.cyto.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Houghton-Trivino N, Salgado DM, Rodriguez JA, Bosch I, Castellanos JE. Levels of soluble ST2 in serum associated with severity of dengue due to tumour necrosis factor alpha stimulation. J Gen Virol. 2010;91:697–706. doi: 10.1099/vir.0.012971-0. [DOI] [PubMed] [Google Scholar]
- 127.Guerrero CD, Arrieta AF, Ramirez ND, Rodriguez LS, Vega R, Bosch I, et al. High plasma levels of soluble ST2 but not its ligand IL-33 is associated with severe forms of pediatric dengue. Cytokine. 2013;61:766–771. doi: 10.1016/j.cyto.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 128.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 130.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Investig. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 133.Shah RV, Januzzi JL., Jr ST2: a novel remodeling biomarker in acute and chronic heart failure. Curr Heart Fail Rep. 2010;7:9–14. doi: 10.1007/s11897-010-0005-9. [DOI] [PubMed] [Google Scholar]
- 134.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hoogerwerf JJ, Tanck MW, van Zoelen MA, Wittebole X, Laterre PF, van der Poll T. Soluble ST2 plasma concentrations predict mortality in severe sepsis. Intensive Care Med. 2010;36:630–637. doi: 10.1007/s00134-010-1773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lecart S, Lecointe N, Subramaniam A, Alkan S, Ni D, Chen R, et al. Activated, but not resting human Th2 cells, in contrast to Th1 and T regulatory cells, produce soluble ST2 and express low levels of ST2L at the cell surface. Eur J Immunol. 2002;32:2979–2987. doi: 10.1002/1521-4141(2002010)32:10<2979::AID-IMMU2979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 137.Pastorelli L, Garg RR, Hoang SB, Spina L, Mattioli B, Scarpa M, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci USA. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mok MY, Huang FP, Ip WK, Lo Y, Wong FY, Chan EY, et al. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2010;49:520–527. doi: 10.1093/rheumatology/kep402. [DOI] [PubMed] [Google Scholar]
- 139.Sahlander K, Larsson K, Palmberg L. Increased serum levels of soluble ST2 in birch pollen atopics and individuals working in laboratory animal facilities. J Occup Environ Med/Am Coll Occup Environ Med. 2010;52:214–218. doi: 10.1097/JOM.0b013e3181d09868. [DOI] [PubMed] [Google Scholar]
- 140.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20:154–167. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 141.Tajima S, Oshikawa K, Tominaga S, Sugiyama Y. The increase in serum soluble ST2 protein upon acute exacerbation of idiopathic pulmonary fibrosis. Chest. 2003;124:1206–1214. doi: 10.1378/chest.124.4.1206. [DOI] [PubMed] [Google Scholar]
- 142.Oshikawa K, Kuroiwa K, Tokunaga T, Kato T, Hagihara SI, Tominaga SI, et al. Acute eosinophilic pneumonia with increased soluble ST2 in serum and bronchoalveolar lavage fluid. Respir Med. 2001;95:532–533. doi: 10.1053/rmed.2001.1080. [DOI] [PubMed] [Google Scholar]
- 143.Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 144.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 145.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–781. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kurokawa M, Matsukura S, Kawaguchi M, Ieki K, Suzuki S, Odaka M, et al. Expression and effects of IL-33 and ST2 in allergic bronchial asthma: IL-33 induces eotaxin production in lung fibroblasts. Intl Arch Allergy Immunol. 2011;155:12–20. doi: 10.1159/000327259. [DOI] [PubMed] [Google Scholar]
- 147.Curry AE, Vogel I, Skogstrand K, Drews C, Schendel DE, Flanders WD, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77:152–160. doi: 10.1016/j.jri.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 148.Harper M, Li L, Zhao Y, Klebanoff MA, Thorp JM, Jr, Sorokin Y, et al. Change in mononuclear leukocyte responsiveness in midpregnancy and subsequent preterm birth. Obstet Gynecol. 2013;121:805–811. doi: 10.1097/AOG.0b013e3182878a80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Christian LM, Porter K. Longitudinal changes in serum proinflammatory markers across pregnancy and postpartum: effects of maternal body mass index. Cytokine. 2014;70:134–140. doi: 10.1016/j.cyto.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young BC, Stanic AK, Panda B, Rueda BR, Panda A. Longitudinal expression of Toll-like receptors on dendritic cells in uncomplicated pregnancy and postpartum. Am J Obstet Gynecol. 2014;210:445.e1–e6. doi: 10.1016/j.ajog.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. J Reprod Med. 1990;35:235–238. [PubMed] [Google Scholar]
- 152.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 153.Maymon E, Ghezzi F, Edwin SS, Mazor M, Yoon BH, Gomez R, et al. The tumor necrosis factor alpha and its soluble receptor profile in term and preterm parturition. Am J Obstet Gynecol. 1999;181:1142–1148. doi: 10.1016/s0002-9378(99)70097-9. [DOI] [PubMed] [Google Scholar]
- 154.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394.e1–e24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hassan SS, Romero R, Haddad R, Hendler I, Khalek N, Tromp G, et al. The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol. 2006;195:778–786. doi: 10.1016/j.ajog.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 156.Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth. 2007;7:S7. doi: 10.1186/1471-2393-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 158.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A role for the inflammasome in spontaneous labor at term. Am J Reprod Immunol. doi: 10.1111/aji.12440. Epub 2016 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oggé G, Romero R, Chaiworapongsa T, Gervasi MT, Pacora P, Erez O, et al. Leukocytes of pregnant women with small-for-gestational-age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Fetal Neonatal Med. 2010;23:476–487. doi: 10.3109/14767050903216033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oggé G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/JPM.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 162.Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94:247–257. doi: 10.1189/jlb.1112603. [DOI] [PubMed] [Google Scholar]
- 163.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Jr, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci (Lond) 2016;130:409–419. doi: 10.1042/CS20150702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sabatier F, Bretelle F, D’Ercole C, Boubli L, Sampol J, Dignat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000;183:1558–1563. doi: 10.1067/mob.2000.108082. [DOI] [PubMed] [Google Scholar]
- 165.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 166.Chaiworapongsa T, Gervasi MT, Refuerzo J, Espinoza J, Yoshimatsu J, Berman S, et al. Maternal lymphocyte subpopulations (CD45RA+ and CD45RO+) in preeclampsia. Am J Obstet Gynecol. 2002;187:889–893. doi: 10.1067/mob.2002.127309. [DOI] [PubMed] [Google Scholar]
- 167.Peraçoli JC1, Bannwart-Castro CF, Romao M, Weel IC, Ribeiro VR, Borges VT, Rudge MV, Witkin SS, Peraçoli MT. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol. 2013;100:129–134. doi: 10.1016/j.jri.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Z, Gao Y, Zhang L, Jia L, Wang P, Zhang L, Li H. Alterations of IL-6, IL-6R and gp130 in early and late onset severe preeclampsia. Hypertens Pregnancy. 2013;32:270–280. doi: 10.3109/10641955.2013.798332. [DOI] [PubMed] [Google Scholar]
- 169.Southcombe JH, Benton SJ, Hu Y, von Dadelszen P, Child T, Snider JV, et al. Measurement of sST2 is comparable to PlGF in the diagnosis of early-onset pre-eclampsia. Pregnancy Hypertens. 2013;3:115–117. doi: 10.1016/j.preghy.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 170.Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11:309–316. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 171.Luppi P, Tse H, Lain KY, Markovic N, Piganelli JD, DeLoia JA. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol. 2006;56:135–144. doi: 10.1111/j.1600-0897.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 172.Mihu D, Sabau L, Costin N, Ciortea R, Malutan A, Mihu CM. Implications of maternal systemic oxidative stress in normal pregnancy and in pregnancy complicated by preeclampsia. J Matern Fetal Neonatal Med. 2012;25:944–951. doi: 10.3109/14767058.2011.600796. [DOI] [PubMed] [Google Scholar]
- 173.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–714. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 174.Jonsson Y, Ruber M, Matthiesen L, Berg G, Nieminen K, Sharma S, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70:83–91. doi: 10.1016/j.jri.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 175.Vitoratos N, Economou E, Iavazzo C, Panoulis K, Creatsas G. Maternal serum levels of TNF-alpha and IL-6 long after delivery in preeclamptic and normotensive pregnant women. Mediators Inflamm. 2010;2010:908649. doi: 10.1155/2010/908649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. 2007;58:21–30. doi: 10.1111/j.1600-0897.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 177.Xiao JP, Yin YX, Gao YF, Lau S, Shen F, Zhao M, et al. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine. 2012;60:856–860. doi: 10.1016/j.cyto.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 178.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 179.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 180.Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199:266.e1–e6. doi: 10.1016/j.ajog.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 181.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lim JH, Kim SY, Park SY, Lee MH, Yang JH, Kim MY, et al. Soluble endoglin and transforming growth factor-beta1 in women who subsequently developed preeclampsia. Prenatal Diagn. 2009;29:471–476. doi: 10.1002/pd.2217. [DOI] [PubMed] [Google Scholar]
- 184.Foidart JM, Munaut C, Chantraine F, Akolekar R, Nicolaides KH. Maternal plasma soluble endoglin at 11–13 weeks’ gestation in pre-eclampsia. Ultrasound Obstet Gynecol. 2010;35:680–687. doi: 10.1002/uog.7621. [DOI] [PubMed] [Google Scholar]
- 185.Korzeniewski SJ, Romero R, Chaiworapongsa T, Chaemsaithong P, Kim CJ, Kim YM, et al. Maternal plasma angiogenic index-1 (placental growth factor/soluble vascular endothelial growth factor receptor-1) is a biomarker for the burden of placental lesions consistent with uteroplacental underperfusion: a longitudinal case-cohort study. Am J Obstet Gynecol. 2016;214:629.e1–e17. doi: 10.1016/j.ajog.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evaluation of a linear mixed-effects model fit of sST2 concentration. Estimated values are plotted against observed plasma sST2 concentration (R2=0.83)
Backward analysis of longitudinal profiles of plasma sST2 concentration. Maternal plasma concentration of sST2 in women with normal pregnancy (black lines) and patients who subsequently developed preeclampsia (red lines) are shown as a function of the number of weeks prior to the disease diagnosis. The gestational-age dependence of the sST2 concentration in women with normal pregnancy (black thick line) and those in the preeclampsia group (red thick line) was estimated using a linear mixed-effect model and a third degree polynomial function. The blue vertical lines on the preeclampsia curve denote statistical significance of the difference between the two groups at the corresponding number of weeks prior to diagnosis.