Abstract
Background
Overweight and obesity are common in pediatric populations. Children with autism spectrum disorder (ASD) and disruptive behavior may be at higher risk.
Objective
This study examined whether children with ASD and disruptive behavior are more likely to be overweight or obese than matched controls.
Methods
Baseline data from medication-free children with ASD who participated in trials conducted by the Research Units on Pediatric Psychopharmacology Autism Network (N=276) were compared to 544 control children from the National Health and Nutrition Examination Survey database matched on age, sex, race, parent education, and era of data collection.
Results
Complete data were available on 276 children with ASD (mean age = 7.9 ± 2.6 years; 84.4% males). In the ASD group, the prevalence was 42.4% for overweight and 21.4% for obesity compared to 26.1% for overweight and 12.0% for obesity among controls (p < 0.001 for each contrast). Within the ASD sample, obesity was associated with minority status and lower daily living skills.
Conclusion
To date, this is the largest case-control study of overweight and obesity in children with ASD and disruptive behavior. Findings suggest children with ASD and disruptive behavior are at increased risk for obesity, underscoring the need for weight management interventions in this population.
Keywords: Prevalence, NHANES, risperidone
Introduction
In addition to the core features of impaired social communication, repetitive behavior, and restricted interests, children with autism spectrum disorder (ASD) may also have gastrointestinal disturbances (McElhanon et al., 2014), feeding problems (Sharp et al., 2013), and disruptive behaviors (e.g., defiance, tantrums, aggression, self-injury, and hyperactivity). Although evidence suggests that children with ASD may be more likely to be overweight or obese compared with the general pediatric population, results are not consistent. To date, 17 studies have investigated this association (Table 1). These studies estimate the prevalence of obesity in children with ASD to be between 10% and 31.8% (Whitely et al, 2004; Phillips et al., 2014). Odds ratios for obesity in children with ASD compared to controls range from 1.16 to 4.83 (De Vinck-Baroody et al., 2015; Broder-Fingert et al., 2014). These wide ranging estimates are likely due to differences in the source of the sample and assessment methods. For example, 53% of studies estimated obesity based on chart review or parent-reported anthropometric parameters and 65% relied on parent-reported ASD diagnosis. Although eleven studies included a control group, only three specified matching procedures. Furthermore, few studies have investigated factors, such as ethnic minority status, intellectual ability, or age – which are associated with obesity in other pediatric populations (Broder-Fingert et al., 2014; Egan et al., 2013). Two recent, large studies from the Autism Treatment Network (ATN) database provide the most methodologically-sound evidence for higher prevalence of obesity in ASD to date (De Vinck-Baroody et al., 2015; Hill et al., 2015), both using the National Health and Nutrition Examination Surveys (NHANES) as a comparison group. De Vinck-Baroody et al. (2015) matched on age, sex, race/ethnicity, and parental education, whereas Hill et al. (2015) did not employ matching procedures. Both studies reported the prevalence of obesity in children with ASD to be approximately 18%. The odds of obesity compared to NHANES comparison groups were 1.16 (p = .003) and 1.10 (p = .12), respectively (De Vinck-Baroody et al., 2015; Hill et al., 2015). Notable factors associated with obesity in children with ASD included Hispanic ethnicity, parental high school education (versus post-secondary), high birth weight, and macrocephaly (De Vinck-Baroody et al., 2015).
Table 1.
Summary of studies examining obesity in children with ASD.
| Study | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||
| Study Characteristics | Broder-Fingert et al., 2014 | Chen et al., 2010 | Curtin et al., 2005 | Curtin et al., 2010 | De Vinck-Baroody et al., 2015 | Dreyer Gillette et al., 2015 | Egan et al., 2013 | Evans et al., 2012 | Hill et al., 2015 | Ho et al., 1997 | Hyman et al., 2012 | Memari et al., 2012 | Phillips et al., 2014 | Rimmer et al., 2010 | Whitely et al., 2004 | Xiong et al., 2009 | Zuckerman et al., 2014 | N | % of Total Studies (17 Total) |
| Design | |||||||||||||||||||
| - Prospective | — | — | — | — | — | — | — | X | — | X | — | X | — | — | — | X | — | 4 | 23.5% |
| - Existing Data/Retrospective | X | X | X | X | X | X | X | — | X | — | X | — | X | X | X | — | X | 13 | 76.5% |
| Anthropometrics | |||||||||||||||||||
| - Direct physical measurement | — | — | — | — | X | — | — | X | X | X | X | X | — | — | — | X | X | 8 | 47.1% |
| - Chart review | X | — | X | X | — | — | X | — | — | — | — | — | — | — | — | — | — | 4 | 23.5% |
| - Parent Report | — | X | — | — | — | X | — | — | — | — | — | — | X | X | X | — | — | 5 | 29.4% |
| ASD Group | |||||||||||||||||||
| - Sample Size | 2976 | 247 | 42 | 454 | 2769 | >900 | 273 | 53 | 5053 | 54 | 362 | 113 | 93 | 159 | 50 | 429 | 376 | ||
| Comparison Group | X | — | — | X | X | X | X | X | X | — | X | — | X | X | X | — | — | 11 | 64.7% |
| - Sample Size | 3696 | — | — | — | — | — | — | 58 | 8844 | — | 559 | — | 8,141 | — | — | — | — | ||
| - Matching Procedures Reported | — | — | — | — | X | — | — | — | — | — | X | — | — | — | X | — | — | 3 | 17.6% |
| Findings | |||||||||||||||||||
| - Prevalence | 23.2% | 23.4% | 19% | 30.4% | 18.2% | 23.9% | 17.6% | 17% | 18% | — | 14–16% | 27.4% | 31.8% | 24.6% | 10% | 18.4% | 17% | ||
| - Odds ratio | 4.83 | — | — | 1.42 | 1.16 | — | — | — | — | — | — | — | — | 2.19 | — | — | — | ||
X, study characteristic or variable reported in study; —, study characteristic or variable not available/omitted in article
Given the prevalence of ASD and evidence of increased risk of obesity, enhanced recognition of vulnerable subgroups is warranted. Childhood obesity may persist into adolescence and adulthood and is associated with several detrimental health outcomes, including impaired glucose tolerance, hyperinsulinemia, dyslipidemia, type 2 diabetes, non-alcoholic fatty liver disease, and cardiovascular disease (Cunningham et al., 2014; Reilly and Kelly, 2011; Weiss et al., 2009; Katzmarzyk et al., 2012; Quattrin et al., 2005). Findings from a large-scale chart review suggest that these adverse outcomes hold true in the ASD population (Croen et al., 2014). Compared to adults without ASD, those with ASD had a 69% higher prevalence of obesity, 42% greater risk of hypertension, and 50% increase in diabetes. Identification of risk factors in children with ASD is a prerequisite for addressing long-term individual health burdens and societal costs of obesity in this population. Available evidence from both clinical and community samples suggests that children with impulsiveness, inattention, and conduct problems without ASD have higher rates of overweight and obesity (Griffiths et al., 2011; Agranat-Meged et al., 2005). Anderson and colleagues (2006) reported that higher body mass index z-scores in children with disruptive behavior problems persist into adulthood. Despite as many as 50% of children with ASD having disruptive behavior (Hartley et al., 2008; Mazurek et al., 2013), no studies have investigated the prevalence and clinical correlates of obesity in children with ASD and disruptive behavior. The aims of the current study were to examine the prevalence of obesity in a well-characterized, treatment-seeking sample of children with ASD accompanied by disruptive behaviors compared to controls and to identify characteristics associated with overweight and obese status in children with ASD.
Methods
Participants
The study sample of 297 children with ASD (age 4 to 17 years) were participants in one of three randomized clinical trials conducted by the National Institute of Mental Health-funded Research Units on Pediatric Psychopharmacology (RUPP) Autism Network conducted between 1999 and 2007 at University of California at Los Angeles, Indiana University, Kennedy Krieger Institute at Johns Hopkins University, the Ohio State University, and Yale University. The studies were approved by each institutional review board and written informed consent was obtained from a parent or legal guardian prior to data collection. Study 1 was a double-blind, placebo-controlled trial of risperidone in children with autistic disorder (N=101) and serious behavioral problems (e.g., tantrums, aggression, and self-injury as evidenced by a score of ≥18 on the Aberrant Behavior Checklist Irritability subscale) (RUPP Autism Network, 2002). Using similar entry criteria, Study 2 enrolled 124 children with ASD who were randomly assigned to risperidone alone or risperidone plus parent training (Aman et al., 2009; Scahill et al., 2012). Study 3 compared methylphenidate to placebo in children with ASD (n=72) accompanied by a per-item mean score of ≥1.5 on the Swanson, Nolan, and Pelham–IV ADHD scale (Swanson, 1992) (RUPP Autism Network, 2005). Thus, in all three trials, disruptive behavior was required for inclusion. The pretreatment assessments were the same across these studies and included evaluation of intelligence quotient (IQ) and adaptive behavior, as well as medical, developmental, and psychiatric histories. Each child was deemed healthy following a physical examination, routine laboratory tests prior to study entry, and collection of anthropometric data. The diagnosis of ASD was conducted by an experienced team at each site and corroborated by the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). In all three trials, children had to be medication-free, with the exception of stable treatment with anticonvulsants for seizures. The duration of washout depended on the half-life of the prior medication (e.g., three to four weeks for long-acting medications such as fluoxetine; two weeks for most other medications). In the risperidone trials, children with a prior adequate trial of risperidone were excluded (defined as at least two weeks of treatment at a dose of ≥1.5 mg per day).
Control sample
NHANES collects cross-sectional data every other year to evaluate the health and nutritional status of children and adults in the United States. Data on height and weight are obtained by direct measurement. Children with ASD were matched on age, sex, race, parent’s highest education, and era of data collection. To match ASD subjects on era, we used NHANES data spanning from 2001 to 2006. Autism-specific information is not included in the NHANES data. To screen out children with a possible diagnosis of ASD, we excluded children from the NHANES sample whose parents endorsed the presence of physical, mental, or emotional problems or placement in special education or early intervention programs.
Variables
Demographic Characteristics
Demographic variables for cases and controls included age, sex, caregiver-reported child race/ethnicity (non-Hispanic Caucasian, non-Hispanic African-American, Hispanic, and other), and primary caregiver education status (less than 9th grade, some high school, high school or GED equivalent, some college, and college degree or higher).
Height and Weight Measures
Height (cm) and weight (kg) were obtained pre-treatment in all RUPP studies. We calculated BMI percentile by age for each participant and converted to age- and sex-adjusted Z-scores based on the 2000 CDC growth charts (Kuczmarski et al., 2002). Overweight was defined as a BMI ≥ 85th percentile (age-sex adjusted Z-score > 1.036). Obesity was defined as a BMI ≥ 95th percentile (age-sex adjusted Z-score > 1.645).
RUPP Study Instruments
Intellectual functioning
Several standardized tests of intelligence were used in these studies. IQ results were dichotomized as < 70 (intellectual disability) or ≥ 70 (normal range) across measures.
Adaptive Functioning
The Vineland Adaptive Behavior Scale is a semi-structured parent interview designed to measure adaptive functioning across three domains: Communication, Daily Living Skills, and Socialization (Sparrow et al., 1984). For each domain, the Vineland yields standard scores (mean=100; SD=15), with higher scores representing greater adaptive functioning.
Behavioral Problems
The Aberrant Behavior Checklist (ABC) is a 58-item, caregiver-completed measure designed to assess behavior problems across 5 subscales (Irritability, Social Withdrawal, Stereotypy, Hyperactivity, Inappropriate Speech) with normative data available for developmentally-disabled populations (Aman et al, 1985; Kaat et al., 2014). Each item is scored from 0 to 3 with higher scores reflecting greater problems. The Children’s Yale-Brown Obsessive Compulsive Scale for Autism Spectrum Disorder (CYBOCS-ASD) is a semi-structured clinician rating for measuring the severity of repetitive behaviors in children with ASD (Scahill et al., 2006). Each of the 5 items is rated 0 to 4 for a range of 0 to 20 with higher scores indicating greater severity.
Feeding Difficulties
Endorsement of a single item question on the medical history form: “does your child have peculiar eating habits” was used as a proxy for feeding difficulties, such as food selectivity.
Statistical analysis
We used a greedy matching algorithm with the %GMATCH macro in SAS (Bergstralh and Kosanke, 2015). In the greedy matching algorithm, cases are matched to controls using the best available match (“nearest neighbor”) in order to minimize the distances between the matched covariates. Unlike other matching algorithms, a greedy match is considered linear because, once the matched control has been selected, the individual is not reconsidered. Our matching procedures sought to pair cases and controls on all variables. We were able to match NHANES controls to subjects with ASD in a 2:1 ratio in all but 8 cases. In these 8 cases, only one control could be matched on all variables.
Statistical analyses were conducted using SAS v 9.3 (Cary, NC, USA). Statistical significance was assessed at the 0.05 level, unless otherwise noted. Descriptive statistics were calculated for all variables of interest and include: means and standard deviations, medians and ranges, or counts and percentages, when appropriate. Prevalence of overweight status and obesity in children was determined by calculating the proportion of participants with age- and sex-adjusted BMI at or above the 85th and at or above the 95th percentile, respectively. Chi-square tests and two-sample t-tests were used for between group comparisons (i.e., ASD vs. controls, obese vs. non-obese). Normality of data was assessed using histograms and density plots and the Anderson-Darling test. Non-normal data were analyzed using equivalent nonparametric tests as needed (i.e., age, BMI percentile). Within the ASD sample, we examined the association of obesity with demographic and clinical characteristics. The strength of association was quantified by odds ratios (OR) and 95% confidence intervals (CI). For the within ASD group analyses, we used odds ratios and 95% CI to evaluate the association of sex, race, IQ, age, adaptive behavior, parental education level, and behavioral problems with obese status.
Results
ASD Sample Description
Of the 297 RUPP study participants, 283 (95.3%) had available pre-treatment weight and height data. Of these, 276 (92.9%) had adequate data for matching (e.g., missing data on parent education) with NHANES controls. As shown in Table 2, mean age at study enrollment was 7.9 years (SD=2.6). Participants were predominately male (84.4%) and non-Hispanic Caucasian (69.9%). Over half of participants (57.4%) had an IQ below 70. Parents reported concern about peculiar eating habits in 58.4% (122 of 209 available responses) of the sample. Scores on the Vineland were in the low to moderate range for adaptive functioning. The mean ABC Irritability subscale indicated a high level of disruptive behavior. The mean score on the CYBOCS-ASD was 14.7 (SD=3.6) suggesting the moderately severe repetitive behavior. Using CDC guidelines, the prevalence of overweight was 42.4% and obesity was 21.4%.
Table 2.
Characteristics of children with ASD and disruptive behavior in three RUPPa Autism Network trials (N = 276).
| Variable | N | % |
|---|---|---|
| Sex (male) | 233 | 84.4 |
| Race/Ethnicity | ||
| Non-Hispanic Caucasian | 193 | 69.9 |
| Non-Hispanic African American | 30 | 10.9 |
| Hispanic | 24 | 8.7 |
| Other | 29 | 10.5 |
| IQ < 70 (n = 258) | 148 | 57.4 |
| Peculiar Eating Habits (n = 209) | 122 | 58.4 |
|
| ||
| Mean | SD | |
|
| ||
| Age (years) | 7.9 | 2.6 |
| Vineland Adaptive Behavior Scales (N=274) | ||
| Communication | 54.1 | 20.9 |
| Daily living | 45.2 | 20.2 |
| Socialization | 55.3 | 15.9 |
| Aberrant Behavior Checklist (N=273) | ||
| Irritability | 25.0 | 9.3 |
| Social Withdrawal | 15.0 | 8.8 |
| Stereotypy | 8.8 | 5.5 |
| Hyperactivity | 33.9 | 8.8 |
| Inappropriate Speech | 5.9 | 3.8 |
| CYBOCS-ASD Total Scoreb (N=276) | 14.7 | 3.6 |
=Research Units on Pediatric Psychopharmacology;
=Children’s Yale-Brown Obsessive Compulsive Scales for ASD
Comparison between ASD and NHANES Controls
NHANES controls (n=544) were compared to 276 study subjects (Table 3). The median (25th – 75th) BMI percentiles were 79.6 (54.9 – 93.6) and 62.8 (34.0 – 86.3) for the ASD sample and controls, respectively. The prevalence of combined overweight and obese children was significantly higher in the ASD sample compared to controls (see Figure 1). As shown in the figure, children with ASD were nearly twice as likely to be obese compared to the NHANES sample.
Table 3.
Demographics, BMI and weight status in children with ASD and NHANESa controls.
| Characteristic | Group
|
p-value | |
|---|---|---|---|
| ASD (N = 276) |
Controls (N = 544) |
||
|
medianb (25th – 75th) |
medianb (25th – 75th) |
||
|
|
|||
| Age (months) | 89 (70 – 112.5) | 90 (69.5 – 113) | 0.865 |
|
|
|||
| N (%) | N (%) | ||
|
|
|||
| Sex | |||
| Female | 43 (15.6%) | 85 (15.6%) | 0.987 |
| Male | 233 (84.4%) | 459 (84.4%) | |
| Race/Ethnicity | |||
| Non-Hispanic White | 193 (69.9%) | 384 (70.6%) | 0.993 |
| Non-Hispanic African American | 30 (10.9%) | 60 (11.0%) | |
| Hispanic | 24 (8.7%) | 46 (8.5%) | |
| Other | 29 (10.5%) | 54 (9.9%) | |
| Parent’s Highest Education | |||
| Less than 9th Grade | 3 (1.1%) | 5 (0.9%) | 1.00 |
| Some High School | 11 (4.0%) | 22 (4.0%) | |
| High School or GED equivalent | 44 (15.9%) | 86 (15.8%) | |
| Some College | 85 (30.8%) | 166 (30.5%) | |
| College Degree or Higher | 133 (48.2%) | 265 (48.7%) | |
| Era | |||
| 1999 – 2003 | 156 (56.5%) | 334 (61.4%) | 0.179 |
| 2004 – 2007 | 120 (43.5%) | 210 (38.6%) | |
|
|
|||
|
medianb (25th – 75th ) |
medianb (25th – 75th ) |
||
|
|
|||
| BMI Percentile | 79.6 (54.9 – 93.6) | 62.8 (34.0 – 86.3) | < 0.001 |
|
|
|||
| N (%) | N (%) | ||
|
|
|||
| Overweight and Obese (≥85th percentile) | 117 (42.4%) | 142 (26.1%) | < 0.001 |
| Obese (≥95th percentile) | 59 (21.4%) | 65 (12.0%) | < 0.001 |
= National Health and Nutrition Examination Survey;
=Comparisons between groups for continuous measurements summarized with median (25th – 75th) were compared using Wilcoxon Rank-Sum test
Figure 1. Prevalence of overweight and obesity in children with ASD versus controls.
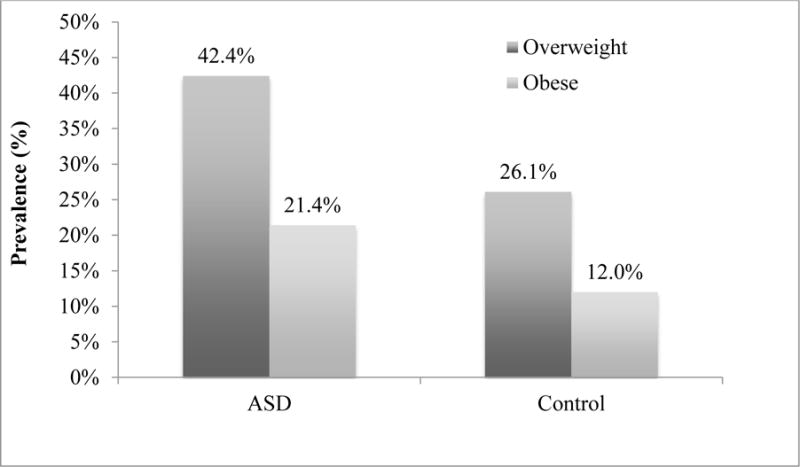
Comparison for overweight: 42.4% in ASD vs. 26.1% in controls; p < 0.001; OR = 2.1; 95% CI [1.5 – 2.8]; Comparison for obesity: 21.4% in ASD vs. 12.0% in controls; p < 0.001; OR = 2.0; 95% CI [1.4 – 3.0]).
Factors Associated with Obesity in Participants in RUPP Trials
Within the ASD sample, minority status (e.g., African American, Hispanic) was associated with an increased likelihood of obesity (47.5% minority status in the obese group versus 25.3% in the non-obese group; OR = 2.66; 95% CI: 1.48 – 4.83). On the Vineland Daily Living score, which measures adaptive everyday living skill, obese children with ASD had lower scores than non-obese children with ASD (40.4 ± 16.2 vs 46.4 ± 20.1; p = 0.04). As shown in Table 4, there were no other significant differences on clinical or demographic characteristics in obese versus non-obese children with ASD.
Table 4.
Factors Associated with Obesity in children with ASD.
| Characteristic | Non-Obese (N = 217) |
Obese (N = 59) |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| Na | n (%) | n (%) | Odds Ratio | (95% CI) | P-value | |
|
|
||||||
| Sex (male) | 183 (84.3%) | 50 (84.8%) | 1.03 | (0.46, 2.26) | 0.938 | |
| Race (minority) | 55 (25.3%) | 28 (47.5%) | 2.66 | (1.48 – 4.83) | 0.001* | |
| IQ (< 70) | 259 | 114 (46.2%) | 34 (61.8%) | 0.79 | (0.43 – 1.46) | 0.452 |
| Peculiar Eating Habits | 209 | 94 (56.2%) | 28 (62.6%) | 1.28 | (0.67, 2.63) | 0.426 |
| Parent Education (less than college degree) | 114 (52.5%) | 29 (49.2%) | 1.03 | (0.92, 1.15) | 0.645 | |
|
|
||||||
| M (SD) | M (SD) | |||||
|
|
||||||
| Age (years) | 7.8 (2.4) | 8.2 (3.1) | (0.96, 1.19) | 0.249 | ||
| Vineland Daily Living Scale | ||||||
| Communication | 274 | 55.3 (21.1) | 49.5 (19.8) | (0.97, 1.00) | 0.060 | |
| Daily Living | 274 | 46.5 (20.1) | 40.6 (20.0) | (0.97, 1.00) | 0.048* | |
| Socialization | 274 | 56.2 (15.7) | 51.9 (16.2) | (0.96, 1.00) | 0.063 | |
| ABC Scales | ||||||
| Irritability | 273 | 25.0 (9.4) | 25.1 (9.3) | (0.97, 1.03) | 0.903 | |
| Social Withdrawal | 273 | 14.6 (8.6) | 16.7 (9.3) | (1.00, 1.06) | 0.097 | |
| Stereotypy | 272 | 8.7 (5.3) | 9.3 (6.0) | (0.97, 1.07) | 0.511 | |
| Hyperactivity | 273 | 33.6 (8.9) | 35.0 (8.4) | (0.98, 1.05) | 0.293 | |
| Inappropriate Speech | 273 | 5.9 (3.7) | 5.7 (4.3) | (0.91, 1.06) | 0.705 | |
| CYBOCS-ASD | 14.6 (3.7) | 14.9 (3.1) | (0.94, 1.11) | 0.621 | ||
N = 276 unless otherwise specified.
p <.05
Discussion
To our knowledge, this is the largest study of overweight and obese status in children with ASD and disruptive behavior. In our sample from three RUPP Autism Network studies, children were nearly twice as likely to be overweight or obese compared to matched controls drawn from NHANES. Within the RUPP study participants, risk for obesity was amplified by minority status, a finding consistent with the extant literature (e.g., Hill et al., 2015). On the Vineland Daily Living domain, obese children also had lower mean scores than non-obese children. This association is unique compared to previous research (e.g., De Vinck-Baroody et al., 2015). This highlights the need to explore the possible interaction between disruptive behavior, adaptive behavior, and obesity in ASD. Collectively, our findings suggest that children with ASD and disruptive are at increased risk for obesity. In addition to replication, future studies can examine factors that contribute to this observed association.
Our study detected a higher prevalence of obesity in children with ASD and disruptive behavior (21.4%), compared to the prevalence of 18% reported in two recent ATN studies (De Vinck-Baroody et al., 2015; Hill et al., 2015). The difference in the rates of overweight and obesity in our sample may be due to a combination of factors. First, in the ATN sample only 20.6% to 34.4% had disruptive behavior problems (De Vinck-Baroody et al., 2015; Hill et al., 2015). By definition, this combined sample from the RUPP trials was enriched with children with disruptive behavior problems. Second, the ATN samples were younger, starting at age 2. Studies in the general pediatric population indicate that the rate of obesity tends to be higher in children over 5 years (Cunningham et al., 2014). Third, to screen out potential children with ASD from the NHANES sample, our matching algorithm excluded children with physical, mental, or emotional problems and children requiring special education or early intervention services. In addition, non-ASD conditions of attention deficit/hyperactivity disorder, learning or behavioral disability, and developmental delay have also been associated with higher rates of obesity than in children without these conditions (Philips et al., 2014). It is possible that prior studies using NHANES as a control may have inadvertently included some children with ASD or these other conditions; thus, reducing the overall magnitude of group differences. One or more of these factors may explain the greater odds ratio of overweight and obese in our sample of 2.0 compared to 1.10–1.16 in the ATN samples (De Vinck-Baroody et al., 2015; Hill et al., 2015).
If children with ASD and disruptive behavior are more likely to be overweight or obese, an important next step is to develop and test effective treatments. Guidelines for the prevention, assessment, and treatment of obesity in the general population of youth are available (Barlow et al., 2007). Findings from several randomized trials support the efficacy of diet modification and behavioral approaches to treat childhood obesity (Foster et al., 2015; Wilson et al., 2003). There are no specific guidelines, however, for prevention or treatment of overweight or obese children with ASD. Assessment and analysis of diet, mealtime behaviors, and meal structure may be useful to identify factors which lead to obesity (e.g., McKenzie et al., 1991). Given the combination of social deficits, restricted interests, lack of social partners for physical activities, and developmental disabilities in children with ASD, successful educational, dietary and exercise programs for obesity in the general pediatric population may not be applicable to children with ASD (Obrusnikova and Cavalier, 2011; Rodger and Umaibalan, 2011). This may be especially true for children with ASD and disruptive behavior. Food selectivity in this population often involves strong preference for processed foods, snacks, and sweets, and a lower intake of fruits and vegetables (Sharp et al., 2013; Berry et al., 2015; Ledford and Gast, 2006). Disruptive behavior may make it difficult for parents to limit carbohydrates, introduce novel foods into the diet, or engage children with ASD in physical activity. Beyond diet and weight management in children with ASD, increased attention on the use and food as reinforcement in behavioral interventions is also warranted (Grondhuis and Aman, 2014).
Antipsychotics are commonly used and are successful for treating disruptive behavior in children with ASD (Gerhard et al., 2009). Use of these medications, however, can lead to unnecessary and unintended weight gain (Correll et al., 2009; Martin et al., 2004; Scahill et al., 2016). At minimum, our findings emphasize the importance of a careful risk-benefit discussion with caregivers about treatment options in children with ASD and disruptive behavior. For example, a recent large-scale randomized trial of parent training showed superiority to parent education for reducing disruptive behavior in young children with ASD, which deserves discussion as an alternative treatment (Bearss et al., 2015). In cases of ASD for whom medication is being considered, routine health screening as well as height and weight monitoring are warranted. Moreover, assessment for weight status and weight management interventions, including attention to diet and exercise, are indicated.
Although this study supports an association between obesity and disruptive behavior in children with ASD, our findings need to be considered against several limitations. First, our findings may not generalize to all children with ASD and disruptive behavior. Our study consisted of treatment-seeking children who participated in NIH-funded medication trials focused on disruptive behavior. At baseline, all study participants were medication-free. In the risperidone trials, children with a prior adequate trial of risperidone were excluded. This exclusion was not applied in the methylphenidate study. Because we did not systematically collect each child’s prior medication history, we cannot rule out the possibility that some children were exposed to medications that promoted weight gain or weight loss. Moreover, we did not collect extensive family histories, family eating habits or measure parents’ height and weight. Thus, the impact of these factors known to affect weight in children is uncertain. Third, the screening item regarding “peculiar eating” was insufficient to evaluate the association of the child’s feeding habits and obesity (e.g., refusal, food selectivity, obesogenic diet). Future research should include a more detailed assessment of diet and mealtime difficulties, physical activity level, and possible biological factors (e.g., gut microbiome) (Galley et al., 2014; Mulle et al., 2013) that may play a role in obesity in this population.
In summary, our findings suggest that disruptive behavior in children with ASD increases the risk of obesity and overweight compared to matched controls. The presence of disruptive behavior in children with ASD may increase the likelihood of use of medications such as risperidone and aripiprazole - which may further contribute to weight gain. These results suggest that tailoring interventions to address both obesity and disruptive behavior in children with ASD is warranted.
Acknowledgments
Dr. Criado participated in study conception and drafting and revision of the article; Dr. Sharp participated in study conception and critically reviewed and revised the manuscript; Dr. C. McCracken analyzed the data; Drs. De Vinck-Baroody, Aman, McDougle, J. McCracken, Arnold, Weitzman, Leventhal, and Vitiello provided critical review and revision of the manuscript; Mr. Dong assisted with data analysis; Dr. Scahill participated in study conception and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors acknowledge the contributions of Epiphany Nyirabahizi, for assembling the data set and preliminary analysis, and Elaine Tierney, for serving as a site PI for two studies included in this sample.
Funding
This work was supported by the National Institute of Mental Health [N01MH80011 to Dr. Aman, N01MH70001 to Dr. McDougle, N01MH70010 to Dr. J McCracken, N01MH70009 to Dr. Scahill, U10MH66768 to The Ohio State University, U10MH66766 to Indiana University, U10MH66764 to Yale University, K23 MH068627 to Dr. Posey, and K24 MH001805 to Dr. J. McCracken]; General Clinical Research Centers, National Center for Research Resources, National Institutes of Health [M01 RR00750 to Indiana University, M01 RR00052 to Johns Hopkins University, M01 RR00034 to The Ohio State University, and M01 RR06022 to Yale University]; and the Korczak Foundation [to Dr. Scahill]. Study medications were provided by Janssen Pharmaceutica and Johnson & Johnson Pharmaceutical Research & Development.
Abbreviations
- ASD
autism spectrum disorder
- ATN
Autism Treatment Network
- NHANES
National Health and Nutrition Examination Surveys
- ADHD
attention deficit/hyperactivity disorder
- BMI
body mass index
- RUPP
Research Units on Pediatric Psychopharmacology
- IQ
intelligence quotient
- ADI-R
Autism Diagnostic Interview-Revised
- ABC
Aberrant Behavior Checklist
- CYBOCS-ASD
Children’s Yale-Brown Obsessive Compulsive Scale for Autism Spectrum Disorder
- OR
odds ratio
- CI
Confidence Interval
Footnotes
Conflict of Interest Statement
Dr. Aman has received research contracts, consulted with, served on advisory boards, or done investigator training for CogState, Inc., CogState Clinical Trials, Ltd., Coronado Biosciences, Forest Research, Hoffman-La Roche, Lumos Pharma, MedAvante, Inc., ProPhase LLC, and Supernus Pharmaceuticals. Dr. J. McCracken has received NIMH research grant and contract funds; consultant income from Roche; research contract support from Seaside Pharmaceuticals and Roche; speaker honoraria from the Tourette Syndrome Association; and study drug and placebo from Shire. Dr. Arnold has received research funding from, Forest, Lilly, Shire, Supernus, and Young Living (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Arbor, Otsuka, Pfizer, Roche, Seaside Therapeutics, and Waypoint. Dr. Vitiello has received salary support from NIH, and consultant fees from the American Physician Institute for Advanced Professional Studies. No other authors have potential conflicts of interest to disclose.
References
- Agranat-Meged AN, Deitcher C, Goldzweig G, et al. Childhood obesity and attention deficit/hyperactivity disorder: a newly described comorbidity in obese hospitalized children. The International Journal of Eating Disorders. 2005;37:357–359. doi: 10.1002/eat.20096. [DOI] [PubMed] [Google Scholar]
- Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(12):1143–1154. doi: 10.1097/CHI.0b013e3181bfd669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, et al. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89(5):485–491. [PubMed] [Google Scholar]
- Anderson SE, Cohen P, Naumova EN, et al. Relationship of childhood behavior disorders to weight gain from childhood into adulthood. Ambulatory Pediatrics. 2006;6:297–301. doi: 10.1016/j.ambp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Barlow SE, Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Bergstralh E, Kosanke J. Locally Written SAS Macros. 2003 Available at: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros (accessed 20 June 2015)
- Berry RC, Novak P, Withrow N, et al. Nutrition Management of Gastrointestinal Symptoms in Children with Autism Spectrum Disorder: Guideline from an Expert Panel. Journal of the Academy of Nutrition and Dietetics. 2015;115(12):1919–1927. doi: 10.1016/j.jand.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Broder-Fingert S, Brazauskas K, Lindgren K, et al. Prevalence of overweight and obesity in a large clinical sample of children with autism. Academic Pediatrics. 2014;14(4):408–414. doi: 10.1016/j.acap.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Chen A, Kim SE, Houtrow AJ, et al. Prevalence of Obesity among Children with Chronic Conditions. Obesity. 2010;18(1):210–213. doi: 10.1038/oby.2009.185. [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin C, Anderson SE, Must A, et al. The Prevalence of Obesity in Children with Autism: A secondary data analysis using Nationally Representative Data from the National Survey of Children’s Health. BMC Pediatrics. 2010;10:11. doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin C, Bandini LG, Perrin EC, et al. Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: a chart review. BMC Pediatrics. 2005;5:48. doi: 10.1186/1471-2431-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, et al. Psychiatric and Medical Conditions Among Adults with ASD. Paper presented at the 14th Annual Meeting of the International Society for Autism Research (INSAR); Atlanta, GA. 2014. Available at: https://imfar.confex.com/imfar/2014/webprogram/start.html. (accessed 22 October 2014) [Google Scholar]
- Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. New England Journal of Medicine. 2014;370(5):403–411. doi: 10.1056/NEJMoa1309753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vinck-Baroody O, Shui A, Macklin EA, et al. Overweight and Obesity in a Sample of Children With Autism Spectrum Disorder. Academic Pediatrics. 2015;15(4):396–404. doi: 10.1016/j.acap.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Dreyer Gillette ML, Borner KB, Nadler CB, et al. Prevalence and Health Correlates of Overweight and Obesity in Children with Autism Spectrum Disorder. Journal of Developmental and Behavioral Pediatrics. 2015;36(7):489–496. doi: 10.1097/DBP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Egan AM, Dreyer ML, Odar CC, et al. Obesity in young children with autism spectrum disorders: prevalence and associated factors. Childhood Obesity. 2013;9(2):125–131. doi: 10.1089/chi.2012.0028. [DOI] [PubMed] [Google Scholar]
- Evans EW, Must A, Anderson SE, et al. Dietary Patterns and Body Mass Index in Children with Autism and Typically Developing Children. Research in Autism Spectrum Disorders. 2012;6(1):399–405. doi: 10.1016/j.rasd.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BA, Farragher J, Parker P, et al. Treatment Interventions for Early Childhood Obesity: A Systematic Review. Academic Pediatrics. 2015;15(4):353–361. doi: 10.1016/j.acap.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley JD, Bailey M, Kamp Dush C, et al. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PloS One. 2014;9(11):e113026. doi: 10.1371/journal.pone.0113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard T, Chavez B, Olfson M, et al. National patterns in the outpatient pharmacological management of children and adolescents with autism spectrum disorder. Journal of Clinical Psychopharmacology. 2009;29(3):307–310. doi: 10.1097/JCP.0b013e3181a20c8a. [DOI] [PubMed] [Google Scholar]
- Griffiths LJ, Dezateux C, Hill A. Is obesity associated with emotional and behavioural problems in children? Findings from the Millennium Cohort Study. International Journal of Pediatric Obesity. 2011;6(2–2):e423–e432. doi: 10.3109/17477166.2010.526221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondhuis S, Aman MG. Overweight and obesity in youth with developmental disabilities. Journal of Intellectual Disability Research. 2014;58(9):787–799. doi: 10.1111/jir.12090. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Sikora DM, McCoy R. Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. Journal of Intellectual Disability Research. 2008;52(10):819–829. doi: 10.1111/j.1365-2788.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AP, Zuckerman KE, Fombonne E. Obesity and Autism. Pediatrics. 2015;136(6):1051–1061. doi: 10.1542/peds.2015-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HH, Eaves LC, Peabody D. Nutrient intake and obesity in children with autism. Focus on Autism and Other Developmental Disabilities. 1997;12:187–192. [Google Scholar]
- Hyman SL, Stewart PA, Schmidt B, et al. Nutrient intake from food in children with autism. Pediatrics. 2012;130(Suppl 2 (Supplement)):S145–S153. doi: 10.1542/peds.2012-0900L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(5):1103–1116. doi: 10.1007/s10803-013-1970-0. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Shen W, Baxter-Jones A, et al. Adiposity in children and adolescents: correlates and clinical consequences of fat stored in specific body depots. Pediatric Obesity. 2012;7(5):e42–e61. doi: 10.1111/j.2047-6310.2012.00073.x. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: Methods and development. National Center for Health Statistics. Vital and Health Statistics. 2002;11(246):1–190. [PubMed] [Google Scholar]
- Ledford JR, Gast DL. Feeding problems in children with autism spectrum disorders: a review. Focus on Autism and Other Developmental Disabilities. 2006;21:153–166. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–686. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Gannon K, Cabral HJ, et al. Association between clinically meaningful behavior problems and overweight in children. Pediatrics. 2003;112:1138–1145. doi: 10.1542/peds.112.5.1138. [DOI] [PubMed] [Google Scholar]
- Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2011;21(6):517–535. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- Martin A, Scahill L, Anderson GM, et al. Weight and leptin changes among risperidone-treated youths with autism: 6-month prospective data. The American Journal of Psychiatry. 2004;161(6):1125–1127. doi: 10.1176/appi.ajp.161.6.1125. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Kanne SM, Wodka EL. Physical aggression in children and adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders. 2013;7:455–465. [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, et al. Gastrointestinal symptoms in autism spectrum disorders: A meta-analysis. Pediatrics. 2014;133(5):872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- McKenzie TL, Sallis JF, Nader PR, et al. BEACHES: an observational system for assessing children’s eating and physical activity behaviors and associated events. Journal of Applied Behavior Analysis. 1991;24(1):141–151. doi: 10.1901/jaba.1991.24-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari AH, Kordi R, Ziaee V, et al. Weight status in Iranian children with autism spectrum disorders: Investigation of underweight, overweight and obesity. R Research in Autism Spectrum Disorders. 2012;6:234–239. [Google Scholar]
- Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Current Psychiatry Reports. 2013;15(2):337. doi: 10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrusnikova I, Cavalier AR. Perceived barriers and facilitators of participation in after-school physical activity by children with autism spectrum disorders. Journal of Developmental and Physical Disabilities. 2011;23:195–211. [Google Scholar]
- Pérez-Bonaventura I, Granero R, Ezpeleta L. The relationship between weight status and emotional and behavioral problems in Spanish preschool children. Journal of Pediatric Psychology. 2015;40(4):455–463. doi: 10.1093/jpepsy/jsu107. [DOI] [PubMed] [Google Scholar]
- Phillips KL, Schieve LA, Visser S, et al. Prevalence and impact of unhealthy weight in a national sample of US adolescents with autism and other learning and behavioral disabilities. Maternal and Child Health Journal. 2014;18(8):1964–1975. doi: 10.1007/s10995-014-1442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrin T, Liu E, Shaw N, et al. Obese children who are referred to the pediatric endocrinologist: characteristics and outcome. Pediatrics. 2005;115(2):348–351. doi: 10.1542/peds.2004-1452. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. International Journal of Obesity. 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. New England Journal of Medicine. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- Research Units on Pediatric Psychopharmacology Autism Network. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry. 2005;62(11):1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Rimmer J, Yamaki K, Lowry BM, et al. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. Journal of Intellectual Disability Research. 2010;54(9):787–794. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- Rodger S, Umaibalan V (Journal of Intellectual Disability Research) The routines and rituals of families of typically developing children compared with families of children with autism spectrum disorder: An exploratory study. British Journal of Occupational Therapy. 74:20–26. [Google Scholar]
- Scahill L, Bearss K, Swiezy N, et al. Parent Training versus Parent Education for Disruptive Behavior in Children with Autism Spectrum Disorder. Presented at: the Annual Meeting of the American Academy of Child & Adolescent Psychiatry; San Antonio, TX. 2015. [DOI] [PubMed] [Google Scholar]
- Scahill L, Jeon S, Boorin SG, et al. Weight gain and metabolic consequences of risperidone in young children with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(5):415–423. doi: 10.1016/j.jaac.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, McDougle CJ, Aman MG, et al. Effects of risperidone and parent training on adaptive functioning in children with pervasive developmental disorders and serious behavioral problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(2):136–146. doi: 10.1016/j.jaac.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill L, McDougle CJ, Williams SK, et al. Children’s Yale-Brown Obsessive Compulsive Scale modified for pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1114–1123. doi: 10.1097/01.chi.0000220854.79144.e7. [DOI] [PubMed] [Google Scholar]
- Sharp WG, Berry RC, McCracken C, et al. Feeding Problems and Nutrient Intake in Children with Autism Spectrum Disorders: A Meta-analysis and Comprehensive Review of the Literature. Journal of Autism and Developmental Disorders. 2013;43(9):2159–2173. doi: 10.1007/s10803-013-1771-5. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Carrey NJ, Wiggins DM, et al. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Swanson JM. School-Based Assessments and Interventions for ADD Students. Irvine, CA: KC Publishing; 1992. [Google Scholar]
- Szatmari P, Georgiades S, Duku E, et al. Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry. 2015;72(3):276–283. doi: 10.1001/jamapsychiatry.2014.2463. [DOI] [PubMed] [Google Scholar]
- Weiss R, Shaw M, Savoye M, et al. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Pediatric Diabetes. 2009;10:360–367. doi: 10.1111/j.1399-5448.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- Whitely P, Dodou K, Todd L, et al. Body Mass Index of Children from the United Kingdom diagnosed with Developmental Disorders. Pediatrics International. 2004;46(5):531–533. doi: 10.1111/j.1442-200x.2004.01946.x. [DOI] [PubMed] [Google Scholar]
- Williams SK, Scahill L, Vitiellio B, et al. Risperidone and Adaptive Behavior in Children with Autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(4):431–439. doi: 10.1097/01.chi.0000196423.80717.32. [DOI] [PubMed] [Google Scholar]
- Wilson P, O’Meara S, Summerbell C, et al. The prevention and treatment of childhood obesity. Quality & Safety in Health Care. 2003;12(1):65–74. doi: 10.1136/qhc.12.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N, Ji C, Li Y, et al. The Physical Status of Children with Autism in China. Research in Developmental Disabilities. 2009;30:70–76. doi: 10.1016/j.ridd.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Zuckerman KE, Hill AP, Guion K, et al. Overweight and obesity: prevalence and correlates in a large clinical sample of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(7):1708–1719. doi: 10.1007/s10803-014-2050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


