Abstract
Transforming growth factor beta 1 (TGFbeta-1) and connective tissue growth factor (CCN2) are important mediators of tissue repair and fibrosis, with CCN2 functioning as a downstream mediator of TGFβ-1. Substance P (SP) is also linked to collagen production in tenocytes. A link between SP, TGFbeta-1 and CCN2 has yet to be established in tenocytes or fibrogenic processes. We sought to determine if SP induces tenocyte proliferation, CCN2 or collagen production via TGFbeta-1 signaling or independently in rat primary tenocytes. Tenocytes were isolated from rat tendons, cultured and stimulated by SP and/or TGFbeta-1. Cultured cells expressed proteins characteristic of tenocytes (vimentin and tenomodulin) and underwent increased proliferation dose-dependently after SP and TGFbeta-1 treatments, alone or combined (more than SP alone when combined). SP induced TGFbeta-1 expression in tenocytes in both dose- and time-dependent manners. SP and TGFbeta-1, alone or combined, stimulated CCN2 expression in tenocytes and their supernatants after both 24 and 48 hours of stimulation; a response blocked with addition of a TGFbeta-1 receptor inhibitor. In contrast, SP potentiated collagen type I secretion by tenocytes, a response abrogated by the TGFbeta-1 receptor inhibitor after 48 hours of stimulation, but not after the shorter 24 hours of stimulation. Our findings suggest that both SP and TGFbeta-1 can stimulate tenocyte fibrogenic processes, albeit differently. TGFbeta-1 pathway signaling was involved in CCN2 production at all time points examined, while SP induced collagen type I production independently prior to the onset of signaling through the TGFbeta-1 pathway.
Keywords: tendons, proliferation, CTGF, Substance P, collagen
Introduction
Tendinosis is a chronic condition observed in patients with various musculoskeletal disorders and it is also one of the biggest outcomes of work-related musculoskeletal disorders [1]. The mechanisms behind tendinosis are incompletely understood, although fibrosis and increased collagen deposition are known hallmark features of this condition. TGFβ-1 is a key growth factor and cytokine secreted by several cell types and involved in a number of wound healing as well as pathological fibrogenic processes, including induction of fibroblast proliferation and collagen synthesis [2–6]. Connective tissue growth factor (CTGF/CCN2) is considered a key downstream mediator of TGFβ-1 signaling in fibroblasts, myoblasts and osteoblasts [2, 7–11], although this has not been determined specifically in tenocytes. A recent systematic review indicates suggest compelling roles for TGFβ-1 and CCN2 in tendon disease, yet a paucity of studies analyzing their dynamic expression and interaction in tendon cells [12].
The importance of neurochemical modulators such as Substance P (SP) in tissue fibrosis has emerged in the last decade. SP is primarily known as a neuropeptide involved in pain transmission. More recently, SP has been shown as involved in inflammation via its production by many immune cell subtypes [13–15], and in fibrotic disorders, such as in lungs and intestines via its production by fibroblasts [16, 17]. In musculoskeletal tissues, SP has been implicated in the pathogenesis of tendinosis in patients [18], and in tendon loading models in rats and rabbits in which loading-induced increases in endogenous SP occur prior to an increase in tenocyte hypercellularity [14, 19–21]. TGFβ-1 and SP can work in concert for enhanced fibroblast proliferation or collagen production (although not yet investigated specifically in tenocytes), either via TGFβ-1 mediated SP receptor regulation [22], transcriptional modulation of each other [16, 23, 24], or through cooperative signaling of these two molecules [17, 25]. Several studies have shown that SP has: 1) potent proliferation effects on fibroblasts (dermal, colonic and cardiac) and tenocytes, and increased collagen type I production by these same cell types, through neurokinin 1 receptor (NK-1R) signaling [17, 20, 26]; 2) and anti-apoptotic effects on tenocytes via Akt specific pathways [27]. However, despite strong data showing key roles for CCN2 in TGFβ-1 induced tissue fibrosis [7, 11, 28], and that inhibition of CTGF can reverse the process of fibrosis [28], a link between SP, TGFβ-1 and CCN2 has yet to be established in tenocytes and fibrogenic tendinopathy processes.
Therefore, we sought for the first time to examine the interplay of three pro-fibrotic molecules, TGFβ-1, SP and CCN2, in cultured primary tenocytes. Specifically, these studies were designed to demonstrate in primary rat tenocyte cultures if SP induces primary tenocyte proliferation, and CCN2 or collagen production through TGFβ-1 signaling or via an independent pathway. We hypothesized that SP is able to enhance the expression of at least one of these fibrogenic processes independent of the TGFβ-1 signaling pathway, although others processes were expected to show synergism. If this is the case, then successful anti-fibrotic therapies need to consider multiple pathways to achieve the most efficacious outcome.
Methods and Methods
Tissue Collection
Samples of rat tendon tissue were obtained under sterile conditions from flexor digitorum tendons and Achilles tendons of twelve normal, young adult, female Sprague-Dawley rats. Females were used due to convenience. The Temple University Institutional Animal Care and Use Committee approved all experiments in compliance with NIH guidelines for the care and use of laboratory animals.
Isolation and Culture of Primary Tenocytes from Rat Flexor Digitorum and Achilles Tendons
The tendon cell culture was performed using a modification of previously described methods [29]. Briefly, tendon tissues were obtained from forelimbs (flexor digitorum tendons) and hindlimbs (Achilles tendons). Collected tendons were washed with sterile 1X Hank’s Balanced Salt Solution (HBSS; catalog # 2018-05, Gibco, Thermo Fisher Scientific, Waltham, MA) with 1% penicillin/streptomycin solution (30-002-Cl, Corning, Manassas, VA) and dissected using a scalpel and scissors to remove muscles and connective tissues. Next, tendons were washed three times with Dulbecco’s Modified Eagle Medium (D-MEM, 10-013-CV, Corning) + 1% penicillin/streptomycin and cut longitudinally into 3 mm pieces. Tendon fragments were enzymatically digested at 37°C using collagenase (2 mg/mL, Clostridopeptidase A, C-0130, Sigma, Saint Louis, MO) diluted in D-MEM. The digested product was centrifuged at 800 × g for 10 min, the supernatant discarded, and the pellet re-suspended and cultured in D-MEM supplemented with 10% fetal bovine serum (FBS; S11150, Atlanta Biologicals, Lawrenceville, GA), 1% penicillin/streptomycin and 2% L-Glutamine (25-005-CI, Sigma) at 37°C in a humidified atmosphere of 5% CO2. The media was changed every third day. Cells at 90% confluence were harvested using 0.05% trypsin with EDTA and sub-cultured to expand their numbers for later plating according to the desired assay. Only cells from the third through fifth passages (mainly third) were used for experiments in this study. In all experiments, the concentration of serum was reduced from 10% to 1–2% for 24 hours prior to cell treatment to eliminate any unwanted effects of serum. Serum-restricted cells appeared healthy and intact for the duration of all experiments (Figure 1 and Supplementary Figure 1).
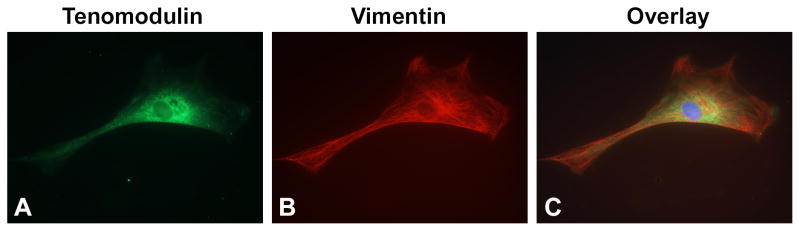
Figure 1.
Vimentin and Tenomodulin expression in primary cultures of rat tenocytes. Cells were plated into 96-well plates in D-MEM with 10% FBS overnight, then fixed and immunostained. (A) Most (95%) tenocytes were elongated and immunoreactive for tenomodulin (green), a glycoprotein predominantly expressed in tendons and ligaments. (B) Tenocyte cells were also immunoreactive for vimentin (red), an intermediate filament found in mesenchymal cells and tenocytes. (C) Merger of images in A and B. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Photos were taken using a 60X objective.
Characterization of Isolated Tenocytes
Passage 3 tenocytes were characterized by seeding 3,000 cells per well on 2-well chamber slides (154461, Lab-Tek™, Naperville, IL) in D-MEM with 10% FBS overnight to allow for cell adherence. After overnight incubation, media was removed and cells were washed with 1X phosphate-buffered saline (PBS). Cells were fixed in 4% paraformaldehyde for 15–20 min followed by two washes with 1X wash buffer (1X PBS containing 0.05% Tween-20, Sigma). Next, cells were permeabilized with 0.1% Triton-X100 in 1X PBS for 1–5 min, rinsed twice with 1X wash buffer, and blocked with 2.5% bovine serum albumin (BSA, BP1605-100, Thermo Fisher Scientific) in 1X PBS for 30 minutes at room temperature. After blocking, the chamber slides were incubated with a mouse monoclonal anti-vimentin antibody (a type III intermediate filament protein expressed in mesenchymal cells; 1:100; M0725, Dako, Glostrup, Denmark) and a goat polyclonal anti-tenomodulin antibody (a type II transmembrane glycoprotein that is expressed at high levels in tenocytes; 1:100; sc-49325, Santa Cruz Biotechnology, Santa Cruz, CA) for 60 min at 37°C. Then, chambers were washed 3 × 10 min with wash buffer and incubated with TRITC-labeled donkey anti-mouse antibody (red fluorescence, 1:2000; 610-709-124, Rockland Immunochemicals, Limerick, PA) and FITC-labeled donkey anti-goat antibody (green fluorescence, 1:2000; 605-702-002, Rockland Immunochemicals) for 1 hour at room temperature. Cells were then rinsed 3 × 10 min with wash buffer, mounted with 4,6-diamidino-2-phenylindole (H-1200, DAPI-Vectashield, Vector Laboratories, Burlingame, CA), cover-slipped and examined for fluorescence. This experiment was repeated three times.
SP and/or TGFβ-1 Treatment of Cultured Tenocytes
Isolated tenocyte cells were serum restricted in 1–2% FBS for 24 hours. Cells were then treated with a dose titration of TGFβ-1 (616455, EMD Millipore, Temecula, CA) or SP (1156, Tocris Bioscience, R&D, Minneapolis, MN), with the highest doses starting at 10 ng/mL and 100 nM, respectively. For collagen deposition assessment, cells were treated in the presence of ascorbic acid (50 μg/mL). For proliferation and dose response experiments, further dilutions were made in the reconstitution buffer (PBS + 1% BSA), with results compared to vehicle control treated cells (PBS + 1% BSA only). Unit concentrations of TGFβ-1 (ng/mL) or SP (nM) were reported as in previous publications [10, 20, 22]. Cells were also treated with TGFβ-1 and SP combined at doses indicated in the results and figures to explore possible synergistic effects. During the treatment period of up to 72 hours, media was changed and cells were re-treated every 24 hours. Cells were also monitored visually for effects of serum restriction in 1–2% FBS on cell viability; the cells continued to appear healthy and intact (Supplemental Figure 1).
Cell Proliferation Assays
Cell proliferation was determined using the CyQUANT NF Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR) according to the manufacturer’s protocol. Briefly, the same number of cells were plated per well (2,000 cells/well) into a 96 well plate (Falcon, Beckton Dickinson Labware, Franklin Lakes, NJ) in D-MEM/10% FBS, and allowed to attach overnight only. The following day, cells were serum restricted in 1% FBS for 24 hours and then treated with a dose titration of SP, TGFβ-1, or their combination, for 72 hours at doses reported in the results and figures. Vehicle controls (cells treated with PBS + 1% BSA only) were included in each replication. During the 72 hour treatment period, media was changed and cells re-treated every 24 hours. After 72 hours, the media was aspirated and replaced with DNA binding dye solution according to the manufacturer’s protocol. Cells were incubated at 37°C for 30 min and samples were measured using a Wallac 1420 fluorimeter (Wallac, Shelton, CT). Cell numbers were calculated based on a standard curve generated as follows: a specific number of tenocytes (0 – 70,000 cell/well) were plated in a 96 well plate for 8 hours in D-MEM + 10% FBS and incubated at 37°C with 5% CO2. Next, the culture media was aspirated and replaced with DNA binding dye solution. Cells were incubated at 37°C for 30 min before measuring the relative fluorescence unit values for each sample with known cell numbers using the fluorimeter. The CyQUANT proliferation assay was repeated in six independent experiments per condition.
The proliferation assays were further repeated using bromodeoxyuridine (BrdU) incorporation in three independent experiments per condition. Tenocytes were seeded at 2,000 cells/well in 96 well plates in DMEM + 10% FBS and incubated overnight at 37°C to allow for cell adherence. The following day, the media was changed to 1% FBS and cells treated with a dose range of SubP, TGFβ-1, or their combination, with the highest doses being 10 ng/mL and 100 nM, respectively. Cells were incubated for 72 hours for each replicate assay. For BrdU incorporation, the assay was performed according to the manufacturer’s instructions (11647229001, Roche Diagnostics, Indianapolis, IN). Briefly, BrdU was added to the cells for 6 hours prior to the 72 hour endpoint, and cells were incubated at 37°C to allow for DNA incorporation of BrdU. The media was then removed and cells were fixed with the provided fixation buffer. Anti-BrdU-peroxidase antibody was used to detect incorporated BrdU as a relative indicator of cell division. Absorbance was measured at 450 nm after incubation with peroxidase substrate.
Quantitative Cell-based Immunoassay for CCN2 and Collagen Type I
Rat tenocytes were seeded at 10,000 cells/well (96-well plates) and allowed to seed at room temperature for 1 hour to reduce any edge effect, before incubating at 37°C overnight. Cells were serum restricted (1% FBS) before being stimulated with dose titrations of SP, TGFβ-1, or their combination, under continued serum-restricted conditions. Media was changed at 24 hours and cells re-treated for another 24 hours. Ascorbic acid (20 micrograms/ml) was added with all treatment conditions to be assayed for collagen type I. CCN2 or collagen type I analysis was performed at 48 hours in three to six independent replications per condition (as indicated in the figure legends) using quantitative cell-based immunoassay methodology [30], and In Cell Western Assay Kits I and II (#926-31070 and #926-31072; LI-COR Biosciences, Lincoln, Nebraska). Each kit included Odyssey Blocking Buffer, IRDye® 800CW secondary antibody for detection of the specific protein target in the 800 nm channel, and CellTag 700 used in the 700 nm channel to normalize for well-to-well variations in cell number. Briefly, cells were washed three times with PBS and fixed in 95% ethanol for 20 minutes at room temperature. Cells were permeabilized by washing gently 5 times, 5 minutes each, with PBS/0.1% Triton X-100, and then blocked with Blocking Buffer for 1 hour at room temperature. Blocking buffer was removed, anti-CCN2 antibody added (1:2000; sc-14939, Santa Cruz Biotechnology), and plates were incubated at room temperature for 2 hours with gentle shaking. Plates were then washed four times with PBS + 0.05% Tween-20. Anti-goat IRDye800 (926-32224, LI-COR, diluted 1:800 in Odyssey blocking buffer) was added. CellTag 700 (diluted 1:500) was added at the same time as secondary antibody for cell normalization. Plates were then incubated in the dark for 1 hour at room temperature, washed four times with PBS + 0.05% Tween-20, 100 microliters of PBS added, and detection performed using an Odyssey Imager and software. For collagen type I analysis, the method was similar, except that an anti-collagen type I antibody was used (Abcam, Cambridge, MA, AB34710, 1:2000). Data from the 800 channel for each primary antibody was normalized to data from the Cell Tag 700 channel.
Several control wells were included on each 96-well assay plate: 6 vehicle control wells (PBS + 1% BSA), 6 wells received IgG isotype control antibody in blocking buffer versus the primary antibody, 6 wells with secondary antibody only; and 6 wells in which no primary or secondary antibodies or CellTag 700 stain were added so that background staining could be determined. Specificity of the selected primary antibodies was also determined by Western blot, and results compared to prior publications from our lab showing specificity of the same primary antibodies [31].
Western Blotting for TGFβ-1, CCN2 or Collagen Type I
Equal numbers of passage 3–5 tenocytes were plated and grown to 90% confluence, before being serum restricted in 1–2% FBS for 24 hours and then treated with SP, TGFβ-1, or their combination, at doses indicated in the results and figures. Then, lysates of tenocytes after treatment with RIPA buffer or conditioned medium (tenocyte supernatants which were not placed in RIPA buffer) were collected from each well after 24, 48 or 72 hours of stimulation. Protein concentration was determined using a Pierce™ BCA Protein Assay Kit (23225, Thermo Fisher Scientific) according to manufacturer’s instructions. Equal amounts of protein (50μg) or supernatant (20 to 50 microliters) per sample were diluted in 4× Laemmli sample buffer (161-0747, Bio-Rad, Hercules, CA, which uses lithium dodecyl sulfate as the detergent) and boiled for 5 min, before loading. Then samples were resolved with 10% SDS-PAGE gels for the TGFβ-1 and CCN2 detection, versus 4–20% gradient SDS–PAGE gels for collagen type I detection. Vehicle control samples (cells treated with PBS + 1% BSA or DMSO (the latter for the TGFβ1-RI (Transforming Growth Factor-β Type I Receptor Kinase Inhibitor) experiments described later)) were also included. Protein samples were electroblotted to a nitrocellulose membrane at 100 V for 75 min. After membranes were blocked in Odyssey Blocking Buffer (with TBS) for 1 hour at room temperature, blots were incubated overnight at 4°C with anti-CCN2 (1:100 dilution; SC-14939, Santa Cruz Biotechnology), anti-collagen type I (using Abcam, Cambridge, MA, #ab34710, 1:2000), or anti-TGFβ-1 (R&D, catalog # MAB240, 1:100 dilution). After four washes of the membrane in TBS + 0.05% Tween 20 for 5 minutes each, blots were incubated with the appropriate secondary antibodies, such as donkey anti-goat-IRDye® 680 (926-32224, Li-COR) or donkey anti-mouse-IRDye 680LT (926-32212, Li-COR) at concentrations of 1:10,000 each, for 1 hour at room temperature. The membrane was washed as before and bands were detected using the Odyssey Infrared Imaging System. Quantification of the immunoblots was performed using either myImageAnalysis v2.0 (Thermo Scientific) or ImageJ 1.48v (NIH Image) software, with results normalized to vehicle control levels as appropriate for the experiment (PBS + 1% BSA, or DMSO). Western blot data reported are the result of three to six independent experiments per condition, as indicated in the figure legends.
ELISA for TGFβ-1, CCN2 or Collagen Type I in Supernatants
Equal numbers of passage 3–5 tenocytes were plated (50,000 Cells/35 mm dish) and grown to 90% confluence, before being serum-restricted in 1–2% FBS for 24 hours and then treated with a concentration of 100 nM of SP (1156, Tocris) and/or 10 ng/ml of TGFβ-1 (616455, EMD Millipore, Temecula, CA) in the absence or presence of a TGFβ receptor I kinase inhibitor (TGFβ-RI), as described further below. After 24 or 48 hours of stimulation with the various treatments, cell culture conditioned media (supernatants) were collected. Commercially available ELISA kits were used to measure TGFβ-1 levels (ADI-900-155, ENZO, Life Sciences Inc., Farmingdale, NY), CCN2/CTGF levels (024398, US Biological, Salem, MA); or collagen type I levels (LS-F5638, Lifespan BioSciences Inc, Seattle, WA), according to the manufacturers’ protocols using 100 microliters of conditioned media per well, and individual samples tested in duplicate. ELISA results are presented as picograms of protein levels per ml of conditioned media, as previously described [13, 16, 32, 33]. Results presented represent findings from three to seven independent experiments per condition, as indicated in the figure legends. Vehicle control samples were included, and were cells treated with either PBS + 1% BSA or DMSO; when examined the effects of blocking with a TGFβ-RI (which was dissolved in DMSO).
Blocking the Activity of TGFβ Receptor I Kinase
Rat tenocyte cultures were serum restricted in 1–2% FBS for 24 hours. Then, cells were treated with a TGFβ-RI (Transforming Growth Factor-β Type I Receptor Kinase Inhibitor; 500 nM; #616451, Calbiochem, Merck KGaA, Darmstadt, Germany) as previously described [10, 34]. Cultures were treated with a standard concentration of 10 ng/mL of TGFβ-1, 100 nM of SP or their combination, in the absence or presence of the TGFβ-RI, or with the TGFβ-RI alone. Since TGFβ-RI was dissolved in DMSO, supernatants from vehicle control cells treated with DMSO were included. After the 24 or 48 hour treatments, supernatants were collected and assayed for secreted levels of CCN2 or collagen type I using Western blot and/or ELISA.
Statistical Analyses
GraphPad PRISM v.6.02 was used for all statistical analyses. All data are expressed as the mean ± standard error of the mean (SEM). A p value of < 0.05 was considered significant. Two-way ANOVAs were used for: cell proliferation assays using the factors dose and treatment; for many of the Western blot assays using the factors dose and time (TGFβ-1) or time and treatment (CCN2); for the quantitative cell-based assays using the factors dose and treatment; and for ELISAs using the factors time and treatment (TGFβ-1, CCN2, collagen type I). One-way ANOVA was used to compare results from other experiments. The Fisher’s PLSD method was used for post hoc tests. The ANOVA results and p values are indicated in the figures and defined in figure legends to enhance brevity.
Results
Confirmation of Tenocyte Phenotype
Over 95% of the cultured tenocytes (plated and cultured overnight) showed a clear elongated fibroblastic appearance under the microscope (Fig. 1). These tenocytes (95%) were immunopositive for both tenomodulin and vimentin in passages 3 through 5, thereby indicating a tenocyte phenotype [35, 36]. This phenotype was maintained under serum-restricted conditions through 72 hours (Supplemental Fig. 1).
Both SP and TGFβ-1 Treatments Increase Tenocyte Proliferation
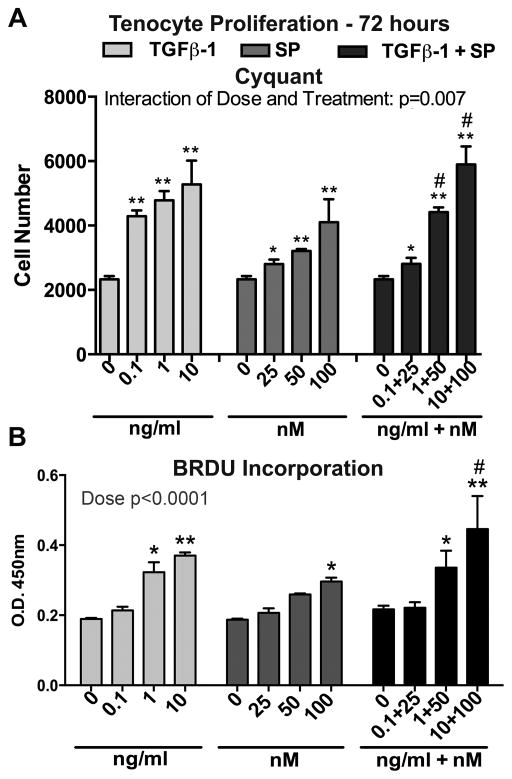
We next evaluated proliferation of tenocytes incubated with either SP or TGFβ-1 at various concentrations for 72 hours under serum-restricted conditions (1% FBS). Both TGFβ-1 and SP treatments alone significantly stimulated tenocyte proliferation in a dose-dependent manner with the highest dose of each treatment (10 ng/mL and 100 nM, respectively) inducing the greatest effect, compared to 72 hour vehicle controls (treated with PBS and 1% BSA only) (Fig. 2A). Co-treatment at the two highest doses of TGF β-1 and SP induced more proliferation than the two highest doses of SP alone, although not higher than similarly dosed TGFβ-1 treatment alone (Fig. 2A). Similar results were achieved by BrdU Incorporation assay (Fig. 2B).
Figure 2.
Both Substance P (SP) and Transforming Growth Factor Beta 1 (TGFβ-1) increased proliferation in cultured rat tenocytes. After allowing equal numbers of cells to adhere overnight in 10% FBS, cells were serum restricted (1% FBS) for 24 hours, and then treated with TGFβ-1, SP, or their combination, at the indicated concentrations for 72 hours. (A) Proliferation was evaluated by CyQUANT® NF Cell Proliferation Assay and normalized to cell number using a standard curve. Data are the means of six independent experiments ± standard error (SEM). (B) BRDU incorporation was assayed. Data are the means of three independent experiments. Two-way ANOVA results shown. * and **: p<0.05 and p<0.01, respectively, compared to vehicle controls (PBS and 1% BSA only; indicated as “0”); #: p<0.05, compared to SP only treatment.
SP Increases TGFβ-1 Secretion in Primary Tenocyte Cultures
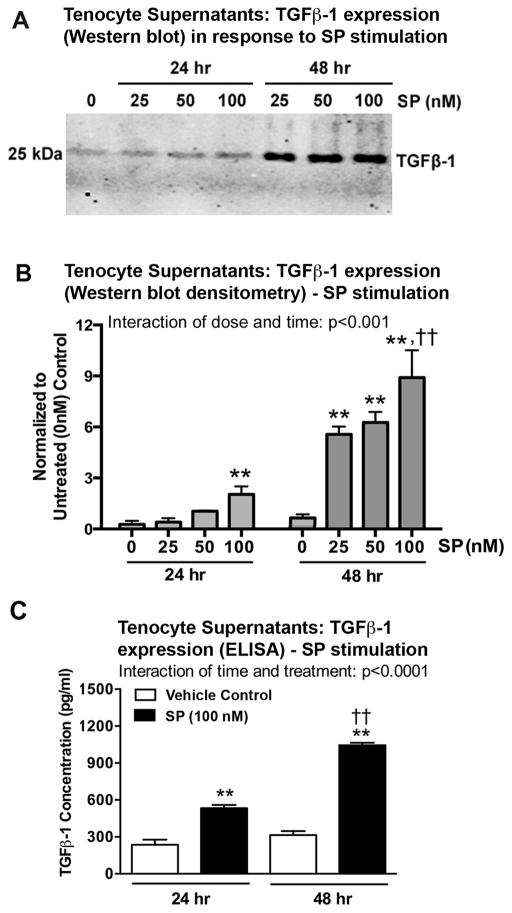
We next examined secreted TGFβ-1 levels in tenocyte supernatants after 24 or 48 hour treatments using different doses of SP (Fig. 3). Western blot analysis showed a dose-dependent increase in TGFβ-1 expression after SP stimulation for each time period, with highest after 48 hours of treatment with 100 nM of SP, compared to time-matched vehicle controls, compared to lower doses of SP treatment for 48 hours, and compared to 24 hours of treatment with 100 nM of SP (Fig. 3A, B). ELISA confirmed that treatment of tenocytes with a 100 nM dose of SP for 24 or 48 hours increased TGFβ-1 expression, compared to time-matched vehicle controls (PBS and 1% BSA only) (Fig. 3C). ELISA also showed the same time-dependent response in TGFβ-1 expression after SP treatment (48 hour > 24 hour treatment) (Fig. 3D).
Figure 3.
SP increases TGFβ-1 expression in supernatants of cultured rat tenocytes in dose and time dependent manners. Equal numbers of passage 3–5 tenocytes were plated and grown to 90% confluence, serum restricted (1–2% FBS) for 24 hours, and treated with SP at indicated concentrations for 24 or 48 hours. (A) Representative Western blot of tenocyte supernatants probed with anti-TGFβ-1. (B) Densitometric results of Western blots from four independent experiments ± SEM. TGFβ-1 band was normalized to time-matched vehicle control levels (PBS and 1% BSA only; indicated as “0”). (C) TGFβ-1 expression in tenocyte supernatants was assessed using ELISA at 24 and 48 hours after onset of SP treatment (using the 100 nM dose). Data are the means of eight independent experiments ± SEM. ANOVA results shown in panels B–D. **: p<0.01, compared to time-matched vehicle controls. ††: p<0.01, compared to 24-hour time-point.
SP and TGFβ-1 Treatments Increase CCN2 Expression in Primary Tenocyte Cultures
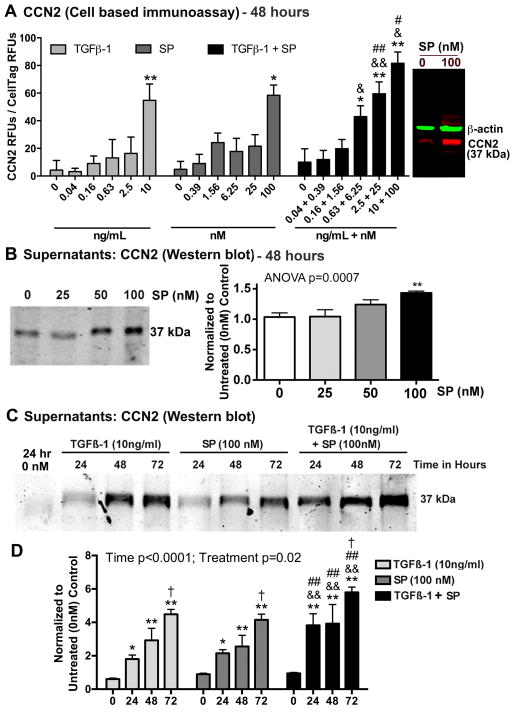
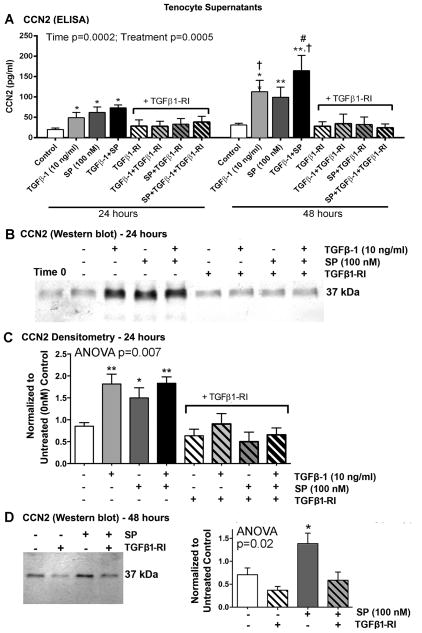
We first established a dose response for stimulation of CCN2 expression in cultured tenocytes with SP, TGFβ-1 or their combination, after 48 hours of stimulation, using quantitative cell-based immunoassay methodology (Fig. 4A). CCN2 expression was significantly increased in primary tenocytes after incubation with TGFβ-1 and SP at the doses of 10 ng/mL and 100 nM, respectively, compared to levels in 48 hour vehicle controls (PBS and 1% BSA only). The combined treatment enhanced CCN2 expression at several dose combinations, with the highest expression with the TGFβ-1 (10 ng/mL) plus SP (100 nM) combination after normalization to the live cell stain, compared to either treatment alone at the same doses (Fig. 4A). Importantly, the isotype control antibody did not show significant signal above the background stain of secondary IR800dye alone (data not shown). The specificity of the anti-CCN2 antibody was examined using Western blot assay (Fig. 4A right side) and appropriately showed a molecular weight of 37 kDa) relative to beta-actin (42 kDa). This gel also showed an increase in CCN2 expression after 48 hours of treatment with 100 nM of SP, compared to time matched vehicle controls.
Figure 4.
Both SP and TGFβ-1 increase expression of CCN2 in cultured rat tenocytes in dose dependent manners. Equal numbers of passage 3–5 tenocytes were plated and grown to 90% confluence, serum restricted (1–2% FBS) for 24 hours, before treatment with TGFβ-1, SP, or their combination, at indicated concentrations for 24, 48 or 72 hours. (A) CCN2 production was assessed after 48 hours using quantitative cell-based immunoassay methodology. Data are the means of six independent experiments ± SEM. Gel figure on right is a representative Western blot showing specificity of antibody used for this assay. Molecular weight of CCN2 detected was 37 kDa and β-actin was 42 kDa. (B) Representative Western blot of tenocyte supernatants probed with anti-CCN2 after stimulating with SP at indicated concentrations for 48 hours. Right: Densitometric results from three independent experiments ± SEM. CCN2 band was normalized to 48 hour vehicle control levels (PBS and 1% BSA; “0”). (C) Representative Western blot of tenocyte supernatants probed with anti-CCN2, after stimulation with TGFβ-1, SP, or their combination for indicated hours. (D) Densitometric results of Western blots from four independent experiments ± SEM. CCN2 band was normalized to time-matched vehicle control levels (PBS and 1% BSA). ANOVA results shown panels A, C, and D. * and **: p<0.05 and p<0.01, respectively, compared to vehicle controls; & and &&: p<0.05 and p<0.01, compared to TGFβ-1 treatment; # and ##: p<0.05 and p<0.01, compared to SP treatment; †: p<0.05, compared to 24-hour time-point.
Using the same anti-CCN2 antibody as above, we also established a dose response for stimulation of CCN2 expression in tenocyte supernatants after 48 hours of stimulation using Western blot analysis. We observed a significant increase in CCN2 secretion using the 100 nM dose of SP, compared to levels in 48 hour vehicle controls (Fig. 4B).
Therefore, we used the TGFβ-1 (10 ng/mL) and SP (100 nM) doses for subsequent experiments (except for the dose-response experiment for collagen type I).
We next analyzed the ability of TGFβ-1 (10 ng/mL) and/or SP (100 nM) to up-regulate CCN2 expression in tenocyte supernatants after 24, 48 or 72 hours of stimulation. Western blot analysis revealed that treatment with either TGFβ-1 or SP increased CCN2 protein expression in supernatants at all time points, compared to time-matched vehicle controls (treated with PBS and 1% BSA only; Fig. 4C and D). The combined treatment had a greater effect than either treatment alone at all time points examined (Fig. 4D). Levels of CCN2 in 24 hour vehicle controls are shown in Figure 4C and D; additional 24 hour vehicle control levels are shown in Figure 5B and C; 48 hour vehicle control levels are shown in Figure 4A (gel at right), Figure 4B and Figure 5D; 72 hour vehicle control levels of CCN2 were similar to 48 hours (Fig. 4D).
Figure 5.
SP induces CCN2 expression via the TGFβ-1 signaling pathway in rat tenocyte supernatants. Equal numbers of passage 3–5 tenocytes were plated and grown to 90% confluence, serum restricted (1–2% FBS) for 24 hours, and then treated with TGFβ-1 (10 ng/ml), SP (100 nM), or DMSO (vehicle for TGFβ1-RI, indicated as Control or -) in the absence or presence of TGFβ1-RI (500 nM) for 24 or 48 hours. (A) CCN2 production assessed using ELISA. Data are means of seven independent experiments ± SEM. (B) Representative Western blot of tenocyte supernatants probed with anti-CCN2 after stimulating for 24 hours. (C) Densitometric results of five independent experiments ± SEM, after stimulating for 24 hours. CCN2 band was normalized to 24 hour vehicle control levels (DMSO). (D) Left: Representative Western blot of tenocyte supernatants probed with anti-CCN2 after stimulating for 48 hours. Right: Densitometric results of three independent experiments ± SEM. CCN2 band was normalized to 48 hour vehicle control levels (DMSO). ANOVA results shown in panels A, C and D. * and **: p<0.01, respectively, compared to vehicle control levels; #: p<0.05, compared to SP treatment; †: p<0.05, compared to 24 hour time-point.
SP Increases CCN2 Secretion in Primary Tenocyte Cultures via the TGFβ-1 Pathway
Since it has been shown that TGFβ-1 induces the expression of CCN2 in fibroblasts [11], and since we earlier showed that SP stimulation enhances TGFβ-1 expression in tenocytes (Fig. 3), we next tested CCN2 expression in tenocyte supernatants after 24 or 48 hours of stimulation with TGFβ-1 (10 ng/mL), SP (100 nM) or their combination, in the absence or presence of a specific TGFβ1 receptor I inhibitor (TGFβ1-RI, 500 nM) (Fig. 5). TGFβ-1 (10 ng/ml) stimulation with or without the inhibitor was used as a positive control. ELISA showed that stimulation with TGFβ-1, SP or their combination increased CCN2 expression after both 24 and 48 hours of stimulation, compared to vehicle controls collected at both 24 or 48 hours (DMSO used since the TGFβ1-RI was dissolved in DMSO) (Fig. 5A). CNN2 expression levels were highest after 48 hours of stimulation with TGFβ-1 alone, or TGFβ-1 and SP combined, compared to the 24 hour combined treatment (Fig. 5A right). Levels of CCN2 were also increased in TGFβ-1 and SP co-treated wells at 48 hours, compared SP treatment alone after 48 hours of stimulation. Inclusion of the TGFβ-RI blocked responses to all treatments at both time-points indicating that CCN2 production was dependent on the TGFβ-1 signaling pathway.
Western blot analysis was used to confirm the ELISA results. Both TGFβ-1 and SP increased CCN2 expression at the 24 hour time-point, compared to levels in 24 hour vehicle controls (DMSO); the addition of TGFβ-RI blocked this effect (Fig. 5B and C). Similarly, 48 hours of stimulation with SP increased CCN2 expression, compared to levels in 48 hour vehicle controls; the addition of TGFβ-RI blocked this effect (Fig. 5D).
SP and TGFβ-1 Treatments Increase Collagen Type I Expression in Primary Tenocyte Cultures
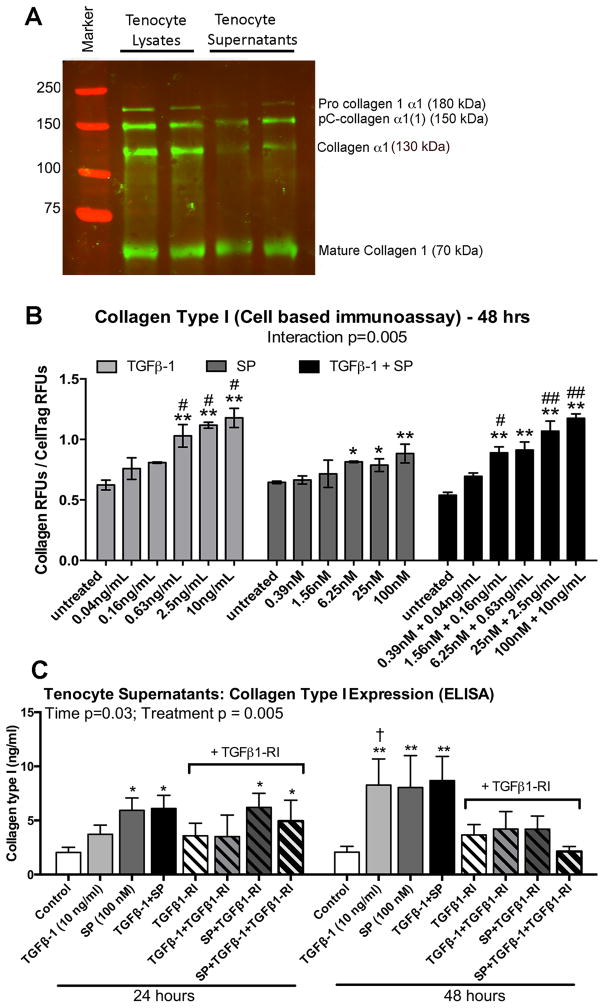
We first tested the specificity of an anti-collagen type I antibody (Abcam #34710) in tenocyte lysates and supernatants (Fig. 6A). This antibody detected multiple bands of collagen type I present due to post-translational modifications and protein solubility: bands of procollagen 1 alpha 1 (180 kDa; the unprocessed isoform of pro-collagen alpha 1); partially cleaved collagen alpha 1 (150 kDa, pC-collagen alpha 1); collagen alpha 1 (130 kDa, the fully processed alpha 1 collagen); and the fully cleaved mature collagen isoform (70 kDa) [37, 38].
Figure 6.
Effects of SP and TGFβ-1 stimulation on collagen type I production in cultured rat tenocytes. (A) Representative Western blot of tenocyte lysates and supernatants probed with anti-collagen type I from Abcam (catalog # 24710), after culturing tenocytes and stimulating with SP (100 nM) for 48 hours. (B) Equal numbers of passage 3–5 cells were plated and grown to 90% confluence, serum restricted (1% FBS) for 24 hours, and then treated for 48 hours with TGFβ-1, SP, or their combination, at indicated concentrations. Collagen type I production was assessed using quantitative cell-based immunoassay methodology and the same antibody as used in panel A. Data are means of three independent experiments ± SEM. (C) Rat tenocytes cultured and treated similarly described in Figure Legend 5 for 24 and 48 hours. Supernatants from equal numbers of cells/well were assayed for collagen type I using ELISA. Data are the means of seven independent experiments ± SEM. * and **: p<0.05 and p<0.01, respectively, compared to time-matched vehicle control levels; # and ##: p<0.05 and p<0.01, compared to SP treated; †: p<0.05, compared to 24-hour treatment time-point.
Using this same antibody, we next established a dose response for stimulation of collagen type I expression in cultured tenocytes after stimulation for 48 hours with TGFβ-1, SP, or their combination, under serum-restricted conditions (1% FBS), using quantitative cell-based immunoassay methodology (Fig. 6B). We observed that production of collagen type I was significantly increased in the cultured tenocytes after 48 hours of incubation with TGFβ-1 at several doses (0.63 to 10 ng/mL), with SP at several doses (6.25 to 100 nM), and with several of the combined treatment doses, after normalization to the live cell stain and compared to levels in 48 hour PBS-only treated controls. Several of TGFβ-1 and combined treatment doses showed higher collagen type I production than SP treatment at similar doses. Therefore, we continued with the TGFβ-1 (10 ng/mL) and SP (100 nM) dose levels for the next experiment.
SP Induces Collagen Type I Secretion by Primary Tenocytes both Dependently and Independently of the TGFβ-1 Pathway, Dependent on Time of Stimulation
We assayed collagen type I levels in tenocyte supernatants using an ELISA kit that recognizes collagen type 1 alpha, after 24 or 48 hours of stimulation with TGFβ-1 (10 ng/mL), SP (100 nM), or their combination (Fig. 6C). This stimulation was performed in the absence or presence of a specific TGFβ1 receptor I inhibitor (TGFβ1-RI, 500 nM). TGFβ-1 stimulation of collagen type I (at the 10 ng/ml dose) with or without the inhibitor was used as a positive control. TGFβ-1 did not increase collagen type I production and secretion into tenocyte supernatants after 24 hours of stimulation, although TGFβ-1 stimulation did increase collagen type I in supernatants at 48 hours. The 48 hour effect was blocked by addition of TGFβ1-RI. In contrast, SP treatment alone increased collagen type I production and secretion into tenocyte supernatants at both time points, compared to time-matched vehicle controls (DMSO treated since TGFβ1-RI was dissolved in DMSO), and at 24 hours of stimulation, compared to TGFβ-1 alone stimulation. Co-treatment of samples with TGFβ-RI and SP (100 nM) for 24 hours did not reduce the SP induced secretion of collagen type I (Fig. 7, left side), indicating that this effect was occurring independently of TGFβ-1 pathway signaling. Collagen type I secretion was also increased after 48 hours of stimulation with SP (as well as with TGFβ-1 or their combination), an increase blocked in each case with TGFβ-RI treatment (Fig. 7, right side), indicating that this effect was now dependent on TGFβ-1 pathway signaling. At each time point, co-treatment with TGFβ-1 and SP did not increase collagen type I production and secretion further than either treatment alone.
Discussion
In this study, we show for the first time that SP treatment directly increased TGFβ-1 expression in primary tenocytes, as well as CCN2 production and secretion by tenocytes. We also observed that a combined treatment of SP and TGFβ-1 induced the greatest effect on proliferation and CCN2 expression by tenocytes at several dose combinations and treatment timepoints. The addition of a TGFβ receptor I kinase inhibitor (TGFβ-RI) blocked the effect of SP on CCN2 expression at both time points examined (24 and 48 hours of stimulation). Both SP and TGFβ-1 treatments also increased collagen type I production in tenocytes and their supernatants, although there was a temporal difference, with SP stimulation increasing collagen type I production 24 hours prior to that seen with TGFβ-1 stimulation of primary tenocytes. Blocking TGFβ-1 signaling with a TGFβ-RI had no effect on SP induction of collagen type I expression at the 24 hour time point, suggesting that an early exposure to SP enhances collagen type I expression independent of the TGFβ-1 signaling pathway. In contrast, after 48 hours of stimulation, potentiation of collagen type I production by SP was dependent on TGFβ-1 pathway signaling.
Substance P (SP) is a nociceptor-related neuropeptide expressed by subsets of neurons and their axons [14, 39], as well as by fibroblasts and tenocytes [14, 29, 40, 41]. Increased SP production or release by several cell types in animal models has been linked to increased pain behaviors, including those occurring with tendinosis [14, 39, 42, 43]. An increased release of SP from nerve endings following tissue injury is associated with neurogenic inflammation and fibroblast proliferation [44], and exogenous administration of SP into paratendinous tissues stimulates fibroblast and tendon-derived stem cell proliferation [45–47]. Treatment of cultured human Achilles tendon cells with SP is known to increase tenocyte proliferation through its preferred receptor, the neurokinin-1 receptor, followed by phosphorylation of extracellular-signal-regulated kinases 1 and 2 (ERK1/2), a common mitogenic signaling pathway [20]. TGFβ-1 is also known to increase tenocyte proliferation [48]. We first confirmed in our model that SP and TGFβ-1 individually enhanced tenocyte proliferation in dose-dependent manners. The combination of SP and TGFβ-1 enhanced tenocyte proliferation more than either treatment alone, similar to 3T3 fibroblast cells in which SP augments fibrogenic cytokine-induced proliferation by this cell type, including the proliferative action of TGFβ-1 [25].
Past studies have shown that dermal fibroblasts derived from normal human skin cultured with SP show increased TGFβ-1 mRNA expression [23]. SP alone, without any co-mediator, increases TGFβ-1 protein production in several human epithelial cell lines [16]. SP also plays a significant role in the pathogenesis of intestinal fibrosis via its stimulatory effect on TGFβ-1 production [17]. Our findings of SP induction of TGFβ-1 production extend data from these other cell types to include tenocytes.
CCN2 is a cysteine-rich secreted protein that belongs to the CCN (CTGF, Cyr61 and Nov) family of early genes that regulate proliferation and differentiation of several connective tissue cells [11]. CCN2 is induced by TGFβ and is generally considered a downstream mediator of the effects of TGFβ-1 [2, 11, 49]. Since studies in the murine mesenchymal stem cell line C3H10T1/2 show that TGFβ-1-induced CCN2 expression leads to fibroblast proliferation and extracellular matrix deposition [49], we hypothesized that SP would signal through the well-established TGFβ-1/CCN2 fibrogenic pathway [10, 28, 34, 49–52] to increase extracellular matrix protein production. We observed that TGFβ-1 treatment induced CCN2 expression in primary tenocytes and their supernatants, as expected [2, 3, 11, 28]. Additionally, SP enhanced CCN2 production in the cultured tenocytes. This novel finding (effects of SP on CCN2 expression has not previously been reported in either tenocytes or fibroblasts) was confirmed using multiple methods (quantitative cell-based immunoassay, Western blot analysis and ELISA). Furthermore, the combination of SP and TGFβ-1 enhanced CCN2 production and secretion from the cultured tenocytes after 48 and 72 hours of stimulation, compared to either treatment alone. We next sought to determine if SP exerts its effect in tenocytes through the TGFβ-1/CCN2 signaling pathway and found that addition of a TGFβ-1 receptor inhibitor blocked SP enhancement of CCN2 production after both 24 and 48 hours of stimulation, indicating that SP signals through some part of this pathway in tenocytes. Future studies should examine if SP has similarly induction effects on CCN2 in fibroblast populations.
We next observed that TGFβ-1 stimulation for 48 hours induced collagen type I production in the primary tenocytes, as expected [2, 53]. Addition of the TGFβ1-RI blocked the 48 hour stimulation response. In contrast, the shorter 24 hour stimulation by SP, but not TGFβ-1, induced collagen type I production in a response that was not abrogated by addition of a TGFβ1-RI. These observations show the presence of both TGFβ-1 dependent and -independent pathways (that were temporally different) in tenocytes by which SP can contribute to collagen matrix production. These findings are similar to those from a skin explant study showing increased collagen type I expression after SP exposure [44]. However, they differ from in vitro experiments using colonic fibroblasts in which SP, in the presence of TGFβ-1 and insulin like growth factor (IGF-1), stimulated collagen synthesis, while SP alone did not [17]. In contrast, lung fibroblasts show reduced collagen expression after SP exposure [54], while cardiac fibroblasts show increased proliferation yet no changes in collagen synthesis following SP stimulation [26]. Thus, different cell types respond differently to SP, warranting its investigation in cells from tissues known to undergo fibrosis such as tendons.
Future studies will need to focus on analysis of downstream signaling pathways initiated by SP both independent of and in combination with TGFβ-1 in primary cultures of rat tenocytes. Issues that need to be addressed include: 1) whether the SP-induced increases in CCN2 and/or collagen synthesis occurs via activation of ERK1/2 signaling since it is a shared common pathway for TGFβ-1 and SP signaling [20], or 2) whether SMAD signaling (SMAD2/3) is also involved since SMAD3 and ERK1/2 coordinately mediate TGFβ-1 induced release of CCN2 by fibroblasts [55]. We have previously shown that the induction of CCN2 expression by TGFβ-1 in primary cultures of osteoblasts relies on the simultaneous activation of SMAD2/3 and ERK1/2 signaling, since blocking either of these two signaling molecules prevents TGFβ-1 mediated induction of CCN2 by osteoblasts [10]. Src can also act as a downstream signaling effector of TGFβ-1 in some cell types [56], as well as Neurokinin 1 receptor (the preferred receptor of SP) trafficking to endosomes [57]. The AKT signaling pathway may be another target to examine in our tenocyte culture system, as Koon and colleagues have shown that SP stimulates fibroblast migration and increases collagen synthesis in the presence of TGFβ-1 and insulin-like growth factor 1 in an AKT-dependent manner [17]. Future experiments will examine the role of these signaling pathways via selective inhibition of key signaling factors in the transcriptional regulation of CCN2 and/or collagen type I induced by SP treatment.
In summary, this is the first report to our knowledge, to demonstrate that SP treatment of primary tenocytes induces CCN2 production, and that SP signals via both TGFβ-dependent and independent pathways to enhance collagen production by tenocytes. These data suggest that both SP and TGFβ-1 may be involved in tendinosis observed in overuse animal models and patients. Further examination of this point is needed to determine if successful anti-fibrotic therapies for work-related musculoskeletal disorders and other fibrotic disorders need to block multiple pathways to achieve the most efficacious outcome.
Supplementary Material
Supplemental Figure 1. Reduction of fetal bovine serum (FBS) from 10% to 1–2% in growth media did not affect cultured tenocyte viability. Tenocytes cultured in 1–2% FBS for 24, 48 and 72 hours are shown in right three panels. Photos were taken using a 10X objective using phase contrast imaging with no staining.
Acknowledgments
The authors would like to thank Mario C Rico for his help with the collagen Western blots. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR056019 to MFB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.BLS. Nonfatal occupational injuries and illnesses requiring days away from work, 2012. U.S. Bureau of Labor Statistics; Washington, DC: 2013. Nov 26, http://www.bls.gov/news.release/pdf/osh2.pdfUSDL-13-2257. [Google Scholar]
- 2.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald EM, Cohn RD. TGFbeta signaling: its role in fibrosis formation and myopathies. Current opinion in rheumatology. 2012;24(6):628–634. doi: 10.1097/BOR.0b013e328358df34. [DOI] [PubMed] [Google Scholar]
- 4.Nall AV, Brownlee RE, Colvin CP, Schultz G, Fein D, Cassisi NJ, Nguyen T, Kalra A. Transforming growth factor beta 1 improves wound healing and random flap survival in normal and irradiated rats. Arch Otolaryngol Head Neck Surg. 1996;122(2):171–177. doi: 10.1001/archotol.1996.01890140057011. [DOI] [PubMed] [Google Scholar]
- 5.Chang J, Thunder R, Most D, Longaker MT, Lineaweaver WC. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105(1):148–155. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Xia C, Yang X, Wang YZ, Sun K, Ji L, Tian S. Tendon healing in vivo and in vitro: neutralizing antibody to TGF-beta improves range of motion after flexor tendon repair. Orthopedics. 2010;33(11):809. doi: 10.3928/01477447-20100924-06. [DOI] [PubMed] [Google Scholar]
- 7.Leask A. Transcriptional profiling of the scleroderma fibroblast reveals a potential role for connective tissue growth factor (CTGF) in pathological fibrosis. The Keio journal of medicine. 2004;53(2):74–77. doi: 10.2302/kjm.53.74. [DOI] [PubMed] [Google Scholar]
- 8.Arnott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. Journal of cellular physiology. 2007;210(3):843–852. doi: 10.1002/jcp.20917. [DOI] [PubMed] [Google Scholar]
- 9.Sobral LM, Montan PF, Martelli-Junior H, Graner E, Coletta RD. Opposite effects of TGF-beta1 and IFN-gamma on transdifferentiation of myofibroblast in human gingival cell cultures. J Clin Periodontol. 2007;34(5):397–406. doi: 10.1111/j.1600-051X.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 10.Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. Molecular requirements for induction of CTGF expression by TGF-beta1 in primary osteoblasts. Bone. 2008;42(5):871–885. doi: 10.1016/j.bone.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grotendorst GR. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine & growth factor reviews. 1997;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 12.Morita W, Snelling SJ, Dakin SG, Carr AJ. Profibrotic mediators in tendon disease: a systematic review. Arthritis Res Ther. 2016;18(1):269. doi: 10.1186/s13075-016-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40(4):251–263. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, Litvin J, Barbe MF. Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res. 2010;28(3):298–307. doi: 10.1002/jor.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum A, Setiawan T, Hang L, Stoyanoff K, Weinstock JV. Interleukin-12 (IL-12) and IL-23 induction of substance p synthesis in murine T cells and macrophages is subject to IL-10 and transforming growth factor beta regulation. Infect Immun. 2008;76(8):3651–3656. doi: 10.1128/IAI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaraee R, Ghazanfari T. Substance P potentiates TGFbeta-1 production in lung epithelial cell lines. Iranian journal of allergy, asthma, and immunology. 2009;8(1):19–24. [PubMed] [Google Scholar]
- 17.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, Lu B, Gerard N, Pothoulakis C. Substance P modulates colitis-associated fibrosis. The American journal of pathology. 2010;177(5):2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riley G. Tendinopathy--from basic science to treatment. Nature clinical practice Rheumatology. 2008;4(2):82–89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 19.Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder. A pilot study Cells, tissues, organs. 1999;165(1):30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- 20.Backman LJ, Fong G, Andersson G, Scott A, Danielson P. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PloS one. 2011;6(11):e27209. doi: 10.1371/journal.pone.0027209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher PW, Zhao Y, Rico MC, Massicotte VS, Wade CK, Litvin J, Bove GM, Popoff SN, Barbe MF. Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J Cell Commun Signal. 2015:1–18. doi: 10.1007/s12079-015-0263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beinborn M, Blum A, Hang L, Setiawan T, Schroeder JC, Stoyanoff K, Leung J, Weinstock JV. TGF-beta regulates T-cell neurokinin-1 receptor internalization and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(9):4293–4298. doi: 10.1073/pnas.0905877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai XN, Wang ZG, Zhu JM, Wang LL. Effect of substance P on gene expression of transforming growth factor beta-1 and its receptors in rat’s fibroblasts. Chinese journal of traumatology = Zhonghua chuang shang za zhi/Chinese Medical Association. 2003;6(6):350–354. [PubMed] [Google Scholar]
- 24.Kant V, Gopal A, Kumar D, Bag S, Kurade NP, Kumar A, Tandan SK, Kumar D. Topically applied substance P enhanced healing of open excision wound in rats. European journal of pharmacology. 2013;715(1–3):345–353. doi: 10.1016/j.ejphar.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 25.Katayama I, Nishioka K. Substance P augments fibrogenic cytokine-induced fibroblast proliferation: possible involvement of neuropeptide in tissue fibrosis. Journal of dermatological science. 1997;15(3):201–206. doi: 10.1016/s0923-1811(97)00608-7. [DOI] [PubMed] [Google Scholar]
- 26.Dehlin HM, Manteufel EJ, Monroe AL, Reimer MH, Jr, Levick SP. Substance P acting via the neurokinin-1 receptor regulates adverse myocardial remodeling in a rat model of hypertension. International journal of cardiology. 2013;168(5):4643–4651. doi: 10.1016/j.ijcard.2013.07.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Backman LJ, Danielson P. Akt-mediated anti-apoptotic effects of substance P in Anti-Fas-induced apoptosis of human tenocytes. J Cell Mol Med. 2013;17(6):723–733. doi: 10.1111/jcmm.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis & tissue repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Backman LJ, Andersson G, Wennstig G, Forsgren S, Danielson P. Endogenous substance P production in the Achilles tendon increases with loading in an in vivo model of tendinopathy-peptidergic elevation preceding tendinosis-like tissue changes. J Musculoskelet Neuronal Interact. 2011;11(2):133–140. [PubMed] [Google Scholar]
- 30.Jones B, Bucks C, Wilkinson P, Pratta M, Farrell F, Sivakumar P. Development of cell-based immunoassays to measure type I collagen in cultured fibroblasts. Int J Biochem Cell Biol. 2010;42(11):1808–1815. doi: 10.1016/j.biocel.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Abdelmagid SM, Barr AE, Rico M, Amin M, Litvin J, Popoff SN, Safadi FF, Barbe MF. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: role of force and inflammation. PloS one. 2012;7(5):e38359. doi: 10.1371/journal.pone.0038359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta J, Robbins J, Jilling T, Seth P. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther. 2011;11(3):311–316. doi: 10.4161/cbt.11.3.14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marriott I, Bost KL. Substance P diminishes lipopolysaccharide and interferon-gamma-induced TGF-beta 1 production by cultured murine macrophages. Cell Immunol. 1998;183(2):113–120. doi: 10.1006/cimm.1998.1248. [DOI] [PubMed] [Google Scholar]
- 34.Arnott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-beta1-induced extracellular matrix production in osteoblasts. J Cell Physiol. 2007;210(3):843–852. doi: 10.1002/jcp.20917. [DOI] [PubMed] [Google Scholar]
- 35.Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials. 2010;31(27):6952–6958. doi: 10.1016/j.biomaterials.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 36.Fong G, Backman LJ, Andersson G, Scott A, Danielson P. Human tenocytes are stimulated to proliferate by acetylcholine through an EGFR signalling pathway. Cell Tissue Res. 2013;351(3):465–475. doi: 10.1007/s00441-012-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alford AI, Golicz AZ, Cathey AL, Reddy AB. Thrombospondin-2 facilitates assembly of a type-I collagen-rich matrix in marrow stromal cells undergoing osteoblastic differentiation. Connect Tissue Res. 2013;54(4–5):275–282. doi: 10.3109/03008207.2013.811236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Shen Y, Ding Y, Liu Y, Su D, Liang X. Hrd1 participates in the regulation of collagen I synthesis in renal fibrosis. Mol Cell Biochem. 2014;386(1–2):35–44. doi: 10.1007/s11010-013-1843-z. [DOI] [PubMed] [Google Scholar]
- 39.Elliott MB, Barr AE, Clark BD, Amin M, Amin S, Barbe MF. High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain. Neuroscience. 2009;158(2):922–931. doi: 10.1016/j.neuroscience.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spang C, Alfredson H, Ferguson M, Roos B, Bagge J, Forsgren S. The plantaris tendon in association with mid-portion Achilles tendinosis - tendinosis-like morphological features and presence of a non-neuronal cholinergic system. Histol Histopathol. 2013;28(5):623–632. doi: 10.14670/HH-28.623. [DOI] [PubMed] [Google Scholar]
- 41.Song Y, Stal PS, Yu JG, Forsgren S. Bilateral increase in expression and concentration of tachykinin in a unilateral rabbit muscle overuse model that leads to myositis. BMC Musculoskelet Disord. 2013;14:134. doi: 10.1186/1471-2474-14-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;38(4):757–764. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 43.Ackermann PW. Neuronal regulation of tendon homoeostasis. Int J Exp Pathol. 2013;94(4):271–286. doi: 10.1111/iep.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheret J, Lebonvallet N, Buhe V, Carre JL, Misery L, Le Gall-Ianotto C. Influence of sensory neuropeptides on human cutaneous wound healing process. Journal of dermatological science. 2014;74(3):193–203. doi: 10.1016/j.jdermsci.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Andersson G, Forsgren S, Scott A, Gaida JE, Stjernfeldt JE, Lorentzon R, Alfredson H, Backman C, Danielson P. Tenocyte hypercellularity and vascular proliferation in a rabbit model of tendinopathy: contralateral effects suggest the involvement of central neuronal mechanisms. Br J Sports Med. 2011;45(5):399–406. doi: 10.1136/bjsm.2009.068122. [DOI] [PubMed] [Google Scholar]
- 46.Burssens P, Steyaert A, Forsyth R, van Ovost EJ, Depaepe Y, Verdonk R. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005;26(10):832–839. doi: 10.1177/107110070502601008. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y, Zhou B, Tang K. The effects of substance p on tendinopathy are dose-dependent: an in vitro and in vivo model study. J Nutr Health Aging. 2015;19(5):555–561. doi: 10.1007/s12603-014-0576-3. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z, Sun Y, Yang S, Cui Q, Li Z. FAK activity is required for HGF to suppress TGF-beta1-induced cellular proliferation. In Vitro Cell Dev Biol Anim. 2015;51(9):941–949. doi: 10.1007/s11626-015-9914-y. [DOI] [PubMed] [Google Scholar]
- 49.Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN. Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. Journal of cellular physiology. 2007;210(2):398–410. doi: 10.1002/jcp.20850. [DOI] [PubMed] [Google Scholar]
- 50.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13(13):1774–1786. [PubMed] [Google Scholar]
- 51.Ito Y, Goldschmeding R, Kasuga H, Claessen N, Nakayama M, Yuzawa Y, Sawai A, Matsuo S, Weening JJ, Aten J. Expression patterns of connective tissue growth factor and of TGF-beta isoforms during glomerular injury recapitulate glomerulogenesis. Am J Physiol Renal Physiol. 2010;299(3):F545–558. doi: 10.1152/ajprenal.00120.2009. [DOI] [PubMed] [Google Scholar]
- 52.Booth AJ, Csencsits-Smith K, Wood SC, Lu G, Lipson KE, Bishop DK. Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(2):220–230. doi: 10.1111/j.1600-6143.2009.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kjaer M, Langberg H, Heinemeier K, Bayer ML, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard MR, Magnusson SP. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19(4):500–510. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 54.Ramos C, Montano M, Cisneros J, Sommer B, Delgado J, Gonzalez-Avila G. Substance P up-regulates matrix metalloproteinase-1 and down-regulates collagen in human lung fibroblast. Exp Lung Res. 2007;33(3–4):151–167. doi: 10.1080/01902140701364409. [DOI] [PubMed] [Google Scholar]
- 55.Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124(6):1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Arnott JA, Rehman S, Delong WG, Jr, Sanjay A, Safadi FF, Popoff SN. Src is a major signaling component for CTGF induction by TGF-beta1 in osteoblasts. Journal of cellular physiology. 2010;224(3):691–701. doi: 10.1002/jcp.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiological reviews. 2014;94(1):265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Reduction of fetal bovine serum (FBS) from 10% to 1–2% in growth media did not affect cultured tenocyte viability. Tenocytes cultured in 1–2% FBS for 24, 48 and 72 hours are shown in right three panels. Photos were taken using a 10X objective using phase contrast imaging with no staining.