Abstract
Introduction
Inquiries from healthcare providers and patients about the gluten and aluminum content of Synthroid® (levothyroxine sodium tablets) have increased. The objective of this study was to measure and evaluate the gluten content of the raw materials used in the manufacturing of Synthroid. Additionally, this study determined the aluminum content in different strengths of Synthroid tablets by estimating the amount of aluminum in the raw materials used in the manufacturing of Synthroid.
Methods
Gluten levels of three lots of the active pharmaceutical ingredient (API) and one lot of each excipient from different vendors were examined. The ingredients in all current Synthroid formulations (strengths) were evaluated for their quantity of aluminum.
Results
Gluten concentrations were below the lowest limit of detection (<3.0 ppm) for all tested lots of the API and excipients of Synthroid tablets. Aluminum content varied across tablet strengths (range 19–137 µg/tablet). Gluten levels of the API and excipients were found to be below the lowest level of detection and are considered gluten-free based on the US Food and Drug Administration (FDA) definition for food products. Across the various tablet strengths of Synthroid, the maximum aluminum levels were well below the FDA-determined minimal risk level for chronic oral aluminum exposure (1 mg/kg/day).
Conclusion
These data demonstrate that Synthroid tablets are not a source for dietary gluten and are a minimal source of aluminum.
Funding
AbbVie Inc.
Keywords: Aluminum, Chemical analysis, Endocrinology, Excipients, Gluten, Hypothyroidism, Levothyroxine, Synthroid
Introduction
Synthroid® (levothyroxine sodium tablets) is indicated as replacement or supplemental therapy in congenital or acquired hypothyroidism [1]. The most common cause of hypothyroidism in the USA is autoimmunity (Hashimoto’s disease) [2]. In adults, the incidence of hypothyroidism is estimated to be 3.5 per 1000 per year in women and 0.6 per 1000 per year in men [3]. Patients with thyroid disorders may be at heightened risk for intolerance to gluten, as Celiac disease has, in a number of studies, been shown to have associations with autoimmune thyroid diseases such as Hashimoto’s [4–8]. In studies of patients with Celiac disease, the percentage of patients who also had autoimmune thyroid diseases ranged from 3.5% to 5.4% [4, 5, 7].
Recently, a methodology and database designed to identify medications that may contain gluten were developed to assist healthcare providers who treat gluten-intolerant individuals [9]. Another illustration of patients’ concerns about gluten in medications is the fact that 1340 inquiries regarding gluten content in Synthroid were received by AbbVie Inc. in 2013, representing 52% of all requests for information about Synthroid during that year.
Exposure to aluminum occurs primarily through the consumption of food items [10]. Aluminum is a known constituent of the colorants used in the manufacturing of Synthroid formulations. Multiple studies have discredited initial reports from the 1960s and 1970s of a possible association of aluminum with Alzheimer’s disease [11, 12]. Nevertheless, occasional inquiries on the aluminum content of Synthroid based on this outdated hypothesis continue to arise.
The objectives of this study were to measure and evaluate the potential gluten content of raw materials used in the manufacturing of Synthroid. Additionally, this study determined the aluminum content in different strengths of those tablets by estimating the amount of aluminum in the raw materials used in the manufacturing of Synthroid.
Methods
Gluten Content Determination
Allergen and other information was requested from the suppliers of the excipients used in the manufacturing of Synthroid and reviewed for the presence of gluten. Three lots of the active pharmaceutical ingredient (API) and one lot of the excipients from each of the suppliers were tested for the presence of gluten using an enzyme immunoassay test method kit (US-RIDASCREEN® Gliadin Test Kit, R-Biopharm AG, Darmstadt, Germany). The lowest limit of detection with this method was 3.0 ppm.
Aluminum Content Determination
The ingredients in the levothyroxine sodium formulations, including colorants, were evaluated to estimate the quantity of aluminum in various tablet strengths. The quantitation of aluminum in the colorants was based on the composition information provided by the colorant manufacturers. For the other raw material used in the manufacturing of Synthroid, aluminum content was estimated on the basis of the raw material specifications, information from the literature, and whether the raw material was plant- or mineral-based. The total aluminum level in the talc was evaluated in two different ways; the first calculations were based on the worst theoretical case based on the explicit limit of not more than 2% for aluminum stated in the US Pharmacopeia monograph, and the second calculations were based on the maximum observed levels (0.6%) tested since 2010. In calculating the contributions of the individual raw materials to the overall aluminum content, it was generally assumed that the maximum possible amount of aluminum was present from each ingredient. Therefore, it is likely that the actual amounts of aluminum present in the tablets are lower than the calculated totals.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Results
Gluten Content
All of the tested lots of API and excipients of Synthroid had gluten concentrations that were below the lowest limit of detection (<3.0 ppm; Table 1).
Table 1.
Gluten test results of raw materials used in the manufacture of Synthroid® tablets
| Raw material/excipient | Gluten concentration (ppm) |
|---|---|
| Levothyroxine sodium, USP | <3.0 |
| Talc, USP | <3.0 |
| Lactose monohydrate, NF | <3.0 |
| Acacia, NF | <3.0 |
| Sugar, confectioner’s, NF | <3.0 |
| Magnesium stearate, NF/EP | <3.0 |
| Povidone, USP | <3.0 |
| Dye, Yellow D&C No. 10, Aluminum Lake | <3.0 |
| Dye, Blue FD&C No. 1, Aluminum Lake | <3.0 |
| Dye, Yellow FD&C No. 6, Aluminum Lake | <3.0 |
| Dye, Blue FD&C No. 2, Aluminum Lake | <3.0 |
| Dye, Red FD&C No. 40, Aluminum Lake | <3.0 |
| Dye, Red Lake Blend | <3.0 |
| Dye, Brown Lake Blend | <3.0 |
| Dye, Olive Lake blend | <3.0 |
EP European Pharmacopoeia, FD&C Federal Food, Drug, and Cosmetic Act, NF National Formulary, USP US Pharmacopeia
Aluminum Content
Each of the colorants used in the various tablet formulations has a different amount of aluminum present as a substrate for the colorant (i.e., Aluminum Lake dyes).
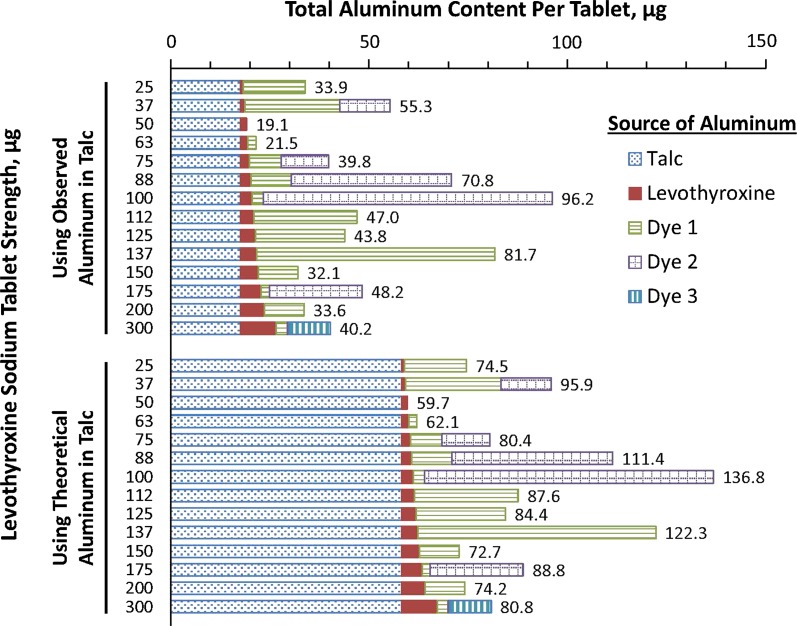
The estimated maximum aluminum levels in the drug product ranged from 19 to 137 µg per tablet across the various tablet strengths (levothyroxine sodium 25, 37, 50, 63, 75, 88, 100, 112, 125, 137, 150, 175, 200, and 300 µg; Fig. 1). This is below the minimal risk level (MRL) for chronic oral aluminum intake of 1 mg per kg of body weight per day (Table 2).
Fig. 1.
Materials and aluminum content (µg) in Synthroid® tablets of various strengths. Values listed are the µg amount of aluminum in each tablet strength. “Observed” is the actual value for a specific lot as reported by the vendor on the certificate of analysis. “Theoretical” is based on the upper limit for the aluminum content and therefore reflects the maximum possible aluminum content in a lot of talc. Dyes 1, 2, and 3 were not necessarily the same among tablets of different strengths; the number of dyes per tablet ranged from 0 to 3. All other ingredients were estimated to contribute <0.2 µg of aluminum to each tablet
Table 2.
US regulations and guidelines applicable to aluminum and compounds in water
| Agency | Description | Information |
|---|---|---|
| EPA | Designated as hazardous substances in accordance with Section 311(b)(2)(A) of the Clean Water Act for aluminum sulfate | Yes |
| Drinking water standards and health advisories | 0.05–0.2 mg/L | |
| National primary drinking water standards | No data | |
| National secondary drinking water standards for aluminum | 0.05–0.2 mg/L | |
| Reportable quantities of hazardous substances designated pursuant to Section 311 of the Clean Water Act for aluminum sulfate | 5000 lb | |
| Water quality criteria for human health for aluminum | ||
| Freshwater criterion maximum concentration | 750 μg/L | |
| Freshwater criterion continuous concentration | 87 μg/L | |
| FDA | Bottled drinking water for aluminum | 0.2 mg/L |
EPA Environmental Protection Agency, FDA US Food and Drug Administration
Discussion
Inquiries related to the presence of gluten and aluminum in Synthroid have increased. As a result of this volume of inquiries received by AbbVie Inc. from healthcare providers and patients, this study was conducted to determine both the gluten content of raw materials used in the manufacturing of Synthroid and to quantify the aluminum content in different strengths of those tablets.
Gluten is of concern in patients with autoimmune thyroiditis requiring levothyroxine sodium who may have gluten intolerance from autoimmune Celiac disease [4, 5]. Inquiries regarding the association of aluminum consumption and Alzheimer’s disease occasionally surface despite the lack of substantiation in modern studies.
In the present study, the excipient suppliers reported that their materials were gluten-free. When testing the excipients used in the manufacturing process for Synthroid and the API (levothyroxine sodium) of those tablets, there were no quantifiable levels of gluten detected. The US Food and Drug Administration (FDA) does not enforce labeling of the gluten content in medications [9], so there is no drug-specific guidance; however, a threshold of less than 20 ppm to define food products as gluten-free has been established by the FDA [13]. These results demonstrate that Synthroid tablets are gluten-free as defined by the threshold of less than 20 ppm that the FDA has established for food products.
The true threshold of gluten exposure exacerbating Celiac disease is unknown but, after evaluation of available dose–response data, the tolerable daily intake level for gluten in individuals with Celiac disease was determined in a safety assessment by the FDA Office of Food Safety in May 2011 to be 0.4 mg gluten/day for adverse morphological effects and 0.015 mg gluten/day for adverse clinical effects [14]. The finding that gluten levels in the excipients and API were below detectable levels provides evidence that Synthroid is unlikely to exacerbate Celiac disease in patients who may be receiving levothyroxine sodium to treat their hypothyroidism.
Aluminum is ubiquitous and is naturally released to the environment [15]. The general population is primarily exposed to aluminum through the consumption of food items, as well as over-the-counter medicinals such as antacids and buffered aspirin; aluminum also is found in a number of topically applied consumer products such as antiperspirants, first-aid antibiotics, and antiseptics [15]. On the basis of the FDA’s 1993 Total Diet Study dietary exposure model and the 1987–1988 US Department of Agriculture (USDA) Nationwide Food Consumption Survey, aluminum intakes of between 0.10 and 0.12 mg aluminum/kg/day for adults (equivalent to 7.0–8.4 mg/day in a 70-kg individual) appear likely [10]. In the present analysis, the levels of aluminum in the raw materials and in the various strengths of tablet formulations of levothyroxine sodium were far below the MRL of 1 mg/kg/day (equivalent to 70 mg/day in a 70-kg individual) [10]. As a worst-case estimate assuming a 100-µg tablet containing 137 µg of aluminum, an individual weighing 70 kg would need to consume at least 511 tablets daily to reach the MRL for aluminum. Therefore, Synthroid is not a significant source of aluminum exposure compared with estimated daily aluminum exposure from other sources. Theoretically, the levothyroxine sodium API could contribute to the overall aluminum content via exposure to water (solvent) during synthesis, with the amount dependent on the particular formulation strength. However, the majority of the aluminum content of a Synthroid tablet, on a weight basis, is from the colorants and from the talc raw material, with all other ingredients estimated to contribute less than 0.2 µg of aluminum to each tablet (see Fig. 1).
Limitations
In the present study, the suppliers of the excipients reported that their materials were not derived from sources that contain gluten. The possibility of gluten making its way into the manufacturing process was mitigated by measuring both the API as well as the excipients used in the manufacturing of Synthroid, which confirmed a lack of measurable gluten. While random selection of batches does not guarantee that all batches would display similar results, it is unlikely that significant variance exists in the production of Synthroid; therefore, these batches could be considered representative.
Aluminum measurements were based on estimates of known quantities of raw ingredients and estimations of content from secondary sources (talc and water). Worst-case estimates were utilized to calculate concentrations of aluminum, finding levels significantly lower than the established MRL.
Conclusions
Across the manufacturing process and production of Synthroid, no gluten or gluten derivatives are used. Testing of the API and excipients demonstrated that gluten levels were below the lowest level of detection and, on the basis of the FDA definition for food products, would be considered gluten-free. Across the various tablet strengths of Synthroid, the maximum aluminum levels were well below the FDA-determined MRL for chronic oral exposure to aluminum. These data demonstrate that it is unlikely Synthroid would present a significant risk of exposure for patients prescribed levothyroxine sodium who are gluten intolerant or concerned by aluminum intake.
Acknowledgements
Medical writing support was provided by Michael J. Theisen, PhD, and Patrick Little, PhD, of Complete Publication Solutions, LLC (North Wales, PA). AbbVie funded the research, medical writing support, and the article processing charges associated with this publication. All authors contributed to the development of the content; the authors maintained control over the final content. AbbVie funded the study, contributed to its design, and was involved in the collection, analysis, and interpretation of the data, and in the writing, review, and approval of the publication. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. This work has been previously presented, in part, at the following meetings: Endocrine Society 97th Annual Meeting and Exposition, March 5–8, 2015, San Diego, CA, USA; American Association of Clinical Endocrinologists 24th Annual Scientific and Clinical Congress, May 13–17, 2015, Nashville, TN, USA; and 15th International Thyroid Congress, October 18–23, 2015, Lake Buena Vista, FL, USA.
Disclosures
Ramon Espaillat is an employee of Abbvie and may own Abbvie stocks or options. Michael F. Jarvis is an employee of Abbvie and may own Abbvie stocks or options. Cory Torkelson is an employee of Abbvie and may own Abbvie stocks or options. Brent Sinclair is an employee of Abbvie and may own Abbvie stocks or options.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/C998F0605088BD00.
An erratum to this article is available at https://doi.org/10.1007/s12325-017-0608-6.
Change history
8/30/2017
An erratum to this article has been published.
References
- 1.Synthroid® (levothyroxine sodium). Full prescribing information, AbbVie, North Chicago. 2012.
- 2.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 3.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43(1):55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 4.Collin P, Reunala T, Pukkala E, Laippala P, Keyrilainen O, Pasternack A. Coeliac disease–associated disorders and survival. Gut. 1994;35(9):1215–1218. doi: 10.1136/gut.35.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper BT, Holmes GK, Cooke WT. Coeliac disease and immunological disorders. Br Med J. 1978;1(6112):537–539. doi: 10.1136/bmj.1.6112.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakanen M, Luotola K, Salmi J, Laippala P, Kaukinen K, Collin P. Clinical and subclinical autoimmune thyroid disease in adult celiac disease. Dig Dis Sci. 2001;46(12):2631–2635. doi: 10.1023/A:1012754824553. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster-Smith MJ, Perrin J, Swarbrick ET, Wright JT. Coeliac disease and autoimmunity. Postgrad Med J. 1974;50(579):45–48. doi: 10.1136/pgmj.50.579.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sategna-Guidetti C, Bruno M, Mazza E, et al. Autoimmune thyroid diseases and coeliac disease. Eur J Gastroenterol Hepatol. 1998;10(11):927–931. doi: 10.1097/00042737-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Cruz JE, Cocchio C, Lai PT, Hermes-DeSantis E. Gluten content of medications. Am J Health Syst Pharm. 2015;72(1):54–60. doi: 10.2146/ajhp140153. [DOI] [PubMed] [Google Scholar]
- 10.Toxicological Profile for Aluminum, Atlanta, GA: Agency for Toxic Substances and Disease Registry, US Department of Health and Human Services, Public Health Service. 2008. [PubMed]
- 11.Lidsky TI. Is the aluminum hypothesis dead? J Occup Environ Med. 2014;56(5 Suppl):S73–S79. doi: 10.1097/JOM.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiyama H, Hosokawa M, Kametani F, et al. Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in AβPP and AβPP/tau transgenic mice. Neuropathology. 2012;32(4):390–397. doi: 10.1111/j.1440-1789.2011.01274.x. [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. Questions and answers: gluten-free food labeling final rule. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Allergens/ucm362880.htm. Accessed 30 July 2015.
- 14.Office of Food Safety, Center of Food Safety and Applied Nutrition, US Food and Drug Administration. Health hazard assessment for gluten exposure in individuals with celiac disease: determination of tolerable daily intake levels and levels of concern for gluten. 2011.
- 15.Krewski D, Yokel RA, Nieboer E, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10(suppl 1):1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]