Abstract
Background
Brain bioenergetic anomalies and redox dysregulation have been implicated in the pathophysiology of psychotic disorders. The present study examined brain energy-related metabolites and the balance between nicotinamide adenine dinucleotide metabolites (oxidized NAD+ and reduced NADH) using 31P-magnetic resonance spectroscopy (31P-MRS) in unaffected siblings, compared to first episode psychosis (FEP) patients and healthy controls.
Methods
21 unaffected siblings, 32 FEP patients (including schizophrenia spectrum and affective psychoses), and 21 controls underwent 31P-MRS in the frontal lobe (6×6×4 cm3) on a 4T MR scanner, using custom-designed dual-tuned surface coil with outer volume suppression. Brain parenchymal pH and steady-state metabolite ratios of high energy phosphate compounds were measured. NAD+ and NADH levels were determined using a 31P-MRS fitting algorithm. 13 unaffected sibling-patient pairs were related; other patients and siblings were unrelated. ANCOVA analyses were used to examine 31P-MRS measures, with age and gender as covariates.
Results
The phosphocreatine/adenosine triphosphate ratio was significantly reduced in both unaffected siblings and FEP patients, compared to controls. NAD+/NADH ratio was significantly reduced in patients compared to siblings and controls, with siblings showing a reduction in NAD+/NADH compared to controls that was not statistically significant. Compared to patients and controls, siblings showed significantly reduced levels of NAD+. Siblings did not differ from patients or controls on brain pH.
Discussion
Our results indicate that unaffected siblings show some, but not all the same abnormalities in brain energy metabolites and redox state as FEP patients. Thus, 31P-MRS studies may identify factors related both to risk and expression of psychosis.
Keywords: unaffected relatives, schizophrenia, bipolar disorder, 31P magnetic resonance spectroscopy, energy metabolism, oxidative stress
1. Introduction
Psychotic disorders are common and severe psychiatric disorders with multifactorial etiology. Schizophrenia and bipolar disorder, in particular, are strongly heritable with some overlapping genetic risk; first-degree relatives of patients with these disorders have an approximately 10 fold increased risk of developing illness compared to the general population (Erlenmeyer-Kimling et al., 1995; International Schizophrenia Consortium et al., 2009; Ivleva et al., 2010; Kendler and Gardner, 1997). Some neurobiological correlates of psychotic disorders are manifest in unaffected relatives, often to a reduced extent, compared to healthy individuals (Byun et al., 2012; Skudlarski et al., 2013). Unaffected relatives of patients with psychotic disorders may share predisposing factors for illness, without sharing confounds of chronic illness and its treatment. Studying neurobiological abnormalities of psychotic disorders in unaffected relatives can therefore contribute to finding heritable markers of disease risk (Gottesman and Gould, 2003), and advance our understanding of factors underlying these disorders.
Studies using 31P magnetic resonance spectroscopy (MRS) have identified abnormalities in brain bioenergetics in psychotic disorders (Du et al., 2014; Nenadic et al., 2012; Pettegrew et al., 1991; Smesny et al., 2007; Yuksel et al., 2015a). 31P MRS allows the measurement of high energy phosphate (HEP) metabolite levels, including phosphocreatine (PCr) and adenosine triphosphate (ATP) as well as inorganic phosphate (Pi), and the phospholipid metabolites, phosphomonoesters (PME) and phosphodiesters (PDE). Brain parenchymal pH can also be measured in 31P MRS using the chemical shift difference between PCr and Pi. Altered levels of these brain metabolites have been found in patients with psychotic disorders, revealing energy metabolism abnormalities and perturbed membrane phospholipid metabolism in the brain (Du et al., 2014; Nenadic et al., 2012; Pettegrew et al., 1991; Smesny et al., 2007; Yuksel et al., 2015a; Yuksel et al., 2015b). In a previous study using a 31P magnetization transfer MRS approach (Du et al., 2014), we found new evidence of dysfunctional brain bioenergetics, specifically, a significant reduction in the forward rate constant of a critical enzyme involved in energy metabolism, creatine kinase, in the frontal lobe of patients with schizophrenia. In addition, we reported that brain parenchymal pH measured by 31P MRS was significantly reduced in patients with schizophrenia, possibly indicating a reduced ratio of oxidative phosphorylation to glycolysis, leading to lactic acid build up. Despite intriguing observations, however, no abnormalities in HEPs or phospholipid metabolites have been reported consistently enough in the 31P MRS literature (Yuksel et al., 2015b). In particular, there are few studies that have used 31P MRS to investigate brain abnormalities in unaffected relatives of patients with psychotic disorders (Keshavan et al., 2003; Klemm et al., 2001).
A recent advance in the 31P MRS literature is the ability to quantify nicotinamide adenine dinucleotide (NAD+) and its reduced form NADH (Kim et al., 2017; Lu et al., 2016), which play crucial roles in redox reactions related to energy metabolism and antioxidant activity. We recently demonstrated the feasibility of a novel 31P MRS approach to quantify phosphorus NAD metabolite resonance signals in the brain (Kim et al., 2017). The balance between the redox pair of NAD+ and NADH concentrations reflects the oxidative state of cells, and the NAD+/NADH ratio may be used as a measure of oxidative stress (Blacker et al., 2014). A converging body of evidence has suggested a role for oxidative stress and altered antioxidant defenses in psychotic disorders (Yao and Keshavan, 2011). In a recent study, we found evidence of a significant reduction in NAD+/NADH measured by 31P MRS, reflecting redox imbalance, in the brain of patients with first episode and chronic schizophrenia (Kim et al., 2017), compared to healthy controls. To our knowledge, no studies have previously measured NAD metabolites using 31P MRS in unaffected relatives of patients with psychotic disorders.
In the present study, we examined whether HEP metabolite abnormalities and redox imbalance measured by in vivo 31P MRS are present in unaffected siblings of patients with first episode psychosis (FEP), as they are in patients with psychosis. Studying both brain HEP metabolites, including PCr, ATP and Pi, in addition to NAD metabolites allows us to examine two aspects of mitochondrial pathways. While NAD+/NADH informs us specifically on oxidative state, these brain metabolites are all at the intersection of multiple critical biochemical pathways within cells, necessary for mitochondrial function and energy metabolism. We hypothesized that unaffected siblings of patients with FEP would show abnormalities in measures of HEP metabolites, NAD+/NADH and pH in the frontal lobe, compared to healthy controls, but that abnormalities observed in unaffected siblings would be of lesser magnitude than those seen in patients. We studied the prefrontal cortex, because it has been implicated in bioenergetic compromise, neurobiochemical (Deicken et al., 1994; Stanley et al., 1995) changes, and other abnormalities in these disorders (Lewandowski et al., 2011).
2. Methods
2.1. Participants
We assessed 32 patients with FEP, 21 unaffected siblings and 21 healthy controls. Of the 32 patients, 4 were diagnosed with schizophrenia, 4 with schizoaffective disorder, 17 with bipolar disorder with psychotic features, 5 with psychosis not otherwise specified (NOS), and 2 with major depressive disorder with psychotic features. Patients were recruited from McLean OnTrack, a FEP outpatient program, and were included within one year of the onset of psychotic symptoms. Patients were excluded if symptoms could be attributable to a general medical condition or substance use, or they had a diagnosis of current substance use disorder. There were no selection criteria based on severity of clinical symptomatology, however, patients were not hospitalized at the time of the study. Unaffected siblings had no history of a psychotic or bipolar disorder, and were not taking psychoactive medications. There were 13 unaffected sibling-patient pairs in the sample who were related, while other patients and unaffected siblings were unrelated. Proband diagnoses for unaffected siblings were obtained by diagnostic interview and review of hospital charts, and included 3 with schizophrenia, 3 with schizoaffective disorder, 14 with bipolar disorder with psychotic features, and 1 with psychosis NOS. Unaffected siblings were recruited through participating patients, a support group for family members in McLean OnTrack and flyers at McLean Hospital. Healthy controls, recruited via flyers on local college campuses, had no psychiatric diagnoses and no history of the same in first-degree relatives. Patients, siblings and controls were excluded if they had a history of significant medical or neurologic illness, history of significant head trauma, contraindication to MR scan (including claustrophobia) or pregnancy. Of note, the unaffected sibling data we present here is entirely new, but 28 patients and 11 controls in this analysis overlap with the study population in our recent study (Kim et al., 2017), and we include them for comparison purposes. The study was approved by the McLean Hospital Institutional Review Board, and participants provided written informed consent.
2.2. Clinical Assessments
The Structured Clinical Interview for DSM-IV-TR (First et al., 1995) was used for diagnosis ascertainment of all subjects. The following rating scales were also administered as appropriate to patients, siblings and/or controls: Wisconsin Schizotypy Scales-short form (Gross et al., 2015), Symptom Checklist-90-Revised (SCL-90-R) (Derogatis et al., 1973), Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), State-Trait Anxiety Inventory (STAI) (Spielberger et al., 1983), Edinburgh Handedness inventory (Oldfield, 1971), and MATRICS Consensus Cognitive Battery (MCCB) (Marder and Fenton, 2004).
2.3. Magnetic resonance imaging and 31P MRS Experiments
31P MRS was performed on a 4T whole-body MR scanner interfaced with Varian INOVA console. Brain anatomic imaging and 31P MRS were acquired from the frontal lobe of the brain by a specially designed half helmet dual-tuned surface coil placed on the forehead (supplementary Figure S1). 31P MRS measures, HEP and phospholipid metabolites, and NAD analyses were extracted from a 31P MRS study using a magnetization transfer approach (31P-MT-MRS) to quantify creatine kinase and ATP synthetase reaction rates (Du et al., 2014; Kim et al., 2017). Details of the 31P MRS techniques have been described previously (Du et al., 2014; Kim et al., 2017), and more information is provided in the supplementary material.
2.4. 31P spectrum Processing andQuantification of pH
Two methods were applied for 31P spectra processing to assess 1) HEP and phospholipid metabolites, and 2) NAD metabolites. First, metabolite levels (PCr, Pi, PDE and PME) using β-ATP as an internal reference were analyzed from spectra acquired without magnetization transfer (Figure 1a) in the time domain with the AMARES algorithm (jMRUI; http://mrui.uab.es/mrui/) (van den Boogaart et al., 1996). This strategy eliminates the magnetization transfer effect for PCr and Pi quantifications, and the spectra (Figure 1a) with 26 signal averages have enough SNR for metabolite quantification. Details on this spectrum processing are previously described (Du et al., 2014). Briefly, the initial 4 points of FID were truncated to remove the broad component. Eight resonance peaks (PME; Pi; PDE; PCr; and three adenosine triphosphates (α-, β -, γ-ATP)) were included in the basis set.
Figure 1. In vivo representative 31P spectra obtained from the frontal lobe of the brain in a healthy participant.
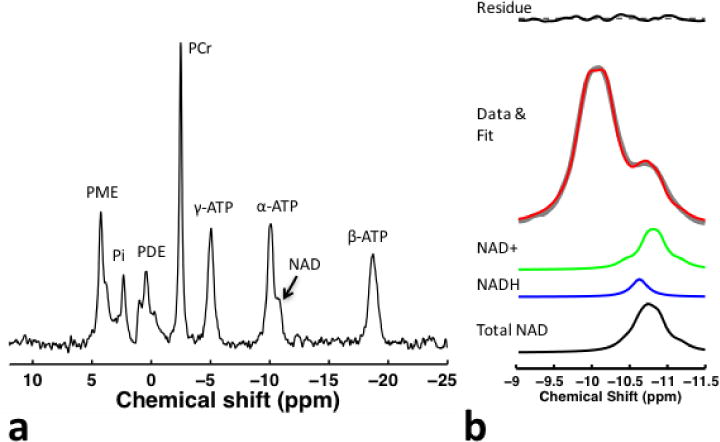
(a) Control spectra (26 signal averages) without saturating γ-ATP resonance in fully relaxation conditions (TR=14 sec) is shown. (b) For NAD+ and NADH quantification, we summed a series of spectra (total of 182 signal averages) that consists of spectra obtained with and without applying saturation pulse, since the saturation pulse had no impact on NAD resonance. High SNR spectra enabled high fitting accuracy of NAD resonance (i.e., fit error < 5 %).
Second, NAD+ and NADH quantification was performed on the summed spectra (Figure 1b) in order to enhance SNR of NAD for higher fitting accuracy. The approach used prior information of chemical shift and strong second-order coupling effects, from which 31P spectra of NAD at 4T can be predicted (Kim et al., 2017). We developed in-house MATLAB software (version 7.9, R2009b) for simulating the 31P spectra containing NAD+, NADH and α-ATP resonances. We previously performed a series of simulation, phantom, and test-retest studies to validate the quantification approach used in this study (Kim et al., 2017). Details of the NAD+ and NADH quantification methods have been described previously (Kim et al., 2017), and more information is provided in the supplementary material. Unknown parameters (i.e., signal intensity and line-width of α-ATP, NAD+, and NADH) were determined using nonlinear least-square fitting of the model output to the resonance signals of NAD+, NADH and α- ATP obtained from in vivo 31P MRS experiments. The fitting errors were calculated by dividing the standard deviation of residual signal by the mean value of the fitted signal. The concentrations were determined using α-ATP signal as an internal reference, with its brain concentration set to 2.8 mM (Zhu et al., 2015), in order to remain consistent with and make comparisons with previous reports (Zhu et al., 2015).
Brain pH was calculated using the chemical shift difference in parts per million between Pi and PCr (Petroff et al., 1985).
2.6. Statistical analyses
We performed statistical analyses using SPSS (PASW) version 22 (SPSS, Chicago, IL). We used chi-square tests for categorical variables and ANOVAs for continuous variables to compare demographic and clinical characteristics among patient, sibling and control groups. We used mixed model ANCOVA analyses with contrasts to examine 31P MRS measures with diagnosis as a between-subjects factor, age and gender as covariates, and patient-sibling pairs as a random factor. Age was included as a covariate given the age dependence of NAD metabolite measures previously reported in the literature (Zhu et al., 2015). Our primary outcome measures were pH, PCr/β-ATP and Pi/β-ATP ratios, and the NAD+/NADH ratio. We also examined phospholipid metabolite ratios, NAD+, and NADH. We carried out exploratory bivariate correlation analyses to examine the relationship of Wisconsin Schizotypy positive and negative factor scores, and SCL-90-R total scores to our primary outcome measures in siblings. All hypothesis tests were two-sided and conducted with a significance level of alpha=0.05.
3. Results
3.1. Clinical Characteristics
Demographic and clinical characteristics of study participants are given in Table 1. There were no significant differences between siblings and controls on measures of psychological distress or psychosis-proneness, including Wisconsin schizotypy positive (F=1.72, P=0.09) and negative (F=−1.5, P=0.13) factor scores, BDI-II (F=0.94, P=0.35) or STAI (F=−0.42, P=0.67) total scores. Medication use is given in Table 1. At the time of the study, there were 10 patients (31.2%) who were taking both antipsychotic and mood stabilizer medications, 1 patient (3.1%) taking 2 antipsychotic medications and 3 patients (9.4%) who were not taking any psychiatric medications.
Table 1.
Demographic and Clinical Characteristics of Unaffected Siblings, Patients with First Episode Psychosis, and Healthy Controls
| Characteristic | Patients (n=32) |
Siblings (n=21) |
Controls (n=21) |
|---|---|---|---|
| Age, mean (SD) | 22.6 (2.7)a | 23.5 (5.6)b | 22.6 (3.5)c |
| Sex (Male), No.(%) *** | 27 (84.4) | 7 (33.3) | 9 (42.9) |
| Race and Ethnicity, No.(%) | |||
| Caucasian | 27 (84.4) | 19 (90.5) | 13 (61.9) |
| African American | 1 (3.1) | 0 (0.0) | 1 (4.8) |
| Hispanic | 1 (3.1) | 1 (4.8) | 3 (14.3) |
| Asian | 2 (6.3) | 2 (9.5) | 4 (19) |
| Left-handed, No.(%) | 5 (17.2) | 3 (14.3) | 2 (9.5) |
| Education, mean (SD) d * | 4.6 (1.4) | 5.3 (1.6) | 5.7 (1.4) |
| Body Mass Index, mean (SD) ** | 25.6 (3.6) | 23.9 (3.4) | 22.5 (3.1) |
| Current smoking, No.(%) ** | 7 (21.9) | 0 (0) | 0 (0) |
| Lifetime Hospitalizations, No.(%) | 1.5 (1.3) | ||
| Prior suicide attempt, No.(%) | 6 (18.8) | ||
| PANSS total score, mean (SD) | 48.9 (13.4) | ||
| Global Assessment of Functioning *** | 58.7 (14.6) | 87.5 (6.6) | |
| MCCB composite total score, mean (SD) e *** | 37.0 (13.9) | 47.7 (11.9) | 55.3 (12.0) |
| SCL-90-R Positive Symptom Distress Index, mean (SD) | 45.3 (12.8) | 37.7 (17.0) | |
| SCL-90-R Global Severity Index, mean (SD) | 45.5 (9.32) | 42.8 (9.7) | |
| Medications, No.(%) | |||
| Atypical antipsychotic | 16 (50.0) | ||
| Clozapine | 2 (6.3) | ||
| Typical antipsychotic | 1 (3.1) | ||
| Lithium | 15 (46.9) | ||
| Mood stabilizer | 19 (59.4) | ||
| Chlorpromazine equivalent, mean (SD), mg/d | 136.0 (187.24) |
Abbreviations: PANSS, Positive and Negative Syndrome Scale; MCCB, MATRICS Consensus Cognitive Battery; SCL-90-R, Symptom Checklist-90-Revised
age range= 18–29 years.
age range= 18–37 years.
age range= 18–28 years.
Education range: 3= high school, 4= some college, 5= 2-year college, 6= 4-year college graduate, 7= some graduate or professional school, and 8= completed graduate or professional school.
MCCB data available for subset of patients (26), relatives (13) and controls (11).
P<0.05.
P< 0.01
P<0.001
3.2. Phosphate Metabolite Ratios and pH Measurements
The mean (SD) PCr SNR did not differ (P=0.28) between patients (52.3(20.0)), siblings (61.2 (18.7)) and controls (59.6 (24.3)). The PCr/β-ATP ratio differed significantly among groups (F=4.56, P=0.01), with siblings and patients showing significantly lower ratios compared to controls (P=0.007, and P=0.02, respectively), while there were no significant differences between patients and siblings (P=0.60) (Table 2). There were no other significant differences among groups for ratios of metabolites to β-ATP, nor for intracellular pH.
Table 2.
31P Magnetic Resonance Spectroscopy in the Frontal Lobe of Unaffected Siblings, Patients with First Episode Psychosis, and Healthy Controls
| Variable | Mean (SD)
|
P Value | ||
|---|---|---|---|---|
| Patients | Siblings | Controls | ||
| n=32 | n=21 | n=20 | ||
|
|
||||
| Intracellular pH | 7.030 (0.016) | 7.025 (0.019) | 7.020 (0.016) | 0.21 |
| Phosphocreatine/β-ATP | 1.20 (0.17) | 1.17 (0.20) | 1.39 (0.36) | 0.01 |
| Inorganic phosphate/β-ATP | 0.39 (0.12) | 0.37 (0.08) | 0.44 (0.12) | 0.15 |
| Phosphodiester/β-ATP | 0.74 (0.16) | 0.65 (0.09) | 0.77 (0.23) | 0.11 |
| Phosphomonoester/β-ATP | 1.05 (0.22) | 0.96 (0.18) | 1.11 (0.28) | 0.12 |
| n=31 | n=18 | n=20 | ||
|
|
||||
| NAD+/NADH | 3.35 (1.10) | 4.74 (1.55) | 5.20 (1.44) | <0.001 |
| NAD+ (mM) | 0.32 (0.05) | 0.28 (0.03) | 0.31 (0.03) | 0.01 |
| NADH (mM) | 0.10 (0.03) | 0.06 (0.02) | 0.06 (0.02) | 0.001 |
Abbreviations: β-ATP, β-adenosine triphosphate; NAD, Nicotinamide adenine dinucleotide.
3.3. NAD+/NADH
Figure 1a presents 31P spectra acquired from a healthy control, and Figure 1b shows the relevant spectral region with NAD+ and NADH fitting results and residual signal. The mean (SD) NAD resonances (NAD+ and NADH) SNR was not significantly different (P = 0.76) between patients (29.8 (12.1)), siblings (32.2 (9.2)) and controls (31.6 (12.8)).
NAD+/NADH ratio differed among groups (F=10.91, P< 0.001), with significant differences between both patients and healthy controls (P< 0.001) and patients and siblings (P<0.001), but not between siblings and controls (P=0.29) (Table 2). NAD+ levels also significantly differed between groups (F= 4.95, P= 0.01), with siblings showing lower levels of NAD+ compared to patients (P=0.005) and controls (P=0.01), but with no differences between patients and controls (P=0.75). NADH differed among groups (F=16.91, P< 0.001), with patients displaying higher levels of NADH compared to controls (P< 0.001) and siblings (P< 0.001), while there were no differences between siblings and controls (P=0.96).
3.4. Additional Analyses
The SCL-90-R global severity index was significantly correlated with pH (r=0.48, P=0.03) in the sibling group. In addition, in the sibling group, the SCL-90-R positive symptom distress index was significantly correlated with PCr/β-ATP ratio (r=0.62, P=0.003) and Pi/β-ATP ratio (r=0.57, P=0.009). No other significant correlations were found between spectroscopic parameters and measures of psychological distress and psychosis-proneness in siblings.
4. Discussion
The results suggest abnormalities in brain energy metabolism and redox regulation in both unaffected siblings at high genetic risk for psychosis and patients with FEP. Consistent with our recent study (Kim et al., 2017), patients with FEP showed significant reductions in NAD+/NADH ratios with elevated levels of NADH, compared to siblings and controls. In unaffected siblings, while we did not find the same striking evidence for redox dysregulation, there was evidence for altered balance between the NAD+ and NADH redox pair. Situated between patients and controls, siblings showed a NAD+/NADH ratio of 4.47, lower than that observed for controls, 5.20, although the difference was not statistically significant. Interestingly, levels of NAD were significantly lower in siblings, compared to both patients and controls. Siblings appear to be in the normal range with regard to maintaining a stable oxidative state in cells, however, it is possible that the decrease in NAD+ levels represents a vulnerability in metabolic pathways and cellular signaling events, related to genetic risk for psychosis. There is emerging evidence for oxidative stress in both early (Flatow et al., 2013; Fournier et al., 2014; Kim et al., 2017) and chronic (Kim et al., 2017; Yao and Keshavan, 2011) stages of psychotic illness, and oxidative stress may have wide-ranging implications for oxidative damage in the brain. An alteration in NAD+/NADH may affect several critical enzymatic reactions, which are required to maintain normal brain function, including neurotransmission and myelin production (Andreazza, 2012).
The decrease in PCr/β-ATP ratio in siblings and patients, compared to controls, further suggests compromised metabolic pathways involved in energy production and use in both individuals at genetically high risk for psychosis and those with psychosis. PCr acts as a reservoir by storing HEP bonds, critical to maintaining ATP levels during increased energy demand (Beard and Braissant, 2010; Saks et al., 1996), and affecting metabolic pathways and neurotransmission. Using the same 31P MRS approach in chronic patients with schizophrenia, we did not previously find a decrease in PCr, suggesting that this abnormality may be present early in illness. Prior studies examining 31P MRS measures in unaffected relatives, namely children, of patients with schizophrenia (Keshavan et al., 2003; Klemm et al., 2001; Rzanny et al., 2003), have not reported significant alterations in PCr, ATP or Pi; these studies showed abnormalities in phospholipid metabolites, which we did not find. However, 1H MRS studies in unaffected relatives of patients with schizophrenia have revealed decreases in PCr and Cr in different brain regions, including the striatum (Keshavan et al., 2009) and thalamus (Yoo et al., 2009).
Taken together, the pattern of findings show that unaffected siblings at genetic risk for psychosis share some abnormalities with FEP patients, but not all, in the two aspects of metabolic pathways studied. It is intriguing that PCr/β-ATP was decreased in both sibling and patient groups, but that the pattern of NAD+/NADH balance was somewhat different between these two groups. This could suggest that redox dysregulation and possibly oxidative stress plays a critical role in the expression of psychosis. Future prospective studies may determine whether at risk individuals who develop psychosis, show progression to significant redox imbalance in the brain.
It is important to point out that we chose a dimensional approach to studying psychosis, including siblings of patients with schizophrenia spectrum disorders as well as affective psychoses. Reinforcing the concept of using a transdiagnostic approach, particularly at an early stage of illness, we have found a high diagnostic switch rate of 50.5% during treatment in our FEP program, McLean OnTrack, with psychotic disorder diagnoses evolving over time (Shinn et al., 2017). This rate of diagnostic shift is consistent with changes in diagnoses found in a large study of first-admission patients with psychotic disorders, followed over a period of 10 years (Bromet et al., 2011).
Of note, in our study, unaffected siblings were high functioning, and not significantly different from controls on measures of psychological distress, global cognitive functioning, depressive and anxiety symptoms, or psychosis-proneness. Our sample of unaffected siblings represented individuals at high genetic risk, but not necessarily at high clinical risk for psychosis. It is possible that siblings, unaffected by psychosis, but with high clinical risk for psychosis and more psychopathology would have shown a more similar pattern to patients on measures of NAD metabolite levels, compared to controls. In addition, siblings were still within the age range for increased risk of developing psychosis, and follow-up would be necessary to determine whether psychopathology evolved over time. Interestingly, we did find that siblings with more psychological distress had increased pH, and increased PCr/β-ATP and Pi/β-ATP ratios; these associations may reflect compensatory responses to metabolic stressors.
This study has limitations to consider. First, the low SNR of 31P MRS forces us to obtain data from a large brain region in the frontal lobes. Outer-volume suppression ensured exclusion of signal from HEP-rich extra-cranial muscle. Second, because we used a proton quadrature surface coil for structural imaging, our results are not corrected for voxel composition of white and gray matter. Third, we chose to study metabolite concentration ratios, rather than metabolite concentrations, using internal referencing, a widely used method. Obtaining absolute metabolite concentrations using an external reference with known concentration and matched electromagnetic properties is time consuming, and can be confounded by issues such as radiofrequency coil loading. Although internal referencing may be a more reliable method, we could not then directly examine ATP concentrations, which may be abnormal when there is bioenergetic dysfunction. Our results, however, do not suggest significant abnormalities in ATP with normal Pi/ATP, PME/ATP and PDE/ATP ratios, consistent with past studies in psychotic disorders (Stanley et al., 1995; Volz et al., 1998). Fourth, the reduction found in PCr should be interpreted with caution in view of the discrepancies between 31P MRS studies in both patients with first episode and chronic psychotic disorders, which may be due in part to differences in methodology (Yuksel et al., 2015b). Fifth, with regards to NAD metabolite measures, while NAD+/NADH is the generally accepted index of redox state of cells (Blacker et al., 2014), NAD+/NADH alterations may also reflect upstream changes in other related redox pairs, including nicotinamide adenine dinucleotide phosphate (NADP+)/reduced NADPH and reduced glutathione/oxidized glutathione. The contributions from these other redox pairs to the NAD 31P metabolite signals are likely low, as NADP+/NADPH intracellular concentrations are small (~ 10%) (Blacker et al., 2014), compared to NAD+ and NADH. Finally, as energy metabolism and redox state are dynamic phenomena, a longitudinal study design would be needed to examine how state fluctuations may relate to the differences observed between patients, siblings and controls.
Overall, this study provides new evidence on brain energy related metabolites and redox balance in individuals at high genetic risk for psychosis, suggesting that compromised energy metabolism and altered NAD biology are observed in those vulnerable to psychosis. As expected, patients with FEP showed greater dysregulation in energy metabolism and oxidative state markers. Findings highlight the need for targeting bioenergetics and the oxidative state in early disease stages to document and improve metabolic status in the brain of patients with psychosis.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreazza AC. Combining redox-proteomics and epigenomics to explain the involvement of oxidative stress in psychiatric disorders. Mol Biosyst. 2012;8(10):2503–2512. doi: 10.1039/c2mb25118c. [DOI] [PubMed] [Google Scholar]
- Beard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010;115(2):297–313. doi: 10.1111/j.1471-4159.2010.06935.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory second edition (BDI-II) The Psychological Corporation; 1996. [Google Scholar]
- Blacker TS, Mann ZF, Gale JE, Ziegler M, Bain AJ, Szabadkai G, Duchen MR. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat Commun. 2014;5:3936. doi: 10.1038/ncomms4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang SW. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186–1194. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun MS, Kim JS, Jung WH, Jang JH, Choi JS, Kim SN, Choi CH, Chung CK, An SK, Kwon JS. Regional cortical thinning in subjects with high genetic loading for schizophrenia. Schizophr Res. 2012;141(2–3):197–203. doi: 10.1016/j.schres.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Calabrese G, Merrin EL, Meyerhoff DJ, Dillon WP, Weiner MW, Fein G. 31phosphorus magnetic resonance spectroscopy of the frontal and parietal lobes in chronic schizophrenia. Biol Psychiatry. 1994;36(8):503–510. doi: 10.1016/0006-3223(94)90613-0. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale–preliminary report. Psychopharmacol Bull. 1973;9(1):13–28. [PubMed] [Google Scholar]
- Du F, Cooper AJ, Thida T, Sehovic S, Lukas SE, Cohen BM, Zhang X, Ongur D. In vivo evidence for cerebral bioenergetic abnormalities in schizophrenia measured using 31P magnetization transfer spectroscopy. JAMA Psychiatry. 2014;71(1):19–27. doi: 10.1001/jamapsychiatry.2013.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Squires-Wheeler E, Adamo UH, Bassett AS, Cornblatt BA, Kestenbaum CJ, Rock D, Roberts SA, Gottesman II. The New York High-Risk Project. Psychoses and cluster A personality disorders in offspring of schizophrenic parents at 23 years of follow-up. Arch Gen psychiatry. 1995;52(10):857–865. doi: 10.1001/archpsyc.1995.03950220067013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Sptizer RL, Gibbon M, Williams JBW. Structured clinical interview or DSM-IV Axis I Disorders. New York State Pscyhiatric Institute, Biometrics Research; New York: 1995. [Google Scholar]
- Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Ferrari C, Baumann PS, Polari A, Monin A, Bellier-Teichmann T, Wulff J, Pappan KL, Cuenod M, Conus P, Do KQ. Impaired metabolic reactivity to oxidative stress in early psychosis patients. Schizophr Bull. 2014;40(5):973–983. doi: 10.1093/schbul/sbu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gross GM, Silvia PJ, Barrantes-Vidal N, Kwapil TR. The dimensional structure of short forms of the Wisconsin Schizotypy Scales. Schizophr Res. 2015;166(1–3):80–85. doi: 10.1016/j.schres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neuroscience and biobehavioral reviews. 2010;34(6):897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31P MRS study. Mol Psychiatry. 2003;8(3):316–323. doi: 10.1038/sj.mp.4001325. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a 1H spectroscopy study. Schizophr Res. 2009;115(1):88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 1997;27(2):411–419. doi: 10.1017/s003329179600445x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cohen BM, Chen X, Lukas SE, Shinn AK, Yuksel AC, Li T, Du F, Ongur D. Redox Dysregulation in Schizophrenia Revealed by in vivo NAD+/NADH Measurement. Schizophr Bull. 2017;43(1):197–204. doi: 10.1093/schbul/sbw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm S, Rzanny R, Riehemann S, Volz HP, Schmidt B, Gerhard UJ, Filz C, Schonberg A, Mentzel HJ, Kaiser WA, Blanz B. Cerebral phosphate metabolism in first-degree relatives of patients with schizophrenia. Am J Psychiatry. 2001;158(6):958–960. doi: 10.1176/appi.ajp.158.6.958. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41(2):225–241. doi: 10.1017/S0033291710001042. [DOI] [PubMed] [Google Scholar]
- Lu M, Zhu XH, Chen W. In vivo (31) P MRS assessment of intracellular NAD metabolites and NAD(+)/NADH redox state in human brain at 4 T. NMR Biomed. 2016;29(7):1010–1017. doi: 10.1002/nbm.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72(1):5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Langbein K, Weisbrod M, Maitra R, Rzanny R, Gussew A, Reichenbach JR, Sauer H, Smesny S. 31P-MR spectroscopy in monozygotic twins discordant for schizophrenia or schizoaffective disorder. Schizophr Res. 2012;134(2–3):296–297. doi: 10.1016/j.schres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Prichard JW, Behar KL, Alger JR, den Hollander JA, Shulman RG. Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology. 1985;35(6):781–788. doi: 10.1212/wnl.35.6.781. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Keshavan MS, Panchalingam K, Strychor S, Kaplan DB, Tretta MG, Allen M. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48(6):563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- Rzanny R, Klemm S, Reichenbach JR, Pfleiderer SO, Schmidt B, Volz HP, Blanz B, Kaiser WA. 31P-MR spectroscopy in children and adolescents with a familial risk of schizophrenia. Eur Radiol. 2003;13(4):763–770. doi: 10.1007/s00330-002-1565-1. [DOI] [PubMed] [Google Scholar]
- Saks VA, Ventura-Clapier R, Aliev MK. Metabolic control and metabolic capacity: two aspects of creatine kinase functioning in the cells. Biochim Biophys Acta. 1996;1274(3):81–88. doi: 10.1016/0005-2728(96)00011-4. [DOI] [PubMed] [Google Scholar]
- Shinn AK, Bolton KW, Karmacharya R, Lewandowski KE, Yuksel C, Baker JT, Chouinard VA, Pingali SM, Bye H, Cederbaum K, Ongur D. McLean OnTrack: a transdiagnostic program for early intervention in first-episode psychosis. Early Interv Psychiatry. 2017;11(1):83–90. doi: 10.1111/eip.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O’Neil K, Pearlson GD. Diffusion Tensor Imaging White Matter Endophenotypes in Patients With Schizophrenia or Psychotic Bipolar Disorder and Their Relatives. Am J Psychiatry. 2013;170(8):886–98. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- Smesny S, Rosburg T, Nenadic I, Fenk KP, Kunstmann S, Rzanny R, Volz HP, Sauer H. Metabolic mapping using 2D 31P-MR spectroscopy reveals frontal and thalamic metabolic abnormalities in schizophrenia. NeuroImage. 2007;35(2):729–737. doi: 10.1016/j.neuroimage.2006.12.023. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory. Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Stanley JA, Williamson PC, Drost DJ, Carr TJ, Rylett RJ, Malla A, Thompson RT. An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry. 1995;52(5):399–406. doi: 10.1001/archpsyc.1995.03950170073010. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A, Van Hecke A, Van Huffel P, Graveron-Demilly S, van Ormondt D, de Beer R. MRUI: a graphical user interface for accurate routine MRS data analysis; 13th Annual Meeting of ESMRMB; Prague. 1996. p. 318. [Google Scholar]
- Volz HP, Hubner G, Rzanny R, Rossger G, Preussler B, Eichhorn M, Kreitschmann-Andermahr I, Kaiser WA, Sauer H. High-energy phosphates in the frontal lobe correlate with Wisconsin Card Sort Test performance in controls, not in schizophrenics: a 31phosphorus magnetic resonance spectroscopic and neuropsychological investigation. Schizophr Res. 1998;31(1):37–47. doi: 10.1016/s0920-9964(97)00157-6. [DOI] [PubMed] [Google Scholar]
- Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15(7):2011–2035. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SY, Yeon S, Choi CH, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS. Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res. 2009;111(1–3):86–93. doi: 10.1016/j.schres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Du F, Ravichandran C, Goldbach JR, Thida T, Lin P, Dora B, Gelda J, O’Connor L, Sehovic S, Gruber S, Ongur D, Cohen BM. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol Psychiatry. 2015a;20(9):1079–1084. doi: 10.1038/mp.2015.13. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Tegin C, O’Connor L, Du F, Ahat E, Cohen BM, Ongur D. Phosphorus magnetic resonance spectroscopy studies in schizophrenia. J Psychiatr Res. 2015b;68:157–166. doi: 10.1016/j.jpsychires.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A. 2015;112(9):2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


