Abstract
Bone fractures are among the most common orthopaedic problems that affect individuals of all ages. Immediately after injury, activated macrophages dynamically contribute to and regulate an acute inflammatory response that involves other cells at the injury site, including mesenchymal stem cells (MSCs). These macrophages and MSCs work in concert to modulate bone healing. In this study, we co-cultured undifferentiated M0, pro-inflammatory M1, and anti-inflammatory M2 macrophages with primary murine MSCs in vitro to determine the cross-talk between polarized macrophages and MSCs and their effects on osteogenesis. After 4 weeks of co-culture, MSCs grown with macrophages, especially M1 macrophages, had enhanced bone mineralization compared to MSCs grown alone. The level of bone formation after 4 weeks of culture was closely associated with prostaglandin E2 (PGE2) secretion early in osteogenesis. Treatment with celecoxib, a cyclooxygenase-2 (COX-2) selective inhibitor, significantly reduced bone mineralization in all co-cultures but most dramatically in the M1-MSC co-culture. We also found that the presence of macrophages reduced the secretion of osteoprotegerin (OPG), the decoy RANKL receptor, suggesting that macrophages may indirectly modulate osteoclast activity in addition to enhancing bone formation. Taken together, these findings suggest that an initial pro-inflammatory phase modulated by M1 macrophages promotes osteogenesis in MSCs via the COX-2-PGE2 pathway.
Keywords: polarized macrophages, mesenchymal stem cells, osteogenesis, fracture healing
INTRODUCTION
Bone fractures are among the most common orthopaedic problems that affect individuals of all ages. In the United States alone, nearly 3.5 million emergency department visits and 900,000 hospitalizations result from bone fractures annually1. Five to ten percent of bone fractures result in delayed healing or nonunion, and these failures may require extensive surgery to achieve complete bone healing, presenting a multi-billion dollar cost to society2,3. In addition to surgical interventions, strategies to enhance bone regeneration in these situations are limited. Thus, it is imperative to gain a better understanding of bone repair at the cellular-molecular level for optimal bone healing.
After acute bone injury, an inflammatory cascade is initiated by local tissue macrophages and polymorphonuclear neutrophils (PMNs) that secrete chemokines (e.g. CCL2 and IL-6) to attract circulating monocytes and macrophages4,5. These recruited macrophages contribute to osteogenesis and initiate bone repair by clearing debris, stimulating angiogenesis, attracting and promoting osteogenic differentiation of mesenchymal stem cells (MSCs), and increasing extracellular matrix synthesis5–7.
As a result of this inflammatory milieu, different macrophage phenotypes arise: undifferentiated M0, pro-inflammatory M1, and anti-inflammatory M2. Given the plasticity of macrophages and their tissue- and context-specific phenotypes, there is debate regarding the existence of the undifferentiated M0 phenotype in vivo; however, polarized macrophages have been shown to drive MSC differentiation towards osteoblasts and promote bone mineralization8–11. M2 macrophages and their cytokines have been shown to support the growth of human MSCs (hMSCs) while M1 macrophages and their pro-inflammatory cytokines inhibit hMSC growth12. However, other studies have shown that direct M1 interaction with murine MSCs and MC3T3 cells (osteoprogenitor cell line) increases osteogenesis and bone mineralization, especially when M1 macrophages are induced to the M2 phenotype after 72 hours of co-culture10,13. These data reveal the importance of acute initial inflammation in bone healing but also highlight the need to further investigate the complex dynamics between polarized macrophages and MSCs.
In clinical practice, non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of musculoskeletal pain. NSAIDs such as naproxen are non-selective cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) inhibitors. COX-1 is constitutively active and plays a homeostatic role in the gastrointestinal system, kidney, and platelet function whereas COX-2 is a stress response gene that is responsible for the conversion of arachidonic acid to prostaglandins like prostaglandin E2 (PGE2) during inflammation14.
Various clinical and preclinical studies suggest that cycloogxygenases play a key role in bone repair, and thus, the use of NSAIDs may negatively alter the natural bone healing process. Non-selective NSAIDs have been shown to delay or even completely inhibit fracture healing in rats15,16. In a retrospective review of human femoral fractures, Giannoudis et al. found that there was a higher incidence of non-unions in patients who had used NSAIDs than those who did not17. COX-2 activity has been specifically identified to be involved in maximal induction of osteogenesis18. Zhang et al. showed that osteogenesis was impaired in COX-2 knockout mice, and that addition of PGE2, a COX-2 metabolite, completely rescued the COX-2-deficient phenotype18. However, controversy over the effect of COX-2 inhibitors and other NSAIDs in clinical practice still persists even after decades of research. Several spinal fusion studies have shown no difference in fracture healing with the treatment of COX-2 selective inhibitors, but impaired healing from other causes such as smoking19–22. With these conflicting results, it is apparent that a better understanding is required to appreciate the nuances of COX-2 activity in musculoskeletal health.
In this study, we utilized a co-culture system to elucidate the cross-talk among primary murine undifferentiated M0, polarized macrophages (M1 and M2), and primary MSCs in osteogenesis and bone mineralization. We found that polarized macrophages, especially pro-inflammatory M1 macrophages, promote bone mineralization in MSCs after 4 weeks of co-culture via the COX-2-PGE2 pathway. Further, we found that macrophages also reduce OPG secretion and thus may indirectly affect osteoclast activity via the OPG-RANKL axis in addition to enhancing bone formation. These findings highlight the importance of a robust, initial pro-inflammatory phase mediated by M1 macrophages in a macrophage-MSC co-culture system and have potential for optimizing fracture therapies.
METHODS
Primary Mouse Bone Marrow Macrophage Isolation
The animal protocol was approved by the Stanford University Animal Care Committee. Primary bone marrow macrophages were isolated from the femora and tibiae of 8-week-old Jackson male C57BL-6J mice (The Jackson Laboratory, Bar Harbor, ME). Using a 25-gauge needle, the bone marrow was flushed into a 50 mL centrifuge tube with 5 mL of basal medium [RPM1 1640 (Life Technologies, Pleasanton, CA), 10% FBS, 1X Antibiotic-Antimycotic]. The cells were then filtered through a 70 μm cell strainer, spun down at 400g for 10 minutes, and resuspended in 1 mL of ice-cold red blood cell lysis buffer (Sigma Aldrich) for 2 minutes at 4°C. After addition of 20 mL basal medium, cells were spun down at 400g for 10 minutes and resuspended in 5 mL of macrophage media [RPMI 1640, 30% leukocyte-conditioned medium,10 ng/mL macrophage colony-stimulating factor (M-CSF), antibiotics]. Cells were counted and placed into T-175 flasks (BD, Franklin Lakes, NJ) at a concentration of 50 × 106 cells/flask in 25 mL macrophage media. After 5 to 7 days in culture, the macrophages were lifted with trypsin-EDTA (Life Technologies) and gentle scraping and frozen in liquid nitrogen for subsequent experiments.
Primary Mouse Mesenchymal Stem Cell Isolation
Primary mouse MSCs were isolated from the femora and tibiae of 8-week-old Jackson male C57BL-6J mice. Using a 27-gauge needle, the bone marrow was flushed into a 10cm dish and resuspended in MSC growth media (α-MEM, 10% heat inactivated MSC qualified FBS, 1X Anti-Anti). The cells were filtered through a 70 μm cell strainer, spun down at 400g for 5 minutes, resuspended in media, and plated onto T-175 flasks. The cells were then incubated overnight in 37°C at 5% CO2, and the media was changed with 20 mL fresh media twice a week for 3–4 weeks until the cells were confluent. Cells were washed with 10 mL PBS, detached with 5 mL trypsin, and incubated for 2 minutes. Detached cells were flushed with 10 mL media into a centrifuge tube and spun at 400g for 5 minutes. Cells were resuspended and plated at a 4,000 cells/cm2 density. This subculture protocol was repeated twice until pure MSCs were isolated in passage 4. The immunophenotypes (Sca1+/CD73+/CD90.2+/CD105+/CD34−/CD45−) of pure MSCs at passage 4 were confirmed by flow cytometry and were used for this study23.
Macrophage Polarization
Macrophage polarization was performed following our previously established protocols13,24. M0 cells were grown in macrophage media. 100 ng/mL LPS (Sigma Aldrich, St. Louis, MO, USA) or 20 ng/mL IL-4 (R&D Systems, Minneapolis, MN, USA) were added to the macrophage culture media to polarize M0 macrophages into M1 or M2 phenotypes, respectively. Macrophages were polarized for 24 hours. This protocol has been shown to reliably produce M0, M1 and M2 macrophages as previously assessed by flow cytometry, qRT-PCR and cytokine secretion profile13,24. After 24 hours of polarization by their respective stimuli, M0, M1, and M2 macrophages were co-cultured with MSCs in fresh mixed osteogenic-macrophage media that did not contain LPS or IL-4.
Macrophage-MSC Direct Co-Culture
Primary polarized macrophages and primary MSCs were seeded together at a 1:1 macrophage:MSC ratio (104 cells/well each in a 24-well plate) and at a 5:1 ratio (5×104 macrophages and 104 MSCs in each well). Co-cultures were cultured for 4 weeks in mixed osteogenic-macrophage media comprised of 50% macrophage media and 50% osteogenic media (Dulbecco’s modified Eagle’s medium, 10% FBS, 1% Penstrep, 100 μg/ml ascorbic acid, and 10 mM β-glycerophosphate, 100 nM dexamethasone). Control groups included MSCs grown alone in normal MSC growth media (MSC-GM) and MSCs grown alone in mixed osteogenic-macrophage media (MSC-MM). To determine the effect of PGE2 inhibition on bone mineralization, co-cultures were treated with 25μM of celexocib (Sigma, resuspended in sterile DMSO), a COX-2 selective inhibitor, for the first week of the 4-week culture.
Measuring Osteogenesis
To assess the osteogenic potential of the co-cultures, Alkaline Phosphatase (ALP) activity assays were conducted on cell lysates at 2 weeks of co-culture using the QuantiChrom Alkaline Phosphatase Assay Kit (DALP-250, BioAssay Systems, Hayward, CA, USA) following manufacturer’s protocol. At 4 weeks, bone mineralization was measured using Alizarin Red staining (pH 4.2; Sigma-Aldrich). Results were imaged for qualitative data. To quantify Alizarin Red stain, each well had 10% cetylpyridinium chloride solution added, and samples were measured via spectrophotometry at absorbance 562 nm in triplicate using a SpectraMax M2e spectrophotometer (Molecular Devices, Sunnyvale, CA, USA).
Enzyme-Linked Immunosorbent Assays (ELISAs)
ELISAs (R&D Systems, Biomedical Technologies, Stoughton, MA, USA) for prostaglandin E2 (PGE2) and osteoprotegerin (OPG) were performed on supernatants taken at 2 and 3 weeks, respectively, following manufacturer’s protocol.
Statistical Analysis
To compare different effects in osteogenesis of polarized macrophages (M0, M1, M2) on MSCs, one-way analysis of variance (ANOVA) followed by Tukey’s test was performed via GraphPad Prism. We used p<0.05 as the threshold for statistical significance. Data was presented as mean ± standard deviation.
RESULTS
Macrophages, especially pro-inflammatory M1 macrophages, enhance bone mineralization
Initially, macrophages and MSCs were co-cultured at a 1:1 ratio (10k macrophages: 10k MSCs). After 4 weeks of culture, there was no significant difference in bone mineralization among MSCs grown alone and all co-culture groups (data not shown), which is consistent with findings observed by Loi et al. in a similar macrophage-MC3T3 (osteoprogenitor cell line) co-culture system25. Thus, we increased the macrophage:MSC seeding density to 5:1 (50k macrophages:10k MSCs) based on work done by Nicolaidou et al. in which increasing the ratio of non-polarized human macrophages:human MSCs allowed for increased bone mineralization, especially when macrophages and MSCs had direct cell-to-cell contact in co-culture11. At this higher seeding density, there was increased bone mineralization in all macrophage-MSC co-cultures regardless of macrophage phenotype (Figure 1B). All cultures containing macrophages had significantly enhanced bone mineralization compared to MSCs grown alone in mixed osteogenic-macrophage media (MSC-MM vs. M0-MSC p= 0.0060, MSC-MM vs. M1-MSC p< 0.0001, MSC-MM vs. M2-MSC p< 0.0001)(Figure 1B). The effect was most prominent in pro-inflammatory M1-MSC co-cultures, which had significantly higher bone formation compared to both MSCs grown alone and non-polarized M0-MSC co-cultures (p= 0.0060)(Figure 1B).
Figure 1. Polarized macrophages, especially pro-inflammatory M1 macrophages, enhance bone mineralization.

Macrophages and MSCs were plated at an initial seeding density of 50k macrophages: 10k MSCs. At 2 weeks of cultures, ALP activity was low in all groups, but was the lowest in M1-MSC (A). Both M0-MSC and M2-MSC had significantly higher ALP activity than M1-MSC and MSCs grown alone (A). After 4 weeks of culture, all macrophage-MSC co-cultures had enhanced bone mineralization by Alizarin Red staining compared to MSCs grown alone in mixed osteogenic-macrophage media (B). The effect was most prominent in the M1-MSC group, which had significantly more bone mineralization than M0-MSC and MSCs grown alone (B). Despite early inhibition of osteogenesis in M1-MSC, M1-MSC exhibited the most bone formation at 4 weeks (B).
*p=0.05; **p=0.01; ***p=0.005; n=4
M1-MSC co-cultures exhibit low ALP activity early in osteogenesis
At 2 weeks of culture, MSCs co-cultured with M1 macrophages had reduced alkaline phosphatase (ALP) activity (1.28 ± 0.42 μmol/L*min) compared to MSCs grown alone, M0-MSC, and M2-MSC groups (2.38 ± 1.10 μmol/L*min, 9.30 ± 3.02 μmol/L*min, 8.78 ± 1.00 μmol/L*min, respectively) (Figure 1A). Both the undifferentiated M0-MSC and anti-inflammatory M2-MSC groups had significantly higher ALP Activity compared to M1-MSC and MSCs grown alone (Figure 1A). Despite low ALP activity early in co-culture, the M1-MSC culture had the greatest bone formation by 4 weeks (Figure 1B).
Macrophage-MSC co-cultures have elevated levels of PGE2 early in osteogenesis
Macrophage-MSC co-cultures demonstrated elevated levels of PGE2 early in osteogenesis (Figure 2A). After 2 weeks of culture, co-cultures with either M1 or M2 macrophages exhibited more PGE2 protein secretion than in MSCs grown alone and in M0-MSC co-cultures (Figure 2A). M1-MSC had the highest PGE2 secretion (442.56 ± 76.74 pg/mL) while M0-MSC and M2-MSC had lower PGE2 present in the supernatant (330.99 ± 70.55 pg/mL and 409.00 ± 69.22 pg/mL, respectively) (Figure 2A). M1-MSC PGE2 secretion was not statistically significant compared to M2-MSC (p=0.44) (Figure 2A). PGE2 protein secretion is reported in absolute value of pg/mL.
Figure 2. Polarized macrophage-MSC co-cultures have elevated levels of PGE2 early in osteogenesis.
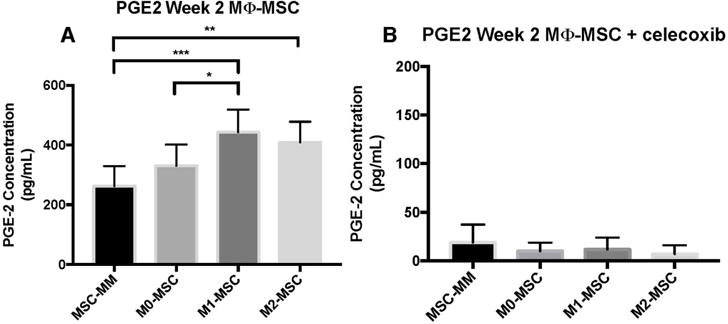
After 2 weeks of culture, all macrophage-MSC co-cultures demonstrated elevated levels of PGE2 compared to MSCs grown alone (A). M1-MSC had significantly higher PGE2 than M0-MSC but not significantly higher PGE2 than M2-MSC (p=0.44) (A). Interestingly, PGE2 protein expression at 2 weeks did not correlate with ALP activity at 2 weeks; trends more closely resemble bone mineralization at 4 weeks (A). Co-cultures were treated with 25μM of celexocib for the first week of culture, and PGE2 protein expression was nearly abolished at 2 weeks of culture (1 week post-celecoxib treatment) (B).
*p=0.05; **p=0.01; ***p=0.005; n=4
Inhibition of COX-2 reduces bone mineralization in macrophage-MSC co-cultures, especially in M1-MSC co-cultures
As increased PGE2 protein secretion was associated with increased bone mineralization, co-cultures were treated with celecoxib, a selective COX-2 inhibitor, to determine the effect PGE2 reduction on bone mineralization. With 1-week treatment of celecoxib at 25μM, PGE2 protein secretion was abolished with PGE2 levels less than 20 pg/mL after 2 weeks of culture (1 week post-celecoxib treatment) (Figure 2B). After 4 weeks of culture, all co-culture groups had reduced bone mineralization compared to MSCs grown alone (p< 0.0001), especially in the M1-MSC group (Figure 3B). There was no significant difference in ALP activity across co-culture groups and MSCs grown alone at 2 weeks of culture (Figure 3A).
Figure 3. Inhibition of COX-2 reduces bone mineralization in macrophage-MSC co-cultures, especially in M1-MSC.

All macrophage-MSC co-cultures and MSCs grown alone were treated with celecoxib (25μM) for 1 week and left to grow for 4 weeks (3 weeks post-celecoxib treatment). At 2 weeks of culture, there was no difference in ALP activity across all co-cultures and MSCs grown alone (A). All macrophage-MSC groups treated with celecoxib had reduced bone mineralization at 4 weeks compared to MSCs grown alone (B). Bone mineralization was the lowest in M1-MSC compared to both M0-MSC and M2-MSC (B)
***p=0.005; n=4
Osteoprotegerin (OPG) is negatively regulated by macrophages in macrophage-MSC co-culture
After 3 weeks of culture, OPG, the decoy receptor for RANKL, protein secretion was decreased in all non-celecoxib-treated co-culture groups compared to MSCs grown alone (Figure 4). OPG secretion was significantly lower in both polarized macrophage co-culture groups (M1-MSC and M2-MSC) compared to the undifferentiated M0-MSC group and MSCs grown alone (Figure 4). RANKL protein secretion was also measured but was not detectable (< 31.3 pg/mL) after 3 weeks of culture. This suggests that macrophages, especially polarized macrophages, decrease OPG secretion in co-culture and thus may regulate osteoclast activity in addition to enhancing bone mineralization.
Figure 4. Osteoprotegerin (OPG) is negatively regulated by macrophages, especially polarized macrophages, in macrophage-MSC co-culture.

After 3 weeks of co-culture, osteoprotegerin (OPG), the RANKL decoy receptor, was reduced in all macrophage-MSC co-culture groups compared to MSCs grown alone. This result suggests that macrophages decrease OPG secretion and may indirectly modulate osteoclast activity via the OPG-RANKL axis.
***p=0.005; n=2
DISCUSSION
In this study, we examined the effects of polarized macrophages on MSC differentiation and bone mineralization. Our data showed that macrophages, especially pro-inflammatory M1 macrophages, promote osteogenesis and bone mineralization of MSCs early in co-culture via the COX-2-PGE2-pathway. In our co-culture system, all MSCs grown in the presence of macrophages exhibited high levels of PGE2 and low ALP activity at 2 weeks. However, despite low ALP activity at 2 weeks of co-culture, all macrophage-MSC co-cultures, especially M1-MSC, had enhanced bone mineralization compared to MSCs grown alone at 4 weeks of culture. Treatment with celecoxib, a selective COX-2 inhibitor, significantly reduced bone formation in all macrophage-MSC co-cultures with the most dramatic effect seen in the M1-MSC group (44.0% reduction compared to MSCs grown alone). These results highlight the importance of an initial, transient inflammatory phase mediated by macrophage-MSC cross-talk for optimal bone healing.
It is well established that MSCs activate macrophages toward the anti-inflammatory M2 phenotype and exert an immunosuppressive response for the resolution of inflammation26–30. These immune modulatory effects require direct cell-cell contact with immune cells while secreted factors from MSCs can also influence the local environment26,31,32. Nemeth et al. showed that activation of TLR4 and TNFR1 by LPS and TNFα on MSCs led to activation of NFκB signaling, which resulted in increased COX-2 activity and PGE2 production33. PGE2 binds EP2 and EP4 receptors on macrophages that increase the production of anti-inflammatory IL-10. Similarly, activation of TLR4 on macrophages induces signaling through the COX-2 and PGE2 regulatory loop via STAT3 and increases the secretion of oncostatin M (OSM), a cytokine of the IL-6 family10,11,33. As both MSCs and macrophages can secrete PGE2 and modulate COX2 activity, this dynamic crosstalk might work to enhance osteogenesis by inducing osteoblast differentiation and increasing matrix mineralization possibly via autocrine and paracrine signaling10,34–36.
In our study, exposing MSCs to a high density of pro-inflammatory M1 macrophages may have best stimulated MSCs to reach their full pro-osteogenic potential and immune modulatory effect by reciprocally modulating macrophage phenotypes from M1 to M2 early in osteogenesis. Compared to the M2-MSC co-cultures, having a strong initial environment of M1 pro-inflammatory signaling may have allowed for a more potent activation of MSCs to secrete higher levels of PGE2 and other anti-inflammatory signals that enhance osteoblast differentiation and modulate an ideal transition of macrophages from the M1 to M2 phenotype37. Given the known tendency for macrophages to assume the M2 phenotype in the presence of MSCs, the differences in bone mineralization between the M1-MSC and M2-MSC groups highlight that these immunomodulatory effects are exerted early in osteogenesis and may be due to a more pro-osteogenic and precisely-timed M1 to M2 transition13,26,28–30. Further, by co-culturing M1 macrophages at a 5:1 macrophage:MSC ratio, we provided a more robust pro-inflammatory environment that likely modeled the physiologic conditions present at the fracture site more closely than the 1:1 macrophage:MSC ratio5,11. In a polarized macrophage-MC3T3 (osteoprogenitor cell line) co-culture system, our group has previously shown that enhanced osteogenesis can be achieved by transitioning M1 macrophages to the M2 phenotype via addition of IL-4 after 72 hours of co-culture13. Similarly, we have also observed that adding IL-4 to primary M1-MSC co-cultures at 72 hours and 96 hours allows for increased bone mineralization (unpublished data). Taken together, the dynamic interplay between polarized macrophages, especially pro-inflammatory M1 macrophages, and MSCs are key to optimal bone formation.
Further, the presence of M1 macrophages reduced ALP activity at 2 weeks of co-culture. The low ALP activity seen in the M1-MSC co-culture without celecoxib (Figure 1A) may be because ALP activity peaked earlier in co-culture and thus had subsided by week 2. We chose the 2 week time point as (1) our group has previously shown that ALP activity is the main osteoprogenitor marker early in differentiation (Day 5–14) in monocultures of MSCs and (2) that ALP activity is elevated in polarized macrophage-MC3T3 (pre-osteoblast cell line) co-cultures at 2 weeks13,38. However, since we propose that M1 macrophages exert their pro-osteogenic effect on MSCs early in osteogenesis, we may have missed the apex of ALP activity by measuring at 2 weeks rather than an earlier time point. The pro-inflammatory environment mediated by the M1 macrophages may have more strongly stimulated MSCs to produce PGE2 and initiate the osteoblastic program earlier in culture than in the M0-MSC and M2-MSC co-cultures. Moreover, we seeded our co-cultures at a 5:1 macrophage:MSC ratio (50k macrophages:10k MSCs), which likely further enhanced the pro-inflammatory setting mediated by the M1 macrophages. It has also been shown that LPS-stimulated macrophages can express ALP, so this additional source of ALP could have disrupted the expected ALP activity profile via macrophage-MSC cross-talk39.
As we measured ALP at a single time point, we cannot say for certain how ALP activity evolved throughout co-culture. Despite the low ALP activity measured at 2 weeks, the M1-MSC group exhibited the most bone mineralization at 4 weeks (Figure 1B). Interestingly, the PGE2 secretion data at 2 weeks of culture more closely correlated with the bone mineralization trends at 4 weeks than with ALP activity at 2 weeks; PGE2 secretion was highest in the M1-MSC co-culture groups while ALP activity was the lowest in M1-MSC groups at 2 weeks (Figure 2A and Figure 1). These data highlight the importance of early PGE2 secretion in our co-culture system for ultimate bone mineralization, the most clinically important endpoint in the setting of fracture healing.
Our study is consistent with previous work showing that the inhibition of COX-2 and PGE2 leads to impaired bone healing19,40–43. However, the effect of COX-2 inhibition on bone formation may be more nuanced and context-specific than previously stated. Yoon et al. proposed that bone formation processes may differ in inflammatory and non-inflammatory states: they found that human MSCs pre-treated with IL-1β as an inflammatory stimulus had impaired bone formation and osteoblastic differentiation when treated with continuous celecoxib for 2 weeks19. On the other hand, MSCs that were not treated with IL-1β did not have reduced bone mineralization19. Similarly, in our study, we observed that the pro-inflammatory M1-MSC co-culture group had the most bone formation after 4 weeks of culture, and with disruption of the COX-2-PGE2 pathway by celecoxib, the M1-MSC co-culture had the most dramatic reduction in bone mineralization (Figure 1B, Figure 3B). In the celecoxib-treated cultures, the MSC-only control had enhanced matrix mineralization with celecoxib treatment while osteogenesis was reduced in all macrophage-MSC co-cultures (Figure 3B). Consistent with preliminary data from our group (data not shown), celecoxib seems to enhance osteogenesis of MSCs that are in an inflammation-free environment, suggesting that the role of COX-2 in bone formation is likely context-specific in an inflammatory response. These findings offer a mechanism for different pathways driving osteogenesis in inflammatory (e.g. injury) versus non-inflammatory (e.g. normal skeletogenesis, fetal bone development) states. Thus, COX-2 inhibition may only be detrimental in inflammatory states and further investigation is warranted for the role of COX-2 inhibition in other contexts.
Bone healing is regulated by pathways other than COX-2-PGE2 since near abolishment of PGE2 did not entirely inhibit bone formation in our co-culture system. These other pathways may be independent of the COX-2-PGE2 pathway or, more likely, intertwined with this signaling cascade. In a study comparing the effect of acetaminophen and celecoxib on bone fracture healing in rats, only celecoxib impaired fracture healing while acetaminophen, which may also in part inhibit the COX-2 pathway, had no negative effect on fracture healing by mechanical and radiographic observations44. Moreover, the Wnt/β-catenin pathway is an important regulator of embryonic skeletonogenesis and positively modulates osteoblasts in a time-dependent manner during normal bone repair45. In response to mechanical loading, the Wnt/β-catenin signaling pathway is activated, and this activation works in concert with increased COX-2 and PGE2 secretion in osteocytes46,47. These and other observations suggest more complex mechanisms underlying fracture healing.
We also found that the presence of macrophages reduced the secretion of OPG, the decoy RANKL receptor. OPG is known to be secreted by both macrophages and MSCs, so it is surprising that OPG protein in the supernatant was most dramatically reduced in the polarized macrophage (M1 and M2) co-cultures with greater than 60% reduction compared to MSCs grown alone and a statistically significant reduction compared to undifferentiated M0-MSCs48,49. These findings suggest that the inflammatory environment mediated by macrophages negatively regulate OPG secretion in our co-culture system and thus may affect osteoclast activity along the OPG-RANKL axis. As both osteoclast and osteoblast activity are important for bone healing and remodeling, future studies should investigate how macrophages affect this osteoclast-osteoblast balance throughout the fracture healing process and in a variety of disease contexts.
This study has several limitations. First, in the present study, osteogenic markers were limited to ALP activity and Alizarin Red staining. Other groups have already shown that COX-2 regulates cbfa-1 and osterix in vivo18. Also, in the clinical setting, the most important outcome in a fracture would be formation of bone and healing of the fracture/defect, and thus, we highlight bone mineralization as measured by Alizarin Red staining. Second, we did not perform cell viability assays to determine the number of macrophages present at different time points in the co-culture. Previous work by our group has shown that macrophages plated with MSCs at a seeding density of 104 cells/well (24-well plate) survived co-culture for at least 10 days13. As such, we did not count cell numbers and did not normalize PGE2 protein levels with cell number. It is possible that the differential survival of the various macrophages phenotypes could have impacted our co-culture system. Others have shown that M0 and M2 macrophages survive longer than M1 macrophages, which have significantly fewer viable cells within the first week of culture50. Given this knowledge, the enhanced bone mineralization observed in the M1-MSC co-cultures is especially impressive as there are likely fewer M1 macrophages present as the co-culture progresses. This finding further supports our assertion that M1 macrophages exert their pro-osteogenic effect early in co-culture. Moreover, the celecoxib-treated and non-celecoxib-treated co-culture experiments were conducted separately and thus are not directly comparable. However, we controlled for any potential technical variations by conducting the experiments under same experimental conditions, by same personnel and using the same batch of cells, and highlight the different trends seen among experimental groups. Lastly, our current co-culture system does not allow us to definitively confirm the proposed ideal M1 to M2 transition that may mediate enhanced bone formation. Other groups have shown that MSCs have an immunosuppressive role and modulate macrophages toward an M2 phenotype while our group has preliminary data (data not shown) that suggests that a M1 to M2 transition at 72 hours or 96 hours can enhance osteogenesis of macrophage-MSC co-cultures27–30. Future studies are needed to characterize macrophage polarization and phenotype throughout co-culture. Despite these limitations, our study demonstrates that a strong, transient pro-inflammatory induction signal by M1 macrophages rather than continuous immune modulation plays a key role in mediating bone formation via the COX-2-PGE2 pathway.
CONCLUSION
In summary, polarized macrophages, especially pro-inflammatory M1 macrophages, enhance MSC osteogenesis and bone formation early in osteogenesis via the COX-2-PGE2 pathway. By promoting an initial inflammatory environment, M1 macrophages likely enable MSCs to elicit a robust response that promotes differentiation toward the osteoblast lineage, allowing for increased bone formation. These observations highlight the importance of an initial, transient inflammatory phase during fracture healing. Our results are consistent with current clinical practice of avoiding NSAID use early in acute bone injury and additionally provide mechanistic insight into why selective COX-2 inhibitors and NSAIDs generally may be detrimental to bone healing. Our findings also highlight the role of precise temporal immune modulation in optimizing bone repair for a variety of osteogenic disease processes and orthopaedic complications. As the role of COX-2 and PGE2 extends beyond musculoskeletal maladies into cancer biology, endocrinology, and cardiovascular health, it is imperative to gain a granular understanding of the nuances of COX-2 and PGE2 activity in all systems of the body. Moreover, all wound healing processes start with an inflammatory phase, so studying the complex role of macrophages in regulating inflammation and their interactions with MSCs provides an opportunity for developing treatments in other inflammatory processes, such as cardiac and neural tissue regeneration.
Statement of Clinical Significance.
Understanding the complex interactions between macrophages and MSCs provide opportunities to optimize bone healing and other regenerative processes via modulation of the inflammatory response. This study provides one possible biological mechanism for the adverse effects of non-steroidal anti-inflammatory drugs on fracture healing and bone regeneration.
Acknowledgments
This work was supported by the NIH grants 2R01 AR055650 and 1R01 AR063717, the Ellenburg Chair in Surgery, the Howard Hughes Medical Institute, and the Stanford Medical Scholars Research Program.
Footnotes
Author contributions: LL designed and performed experiments, analyzed data, and wrote the paper; FL and KN assisted with experiments; TL, JP, EG, AN, LC, and EJ contributed to critical revision of the manuscript. ZY and SG supervised the project.
References
- 1.National Hospital Ambulatory Medical Care Survey: 2011 Emergency Department Summary Tables. Available from: http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf.
- 2.Gómez-Barrena E, Rosset P, Lozano D, et al. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone. 2015;70:93–101. doi: 10.1016/j.bone.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Office of the Surgeon General (US) The Burden of Bone Disease: A Report of the Surgeon General. 2004 Available from: http://www.ncbi.nlm.nih.gov/books/NBK45502/
- 4.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–5. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claes LC, Recknage S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 6.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 7.Ren PG, Lee SW, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29:4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs functional differentiation. 2014 doi: 10.3389/fimmu.2014.00514. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4201108/ [DOI] [PMC free article] [PubMed]
- 10.Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 11.Nicolaidou V, Wong M, Redpath AN, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039871. e39871.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–9. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 13.Loi F, Córdova LA, Zhang R, et al. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016:1–11. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raisz LG. Prostaglandins and bone: physiology and pathophysiology. Osteoarthr Cartil. 1999;7:419–421. doi: 10.1053/joca.1998.0230. [DOI] [PubMed] [Google Scholar]
- 15.Altman RD, Latta LL, Keer R, et al. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma. 1995;9:392–400. doi: 10.1097/00005131-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 17.Pountos I, Georgouli T, Calori GM, Giannoudis PV. Do nonsteroidal antiinflammatory drugs affect bone healing? A critical analysis. ScientificWorldJournal. 2012 doi: 10.1100/2012/606404. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3259713/ [DOI] [PMC free article] [PubMed]
- 18.Zhang X, Schwarz EM, Young DA, et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon DS, Yoo JH, Kim YH, et al. The Effects of COX-2 Inhibitor During Osteogenic Differentiation of Bone Marrow-Derived Human Mesenchymal Stem Cells. Stem Cells Dev. 2010;19:1523–1533. doi: 10.1089/scd.2009.0393. [DOI] [PubMed] [Google Scholar]
- 20.Long J, Lewis S, Kuklo T, et al. The Effect of Cyclooxygenase-2 Inhibitors on Spinal Fusion. J Bone Jt Surg. 2002;84 doi: 10.2106/00004623-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Glassman SD, Rose SM, Dimar JR, et al. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine (Phila Pa 1976) 1998;23:834–8. doi: 10.1097/00007632-199804010-00020. [DOI] [PubMed] [Google Scholar]
- 22.Andersen T, Christensen FB, Laursen M, et al. Smoking as a predictor of negative outcome in lumbar spinal fusion. Spine (Phila Pa 1976) 2001;26:2623–8. doi: 10.1097/00007632-200112010-00018. [DOI] [PubMed] [Google Scholar]
- 23.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 24.Rao AJ, Gibon E, Ma T, et al. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8(7):2815–23. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibon E, Loi F, Crdova LA, et al. Aging Affects Bone Marrow Macrophage Polarization: Relevance to Bone Healing. Regen Eng Transl Med. 2016;2:98–104. doi: 10.1007/s40883-016-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2012;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 27.Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical Cord-Derived Mesenchymal Stromal Cells Modulate Monocyte Function to Suppress T Cell Proliferation. J Immunol. 2010;185:6617–6623. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- 28.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–95. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 29.Maggini J, Mirkin G, Bognanni I, et al. Mouse Bone Marrow-Derived Mesenchymal Stromal Cells Turn Activated Macrophages into a Regulatory-Like Profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells. 2011;29:1572–9. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 32.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the antiinflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouffi C, Bony C, Courties G, et al. IL-6-Dependent PGE2 Secretion by Mesenchymal Stem Cells Inhibits Local Inflammation in Experimental Arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsatsanis C, Androulidaki A, Dermitzaki E, et al. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-α release from macrophages via induction of COX-2 and PGE2. J Cell Physiol. 2007;210:774–783. doi: 10.1002/jcp.20900. [DOI] [PubMed] [Google Scholar]
- 36.Gu W, Song L, Li XM, et al. Mesenchymal stem cells alleviate airway inflammation and emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733. doi: 10.1038/srep08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.YlÖstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human Mesenchymal Stem/Stromal Cells Cultured as Spheroids are Self-activated to Produce Prostaglandin E2 that Directs Stimulated Macrophages into an Anti-inflammatory Phenotype. Stem Cells. 2012;30:2283–2296. doi: 10.1002/stem.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Z, Nelson ER, Smith RL, Goodman SB. The Sequential Expression Profiles of Growth Factors from Osteroprogenitors to Osteoblasts In Vitro. Tissue Eng. 2007;13:2311–2320. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- 39.Reale M, Felaco M, Grilli A, et al. Induction of alkaline phosphatase generation by il-1β and LPS on human neutrophils and macrophages and lack of inhibition by interleukin-1 receptor antagonist. Inflammopharmacology. 1994;3:25–34. [Google Scholar]
- 40.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-Oxygenase 2 Function Is Essential for Bone Fracture Healing. J Bone Miner Res. 2002;17:963–976. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 41.Goodman S, Ma T, Trindade M, et al. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20:1164–1169. doi: 10.1016/S0736-0266(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 42.Murnaghan M, Li G, Marsh DR. Nonsteroidal Anti-Inflammatory Drug-Induced Fracture Nonunion: An Inhibition of Angiogenesis? J Bone Jt Surg. 2006;88:140. doi: 10.2106/JBJS.F.00454. [DOI] [PubMed] [Google Scholar]
- 43.Cottrell J, O’Connor JP. Effect of Non-Steroidal Anti-Inflammatory Drugs on Bone Healing. Pharmaceuticals. 2010;3:1668–1693. doi: 10.3390/ph3051668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergenstock M, Min W, Simon AM, et al. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–23. doi: 10.1097/01.bot.0000184144.98071.5d. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Whetstone HC, Lin AC, et al. Beta-Catenin Signaling Plays a Disparate Role in Different Phases of Fracture Repair: Implications for Therapy to Improve Bone Healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21:294–301. doi: 10.1016/j.tem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada N, Tsujimura T, Ueda H, et al. Down-regulation of osteoprotegerin production in bone marrow macrophages by macrophage colony-stimulating factor. Cytokine. 2005;31:288–297. doi: 10.1016/j.cyto.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Sharaf-Eldin WE, Abu-Shahba N, Mahmoud M, El-Badri N. The Modulatory Effects of Mesenchymal Stem Cells on Osteoclastogenesis. Stem Cells Int. 2016;2016:1908365. doi: 10.1155/2016/1908365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SC, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]


