Summary
Objective
Valproic acid (VA) is a histone deacetylase (HDAC) inhibitor that has antiproliferative effects on several types of cancer, including thyroid cancer. In addition, VA has been reported to upregulate the sodium-iodine symporter in thyroid cancer cells and increases radioiodine uptake in preclinical studies. The aim of this study was to assess the antiproliferative effects of VA and to evaluate if VA can increase the radioiodine uptake in patients with advanced, radioiodine-negative thyroid cancer.
Design
An open-label Simon two-stage phase II trial.
Patients and Measurements
VA was administered orally, and doses were adjusted to maintain serum trough levels between 50 and 100 mg/l for 10 weeks, followed by injections of recombinant human thyroid–stimulating hormone and a radioiodine uptake scan. Anatomical imaging studies were performed at week 16 to assess tumour response and radioiodine therapy in patients with increased radioiodine uptake.
Results
Thirteen patients with a median age of 66 years (50–78 years) were enrolled and evaluated. Seven patients had papillary thyroid cancer (PTC), two had follicular variant PTC, two had follicular thyroid cancer, and two had Hürthle cell carcinoma. None of the 10 patients who completed the 10-week treatment had increased radioiodine uptake at their tumour sites. Three patients were taken off the study prior to the 10-week radioiodine uptake scan: one with grade-3 hepatic toxicity, one with disease progression, and one for noncompliance. Four of 13 patients had decreased stimulated serum thyroglobulin with VA treatment. None of the patients had complete or partial responses based on Response Evaluation Criteria in Solid Tumors (RECIST), and six patients had disease progression.
Conclusions
VA does not increase radioiodine uptake and does not have anticancer activity in patients with advanced, radioiodine-negative thyroid cancer of follicular cell origin.
Keywords: thyroid cancer, valproic acid, histone deacetylase inhibitor, radioiodine
Introduction
Thyroid cancer is the fastest growing cancer diagnosis in the United States.1 Although most patients with thyroid cancer have an excellent prognosis, approximately 10–15% present with an aggressive form of the disease, and the mortality rate in these patients at 5–10 years following initial treatment is up to 30%.2 In patients with progressive or recurrent differentiated thyroid cancer, one-third have or will develop dedifferentiated tumours (loss of differentiation).3 Tumour dedifferentiation results in more aggressive tumour growth, metastasis, decreased or loss of iodine uptake in the tumour, and cancers that are often refractory to conventional treatments, including surgical resection, radioactive iodine (RAI), and thyroid hormone for TSH suppression Patients with RAI non-avid, metastatic thyroid cancer of follicular cell origin have a ten-year survival rate of only 10%, compared to 56% in those with RAI avid, metastatic thyroid cancer4. In addition, up to 20% of patients with differentiated thyroid cancer have evidence of residual disease, as indicated by detectable serum thyroglobulin levels, but have negative RAI scans.5
Valproic acid (VA), a histone deacetylase (HDAC) inhibitor, has long been used as an anticonvulsant to treat seizures in patients with epilepsy. It has also been used to treat bipolar disorder. Recent studies have shown that HDAC inhibitors have promising effects in the treatment of cancer by inhibiting cancer cell proliferation, inducing apoptosis, cell cycle arrest, and differentiation in brain, haematological, endometrial, ovarian, and prostate cancers.6–9 Preclinical studies also suggest that VA has a similar antiproliferative and differentiating effect in human thyroid cancer cells.10–15 VA interacts with the catalytic site of histone/acetyltransferases to disrupt its activity. The post-translational modification process of acetylation is critical to gene transcription, with increased acetylation resulting in increased gene transcription. Because acetylation affects the chromatin structure and nucleosomal packaging, it also affects gene expression through this mechanism. Approximately 2–5% of transcribed genes are significantly affected after treatment with HDAC inhibitors. In thyroid cancer, VA, at clinically achievable concentrations, has antiproliferative and differentiating effects. At concentrations of 0.5 to 2.0 mM, VA inhibits growth by 55 to 80%.10–15. VA also upregulates sodium-iodine symporter (NIS) gene expression in dedifferentiated thyroid cancer cells.13–15 The NIS gene encodes the Na+/I− symporter, which is located on the basolateral membrane of follicular thyroid cells and is responsible for iodide uptake. Downregulated NIS expression is the predominant mechanism of dedifferentiation in thyroid cancer cells of follicular cell origin that makes RAI therapy ineffective. The prognosis for patients with thyroid cancer is related to the ability of the cancer to accumulate RAI, since well-differentiated thyroid cancers usually respond to treatment with RAI. In preclinical studies, treatment with VA increases iodide accumulation in poorly differentiated thyroid cancer cells by increasing the expression of the NIS gene.
We hypothesize that VA may inhibit proliferation and induce differentiation in thyroid cancer cells so that I-131 may detect residual disease and be more effective for RAI therapy in patients with thyroid cancer of follicular cell origin. The study objectives were to determine if VA treatment results in (1) tumour response, as demonstrated by a decrease in tumour size and or serum thyroglobulin levels, and (2) increased RAI uptake in patients with advanced, RAI-resistant thyroid cancer of follicular cell origin.
Materials and Methods
Eligibility criteria
The main eligibility criteria for the study are summarized in Table 1. We included adult patients with a life expectancy greater than three months and histological proof of thyroid cancer of follicular cell origin who failed conventional treatments, and had elevated serum thyroglobulin, with or without measurable disease, fulfilling Response Evaluation Criteria in Solid Tumors (RECIST) 1.0.16 RAI-resistant thyroid cancer was defined as a thyroid cancer that does not respond to RAI treatment(s) based on tumour size and/or thyroglobulin level. Patients also needed to have no RAI uptake (<1%) within 18 months prior to enrollment. Patients with anti-thyroglobulin antibodies were eligible if they had measurable disease, as determined by anatomical imaging. These included inoperable loco-regional tumours and/or metastatic sites. If a patient had no measurable disease on anatomical imaging, the serum thyroglobulin level had to be ≥100 μg/l in the absence of anti-thyroglobulin antibodies to be eligible for the study because elevated serum thyroglobulin after RAI therapy is an accurate marker of persistent/recurrent disease and is associated with overall survival.17 The protocol was approved by the National Cancer Institute (NCI) Institutional Review Board (IRB). All participating patients provided written informed consent.
Table 1.
Eligibility criteria for the study
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥ 18 years | Allergy to valproic acid. |
| Life expectancy of > 3 months | Current coexisting malignancy other than basal cell carcinoma. |
| Clinical performance status of ECOG1 ≤ 1 | Women of child-bearing potential who are pregnant or breastfeeding4. |
| Advanced/poorly differentiated thyroid cancers of follicular cell origin that have no uptake (<1%) on radioiodine scan or are unresponsive to radioiodine therapy2. | Active systemic infections, coagulation disorders or other major medical illnesses |
| Patients with inoperable disease | Patients with Seizure disorder |
| Thyroglobulin (Tg) levels greater than or equal to 100 μg/l in the absence of Tg antibodies. | Patients with brain metastases |
| Within 18 months of enrollment, patients must have had an RAI scan, showing no or therapeutically insignificant RAI uptake (≤1%) | Patients taking tolbutamide, warfarin, zidovudine, benzodiazapines, clonazepam, diazepam |
| Initial therapy must have included total/near-total thyroidectomy and RAI ablation therapy. | |
| Laboratory results must be within the specified parameters3 before entry. | |
| No chemotherapy, radiotherapy, or biological therapy for their malignancy in the month prior to VA treatment and must have recovered from all side effects of therapeutic and diagnostic interventions. |
ECOG: Eastern Cooperative Oncology Group
Unresponsiveness to radioiodine therapy is defined as a patient’s thyroglobulin not falling to less than 2μg/l within 6 months after previous radioiodine ablative treatment.
-
-Absolute Neutrophil Count > 750 cells/mm3
-
-Haemoglobin > 80 g/l
-
-Platelet count > 75000/mm3
-
-Creatinine < 1.5 times upper limit of normal (ULN)
-
-Total protein > 64 g/l
-
-Total bilirubin should be < 1.5 times ULN.
-
-Aspartate and alanine aminotransferase < 1.5 times ULN.
-
-Amylase < 1.5 times ULN
-
-Ammonia < 1.5 times ULN
A negative pregnancy test was required for all females with child-bearing potential.
Study design
We conducted an open-label phase II clinical trial to assess the efficacy of VA therapy in patients with incurable differentiated thyroid cancer. The primary objective of the study was to determine the antitumour activity of VA as evidenced by tumour response per RECIST and/or a decrease in thyroglobulin level in patients with nonmedullary thyroid cancer that was refractory to RAI. The secondary objective was to determine if VA can induce differentiation of thyroid cancer cells and, therefore, increase uptake of RAI by the tumour cells. Patients received VA on an outpatient basis for at least 16 weeks with TSH suppression therapy. The administration of VA started with 500 mg orally each day for three days, followed by 500 mg twice daily. Serum VA levels were checked weekly from weeks 2 to 10 of treatment, and at week 12 for the patients on Schedule 2 (Fig. 1). The dose of VA was titrated as noted below to maintain serum trough levels between 50 and 100 mg/l. Persistent grade-2 toxicities or any toxicities greater than or equal to grade 3 required dose adjustments and temporary discontinuation of VA until toxicity resolved to grade 1 or baseline, respectively. Following the resolution of toxicities, the dose would be reduced. Patients who exhibited increased RAI uptake based on a scan post-VA therapy at week 10 would continue VA for 6 more weeks, for a total of 16 weeks, until I-131 treatment. Because 6-weeks following a RAI uptake scan is sufficient to clear the iodine in most patients,18, 19 we did not anticipate the stunning effect due to previous iodine exposure. Patients who did not exhibit increased RAI uptake post-VA therapy at week 10 continued the treatment for 6 more weeks, followed by an evaluation of tumour response. VA was discontinued if there was no response based on RECIST or if there was no reduction in the thyroglobulin level from baseline. Depending on the results of the scan, patients would continue VA and prepare for RAI therapy, or would continue with VA (Fig. 1). The activity of I-131 would be determined by dosimetry performed during the diagnostic study. The RAI therapeutic dose was determined by dosimetry performed at week 10, not to exceed a total cumulative RAI dose of 600 mCi. Patient safety was monitored by scheduled clinic visits and laboratory testing every two weeks for four months after the first dose was administered.
Figure 1.
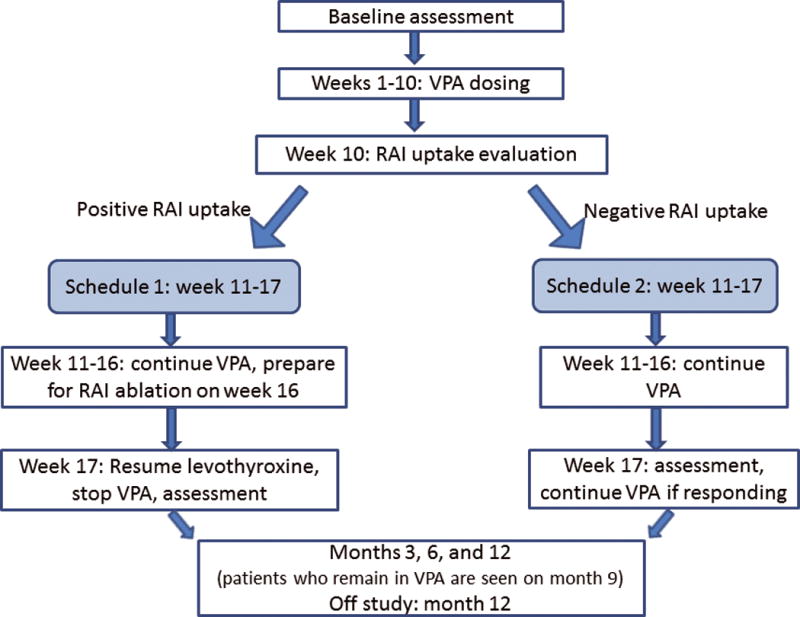
Study schema. Schedule 1 was for patients with increased or positive RAI uptake and Schedule 2 was for those without increased RAI uptake.
Evaluation of tumour response
All patients underwent a Thyrogen® (thyrotropin alfa for injection) RAI scan at entry to the study and after completion of 10 weeks of VA treatment. A low iodine diet was prescribed to all patients two weeks prior to the scan. Thyrogen® (0.9 mg) was injected intramuscularly every 24 hours for two doses. An I-131 scan was performed at 24 and 48 hours following the last dose of Thyrogen®. Whole body, local neck and chest images were obtained anteriorly and posteriorly to visually assess for I-131 uptake. In visually negative scans, geometric mean counts of representative tumours, as identified by anatomical imaging studies, were background corrected and compared to 30–70 μCi I-131 standard imaged in a Plexiglas neck phantom or the neck phantom plus added Plexiglas when lesions were located in the chest. The standard and the patient I-131 dose were calibrated to the same date and time of administration allowing a simple and direct calculation of the percent uptake in the lesion at any time point after administration. The camera method was compared to gamma probe radioactive iodine uptake in clinical patients with thyroid abnormalities and gave virtually identical results to the probe method.
Baseline radiographic studies included ultrasonography of the neck, noncontrast computed tomography or magnetic resonance imaging of the neck and chest, and 18F-Fluorodeoxyglucose positron emission tomography scan. These studies, as well as serum thyroglobulin levels, were repeated to evaluate tumour response at weeks 16 and 30 in all patients.
Statistical analyses
The study used a Simon two-stage optimal design20. The two-stage optimal design minimizes the expected sample size if the regimen has low therapeutic activity, allowing early trial termination in case of a low response rate being observed. An interim analysis was performed when 13 patients had been evaluated for response (stage I). If patients did not have complete/partial responses during that time, the study would close to accrual. The study would proceed to stage II if at least one response was seen among the first 13 patients. At the end of study, if the cumulative number of responses among the 20 evaluable patients was 3 or more, valproic acid would be considered efficacious, while this would not be the case if 1–2 responses were observed.
Under the null hypothesis of a true response rate that does not exceed 5% (p0 = 0.05), the two-stage design would have a type I error (alpha) not more than 7.4%, and if the true response rate was at least 25% (p1 = 0.25), the study would have at least 90% power (beta = 0.10). If the accrual is stopped at the first stage due to futility, the upper bound of a 95% confidence interval for the response rate would be less than 25%. We planned to enrol up to 25 patients with the expectation that 20 (80%) would be evaluated for response.
Results
There were a total of 13 patients enrolled in the study, four women and nine men, with a median age at diagnosis of 55 years (range: 8–67 years). The median age at the study’s enrollment was 62 years (range: 49–78 years). Nine patients were Caucasian. The remaining were black (n = 2) and undesignated (n = 2). At the time of enrollment, 12 patients had metastatic disease in the lung and/or mediastinum (n = 11, 84.5%), and bone (n = 5, 38.5%). Seven patients had locoregional recurrent disease. One patient had elevated serum thyroglobulin without measurable disease on anatomical imaging. Histology showed papillary thyroid cancer (n = 9, 69.2%), follicular thyroid cancer (n = 2, 15.4%), and Hürthle cell cancer (n = 2, 15.4%). Prior therapy other than thyroid hormone for TSH suppression and RAI therapy included cytotoxic chemotherapy (n = 1), multikinase inhibitors (n = 2), external beam radiation (n = 3), and experimental oncolytic viral therapy (n = 1).
Safety
There were two grade-4 adverse events: hyponatraemia (n = 1) and hypercalcaemia (n = 1). Ten grade-3 adverse events were reported in five patients. The most common adverse events were grades 1 and 2 leukopenia (n = 6). Three patients were taken off the study prior to the 10-week RAI uptake scan: one with grade-3 hepatic toxicity, one with disease progression and deterioration of health status after six weeks of treatment, and one due to noncompliance. The adverse events are summarized in Table 2.
Table 2.
Adverse Events in Patients with Radioiodine-Resistant Thyroid Cancer Treated with Valproic Acid
| Adverse events (number of patients) |
Number of adverse events by grade (CTCAE) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Elevated ALT and AST (n = 1) | 2 | 2 | |||
| Elevated alkaline phosphatase (n = 1) | 1 | 1 | |||
| Fatigue/weakness (n = 2) | 2 | 2 | |||
| Hyponatraemia (n = 1) | 1 | 1 | 2 | ||
| Hypercalcaemia (n = 1) | 2 | 1 | 3 | ||
| Hypermagnesaemia (n = 1) | 1 | 1 | |||
| Hyperglycaemia (n = 1) | 1 | 1 | |||
| Dyspnoea and hypoxia (n = 2) | 2 | 2 | |||
| Pleural effusion (n = 2) | 1 | 1 | 2 | ||
| Cough (n = 2) | 1 | 1 | 2 | ||
| Hypotension (n = 1) | 1 | 1 | |||
| Leukopenia (n = 6) | 4 | 4 | 8 | ||
| Thrombocytopenia (n = 3) | 2 | 1 | 3 | ||
| Azotemia (n = 1) | 1 | 1 | |||
Tumour response
None of the patients had partial or complete tumour responses. Six had disease progression. Four patients’ diseases were stable after four months of treatment. Four of 13 patients had decreased serum thyroglobulin with VA treatment. Two patients had thyroglobulin antibodies: one had a 38.4% decrease in the serum thyroglobulin level, with a 41% decrease in thyroglobulin antibody level, while the other developed a high level of thyroglobulin antibodies after six weeks of treatment with stable thyroglobulin level. Of four patients who had decreased serum thyroglobulin levels, the average drop compared to baseline was 27.5% (range: 11–38.4%). The remaining patients had either serum thyroglobulin level (n = 3) increases or thyroglobulin levels exceeding the upper limit of the detection range (>900 μg/l) in both measurements (n = 4).
Radioiodine uptake
None of the patients (n = 10) who underwent a RAI uptake scan after 10 weeks of VA treatment demonstrated increased RAI uptake compared to the baseline scan. All had less than 1% RAI uptake at their tumour sites. Thus, no patients received RAI therapy.
Discussion
We conducted an open-label phase II clinical trial to study the efficacy of VA therapy in 13 patients with incurable differentiated thyroid cancer. The primary objective of the study was to determine the antitumour activity of VA as evidenced by measurable tumour response and/or decreased thyroglobulin levels in patients. We found that the use of VA did not result in decreased tumour sizes. Six patients had disease progression. We observed a modest decrease in serum thyroglobulin levels in only four patients, with no corresponding tumour response. The secondary objective was to determine if VA can increase RAI uptake by the tumour cells. We did not find increased RAI uptake at the tumour sites in any patients. VA was well tolerated in most patients. However, severe liver toxicity resulting in early withdrawal occurred in one patient. The most common adverse event was leukopenia. Increased serum calcium and a reduction in bone mineral density are associated with VA treatment as a result of increased bone turnover21.
VA is an HDAC inhibitor that has long been used to treat epilepsy and bipolar disorder. Similar to suberoylanilide hydroxamic acid (vorinostat), panobinostat, and trichostatin A, VA is a broad-spectrum HDAC inhibitor, while romidepsin, apcidin, and entinostat are class-I–specific inhibitors.22 Preclinical studies demonstrated the antiproliferative and differentiation effects of VA in human thyroid cancer cells10–15, which were similar to the effects of other HDAC inhibitors on several solid cancers.6–9 A phase I study of vorinostat in patients with advanced thyroid cancer showed partial response in one patient and stable disease in the remaining five patients.23 However, no patients (n = 16) with advanced thyroid cancer achieved partial or complete response in a subsequent phase II clinical trial.24 Of 20 patients with advanced thyroid cancer treated with romidepsin in a phase II clinical trial, none developed a partial or complete response.25 Although we observed a modest reduction in serum thyroglobulin levels in four patients, none of our patients had objective tumour responses while receiving therapeutic dosages of VA. It is possible that VA stabilized tumour growth in 4 patients with stable disease during 4 months of treatment.
Because increased expression of NIS was observed in dedifferentiated thyroid cancer cells treated with HDAC inhibitors, we were interested in determining if VA could induce RAI uptake in patients with RAI-resistant thyroid cancer. Previous phase I and II studies of vorinostat showed re-induction of RAI uptake in two out of six patients.23, 24 Romidepsin restored RAI avidity in 2 of the 20 patients treated.25 Unfortunately, none of the 10 patients who were treated with VA for 10 weeks had increased RAI uptake. It is possible that RAI uptake could be seen if a therapeutic dose of RAI was given. However, only a small number of patients with a negative RAI uptake scan have meaningful uptake following a therapeutic, high dose of RAI26. In this study, patients had TSH suppressive therapy during VA treatment because of the clinical benefit in patients with advanced thyroid cancer.19 We elected to continue TSH suppression because the efficacy of VA in thyroid cancer growth and differentiation was not known and discontinuation of TSH suppression could lead to tumour progression. This is consistent with other clinical trials of drugs that increased RAI uptake, in some patients, that also did not discontinue TSH suppressive therapy.27, 28 Furthermore, the additional increased in vitro NIS expression and RAI uptake in thyroid cancer cells treated with HDAC inhibitor occurred within 48 hours following TSH administration29. This suggests that short term exposure to a supra-physiological level of TSH following Thyrogen® injections may be sufficient to induce NIS expression and RAI uptake in thyroid cancer cells treated with a HDAC inhibitor. Other medications that can induce the expression of NIS and RAI uptake include retinoic acid and selumitinib. The effectiveness of retinoic acid in patients with RAI refractory thyroid cancer remains controversial. Improved RAI uptake was reported in 21% (n=3/14) of patients with a 50% decrease in serum thyroglobulin levels from baseline in patients who responded to treatment.27 However, other studies did not find clinical benefit of retinoic acid in patients with RAI refractory thyroid cancer.30, 31 Selumitinib was recently shown to increase RAI uptake in 60% of patients (n=12/20). All patients treated with RAI had decreased serum thyroglobulin levels.28
There are several proposed mechanisms of resistance to HDAC inhibitors. The redox pathway plays an important role in the resistance of normal and cancer cells to HDAC inhibitors. There is a negative correlation between the sensitivity to HDAC inhibitors and the levels of thioredoxin, a thiol reductase that is a scavenger of reactive oxidative species.32 High levels of thioredoxin protect cells from damage caused by reactive oxidative species generation, resulting in resistance to HDAC inhibitors. Other mechanisms include constitutive activation of the nuclear factor kB (NF-kB) signalling pathway, aberrant activation of the signal transducers and activators of transcription (STAT) pathways, and increased multidrug resistance gene expression.33
In summary, we found only a modest biochemical response in 40% of patients with advanced, RAI-resistant thyroid cancer of follicular cell origin who were treated with VA. However, none of the patients had objective tumour responses. Despite promising preclinical data, VA did not result in increased RAI uptake in any of the tumour sites. Thus, VA is an ineffective single-agent treatment for patients with RAI-resistant thyroid cancer.
Acknowledgments
The research activities performed in this manuscript were supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of interest statement:
All authors declare that no competing financial interests exist.
Name of author to whom proofs will be sent: Naris Nilubol
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. Jama. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA. Perspective: National Cancer Institute summary report about estimated exposures and thyroid doses received from iodine 131 in fallout after Nevada atmospheric nuclear bomb tests. CA Cancer J Clin. 1998;48:285–298. doi: 10.3322/canjclin.48.5.285. [DOI] [PubMed] [Google Scholar]
- 3.Goretzki PE, Simon D, Frilling A, et al. Surgical reintervention for differentiated thyroid cancer. Br J Surg. 1993;80:1009–1012. doi: 10.1002/bjs.1800800826. [DOI] [PubMed] [Google Scholar]
- 4.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 5.Ma C, Xie J, Kuang A. Is empiric 131I therapy justified for patients with positive thyroglobulin and negative 131I whole-body scanning results? J Nucl Med. 2005;46:1164–1170. [PubMed] [Google Scholar]
- 6.Munster P, Marchion D, Bicaku E, et al. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res. 2009;15:2488–2496. doi: 10.1158/1078-0432.CCR-08-1930. [DOI] [PubMed] [Google Scholar]
- 7.Rocca A, Minucci S, Tosti G, et al. A phase I-II study of the histone deacetylase inhibitor valproic acid plus chemoimmunotherapy in patients with advanced melanoma. Br J Cancer. 2009;100:28–36. doi: 10.1038/sj.bjc.6604817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, et al. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–222. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Michaelis M, Doerr HW, Cinatl J., Jr Valproic acid as anti-cancer drug. Curr Pharm Des. 2007;13:3378–3393. [PubMed] [Google Scholar]
- 10.Catalano MG, Pugliese M, Poli R, et al. Effects of the histone deacetylase inhibitor valproic acid on the sensitivity of anaplastic thyroid cancer cell lines to imatinib. Oncol Rep. 2009;21:515–521. [PubMed] [Google Scholar]
- 11.de Vries L, Karasik A, Landau Z, et al. Endocrine effects of valproate in adolescent girls with epilepsy. Epilepsia. 2007;48:470–477. doi: 10.1111/j.1528-1167.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 12.Catalano MG, Fortunati N, Pugliese M, et al. Valproic acid, a histone deacetylase inhibitor, enhances sensitivity to doxorubicin in anaplastic thyroid cancer cells. J Endocrinol. 2006;191:465–472. doi: 10.1677/joe.1.06970. [DOI] [PubMed] [Google Scholar]
- 13.Shen WT, Wong TS, Chung WY, et al. Valproic acid inhibits growth, induces apoptosis, and modulates apoptosis-regulatory and differentiation gene expression in human thyroid cancer cells. Surgery. 2005;138:979–984. doi: 10.1016/j.surg.2005.09.019. discussion 984–975. [DOI] [PubMed] [Google Scholar]
- 14.Catalano MG, Fortunati N, Pugliese M, et al. Valproic acid induces apoptosis and cell cycle arrest in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2005;90:1383–1389. doi: 10.1210/jc.2004-1355. [DOI] [PubMed] [Google Scholar]
- 15.Fortunati N, Catalano MG, Arena K, et al. Valproic acid induces the expression of the Na+/I− symporter and iodine uptake in poorly differentiated thyroid cancer cells. J Clin Endocrinol Metab. 2004;89:1006–1009. doi: 10.1210/jc.2003-031407. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Piccardo A, Arecco F, Puntoni M, et al. Focus on high-risk DTC patients: high postoperative serum thyroglobulin level is a strong predictor of disease persistence and is associated to progression-free survival and overall survival. Clin Nucl Med. 2013;38:18–24. doi: 10.1097/RLU.0b013e318266d4d8. [DOI] [PubMed] [Google Scholar]
- 18.Padovani RP, Kasamatsu TS, Nakabashi CC, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22:926–930. doi: 10.1089/thy.2012.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y, Kondo I, Ishida S, et al. Decreased bone mass and increased bone turnover with valproate therapy in adults with epilepsy. Neurology. 2001;57:445–449. doi: 10.1212/wnl.57.3.445. [DOI] [PubMed] [Google Scholar]
- 22.Wu D, Huang Q, Zhang Y, et al. Screening of selective histone deacetylase inhibitors by proteochemometric modeling. BMC Bioinformatics. 2012;13:212. doi: 10.1186/1471-2105-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94:164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman EJ, Su YB, Lyall A, et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013;23:593–599. doi: 10.1089/thy.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fatourechi V, Hay ID, Javedan H, et al. Lack of impact of radioiodine therapy in tg-positive, diagnostic whole-body scan-negative patients with follicular cell-derived thyroid cancer. J Clin Endocrinol Metab. 2002;87:1521–1526. doi: 10.1210/jcem.87.4.8373. [DOI] [PubMed] [Google Scholar]
- 27.Oh SW, Moon SH, Park do J, et al. Combined therapy with 131I and retinoic acid in Korean patients with radioiodine-refractory papillary thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38:1798–1805. doi: 10.1007/s00259-011-1849-2. [DOI] [PubMed] [Google Scholar]
- 28.Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng W, Liu R, Zhu G, et al. Robust Thyroid Gene Expression and Radioiodine Uptake Induced by Simultaneous Suppression of BRAF V600E and Histone Deacetylase in Thyroid Cancer Cells. J Clin Endocrinol Metab. 2016;101:962–971. doi: 10.1210/jc.2015-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Courbon F, Zerdoud S, Bastie D, et al. Defective efficacy of retinoic acid treatment in patients with metastatic thyroid carcinoma. Thyroid. 2006;16:1025–1031. doi: 10.1089/thy.2006.16.1025. [DOI] [PubMed] [Google Scholar]
- 31.Short SC, Suovuori A, Cook G, et al. A phase II study using retinoids as redifferentiation agents to increase iodine uptake in metastatic thyroid cancer. Clin Oncol (R Coll Radiol) 2004;16:569–574. doi: 10.1016/j.clon.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Marks PA. Thioredoxin in cancer–role of histone deacetylase inhibitors. Semin Cancer Biol. 2006;16:436–443. doi: 10.1016/j.semcancer.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JH, Choy ML, Marks PA. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012;116:39–86. doi: 10.1016/B978-0-12-394387-3.00002-1. [DOI] [PubMed] [Google Scholar]


