Abstract
Introduction
In settings where HIV is prevalent in heterosexual population, pregnancy and postpartum breastfeeding periods can be associated with substantial HIV acquisition risk. Pre-exposure prophylaxis (PrEP) with daily oral tenofovir disoproxil fumarate (TDF)/emtricitabine is an attractive HIV prevention option for women who are lactating but data are limited on its safety during the lactation period.
Areas covered
We provide a concise synthesis and summary of current evidence on the safety of TDF-based PrEP during breastfeeding. We conducted a review, searching multiple databases and major PrEP conferences for primary studies with TDF-based PrEP exposure during postpartum breastfeeding.
Expert opinion
TDF-based oral PrEP is an effective female-controlled HIV prevention option. There is evidence supporting the safety of TDF use for infant outcomes during breastfeeding in antiretroviral treatment regimens for HIV and hepatitis B virus, and more limited, but consistently safe, data from use of TDF as PrEP. The potential for risk is arguably outweighed for at-risk individuals by HIV prevention benefits, including indirect protection to the infant as a result of preventing HIV in the breastfeeding mother. As PrEP delivery is scaled up in heterosexual populations in high HIV prevalence settings and for at-risk persons in other settings, implementation science studies can provide a framework to increase the accrual of safety, acceptability, and use data related to PrEP during lactation.
1 Introduction
In settings where HIV is prevalent in heterosexual populations, women have higher than acceptable HIV risk during pregnancy and lactation, with reported pooled cumulative HIV incidence of 3.8 per 100 woman-years (4.7/100 woman-years during pregnancy and 2.9/100 woman-years during lactation period) in one review (1, 2). Elevated risk during these periods in many of these settings is because the general HIV risk is higher than acceptable but may also result from infrequent condom use, unknown partner’s HIV status, and biologic changes in women during pregnancy and lactation or changes in their partner’s sexual partnerships that increase susceptibility (3–10).
Pre-exposure prophylaxis (PrEP) using either daily oral tenofovir disoproxil fumarate (TDF) or co-formulated TDF/emtricitabine (TDF/FTC) is an effective HIV prevention option for individuals at substantial risk for HIV including women who are or might become pregnant or are breastfeeding (11–14). However, clinical trials that established the efficacy of PrEP for HIV prevention excluded pregnant and lactating women, and uncertainty about the safety of PrEP during lactation has the potential to limit large-scale PrEP implementation among women in settings and populations where both HIV infection and breastfeeding are common. In this review, we provide a concise synthesis and summary of current data on the clinical safety of using TDF-based PrEP during lactation.
2 Mechanism of action and clinical pharmacology
TDF is a prodrug for tenofovir (Box 1), an acyclic nucleotide analogue reverse transcriptase inhibitor (15). Tenofovir is a potent competitive inhibitor of HIV and hepatitis B virus (HBV) reverse transcriptase that is additive or synergistic when combined with other antiretroviral agents inhibiting viral replication (15); for both HIV and HBV, it has a high genetic barrier for the development of viral resistance mutations. It has a long elimination and intracellular half-life (~17 and >60 hours, respectively), allowing for once-daily dosing. Tenofovir has poor bioavailability but after oral administration as TDF, it is rapidly converted to tenofovir which is subsequently metabolized intracellularly to tenofovir diphosphate, its active metabolite (16). Tenofovir readily crosses into placenta and amniotic fluid but only appears in suboptimal levels in breast milk (17–19). For breastfeeding women taking oral TDF PrEP, breastmilk will exclusively contain tenofovir in an unconjugated form, and, because of its poor oral bioavailability, negligible tenofovir concentrations would be expected to be excreted into breastmilk and subsequently available to the infants through breastfeeding. Studies of pregnant women on TDF for treatment of HIV infection have reported tenofovir umbilical cord levels >70–100% compared to maternal plasma concentration indicating high placental transfer (20–22). TDF is classified as US Food and Drug Administration Pregnancy Category B meaning that animal reproduction studies fail to demonstrate a risk to the fetus, and adequate, but well-controlled, studies of pregnant women have not been conducted. FTC, a nucleotide reverse transcriptase inhibitor, is structurally similar to lamuvidine, which has been extensively used and studied for HIV treatment and prevention of mother to child HIV transmission as well as for the treatment of HBV infection. FTC has shown to have good placental transfer (>80% of plasma concentration)(19), and readily crosses into the breast milk (18). Both TDF and FTC are not inducers or substrates for cytochrome P450 enzymes but are primarily eliminated unchanged in urine by a combination of glomerular filtration and active proximal tubular secretion (16). TDF, alone or with FTC, when used as PrEP is generally safe and well tolerated. Small but nonprogressive decreases in estimated creatinine clearance levels and decreases in bone mineral density can occur in a minority of persons taking TDF as PrEP but these quickly resolve within weeks of discontinuing PrEP (23–26).
Box 1. Drug Summary.
| Drug name | Tenofovir Disoproxil Fumarate (TDF), alone or formulated with emtricitabine (FTC) |
| Phase | US FDA approval of emtricitabine FTC/-TDF for prevention of HIV infection: 2012 |
| Indication | Prevention and Treatment of HIV infection |
| Pharmacology description | TTDF is an oral prodrug of tenofovir, an acyclic nucleotide (nucleoside monophosphate) analogue with activity against retroviruses, including HIV, and hepadnaviruses. FTC is a synthetic nucleoside analogue of cytidine with activity against HIV reverse transcriptase. |
| Route of administration | Oral |
| Chemical structure |
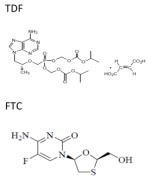
|
| Pivotal trial for safety and efficacy against HIV acquisition in heterosexual women | The Partners PrEP Study was a randomized, double-blind, placebo-controlled 3 arm trial of daily TDF alone or in combination with FTC in 4747 serodiscordant heterosexual couples in Kenya and Uganda [Reference 6]. |
3 Clinical application of TDF-based PrEP for HIV prevention
PrEP has been shown to protect against HIV acquisition by 44–75% in randomized comparisons versus placebo and by >90% in persons adherent to PrEP as prescribed (11, 27, 28). PrEP is effective in both men and women when taken with sufficient adherence including in groups with substantial HIV risk (e.g. young women, HIV serodiscordant couples, men who have sex with men, injection drug users) (11, 13, 14). In 2012, the US FDA approved daily oral FTC/TDF for HIV prevention in persons with heightened risk for HIV in combination with other HIV prevention strategies (29), and other nations have subsequently done the same. Normative organizations including the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) issued guidelines recommending PrEP be offered as a prevention options to persons at substantial risk for HIV acquisition (30, 31). TDF alone is also effective for HIV prevention and is recommendation as an alternative to FTC/TDF by WHO. PrEP is not for life long use but recommended for daily use during periods of substantial HIV risk. Given the extremely low concentration of PrEP medications (i.e., tenofovir and emtricitabine) that the breastfed infant would receive via breast milk, oral PrEP to the lactating HIV-uninfected mother will be not be a direct PrEP to the breastfeeding infant but preventing HIV infection in the mother indirectly prevents any infection in her child.
4 Safety evaluation
We conducted a literature review to identify published reports in English on HIV-uninfected lactating women who received oral PrEP with TDF alone or in combination with FTC during breastfeeding. A search of PubMed electronic databases was performed using combination search terms (tenofovir and lactation; tenofovir PrEP and breastfeeding; tenofovir and breast milk; PrEP in breastfeeding; and preexposure prophylaxis and breastfeeding) through March 2017. Manual searches of references from relevant articles and abstracts from relevant conferences between 2010 to 2017 including the Conference on Retroviruses and Opportunistic Infections was also performed. Because of the extensive data on the safety of FTC in treatment and prevention of mother-to-child transmission of HIV, the focus of this review is on the TDF effects on infant growth outcomes when used as PrEP by HIV uninfected women breastfeeding a child.
Overall, 85 records were identified from the literature search, with 55 unique records retained after removal of duplicate records. Screening of titles identified only 1 study that addressed the primary focus of this review (18), use of TDF-based PrEP in lactation. The remaining 54 unique reports were not included in the primary review because they, were reviews/policy documents (n=28), or not relevant to topic of focus (n=13), or were in animals (n=2) or vaginal TDF gel study (n=1) or were either pharmacokinetic or safety studies of TDF ART during pregnancy or lactation (n=10). An additional 7 studies of TDF use HIV uninfected women identified through manual search of published articles were reviewed: 5 from hepatitis B virus (HBV) monoinfected women (32–36) and 2 PrEP studies of HIV-uninfected women (11, 28).
4.1 HIV-uninfected women using TDF-based PrEP during breastfeeding
There are limited data on PrEP in HIV-uninfected women during breastfeeding. In PrEP clinical trials, medication was discontinued for those who became pregnant and was not reinitiated in women who were breastfeeding a child. Only one small pharmacokinetics study reported PrEP use during breastfeeding (18). This study which included 50 HIV-uninfected mother-infant dyads, half with infants aged <12 weeks and half with infants aged 13–24 weeks, evaluated the transfer of tenofovir and emtricitabine in breast milk and the extent of infant exposure when daily FTC/TDF was used as PrEP by lactating HIV uninfected women. PrEP was administered to women through daily directly observed therapy for ten consecutive days and then discontinued thereafter. Non-fasting peak and trough samples of maternal plasma and breast milk were obtained at concentration steady states on days 7 and 10, and a single infant plasma sample was obtained on day 7. In that study, tenofovir was excreted into breast milk in very small quantities and tenofovir was unquantifiable in 94% of 49 infant samples. Based on the concentration in breast milk, the doses of tenofovir a breastfeeding infant would be expected ingest daily from breastfeeding were estimated to be the equivalent of <0.01% of the proposed pediatric doses for treatment of infant HIV infection and for prevention of infant postnatal HIV infection. As has been reported for lamivudine (37), emtricitabine levels were somewhat higher in breastmilk and detectable in infant plasma but the concentrations were still small and represented 200-fold lower (or 0.5%) than those achieved with pediatric therapeutic dosing. No significant adverse events were noted in mothers or infants in that 10-day study.
In addition to these limited data on TDF-based PrEP exposure during lactation, there are encouraging safety data available from infants born to HIV-uninfected women with first trimester TDF exposure in PrEP trials as well as HIV-uninfected women using TDF for prevention of vertical HBV transmission. In the Partners PrEP Study (11), there was no evidence to suggest growth restriction based on z-score computed from serial weight, height, or head circumference or infant creatinine at age 1 and 3 months (38). Similarly, no substantial adverse infant growth outcomes have been seen in data from HBV mono-infected women using TDF during pregnancy or breastfeeding (32–36), although most of these studies discouraged women from breastfeeding while on treatment.
4.2 Corollary information from HIV-infected women using TDF-based HIV treatment and from use of TDF during pregnancy
Although data on TDF PrEP use during breastfeeding are very limited, the safety of TDF and FTC during breastfeeding can be extrapolated from research among HIV-infected women taking antiretroviral therapy for HIV treatment and for prevention of maternal-to-child HIV transmission. TDF and FTC are recommended by global treatment guidelines for use in pregnant women.
Data on use of TDF and FTC in pregnancy and lactation among HIV infected women for HIV treatment have been reviewed extensively (39). Briefly, data have been generally reassuring (39–44). The Antiretroviral Pregnancy Registry database that includes data from >3000 infants exposed to TDF and FTC in first trimester in the U.S sufficient to rule up to 2-fold increase in risk of birth defects, no evidence of elevation in risk of congenital anomalies than would be expected in general population (45). Infant outcome studies have suggested no substantive differences in infant adverse growth outcomes at birth or 12 months between children exposed to TDF versus non-TDF-exposed children (41, 42, 44, 46). Studies of markers of bone turnover or markers of renal injury in infants have found no substantial difference between TDF and non-TDF-exposed infants (40, 41, 44). Recently, the PROMISE study observed lower rates of very preterm birth and neonatal mortality with ZDV/FTC/LPV/r than TDF/FTC/LPV/r, although this may have been related to concurrent TDF and LPV/r use (47). Taken together, these data provide additional reassurance about the use of TDF and FTC when PrEP is used during lactation.
5 Comparison with safety of potential alternative PrEP drugs
Currently, fixed-dose, oral co-formulation of FTC/TDF (Truvada®) is the approved regimen for PrEP in the U.S. The World Health Organization recommends TDF-containing medications as PrEP, which includes TDF combined with FTC as well as potentially TDF alone and TDF combined with lamivudine. However, new PrEP drugs and formulations including other oral agents (maraviroc), intravaginal rings (dapivirine, tenofovir), and longer-acting injectable agents (rilpivirine, cabotegravir) are currently in the pipeline and may provide alternative strategies to daily oral PrEP in the future.
6 Conclusions
Data on infants breastfed by HIV-uninfected women using TDF-based PrEP are limited. However, available information suggests tenofovir is excreted in breast milk in very small concentrations that are unlikely to be of substantial clinical consequence in breastfeeding infants of HIV-uninfected women using TDF-based PrEP. Extrapolation of available data from TDF use in HIV-infected women and uninfected women during pregnancy and lactation are also reassuring in terms of maternal, pregnancy, and infant growth outcomes.
Taken together, accumulated data support implementing PrEP in women at substantial HIV risk during lactation but additional surveillance is still important. Thus, there is need for continued accrual of data on maternal and infant safety, infant growth, and pregnancy outcome information in PrEP research studies and demonstration projects, in which women use PrEP through pregnancy and lactation.
7 Expert opinion
Women living in regions with high HIV prevalence are at high risk of HIV acquisition in pregnancy and postpartum because they infrequently use condoms, do not know their partner’s HIV status, and have biologic changes or changes in their partner’s sexual partnerships that increase susceptibility. For young women in Africa, where breastfeeding lasts often beyond the second year in most settings, women spend a significant part of their life either pregnant or breastfeeding a child. Indeed, over 40% of new infant HIV infections worldwide are estimated to be due to maternal HIV acquisition in pregnancy and postpartum (48). Therefore, urgent implementation of effective HIV prevention options that do not require negotiations for safe sex or interfere with pregnancy or breastfeeding is a priority not only for women’s health but also as an indirect protection to her child by preventing maternal HIV during breastfeeding in the first place.
PrEP with TDF alone or when coformulated with FTC is a potent prevention option for use during periods of elevated HIV risk including during breastfeeding. A recent PK study in HIV-uninfected mother-infant dyads showing that tenofovir is excreted in breast milk in very small quantities with trivial infant absorption is reassuring – those data strongly suggest that PrEP can be safely used during breastfeeding without exposing an infant to pharmacologically important concentrations of PrEP medication.
Current WHO guidelines permit use of PrEP during pregnancy and lactation. The benefits of breastfeeding are well documented, associated with survival particularly in resources limited settings (49, 50), as well as cognition and bonding. Women in Africa at substantial risk for infection should not have choose to whether to breastfeed their child or protect themselves from acquiring HIV. In summary, TDF-based PrEP is a potent female-controlled HIV prevention option, for which the potential risks arguably are outweighed, at the public health level and for at-risk individuals, by its HIV prevention benefits that include indirect protection for infant from vertical HIV transmission as a result of preventing acute maternal infection during lactation.
Acknowledgments
Funding support: This work was supported by funding from the National Institutes of Mental Health (2R01 MH095507), The Bill and Melinda Gates Foundation (OPP1056051), and the Center for AIDS Research University of Washington (P30 AI027757).
References
*of importance,
**of considerable importance.
Uncategorized References
- 1.UNAIDS. [Accessed December 21, 2015];The Gap Report. 2014 Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf.
- 2**.Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during Pregnancy and Postpartum and Risk of Mother-to-Child HIV Transmission: A Systematic Review and Meta-Analysis. PLoS Med. 2014;11(2):e1001608. doi: 10.1371/journal.pmed.1001608. Provides critical evidence of substantial HIV risk in pregnancy and lactation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Schacht C, Hoffman HJ, Mabunda N, Lucas C, Alons CL, Madonela A, et al. High Rates of HIV Seroconversion in Pregnant Women and Low Reported Levels of HIV Testing among Male Partners in Southern Mozambique: Results from a Mixed Methods Study. PLoS ONE. 2014;9(12):e115014. doi: 10.1371/journal.pone.0115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating MA, Hamela G, Miller WC, Moses A, Hoffman IF, Hosseinipour MC. High HIV Incidence and Sexual Behavior Change among Pregnant Women in Lilongwe, Malawi: Implications for the Risk of HIV Acquisition. PLOS ONE. 2012;7(6):e39109. doi: 10.1371/journal.pone.0039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinuthia J, Richardson BA, Drake AL, Matemo D, Unger JA, McClelland RS, et al. Sexual Behavior and Vaginal Practices During Pregnancy and Postpartum: Implications for HIV Prevention Strategies. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2017;74(2):142–9. doi: 10.1097/QAI.0000000000001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nel A, Mabude Z, Smit J, Kotze P, Arbuckle D, Wu J, et al. HIV Incidence Remains High in KwaZulu-Natal, South Africa: Evidence from Three Districts. PLOS ONE. 2012;7(4):e35278. doi: 10.1371/journal.pone.0035278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peltzer K, Mlambo G. Sexual HIV risk behaviour and associated factors among pregnant women in Mpumalanga, South Africa. BMC Pregnancy and Childbirth. 2013;13:57. doi: 10.1186/1471-2393-13-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson K, Weinberg A. Dynamics of regulatory T-cells during pregnancy: effect of HIV infection and correlations with other immune parameters. PLoS One. 2011;6(11):e28172. doi: 10.1371/journal.pone.0028172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheffield JS, Wendel GD, Jr, McIntire DD, Norgard MV. The effect of progesterone levels and pregnancy on HIV-1 coreceptor expression. Reprod Sci. 2009;16(1):20–31. doi: 10.1177/1933719108325510. [DOI] [PubMed] [Google Scholar]
- 10.Truong HM, Sim MS, Dillon M, Uittenbogaart CH, Dickover R, Plaeger SF, et al. Correlation of immune activation during late pregnancy and early postpartum with increases in plasma HIV RNA, CD4/CD8 T cells, and serum activation markers. Clin Vaccine Immunol. 2010;17(12):2024–8. doi: 10.1128/CVI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baeten JM, Heffron R, Kidoguchi L, Mugo NR, Katabira E, Bukusi EA, et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1–Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. doi: 10.1371/journal.pmed.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murnane PM, Celum C, Mugo N, Campbell JD, Donnell D, Bukusi E, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–60. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson KA, Baeten JM, Mugo NR, Bekker L-G, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Current Opinion in HIV and AIDS. 2016;11(1):18–26. doi: 10.1097/COH.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, et al. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8(5–6):261–72. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 16.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 17.Mirochnick M, Taha T, Kreitchmann R, Nielsen-Saines K, Kumwenda N, Joao E, et al. Pharmacokinetics and Safety of Tenofovir in HIV-Infected Women During Labor and Their Infants During the First Week of Life. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;65(1):33–41. doi: 10.1097/QAI.0b013e3182a921eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Mugwanya KK, Hendrix CW, Mugo NR, Marzinke M, Katabira ET, Ngure K, et al. Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption. PLOS Medicine. 2016;13(9):e1002132. doi: 10.1371/journal.pmed.1002132. The only published evidence on use of TDF-based PrEP during breastfeeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flynn PM, Mirochnick M, Shapiro DE, Bardeguez A, Rodman J, Robbins B, et al. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 2011;55(12):5914–22. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn PM, Mirochnick M, Shapiro DE, Bardeguez A, Rodman J, Robbins B, et al. Pharmacokinetics and safety of single-dose tenofovir disoproxil fumarate and emtricitabine in HIV-1-infected pregnant women and their infants. Antimicrob Agents Chemother. 2011;55(12):5914–22. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Best BM, Burchett S, Li H, Stek A, Hu C, Wang J, et al. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med. 2015;16(8):502–11. doi: 10.1111/hiv.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colbers AP, Hawkins DA, Gingelmaier A, Kabeya K, Rockstroh JK, Wyen C, et al. The pharmacokinetics, safety and efficacy of tenofovir and emtricitabine in HIV-1-infected pregnant women. AIDS. 2013;27(5):739–48. doi: 10.1097/QAD.0b013e32835c208b. [DOI] [PubMed] [Google Scholar]
- 23.Mugwanya KK, Wyatt C, Celum C, Donnell D, Kiarie J, Ronald A, et al. Reversibility of Glomerular Renal Function Decline in HIV-Uninfected Men and Women Discontinuing Emtricitabine-Tenofovir Disoproxil Fumarate Pre-Exposure Prophylaxis. J Acquir Immune Defic Syndr. 2016;71(4):374–80. [Google Scholar]
- 24.Mugwanya KK, Wyatt C, Celum C, et al. Changes in glomerular kidney function among hiv-1–uninfected men and women receiving emtricitabine–tenofovir disoproxil fumarate preexposure prophylaxis: A randomized clinical trial. JAMA Internal Medicine. 2015;175(2):246–54. doi: 10.1001/jamainternmed.2014.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulligan K, Glidden DV, Anderson PL, Liu A, McMahan V, Gonzales P, et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis. 2015;61(4):572–80. doi: 10.1093/cid/civ324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasonde M, Niska RW, Rose C, Henderson FL, Segolodi TM, Turner K, et al. Bone Mineral Density Changes among HIV-Uninfected Young Adults in a Randomised Trial of Pre-Exposure Prophylaxis with Tenofovir-Emtricitabine or Placebo in Botswana. PLoS ONE. 2014;9(3):e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381(9883):2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 28.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 29.Food and Drug Administration. [Accessed September 12, 2015];Truvada approved to reduce the risk of sexually transmitted HIV in people who are not infected with the virus. 2012 http://www.fda.gov/downloads/ForConsumers/ConsumerUpdates/UCM311828.pdf.
- 30.World Health Organization. [Accessed October 27, 2015];Guideline on When To Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis For HIV. 2015 Sep; Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ [PubMed]
- 31.Consensus Committee SAHCS. Southern African guidelines for the safe use of pre-exposure prophylaxis in men who have sex with men who are at risk for HIV infection. 2012 [Google Scholar]
- 32*.Celen MK, Mert D, Ay M, Dal T, Kaya S, Yildirim N, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy for the prevention of vertical transmission of HBV infection. World Journal of Gastroenterology: WJG. 2013;19(48):9377–82. doi: 10.3748/wjg.v19.i48.9377. Provides corollary information from mon-HBV infected women who used TDF during pregnancy and lactation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Chen HL, Lee CN, Chang CH, Ni YH, Shyu MK, Chen SM, et al. Efficacy of maternal tenofovir disoproxil fumarate in interrupting mother-to-infant transmission of hepatitis B virus. Hepatology. 2015;62(2):375–86. doi: 10.1002/hep.27837. Provides corollary information from mon-HBV infected women who used TDF during pregnancy and lactation. [DOI] [PubMed] [Google Scholar]
- 34*.Greenup AJ, Tan PK, Nguyen V, Glass A, Davison S, Chatterjee U, et al. Efficacy and safety of tenofovir disoproxil fumarate in pregnancy to prevent perinatal transmission of hepatitis B virus. J Hepatol. 2014;61(3):502–7. doi: 10.1016/j.jhep.2014.04.038. Provides corollary information from mon-HBV infected women who used TDF during pregnancy and lactation. [DOI] [PubMed] [Google Scholar]
- 35*.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N Engl J Med. 2016;374(24):2324–34. doi: 10.1056/NEJMoa1508660. Provides corollary information from mon-HBV infected women who used TDF during pregnancy and lactation. [DOI] [PubMed] [Google Scholar]
- 36*.Samadi Kochaksaraei G, Castillo E, Osman M, Simmonds K, Scott AN, Oshiomogho JI, et al. Clinical course of 161 untreated and tenofovir-treated chronic hepatitis B pregnant patients in a low hepatitis B virus endemic region. J Viral Hepat. 2016;23(1):15–22. doi: 10.1111/jvh.12436. Provides corollary information from mon-HBV infected women who used TDF during pregnancy and lactation. [DOI] [PubMed] [Google Scholar]
- 37.Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, Masaba R, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53(3):1170–6. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mugo NR, Hong T, Celum C, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for hiv prevention: A randomized clinical trial. JAMA. 2014;312(4):362–71. doi: 10.1001/jama.2014.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Mofenson LM, Baggaley RC, Mameletzis I. Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding. AIDS. 2017;31(2):213–32. doi: 10.1097/QAD.0000000000001313. Provides comprehensive review of available evidence of safety TDF use in pregnancy and lactation. [DOI] [PubMed] [Google Scholar]
- 40.Jao J, Abrams EJ, Phillips T, Petro G, Zerbe A, Myer L. In Utero Tenofovir Exposure Is not Associated With Fetal Long Bone Growth. Clin Infect Dis. 2016;62(12):1604–9. doi: 10.1093/cid/ciw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Liotta G, Floridia M, Andreotti M, Jere H, Sagno JB, Marazzi MC, et al. Growth indices in breastfed infants pre and postnatally exposed to tenofovir compared with tenofovir-unexposed infants. AIDS. 2016;30(3):525–7. doi: 10.1097/QAD.0000000000000944. Provides corollary information from HIV-infected women who used TDF during pregnancy and lactation and infant adverse outcomes. [DOI] [PubMed] [Google Scholar]
- 42*.Ransom CE, Huo Y, Patel K, Scott GB, Watts HD, Williams P, et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64(4):374–81. doi: 10.1097/QAI.0b013e3182a7adb2. Provides corollary information from HIV-infected women who used TDF during pregnancy and lactation and infant adverse outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Siberry GK, Williams PL, Mendez H, Seage GR, 3rd, Jacobson DL, Hazra R, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26(9):1151–9. doi: 10.1097/QAD.0b013e328352d135. Provides corollary information from HIV-infected women who used TDF during pregnancy and lactation and infant adverse outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and Infant Outcomes among HIV-Infected Women Taking Long-Term ART with and without Tenofovir in the DART Trial. PLOS Medicine. 2012;9(5):e1001217. doi: 10.1371/journal.pmed.1001217. Provides corollary information from HIV-infected women who used TDF during pregnancy and lactation and infant adverse outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang L, Kourtis AP, Ellington S, Legardy-Williams J, Bulterys M. Safety of tenofovir during pregnancy for the mother and fetus: a systematic review. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2013;57(12):1773–81. doi: 10.1093/cid/cit601. [DOI] [PubMed] [Google Scholar]
- 46.Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag-Bonnet N, Faye A, et al. Association between Prenatal Exposure to Antiretroviral Therapy and Birth Defects: An Analysis of the French Perinatal Cohort Study (ANRS CO1/CO11) PLoS Med. 2014;11(4):e1001635. doi: 10.1371/journal.pmed.1001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med. 2016;375(18):1726–37. doi: 10.1056/NEJMoa1511691. Provides corollary information from HIV-infected women who used TDF during pregnancy and lactation and infant adverse outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lockman S, Creek T. Acute maternal HIV infection during pregnancy and breast-feeding: substantial risk to infants. The Journal of infectious diseases. 2009;200(5):667–9. doi: 10.1086/605124. [DOI] [PubMed] [Google Scholar]
- 49.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355(9202):451–5. [PubMed] [Google Scholar]
- 50.Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, et al. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):3–13. doi: 10.1111/apa.13147. [DOI] [PubMed] [Google Scholar]


