Abstract
Background
Lithium augmentation of antidepressants is an effective strategy in treatment-resistant depression. The proteohormone ghrelin is thought to be involved in the pathophysiology of depression. The purpose of this study was to investigate the association of treatment response with the course of ghrelin levels during lithium augmentation.
Method
Ghrelin serum concentrations and severity of depression were measured in 85 acute depressive patients before and after 4 weeks of lithium augmentation.
Results
In a linear mixed model analysis, we found a significant effect of response*time interaction (F1.81=9.48; P=.0028): under treatment, ghrelin levels increased in nonresponders and slightly decreased in responders to lithium augmentation. The covariate female gender had a significant positive effect (F1.83=4.69; P=.033), whereas time, response, appetite, and body mass index (kg/m2) did not show any significant effect on ghrelin levels (P>.05).
Conclusion
This is the first study showing that the course of ghrelin levels separates responders and nonresponders to lithium augmentation. Present results support the hypothesis that ghrelin serum concentrations might be involved in response to pharmacological treatment of depression.
Keywords: ghrelin, lithium augmentation, response, depression, therapy resistant depression
Introduction
The role of the proteohormone ghrelin in the pathophysiology of depression is currently discussed. While ghrelin is secreted mainly at the fundus of the stomach, only small amounts are produced in the central nervous system. Ghrelin is able to cross the blood brain barrier and stimulates appetite and food intake by binding to its receptor (GHS-R1a) in the hypothalamus. GHS-R1a is expressed in numerous neuronal populations, and therefore the physiological role of ghrelin might include learning, memory, anxiety, depression, and neuroprotection (Dos Santos et al., 2013). A recent observation showed that plasma acylated ghrelin is associated with neural abnormalities in the ventral tegmental area, thereby modulating the reward system, which has been implicated in the pathophysiology of depression. Ghrelin modulates monoamine neurotransmission and the HPA axis, which both play an important role in the pathophysiology of depression. Furthermore, a polymorphism on ghrelin’s encoding gene was described to be associated with depression (Nakashima et al., 2008). An increase in ghrelin levels occurs not only in states of anergia but also follows acute and chronic stress. In summary, ghrelin signalling could play an important role in the pathophysiology of major depressive disorder (MDD), which still needs to be clarified.
Meta-analytical data show lithium augmentation (LA) of antidepressants as an effective strategy in MDD. The mechanisms underlying therapeutic efficacy of LA are still poorly understood, and individual response to LA cannot yet be predicted (Bauer et al., 2014). To the best of our knowledge, the course of ghrelin serum levels during lithium treatment and the association of treatment response with the course of ghrelin serum levels in the treatment of depression have not yet been investigated.
The purpose of this study was to investigate the association of treatment response with the course of ghrelin levels during LA by measuring ghrelin before and during LA in patients with MDD in a prospective cohort study.
We hypothesized that ghrelin is influenced by LA and that there is a different effect of LA in responders and nonresponders on ghrelin levels.
Methods
The study population was a subsample of a prospective cohort study investigating treatment response and side effect burden of LA in MDD. In this subsample, data on serum levels of ghrelin (baseline and study endpoint) were available in 85 patients (71 in- and 14 outpatients). The inclusion criteria were: MDD (according to DSM-IV cirteria) (American Psychiatric Association, 1994), age older than 18 years, indication for an antidepressant pharmacotherapy, insufficient response to an adequate antidepressant pretreatment and clinical indication for lithium augmentation, a Hamilton Depression Rating Scale (HDRS-17; Hamilton 1960) score ≥12, and written informed consent. The respective diagnosis was confirmed using the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). Patients were recruited between December 2008 and December 2012 in 12 psychiatric departments of the Berlin Research Network on Depression. The local ethics committee approved the study, and written informed consent was obtained from all subjects.
Ghrelin serum concentrations (nonfasting) were first measured in medicated patients before LA (baseline) and then after 4 weeks of co-medication with lithium (endpoint). Severity of depression (measured with HDRS-17) and body mass index (BMI, kg/m2) were measured on both occasions. Clinical response was defined as a HDRS-17 decrease of 50% or more. All patients received individual doses of lithium carbonate adapted to their individual lithium serum levels.
Endogenous ghrelin levels were measured in the rethawed serum samples using RayBio Ghrelin Enzyme Immunoassay (EIA) Kit, an in vitro quantitative assay based on the principle of competitive enzyme immunoassay. The detection range was 0.1 to 1.000 ng/mL, the intra-assay CV was <10%, and the inter-assay CV was <15%, according to the manufacturer’s specifications (RayBio Human Ghrelin EIA Kit Protocol, 2013).
We used a linear mixed model for statistical analysis to investigate the effect of LA (time) as an explanatory factor for change in ghrelin levels. The differential effect of response to LA on change in ghrelin levels is represented by a response*time interaction term. Linear mixed-effects models have the advantage of allowing the investigation of variability between patients (heterogeneity) and simultaneously adjusting for the within-subject correlation. In the present analysis, random effects were permitted for the intercepts. We entered time, gender, BMI, appetite, response, and response*time interaction as fixed effects into the model. To control for the effect of appetite on ghrelin levels, we used the HRDS-17 item “12. Somatic symptoms (gastrointestinal)”. Model regression coefficients are reported together with their 95% CI. We used SAS software, version 9.4. to perform the linear mixed model analysis and SPSS (version 21) for descriptive statistics, paired sample t test, and repeated-measures ANOVA-rm (Table 1). Significance level was set at .05 in all analyses.
Table 1.
Clinical Data and Results
| n (%) | Responder | Nonresponder | P Value* | ||
|---|---|---|---|---|---|
| Patients | 85 (100) | ||||
| Female | 53 (62.4) | 18 (52.9) | 35 (68.6) | ||
| Male | 32 (37.6) | 16 (47.1) | 16 (31.4) | >.1 | |
| Response | 34 (40.0) | ||||
| Nonresponse | 51 (60.0) | ||||
| Psychotropic comedication | |||||
| Antidepressants | |||||
| SSRI | 47 (55.3) | 16 (47.1) | 31 (60.8) | >.1 | |
| SNRI | 21 (24.7) | 12 (35.3) | 9 (17.6) | .65 | |
| TCA | 9 (10.6) | 4 (11.8) | 5 (9.8) | >.1 | |
| NDRI | 4 (4.7) | 0 (0.0) | 4 (7.8) | .94 | |
| Valdoxan | 1 (1.2) | 0 (0.0) | 1 (1.9) | >.1 | |
| NaSSA | 12 (14.1) | 5 (14.7) | 7 (13.7) | >.1 | |
| MAO-I | 2 (2.4) | 1 (2.9) | 1 (1.9) | >.1 | |
| Atypical antipsychotics | 22 (25.9) | 9 (26.5) | 13 (25.5) | >.1 | |
| Antiepileptic drugs | 8 (9.4) | 2 (5.9) | 6 (11.8) | >.1 | |
| Benzodiazepines | 19 (22.4) | 10 (29.4) | 9 (17.6) | >.1 | |
| Low-potency antipsychotics | 4 (4.7) | 0 (0.0) | 4 (7.8) | .94 | |
| Mean (SD) | Responder mean (SD) | Nonresponder mean (SD) | P Value** | ||
| Age | 85 (100) | 48.31 ± 15.27 | 50.00 ± 15.56 | 47.18 ± 15.12 | >.1 |
| Lithium serum level (mmol/L) last visit | 77 (90.6) | 0.713 ± 0.152 | 0.704 ± 0.146 | 0.719 ± 0.157 | >.1 |
| HRDS-17 score baseline | 85 (100) | 21.32 ± 5.03 | 19.73 ± 4.94 | 22.35 ± 4.85 | >.1 |
| HDRS-17 score last visit | 85 (100) | 12.84 ± 7.59 | 5.79 ± 2.95 | 17.53 ± 5.91 | <.01 |
| BMI baseline | 84 (98.8) | 25.54 ± 5.74 | 26.27 ± 6.26 | 26.64 ± 6.14 | >.1 |
| BMI last visit | 85 (100) | 26.00 ± 5.69 | 25.15 ± 5.44 | 25.58 ± 5.39 | >.1 |
| Ghrelin serum level (ng/mL) baseline | 84 (98.8) | 5.71 ± 6.20 | 6.50 ± 5.44 | 5.46 ± 4.90 | >.1 |
| Ghrelin serum level (ng/mL) last visit | 85 (100) | 6.42 ± 6.99 | 5.12 ± 6.71 | 7.05 ± 8.07 | <.05 |
| Before lithium augmentation | After lithium augmentation | P Value*** | |||
| Ghrelin serum level (ng/mL) | 84 (98.8) | 5.71 ± 6.20 | 85 (100) | 6.42 ± 6.99 | <.05 |
| Ghrelin serum level (ng/mL) responder | 36 (42.9) | 6.50 ± 5.44 | 36 (42.4) | 5.46 ± 4.90 | >.1 |
| Ghrelin serum level (ng/mL) nonresponder | 48 (57.1) | 5.12 ± 6.71 | 49 (57.6) | 7.05 ± 8.07 | <.05 |
| HRDS-17 score | 85 (100) | 21.32 ± 5.03 | 85 (100) | 12.84 ± 7.59 | <.05 |
| BMI | 84 (98.8) | 25.54 ± 5.74 | 85 (100) | 26.00 ± 5.69 | <.05+ |
| BMI responder | 34 (40.4) | 26.27 ± 6.26 | 34 (40.0) | 26.64 ± 6.14 | .052 |
| BMI nonresponder | 50 (59.5) | 25.15 ± 5.44 | 51 (60.0) | 25.58 ± 5.39 | <.05 |
Abbreviations: BMI, body mass index; HDRS-17, Hamilton Depression Rating Scale; MAO-I, mono amine oxidase inhibitor (tranylcypromine); NaSSA, noradrenergic and specifically serotonergic antidepressant (mirtazapine); NDRI, norepinephrine and dopamine reuptake inhibitor (bupropione); reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
*Result of Chi-square test; **results of independent t test; all parameters were normally distributed;
***results of paired sample t test; all parameters were normally distributed; the comparison of ghrelin and BMI before and after lithium augmentation (LA) was conducted with 84 patients, whereas mean and SD for the observation after LA are displayed for 85 patients in this table to present all data that were used for linear mixed model analysis.
+No significant effect of response on the course of BMI during LA was found in an ANOVA-rm (P>.05).
Results
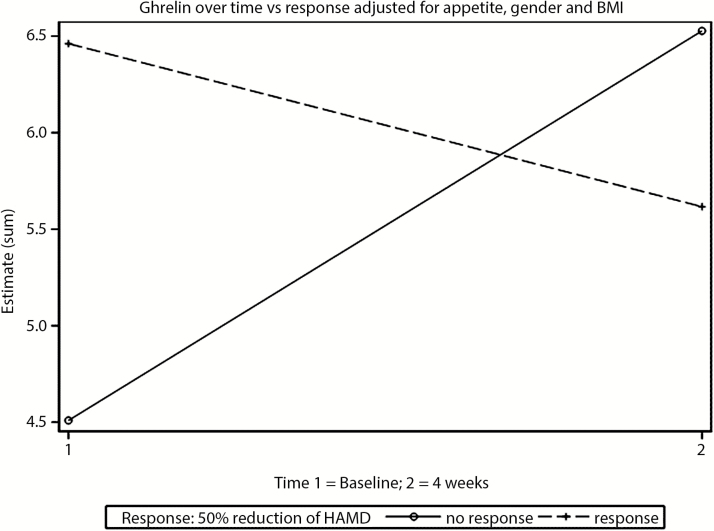
Table 1 provides information on measured ghrelin levels and clinical data as well as a basic statistical analysis of group characteristics (responders vs nonresponders) and the data that was used for linear mixed model analysis. In the following text and in Figure 1, we present estimated values of ghrelin levels as a result of the linear mixed model analysis. These estimates describe the influence of the respective patient characteristics on ghrelin levels. The result of the linear mixed model analysis is the main result of the study. For n=85 patients, 169 observations of ghrelin levels were used for linear mixed model analysis (a baseline measurement for 1 patient was missing). A significant effect on ghrelin levels was found for response*time interaction (F1.81=9.48; P=.0028) and gender (F1.83=4.69; P=.033). In this model, BMI (F1.91=0.22; P=.64), response (F1.84=0.14; P=.71), appetite (F1.113=0.95; P=.33), and time (equal to “treatment effect of LA”; F1.83=1.58; P=.21) did not show a significant effect on ghrelin levels. The main finding of the study (significant response*time interaction) is demonstrated in Figure 1 by the crossed regression graphs. In this analysis, the estimated ghrelin levels do not differ significantly between responders and nonresponders at baseline and after LA (overlapping 95% CI). The estimated ghrelin levels at baseline were 4.51 ng/mL (95% CI: 2.61–6.41; P<.0001) in nonresponders and 6.45 ng/mL (95% CI: 4.30–8.61; P<.0001) in responders. The estimated ghrelin levels at endpoint were 6.53 ng/mL (95% CI: 4.65–8.41; P<.0001) in nonresponders and 5.62 ng/mL (95% CI: 3.41–7.83; P<.0001) in responders (Figure 1.). The estimated ghrelin levels in men were 3.01 ng/mL lower compared with women (95% CI: -5.77 to 0.25; P=.033).
Figure 1.
Course of ghrelin levels in responders and nonresponders adjusted for gender and BMI: ghrelin increases in nonresponders and decreases slightly in responders to lithium augmentation. Ghrelin serum levels are presented as estimated means in ng/mL.
Discussion
This is the first study investigating the course of ghrelin serum levels and the effect of treatment response on ghrelin levels during LA in patients with MDD. We found that during LA, ghrelin levels increase in nonresponders and slightly decrease in responders.
Our findings are in line with a study that found higher ghrelin serum levels in nonresponders with MDD to antidepressant treatment when compared with responders and normal controls (Ishitobi et al., 2012). These findings strengthen evidence that ghrelin might separate subgroups of depressive patients that respond to pharmacological treatment of depression. As depression-related stress continues in nonresponders but not (or less severely) in responders, our results are in line with studies reporting a stress-dependent increase in ghrelin levels (Rouach et al., 2007) and higher ghrelin serum levels in depressed individuals compared with healthy controls (Gecici et al., 2005).
The observed gender effect in our study is in line with previous studies reporting higher ghrelin levels in females than males in both healthy subjects (Chan et al., 2004) and depressed patients (Kluge et al., 2009).
Our finding of an increase of ghrelin levels in nonresponders and a decrease in responders is in line with a preclinical study that reported an antidepressant-like effect following inhibition of ghrelin secretion after central or peripheral injection of ghrelin antisense DNA in rats (Kanehisa et al., 2006), suggesting a depressiogenic effect of ghrelin. A depressiogenic effect of ghrelin might theoretically be explained by the suppressive effect of ghrelin on serotonin release as well as its stimulatory action on HPA axis (Kluge et al., 2007). On the other hand, the majority of data, in particular from animal studies, revealed contrasting results (reviewed in Spencer et al., 2015; Wittekind and Kluge, 2015) and reported antidepressive and anxiolytic effects of ghrelin infusion (Lutter et al., 2008; Carlini et al., 2012), which might theoretically be explained by a stimulating effect of ghrelin on norepinephrine neurotransmission (Date et al., 2006) or via inhibition of glycogen-synthase-kinase 3 beta (Zhang et al., 2013). In this context, the ghrelin upregulation in nonresponders observed in our study could also be interpreted as an intrinsic or pharmacologically induced attempt to counteract depressive symptomatology. However, in a small sample, ghrelin infusion in patients with depression did not have a significant effect on depressive symptoms (Kluge et al., 2011). It is important to note that studies on the effect of ghrelin application have investigated short-term effects (15 minutes until a few days) after short-term application, whereas our study investigated long-term effects (4 weeks) after long-term application; thus, comparability of study results is limited.
Another explanation for the increase of ghrelin levels in nonresponders and the decrease in responders could be that we “only” observe secondary effects of depressive symptomatology and successful antidepressant treatment with LA: one of the main symptoms of MDD is reduced appetite and consecutively reduced food intake. Starvation is known to be a strong cue for ghrelin release, which could contribute to the observed ghrelin increase in nonresponders. Responders start to eat normally because of the relief of depressive symptomatology, which consecutively results in a decrease of ghrelin levels. Noteworthy, the result of our analysis is corrected for the effect of appetite on ghrelin levels, and we did not find a significant effect of response on BMI, which therefore does not support this hypothesis. Furthermore, other studies rather report lower (Barim et al., 2009) or unchanged (Kluge et al., 2009) ghrelin serum levels in depressed subjects compared with healthy controls.
However, in our study, we did not find a correlation between severity of depression and ghrelin serum levels (data not shown). In summary, the course of ghrelin levels observed in our study could be interpreted in the context of either an antidepressive or a depressiogenic effect of ghrelin or rather reflect a secondary effect of depressive symptomatology, that is, reduced appetite and food intake.
Lithium has well-known neuroprotective effects (Bauer et al., 2014) and acts as a glycogen-synthase-kinase 3 beta inhibitor (Jope and Bijur, 2002). Interestingly, preclinical studies suggest that ghrelin can function as a neuroprotective agent that inhibits apoptotic pathways (Zhang, 2013) and that inactivation of GSK-3B contributes to the antiapoptotic effects of ghrelin. In the current analysis, we found ghrelin influenced by LA. In previously published analyses, we found that serum levels of brain-derived neurotrophic factor (Ricken et al., 2013) and fibroblast growth factor 23 (Fakhri et al., 2014), both discussed in the context of neuroprotection, increased during LA. Therefore, one might speculate that ghrelin, brain-derived neurotrophic factor, and fibroblast growth factor 23 signalling are potentially involved in the neuroprotective action of lithium.
Previous studies investigating ghrelin levels during antidepressant treatment found an increase of serum ghrelin levels during treatment with maprotiline (Pinar et al., 2008) and amitriptyline (Huang et al., 2013), while a decrease in ghrelin serum levels was reported during treatment with citalopram (Barim et al., 2009) and mirtazapine (Schmid et al., 2006), electroconvulsive therapy (Kurt et al., 2007), or various antidepressive treatment strategies (Nakashima et al., 2014). These heterogeneous findings could be due to different treatment strategies, small sample sizes, and different response rates. Limitations of these previous studies are small sample sizes and that ghrelin levels were not available together with longitudinally assessed clinical response data. The main strengths of our study are the relatively large sample size and the prospective study design. Our study extends existing data by providing (1) the first investigation of ghrelin serum levels during lithium treatment and (2) the first longitudinal investigation of the effect of treatment response on the course of ghrelin levels in patients with MDD.
Limitations of our study include the lack of a control group and a heterogeneous psychopharamcological comedication. We therefore cannot say if the observed changes in ghrelin levels are a lithium-specific effect. However, psychopharmacological comedication was stable during and before LA, and we did not find significant differences in this medication between responders and nonresponders, so that the influence of this confounding variable is limited. Also, we measured nonfasting ghrelin levels that were not taken after a standard meal and we did not obtain information on the time of the day when the blood samples were taken. Ghrelin levels strongly depend on food intake, appetite, and the anticipation of food, and its secretion has a circadian rhythm (Kirsz and Zieba, 2011) that increases variation of ghrelin levels. Nevertheless, we think that the large sample size could sufficiently compensate this confounder. Furthermore, we only have a pre- and postmeasurement of ghrelin levels, so we cannot distinguish between cause and effect (i.e., do ghrelin levels influence treatment response or does treatment response influence ghrelin levels?). To investigate this, a series of ghrelin and psychometric measurements with shorter time intervals is recommended for future studies.
In conclusion, the present results support the hypothesis that ghrelin serum concentrations might be involved in the response to pharmacological treatment of depression. Based on our data, one might speculate that an increase of the ghrelin serum concentration might be an indicator of nonresponse to LA in patients suffering from treatment-resistant depression. Further studies are needed to separate specific effects of different antidepressants on ghrelin concentration but also to address ghrelin downstream mechanisms.
Statement of Interest
Roland Ricken received an unrestricted research grant from Aristo. Thomas Stamm received speaker honoraria from Lundbeck and BristolMyersSquibb. He is a consultant for Servier. Hubertus Himmerich received speaker honoraria from AstraZeneca and Servier, consulting fees from Bristol-Myers Squibb, and chemical substances for study support from AstraZeneca, Novartis, and Wyeth. Stefan Borgwardt received contributions or honoraria from Janssen-Cilag, Lilly, Takeda, Lundbeck, and Pfizer. Michael Bauer has received grants or research support from German Research Foundation, European Commission (FP7), American Foundation for Suicide Prevention, and German Federal Ministry for Education and Research. He has received speaker honoraria from AstraZeneca, GlaxoSmithKline, Lilly, Lundbeck, Otsuka, and Pfizer. He is a consultant for AstraZeneca, Bristol-Myers Squibb, Ferrer Internacional, Janssen, Lilly, Novartis, Takeda, Otsuka, and Lundbeck. Samuel Elstner received honoraria from Novartis and Janssen-Cilag. Mazda Adli has received grants or research support from Aristo, Servier, and Bristol-Myers Squibb; honoraria for speaking from Deutsche Bank, the Johanniter Order, East German Savings Banks Association, Pusch Wahl Legal Lawyers, HRM Forum, Helios Media, Lundbeck, Bristol-Myers Squibb, Boehringer Ingelheim, Servier, Aristo, Viiv, and Gilead; travel grants from the Alfred Herrhausen Society, Lundbeck, and Servier; and has been a consultant to Deutsche Bank, Bristol-Myers Squibb, Aristo, Merz, and Lundbeck. Sandra Bopp, Peter Schlattmann, Rainer Hellweg, Brigitte Schulz-Ratei, Philipp Sterzer, Alexandra Lingesleben, Christoph Richter, Undine E. Lang, and Andreas Heinz wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
We thank Professor Florian Lang and Hajar Fakhri at Institute of Physiology I, Eberhard Karls Universität Tübingen for performance of ghrelin measurement and Maximilian Berger and Sarah Enge (Charité - Universitätsmedizin Berlin, Department of Psychiatry and Psychotherapy, Campus Charité Mitte) for help with submission of the paper.
This work was supported by sources of the Mood Disorders Research Unit of Charité Universitätsmedizin Berlin, Department of Psychiatry and Psychotherapie, Campus Charité Mitte.
References
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. [Google Scholar]
- Barim AO, Aydin S, Colak R, Dag E, Deniz O, Sahin I (2009) Ghrelin, paraoxonase and arylesterase levels in depressive patients before and after citalopram treatment. Clin Biochem 42:1076–1081. [DOI] [PubMed] [Google Scholar]
- Bauer M, Adli M, Ricken R, Severus E, Pilhatsch M (2014) Role of lithium augmentation in the management of major depressive disorder. CNS Drugs 28(4):331–42. [DOI] [PubMed] [Google Scholar]
- Chan JL, Bullen J, Lee JH, Yiannakouris N, Mantzoros CS (2004) Ghrelin levels are not regulated by recombinant leptin administration and/or three days of fasting in healthy subjects. J Clin Endocrinol Metab 89(1):335–43. [DOI] [PubMed] [Google Scholar]
- Carlini VP, Machado DG, Buteler F, Ghersi M, Ponzio MF, Martini AC, Schiöth HB, de Cuneo MF, Rodrigues AL, de Barioglio SR (2012) Acute ghrelin administration reverses depressive-like behavior induced by bilateral olfactory bulbectomy in mice. Peptides 35(2):160–5. [DOI] [PubMed] [Google Scholar]
- Dos Santos VV, Rodrigues AL, De Lima TC, de Barioglio SR, Raisman-Vozari R, Prediger RD (2013) Ghrelin as a neuroprotective and palliative agent in Alzheimer’s and Parkinson’s disease. Current pharmaceutical design 19:6773–6790. [DOI] [PubMed] [Google Scholar]
- Date Y, Shimbara T, Koda S, Toshinai K, Ida T, Murakami N, Miyazato M, Kokame K, Ishizuka Y, Ishida Y, Kageyama H, Shioda S, Kangawa K, Nakazato M (2006) Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metabolism 4:323–331. [DOI] [PubMed] [Google Scholar]
- Fakhri H, Ricken R, Adli M, Fajol A, Walter M, Föller M, Lang F, Lang UE, Lange C (2014) Impact of Lithium Treatment on FGF-23 Serum Concentrations in Depressive Patients. J Clin Psychopharmacol 19. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gecici O, Serteser M, Emül M, Demirel R (2005) Serum ghrelin and leptin levels in major depressive disorders. Neurol Psychiatry Brain Res 12:47–52. [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Jiang SM, Jia L, You LQ, Huang YX, Gong YM, Wang GQ (2013) Effect of amitriptyline on gastrointestinal function and brain-gut peptides: a double-blind trial. World J Gastroenterol 19:4214–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitobi Y, Kohno K, Kanehisa M, Inoue A, Imanaga J, Maruyama Y, Ninomiya T, Higuma H, Okamoto S, Tanaka Y, Tsuru J, Hanada H, Isogawa K, Akiyoshi J (2012) Serum ghrelin levels and the effects of antidepressants in major depressive disorder and panic disorder. Neuropsychobiology 66:185–192. [DOI] [PubMed] [Google Scholar]
- Jope RS, Bijur GN (2002): Mood stabilizers, glycogen synthase kinase 3-beta and cell survival. Mol Psychiatry 7(suppl 1):35–45. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Akiyoshi J, Kitaichi T, Matsushita H, Tanaka Y, Kodama K, Hanada H, Isogawa K (2006) Administration of antisense DNA for ghrelin causes an antidepressant and anxiolytic response in rats. Prog Neuropsychopharmacol Biol Psychiatry 30:1403–1407. [DOI] [PubMed] [Google Scholar]
- Kirsz K, Zieba DA (2011) Ghrelin-mediated appetite regulation in the central nervous system. Peptides 32(11):2256–64. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schüssler P, Zuber V, Yassouridis A, Steiger A (2007) Ghrelin administered in the early morning increases secretion of cortisol and growth hormone without affecting sleep. Psychoneuroendocrinology 32:287–292. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schussler P, Schmid D, Uhr M, Kleyer S, Yassouridis A, Steiger A (2009) Ghrelin plasma levels are not altered in major depression. Neuropsychobiology 59:199–204. [DOI] [PubMed] [Google Scholar]
- Kluge M, Schüssler P, Dresler M, Schmidt D, Yassouridis A, Uhr M, Steiger A (2011) Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J Psychiatr Res 45(3):421–6. [DOI] [PubMed] [Google Scholar]
- Kurt E, Guler O, Serteser M, Cansel N, Ozbulut O, Altinbas K, Alatas G, Savas H, Gecici O (2007) The effects of electroconvulsive therapy on ghrelin, leptin and cholesterol levels in patients with mood disorders. Neurosci Lett 426:49–53. [DOI] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM (2008) The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 11(7):752–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Akiyoshi J, Hatano K, Hanada H, Tanaka Y, Tsuru J, Matsushita H, Kodama K, Isogawa K (2008) Ghrelin gene polymorphism is associated with depression, but not panic disorder. Psychiatr Genet 18(5):257. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Akiyoshi J, Hatano K, Hanada H, Tanaka Y, Tsuru J, Matsushita H, Kodama K, Ozsoy S, Besirli A, Abdulrezzak U, Basturk M (2014) Serum Ghrelin and Leptin Levels in Patients with Depression and the Effects of Treatment. Psychiatry Investig 11(2):167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinar M, Gulsun M, Tasci I, Erdil A, Bolu E, Acikel C, Doruk A (2008) Maprotiline induced weight gain in depressive disorder: changes in circulating ghrelin and adiponectin levels and insulin sensitivity. Prog Neuropsychopharmacol Biol Psychiatry 32:135–139. [DOI] [PubMed] [Google Scholar]
- RayBio ® Human Ghrelin EIA Kit Protocol (2013) RayBiotech, Inc; www.raybiotech.com
- Ricken R, Adli M, Lange C, Krusche E, Stamm TJ, Gaus S, Koehler S, Nase S, Bschor T, Richter C, Steinacher B, Heinz A, Rapp MA, Borgwardt S, Hellweg R, Lang UE (2013) Brain-derived neurotrophic factor serum concentrations in acute depressive patients increase during lithium augmentation of antidepressants. J Clin Psychopharmacol 33(6):806–9. [DOI] [PubMed] [Google Scholar]
- Rouach V, Bloch M, Rosenberg N, Gilad S, Limor R, Stern N, Greenman Y (2007) The acute ghrelin response to a psychological stress challenge does not predict the post-stress urge to eat. Psychoneuroendocrinology 32:693–702. [DOI] [PubMed] [Google Scholar]
- Schmid DA, Wichniak A, Uhr M, Ising M, Brunner H, Held K, Weikel JC, Sonntag A, Steiger A (2006) Changes of sleep architecture, spectral composition of sleep EEG, the nocturnal secretion of cortisol, ACTH, GH, prolactin, melatonin, ghrelin, and leptin, and the DEX-CRH test in depressed patients during treatment with mirtazapine. Neuropsychopharmacology 31:832–844. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–23. [PubMed] [Google Scholar]
- Spencer SJ, Emmerzaal TL, Kozicz T, Andrews ZB (2015) Ghrelin’s Role in the Hypothalamic-Pituitary-Adrenal Axis Stress Response: Implications for Mood Disorders. Biol Psychiatry 78(1):19–27. [DOI] [PubMed] [Google Scholar]
- Wittekind DA, Kluge M (2015) Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology 52:176–94. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Deng C, Huang XF (2013) The role of ghrelin signalling in second-generation antipsychotic-induced weight gain. Psychoneuroendocrinology 38(11):2423–38. [DOI] [PubMed] [Google Scholar]