Abstract
Objectives
Individuals with attention-deficit/hyperactivity disorder (ADHD) often have heightened levels of anxiety, which has been associated with worse performance on working memory tasks. Knowledge of the neural pathways underlying the combined presence of ADHD and anxiety may aid in a better understanding of their co-occurrence. Therefore, we investigated how anxiety modulates the effect of ADHD severity on neural activity during a visuospatial working memory (VSWM) task.
Methods
Neuroimaging data was available for 371 adolescents and young adults participating in the multicentre cohort study NeuroIMAGE (average age 17.1 years). We analysed the effects of ADHD severity, anxiety severity, and their interaction on task accuracy, and on neural activity associated with working memory (VSWM trials minus baseline), and memory load (high memory load trials minus low load trials).
Results
Anxiety significantly modulated the relation between ADHD severity and neural activity in the cerebellum for the working memory contrast, and bilaterally in the striatum and thalamus for the memory load contrast.
Conclusions
We found that ADHD with co-occurring anxiety is associated with lowered neural activity during a VSWM task in regions important for information gating. This fits well with previous theorizing on ADHD with co-occurring anxiety, and illustrates the neurobiological heterogeneity of ADHD.
Keywords: Attention Deficit Hyperactivity Disorder, Anxiety, Working Memory, Magnetic Resonance Imaging, Comorbidity
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is associated with heightened levels of anxiety; up to 50 percent of individuals with ADHD have one or more comorbid anxiety disorders (Schatz and Rostain 2006; Yoshimasu et al. 2012). Higher rates of anxiety disorders are found in both clinical and epidemiological samples of individuals with ADHD, even when controlling for overlap in symptomatology (Angold, Costello, and Erkanli 1999; Pliszka 2000). Given reports of significant effects of co-occurring anxiety on the cognitive functioning of individuals with ADHD (Bloemsma et al. 2013), their clinical presentation (March et al. 2000), and response to medication (M. T. A. Cooperative Group 1999; Bedard and Tannock 2008; Tannock, Ickowicz, and Schachar 1995), some authors have suggested that ADHD with comorbid anxiety represents a distinct subtype of ADHD (Jensen, Martin, and Cantwell 1997).
Visuospatial working memory (VSWM) tasks are well suited to investigate how co-occurring anxiety may influence ADHD-related cognitive impairments. Impaired working memory is a core cognitive deficit in ADHD, with strongest deficits in VSWM (Martinussen et al. 2005). Working memory is also a strong determinant of functional outcomes such as academic achievement (Rennie, Beebe-Frankenberger, and Swanson 2014), which makes it an important research target. Cognitive interference theories predict that high levels of anxiety have adverse effects on the performance of cognitive tasks, as irrelevant thoughts may interfere with information processing (Sarason 1984). Anxiety has been rather inconsistently associated with lowered VSWM performance (Shackman et al. 2006; Aronen et al. 2005; Staugaard 2010), but has also been reported to interact with working memory capacity, such that only individuals with low capacity showed a negative relation between anxiety level and task performance (Owens et al. 2014). Given that ADHD is associated with impaired working memory, such findings suggest that anxiety may be particularly detrimental if co-occurring with ADHD. This is in accordance with several reports that individuals with ADHD and comorbid anxiety are less accurate on working memory tasks than those who have ADHD without anxiety (Jarrett et al. 2012; Bedard and Tannock 2008; Skirbekk et al. 2011; Pliszka 1989), although these findings have not always been replicated (Vance et al. 2013; Manassis et al. 2007).
Neuroimaging data may provide a more sensitive and informative measure of working memory impairments than data on behavioural performance. VSWM is primarily associated with activity of frontoparietal brain regions, in line with the role of these regions in executive functioning and spatial awareness (Curtis 2006). Longitudinal studies of VSWM capacity have further shown that its development is associated with increased neural activity and connectivity of these frontal and parietal, as well as striatal, regions (Darki and Klingberg 2014). The few functional magnetic resonance imaging (fMRI) studies on the relation between ADHD and VSWM have not produced a clear pattern of results, as they reported either higher or lower activity of these neural circuits in individuals with ADHD compared to controls, or no differences at all (Li et al. 2014; Vance et al. 2007; Silk et al. 2005; van Ewijk et al. 2015). Anxiety has been associated mostly, though also inconsistently, with heightened activity in frontal regions during working memory, particularly the dorsolateral prefrontal cortex (DLPFC) (Fales et al. 2008; Park et al. 2016; Denkova et al. 2010; Moon and Jeong 2015; Basten, Stelzel, and Fiebach 2012). To our knowledge, no study to date has investigated whether ADHD and anxiety interact on working-memory related brain activity.
We hypothesized that the neurobiological pathways underlying anxiety severity co-occurring with symptoms of ADHD may differ from those that have been found for anxiety or ADHD severity when studied separately, thereby contributing to the large heterogeneity observed in behavioural and neuroimaging studies. Because of these possible differences, we did not restrict our analyses to brain regions commonly reported to be associated with ADHD or anxiety but employed a whole-brain approach. We carried out the analyses in an adolescent and young adult sample (mean age 17.1 years) consisting of individuals with ADHD, individuals with subthreshold ADHD, and healthy comparison subjects, thus enabling analysis within a wide range of ADHD severity in accordance with the continuous distribution of ADHD within the population (Levy et al. 1997).
Method
Participants and protocol
Participants were selected from the NeuroIMAGE study, a follow-up of the Dutch part of the International Multicenter ADHD Genetics (IMAGE) study (von Rhein et al. 2014). NeuroIMAGE included 365 families with at least one child with ADHD and at least one biological sibling (regardless of ADHD diagnosis) and 148 control families with at least one child without any formal or suspected ADHD diagnosis in any of the family members or their first-degree relatives. To be included in NeuroIMAGE, participants had to be of European Caucasian descent, between ages 5 and 30 years, have an IQ ≥ 70, and have no diagnosis of autism, epilepsy, general learning difficulties, brain disorders, or known genetic disorders. The study was approved by the regional ethics committee (CMO Regio Arnhem – Nijmegen; 2008/163; ABR: NL23894.091.08) and the medical ethical committee of the VU University Medical Center. All participants, and their parents (if the participant was younger than 18 years), signed informed consent; parents signed informed consent for participants under twelve years of age.
For the analyses reported in this paper, 371 participants, from 234 families, had complete data. Of the 371 participants, 122 participants had an ADHD diagnosis, 49 participants had subthreshold ADHD (i.e., had elevated levels of ADHD symptoms without meeting the full criteria for an ADHD diagnosis), and 200 participants were healthy controls. ADHD diagnoses were made in accordance with DSM 5 criteria, on the basis of a combination of a semi-structured interview and the Conners Rating Scales (Conners et al. 1998). Of those with an ADHD diagnosis, 93 (76%) had a history of treatment with stimulant medication. Sixteen (33%) of those with subthreshold ADHD and one healthy control (0.5%) had a history of receiving stimulant medication. Participants were asked to withhold use of their stimulant medication or other psychoactive drugs for 48 hours before measurement. Mean age of this sample was 17.1 years (standard deviation (SD) 3.4), ranging from 7.7 to 29.2 years, with the 10th and 90th percentiles being 13.1 and 21.4 years. 52.3% of the sample was male. More information on the NeuroIMAGE study, its diagnostic algorithm, and its participants, is presented in the Supplementary Information (SI) and in von Rhein et al. (2014).
ADHD outcome measure
In order to retain as much information as possible, we used a continuous measure of ADHD severity, the raw score on subscale N of the Conners Parent Rating Scale (CPRS) (Conners et al. 1998). This score ranged from 0 to 53, with an average of 11.3 (SD 11.5). This measure was chosen because it was available for all participants, from both ADHD families and control families.
Anxiety outcome measure
Anxiety severity was measured with the emotion subscale of the Strengths and Difficulties Questionnaire (SDQ-E) (Goodman 2001). The SDQ is well validated, has good internal consistency, and is reliable across informants and time (Achenbach et al. 2008). It has been shown to be a good dimensional measure of psychopathology in children, with the odds of a psychiatric disorder increasing at a constant rate across the range of scores (Goodman and Goodman 2009). The emotion subscale contains the following items: “Many worries, often seems worried”, “Often unhappy, down-hearted or tearful”, “Often complains of headaches, stomach-aches or sickness”, “Nervous or clingy in new situations, easily loses confidence”, and “Many fears, easily scared”. Ratings on the emotion subscale have been shown to be associated with the presence of common comorbid anxiety disorders, but not depression in an ADHD sample (Bekker, Bruck, and Sciberras 2013). Each item was rated both by the participant (if older than 12 years of age) and a parent on a three-point scale (“not true” (0), “somewhat true” (1), “certainly true” (2)). In order to gain maximum sensitivity to often underreported anxiety symptoms (Pliszka 1992), we selected the highest of either of the two informants’ scores on each item and subsequently summed these five highest item scores together, for a minimum of 0 and a maximum of 10. The average score for the whole sample was 4.16 (SD 2.03). Additional information on the composition of this measure is given in SI, as well as an overview of the scores per item and per informant.
Socio-economic status
As a measure of socio-economic status (SES), the highest, successfully completed education level of the parents was re-coded into a measure reflecting years of education. This scale contained nine levels, ranging from 0 (no formal education) to 17 (university) years of education (Buis 2010). The average of both parents was used, which, in this sample, ranged from 5 to 17 with an average of 12.2 (SD 2.47).
Visuospatial working memory task
To measure VSWM, we used a spatial span task (Klingberg, Forssberg, and Westerberg 2002) described more fully elsewhere (van Ewijk et al. 2014). The task consisted of two trial types (baseline and working memory) and two memory loads (low and high). In working memory trials, either three (low load) or six yellow circles (high load) were sequentially displayed on a 4×4 grid for 500 ms, with a 500 ms inter-stimulus interval. This was followed by a 2000 ms response window, during which the participant was asked whether a probe, consisting of a question mark and a number, was presented in the correct spatial location and the right temporal order of the presentation of cues, as indicated by the number. Participants answered by pressing one of two buttons representing ‘yes’ and ‘no’. We also included baseline trials for the low and high memory load conditions, both consisting of sequentially presented red circles in the four corners of the grid, followed by a probe (always the number 8). Participants were asked to pay attention but not to try to remember the sequence, and to press the ‘no’ button. Feedback on all trials was presented after the response in the form of a green or red coloured bar below the probe (for correct and incorrect responses, respectively). Accuracy on all trial types was determined as percentage correct responses. The task consisted of 4 blocks of 24 trials each, presented in fixed random order, for a total time of approximately 16 minutes.
fMRI analysis
Details on fMRI data acquisition, handling of runs with excessive head motion, and preprocessing are given in SI.
For the within-run analyses, we constructed a general linear model containing regressors for each of the four types of trial (i.e. low and high memory load, for both working memory and baseline trials), modelled from the start of the trial to the onset of the probe, in accordance with the approach by Klingberg et al. (2002). We also included regressors for feedback on correct and incorrect trials in the model, to remove unexplained variance. Further, global signal from cerebral spinal fluid and white matter, and standard and extended motion parameters were included as nuisance covariates. Beta-maps from all runs were concatenated using a single-subject fixed effects model, and the resulting maps were transformed to a study-specific template. Our contrasts of interest were mean working memory (both memory loads averaged, working memory trials minus baseline trials) and memory load difference (high minus low memory load trials), both consisting of the regressors for working memory trials corrected for regressors of the baseline trials of the same load.
Whole-brain analysis was conducted with the use of a variant of FSL’s ‘randomise’ algorithm, which employs nonparametric permutation inference (Winkler et al. 2015). To take into account the family relatedness present in the sample, we specified exchangeability blocks based on family membership, which ensured that only the rearrangements of the data that respect exchangeability were used (Winkler et al. 2015). The main model consisted of ADHD severity, anxiety severity, their interaction, and age, sex, socio-economic status, and scanning location as additional covariates. All regressors were mean-centred. The data were permuted 5000 times. We used cluster mass-based inference, with an initial cluster-forming threshold of z=2.3. We set the whole brain family-wise error rate corrected p-value at p < .01 to correct for the fact that we were looking at whole-brain maps for both the working memory contrast and memory load contrast.
Analysis of task accuracy and cluster data
All additional analyses were performed in R, v3.1.1, with linear mixed effects models. We estimated a random intercept for family, i.e. the intercept was allowed to vary between families, to account for the non-independence of the observations due to the inclusion of siblings in the sample. The linear mixed effects model included memory load (high and low load) as a within-subject factor, and accuracy on baseline trials as a covariate to control for basic processing or motivational effects. All models had age, sex, socio-economic status, and scanner location included as covariates. All continuous variables were standardized, because they were measured on widely differing scales.
“ In a second set of analyses, we assessed the effects of memory load by adding interaction terms between memory load condition (low or high load) and the predictors of interest (ADHD, anxiety, and their interaction) to the initial model.”
We further extracted the mean signal from significant clusters from the whole-brain analysis and calculated Cohen’s f2 as a measure of effect size for the significant predictors, as shown in Table 1. As a post-hoc analysis, we also ran a mixed effects repeated measures model where working memory task accuracy was regressed on the cluster data, corrected for baseline accuracy, in order to couple our results at the neural level to task performance.
Table 1.
Summary of the clusters that showed a significant correlation (PFWE < 0.01) between brain activity and the predictors. The upper part of the table shows the clusters correlating with activity for the mean working memory contrast, the bottom part of the table shows those associated with memory load.
| Mean working memory | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Contrast | Direction | Region | X | Y | Z | Size | Zmax | Cohen’s f2 |
| ADHD | Negative | L Frontal Pole, Superior Frontal Gyrus | −4 | 42 | 56 | 918 | 4.09 | .011 |
| Anxiety | N/A | |||||||
| ADHD * Anxiety | Negative | R Cerebellar Crus I, Crus II VIIIa | 18 | −88 | −34 | 762 | 3.54 | .028 |
| Memory load difference | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Contrast | Direction | Region | X | Y | Z | Size | Zmax | Cohen’s f2 |
| ADHD | N/A | |||||||
| Anxiety | N/A | |||||||
| ADHD * Anxiety | Negative | R Thalamus, Caudate, Putamen | 12 | −18 | 16 | 682 | 4.46 | .047 |
| Negative | L Thalamus, Caudate, Putamen, Pallidum | −20 | 0 | 0 | 516 | 3.93 | .055 | |
Note: X, Y, Z coordinates are in MNI-space in mm, and represent the peak of the cluster. The anatomical labels are according to the Harvard-Oxford atlas. MNI=Montreal Neurological Institute; Zmax= Z-score at the peak of the cluster.
Sensitivity analyses
Because of our dimensional approach to studying ADHD and its interaction with anxiety, we report demographic information on the participants split by ADHD diagnostic status in the SI. We further checked whether the findings were influenced by scanning location, by adding an interaction term between testing location and our predictors of interest and reran the analysis on the data from clusters found to be significant in the main analysis. In addition, while stimulant medication was discontinued 48h before measurement, we also reran the analyses with a covariate for duration of stimulant medication use, in the subset of individuals with an ADHD diagnosis.
Results
Task accuracy
ADHD severity was associated with lower accuracy on the VSWM task, independent of anxiety (b=−0.10, SE=0.05, p=.04), in accordance with a previous report on a subsample used in this study (van Ewijk et al. 2014). There was no conditional main effect of anxiety severity (b=−0.05, SE=0.04, p=.26), nor did anxiety severity modulate the effect of ADHD severity on working memory task accuracy (b=0.01, SE=0.03, p=.77). See Table S2 for the full results.
Memory load was a highly significant predictor of accuracy (b=−0.45, SE=0.06, p<.001), with the average accuracy on low load working memory trials being 79.2% versus 74.3% at high load, confirming the success of the task manipulation. Memory load did not significantly modulate the effect of ADHD severity, anxiety severity, or their interaction, on task accuracy (all p>.19).
Neural activity for mean working memory contrast
The mean working memory contrast was associated with widespread brain activity across superior and middle frontal gyri, superior parietal cortex, thalamus, striatum, and cerebellum. Further information on these clusters is provided in SI.
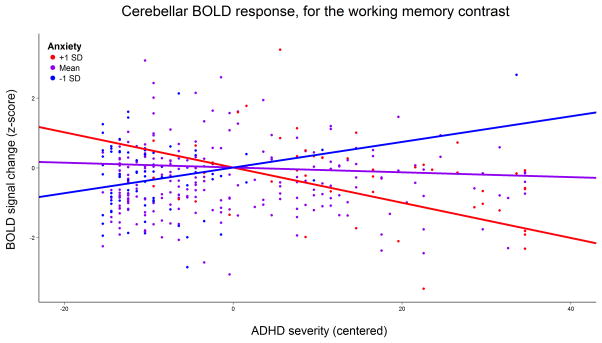
We found ADHD severity to be negatively correlated with working memory-associated brain activity in the left frontal pole. There was no conditional main effect of anxiety severity, but anxiety did modulate the relation between ADHD severity and brain activity in the right cerebellum, such that it correlated positively with ADHD severity for individuals with low levels of anxiety and negatively for individuals with high levels of anxiety (Figure 1). More information on these clusters is reported in Table 1. Figure 2a shows the location of these clusters overlaid on the sample’s average anatomical image.
Figure 1.
Modulating effect of anxiety severity on the association between ADHD severity and blood-oxygen dependent level (BOLD) signal change in the cerebellum while subjects performed the visuospatial working memory task. The x-axis reflects the score on the Conners’ Parent Rating Scale. Regression lines indicate effect of ADHD severity for average anxiety severity (purple line), as well as one standard deviation (SD) above (blue line) and one SD below average anxiety severity (red line).
Figure 2.
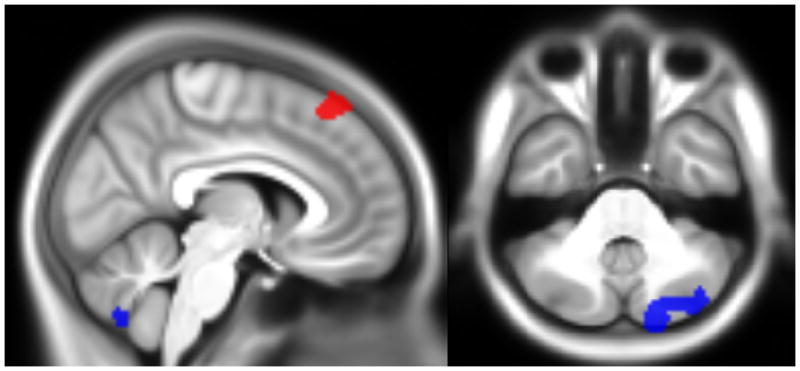
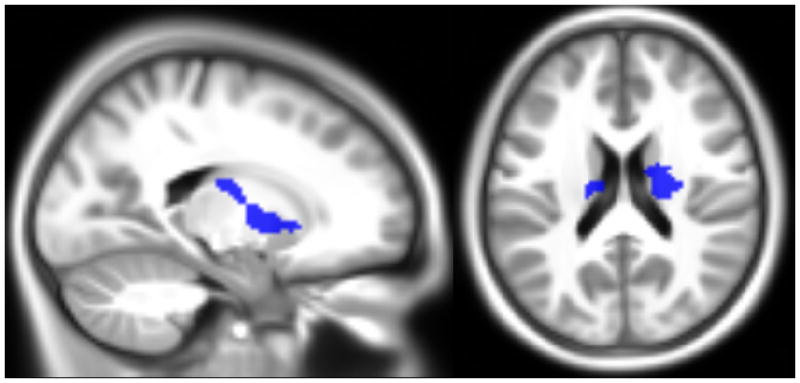
Location of the clusters surviving multiple comparison correction at P<.01, overlaid onto the sample’s average anatomical image. The top part (X=6, Y= −60, Z=−36) shows where there was an effect of ADHD severity (red) and the interaction between anxiety and ADHD (blue) on brain activity for the mean working memory contrast; the bottom part (X=−20, Y=−10, Z=20) shows the clusters with a significant effect of the interaction between anxiety and ADHD for the memory load difference contrast.
Neural activity for memory load difference contrast
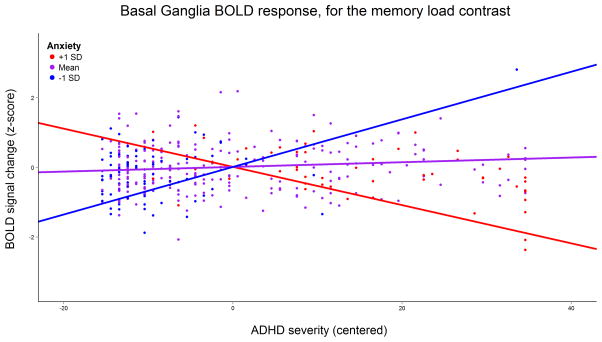
Anxiety severity modulated the association of ADHD severity with brain activity bilaterally in the dorsal striatum and thalamus for the memory load difference contrast. Individuals with high anxiety had a negative correlation between ADHD severity and task difficulty-associated brain activity, whereas those with low anxiety displayed a positive slope (Figure 3). Further information on these clusters is reported in Table 1 and Figure 2b. Post-hoc analysis showed that task accuracy was significantly positively correlated with the mean values of these clusters for the memory load difference contrast (b=0.17, SE=0.05, p<.001). In other words, an increase of basal ganglia activity when the task became more difficult was associated with higher task accuracy.
Figure 3.
Graph of the mean blood-oxygen level dependent (BOLD) signal change at the clusters in the striatum and thalamus (clusters combined), for the memory load contrast. Regression lines indicate effect of ADHD severity for average anxiety severity (purple line), as well as one standard deviation above (blue line) and below (red line) average anxiety severity.
Sensitivity analyses
Results from the sensitivity analyses can be found in SI. There were no interaction effects between the predictors of interest and testing location on brain activity in the significant clusters. Further, the predictors found to be significant in the main analyses remained significant when treatment duration was added as a covariate.
Discussion
We aimed to investigate whether anxiety modulates the association between ADHD severity and neural correlates of VSWM. Such interaction effects would indicate that previously reported differences in working memory performance between people with ADHD with and without comorbid anxiety (Jarrett et al. 2012; Bedard and Tannock 2008; Skirbekk et al. 2011; Pliszka 1989) cannot be attributed solely to additive effects of ADHD and anxiety, and illustrate that their co-occurrence carries significant information that may aid in explaining some of the etiological and phenotypic heterogeneity typical of ADHD research. We found that anxiety severity modulates the association between ADHD severity and brain activity in the cerebellum, basal ganglia and thalamus.
In our sample, ADHD severity was associated with lowered accuracy on VSWM task performance (see also van Ewijk et al. 2014), in accordance with meta-analyses (Martinussen et al. 2005; Willcutt et al. 2005). We also replicated a previous finding of a negative relation between ADHD severity and activity in frontal brain regions underlying VSWM (Silk et al. 2005). Specifically, we found less activity in the left frontal pole and superior frontal gyrus, which are known to respond more to spatial tasks than to object-based tasks (Courtney et al. 1996), when working memory must be continuously updated, and when temporal order must be maintained (Wager and Smith 2003), as was the case in our task. Further, these effects were independent of co-occurring anxiety.
While cognitive interference theories state that anxiety causes irrelevant information to interfere with task performance (Sarason 1984), we did not find effects of anxiety on VSWM brain activation or performance. Eysenck et al. theorized that the worrying of anxious individuals can lead to lowered performance on cognitive tasks by occupying resources, but that this is partly compensated by greater on-task effort (Eysenck and Calvo 1992). This may cause the negative effect of anxiety on working memory performance to only become apparent at higher difficulty levels, or in individuals with low working memory capacity as has been previously reported (Owens et al. 2014), rather than there being a direct relation between anxiety and cognitive functioning (Eysenck et al. 2007). The inconsistent results from previous behavioural and neuroimaging studies, as well as our null findings, attest to a complex relation between anxiety and working memory that is dependent on task and sample characteristics (Jarrett 2016).
Consistent with this reasoning, we did find that anxiety interacts with ADHD severity on neural activity. During the working memory task, higher levels of anxiety were associated with a more negative relation between ADHD severity and neural activity in the cerebellum. The cerebellum is increasingly recognized to be important for the development of cognitive functions (Riva and Giorgi 2000), including working memory (Ford et al. 2014), and has been previously shown to be less active in individuals with ADHD compared to controls during working memory tasks (Valera et al. 2005; Massat et al. 2012). Its specific function during working memory has been characterized as that of a gatekeeper, based on findings that individuals with lesions in the cerebellum are more susceptible to interference by irrelevant information during tasks reliant on VSWM (Baier, Muller, and Dieterich 2014). Less cerebellar activity therefore could indicate that ADHD co-occurring anxiety is associated with lowered information filtering.
The interaction between ADHD and anxiety was further associated with less task difficulty-related brain activity in the caudate nucleus, putamen, and thalamus, supporting the notion that ADHD with co-occurring anxiety may involve lowered information filtering. Activity in the basal ganglia and thalamus during working memory has been shown to predict the extent to which only relevant information is stored, a strong determinant of working memory capacity (McNab and Klingberg 2008). Activation of the basal ganglia allows for the disinhibition of the thalamus and subsequent processing of stimuli for working memory (Gisiger and Boukadoum 2011). Information filtering should become more important with increasing task difficulty, as higher amounts of information may become overwhelming and reduce performance. Results from our post hoc analyses are consistent with this reasoning, as basal ganglia activity due to increased memory load was associated with higher task accuracy. Further, it is also in line with Levy’s theory of ADHD with comorbid anxiety, which states that divergent gating of the basal ganglia leads to increased detection of threatening stimuli, contributing to feelings of anxiety (Levy 2004). The important role of dopamine in this process (Grace et al. 2007) conceivably plays a role in the repeatedly reported finding that individuals with ADHD with comorbid anxiety respond less well to stimulant medications than those without comorbid anxiety (Pliszka 1989; M. T. A. Cooperative Group 1999; Tannock, Ickowicz, and Schachar 1995).
We did not find evidence that anxiety modulates the relation between ADHD severity and task performance. Findings from studies into the relation between working memory performance and ADHD with comorbid anxiety have been mixed, with some reporting significant differences (Jarrett et al. 2012; Bedard and Tannock 2008; Skirbekk et al. 2011; Pliszka 1989) and others reporting null findings (Vance et al. 2013; Manassis et al. 2007). This inconsistency may be partly due to differences in task characteristics between studies; our findings on brain activity associated with varying memory load described above suggest that particularly task difficulty may be an important factor in outcomes of neuropsychological research of ADHD with co-occurring anxiety; problems with information gating may only be captured by measures of task performance when the stimulus rate increases and the information flow gets too high to adequately process. We therefore speculate that at higher task difficulties than present in the current study, less activity of the basal ganglia and cerebellum associated with the interaction between anxiety and ADHD severity may also be associated with overt performance difficulties on working memory tasks.
Strengths of this study include a large sample size and use of dimensional measures, allowing for optimal detection of effects. Furthermore, to our knowledge, we are the first to investigate the effects of ADHD co-occurring anxiety at the neural level, thereby providing information on its neurobiological correlates. The foremost limitation is the observational, cross-sectional design of our study, which prevents strong inferences about any causal relationship between the developmental course of ADHD, anxiety, and the brain. One might also argue that our measure of anxiety is not entirely specific to anxiety, but also measures depression. However, in ADHD samples, this instrument was found to only significantly relate to commonly occurring comorbid anxiety disorders, and not to mood disorders (Bekker, Bruck, and Sciberras 2013), see also SI. Further, due to our recruitment strategy of healthy controls, our sample has limited variation in anxiety in individuals with low ADHD severity, restricting the interpretation how these findings relate to individuals with pure anxiety. Therefore, also given that we are the first to report on the neural correlates of ADHD co-occurring anxiety, future studies in independent samples are needed to replicate our findings. Particularly fMRI studies using tasks that strongly engage the limbic system and experimental studies that manipulate the dopaminergic system may be valuable, to more directly test whether divergent information gating is a key factor in ADHD co-occurring anxiety.
In conclusion, we found that ADHD with co-occurring anxiety is associated with patterns of brain activity beyond the additive effects of ADHD and anxiety. This indicates that the combined presence of symptoms of ADHD and anxiety may be due to partly different neurobiological correlates than either separately. Given their common co-occurrence, these interaction effects may form a source of heterogeneity that contributes to inconsistent findings in studies of ADHD and anxiety. The involvement of brain regions important for gating of information could suggest that measures of the combined presence of ADHD and anxiety may capture problems with filtering of information, in line with Levy’s biological theory of ADHD with comorbid anxiety (Levy 2004), whereas at lower levels of anxiety, other mechanisms may be at play that contribute to any negative relation between ADHD severity and working memory performance. Further research on the neurobiological correlates of ADHD and co-occurring anxiety may not only aid in resolving some of the heterogeneity of ADHD, but ultimately also have clinical implications by enabling a more effective treatment tailored to the individual’s profile (M. T. A. Cooperative Group 1999).
Supplementary Material
Acknowledgments
We acknowledge the department of Pediatrics of the VU University Medical Center for having the opportunity to use the mock scanner for preparation of our participants.
Footnotes
Financial support
This work was supported by NIH Grant R01MH62873 (to Stephen V. Faraone), NWO Large Investment Grant 1750102007010 and NWO Brain & Cognition an Integrative Approach grant (433-09-242) (to Jan Buitelaar), and grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. The research leading to these results also received funding from the European Community’s Seventh Framework Programme (FP7/2007– 2013) under grant agreement numbers 278948 (TACTICS), 602450 (IMAGEMEND) and n° 602805 (Aggressotype), and from the European Community’s Horizon 2020 Programme (H2020/2014 – 2020) under grant agreement n° 643051 (MiND). Barbara Franke is supported by a Vici grant from NWO (grant number 016-130-669). In addition, Jan Buitelaar and Barbara Franke are supported by a grant for the ENIGMA Consortium (grant number U54 EB020403) from the BD2K Initiative of a cross-NIH partnership.
Financial disclosure
Dr. Franke has received a speaker fee from Merz.
Dr. Hoekstra has received an unrestricted research grant from Shire and has been member of the advisory boards of Shire and Eli Lilly.
Dr. Oosterlaan has received an unrestricted investigator initiated research grant from Shire pharmaceuticals.
Dr. Buitelaar has been in the past 3 years a consultant to / member of advisory board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, Novartis, Roche and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies.
In the past year, Dr. Faraone received income, travel expenses and/or research support from and/or has been on an Advisory Board for Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Neurovance, Impax, NeuroLifeSciences and research support from the National Institutes of Health (NIH). His institution is seeking a patent for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received consulting fees or was on Advisory Boards or participated in continuing medical education programs sponsored by: Shire, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health and Oxford University Press: Schizophrenia: The Facts.
References
- Achenbach TM, Becker A, Dopfner M, Heiervang E, Roessner V, Steinhausen HC, Rothenberger A. Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: research findings, applications, and future directions. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(3):251–75. doi: 10.1111/j.1469-7610.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of child psychology and psychiatry, and allied disciplines. 1999;40(1):57–87. [PubMed] [Google Scholar]
- Aronen ET, Vuontela Virve, Steenari M-R, Salmi Juha, Carlson Synnöve. Working memory, psychiatric symptoms, and academic performance at school. Neurobiology of Learning and Memory. 2005;83(1):33–42. doi: 10.1016/j.nlm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Baier B, Muller NG, Dieterich M. What part of the cerebellum contributes to a visuospatial working memory task? Annals of Neurology. 2014 doi: 10.1002/ana.24272. [DOI] [PubMed] [Google Scholar]
- Basten U, Stelzel C, Fiebach CJ. Trait anxiety and the neural efficiency of manipulation in working memory. Cognitive, affective & behavioral neuroscience. 2012;12(3):571–88. doi: 10.3758/s13415-012-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard AC, Tannock R. Anxiety, methylphenidate response, and working memory in children with ADHD. Journal of attention disorders. 2008;11(5):546–57. doi: 10.1177/1087054707311213. 1087054707311213 [pii] [DOI] [PubMed] [Google Scholar]
- Bekker J, Bruck D, Sciberras E. Congruent Validity of the Strength and Difficulties Questionnaire to Screen for Comorbidities in Children With ADHD. Journal of attention disorders. 2013 doi: 10.1177/1087054713496462. 1087054713496462 [pii] [DOI] [PubMed] [Google Scholar]
- Bloemsma JM, Boer F, Arnold R, Banaschewski T, Faraone SV, Buitelaar JK, Sergeant JA, Rommelse N, Oosterlaan J. Comorbid anxiety and neurocognitive dysfunctions in children with ADHD. European child & adolescent psychiatry. 2013;22(4):225–34. doi: 10.1007/s00787-012-0339-9. [DOI] [PubMed] [Google Scholar]
- Buis Maarten L. Scaling levels of education. Faculty of Social Sciences, VU-University Amsterdam; 2010. [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of abnormal child psychology. 1998;26(4):257–68. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Ungerleider LG, Keil K, Haxby JV. Object and spatial visual working memory activate separate neural systems in human cortex. Cerebral cortex (New York, NY: 1991) 1996;6(1):39–49. doi: 10.1093/cercor/6.1.39. [DOI] [PubMed] [Google Scholar]
- Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139(1):173–80. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- Darki F, Klingberg T. The Role of Fronto-Parietal and Fronto-Striatal Networks in the Development of Working Memory: A Longitudinal Study. Cerebral cortex (New York, NY: 1991) 2014 doi: 10.1093/cercor/bht352. bht352 [pii] [DOI] [PubMed] [Google Scholar]
- Denkova Ekaterina, Wong Gloria, Dolcos Sanda, Sung Keen, Wang Lihong, Coupland Nicholas, Dolcos Florin. The impact of anxiety-inducing distraction on cognitive performance: a combined brain imaging and personality investigation. PloS one. 2010;5(11):e14150. doi: 10.1371/journal.pone.0014150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck Michael W, Derakshan Nazanin, Santos Rita, Calvo Manuel G. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Eysenck Michael W, Calvo Manuel G. Anxiety and performance: The processing efficiency theory. Cognition & Emotion. 1992;6(6):409–34. [Google Scholar]
- Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JR, Braver TS. Anxiety and cognitive efficiency: differential modulation of transient and sustained neural activity during a working memory task. Cognitive, affective & behavioral neuroscience. 2008;8(3):239–53. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- Ford BQ, Mauss IB, Troy AS, Smolen A, Hankin B. Emotion Regulation Moderates the Risk Associated With the 5-HTT Gene and Stress in Children. Emotion (Washington, DC) 2014 doi: 10.1037/a0036835. 2014-21029-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisiger T, Boukadoum M. Mechanisms Gating the Flow of Information in the Cortex: What They Might Look Like and What Their Uses may be. Frontiers in computational neuroscience. 2011;5:1. doi: 10.3389/fncom.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A, Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(4):400–3. doi: 10.1097/CHI.0b013e3181985068. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(11):1337–45. doi: 10.1097/00004583-200111000-00015. S0890-8567(09)60543-8 [pii] [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Jarrett MA. Attention-deficit/hyperactivity disorder (ADHD) symptoms, anxiety symptoms, and executive functioning in emerging adults. Psychol Assess. 2016;28(2):245–50. doi: 10.1037/pas0000190. [DOI] [PubMed] [Google Scholar]
- Jarrett MA, Wolff JC, Davis TE, 3rd, Cowart MJ, Ollendick TH. Characteristics of Children With ADHD and Comorbid Anxiety. Journal of attention disorders. 2012 doi: 10.1177/1087054712452914. 1087054712452914 [pii] [DOI] [PubMed] [Google Scholar]
- Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(8):1065–79. doi: 10.1097/00004583-199708000-00014. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of cognitive neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Levy F. Synaptic gating and ADHD: a biological theory of comorbidity of ADHD and anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(9):1589–96. doi: 10.1038/sj.npp.1300469. [DOI] [PubMed] [Google Scholar]
- Levy F, Hay DA, McStephen M, Wood C, Waldman I. Attention-deficit hyperactivity disorder: a category or a continuum? Genetic analysis of a large-scale twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(6):737–44. doi: 10.1097/00004583-199706000-00009. [DOI] [PubMed] [Google Scholar]
- Li Y, Li F, He N, Guo L, Huang X, Lui S, Gong Q. Neural hyperactivity related to working memory in drug-naive boys with attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:116–22. doi: 10.1016/j.pnpbp.2014.03.013. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: the Multimodal Treatment Study of children with Attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56(12):1088–96. doi: 10.1001/archpsyc.56.12.1088. [DOI] [PubMed] [Google Scholar]
- Manassis Katharina, Tannock Rosemary, Young Arlene, Francis-John Shonna. Cognition in anxious children with attention deficit hyperactivity disorder: a comparison with clinical and normal children. Behavioral and Brain Functions. 2007;3(1):1–10. doi: 10.1186/1744-9081-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Swanson JM, Arnold LE, Hoza B, Conners CK, Hinshaw SP, Hechtman L, et al. Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD (MTA) Journal of abnormal child psychology. 2000;28(6):527–41. doi: 10.1023/a:1005179014321. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44(4):377–84. doi: 10.1097/01.chi.0000153228.72591.73. S0890-8567(09)61489-1 [pii] [DOI] [PubMed] [Google Scholar]
- Massat Isabelle, Slama Hichem, Kavec Martin, Linotte Sylvie, Mary Alison, Baleriaux Daniele, Metens Thierry, Mendlewicz Julien, Peigneux Philippe. Working Memory-Related Functional Brain Patterns in Never Medicated Children with ADHD. PloS one. 2012;7(11):e49392. doi: 10.1371/journal.pone.0049392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature neuroscience. 2008;11(1):103–7. doi: 10.1038/nn2024. nn2024 [pii] [DOI] [PubMed] [Google Scholar]
- Moon Chung-Man, Jeong Gwang-Woo. Functional neuroanatomy on the working memory under emotional distraction in patients with generalized anxiety disorder. Psychiatry and clinical neurosciences. 2015;69(10):609–19. doi: 10.1111/pcn.12295. [DOI] [PubMed] [Google Scholar]
- Owens M, Stevenson J, Hadwin JA, Norgate R. When does anxiety help or hinder cognitive test performance? The role of working memory capacity. British journal of psychology (London, England : 1953) 2014;105(1):92–101. doi: 10.1111/bjop.12009. [DOI] [PubMed] [Google Scholar]
- Park Jong-Il, Kim Gwang-Won, Jeong Gwang-Woo, Chung Ho Gyung, Yang Jong-Chul. Brain Activation Patterns Associated with the Effects of Emotional Distracters during Working Memory Maintenance in Patients with Generalized Anxiety Disorder. Psychiatry investigation. 2016;13(1):152–6. doi: 10.4306/pi.2016.13.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR. Comorbidity of attention-deficit hyperactivity disorder and overanxious disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):197–203. doi: 10.1097/00004583-199203000-00003. S0890-8567(09)64439-7 [pii] [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Patterns of psychiatric comorbidity with attention-deficit/hyperactivity disorder. Child and adolescent psychiatric clinics of North America. 2000;9(3):525–40. vii. [PubMed] [Google Scholar]
- Pliszka Steven R. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(6):882–7. doi: 10.1097/00004583-198911000-00012. [DOI] [PubMed] [Google Scholar]
- Rennie B, Beebe-Frankenberger M, Swanson HL. A longitudinal study of neuropsychological functioning and academic achievement in children with and without signs of attention-deficit/hyperactivity disorder. Journal of clinical and experimental neuropsychology. 2014;36(6):621–35. doi: 10.1080/13803395.2014.921284. [DOI] [PubMed] [Google Scholar]
- Riva Daria, Giorgi Cesare. The cerebellum contributes to higher functions during development. Brain. 2000;123(5):1051–61. doi: 10.1093/brain/123.5.1051. [DOI] [PubMed] [Google Scholar]
- Sarason Irwin G. Stress, anxiety, and cognitive interference: Reactions to tests. Journal of personality and social psychology. 1984;46(4):929–38. doi: 10.1037/0022-3514.46.4.929. [DOI] [PubMed] [Google Scholar]
- Schatz DB, Rostain AL. ADHD with comorbid anxiety: a review of the current literature. Journal of attention disorders. 2006;10(2):141–9. doi: 10.1177/1087054706286698. 10/2/141 [pii] [DOI] [PubMed] [Google Scholar]
- Shackman Alexander J, Sarinopoulos Issidoros, Maxwell Jeffrey S, Pizzagalli Diego A, Lavric Aureliu, Davidson Richard J. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6(1):40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Silk T, Vance A, Rinehart N, Egan G, O’Boyle M, Bradshaw JL, Cunnington R. Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. The British Journal of Psychiatry. 2005;187(3):282–3. doi: 10.1192/bjp.187.3.282. [DOI] [PubMed] [Google Scholar]
- Skirbekk Benedicte, Hansen Berit Hjelde, Oerbeck Beate, Kristensen Hanne. The relationship between sluggish cognitive tempo, subtypes of attention-deficit/hyperactivity disorder, and anxiety disorders. Journal of abnormal child psychology. 2011;39(4):513–25. doi: 10.1007/s10802-011-9488-4. [DOI] [PubMed] [Google Scholar]
- Staugaard Søren Risløv. Threatening faces and social anxiety: a literature review. Clinical psychology review. 2010;30(6):669–90. doi: 10.1016/j.cpr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Tannock R, Ickowicz A, Schachar R. Differential effects of methylphenidate on working memory in ADHD children with and without comorbid anxiety. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34(7):886–96. doi: 10.1097/00004583-199507000-00012. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ. Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biological psychiatry. 2005;57(5):439–47. doi: 10.1016/j.biopsych.2004.11.034. S0006-3223(04)01257-0 [pii] [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Luman M, Rommelse NN, Hartman CA, Hoekstra P, Franke B, Buitelaar JK, Oosterlaan J. Visuospatial working memory in ADHD patients, unaffected siblings, and healthy controls. Journal of attention disorders. 2014;18(4):369–78. doi: 10.1177/1087054713482582. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Weeda WD, Heslenfeld DJ, Luman M, Hartman CA, Hoekstra PJ, Faraone SV, Franke B, Buitelaar JK, Oosterlaan J. Neural correlates of visuospatial working memory in attention-deficit/hyperactivity disorder and healthy controls. Psychiatry Res. 2015;233(2):233–42. doi: 10.1016/j.pscychresns.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Vance A, Ferrin M, Winther J, Gomez R. Examination of spatial working memory performance in children and adolescents with attention deficit hyperactivity disorder, combined type (ADHD-CT) and anxiety. Journal of abnormal child psychology. 2013;41(6):891–900. doi: 10.1007/s10802-013-9721-4. [DOI] [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Molecular psychiatry. 2007;12(9):826–32. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J, Heslenfeld D, et al. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. European child & adolescent psychiatry. 2014 doi: 10.1007/s00787-014-0573-4. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, affective & behavioral neuroscience. 2003;3(4):255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Willcutt Erik G, Doyle Alysa E, Nigg Joel T, Faraone Stephen V, Pennington Bruce F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biological psychiatry. 2005;57(11):1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Vidaurre D, Nichols TE, Smith SM. Multi-level block permutation. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.05.092. S1053-8119(15)00508-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Barbaresi WJ, Colligan RC, Voigt RG, Killian JM, Weaver AL, Katusic SK. Childhood ADHD is strongly associated with a broad range of psychiatric disorders during adolescence: a population-based birth cohort study. Journal of child psychology and psychiatry, and allied disciplines. 2012;53(10):1036–43. doi: 10.1111/j.1469-7610.2012.02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.