Abstract
Long-standing theory predicts that the intensity of consumer-prey interactions declines with increasing latitude, yet for plant-herbivore interactions, latitudinal changes in herbivory rates and plant palatability have received variable support. The topic is of growing interest given that lower-latitude species are moving poleward at an accelerating rate due to climate change, and predicting local interactions will depend partly on whether latitudinal gradients occur in these critical biotic interactions. Here, we assayed the palatability of 50 seaweeds collected from polar (Antarctica), temperate (northeastern Pacific; California), and tropical (central Pacific; Fiji) locations to two herbivores native to the tropical and subtropical Atlantic, the generalist crab Mithraculus sculptus and sea urchin Echinometra lucunter. Red seaweeds (Rhodophyta) of polar and temperate origin were more readily consumed by urchins than were tropical reds. The decline in palatability with decreasing latitude is explained by shifts in tissue organic content along with the quantity and quality of secondary metabolites, degree of calcification or both. We detected no latitudinal shift in palatability of red seaweeds to crabs, nor any latitudinal shifts in palatability of brown seaweeds (Phaeophyta) to either crabs or urchins. Our results suggest that evolutionary pressure from tropical herbivores favored red seaweeds with lower palatability, either through the production of greater levels of chemical defenses, calcification or both. Moreover, our results tentatively suggest that the ‘tropicalization’ of temperate habitats is facilitated by the migration of tropical herbivores into temperate areas dominated by weakly defended and more nutritious foods, and that the removal of these competing seaweeds may facilitate the invasion of better-defended tropical seaweeds.
Keywords: latitudinal gradient, biotic interactions, seaweeds, herbivore, diffuse coevolution, macroalgae
INTRODUCTION
Interactions between plants and herbivores play central roles in structuring ecosystems, determining spatial patterns of biodiversity, and cycling nutrients and materials through ecosystems (Hunter 2016). Because tropical latitudes have greater herbivore diversity, elevated temperatures and relatively low seasonality compared to more temperate latitudes, ecologists have long hypothesized that plant-herbivore interactions are stronger, and that evolution favors plants that are more resistant or tolerant to herbivores (Dobzhansky 1950, Schemske et al. 2009). However, recent meta-analyses have questioned the universality of latitudinal gradients in herbivore impact and plant defenses (Hillebrand 2009, Schemske et al. 2009, Moles et al. 2011, Poore et al. 2012), strongly suggesting that we still require assessments of latitudinal gradients in a diversity of geographic areas and systems.
As an example, tissue palatability – a quantifiable trait that integrates nutritional content, morphological and chemical defenses, and consumer feeding behavior (Forbey et al. 2013) –is generally lower in the tropics than at higher latitudes for most vascular plants (e.g., Basset 1994, Pennings et al. 2001, Morrison and Hay 2012, Anstett et al. 2015; see meta-analysis of Moles et al. 2011 for counterexample), but evidence supporting a latitudinal decline in palatability of non-vascular plants (seaweeds) is equivocal. In the only direct test of a latitudinal gradient in seaweed palatability, Bolser and Hay (1996) demonstrated that the tissues and lipophilic extracts of temperate Atlantic seaweed are more palatable than those of tropical Atlantic seaweeds. Moreover, recent studies documented that some seaweeds in Antarctic and Arctic habitats produce chemical and/or structural defenses against local herbivores (Amsler et al. 2005, Wessels et al. 2006, Wiencke and Amsler 2012). If defended seaweeds are common in polar latitudes, then this would be difficult to reconcile with latitudinal declines in palatability (Schemske et al. 2009). Addressing these apparently conflicting lines of evidence requires direct tests of plant palatability across tropical, temperate and polar regions, a task that to our knowledge, has not been previously attempted.
The urgency of this question is clear, given that lower-latitude plants and herbivores are moving into higher latitudes at accelerating rates because of climate change (Parmesan 2006, Vergés et al. 2014, 2016), and predicting local plant-herbivore interactions will depend partly on the strength of latitudinal gradients in herbivore impact and plant palatability.
Here, we tested for latitudinal differences in palatability by assaying tissue palatability of 50 seaweeds collected from polar (Antarctica), temperate (California; Pacific Ocean) and tropical (Fiji; Pacific Ocean) regions to two generalist herbivores, the urchin Echinometra lucunter and crab Mithraculus sculptus, that are native to the tropical and subtropical Atlantic Ocean and naïve to assayed seaweeds. We also assessed latitudinal gradients in several nutritional (ash-free dry mass, protein content, carbon to nitrogen ratio) and chemical (phenolic) traits that may affect tissue palatability. All results were assessed correcting for phylogenetic relationships, which we evaluated using nuclear (18S) and chloroplast (rbcL) loci.
METHODS
Organism Collection and Maintenance
Thirty-one Rhodophyta (hereafter red) and 19 Phaeophyta (hereafter brown) seaweeds were collected from: 1) the Fiji Islands (18.00° S, 179.00° E), 2) near San Diego and Bodega Bay, California (32.72° N, 117.16° W, and 38.32° N, 123.04° W), and 3) near Palmer Station, Antarctica (64.77° S, 64.05° W). These seaweeds represented a haphazardly-selected subset of species that were common in the local communities. Our collection strategy did not target chemically-defended species, but our collection of species does include genera that are known to be chemically-rich (e.g., Plocamium, Dictyota, Dichotomaria). Tropical seaweeds were collected primarily from shallow reef flats where seaweeds are more abundant and herbivory rates are lower than on deeper reef slopes (Rasher and Hay, personal communication; Hay 1984). Seaweeds were blotted/spun dry and frozen until lyophilized. Prior to and between use for our research, all tissues were stored at −20 to −80°C in frozen or freeze-dried states. Because of the logistical challenges of collecting Antarctic seaweeds, we opportunistically used species that had been collected and frozen for other projects. Consequently, we collected Antarctic samples from 1996 to 2011, and Fijian and Californian samples in 2013 and 2014. From the Florida Keys (25°N 80.5°W), we collected adults of the crab Mithraculus sculptus (Lamarck, 1818) and juveniles (2.25–3.25 cm test diameter) of the urchin Echinometra lucunter (Linnaeus, 1758) in April 2014, and a licensed fisherman collected additional M. sculptus in August 2014. Both species were maintained in separate 160 L recirculating systems at 25 (±1) °C and 32 (±3) ppt salinity at Grice Marine Laboratory for eight months while assays were conducted. Individuals were kept within 700 mL plastic containers with two mesh-covered holes 6 cm in diameter (mesh size: 1×1 mm) to allow for seawater circulation, and fed locally abundant and palatable seaweeds (Gracilaria vermiculophylla and Ulva spp.) ad libitum.
Palatability to generalist consumers
We quantified the relative palatability of 50 seaweeds by offering each generalist herbivore a pairwise choice between each test seaweed and a control in artificial feeding assays with finely ground lyophilized tissue. We used two tropical Caribbean herbivores in these assays because Caribbean herbivores would not have recent experience with Pacific and Southern Ocean species. Additionally, previous studies indicated that tropical fishes and urchins tend to have greater feeding tolerance to chemical defenses of tropical seaweeds relative to temperate herbivores (Cronin et al. 1997, Craft et al. 2013). Thus, we would expect tropical consumers would provide a more conservative test of latitudinal shifts in chemical defense than would temperate herbivores. The control seaweed was a mixture of Ulva lactuca and U. intestinalis (Chlorophyta), abundant and palatable seaweeds collected locally from Charleston, SC in the winters of 2013 and 2014. Control seaweed was frozen, lyophilized, stored at −20°C and prepared similarly to all test seaweeds. Because all test seaweeds were in the Divisions Rhodophyta or Phaeophyta, the use of a chlorophyte seemed appropriate for preventing biases in choice comparisons. We used freeze-dried tissue in order to standardize food quality and allow us to assay seaweeds that were collected 1000s of kilometers and months apart. All feeding assays used a dry to wet mass ratio of 0.081, which is within the natural range for our samples (see metadata at Dryad). For each assay, 8 g of freeze-dried and ground powder (ground via Wiley Mill) of one experimental seaweed and the Ulva control were rehydrated with 28 mL distilled water and mixed with 72 mL molten agar (2% by weight). Seaweed mixes were then poured into side-by side lanes in a mold on window screen (1×2 mm squares) in a thickness of approximately 2 mm (Hay et al. 1998). After cooling, the screen was then cut into strips with approximately 80 squares of each food type separated by 2 cm.
Individual strips were then isolated with approximately 30 separate crabs and 50 separate urchins, and removed before the entirety of either food was consumed or until 24 to 30 hours had elapsed. Replicates in which less than 10% or greater than 95% of all food offered was consumed were removed before statistical analysis because of their low power to infer feeding choice. Our sample size for each seaweed-herbivore combination ranged from n = 10 to 46. Palatability of each seaweed to each herbivore was quantified as the proportion of the experimental seaweed consumed divided by the total consumption of experimental and control seaweed within a replicate (%T; see also Craft et al. 2013).
Nutritional Data and Analysis
We determined organic content (ash-free dry mass of freeze-dried tissue) in five technical replicates per seaweed species. Samples (10–20 mg each) were dried to a constant mass at 60 °C and weighed. Samples were then placed in a muffle furnace at 500 °C for 6 to 7 hours and then reweighed. Ash-free dry mass was expressed as a percentage of total dry mass. We measured NaOH-soluble protein content in triplicate using the Bradford colorimetric assay (1976). Freeze-dried seaweeds were ground via mortar and pestle and 10 (±0.1) mg was added to an Eppendorf tube. Each tube was filled with 0.5 mL of 1M NaOH, vortexed, and left to sit at room temperature for 24 hours. The supernatant (20 uL) was added to a polystyrene cuvette, mixed with 1 mL of Bradford reagent, and kept at room temperature for 5 minutes before absorbance (595nm) was quantified on a Milton Roy Spectronic 601 spectrophotometer. Absorbance values were converted to protein concentrations using a standard curve based on a dilution series of bovine serum albumin. We determined phenolic content in triplicate using the Folin-Ciocalteau method (following Reynolds and Sotka 2011). To estimate carbon and nitrogen content, freeze-dried seaweed samples were pulverized via bead beating (Mini-Beadbeater, Biospec Products) and triplicate samples of 5.5 (±0.5) mg were analyzed using a NCS 2500 Series Elemental Analyzer (CE Instruments).
Molecular phylogenies
Because we wished to ensure accurate phylogenetic correction of comparative data, we constructed novel molecular phylogenies of these seaweeds using one plastid and one nuclear marker. Lyophilized seaweed tissue was pulverized via bead beating and total genomic DNA extracted using the Nucleospin Plant II kit (Macherey-Nagel, Düren, Germany). We PCR amplified the conserved 18S rRNA and the more variable rbcL (i.e., large subunit of RuBisCO) using primers from Hadziavdic et al. 2014, Draisma et al. 2001 and Hommersand et al. 1994 (Appendix S1: Table S2). PCR products were cleaned using ExoSAP-IT (Affymetrix) and sent to Eurofins MWG Operon LLC (Louisville, KY) for Sanger sequencing. Sequences were edited manually using Sequencher® (Version 5.3). We generated sequences for both loci for most species (Accession Numbers KY987557- 639), and complemented these with archived sequences at NCBI (Appendix S1: Table S3–4). Because 18S is highly conserved and poorly resolves relationships at or below genera, we only used 18S data when rbcL sequences were available for that species.
Phylogenies for red and brown seaweeds were generated independently. We haphazardly chose three browns (Petalonia binghamiae, Eisenia sp. and Macrocystis pyrifera) to include as outgroups for the reds phylogenies and three reds (Gracilaria edulis, Gelidium sp. and Gelidium coulteri) to include as outgroups for the browns phylogenies. All sequences were aligned using the G-INS-1 progressive method in the MAFFT v7 online server (Katoh and Standley 2013) and uploaded into the online GBlock server (Talavera and Castresana 2007) and the least stringent options checked to remove regions that were poorly aligned. The most parsimonious (MP) trees of the concatenated dataset were determined using a heuristic search with random addition in PAUP* v4.0 (Swofford 2003) and a strict consensus tree formed with 10,000 bootstrap replicates (Appendix S1: Figure S1). A maximum-likelihood (ML) tree with 10,000 rapid bootstrap replicates was constructed via RAxML GUI (Silvestro 2012, Stamatakis 2014) with Macrocystis pyrifera and Gracilaria edulis selected as the single outgroup sequence for reds and browns phylogeny (respectively), and a partition splitting 18S and rbcL fragments set prior to tree construction. A generalized time-reversible (GTR) model of evolution with gamma-distributed evolutionary rates (G) and invariable sites (I) for browns and GTR + G for reds was selected using the Akaike information criterion (AIC) in jModelTest v2 (Darriba et al. 2012). Tree types (Strict MP and ML) were visualized in FigTree v1.4.2 (Appendix S1: Figure S2).
Analysis
Our core hypotheses centered on the interactive effect of latitudinal distribution and seaweed traits on tissue palatability. The effect of latitudinal distribution was assessed in two ways. First, we collected seaweeds from three locations (Fiji, California, and Antarctica) that span tropical, temperate, and polar regions, respectively, and analyzed palatability to two allopatric herbivores and tissue traits using a series of ANOVAs using collection site as a fixed-effect categorical variable. A three-way ANOVA on palatability indicated a significant interaction between herbivore, seaweed division (Rhodophyta vs Phaeophyta), and collection site. For subsequent tests of the effect of collection site, we separately analyzed combinations of herbivore and divisions (i.e. crab-reds, urchin-reds, crab-browns, and urchin-browns). The effect of collection site on nutritional traits and palatability was assessed through a series of one-way ANOVAs followed by Tukey’s post-hoc tests.
Second, we used the mean latitude of the geographical distribution of each genus (Figure 1) as a continuous independent variable in linear regressions. Geographic occurrence records for each genus were downloaded from the Global Biodiversity Information Facility (GBIF.org; February 2015). All statistical tests performed met the appropriate assumptions, were performed in R version 3.0.2 (2013) or later, and used 31 rhodophytes and 19 phaeophytes.
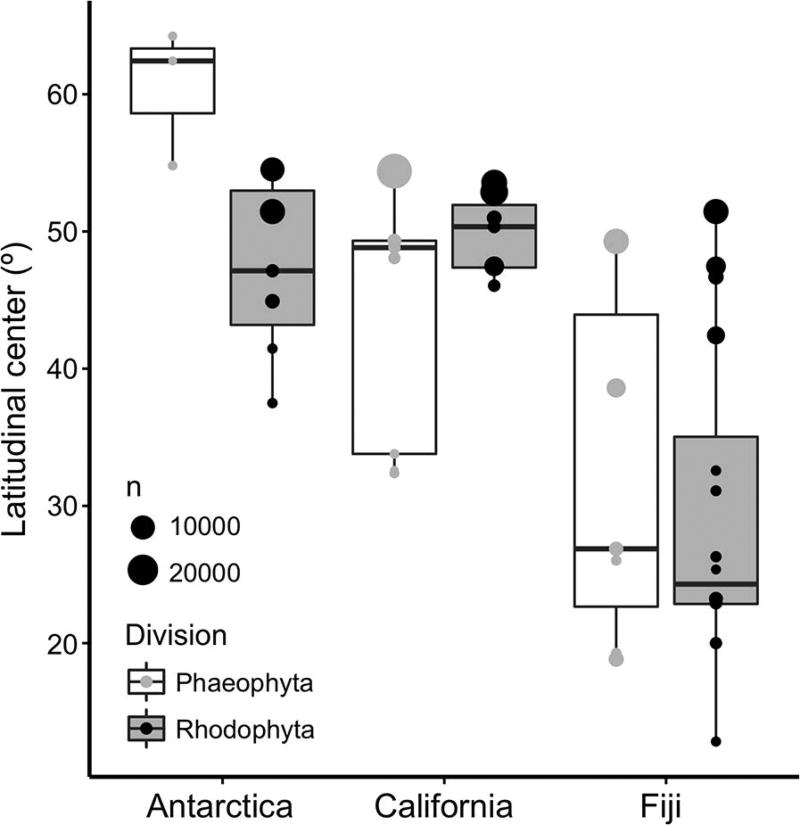
Figure 1.
Average latitudinal center of seaweed genera collected from tropical (Fiji), temperate (California), and polar (Antarctic), split across Division (Rhodophyta versus Phaeophyta). Size of points indicates the number of GBIF records for each genus. P-values for ANOVA on latitudinal center: Site (F2,43 = 22.2; p <0.001), Division (F1,43 = 0.5; p = 0.478); Site*Division (F2,43 = 2.5; p = 0.096). Tukey’s HSD posthoc indicates that Fiji < California = Antarctica. Boxplots indicate quartile distribution, mean, maximum and minimum of latitude.
We assessed the effect of latitude that remained after accounting for phylogenetic effects (Figure 2) using phylogenetically-independent contrasts (PIC; Felsenstein 1985). We analyzed PICs on seaweeds within a single division because the long branch between divisions obscured phylogenetic signals. Following Legendre and Desdevises (2009), all PIC regressions were computed through the origin and permutated 10,000 times. All PIC analyses were pursued using the ape package in R (Paradis et al. 2004). We evaluated PICs with all species except the four red seaweeds for which we lacked sequences (Galaxaura sp., Amphiroa crassa, Neurymenia fraxinifolia, and Actinotrichia sp.).
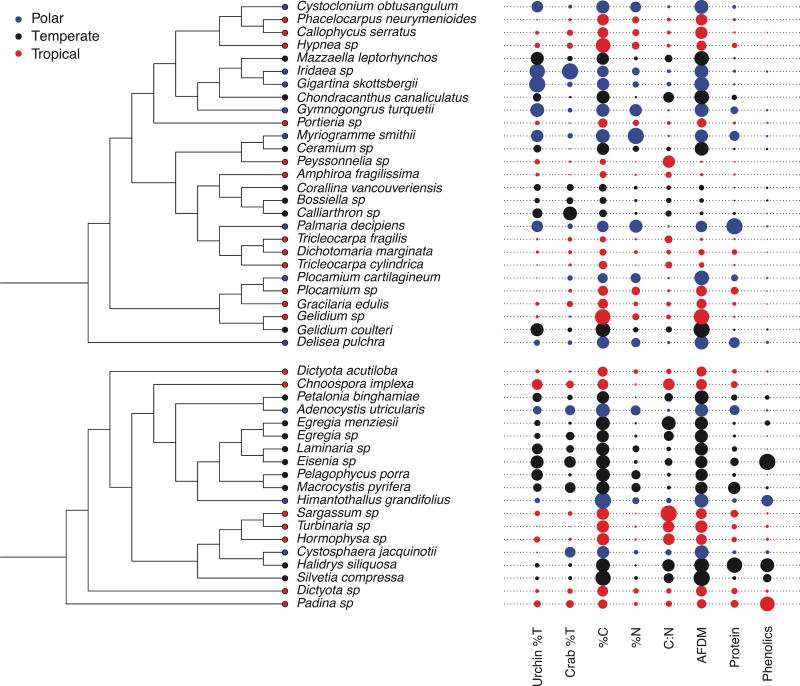
Figure 2.
Maximum likelihood phylogenies based on 18S and rbcL sequences and corresponding trait values for Rhodophyta and Phaeophyta. Circle size represents proportion of the maximum value for that trait.
Because organic content (AFDM), latitudinal origin and the palatability of rhodophytes to urchins strongly covaried, we performed a residual analysis to assess whether the effect of latitudinal origin remained after palatability was regressed onto AFDM. We repeated the analysis after removing the heavily calcified seaweeds to determine if the presence of calcified tropical and temperate seaweeds biased our results.
RESULTS
The global distributions of seaweed genera collected from Fiji (18°S) had a significantly lower mean latitude than did seaweeds collected from either California (32–38°N) or Antarctica (66°S), which were statistically indistinguishable (Figure 1), demonstrating that our field collections effectively captured tropical versus non-tropical seaweeds. The geographic separation of tropical (Fiji) vs. non-tropical (California and Antarctica) seaweeds was greater among reds compared to browns.
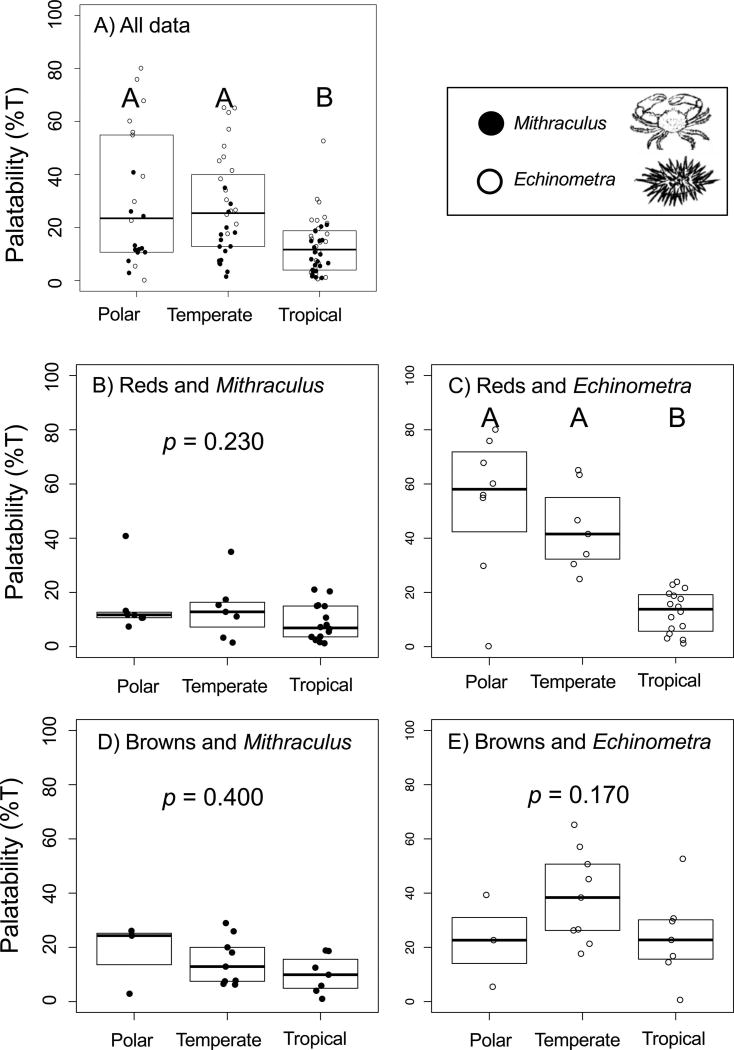
Overall, generalist crabs and urchins tended to prefer tissue from non-tropical seaweeds collected in Antarctica and California than from tropical seaweeds collected in Fiji (Figure 3A; Appendix S1: Table S1). This pattern was not consistent across herbivore nor seaweed division; rather, the pattern was largely driven by the response of Echinometra urchins to reds (Figure 3C). Urchins did not distinguish among browns from the three collection sites (Figure 3E) and crabs did not distinguish among collection sites when offered either reds or browns (Figure 3B, D). These one-way effects of collection site on palatability were confirmed by ML-PIC and MP-PIC (Table 1). Similarly, we found that the palatability of reds to urchins increased with increased latitudinal mean of their global distributions using linear regression on raw and ML-PIC data (Table 2A). There were no significant effects of mean global distribution on palatability for any other seaweed-herbivore combinations (crab-browns; crab-reds; urchin-browns; Table 2A,B).
Figure 3.
Palatability of seaweeds across a broad latitudinal gradient when analyzed by A) all seaweeds and herbivores together, B–C) only Rhodophyta D–E) only Phaeophyta. Seaweeds were collected from tropical (Fiji), temperate (California), and polar (Antarctic) sites and offered to crabs Mithraculus sculptus (closed circles) and urchins Echinometra lucunter (open circles). Palatability was determined by calculating the mean percentage of test seaweeds consumed (n = 10–46 per assay). Boxplots indicate quartile distribution and mean palatability; points indicate each seaweed-herbivore combination (analyzed by ANOVA in Table 1). Letters indicate results of a post-hoc Tukey’s HSD test.
Table 1.
ANOVAs of the effect of collection site (Polar, Temperate, Tropical) on averaged values of palatability and other plant traits in A) Rhodophyta seaweeds and B) Phaeophyta seaweeds. F-TEST indicates results from raw means; PIC tests reflect phylogenetic independent contrasts using Maximum Likelihood (ML) and Maximum Parsimony (MP) trees. All plant nutrient values were tested in percentage of dry mass. Palatability results are plotted in Figure 2 while plant traits are plotted in Appendix S1: Figure S3. Shaded cells indicate p<0.05.
| A) Rhodophyta – ANOVAs | ||||||
|---|---|---|---|---|---|---|
| F-TEST | ML PIC | MP PIC | ||||
| F | p-value | Adj R2 | p-value | Adj R2 | p-value | |
| Palatability (Urchin) | 20.0 | <0.001 | 0.425 | <0.001 | 0.415 | <0.001 |
| Palatability (Crab) | 1.5 | 0.233 | 0.074 | 0.112 | 0.091 | 0.105 |
| Carbon | 3.6 | 0.042 | 0.087 | 0.048 | 0.177 | 0.015 |
| Nitrogen | 19.8 | <0.001 | 0.510 | <0.001 | 0.477 | <0.001 |
| C:N | 4.3 | 0.024 | 0.253 | 0.005 | 0.189 | 0.015 |
| AFDM | 5.9 | 0.007 | 0.241 | 0.004 | 0.282 | 0.002 |
| Protein | 6.7 | 0.004 | 0.206 | 0.012 | 0.248 | 0.005 |
| Phenolics | 1.9 | 0.174 | 0.136 | 0.030 | 0.108 | 0.054 |
| B) Phaeophyta–ANOVAs | ||||||
| F-TEST | ML PIC | MP PIC | ||||
| F | p-value | Adj R2 | p-value | Adj R2 | p-value | |
| Palatability (Urchin) | 2.0 | 0.170 | −0.043 | 0.509 | 0.004 | 0.315 |
| Palatability (Crab) | 1.0 | 0.396 | −0.054 | 0.694 | −0.009 | 0.419 |
| Carbon | 10.0 | 0.002 | −0.051 | 0.442 | 0.118 | 0.090 |
| Nitrogen | 2.9 | 0.087 | −0.062 | 0.342 | 0.054 | 0.219 |
| C:N | 1.6 | 0.242 | −0.057 | 0.464 | 0.068 | 0.157 |
| AFDM | 15.1 | <0.001 | −0.060 | 0.365 | 0.241 | 0.030 |
| Protein | 0.1 | 0.890 | −0.060 | 0.981 | −0.053 | 0.726 |
| Phenolics | 0.3 | 0.733 | −0.056 | 0.799 | 0.001 | 0.297 |
Table 2.
Linear regressions of effect of mean latitudinal center of each seaweed genus against averaged values of palatability and other plant traits in A) Rhodophyta seaweeds and B) Phaeophyta seaweeds. Linear regression indicates results from raw means; PIC tests reflect phylogenetic independent contrasts using Maximum Likelihood (ML) and Maximum Parsimony (MP) trees. All plant nutrient values were tested in percentage of dry mass. Shaded cells indicate p<0.05.
| A) Rhodophyta | ||||||
|---|---|---|---|---|---|---|
| Linear regression | ML PIC | MP PIC | ||||
| F | p-value | Adj R2 | p-value | Adj R2 | p-value | |
| Palatability (Urchin) | 11.1 | 0.002 | 0.195 | 0.016 | 0.048 | 0.135 |
| Palatability (Crab) | 0.6 | .439 | −0.042 | 0.914 | −0.034 | 0.771 |
| Carbon | 5.9 | 0.022 | 0.031 | 0.117 | −0.005 | 0.337 |
| Nitrogen | 5.4 | 0.028 | 0.013 | 0.114 | −0.022 | 0.480 |
| C:N | 1.8 | 0.188 | −0.039 | 0.768 | −0.036 | 0.713 |
| AFDM | 9.1 | 0.005 | 0.143 | 0.012 | 0.136 | 0.031 |
| Protein | 0.5 | 0.483 | 0.015 | 0.255 | −0.035 | 0.699 |
| Phenolics | 1.2 | 0.278 | −0.040 | 0.768 | −0.009 | 0.387 |
| B) Phaeophyta | ||||||
| Linear regression | ML PIC | MP PIC | ||||
| F | p-value | Adj R2 | p-value | Adj R2 | p-value | |
| Palatability (Urchin) | 0.2 | 0.670 | 0.103 | 0.089 | 0.048 | 0.371 |
| Palatability (Crab) | 1.3 | 0.263 | −0.061 | 0.783 | −0.012 | 0.236 |
| Carbon | 3.9 | 0.065 | −0.056 | 0.885 | 0.136 | 0.074 |
| Nitrogen | 3.4 | 0.084 | −0.062 | 0.620 | −0.046 | 0.396 |
| C:N | 2.7 | 0.119 | −0.058 | 0.899 | −0.066 | 0.806 |
| AFDM | 3.0 | 0.103 | −0.057 | 0.761 | 0.192 | 0.039 |
| Protein | 0.1 | 0.789 | 0.059 | 0.144 | 0.094 | 0.064 |
| Phenolics | 0.4 | 0.524 | −0.056 | 0.885 | −0.039 | 0.502 |
Among seaweed traits that we assessed, the most consistently supported pattern was an increase in organic content (i.e., ash-free dry mass or AFDM as a percentage of total dry mass) of reds with latitude; AFDM significantly differed among collection site using ANOVA (Table 1A; Appendix S1: Figure S3) and uncorrected and PIC-corrected regressions (Table 2A). A similar latitudinal increase in AFDM was detected using ANOVA in browns on raw data and MP-PIC (Table 1B; Appendix S1: Figure S3), and MP-PIC with mean latitude in linear regressions (Table 2B). Another proxy for organic content, % carbon, showed a latitudinal increase in some but not all statistical approaches (Table 1, Table 2), and there was less statistical support for other nutritional traits (% nitrogen; C:N, protein) to vary with latitude in either reds or browns.
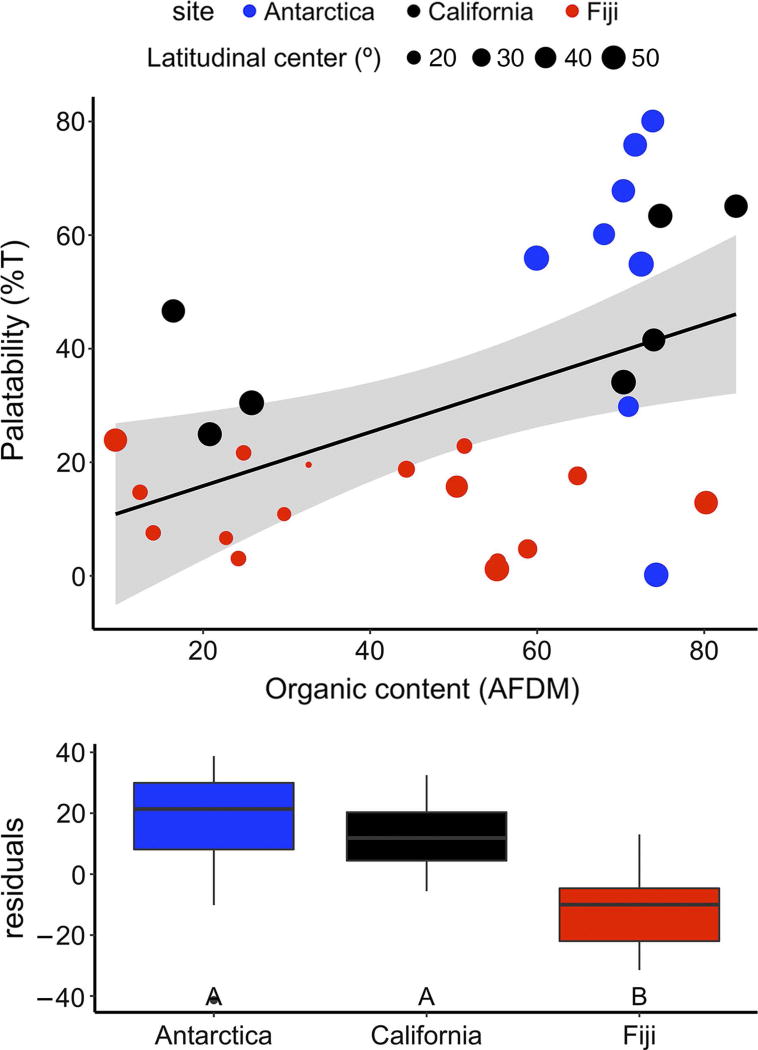
Because organic content (AFDM), latitudinal distribution, and the palatability of reds to urchins strongly covary (Table 2), we used a residual analysis to separate these effects. Reds collected from Antarctica and California tend to be more palatable than expected from their organic content, while Fijian reds tend to be less palatable than expected (Figure 4). However, given that heavily calcified seaweeds were collected from tropical and temperate waters but not polar regions, we removed heavily-calcified reds and repeated the residual analysis. Palatability of fleshy reds still significantly increased with AFDM, but was only marginally explained by latitudinal origin in the residual analysis (p<0.10; Appendix S1: Figure S4).
Figure 4.
Palatability of Rhodophyta seaweeds to urchins depends on both organic content (percent ash-free dry mass per unit dry mass, or AFDM) and collection site. Top panel regresses palatability against AFDM (F1,29 = 8.2; p = 0.008) while the bottom panel shows the boxplot of their residuals against collection site (F2,28 = 8.0; p = 0.002). Point size in top panel indicates average latitudinal distribution of genus. Letters in the bottom panel indicate results of a posthoc Tukey’s HSD test.
DISCUSSION
The present study represents one of the few tests of latitudinal gradients in palatability of any primary producer spanning polar to tropical latitudes, and one of two such studies for marine producers (Bolser and Hay 1996). Consistent with theory (Schemske et al. 2009), reds from temperate and polar latitudes were more palatable to generalist sea urchins when compared to tropical reds. However, these results are dependent on both seaweed and herbivore identity, as we detected no latitudinal shift in palatability of reds to crabs, nor any latitudinal shifts in palatability of browns to either crabs or urchins.
The latitudinal decline in the palatability of reds may be explained by latitudinal shifts in tissue organic content, secondary metabolites, calcification, or some combination of these traits. Non-tropical reds contain greater organic content (AFDM) than tropical reds, and their greater palatability is consistent with the notion that marine generalist herbivores prefer tissues with greater organic content (Vadas 1977). Yet, after accounting for the positive effect of organic content on palatability in initial analyses, a residual effect of latitudinal origin on palatability remained. This residual decline may reflect greater chemical defenses. Indeed, lipophilic extracts of temperate versus tropical congeneric pairs of seaweeds explained ~60% of the variability in palatability to urchin grazers (Bolser and Hay 1996). Similarly, Siska et al. (2002) and Long et al. (2011) determined that polar extracts better explained latitudinal patterns in marsh plant palatability than did nutritional plant traits.
When only fleshy red seaweeds were analyzed, organic content remained significantly correlated with palatability; however, the residual effect of latitude became marginal. Therefore, the residual decline in palatability may also reflect the greater frequency of calcification in temperate and tropical seaweeds relative to polar seaweeds that were assayed, as crustose and calcareous seaweeds tend to be more resistant to herbivory than fleshy seaweeds (Littler et al. 1983), or reflect a combination of calcification and chemical defense employed by lower-latitude seaweeds to deter grazers. Paul and Hay (1986) found that significantly more calcified seaweeds produced secondary metabolites compared to fleshy seaweeds suggesting that tropical seaweeds may be selected to deploy both defenses. Feeding deterrence was typically stronger in foods with secondary metabolites added, or in combinations of secondary metabolites and CaCO3 (Hay et al. 1994, Schupp and Paul 1994). Studies that separate the roles of calcification and secondary metabolites in driving latitudinal gradients in palatability would be valuable.
We detected no latitudinal shift in palatability of reds to crabs, and it is unclear why crabs and urchins responded so differently. Crabs overwhelmingly preferred the control alga (Chlorophyta; Ulva spp.) in nearly all assays, with %T values being significantly lower in crab assays compared to urchin assays (Student’s T-test p<0.001; Figure 3). It is possible that a control alga of lower preference would have generated more variance in palatability among the treatment algae. Mithraculus crabs have low mobility, and often shelter in, and clean, structurally complex organisms like upright encrusting coralline algae and corals. These behaviors appear to have selected for high resistance to algal chemical defenses relative to more mobile crabs (Stachowicz and Hay 1996, 1999).
Somewhat surprisingly given the results of Bolser and Hay (1996), we detected no latitudinal shift in palatability of browns to either crabs or urchins. There could be several, non-exclusive reasons for this. First, the lack of a latitudinal pattern in palatability is consistent with the lack of a latitudinal signal in total phenolics within their tissues, a class of water-soluble secondary metabolites whose members can deter some marine herbivores (Amsler 2008). Second, brown seaweeds may not have been adequately sampled. For example, it is possible that latitudinal patterns would have been detected if we had assayed more than three Antarctic brown seaweeds. The three Antarctic browns are deterrent to sympatric grazers (Amsler et al. 2005) and were relatively deterrent in our study. Third, it is possible that the water-soluble secondary metabolites were not deterrent to our herbivores or that the compounds leached out of the food strips and our assay was not reflective of actual tissue deterrence.
The logistical benefits of using freeze-dried tissue in order to standardize food quality and allow us to assay seaweeds that were collected 1000s of kilometers and months apart come at the cost of minimizing morphological traits. Morphological seaweed traits are associated with herbivory pressure, abiotic environment and phylogenetic lineage, and their relationships with latitude are variable (Hay 1981, Henkel et al. 2007, Santelices et al. 2009), so clearly this would be an interesting question to further pursue. Pennings et al. (2001) compared northern and southern Spartina populations and found that southern Spartina was significantly less palatable in fresh tissue assays (see also Long et al. 2011), but when lyophilized tissue was tested the significance was reduced (Siska et al. 2002). In contrast, when feeding preferences of freshwater macrophytes to herbivores was tested, stronger food preferences were seen in the freeze-dried assays relative to the fresh-tissue assays (Morrison and Hay 2012). In future experiments, it would be valuable to directly compare feeding preferences for fresh tissues, freeze-dried tissues, and their chemical extracts.
Clearly, our test of latitudinal gradients in seaweed palatability is limited by the number of collection sites (one per region) and thus, future tests will require more sites, seaweeds, herbivores and ocean basins. Despite this limitation, we have confidence that latitudinal decline in red palatability toward urchins is genuine and not a function of random variation in seaweed occurrence among sites because the pattern was maintained when collection site or latitudinal midpoint was analyzed.
If our findings are confirmed to be robust across broader regions, this would have at least two important implications. First, evolutionary pressure from tropical macrograzers (e.g., fishes, sea urchins, turtles) may have favored red seaweeds with lower palatability, either through the production of greater levels of chemical defenses, calcification or both. Such selection pressure would also result in less overall variation compared to regions under patchy or less herbivory; indeed, variance of palatability in tropical reds was lower than that of non-tropical reds in our feeding assays with urchins (Figure 3C). This also raises the possibility that in response to increased seaweed defenses, fish and urchin herbivores from lower latitudes evolved greater levels of tolerance to these defenses (Cronin et al. 1997, Craft et al. 2013), a diffuse coevolutionary scenario analogous to that proposed for Australasian reefs (Steinberg et al. 1995).
Second, these results may alter how we interpret the ‘tropicalization’ of temperate plant-herbivore interactions (Vergés et al. 2014, 2016). When increases in seawater temperature facilitate the poleward movement of lower-latitude fishes and urchins, there is typically a dramatic phase shift from seaweed-dominant systems to greater amounts of bare rock, tropical species or both. One way to view these effects is as the outcome of diffuse coevolution that may be occurring in tropical habitats. Tropical herbivores enter areas dominated by weakly defended and more nutritious foods, and the removal of competing seaweeds facilitates the invasion of better-defended tropical species. Thus, the poleward movement of tropical seaweeds may depend on tropical herbivores moving into temperate waters beforehand. Our results also suggest that such tropicalization effects will not be consistent across all herbivore and seaweed species. Consequently, continued studies on latitudinal gradients across taxa are needed if we hope to predict the outcome of inevitable poleward movement of seaweeds and herbivores into temperate (Vergés et al. 2014, 2016) and polar regions (Ducklow et al. 2013), and its impact on ecosystem functioning.
Supplementary Material
Acknowledgments
We would like to thank Dr. B.J. Baker and J. Fries for providing additional samples, C. Gerstenmaier, N. Kollars, E. Jones, M. Smylie, P. Bippus, L. Lees, K. Hill-Spanik and S. Krueger-Hadfield for logistical support, and A. Strand and G. Naylor for discussions. Funding was provided by NSF (OCE-1357386, OCE-1057707, PLR-1341333, PLR-1341339, OCE 0929199), the National Institute of Health (U19TW007401), the Teasley Endowment to the Georgia Institute of Technology, the Smithsonian Hunterdon Oceanographic Fund and the College of Charleston. We thank E. Duffy, M. Graham, and an anonymous reviewer for comments that improved the manuscript.
Footnotes
Authors declare no conflicts of interest.
References
- Amsler CD. Algal Chemical Ecology. Springer-Verlag Berlin Heidelberg; Germany: 2008. [Google Scholar]
- Amsler CD, Iken K, McClintock JB, Amsler MO, Peters KJ, Hubbard JM, et al. Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Marine Ecology Progress Series. 2005;294:141–159. [Google Scholar]
- Anstett DN, Ahern JR, Glinos J, Nawar N, Salminen J, Johnson MTJ. Can genetically based clines in plant defence explain greater herbivory at higher latitudes? Ecology Letters. 2015;18:1376–1386. doi: 10.1111/ele.12532. [DOI] [PubMed] [Google Scholar]
- Basset Y. Palatability of tree foliage to chewing insects: a comparison between a temperate and a tropical site. Acta Œcologica. 1994;15:181–191. [Google Scholar]
- Bolser R, Hay ME. Are tropical plants better defended? Palatability and defenses of temperate vs. tropical seaweeds. Ecology. 1996;77:2269–2286. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Craft JD, Paul VJ, Sotka EE. Biogeographic and phylogenetic effects on feeding resistance of generalist herbivores toward plant chemical defenses. Ecology. 2013;94:18–24. doi: 10.1890/11-0873.1. [DOI] [PubMed] [Google Scholar]
- Cronin G, Paul VJ, Hay ME, Fenical W. Are tropical herbivores more resistant than temperate herbivores to seaweed chemical defenses? Diterpenoid metabolites from Dictyota acutiloba as feeding deterrents for tropical versus temperate fishes and urchins. Journal of Chemical Ecology. 1997;23:289–302. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Evolution in the tropics. American Scientist. 1950;38:209–221. [Google Scholar]
- Draisma SGA, Prud’homme van Reine WF, Stam WT, Olsen JL. A reassessment of phylogenetic relationships within the Phaeophyceae based on rubisco large subunit and ribosomal DNA sequences. Journal of Phycology. 2001;37:586–603. [Google Scholar]
- Ducklow HW, Fraser WR, Meredith MP, Stammerjohn SE, Doney SC, Martinson DG, Sailley SF, Schofield OM, Steinberg DK, Venables HJ, Amsler CD. West Antarctic Peninsula: An ice-dependent coastal marine ecosystem in transition. Oceanography. 2013;26:190–203. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. American Naturalist. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Forbey JS, Dearing MD, Gross E, Orians CM, Sotka EE, Foley WJ. A pharm-ecological perspective of terrestrial and aquatic plant-herbivore interactions. Journal of Chemical Ecology. 2013;139:465–480. doi: 10.1007/s10886-013-0267-2. [DOI] [PubMed] [Google Scholar]
- GBIF.org. GBIF Occurrence Download. 2015 Mar 21; http://doi.org/10.15468/dl.yfdp0c.
- Hadziavdic K, Lekang K, Lanzen A, Jonassen I, Thompson EM, Troedsson C. Characterization of the 18S rRNA gene for designing universal eukaryote specific primers. PLoS One. 2014:9. doi: 10.1371/journal.pone.0087624. http://dx.doi.org/10.1371/journal.pone.0087624. [DOI] [PMC free article] [PubMed]
- Hay ME. The functional morphology of turf-forming seaweeds: persistence in stressful marine habitats. Ecology. 1981;62:739–750. [Google Scholar]
- Hay ME. Predictable spatial escapes from herbivory: how do these affect the evolution of herbivore resistance in tropical marine communities? Oecologia. 1984;64:396–407. doi: 10.1007/BF00379139. [DOI] [PubMed] [Google Scholar]
- Hay ME, Kappel QE, Fenical W. Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology. 1994;75:1714–1726. [Google Scholar]
- Hay ME, Stachowicz JJ, Cruz-Rivera E, Bullard S, Deal MS, Lindquist N. Bioassays with marine and freshwater macroorganisms. In: Haynes KF, Miller JG, editors. Methods in Chemical Ecology, Volume 2, Bioassay Methods. Chapman and Hall; New York, NY, USA: 1998. pp. 39–141. [Google Scholar]
- Henkel SK, Hofmann GE, Whitmer AC. Morphological and genetic variation in Egregia menziesii over a latitudinal gradient. Botanica Marina. 2007;50:159–170. [Google Scholar]
- Hillebrand H. Meta-analysis of grazer control of periphyton biomass across aquatic ecosystems. Journal of Phycology. 2009;45:798–806. doi: 10.1111/j.1529-8817.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- Hommersand MH, Fredericq S, Freshwater DW. Phylogenetic systematics and biogeography of the Gigartinaceae (Gigartinales, Rhodophyta) based on sequence analysis of rbcL. Botanica Marina. 1994;37:193–203. [Google Scholar]
- Hunter MD. The Phytochemical Landscape: Linking Trophic Interactions and Nutrient Dynamics. Princeton University Press; Princeton, NJ, USA: 2016. [Google Scholar]
- Katoh K, Standley DM. MAFFT: Multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre P, Desdevises Y. Independent contrasts and regression through the origin. Journal of Theoretical Biology. 2009;259:727–743. doi: 10.1016/j.jtbi.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Littler MM, Taylor PR, Littler DS. Algal resistance to herbivory on a Caribbean barrier reef. Coral Reefs. 1983;2:111–118. [Google Scholar]
- Long J, Mitchell J, Sotka EE. Local consumers induce resistance differentially between Spartina populations in the field. Ecology. 2011;92:180–188. doi: 10.1890/10-0179.1. [DOI] [PubMed] [Google Scholar]
- Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ. Assessing the evidence for latitudinal gradients in plant defense and herbivory. Functional Ecology. 2011;25:380–388. [Google Scholar]
- Morrison WE, Hay ME. Are lower-latitude plants better defended? Palatability of freshwater macrophytes. Ecology. 2012;93:65–74. doi: 10.1890/11-0725.1. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annual Review of Ecology and Systematics. 2006;37:637–669. [Google Scholar]
- Paul VJ, Hay ME. Seaweed susceptibility to herbivory: chemical and morphological correlates. Marine Ecology Progress Series. 1986;33:255–264. [Google Scholar]
- Pennings SC, Siska EL, Bertness MD. Latitudinal differences in plant palatability in Atlantic coast salt marshes. Ecology. 2001;82:1344–1359. [Google Scholar]
- Poore AG, Campbell AH, Coleman RA, Edgar GJ, Jormalainen V, Reynolds PL, et al. Global patterns in the impact of marine herbivores on benthic primary producers. Ecology Letters. 2012;15:912–922. doi: 10.1111/j.1461-0248.2012.01804.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R version 3.0.2. R Foundation for Statistical Computing; Vienna, Austria: 2013. http://www.R-project.org/ [Google Scholar]
- Reynolds PL, Sotka EE. Nonconsumptive predator effects indirectly influence marine plant biomass and palatability. Journal of Ecology. 2011;99:1272–1281. [Google Scholar]
- Santelices B, Bolton JJ, Meneses I. Marine algal communities. In: Witman JD, Roy K, editors. Macroecology. The University of Chicago Press; Chicago, IL, USA: 2009. pp. 153–194. [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution and Systematics. 2009;40:245–269. [Google Scholar]
- Schupp PJ, Paul VJ. Calcification and secondary metabolites in tropical seaweeds: variable effects on herbivorous fishes. Ecology. 1994;75:1172–1185. [Google Scholar]
- Silvestro M. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity and Evolution. 2012;12:335–337. [Google Scholar]
- Siska EL, Pennings SC, Buck TL, Hanisak MD. Latitudinal variation in palatability of salt-marsh plants: which traits are responsible? Ecology. 2002;83:3369–3381. [Google Scholar]
- Stachowicz JJ, Hay ME. Facultative mutualism between an herbivorous crab and a coralline alga: advantages of eating noxious seaweeds. Oecologia. 1996;105:377–387. doi: 10.1007/BF00328741. [DOI] [PubMed] [Google Scholar]
- Stachowicz JJ, Hay M. Reduced mobility is associated with compensatory feeding and increased diet breadth of marine crabs. Marine Ecology Progress Series. 1999;188:169–178. [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg PD, Estes JA, Winter FC. Evolutionary consequences of food chain length in kelp forest communities. Proceedings of the National Academy of Sciences. 1995;92:8145–8148. doi: 10.1073/pnas.92.18.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP*. [Google Scholar]
- Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Vadas RL. Preferential feeding: an optimization strategy in sea urchins. Ecological Monographs. 1977;47:337–371. [Google Scholar]
- Vergés A, Doropoulos C, Malcolm HA, Skye M, Garcia-Pizá M, Marzinelli EM, et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences. 2016;113:13791–13796. doi: 10.1073/pnas.1610725113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergés A, Steinberg PD, Hay ME, Poore AGB, Campbell AH, Ballesteros E, et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society of London B: Biological Sciences. 2014;281:20140846. doi: 10.1098/rspb.2014.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels H, Hagen W, Molis M, Wiencke C, Karsten U. Intra- and interspecific differences in palatability of Arctic macroalgae from Kongsfjorden (Spitsbergen) for two benthic sympatric invertebrates. Journal of Experimental Marine Biology and Ecology. 2006;329:20–33. [Google Scholar]
- Wiencke C, Amsler CD. Seaweeds and their communities in polar regions Pages. In: Wiencke C, Bischof K, editors. Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization. Springer-Verlag; Berlin, Germany: 2012. pp. 265–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.