Abstract
Introduction
Increased gut permeability (“leaky gut”) has been proposed as a potential contributor to age-related inflammation and gut dysbiosis. However, information on the relationship between a leaky gut and inflammation and physical frailty during aging are limited.
Objective
To investigate the hypothesis that an aging-associated leaky gut is linked to the age-related inflammation and frailty.
Methods
Two cohorts of healthy adults were studied: young (18–30-years-old, n=19) and older (≥70-years-old, n=18). Serum concentrations of the TNF-α and IL6, zonulin (a marker for leaky gut) and high-mobility group box protein (HMGB1, a nuclear protein triggering inflammation) were measured. Correlations of serum levels of zonulin and HMGB1 with strength of plantar flexor muscles and number of steps taken per day were analyzed.
Results
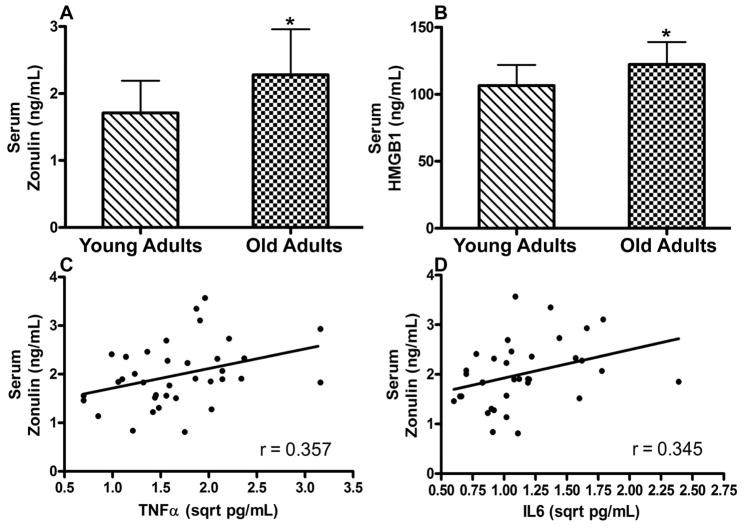
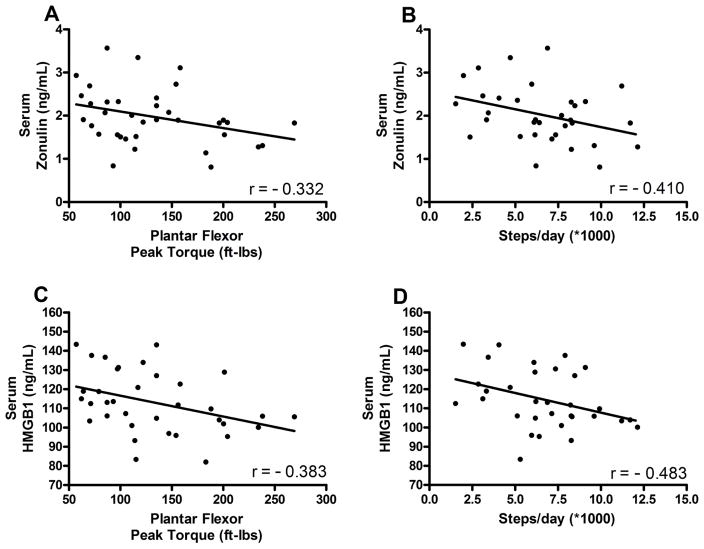
Serum concentration of zonulin and HMGB1 were 22% (p = 0.005) and 16% (p = 0.010) higher in the older vs young adults. Serum zonulin was positively associated with the concentrations of the TNF-α (r=0.357, p=0.032) and IL6 (r=0.345, p=0.043). Importantly, both zonulin and HMGB1 were negatively correlated with skeletal muscle strength (zonulin: r=−0.332, p=0.048; HMGB1: r=−0.383, p=0.023) and habitual physical activity (zonulin: r=−0.410, p=0.016; HMGB1: r=−0.483, p=0.004).
Conclusions
Serum zonulin was associated with both systemic inflammation and two key indices of physical frailty. These data suggest that a leaky gut may play a critical role in the development of age-related inflammation and frailty.
Keywords: aging, leaky gut, zonulin, inflammation, physical frailty
Introduction
Human aging is a continual and progressive process that is associated with a decrease in diverse physiological functions across all organ systems.1 These changes result in an increased vulnerability to infection and disease with an elevated mortality risk.2 Chronic, low-grade inflammation is the most consistent features of chronological aging and various age-related diseases/disorders.3 However, the precise mechanisms underlying systemic inflammation in aging remain poorly understood. The gut houses the majority of the body’s immune cells and the immune system is known to regulate the composition of gut microbiota.4 Advanced age is associated with changes in the gut microbiota.5,6 Moreover, recent data indicate that gut dysbiosis – a disturbance of the density and/or composition of gut microbiota – is associated with systemic inflammation5 as well as the development of inflammation-related geriatric syndromes including cognitive impairment7 and physical frailty.8
Recently, increased gut permeability (i.e. “leaky gut”)9 has been proposed as a potential source of agerelated inflammation. However, few data are available to support this innovative hypothesis. Thus, the objective of our study was to determine if the serum concentration of zonulin, a physiologic regulator of intestinal permeability10, is increased in healthy older people. We also aimed to determine if chronologic age was associated with increases in high-mobility group box protein 1 (HMGB1), a nuclear protein that triggers inflammation and is commonly found in epithelial cells.11 Finally, we evaluated associations of zonulin and HMGB1 with established inflammatory biomarkers of aging as well as physical indices related to frailty.
Methods
Healthy adults aged 18–30 (n=19) and ≥70 years (n=18) were recruited for the study, which was approved by the University of Florida Institutional Review Board (IRB #688-2009). Exclusion criteria designed to recruit healthy individuals free of chronic disease and other details of inclusion/exclusion criteria were previously published, and same cohort of subjects was also used in this study.12 Strength of the plantar flexor muscles was evaluated based on methodology described previously.12 To evaluate physical activity habits, participants wore validated physical activity monitor (SenseWear; BodyMedia, Pittsburgh, PA) for 7 days.13 Fasting venous blood samples were drawn and serum was used to quantify TNF-α (Millipore), IL-6 (R & D Systems), HMGB1 (Antibodies online), and zonulin (Immundiagnostik AG).
Statistical Considerations: For all parameters, primary analyses were completed with outliers removed using Cook’s distance at a threshold ≥0.20. After outliers were removed, data were analyzed initially for normality and homogeneity of variance prior to computation of descriptive statistics. Where the assumption of normality was violated, the square root transformation was utilized to normalize concentrations of circulating analytes. Meeting the assumptions of parametric tests, age-related differences in zonulin and HMGB1 were calculated using Student’s t-test for independent samples. Correlation coefficients of zonulin and HMGB1 with inflammatory and physical indices were then performed using the Pearson test. Where outliers were removed, analyses were repeated and differences reported. For correlation analyses involving IL-6, correlations with outliers included were conducted using Spearman’s test because the distribution did not meet assumptions of normality. All analyses were performed using IBM SPSS Statistics (IBM, Armonk, NY), and data are presented as mean ± SD. A two-sided alpha level of 0.05 was utilized throughout to determine statistical significance. No correction for multiple comparisons was utilized given the exploratory nature of the study.
Results
A total of 19 young and 18 older adults were studied and their pertinent demographic characteristics and other data are summarized in Table 1. Both serum zonulin (p = 0.005) and HMGB1 (p = 0.010) were higher among older adults than young adults (Figure 1). Serum zonulin was correlated with both TNFα (p = 0.032) and IL6 (p = 0.043; Figure 1). In contrast, serum HMGB1 was not significantly correlated with either parameter (TNFα: r = 0.267, p = 0.121; IL6: r = 0.302, p = 0.078). Interestingly, however, both biomarker concentrations were correlated with muscle strength (zonulin: r=−0.332, p=0.048; HMGB1: r=−0.383, p=0.023) and physical activity measured by steps/day (zonulin: r=−0.410, p=0.016; HMGB1: r=−0.483, p=0.004; Figure 2).
Table 1.
Pertinent demographic characteristics of the cohorts
| Young Adults n=19 |
Older Adults n=18 |
|
|---|---|---|
| Age, years | 23.1 ± 3.9 | 76.7 ± 5.2*** |
| Female, n | 8 (42.1%) | 7 (38.9%) |
| Height, cm | 175.1 ± 9.1 | 170.3 ± 10.9 |
| Body Mass, kg | 74.1 ± 17.7 | 76.2 ± 15.5 |
| Body Mass Index, kg* m−2 | 24.0 ± 4.3 | 26.2 ± 4.6 |
| Radial Pulse Rate, bpm | 71.9 ± 9.7 | 61.3 ± 10.4** |
| Systolic BP, mm Hg | 115.9 ± 10.5 | 139.6 ± 18.0*** |
| Diastolic BP, mm Hg | 70.3 ± 9.0 | 73.8 ± 10.4 |
| Serum TNFα, sqrt pg/mL | 0.95 ± 0.25 | 1.36 ± 0.42# |
| Serum interleukin 6, sqrt pg/mL | 1.32 ± 0.41 | 2.01 ± 0.52*** |
| Muscle strength*, ft-l bs | 158.1 ± 58.9 | 105.2 ± 38.4 |
| Physical activity, steps/day | 8431 ± 3358 | 5560 ± 2844& |
TNFα = tumor necrosis factor alpha;
Isokinetic Plantar flexor Peak Torque
p<0.0001
p=0.003
p=0.0013
p=0.0081
Figure 1.
Serum zonulin (A) and HMGB1 (B) concentrations among healthy young (n = 19) and older adults (n = 18). * indicates p < 0.05 for differences between groups. Scatterplots indicating correlation of serum zonulin with tumor necrosis factor α(C, p=0.032) and interleukin 6 (D, p=0.043).
Figure 2.
Scatterplots indicating correlations of serum zonulin (A, B) and HMGB1 (C, D) with plantar flexor muscle strength (A,p=0.048; C,p=0.023) and number of steps taken per day (B, p=0.016; D,p=0.004).
For primary analyses, a total of 3 outliers were removed (two for IL-6, one for HMGB1). For IL-6, removed outliers were 7.9 and 5.1 SD above the group mean. For HMGB1, the removed outlier was 3.2 SD above the group mean. For HGMB1, the group effect remained significant with older adults displaying greater systemic concentrations than younger adults (p = 0.005). With the outlier included, correlations with HMGB1 remained consistent (TNFα: r = 0.233, p = 0.172; peak torque: r = −0.382, p = 0.022; steps/day: r = −0.525, p = 0.001) with the exception of IL-6 which became statistically significant (r = 0.358, p = 0.029). The correlation of zonulin with IL6 also fell just beyond the stated threshold for statistical significance (r = 0.314, p = 0.058) though the overall trend was similar.
Discussion
The human intestinal tract and bacterial microorganisms within it have recently received increasing interest relative to their role in human health and disease. Accumulating evidence supports the notion that the “gut” is an important physiologic site, critical for regulating immune responses, and associated with development of multiple chronic diseases. The central findings of the present study are that 1) serum concentrations of zonulin and HMGB1 are elevated in a cohort of older compared with younger adults, 2) serum zonulin, but not HMGB1, is positively correlated with concentrations of age-related inflammatory cytokines (TNFα and IL6), and 3), concentrations of both zonulin and HMGB1 are negatively correlated with skeletal muscle strength and habitual physical activity. The latter are two key indices of age-related frailty. To our knowledge, this is the first study to indicate that serum concentrations of both zonulin and HMGB1 are elevated in association with advanced age. These findings support the suggestion that intestinal permeability and inflammation may contribute, at least partially, to age-related increases in chronic inflammation and associated disorders/diseases.
Recently, emerging evidence has suggested that the gut microbiota may play an integral role in these agerelated inflammatory changes. Though frequently overlooked in considerations of human health, gut microorganisms encode >150 times more genes than the human genome and are highly involved in numerous metabolic reactions that influence normal host physiology and metabolism. To this end, substantial evidence now indicates that the gut microbiota is substantially involved in regulatory processes both locally and within organs distal from the intestine.14 Accordingly, changes to the gut microbiota appear to have tremendous potential for regulating inflammation and overall health of an organism. Several studies have found that advanced age is associated with gut dysbiosis.7 Moreover, these reports also indicate that dysbiosis is associated with chronic inflammation, as well as, key geriatric syndromes including cognitive impairment and physical frailty. Despite these intriguing findings, evidence related to the mechanism of dysbiosis-related inflammation is sparse. Here we investigated biomarkers related to a “leaky gut” hypothesis suggesting that the intestinal barrier preventing harmful substances from reaching the bloodstream is more permeable in advanced age. The leaky gut is well-associated with inflammatory bowel conditions but is now being proposed as a contributor to a wide variety of health conditions.9 To our knowledge, only one prior study has reported evidence of increased intestinal permeability in older humans. Man and others15 have recently demonstrated that, compared with younger adults, ileal tissues from older adults have increased IL-6 concentrations accompanied by increased intestinal permeability.
The present study is important in two ways: 1) it provides evidence of intestinal permeability using a circulating biomarker, zonulin, which can be easily collected clinically and 2) it supports prior associations of gut dysbiosis with chronic inflammation and frailty by highlighting associations with biomarkers of intestinal permeability (zonulin) and inflammation (HMGB1). These findings are important because they support the suggestion that increases in gut permeability are likely to permit release of bacterial metabolites into the circulation. Thus, it is tempting to suggest that aging-linked dysbiosis would be associated with release of microbial metabolites that would have adverse effects on overall physiological homeostasis. These findings also have important implications for advancing research related to age-related inflammation and dysbiosis, as well as, potentially suggesting a clinically-relevant biomarker for identifying intestinal dysfunction. This was an exploratory study limited by a small sample size and our findings warrant replication in larger cohorts. Moreover, our findings require replication in more diverse cohorts of older adults with a variety of behavioral (i.e. diet/exercise) and medical backgrounds. In conclusion, this study’s findings indicate that serum zonulin and HMGB1 concentrations are elevated in healthy aging and these biomarkers are correlated with several indices of chronic inflammation and physical frailty.
Acknowledgments
Funding: This work was supported by National Institute of Health grants HL33610, HL56921 (M.K. Raizada and C.J. Pepine); UM1 HL087366 to the Cardiovascular Cell Therapy Research Network and the Gatorade Trust through funds distributed by the University of Florida, Department of Medicine (C.J. Pepine and Y.F. Qi); NIH NCATS—University of Florida Clinical and Translational Science UL1TR001427, KL2TR000065 (T.W. Buford, C.J. Pepine); PCORI—OneFlorida Clinical Research Consortium CDRN-1501-26692 (C.J. Pepine); Merck & Co. - Investigator Initiated Study#37268 (T.W. Buford), NIH NIA - University of Florida Claude D. Pepper Older Americans Independence Center P30AG028740 (T.W. Buford) and the Dean’s Office of the College of Medicine, University of Florida, and Florida Heart Foundation Stop Heart Disease (Y.F. Qi).
Footnotes
Conflicts of interest: No
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franceschi C, Motta L, Motta M, et al. The extreme longevity: the state of the art in Italy. Exp Gerontol. 2008;43:45–52. doi: 10.1016/j.exger.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Troen BR. The biology of aging. Mt Sinai J Med. 2003;70:3–22. [PubMed] [Google Scholar]
- 3.Cevenini E, Caruso C, Candore G, et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr Pharm Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 4.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 5.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaneo A, Cattane N, Galluzzi S, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Jackson MA, Jeffery IB, Beaumont M, et al. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8-016-0262-7. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoletti C. Age-associated changes of the intestinal epithelial barrier: local and systemic implications. Expert Rev Gastroenterol Hepatol. 2015;9:1467–1469. doi: 10.1586/17474124.2015.1092872. [DOI] [PubMed] [Google Scholar]
- 10.Sturgeon C, Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 12.Buford TW, Macneil RG, Clough LG, et al. Active muscle regeneration following eccentric contractioninduced injury is similar between healthy young and older adults. J Appl Physiol. 2014;116:1481–90. doi: 10.1152/japplphysiol.01350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackey DC, Manini TM, Schoeller DA, et al. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1108–1113. doi: 10.1093/gerona/glr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man AL, Bertelli E, Rentini S, et al. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci (Lond) 2015;129:515–527. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]