Abstract
Objective
We have previously shown that exogenous administration of the nuclear protein High Mobility Group Box 1 (HMGB1) improves angiogenesis following tissue ischemia. Antagonizing HMGB1 prolongs muscle necrosis and deters regeneration. In this study, we evaluated HMGB1 expression in peripheral arterial disease (PAD), and the mechanisms that promote its release in a murine model of hindlimb ischemia Specifically, we investigated how chloroquine (CQ), a commonly employed disease modifying anti-rheumatic drug promotes HMGB1 release from muscle. We hypothesized that CQ could increase HMGB1 locally and systemically, allowing it to mediate recovery from ischemic injury.
Methods
Muscle biopsies were performed on patients undergoing lower extremity surgery for non-PAD related disease as well as for claudication and critical limb ischemia (CLI). Clinical symptoms and ankle brachial indices (ABIs) were recorded for each patient. HMGB1 was detected in muscle sections using immunohistochemical staining. Unilateral femoral artery ligation (FAL) was performed on both wildtype and inducible HMGB1 knockout mice (iHMGBKO). Wild-type mice were administered intraperitoneal CQ two weeks before and after FAL. Laser Doppler perfusion imaging was used to determine perfusion recovery (LDPI). Serum and tissue levels of HMGB1 were measured at designated time points. In vitro, cultured C2C12 myoblasts were treated with increasing doses of CQ. HMGB1, autophagosome formation, p62/SQSTM1 accumulation, caspase-1 expression and activity and LDH levels were measured in supernatants and cell lysates.
Results
Nuclear expression of HMGB1 was prominent in patients with claudication and CLI (P<.05) compared to controls. CQ treated mice had elevated serum HMGB1 and diffuse HMGB1 staining in muscle (P<.01). In wild-type mice, CQ treatment resulted in higher LDPI ratios in the ischemic limb at 7 days (P<.03), and less fat replacement after two weeks (P<.03). In cultured myoblasts, chloroquine induced autophagosome accumulation, inhibited p62/SQSTM-1 degradation, and activated caspase-1.
Conclusion
HMGB1 is prominently expressed in PAD muscle, but mostly confined to the nucleus. Our in vivo data suggests that HMGB1 mobilization into the sarcoplasm and serum can be increased with chloroquine, possibly through caspase-1 mediated pathways. While HMGB1 can be released by many cell types, these studies suggest that the muscle may be an important additional source that is relevant in PAD.
Introduction
Peripheral arterial disease (PAD) represents an advanced stage of atherosclerosis that predicts cardiovascular events and can result in critical limb ischemia (CLI). In the U.S., PAD has historically been shown to affect approximately 5 million adults over the age of 40, 1 with a prevalence of more than 20% of patients over the age of 70. 2 In a recent analysis of insured patients, the annual incidence of PAD was just over 2%, with a prevalence of nearly 11% in the cohort. 3 In patients who are not candidates for revascularization either with open surgical or endovascular therapies, limb loss is a significant risk.1 While multiple studies highlight the etiology of atherosclerotic occlusive disease leading to PAD, fewer investigations have determined how the biochemical changes associated with chronic muscle ischemia drive tissue regeneration and recovery in PAD. Proangiogenic agents injected directly into ischemic muscle have had mixed results.4–6 Injection of bone marrow derived tissue repair cells may show benefit, but is arduous.7 Therefore, novel, nonsurgical methods of improving muscle regeneration and reperfusion in PAD are still an active area of investigation. 8 Modulation of danger signaling may classify as one such method.
We have previously shown that the damage associated molecular pattern (DAMP) high mobility group box 1 protein (HMGB1) promotes recovery from muscle ischemia. 9, 10 Specifically, HMGB1 is required for endothelial cell tube formation in vitro, and increases vascular density in ischemic skeletal muscle.9 Anti-HMGB1 antibody prolongs necrosis after femoral artery ligation. 10 Others have also demonstrated a potent, pro-angiogenic, and pro-regenerative role for HMGB1 in both ischemic injury and in other muscle pathologies. 11, 12 Therefore, investigating ways in which HMGB1 can be made available in a controlled fashion to improve local regenerative effects may be a novel way to treat recalcitrant PAD.
While we have focused on understanding how HMGB1 affects the cellular components of muscle recovery and angiogenesis, the mechanisms by which HMGB1 is released from the ischemic muscle remains to be determined. It is well known that inflammatory cells can actively secrete HMGB1 and that necrotic cells can passively release it due to cell membrane disruption.13 When released from necrotic cells, HMGB1 accompanies a potential host of other “damage associated” proteins that can lead to a pronounced, uncontrolled inflammatory response. However, recent evidence suggests that HMGB1 release, even from dying cells, can be an active, controlled event, typically involving macroautophagy (herein referred to as autophagy) and/or intracellular inflammatory pathways. 14 Autophagy describes the lysosomal mediated consumption of cytoplasmic constituents to provide the cell with energy during stress. It also represents a “housekeeping” activity that helps to maintain homeostasis within a number of organ systems such as muscle. 15 Autophagy is known to be linked to inflammatory pathways including inflammasome signaling. The inflammasome consists of intracellular pattern recognition receptors that oligomerize to activate caspase-1, thereby releasing members of the interleukin family. Inflammasome signaling also can facilitate HMGB1 release in some cell types.16,17 A number of studies report local effects of HMGB1 on vascular and muscle stem cells including proliferation, angiogenesis and stem cell recruitment. 18, 19 Thus, the controlled release of HMGB1 through inflammasome or autophagic modulation may be a mechanism to regulate recovery from ischemic injury.
Chloroquine (CQ) is a weak base that prevents acidification of lysosomes and inhibits the final stages of autophagic flux. It has been shown to modulate HMGB1 in a murine sepsis model although similar effects in muscle have not been documented.20 In addition to blocking autophagy, CQ has also been shown to “normalize” tumor vasculature and improve chemotherapeutic delivery in cancer.21 It is known that tumor vasculature is abnormally organized, leading to persistent hypoxia. 22 Recently, Maes et al demonstrated a vessel-organizing and strengthening effect of CQ in melanoma, which resulted in less metastasis and cancer invasion.21 While CQ has never been assessed in the management of peripheral vascular disease, its ability to improve the organization of neo-angiogenic vessels and modulate HMGB1 may render it a potential adjunct in the management of arterial insufficiency. In this study, we hypothesized that CQ is protective in the setting of muscle ischemia by increasing local and systemic HMGB1, rendering it available for regenerative effects.
Materials and Methods
Reagents
The reagents used for these experiments were as follows; chloroquine (Sigma-Aldrich; St. Louis, MO) HMGB1 ELISA (Shino-test Corporation, Japan), primary antibodies to HMGB1, CD31, CD45 and β-actin as well as Cy-3 conjugated secondary antibodies (Abcam, Cambridge, MA). Primary antibodies were used at 1:250 or 1:1000 for immunohistochemistry (IHC) or western blot (WB), respectively. Cy-3 conjugated secondary antibody was used at 1:500 or 1:1000 for IHC. Caspase-1 antibody (R&D Systems, Minneapolis, MN; 1:1000) LC3 antibody (Cell Signaling, Danvers, MA; 1:1000), p62/SQSTM-1 antibody (EMD Millipore, Billerica, MA, 1:1000) were used for WB on cell lysates. Recombinant HMGB1 (rHMGB1) was isolated from yeast using a modification of the vector YEpFLAG as described,23 and was used at 1μg/ml in cell culture.9 LDH (ThermoFisher, Waltham, MA) and caspase-1 activity assays (Enzo, Farmingdale, NY) were used according to the manufacturers’ instruction on cell supernatants and lysates, respectively.
Human muscle biopsies
Under the auspices of an existing IRB at the University of Nebraska, patients undergoing lower extremity procedures for either PAD or non-PAD related pathology were approached for consent for gastrocnemius muscle biopsy under anesthesia. The diagnosis of PAD was made based on medical history, physical examination, standard and/or Computerized Tomographic Angiography (CTA), vascular duplex evaluation and ankle brachial index (ABI). Patients were divided into claudicants or critical limb ischemia (CLI) based on clinical history. Control patients were those undergoing procedures for non-PAD related vascular diseases such as abdominal aortic or popliteal artery aneurysms or varicose veins. Gastrocnemius muscle was harvested as described. 24 Briefly, samples were obtained with a 6mm Bergstrom needle after the initiation of adequate anesthesia. The samples were placed into cold methacarn fixative, transferred to ethanol, and paraffin embedded for histology. Four micrometer serial sections through the biopsy were made, and sections were stained using primary antibodies to HMGB1. DAPI was included in the secondary antibody cocktail to identify nuclei. Images were captured using an Olympus Fluoview FV1000 confocal microscope configured on an Olympus IX-81 inverted microscope and Olympus imaging system. Sections incubated only with secondary antibody were used to determine background staining. All images were acquired with the same exposure time for HMGB1 by an investigator who was blinded to the identity of the samples. Four to five images per section were color-separated using Image J (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2016). Image J analysis software was also used to determine the integrated density of HMGB1 staining in a fixed area of the muscle.
Animals
C57BL/6J male mice were purchased from The Jackson Laboratories (Bar Harbor, ME) and used at 10–12 weeks of age. iHMGBKO mice were generated from HMGB1flox/flox mice as previously described.25 In the floxed mice, exons 2 and 3 of the HMGB1 gene were flanked by two lox/p sites that enabled the recombination of the HMGB1 loci in the presence of Cre- recombinase. Tamoxifen (4-OHT: 4-hydroxytamoxifen)-inducible HMGB1 knockout mice (iHMGB1 KO mice) were generated by crossing HMGB1flox/flox mice and mice expressing tamoxifen-inducible Cre- recombinase under the control of the CAG promoter (Cag-Cre-ER). Mice carrying Cag-Cre-ER and floxed HMGB1 genes were treated with tamoxifen for 2 consecutive days at a dose of 120 mg/kg followed by at least 2 more days to allow sufficient recombination prior to their use in studies. iHMGB1KO mice were used at 10 weeks of age.
Femoral artery ligation (FAL)
Unilateral FAL was performed on the right and exposure of the vessels without ligation was performed on the left hindlimb as described. 9 Specifically, on the right hindlimb, the femoral artery and distal external iliac artery are exposed. The proximal femoral artery and vein are isolated and ligated, with preservation of the nerve. We have utilized this procedure over ligation and excision in order to minimize dissection that could potentially affect histologic analysis of the tibialis anterior muscle. In the literature, the C57BL/6J strain tolerates arterial ligation of this nature very well. Digital necrosis is not a salient feature, and muscle regeneration and angiogenesis are robust.26, 27 All surgical procedures were performed after intraperitoneal injection of Nembutal, followed by inhalational, continuous anesthesia. All procedures conformed to the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (available at http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf) and the policies of the Institutional Animal Use and Care Committee of the University of Pittsburgh (Approved - protocol #13021224). At designated time points both serum and tibialis anterior (TA) muscle were harvested at the time of euthanasia. Blood was obtained through cardiac puncture and muscle was snap frozen in cold isobutane for IHC and standard histology. IHC for HMGB1, CD31 (endothelial cell marker) and CD45 (leukocyte marker) were performed as described for the human samples except that muscle sections were 8μm thick. Standard hematoxylin and eosin (H&E) was performed to assess muscle architecture and fat replacement. Animals were treated with intraperitoneal (IP) injection of reagent grade chloroquine (50mg/mouse) or control PBS buffer every other day for two weeks before and after FAL. CQ is known to have delayed onset of effects which is why pretreatment was performed. 21 The dose of CQ was chosen based on literature describing its adjunctive use in cancer models and clinical trials. While some have proposed giving CQ in drinking water, we administered CQ intraperitoneally to better regulate the dose.28 Each dose was administered after exposing mice to inhalational isofluorane for their comfort. In order to minimize the risk of death from overexposure to isofluorane, doses were administered every other day. CQ and its derivatives have a slow onset of action and a long-half-life which was the rationale for every other day dosing.29
Laser Doppler Perfusion Imaging
LDPI (PeriScan PIM III system, Perimed AB, Stockholm, Sweden) was performed under inhalational anesthesia to normalize hemodynamics at the time of each study. Animals were placed on a heating pad to keep them warm, and to normalize the temperature from animal to animal. Three images were obtained at each time point to obtain an average, and the results were expressed as the ratio of the ischemic to nonischemic hindlimbs.
Cell culture
C2C12 myoblasts are derived from Mus Musculus, and were purchased through the American Type Culture collection (ATCC; Manassas, VA: # CRL-1772). Cells were maintained in growth medium containing DMEM and 10% fetal bovine serum. Experiments were performed with 2% horse serum (HS) which induces differentiation and fusion. 30 Incorporation of the radionucleotide was determined using scintigraphy.9 To determine HMGB1 release from cultured myoblasts, cells were first seeded into 6-well plates and treated with indicated doses of CQ. Supernatants were removed after 18 hours and centrifuged to remove cellular debris. ELISA was performed to determine HMGB1 concentration on cell culture supernatants as per the manufacturer’s instructions. Western blots were also performed on concentrated supernatants to confirm HMGB1 release. Cells were subsequently lysed in the presence of protease inhibitors and lysates were separated on SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes which were blocked and immunoblotted for designated antigens. A semi-quantitative expression of each protein as a fraction of beta-actin was performed using the gel-analysis program on Image J.
Statistical Analysis
All data is expressed as mean plus standard error of the mean (SEM). Analysis of variance (ANOVA) was used to compare multiple means. For comparisons between two groups, Student’s t-test was used to assess the means of continuous variables P<0.05. was used to indicate statistical significance.
Results
Expression of nuclear HMGB1 is increased in clinical PAD
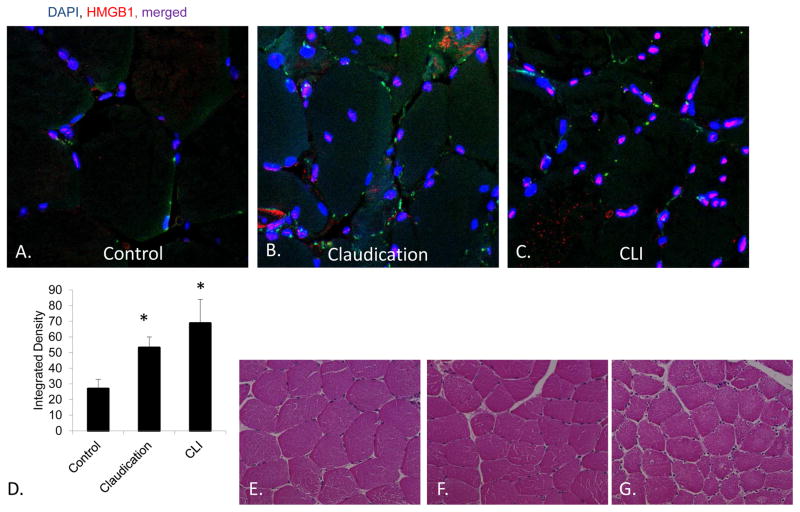
In models of hepatic ischemia re-perfusion injury, sepsis and shock, HMGB1 has been demonstrated to be a potential clinical target to attenuate disease. 31 However, the same has not been demonstrated for PAD. In order to determine whether HMGB1 is a) present in human skeletal muscle and b) has a modified expression in the setting of pathologic ischemia, we assessed its expression in PAD patients. All patients were men except for one female in the claudication group. All but two patients were Caucasian. The mean age was 64.8 ± 5.8 (controls; N=5), 66.5 ± 6.9 (claudicants; N=6) and 68.8 ± 7.8 (CLI; N=6). The ABIs for control patients, claudicants and patients with CLI were 1.05 ± 0.02, 0.50 ± 0.06, 0.24 ± 0.07, respectively. ABIs for patients with critical limb ischemia were significantly lower than control patients (P<.001 for claudicants and CLI patients, N=5–6 patients/group). HMGB1 staining was significantly elevated in patients with both claudication and CLI (Figure 1) and was predominantly nuclear.
Figure 1. HMGB1 expression in peripheral arterial disease (PAD).
HMGB1 staining in human skeletal muscle was performed on serial muscle sections obtained from patients A) without PAD or with chronic limb ischemia in the form of B) claudication or C) critical limb ischemia (CLI). Images were obtained using an Olympus FV1000 scanning confocal microscope with a 40X objective. D) Quantification of HMGB1 integrated density was performed using Image J analysis software on color separated images obtained from human samples *P<.03, N=5–7 patients/group, ANOVA. Standard hematoxylin and eosin staining was performed to assess the general architecture of muscles from E) control, F) claudication and G) CLI patients. Scale bar = 50μm
CQ administration improves global recovery from ischemia by increasing HMGB1 in muscle and serum
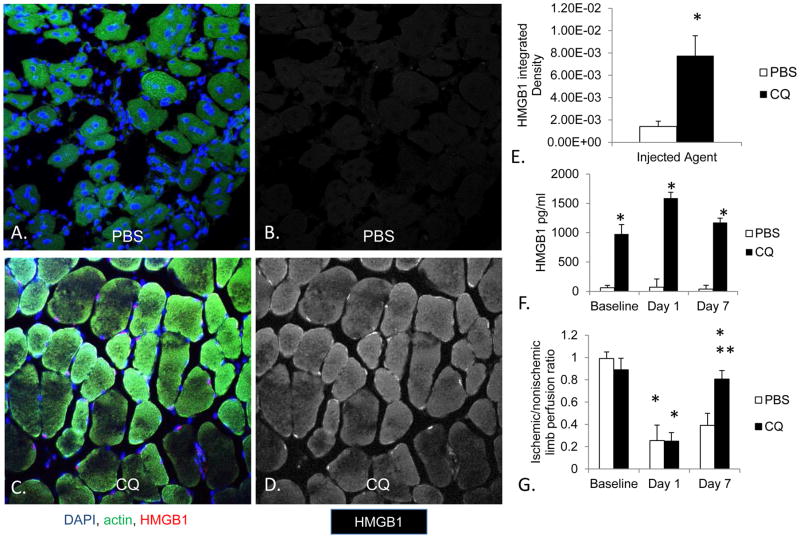
CQ is known to affect HMGB1 levels in other clinical syndromes such as sepsis. 20 We thus hypothesized that CQ would affect HMGB1 expression in muscle ischemia. To confirm that the treatment did not result in baseline differences in perfusion to the hindlimbs, LDPI was performed the day before surgery and 1 and 7 days after arterial ligation. LDPI demonstrated no baseline differences in perfusion to either hindlimb as a function of extended CQ treatment. Similarly, one day after FAL, both groups of animals were rendered ischemic in one limb. Seven days following FAL, mice treated with CQ demonstrated a higher perfusion ratio in ischemic to nonischemic limbs compared to control mice (Figure 2). This finding was associated with significantly more diffuse HMGB1 expression within the TA. HMGB1 was present in higher concentrations within the serum in all CQ treated mice (Figure 2).
Figure 2. CQ effects on muscle and serum expression of HMGB1.
Anterior tibialis muscle from mice pretreated for two weeks with intraperitoneal phosphate buffered saline (PBS) or chloroquine (CQ) before and 7 days after femoral artery ligation (FAL) is shown. The HMGB1 staining and quantification were performed on muscle sections from mice sacrificed 7 days after arterial ligation. Sections were stained for HMGB1 using primary and Cy-3 conjugated secondary antibody (red). Muscle actin (green) and DAPI (blue) are also shown to highlight the fibers and nuclei, respectively. Images were obtained using an Olympus FV1000 scanning confocal microscope with a 40X objective. Merged images (A and C) as well as color-separated images that represent HMGB1 staining (B and D) are shown. Arrows represent areas of HMGB1 staining in both nuclear and sarcoplasmic areas. E) Quantification of HMGB1 integrated density was calculated using Image J analysis using color-separated images. *P< .01, N=4–6 mice/group, t-test. F) Serum was obtained from cardiac puncture at the time of sacrifice before or 1 and 7 days following femoral artery ligation. ELISA was performed on serum samples to assess HMGB1 concentration. *P<.01 CQ to PBS, N= 5–7mice/group, t-test at each time point. G) LDPI was performed to assess perfusion ratios in ischemic/nonischemic limbs following FAL in mice treated with PBS or CQ before and after FAL. *P<0.01 day 1 to baseline and day 7 to day 1,** p<0.03 day 7 CQ to day 7 PBS; N=4–6 mice/group, t-test comparing PBS and CQ. Scale bar = 50μm
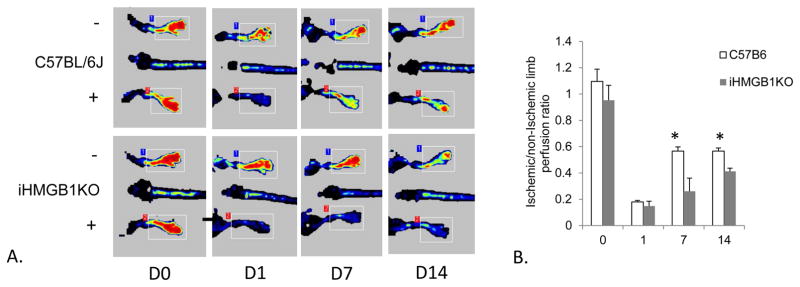
Because CQ treatment was associated with high levels of HMGB1 in the muscle and serum, as well as a higher recovery of perfusion to the ischemic limb, we hypothesized that the beneficial effects of CQ were mediated through HMGB1. We have previously demonstrated that mice treated with anti-HMGB1 antibody have a poor recovery to muscle ischemia,10 but had not evaluated the effects of genetically eradicating HMGB1. While global HMGB1 knockout (KO) mice typically die in utero, inducible HMGB1KO (iHMGB1KO) mice are viable. We therefore performed FAL in iHMGB1KO and wild-type mice to assess the effect on perfusion recovery. Baseline and day one perfusion ratios were similar between control and iHMGB1KO mice. However, the iHMGB1KO mice had significantly attenuated perfusion recovery both 7 and 14 days after surgery, supporting our hypothesis that HMGB1 may be a critical mediator of perfusion recovery (Figure 3).
Figure 3. Perfusion recovery in HMGB1KO mice.
A) Laser Doppler perfusion imaging (LDPI) is shown from wild type C57Bl/6J and inducible HMGB1 knockout (iHMGB1KO) mice before, and 1, 7 and 14 days following femoral artery ligation (FAL). (+) indicates the ligated limb. (-) indicates the nonligated limb with uninterrupted perfusion. B) The mean ischemic/nonischemic perfusion ratio was compared between groups at each time point using t-test. *P<.01, N=3–4 mice/group, t-test iHMGBKO to C57Bl/6J
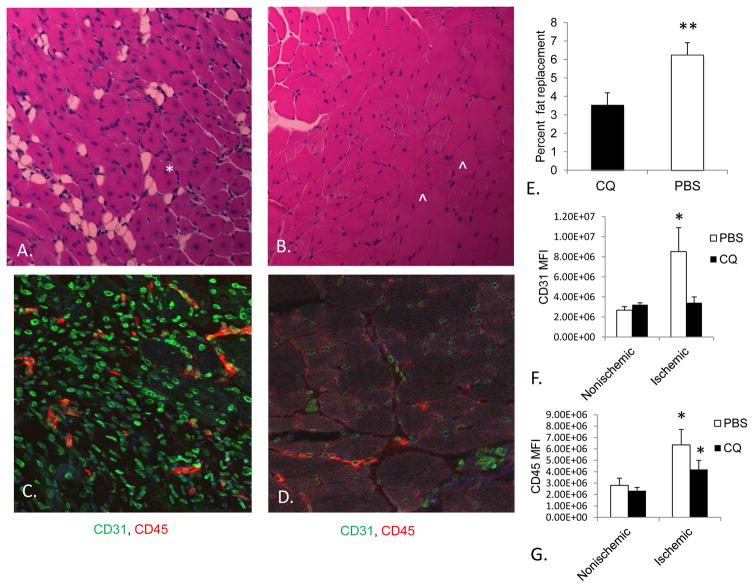
Mice treated with CQ were also evaluated two weeks after ischemic injury to assess muscle regeneration, inflammatory infiltration and vascular density. In previous time course experiments, we have determined that maximal injury is seen 7 days after injury, while recovery is underway by 14 days. We hypothesized that the ischemic muscle from the CQ treated mice would show less evidence of ischemic damage. It is known that CQ has “organizing” effects on angiogenic vessels resulting in less vascular density, but more ordered appearance. 21 Furthermore, in cancer models, mice treated with CQ demonstrate a higher expression of pericytes on angiogenic vessels, which is a sign of maturation and stability. 21 Two weeks following FAL, TA muscle from CQ-treated mice had less fat replacement (Figure 4), and less actively regenerating myocytes in favor of mature myocytes. As expected, CD31 staining in CQ treated mice was less, but more organized than in PBS treated mice. In both groups, inflammatory cell infiltrate was more prominent in the ischemic limb than in the non-ischemic limb, but not significantly different from each other (Figure 4). These results suggested that the improvement in histology were not solely based on anti-inflammatory effects of CQ.
Figure 4. Histologic findings following arterial ligation with and without chloroquine (CQ).
Hematoxylin and eosin (H&E) staining demonstrates fat replacement (arrow) regenerating muscle (asterisk) and mature muscle (arrowhead) in PBS (A) and CQ (B) treated mice after 14 days. Regenerating myocytes are identified by their circular shape and centrally located nuclei. Mature myocytes are identified by peripheral nuclei. Fat is identified by rounded, septate and clear spaces. CD31 (green), and CD45 (red) antigen detection was determined using immunohistochemistry, and imaged on an Olympus FV1000 confocal microscope. Mean Fluorescence Intensity (MFI) was determined for each section for the right and left hindlimbs. The right, ischemic hindlimbs are shown for PBS (C) and CQ (D) treated mice. E) Quantification of fat replacement between PBS and CQ treated groups was calculated by measuring the area of fat and expressing it as a percentage of the total muscle area. Four-six images per animal were used to determine percent fat replacement. **P<.03; N=5–6 mice/group, t-test. Mature myocytes were not quantified but predominated in CQ treated mice. F) Quantification of CD31 Mean Fluorescent Intensity (MFI) *P<0.03, PBS vs. CQ, N=4–6 amice/group, t-test. G) Quantification of CD45 MFI *P<0.03, ischemic vs. nonischemic, N=4–6 mice/group, t-test. Scale bar = 25μm
CQ prevents autophagy-mediated degradation of p62/SQSTM1, activates caspase-1 and initiates the release of HMGB1 in myoblasts
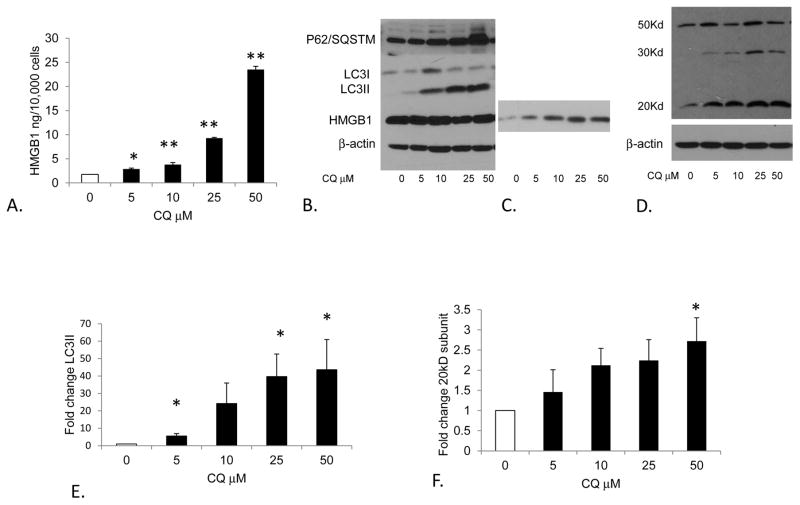
Our results in vivo suggested that treatment with CQ increased the expression and release of HMGB1 from ischemic muscle. To determine if CQ mediated HMGB1 release from muscle cells through inflammasome activation, we treated C2C12 murine myoblasts with increasing doses of CQ and quantified HMGB1 release. HMGB1 was significantly higher in the supernatant of CQ treated cells by both ELISA and western blot (Figure 5). Cell lysates were similarly probed for HMGB1, LC3II (to assess accumulation of autophagosomes), and p62/SQSTM1 which is a ubiquitin-binding scaffold protein that is degraded by autophagy.32 We hypothesized that if autophagy is impaired due to CQ-mediated lysosomal dysfunction, LC3II accumulation would occur due to the inability of autophagosomes to fuse with lysosomes. Similarly, p62/SQSTM1 would accumulate as well. Expectedly, CQ promoted a dose-dependent increase in both LC3II and p62/SQSTM1 in myoblasts. Furthermore, the expression of activated caspase-1 (20kD subunit) was also increased (Figure 5).
Figure 5. Effects of chloroquine (CQ) on cultured C2C12 myoblasts.
A) ELISA was used to determine HMGB1 concentration in the supernatant of CQ treated C2C12 myoblasts. *P<.05, **p<.005 compared to no CQ; N=3 experiments performed in triplicate, t-test. B) Western blot of C2C12 myoblast lysates (B) and supernatants (C) after exposure to CQ at designated concentration for 18 hours. Lysates were assessed for P62/SQSTM1, LC3I-II conversion, HMGB1 and actin expression. Supernatants were concentrated and evaluated for HMGB1 expression. D) WB showing procaspase-1 and activated caspase-1 (20Kd subunit) from C2C12 myoblasts treated with CQ at designated concentration. E) LC3II and F) activated caspase-1 expression normalized to β-actin in response to CQ is shown relative to untreated cells. *P<.05; N=3 experiments performed in triplicate, t-test.
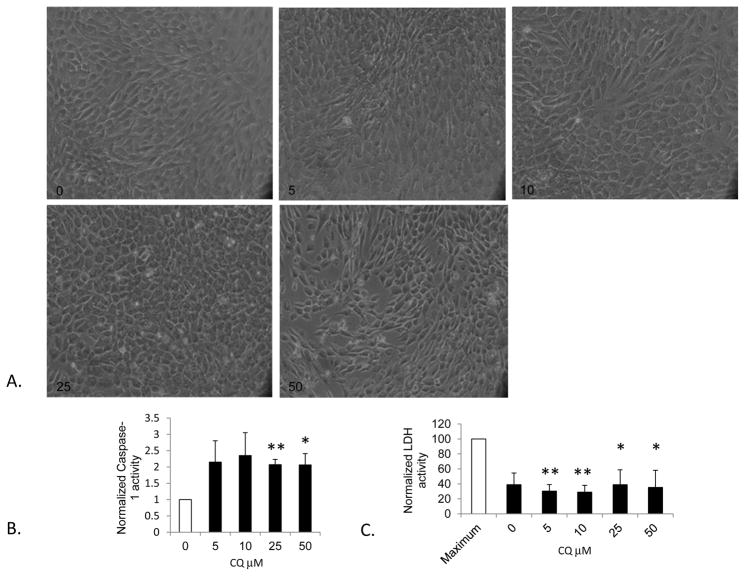
The presence of activated caspase-1 in cultured myoblasts cells suggests that muscle may release HMGB1 through activation of inflammasomes. CQ also increased caspase-1 activity (Figure 6). One potential reason that HMGB1 is released into the supernatant would be cell death. To further investigate this potential, we performed an LDH assay on cell supernatants. This assay demonstrated no increase in LDH activity in CQ treated cells to suggest cell excessive death, which was confirmed by images obtained of the cells taken at each dose. (Figure 6).
Figure 6. Caspase-1 and LDH activity in cultured C2C12 myoblasts exposed to chloroquine (CQ).
A) Images depicting C2C12 myoblast morphology in culture after exposure to CQ for 18 hours showing maintenance of cell numbers up to 25 μM. B) Caspase-1 activity assays were performed on CQ treated cell lysates and expressed as a ratio to untreated (0) samples. *P<.03, **P<.005; N=4 experiments, t-test to no treatment C) Lactate dehydrogenase (LDH) assays to assess cell damage was performed on supernatants and expressed as a function of maximum lysis and cell leakage which is a positive control. Numbers indicate CQ concentration. *P<.05; **P<.01, N=3 experiments performed in triplicate, t-test to maximally lysed samples.
Discussion
CQ is an antimalarial drug with a well-described safety profile. While it has been studied extensively in infectious disease, newer applications have included an adjunctive role in cancer therapy. 33 A common derivative of CQ, hydrochloroquine (HC) is used extensively in rheumatic disorders.34 HC has a specific role in cutaneous lupus erythematosus (CLE) which is characterized by skin ulcers. The dermatologic literature suggests that HC as well as CQ can promote wound healing in CLE. 35 Long term use of HC is associated with ophthalmologic disease, and patients should be monitored for ocular complications. 36 The mechanism by which CQ and its derivatives promote skin ulcer healing is not clear, but modulation of inflammation is likely a critical aspect. CQ has not been investigated in PAD, in which wound healing is an important aspect. In this study, we investigated not only a potential role for CQ in a murine model of muscle ischemia, but also put forth a mechanism of action that involves the danger signal HMGB1. We have also made the novel finding that HMGB1 is prominently expressed in patients with PAD, and thus may be controlled pharmacologically with a drug like CQ.
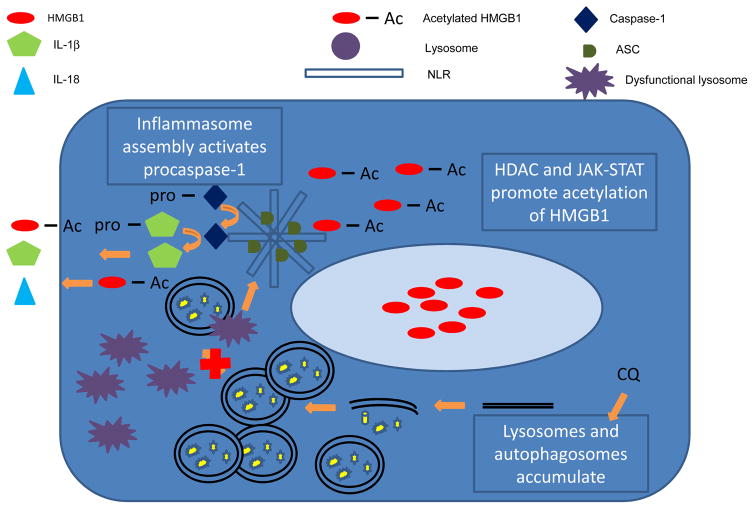
Our data suggests a link between autophagy, inflammasome activation and HMGB1 release which has not been described in muscle (Figure 7). Specifically, degradation of autophagolysosomes is a negative regulator of inflammasome activation. Thus, accumulation of autophagic products in cells would be expected to initiate inflammasome activation and caspase-1 mediated activity. 37 By disrupting lysosomal acidification, CQ promotes intracellular autophagosome accumulation in cells and inflammasome signaling. Inflammasomes are an oligomer of cytosolic pattern recognition receptors such as Nod-like receptors (NLR) that initiates the cleavage of procaspase-1 to activated caspase-1 subunits. Activated caspase-1 can initiate the release of inflammatory cytokines as well as HMGB1 from macrophages and certain parenchymal cells. 37 Lysosomal dysfunction can activate inflammasome-mediated signaling 37 indicating a role for CQ in inflammasome activation.
Figure 7. Mechanisms linking autophagy, HMGB1 and inflammasome signaling.
A proposed mechanism linking autophagy, inflammasome activation and HMGB1 release from myoblasts is shown. It is known that HMGB1 requires acetylation to leave the nucleus, which can be facilitated by histone deacetylases and JAK-STAT signaling. It is also known that in other cell types, inflammasomes activate caspase-1 and initiates release of HMGB1 as well as IL1-β and IL-18. Lysosomal disruption with agents like CQ may initiate inflammasome activation, and promote the release of HMGB1 from myoblasts. This is a novel area of investigation in PAD.
While inflammasomes have not been extensively studied in muscle, recent studies suggest that their activity may play a role in the etiology of certain muscle diseases. Specifically, dysferlin-deficient mice were found to upregulate inflammasome-mediated pathways including NLR in myoblasts, potentially contributing to the development of certain muscular dystrophies. 38 Autophagy in muscle is also a relatively new area of investigation, and is known to be important in homeostasis as well as some disease states. 39
In our studies, CQ treatment resulted in accumulation of LC3II and p62/SQSTM1 indicating inhibition of autophagy. CQ also promoted activated caspase-1 expression and activity, and release of HMGB1 from cultured myoblasts. In vivo, the protective effect of CQ was linked to mobilization of HMGB1 in tissue, suggesting that inflammasome mediated activity may occur vivo. In cancer, release of HMGB1 by dying cells is regulated by autophagy, modulated by chemotherapeutics and potentially beneficial due to its stimulation of the immune system. 40 Similar mechanisms have not been investigated in muscle, but may prove to be an important adjunct in the management of PAD. Our studies would suggest that in the setting of ischemia, disrupting autophagy with chloroquine during injury may promote inflammasome mediated HMGB1 release in a regulated rather than chaotic fashion. Regulated mobilization of nuclear HMGB1 into the extracellular spaces may be a way to promote better recovery from ischemic injury. Additionally, CQ may encourage more efficient perfusion as suggested by our in vivo LDPI data, although these results were notably transient. While it is likely that CQ effects are multifactorial, its regulation of HMGB1 expression is likely to be critical to its mechanism of action. Clinically, adding either CQ or HC to a medical regimen for patients with PAD may help improve recovery for patients who have failed other means of revascularization. In CLE, CQ was administered at a dose of 250mg/day. 35 Clearly, dosing and benefit in PAD would need to be experimentally determined.
Our study has some important weaknesses. While our clinical data is novel and suggests a high expression of HMGB1 in PAD muscle, our numbers are small. We will continue our efforts to acquire more tissue and expand our analysis to detect activated caspase-1 and inflammasome components in the muscle. Also, we showed that neovascularization in muscle tissue from CQ treated animals were better organized. We do not know if this occurred because the arteriogenic response was improved by CQ. This is a focus for future studies. Another important weakness is a relative lack of data demonstrating a strong link between HMGB1 and CQ benefits in vivo. Currently, we are evaluating how CQ administration affects recovery from FAL in iHMGB1KO mice. We would expect that CQ would not have a beneficial role in iHMGBKO if the CQ’s effects are mediated through HMGB1. A muscle specific knockout for HMGB1 is not yet available, but its creation remains an important goal for our investigative team. Finally, while we have determined that CQ appears to mobilize HMGB1 in tissue and in serum, it is not clear whether the effects on FAL are mediated at a tissue or systemic level. A tissue specific HMGB1KO mouse would help make this differentiation.
In conclusion, we demonstrate that CQ, a well known anti-rheumatic may have a novel use in muscle ischemia. Specifically, it may modulate autophagic processes that allow for regulated HMGB1 release, and improved histologic recovery. Critical evaluation of disease states in which CQ is currently used may provide further support for testing CQ in PAD.
Significance.
The nuclear protein High Mobility Group Box 1 (HMGB1)may be modulated to improve recovery from ischemic muscle injury. We investigate HMGB1 expression in PAD in mice, and ways in which HMGB1 expression can be increased by chloroquine.
Type of Research.
Prospective experimental study
Take Home Message.
Chloroquine pretreatment caused improved blood flow recovery and less skeletal muscle damage after hind limb ischemia in mice from increased HMGB1 release and potentially decreased autophagy.
Recommendation.
The authors suggest that chloroquine may decrease skeletal muscle damage from ischemia by increasing release of HMGB1, and may be a potential adjunctive treatment for peripheral arterial disease.
Strength of Recommendation.
Weak
Level of Evidence.
Low to very low
Clinical Relevance Paragraph.
This paper evaluates the expression of High Mobility Group Box-1 (HMGB1) in peripheral arterial disease (PAD). It further evaluates how HMGB1 expression can be increased by chloroquine, a commonly prescribed disease modifying anti-rheumatic drug that improves blood flow in animal models of ischemia. We and others have shown that HMGB1 has strong pro-regenerative effects in muscle, and may be a novel therapeutic target for patients with non-reconstructable PAD. Our study suggests that chloroquine administration may be a promising avenue for patients with severe PAD.
Acknowledgments
Funding: Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) under Award Number K08HL103899 (US), The SVS/ACS Foundation grant (US), the Department of Veterans Affairs, Veterans Health Administration, Office of Biomedical Laboratory Research and Development (ET), NIH T32 HL098036 (JX), and NIH grant R01AG034995 (IP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 2.Pavlovic S, Wiley E, Guzman G, Morris D, Braniecki M. Marjolin ulcer: an overlooked entity. International Wound Journal. 2011;8:419–424. doi: 10.1111/j.1742-481X.2011.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. Journal of Vascular Surgery. 2014;60:686–695. e2. doi: 10.1016/j.jvs.2014.03.290. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional Angiogenesis With Vascular Endothelial Growth Factor in Peripheral Arterial Disease: A Phase II Randomized, Double-Blind, Controlled Study of Adenoviral Delivery of Vascular Endothelial Growth Factor 121 in Patients With Disabling Intermittent Claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- 5.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, Nakagawa K, Hou X, Nagai Y, Hasegawa M, Sugimachi K, Sueishi K. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ Res. 2002;90:966–73. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 6.Powell RJ, Dormandy J, Simons M, Morishita R, Annex BH. Therapeutic angiogenesis for critical limb ischemia: design of the hepatocyte growth factor therapeutic angiogenesis clinical trial. Vasc Med. 2004;9:193–8. doi: 10.1191/1358863x04vm557oa. [DOI] [PubMed] [Google Scholar]
- 7.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Journal of Vascular Surgery. 2009;50:1378–1390. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 8.Eton D, Yu H. Enhanced cell therapy strategy to treat chronic limb-threatening ischemia. Journal of Vascular Surgery. 2010;52:199–204. doi: 10.1016/j.jvs.2009.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Sachdev U, Cui X, Hong G, Namkoong S, Karlsson JM, Baty CJ, Tzeng E. High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. Journal of Vascular Surgery. 55:180–191. doi: 10.1016/j.jvs.2011.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sachdev U, Cui X, Tzeng E. HMGB1 and TLR4 mediate skeletal muscle recovery in a murine model of hindlimb ischemia. Journal of Vascular Surgery. 2013;58:460–469. doi: 10.1016/j.jvs.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Mori R, Straino S, Di Carlo A, Mangoni A, Pompilio G, Palumbo R, Bianchi ME, Capogrossi MC, Germani A. Multiple effects of high mobility group box protein 1 in skeletal muscle regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2377–83. doi: 10.1161/ATVBAHA.107.153429. [DOI] [PubMed] [Google Scholar]
- 12.Riuzzi F, Sorci G, Donato R. The Amphoterin (HMGB1)/Receptor for Advanced Glycation End Products (RAGE) Pair Modulates Myoblast Proliferation, Apoptosis, Adhesiveness, Migration, and Invasiveness: FUNCTIONAL INACTIVATION OF RAGE IN L6 MYOBLASTS RESULTS IN TUMOR FORMATION IN VIVO. Journal of Biological Chemistry. 2006;281:8242–8253. doi: 10.1074/jbc.M509436200. [DOI] [PubMed] [Google Scholar]
- 13.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 14.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clave C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Droge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fesus L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, Gonzalez-Estevez C, Gorski S, Gottlieb RA, Haussinger D, He YW, Heidenreich K, Hill JA, Hoyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jaattela M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovacs AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, Lopez-Otin C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Melendez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Munz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nurnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Talloczy Z, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcategui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandri M. Autophagy in skeletal muscle. FEBS Lett. 584:1411–6. doi: 10.1016/j.febslet.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, Valdes–Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JPY, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti T-D, Dixit VM. Inflammasome-Dependent Release of the Alarmin HMGB1 in Endotoxemia. The Journal of Immunology. 2010;185:4385–4392. doi: 10.4049/jimmunol.1000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–12. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 19.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Cao L, Xie M, Yu Y, Kang R, Yang L, Zhao M, Tang D. Chloroquine Inhibits HMGB1 Inflammatory Signaling and Protects Mice from Lethal Sepsis. Biochemical pharmacology. 2013;86:410–418. doi: 10.1016/j.bcp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, Quaegebeur A, Schoors S, Georgiadou M, Wouters J, Vinckier S, Vankelecom H, Garmyn M, Vion A-C, Radtke F, Boulanger C, Gerhardt H, Dejana E, Dewerchin M, Ghesquière B, Annaert W, Agostinis P, Carmeliet P. Tumor Vessel Normalization by Chloroquine Independent of Autophagy. Cancer Cell. 2014;26:190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 22.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngamkitidechakul C, Twining SS. Buffered non-fermenter system for lab-scale production of secreted recombinant His-tagged proteins in Saccharomyces cerevisiae. Biotechniques. 2002;33:1296–300. doi: 10.2144/02336pt02. [DOI] [PubMed] [Google Scholar]
- 24.Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, Miserlis D, Johanning JM, Pipinos II. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. Journal of Translational Medicine. 2013;11:230–230. doi: 10.1186/1479-5876-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang R, Zhang Q, Hou W, Yan Z, Chen R, Bonaroti J, Bansal P, Billiar TR, Tsung A, Wang Q, Bartlett DL, Whitcomb DC, Chang EB, Zhu X, Wang H, Lu B, Tracey KJ, Cao L, Fan X-G, Lotze MT, Zeh HJ, Iii, Tang D. Intracellular Hmgb1 Inhibits Inflammatory Nucleosome Release and Limits Acute Pancreatitis in Mice. Gastroenterology. 2014;146:1097–1107. e8. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shireman PK, Quinones MP. Differential necrosis despite similar perfusion in mouse strains after ischemia. J Surg Res. 2005;129:242–50. doi: 10.1016/j.jss.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 27.McEnaney RM, Shukla A, Madigan MC, Sachdev U, Tzeng E. P2Y2 nucleotide receptor mediates arteriogenesis in a murine model of hind limb ischemia. Journal of Vascular Surgery. 2016;63:216–225. doi: 10.1016/j.jvs.2014.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boone BA, Orlichenko L, Schapiro NE, Loughran P, Gianfrate GC, Ellis JT, Singhi AD, Kang R, Tang D, Lotze MT, Zeh HJ. The Receptor for Advanced Glycation End Products (RAGE) Enhances Autophagy and Neutrophil Extracellular Traps in Pancreatic Cancer. Cancer gene therapy. 2015;22:326–334. doi: 10.1038/cgt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil K, Suneela D, Chopade S, Joshi P. Design, Synthesis and In Vitro Release Studies of Co-Drugs for Rheumatoid Arthritis. Inflammation & Allergy-Drug Targets (Discontinued) 2015;14:47–52. doi: 10.2174/1871528114666151201200157. [DOI] [PubMed] [Google Scholar]
- 30.Sachdev U, Cui X, Xu J, Xu J, Tzeng E. MyD88 and TRIF mediate divergent inflammatory and regenerative responses to skeletal muscle ischemia. Physiological reports. 2014:2. doi: 10.14814/phy2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T. Methods in Enzymology. Vol. 452. Academic Press; 2009. Chapter 12 Monitoring Autophagic Degradation of p62/SQSTM1; pp. 181–197. [DOI] [PubMed] [Google Scholar]
- 33.Yang ZJ, Chee CE, Huang S, Sinicrope FA. The Role of Autophagy in Cancer: Therapeutic Implications. Molecular Cancer Therapeutics. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahlenberg JM, Fox DA. Advances in the Medical Treatment of Rheumatoid Arthritis. Hand clinics. 2011;27:11–20. doi: 10.1016/j.hcl.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang AY, Piette EW, Foering KP, Tenhave TR, Okawa J, Werth VP. Response to Antimalarials in Cutaneous Lupus Erythematosus A Prospective Analysis. Archives of Dermatology. 2011;147:1261–1267. doi: 10.1001/archdermatol.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peponis V, Kyttaris VC, Chalkiadakis SE, Bonovas S, Sitaras NM. Ocular side effects of anti-rheumatic medications: what a rheumatologist should know. Lupus. 2010;19:675–682. doi: 10.1177/0961203309360539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodgers MA, Bowman JW, Liang Q, Jung JU. Regulation Where Autophagy Intersects the Inflammasome. Antioxidants & Redox Signaling. 2014;20:495–506. doi: 10.1089/ars.2013.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rawat R, Cohen TV, Ampong B, Francia D, Henriques-Pons A, Hoffman EP, Nagaraju K. Inflammasome Up-Regulation and Activation in Dysferlin-Deficient Skeletal Muscle. The American Journal of Pathology. 2010;176:2891–2900. doi: 10.2353/ajpath.2010.090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009;16:175–83. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]