Abstract
Oxygenases, including lipoxygenases and cytochrome P450s, generate an array of structurally diverse oxylipins that modulate distinct biological responses in mammals. Depending on the source of tissues and enzymes, distinct oxylipins are generated with inherent cellular function. Here, we report structurally different forms of 12-HETrE, with distinct biological function in tissues as well as their derived enzymatic source.
Keywords: Dihomo-gamma-linolenic acid, Arachidonic acid, Platelet 12-LOX, 12(S)-HETrE, 12(R)-HETrE, Thrombosis
Introduction
A multitude of dietary or essential fatty acids are produced in nature. When humans ingest these fatty acids, they are often elongated or saturated by enzymes in the body. Each of these enzymatic steps creates a new slightly varied fatty acid, which can be enzymatically modified in various tissue beds to function as bioactive lipids (metabolites). The structural differences inherent in these fatty acids contribute to differences in physiological processes by either potentiating or attenuating biochemical steps in a variety of tissues. Predominantly, the fatty acids themselves are inert and rely on catalysis by oxygenases such as cyclooxygenase (COX), lipoxygenase (LOX), or cytochrome P450 (CYP), to modify polyunsaturated fatty acids (PUFAs) to generate potent signaling lipid mediators that play crucial roles in diverse physiological and pathological pathways[1–4].
One example of this modification is the formation of 12(S)-hydroxy-8,10,14-eicosatrienoic acid (12(S)-HETrE), an endogenous metabolite derived from the platelet-type 12-lipoxygenase (p12-LOX) (a non-heme oxygenase) oxidation of the ω-6 PUFA dihomo-γ-linolenic acid (DGLA). This metabolite was recently discovered to provide protection against thrombotic-mediated events in vivo[5]. Further, this discovery strongly supports 12(S)-HETrE and possibly other oxidized metabolites as playing potentially therapeutic roles in providing cardioprotection in people. However, the chemical structure of platelet 12(S)-HETrE should not be confused with that of the epithelium-derived 12(R)-hydroxy-5,8,14-eicosatrienoic acid (12(R)-HETrE)[6–11]. While the abbreviation would suggest that these metabolites share the same compound structure and only differ based on tissue source of metabolite origin, they are actually structurally disparate. In addition to their structural difference, the physiological effects elicited by each of these metabolites are unique and in fact opposing in nature. Hence, while published work has described two oxidized lipids with the same shortened abbreviation (12-HETrE), the functional difference between these two metabolites and the difference in tissue expression and biological function are striking. Thus, the primary focus of this review is to compare and contrast the two 12-HETrE compounds described in the literature thus far based on their structural backbones, functions, mechanisms, and involvement in diseased states, for the expressed purpose of defining the physiological and chemical differences inherent to each of these metabolites and delineating the physiological tissues in which each is purported to have a regulatory function.
Formation and function of platelet 12-HETrE
Historically, 12(S)-hydroxy-8,10,14-eicosatrienoic acid (12(S)-HETrE), isolated from human platelets treated with 8,11,14-eicosatrien[1-14C]oic acid, a radiolabeled compound of DGLA[12] (presumably derived from platelet-type 12-LOX oxidation) in 1976, was largely neglected or overlooked due to the preponderance of cyclooxygenase-derived products, prostaglandin E1 (PGE1) or thromboxane A1 (TxA1) from DGLA[13–20]. However, recent characterization of 12(S)-HETrE and its potential role in platelet function first described in 2012, signifies its revival and importance not only in platelet function[5, 21], but also its potential biological roles in other cellular types.
In agreement with previously postulated formation of 12(S)-HETrE through the p12-LOX [12], current studies verify that the p12-LOX (which only generates S-stereoisomer products)[22], principally found in platelets, keratinocytes, and some tumor cells[23], is important and required for 12(S)-HETrE generation from DGLA [5, 21]. Ikei et al. were also able to demonstrate that the purified p12-LOX was just as efficient in transforming DGLA to 12(S)-HETrE as to the predominant substrate, arachidonic acid (AA), converted to 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid (12(S)-HETE). Similarly to other prostanoids generated in platelets, the role of 12(S)-HETrE was directly assessed via a number of biochemical assays regulating overall platelet function. Following platelet treatment with 12(S)-HETrE, platelet activation endpoints (aggregation, Rap1 and αIIbβ3 integrin activation) were significantly inhibited. Not only was platelet activation potently inhibited, but integrin-dependent platelet-mediated clot retraction was significantly decreased [21]. This strongly suggests that the attenuation of platelet activation through the 12-LOX oxidation of DGLA to its metabolite could also function in preventing platelet-mediated thrombosis.
More recently, the role of platelet 12(S)-HETrE on thrombosis was investigated in vivo. To mimic physiological thrombotic occlusion of the vessels, the cremaster arterioles of male mice were exposed to laser emission, resulting in a vascular insult of the arteriole endothelium exposing the underlying collagen matrix, and fluorescently labeled platelets and fibrin that make up the thrombus were monitored for accumulation at the site of laser injury. Mice that had been intravenously administered 12(S)-HETrE or DGLA were protected from thrombus accumulation at the site of the arteriole injury compared to the wild-type control [5]. The antiplatelet effects of DGLA in vivo were also shown to be dependent on the presence of functional p12-LOX. The ability of 12(S)-HETrE to inhibit thrombotic occlusion induced by laser injury implicates its potential use as an anti-platelet therapy to treat thrombotic-associated diseases. Determining the extent of stability for 12(S)-HETrE in circulation will be essential as this metabolite is investigated further for its potential in effectively inhibiting thrombosis following IV or possibly oral administration.
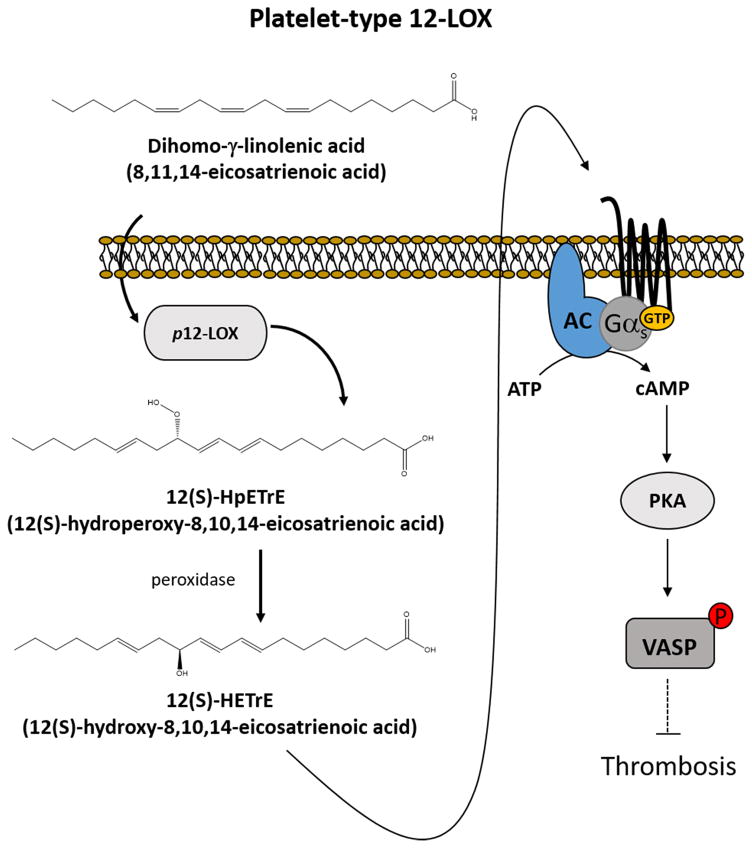
Many prostanoids generated by oxygenases elicit their actions through direct binding of receptors on the surface of cells[24–31]. Similar to other prostanoids such as prostacyclin[4], 12(S)-HETrE was shown recently to bind to the Gαs-linked G protein-coupled receptors (GPCR) resulting in activation of adenylyl cyclase (AC) and formation of cAMP[5] to activate protein kinase A (PKA). This process was shown to be a key step in 12(S)-HETrE mediated anti-platelet effects (Figure 1). Thus, the inhibitory regulatory signal mediated through the Gαs pathway was shown to be activated by 12(S)-HETrE. cAMP and vasodilator-stimulated phosphoprotein (VASP) phosphorylation were significantly increased in the presence of 12(S)-HETrE as shown in figure 1. These observations strongly support the interaction of 12(S)-HETrE with Gαs-linked GPCRs to elicit anti-platelet effects. However, despite evidence of activation of the Gαs signaling pathway in the platelets following 12(S)-HETrE exposure, the identification of 12(S)-HETrE receptor has not yet been determined and remains an area of active investigation.
Fig. 1.
Platelet 12-lipoxygenase (p12-LOX) generates 12(S)-hydroperoxy-8,10,14-eicosatrienoic acid (12(S)-HpETrE) from dihomo-γ-linolenic acid (DGLA; 8,11,14-eicosatrienoic acid), which is readily reduced to 12(S)-hydroxy-8-10-14-eicosatrienoic acid (12(S)-HETrE)). 12(S)-HETrE is released and acts in an autocrine or paracrine manner to inhibit thrombosis through the Gαs signaling pathway. 12(S)-HETrE directly activates the Gαs signaling pathway, which involves the activation of adenylyl cyclase (AC) to generate cAMP from ATP. cAMP binds and activates protein kinase A (PKA), which phosphorylates vasodilator-stimulated phosphoprotein (VASP) to inhibit platelet-mediated thrombosis.
The physiological role of 12(S)-HETrE in the platelet is however unique to what has been observed for the AA-derived p12-LOX product, 12(S)-HETE. 12(S)-HETE was first reported in a cancer cell line to mediate metastasis[32–38] and prolong survival[39–41] through its high affinity binding to an orphan G protein-coupled receptor (GPCR), GPR31 (12-HETER)[42]. In support of G protein-coupled receptor binding, 12(S)-HETE had also been reported to modulate neuronal excitotoxicity through the Gαi/o-protein-coupled receptor[43] by which adenylyl cyclase activity was inhibited to reduce voltage-sensitive calcium channel activity. However, 12(S)-HETE had also been implicated in neuroprotection through its ability to enhance and activate peroxisome proliferator-activated receptor γ (PPARγ), a member of the nuclear hormone receptor family of ligand-dependent transcription factors[44].
Moreover, 12(S)-HETE has been shown to play a role in coagulation through esterification into the lipid membrane following formation in the platelet, resulting in enhanced tissue factor-dependent thrombin generation in the vessel[45]. The exogenous addition of 12(S)-HETE to platelets has also been demonstrated to increase dense granule secretion following stimulation of the thrombin receptor pathway in the human platelet[46]. Hence, while p12-LOX can form a number of oxidized metabolites in the platelet based on the available PUFA substrates, 12(S)-HETE and 12(S)-HETrE appear to exert opposite effects on platelet activation based in part on differential receptor signaling. For instance, 12(S)-HETE had been reported to augment calcium mobilization and PKC activation[47] as well as Rho, ROCK, and myosin light chain 2 (MLC2) activation through 12-HETER[41]; whereas, 12(S)-HETrE has been recently shown to signal through Gαs-GPCR activation pathway, encompassing cAMP formation, and PKA activation through an as-yet-to-be-determined receptor.
Formation and function of 12(R)- and 12(S)-HETrE from e12-LOX, 12R-LOX, and CYP450
Unlike the 12(S)-HETrE generated from p12-LOX, the epidermal, epithelial cells, and neutrophils expressing a combination of epidermis-type 12-LOX (e12-LOX)[48, 49], the epidermis R form of 12-LOX (12R-LOX), uncovered in the late 1990s,[50, 51] and cytochrome P450 (CYP450)[9, 10, 52] are also indirectly involved in the generation of both R and S stereoisomers of 12-HETrE, which are structurally different from the platelet 12(S)-HETrE. The two chiral forms of 12(R)-HETrE and 12(S)-HETrE, 12(R)-hydroxy-5,8,14-eicosatrienoic acid and 12(S)-hydroxy-5,8,14-eicosatrienoic acid, respectively, derived from AA, were identified in the epithelial, epidermal, and neutrophils of mammals[7, 8, 10, 52–58].
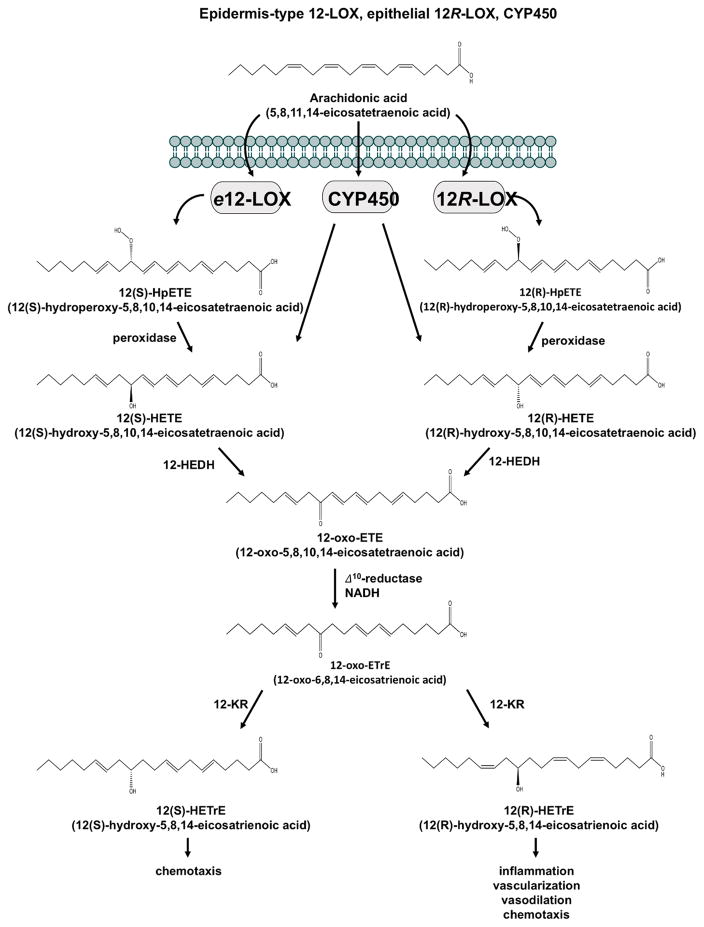
While 12R-LOX and e12-LOX generally form their respective stereoselective R or S enantiomeric 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid (12-HpETE) from AA, which is readily reduced to 12-hydroxy-5,8,10,14-eicosatetraenoic acid (12-HETE), CYP450 isoenzymes generate a mixture of R and S of 12-HETE, with the R enantiomer tending to be the predominate [59, 60]. Both R and S 12-HETE enantiomeric forms are converted to 12-oxo-5,8,10,14-eicosatetraenoic acid (12-oxo-ETE) by 12-hydroxyeicosanoid dehydrogenase (12-HEDH), an enzyme that is also capable of oxidizing other 12-hydroxyeicosanoids. 12-oxo-ETE is further metabolized by the NADPH-dependent cytosolic enzyme, 12-oxoeicosanoid Δ10-reductase (Δ10-reductase), to 12-oxo-6,8,14-eicosatrienoic acid (12-oxo-ETrE or 10,11-dihydro-12-oxo-ETE) and reduced by 12-ketoreductase (12-KR) to either 12(R)-HETrE or 12(S)-HETrE[55, 56, 61] as shown in figure 2.
Fig. 2.
Epidermis-type 12-LOX (e12-LOX) and epithelial 12R-LOX oxidizes arachidonic acid (AA) to their respective S and R enantiomers 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid (12-HpETE), which are reduced to 12-hydroxy-5,8,10,14-eicosatetraenoic acid (12-HETE). CYP450 isoenzymes generate both S and R enantiomers of 12-HETE. 12(S)-HETE and 12(R)-HETE are transformed into 12-oxo-5,8,10,14-eicosatetraenoic acid (12-oxo-ETE) by 12-hydroxyeicosanoid dehydrogenase (12-HEDH) and reduced to 12-oxo-6,8,14-eicosatrienoic acid (12-oxo-ETrE) by 12-oxoeicosanoid Δ10-reductase (Δ10-reductase). 12-ketoreductase (12-KR) converts 12-oxo-ETrE to either 12(R)-hydroxy-5,8,14-eicosatrienoic acid (12(R)-HETrE) or 12(S)-12-hydroxy-5,8,14-eicosatrienoic acid (12(S)-HETrE).
Although both 12(R)- and 12(S)-HETrE were found to be biologically active, 12(R)-HETrE appeared to be the major metabolite formed in quantity compared to 12(S)-HETrE[56, 61]. 12(R)-HETrE has been demonstrated to be directly associated with or to increase vasodilation[52] and inflammation in mammals[53, 62], as well as functioning as a potent chemotactic agent for neutrophils[11]. In addition to inflammation, 12(R)-HETrE had been implicated in vascular permeability and neovascularization in the cornea of the rabbit[10] following hypoxia-induction. Enhanced VEGF expression via ERK1/2 activation[63] was observed also to be concomitant with neovascularization in the corneal epithelial cells[64] following 12(R)-HETrE treatment. Treating coronary endothelial cells treated with 12(R)-HETrE also resulted in NF-κB activation as well as increased c-fos, c-jun, and c-myc oncogene expression[65], indicating 12(R)-HETrE’s angiogenic-induced process involves the NF-κB activation pathway. While binding assays of 12(R)-HETrE to the surface and cytoplasm of the endothelial cells had suggested a putative receptor[66], the 12(R)-HETrE receptor has yet to be identified as a new target for inhibiting angiogenesis and inflammation-associated diseases.
Although 12(S)-HETrE is produced by the 12R-LOX or CYP450 pathway in lower amounts in human neutrophils, monocytes, and macrophrophages[67, 68] and deemed to be biologically inactive in certain cells compared to 12(R)-HETrE[52], it appeared to be biologically more important in neutrophils than 12(R)-HETrE. 12(S)-HETrE was demonstrated to be about 20 times more potent in stimulating cytosolic calcium release from neutrophils than 12(R)-HETrE[55]. Neutrophils treated with 12(S)-HETrE also displayed enhanced chemotactic activity compared to 12(R)-HETrE.
Conclusion
Platelet 12(S)-HETrE is structurally and functionally unique from the 12(R)- and 12(S)-HETrE produced by 12R-LOX, CYP450 isoenzymes, and epithelial 12-LOX. Platelet 12(S)-HETrE had recently been discovered to elicit anti-thrombotic function within the vessels without affecting bleeding. This finding implicates its therapeutic potential in the treatment of cardiovascular diseases. Although platelet 12(S)-HETrE is shown to impinge on the Gαs signaling pathway, the receptor has yet to be identified on the platelets. Current studies are ongoing to identify which Gαs-coupled GPCRs on the surface of platelets functions as the 12(S)-HETrE receptor. Several Gαs-coupled GPCRs are already known to be expressed on the human platelet, including the IP2, EP2, EP4, and DP1 receptors. Using pharmacological, genetic, and screening approaches, it is reasonable to presume that the 12(S)-HETrE receptor will be identified in the near future. These approaches will enable in vivo studies using 12(S)-HETrE derived from DGLA oxidation by 12-LOX to prove which receptor(s) are essential for 12(S)-HETrE-mediated protection from injury-induced platelet activation and thrombosis in the vessel. Following its identification, it will be worthwhile for investigators to follow up on the contrasting concepts laid out in this review in regards to the multiple forms of 12-HETrE to determine if AA-derived 12-HETrE metabolites are also able to signal platelets (and possibly other cells) through the platelet 12-HETrE receptor. The AA-derived 12(S)-HETrE has been demonstrated to induce calcium release in the neutrophils. Thus, this implicates 12(S)-HETrE derived from CYP450 pathway could impinge on either Gαq or Gαi-coupled receptors on leukocytes as well as platelets. Enhanced calcium flux in platelets would potentiate platelet activation in a manner similar to what has been previously published for 12(S)-HETE[46, 69]. It will be of great interest in the future to determine if AA-derived 12(S)-HETrE functions as a procoagulant signal in the human platelet and if so, whether this potential signaling has a physiologically relevant role in regulating platelet reactivity during inflammatory states. Future studies of platelet 12-LOX regulated 12(S)-HETrE formation as well as the other structurally unique forms of 12-HETrE produced by 12R-LOX, CYP450, and epithelial 12-LOX and their receptors will likely uncover a myriad of physiologically relevant signaling events beyond that of cardiovascular health and inflammation.
Highlights.
Structurally distinct 12-HETrE structures derived from platelet 12-LOX and CYP450
12(S)-HETrE derived from platelet 12-LOX oxidation of DGLA is anti-thrombotic
12(R)-HETrE derived from CYP450 oxidation of AA is pro-inflammatory
Acknowledgments
This work was supported in part by the National Institutes of Health (NIH) Office of Dietary Supplement (ODS), GM105671 (M.H.), HL114405 (M.H.), and Ruth L. Kirschstein Institutional National Research Service Awards (NSRA) F31HL129481 (J.Y.).
Footnotes
Authors’ contributions
J. Yeung performed literature search, wrote the manuscript and created figures. M. Holinstat wrote, edited and proofed the manuscript.
Conflict of interest statement
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta. 2015;1851(4):340–55. doi: 10.1016/j.bbalip.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieg P, Furstenberger G. The role of lipoxygenases in epidermis. Biochim Biophys Acta. 2014;1841(3):390–400. doi: 10.1016/j.bbalip.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Munoz-Garcia A, Thomas CP, Keeney DS, Zheng Y, Brash AR. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim Biophys Acta. 2014;1841(3):401–8. doi: 10.1016/j.bbalip.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12-HETrE, a 12-Lipoxygenase Oxylipin of Dihomo-gamma-Linolenic Acid, Inhibits Thrombosis via Galphas Signaling in Platelets. Arterioscler Thromb Vasc Biol. 2016 doi: 10.1161/ATVBAHA.116.308050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du L, Yermalitsky V, Hachey DL, Jagadeesh SG, Falck JR, Keeney DS. A biosynthetic pathway generating 12-hydroxy-5,8,14-eicosatrienoic acid from arachidonic acid is active in mouse skin microsomes. J Pharmacol Exp Ther. 2006;316(1):371–9. doi: 10.1124/jpet.105.093922. [DOI] [PubMed] [Google Scholar]
- 7.Falgueyret JP, Leblanc Y, Riendeau D. Stereoselective carbonyl reductases from rat skin and leukocyte microsomes converting 12-ketoeicosatetraenoic acid to 12(S)-HETE. FEBS Lett. 1990;262(2):197–200. doi: 10.1016/0014-5793(90)80188-o. [DOI] [PubMed] [Google Scholar]
- 8.Masferrer JL, Laniado-Schwartzman M. Novel cytochrome P450-dependent arachidonic acid metabolites and their ocular effects. Prog Clin Biol Res. 1989;312:85–93. [PubMed] [Google Scholar]
- 9.Masferrer JL, Murphy RC, Abraham NG, Laniado-Schwartzman M. Corneal arachidonate metabolism via cytochrome P450: characterization of two novel biologically active metabolites. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:351–4. [PubMed] [Google Scholar]
- 10.Masferrer JL, Murphy RC, Pagano PJ, Dunn MW, Laniado-Schwartzman M. Ocular effects of a novel cytochrome P-450-dependent arachidonic acid metabolite. Invest Ophthalmol Vis Sci. 1989;30(3):454–60. [PubMed] [Google Scholar]
- 11.Masferrer JL, Rimarachin JA, Gerritsen ME, Falck JR, Yadagiri P, Dunn MW, Laniado-Schwartzman M. 12(R)-hydroxyeicosatrienoic acid, a potent chemotactic and angiogenic factor produced by the cornea. Exp Eye Res. 1991;52(4):417–24. doi: 10.1016/0014-4835(91)90037-f. [DOI] [PubMed] [Google Scholar]
- 12.Falardeau P, Hamberg M, Samuelsson B. Metabolism of 8,11,14-eicosatrienoic acid in human platelets. Biochim Biophys Acta. 1976;441(2):193–200. doi: 10.1016/0005-2760(76)90162-4. [DOI] [PubMed] [Google Scholar]
- 13.Lagarde M, Dechavanne M, Rigaud M, Durand J. Basal level of human platelet prostaglandins: PGE1 is more elevated than PGE2. Prostaglandins. 1979;17(5):685–705. doi: 10.1016/s0090-6980(79)80041-6. [DOI] [PubMed] [Google Scholar]
- 14.Lagarde M, Guichardant M, Dechavanne M. Human platelet PGE1 and dihomogammalinolenic acid. Comparison to PGE2 and arachidonic acid. Prog Lipid Res. 1981;20:439–43. doi: 10.1016/0163-7827(81)90078-3. [DOI] [PubMed] [Google Scholar]
- 15.Needleman P, Whitaker MO, Wyche A, Watters K, Sprecher H, Raz A. Manipulation of platelet aggregation by prostaglandins and their fatty acid precursors: pharmacological basis for a therapeutic approach. Prostaglandins. 1980;19(1):165–81. doi: 10.1016/0090-6980(80)90163-x. [DOI] [PubMed] [Google Scholar]
- 16.Ashby B. Cyclic AMP turnover in response to prostaglandins in intact platelets: evidence for separate stimulatory and inhibitory prostaglandin receptors. Second Messengers Phosphoproteins. 1988;12(1):45–57. [PubMed] [Google Scholar]
- 17.Gorman RR, Bunting S, Miller OV. Modulation of human platelet adenylate cyclase by prostacyclin (PGX) Prostaglandins. 1977;13(3):377–88. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- 18.Levin G, Duffin KL, Obukowicz MG, Hummert SL, Fujiwara H, Needleman P, Raz A. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem J. 2002;365(Pt 2):489–96. doi: 10.1042/BJ20011798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tateson JE, Moncada S, Vane JR. Effects of prostacyclin (PGX) on cyclic AMP concentrations in human platelets. Prostaglandins. 1977;13(3):389–97. doi: 10.1016/0090-6980(77)90019-3. [DOI] [PubMed] [Google Scholar]
- 20.Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, Sui de X, Regan JW, Smith WL. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 2007;282(31):22254–66. doi: 10.1074/jbc.M703169200. [DOI] [PubMed] [Google Scholar]
- 21.Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res. 2012;53(12):2546–59. doi: 10.1194/jlr.M026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammarstrom S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, Voorhees JJ. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc Natl Acad Sci U S A. 1975;72(12):5130–4. doi: 10.1073/pnas.72.12.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamberg M. Decomposition of unsaturated fatty acid hydroperoxides by hemoglobin: Structures of major products of 13L-hydroperoxy-9,11-octadecadienoic acid. Lipids. 1975;10(2):87–92. doi: 10.1007/BF02532161. [DOI] [PubMed] [Google Scholar]
- 24.Liu B, Khan WA, Hannun YA, Timar J, Taylor JD, Lundy S, Butovich I, Honn KV. 12(S)-hydroxyeicosatetraenoic acid and 13(S)-hydroxyoctadecadienoic acid regulation of protein kinase C-alpha in melanoma cells: role of receptor-mediated hydrolysis of inositol phospholipids. Proc Natl Acad Sci U S A. 1995;92(20):9323–7. doi: 10.1073/pnas.92.20.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabre JE, Nguyen M, Athirakul K, Coggins K, McNeish JD, Austin S, Parise LK, FitzGerald GA, Coffman TM, Koller BH. Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation. J Clin Invest. 2001;107(5):603–10. doi: 10.1172/JCI10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breyer MD, Breyer RM. G protein-coupled prostanoid receptors and the kidney. Annu Rev Physiol. 2001;63:579–605. doi: 10.1146/annurev.physiol.63.1.579. [DOI] [PubMed] [Google Scholar]
- 27.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 28.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: advances in the study of EP3 receptor signaling. J Biochem. 2002;131(6):781–4. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- 29.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108(1):25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierce KL, Fujino H, Srinivasan D, Regan JW. Activation of FP prostanoid receptor isoforms leads to Rho-mediated changes in cell morphology and in the cell cytoskeleton. J Biol Chem. 1999;274(50):35944–9. doi: 10.1074/jbc.274.50.35944. [DOI] [PubMed] [Google Scholar]
- 31.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108(1):15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chopra H, Timar J, Chen YQ, Rong XH, Grossi IM, Fitzgerald LA, Taylor JD, Honn KV. The lipoxygenase metabolite 12(S)-HETE induces a cytoskeleton-dependent increase in surface expression of integrin alpha IIb beta 3 on melanoma cells. Int J Cancer. 1991;49(5):774–86. doi: 10.1002/ijc.2910490524. [DOI] [PubMed] [Google Scholar]
- 33.Honn KV, Tang DG. Eicosanoid 12(S)-HETE upregulates endothelial cell alpha V beta 3 integrin expression and promotes tumor cell adhesion to vascular endothelium. Adv Exp Med Biol. 1997;400B:765–73. [PubMed] [Google Scholar]
- 34.Silletti S, Timar J, Honn KV, Raz A. Regulation of tumor cell motility by 12(S)-HETE. Adv Exp Med Biol. 1997;400B:683–92. [PubMed] [Google Scholar]
- 35.Steinert BW, Tang DG, Grossi IM, Umbarger LA, Honn KV. Studies on the role of platelet eicosanoid metabolism and integrin alpha IIb beta 3 in tumor-cell-induced platelet aggregation. Int J Cancer. 1993;54(1):92–101. doi: 10.1002/ijc.2910540116. [DOI] [PubMed] [Google Scholar]
- 36.Tang DG, Chen YQ, Diglio CA, Honn KV. Protein kinase C-dependent effects of 12(S)-HETE on endothelial cell vitronectin receptor and fibronectin receptor. J Cell Biol. 1993;121(3):689–704. doi: 10.1083/jcb.121.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang DG, Diglio CA, Honn KV. 12(S)-HETE-induced microvascular endothelial cell retraction results from PKC-dependent rearrangement of cytoskeletal elements and alpha V beta 3 integrins. Prostaglandins. 1993;45(3):249–67. doi: 10.1016/0090-6980(93)90051-8. [DOI] [PubMed] [Google Scholar]
- 38.Tang DG, Grossi IM, Chen YQ, Diglio CA, Honn KV. 12(S)-HETE promotes tumor-cell adhesion by increasing surface expression of alpha V beta 3 integrins on endothelial cells. Int J Cancer. 1993;54(1):102–11. doi: 10.1002/ijc.2910540117. [DOI] [PubMed] [Google Scholar]
- 39.Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res. 2003;63(14):4258–67. [PubMed] [Google Scholar]
- 40.Pidgeon GP, Tang K, Rice RL, Zacharek A, Li L, Taylor JD, Honn KV. Overexpression of leukocyte-type 12-lipoxygenase promotes W256 tumor cell survival by enhancing alphavbeta5 expression. Int J Cancer. 2003;105(4):459–71. doi: 10.1002/ijc.11134. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen CH, Stadler S, Brenner S, Huttary N, Krieger S, Jager W, Dolznig H, Krupitza G. Cancer cell-derived 12(S)-HETE signals via 12-HETE receptor, RHO, ROCK and MLC2 to induce lymph endothelial barrier breaching. Br J Cancer. 2016;115(3):364–70. doi: 10.1038/bjc.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV. Identification of the orphan G protein-coupled receptor GPR31 as a receptor for 12-(S)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286(39):33832–40. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampson AJ, Grimaldi M. 12-hydroxyeicosatetrenoate (12-HETE) attenuates AMPA receptor-mediated neurotoxicity: evidence for a G-protein-coupled HETE receptor. J Neurosci. 2002;22(1):257–64. doi: 10.1523/JNEUROSCI.22-01-00257.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Xu YW, Han J, Liang H, Wang N, Cheng Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARgamma: a possible neuroprotective effect in ischemic brain. J Lipid Res. 2015;56(3):502–14. doi: 10.1194/jlr.M053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kuhn H, Hazen SL, Goodall AH, Hamali HA, Collins PW, O’Donnell VB. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285(10):6891–903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeung J, Apopa PL, Vesci J, Stolla M, Rai G, Simeonov A, Jadhav A, Fernandez-Perez P, Maloney DJ, Boutaud O, Holman TR, Holinstat M. 12-lipoxygenase activity plays an important role in PAR4 and GPVI-mediated platelet reactivity. Thromb Haemost. 2013;110(3):569–81. doi: 10.1160/TH13-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Maher RJ, De Jonckheere JP, Popat RU, Stojakovic S, Hannun YA, Porter AT, Honn KV. 12S)-HETE increases the motility of prostate tumor cells through selective activation of PKC alpha. Adv Exp Med Biol. 1997;400B:707–18. [PubMed] [Google Scholar]
- 48.van Dijk KW, Steketee K, Havekes L, Frants R, Hofker M. Genomic and cDNA cloning of a novel mouse lipoxygenase gene. Biochim Biophys Acta. 1995;1259(1):4–8. doi: 10.1016/0005-2760(95)00158-9. [DOI] [PubMed] [Google Scholar]
- 49.Sun D, Elsea SH, Patel PI, Funk CD. Cloning of a human “epidermal-type” 12-lipoxygenase-related gene and chromosomal localization to 17p13. Cytogenet Cell Genet. 1998;81(1):79–82. doi: 10.1159/000014993. [DOI] [PubMed] [Google Scholar]
- 50.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning, and expression. Proc Natl Acad Sci U S A. 1998;95(12):6744–9. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun D, McDonnell M, Chen XS, Lakkis MM, Li H, Isaacs SN, Elsea SH, Patel PI, Funk CD. Human 12(R)-lipoxygenase and the mouse ortholog. Molecular cloning, expression, and gene chromosomal assignment. J Biol Chem. 1998;273(50):33540–7. doi: 10.1074/jbc.273.50.33540. [DOI] [PubMed] [Google Scholar]
- 52.Murphy RC, Falck JR, Lumin S, Yadagiri P, Zirrolli JA, Balazy M, Masferrer JL, Abraham NG, Schwartzman ML. 12(R)-hydroxyeicosatrienoic acid: a vasodilator cytochrome P-450-dependent arachidonate metabolite from the bovine corneal epithelium. J Biol Chem. 1988;263(32):17197–202. [PubMed] [Google Scholar]
- 53.Conners MS, Schwartzman ML, Quan X, Heilman E, Chauhan K, Falck JR, Godfrey HP. Enhancement of delayed hypersensitivity inflammatory reactions in guinea pig skin by 12(R)-hydroxy-5,8,14-eicosatrienoic acid. J Invest Dermatol. 1995;104(1):47–51. doi: 10.1111/1523-1747.ep12613482. [DOI] [PubMed] [Google Scholar]
- 54.Mastyugin V, Aversa E, Bonazzi A, Vafaes C, Mieyal P, Schwartzman ML. Hypoxia-induced production of 12-hydroxyeicosanoids in the corneal epithelium: involvement of a cytochrome P-4504B1 isoform. J Pharmacol Exp Ther. 1999;289(3):1611–9. [PubMed] [Google Scholar]
- 55.Powell WS, Hashefi M, Falck JR, Chauhan K, Rokach J, Wang SS, Mills E, MacLeod RJ. Effects of oxo and dihydro metabolites of 12-hydroxy-5,8,10,14-eicosatetraenoic acid on chemotaxis and cytosolic calcium levels in human neutrophils. J Leukoc Biol. 1995;57(2):257–63. doi: 10.1002/jlb.57.2.257. [DOI] [PubMed] [Google Scholar]
- 56.Wainwright S, Falck JR, Yadagiri P, Powell WS. Metabolism of 12(S)-hydroxy-5,8,10,14-eicosatetraenoic acid and other hydroxylated fatty acids by the reductase pathway in porcine polymorphonuclear leukocytes. Biochemistry. 1990;29(43):10126–35. doi: 10.1021/bi00495a017. [DOI] [PubMed] [Google Scholar]
- 57.Van Wauwe J, Coene MC, Van Nyen G, Cools W, Goossens J, Le Jeune L, Lauwers W, Janssen PA. NADPH-dependent formation of 15- and 12-hydroxyeicosatrienoic acid from arachidonic acid by rat epidermal microsomes. Eicosanoids. 1991;4(3):155–63. [PubMed] [Google Scholar]
- 58.Yamamoto S, Nishimura M, Conners MS, Stoltz RA, Falck JR, Chauhan K, Laniado-Schwartzman M. Oxidation and keto reduction of 12-hydroxy-5,8,10,14-eicosatetraenoic acids in bovine corneal epithelial microsomes. Biochim Biophys Acta. 1994;1210(2):217–25. doi: 10.1016/0005-2760(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 59.Capdevila J, Yadagiri P, Manna S, Falck JR. Absolute configuration of the hydroxyeicosatetraenoic acids (HETEs) formed during catalytic oxygenation of arachidonic acid by microsomal cytochrome P-450. Biochem Biophys Res Commun. 1986;141(3):1007–11. doi: 10.1016/s0006-291x(86)80144-9. [DOI] [PubMed] [Google Scholar]
- 60.Oliw EH. bis-Allylic hydroxylation of linoleic acid and arachidonic acid by human hepatic monooxygenases. Biochim Biophys Acta. 1993;1166(2–3):258–63. doi: 10.1016/0005-2760(93)90106-j. [DOI] [PubMed] [Google Scholar]
- 61.Wainwright SL, Powell WS. Mechanism for the formation of dihydro metabolites of 12-hydroxyeicosanoids. Conversion of leukotriene B4 and 12-hydroxy-5,8,10,14-eicosatetraenoic acid to 12-oxo intermediates. J Biol Chem. 1991;266(31):20899–906. [PubMed] [Google Scholar]
- 62.Mieyal PA, Dunn MW, Schwartzman ML. Detection of endogenous 12-hydroxyeicosatrienoic acid in human tear film. Invest Ophthalmol Vis Sci. 2001;42(2):328–32. [PubMed] [Google Scholar]
- 63.Mezentsev A, Seta F, Dunn MW, Ono N, Falck JR, Laniado-Schwartzman M. Eicosanoid regulation of vascular endothelial growth factor expression and angiogenesis in microvessel endothelial cells. J Biol Chem. 2002;277(21):18670–6. doi: 10.1074/jbc.M201143200. [DOI] [PubMed] [Google Scholar]
- 64.Seta F, Patil K, Bellner L, Mezentsev A, Kemp R, Dunn MW, Schwartzman ML. Inhibition of VEGF expression and corneal neovascularization by siRNA targeting cytochrome P450 4B1. Prostaglandins Other Lipid Mediat. 2007;84(3–4):116–27. doi: 10.1016/j.prostaglandins.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stoltz RA, Abraham NG, Laniado-Schwartzman M. The role of NF-kappaB in the angiogenic response of coronary microvessel endothelial cells. Proc Natl Acad Sci U S A. 1996;93(7):2832–7. doi: 10.1073/pnas.93.7.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoltz RA, Schwartzman ML. High affinity binding sites for 12(R)-Hydroxyeicosatrienoic acid [12(R)-HETrE] in microvessel endothelial cells. J Ocul Pharmacol Ther. 1997;13(3):191–9. doi: 10.1089/jop.1997.13.191. [DOI] [PubMed] [Google Scholar]
- 67.Schonfeld W, Kasimir S, Knoller J, Jablonski K, Konig W. Metabolism of leukotriene B4 via reduction into dihydro-leukotriene B4 in human monocytes, alveolar macrophages, and U-937 cells. J Leukoc Biol. 1991;50(3):303–12. doi: 10.1002/jlb.50.3.303. [DOI] [PubMed] [Google Scholar]
- 68.Fauler J, Marx KH, Kaever V, Frolich JC. Human monocytes convert leukotriene B4 to two dihydro-leukotriene B4-metabolites. Prostaglandins Leukot Essent Fatty Acids. 1989;37(3):193–6. doi: 10.1016/0952-3278(89)90085-9. [DOI] [PubMed] [Google Scholar]
- 69.Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O, Holinstat M. Protein kinase C regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81(3):420–30. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]