Abstract
Background
Impaired empathic abilities lead to severe negative social consequences and influence the development and treatment of several psychiatric disorders. Furthermore, empathy has been shown to play a crucial role in moral and prosocial behavior. Although the serotonin system has been implicated in modulating empathy and moral behavior, the relative contribution of the various serotonin receptor subtypes is still unknown.
Methods
We investigated the acute effect of psilocybin (0.215 mg/kg p.o.) in healthy human subjects on different facets of empathy and hypothetical moral decision-making using the multifaceted empathy test (n=32) and the moral dilemma task (n=24).
Results
Psilocybin significantly increased emotional, but not cognitive empathy compared with placebo, and the increase in implicit emotional empathy was significantly associated with psilocybin-induced changed meaning of percepts. In contrast, moral decision-making remained unaffected by psilocybin.
Conclusions
These findings provide first evidence that psilocybin has distinct effects on social cognition by enhancing emotional empathy but not moral behavior. Furthermore, together with previous findings, psilocybin appears to promote emotional empathy presumably via activation of serotonin 2A/1A receptors, suggesting that targeting serotonin 2A/1A receptors has implications for potential treatment of dysfunctional social cognition.
Keywords: empathy, moral decision-making, psilocybin, serotonin, 5-HT2A/1A receptors
Significance Statement
Empathy is important for the maintenance of social relationships and plays a crucial role in moral and prosocial behavior. This study investigated the acute effect of the serotonergic hallucinogen psilocybin in healthy human subjects on different facets of empathy and moral decision-making. Psilocybin significantly increased explicit and implicit emotional empathy, compared with placebo, whereas it did not affect cognitive empathy nor moral decision-making. These findings provide first evidence that psilocybin has distinct effects on social cognition by enhancing emotional empathy but not moral behavior. As the psychological effects of psilocybin are primary mediated by serotonin (5-HT) 2A receptor activation and partially modulated by 5-HT1A receptor modulations, our findings suggest the implication of these receptor subtypes in everyday social experience. Therefore, targeting 5-HT2A/1A receptors may have potential beneficial effects in the treatment of mood disorders or psychopathy, which are characterized by deficits in social skills and in particular in the ability to feel with other people.
Introduction
Empathy and moral behavior are fundamental components of human relationships and important for a well-functioning society (Decety and Cowell, 2014). They have been conceptually linked, and it is thought that empathy plays a crucial role in moral and prosocial behavior (Eisenberg, 2000). Both topics have undergone a renaissance in psychological and neuroscience research in the last decade (Christensen and Gomila, 2012; Decety and Cowell, 2014).
Empathy is a multidimensional construct consisting of at least a cognitive and an emotional component (Blair, 2005). Cognitive empathy describes the ability to adopt and understand the mental or emotional state of another person without necessarily sharing the emotional state. The term cognitive empathy can be used interchangeably with Theory of Mind or perspective taking (Blair, 2005). In contrast, emotional empathy is the ability to share the emotional state of another person. Empathy deficits have severe negative social effects such as social withdrawal as a result of experienced difficulties in reciprocal social interactions and communication (Baron-Cohen, 2012). Impairments in empathic abilities are reported in various psychiatric disorders such as major depression disorder (Cusi et al., 2011; Fujino et al., 2014), bipolar disorder (Shamay-Tsoory et al., 2009), borderline personality disorder (Dziobek et al., 2011), and psychopathy (Blair, 2005). The clinical relevance of empathy deficits is highlighted by evidence that a higher number of depressive episodes in major depression disorder patients is associated with a greater reduction in perspective-taking abilities (Cusi et al., 2011), suggesting that empathic abilities may become increasingly impaired with illness progression, which might contribute to a more severe course of depression.
Morality exerts a regulatory role in social decision-making and actions (Decety and Cowell, 2014). To investigate moral judgment and moral decision-making, cognitive neuroscience frequently uses moral dilemma tasks (Christensen and Gomila, 2012). Moral dilemmas are commonly presented as short stories about a situation involving a moral conflict with 2 courses of action: utilitarian action and deontological action. Utilitarian action is the option that produces the highest welfare for the largest number of involved individuals. By contrast, deontological action is the option that respects the rights of persons and does not use persons as a means to an end. Moral dilemmas can be distinguished between personal moral and impersonal moral dilemmas (Greene et al., 2001; Greene et al., 2004). Personal dilemmas are considered more emotionally engaging than impersonal dilemmas, because they contain a course of action where the individual directly implies serious bodily harm to a victim. People tend to rather choose deontological actions in personal moral dilemmas than in impersonal moral dilemmas (Greene et al., 2001). Utilitarian choices are seen as the failure to conform to the social norm to not harm others and are associated with antisocial behavior (Koenigs et al., 2007; Bartels and Pizarro, 2011). Increased utilitarian responses in personal moral dilemmas are found in adults with high-functioning autism (Gleichgerrcht et al., 2013), low-anxious psychopaths (Koenigs et al., 2012), alcohol-dependent patients (Khemiri et al., 2012), poly-substance patients (Carmona-Perera et al., 2012), and patients with lesions in prefrontal cortex (Ciaramelli et al., 2007; Koenigs et al., 2007).
To date, studies investigating the neuropharmacological underpinnings of social experiences and behavior such as empathy and moral decision-making are scarce. Serotonin (5-hydroxytryptamine, 5-HT) seems to be crucially involved in both processes. Recent pharmacological studies in healthy human subjects showed that the 5-HT-releasing agent 3,4-methylenedioxymethamphetamine (MDMA) increased emotional empathy but did not affect cognitive empathy on the multifaceted empathy test (MET) (Hysek et al., 2014; Kuypers et al., 2014; Schmid et al., 2014). Given that pretreatment with the 5-HT1A antagonist pindolol did not attenuate the empathogenic effects of MDMA in the MET, it has been speculated that these effects might be mainly driven by 5-HT2A receptor activation (Kuypers et al., 2014). However, this hypothesis has not yet been tested. Furthermore, recent studies indicated that the 5-HT system is also involved in moral processes. Specifically, manipulating the serotonergic tone by the administration of a single dose of the serotonin reuptake inhibitor citalopram in healthy subjects increased subjects’ aversion to personally harm others in the judgment of personal moral dilemmas but did not affect impersonal moral dilemmas (Crockett et al., 2010). Furthermore, citalopram also increased harm aversion for self and others in moral decision-making to inflict pain on oneself and others for financial gain (Crockett et al., 2015). Given the involvement of serotonin in social processes and the need for improved and more tailored pharmacological treatments of social deficits in psychiatric disorders (Derntl and Habel, 2011), it is important to better understand the specific contribution of different 5-HT receptor subtypes to social experiences (e.g., empathy) and social behavior (e.g., moral decision-making).
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine) is a hallucinogen that produces dose-dependently an altered state of consciousness characterized by changes in sensory perception, emotion, thought, and the sense of self (Hasler et al., 2004; Studerus et al., 2011). Psilocybin binds to 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5, 5-HT6, and 5-HT7 receptors (PDSP database https://pdspdb.unc.edu/pdspWeb/). In humans, psilocybin is rapidly dephosphorylated into the metabolite psilocin (4-N,N-dimethyltryptamine) (Hasler et al., 1997), which acts as a partial agonist at 5-HT2A and 5-HT1A receptors (Vollenweider et al., 1998; Halberstadt and Geyer, 2011). Human and animal studies showed reliably that the core psychological effects of psilocybin are primarily mediated via 5-HT2A receptor activation (Vollenweider et al., 1998; González-Maeso et al., 2007, 2008; Halberstadt and Geyer, 2011). Specifically, the selective 5-HT2A antagonist ketanserin blocked the psilocybin-induced subjective effects in a dose-dependent manner in humans (Vollenweider et al., 1998; Carter et al., 2007; Kometer et al., 2012). Moreover, accumulating evidence suggests that low to moderate doses of psilocybin enhance positive mood and attenuate the processing of negative emotional and social stimuli such as threat-related scenes, negative facial expressions, and social rejection (Kometer et al., 2012; Schmidt et al., 2013; Bernasconi et al., 2014; Kraehenmann et al., 2014; Preller et al., 2016), mainly by 5-HT2A receptor activation (Kometer et al., 2012). For example, psilocybin has been shown to decrease the recognition of negative facial expressions in the reading the mind in the eyes test, an effect that was abolished by ketanserin (Kometer et al., 2012). In addition, psilocybin has also been shown to decrease aggressive behavior in rodents (Kostowski et al., 1972; Uyeno, 1978), suggesting that psilocybin may increase the aversion to harm others. Given the role of serotonin in empathy and moral decision-making and of the 5-HT2A receptor system in the mechanism of action of psilocybin, we hypothesize that psilocybin impairs cognitive empathy for negative stimuli as shown in the reading the mind in the eyes test, whereas it increases emotional empathy. Further, we hypothesize that psilocybin reduces utilitarian choice of action in personal moral dilemmas.
Methods
Participants
Thirty-three healthy human subjects were recruited through advertisements placed at local universities. The MET was conducted in 33 subjects. One participant did not understand the task and had to be excluded from the study; thus, data of 32 participants (17 men, 15 women, mean age 26.72±5.34 years, range 20–38 years) were included in the data analysis. Due to technical reasons, the moral dilemma task (MDT) had been implemented at a later stage during the study and was conducted in 24 subjects immediately after the MET; thus, 24 participants (13 men, 11 women, mean age 26.63 ±5.33 years; range, 20–38) completed both tasks.
Healthy subjects, aged between 20 and 40 years and willing to refrain from consuming illicit psychoactive drugs from at least 2 weeks before the first experimental session until the last experimental session, were included in the study. All participants underwent a physical examination including electrocardiography and detailed blood and urine analyses (drug screening and pregnancy test) at a screening visit. Pregnant women were identified by urine tests and excluded. A self-report drug questionnaire was used to exclude subjects with a history of drug dependence. To exclude subjects with present or antecedent neurologic and psychiatric disorders or a history of major psychiatric disorders in first-degree relatives, we used the mini-international neuropsychiatric interview, a structured psychiatric interview (Sheehan et al., 1998), the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (First et al., 1997), and the John Hopkins Symptom Checklist-90 revised (Derogatis and Unger, 2010). A total of 39 subjects were screened whereby 6 subjects did not meet the inclusion/exclusion criteria and therefore were excluded.
Before participating, all participants gave their written consent after having received detailed written and oral information about the aims of the study and the effects and possible risks of psilocybin administration in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Department of Public Health of the Canton of Zurich, Switzerland, and the use of psilocybin was authorized by the Swiss Federal Office for Public Health, Department of Pharmacology and Narcotics, Berne, Switzerland.
Study Design
This study was designed as a double-blind, randomized, placebo-controlled, within-subject design with 2 experimental sessions. Each participant received psilocybin on one session (0.215 mg/kg body weight, p.o.) and the same amount of identical-looking gelatine capsules containing placebo (mannitol) on the other session. The 2 sessions were separated by at least 10 days.
Study Procedures
Participants were requested to refrain from drinking alcohol the day before the experimental session as well as from drinking alcohol and caffeinated beverages during the experimental days. The absence of acute drug use was assured by urine tests conducted before each experimental session. Participants completed the MET (Dziobek et al., 2008) and the MDT (Harrison et al., 2012) on a computer in a quiet room 160 minutes after substance administration, when the peak perceptual/visual effect of psilocybin had already markedly subsided (Hasler et al., 2004). The Altered States of Consciousness Rating Scale (5D-ASC) (Dittrich, 1998) was used 360 minutes after drug intake when the drug effects have completely subsided to retrospectively quantify the acute subjective effect of psilocybin. The Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988) was applied before and 360 minutes after drug intake to assess the acute effect of psilocybin on mood. Participants were monitored until the drug effects had completely worn off. This was assessed by a trained physician interviewing participants at the end of each session.
Measures
Interpersonal Reactivity Index
The Interpersonal Reactivity Index (IRI) (Davis, 1980, 1983) (German version: Paulus, 2009) is a self-report questionnaire to measure trait empathy and was applied at the screening visit. The short form contains 16 items with a 5-point Likert scale (1=never; 5=always). The questionnaire assesses aspects of cognitive and affective empathy by means of 4 subscales: perspective taking (PT) and fantasy (FS) as cognitive empathy subscales, and empathic concern (EC) and personal distress (PD) as affective empathy subscales. PT measures the tendency to adopt the psychological point of view of others, whereas FS measures the disposition to identify with fictional characters. EC captures the other-oriented tendency to experience feelings of compassion, and concern for others, whereas PD assesses self-oriented tendency to feel unease and discomfort resulting from the emotions of others.
MET
The MET is a PC-assisted test that assesses cognitive empathy as well as explicit and implicit emotional empathy (Dziobek et al., 2008). It consists of 40 photorealistic stimuli showing people in different emotionally charged situations (20 positive, 20 negative). Each picture is presented 3 times with a different question to assess the 3 different components of empathy. Cognitive empathy is operationalized by the question “What is this person feeling?” and participants have to identify the correct mental state from a list of 4 choices. Explicit emotional empathy is operationalized by the question “How concerned are you for this person” (negative valence pictures) and “How happy are you for this person” (positive valence pictures) with a 9-point Likert scale (1=not at all; 9=very much), respectively. To allow for the measurement of emotional empathy while reducing subjects’ tendencies to give socially desirable answers, an implicit emotional empathy condition was also included, which is operationalized by the question “How calm/aroused does this picture make you feel?” with a 9-point Likert scale (1=very calm; 9=very aroused).
MDT
For the MDT, 2 different sets of matched scenarios containing standard hypothetical moral dilemmas were constructed. They were matched according to previously reported emotional ratings and ratio of utilitarian answers (Koenigs et al., 2007; Harrison et al., 2012). Each set consists of 22 vignettes (adapted from Harrison et al., 2012), illustrating 9 personal dilemmas (thereof 2 inevitable scenarios), 9 impersonal dilemmas, and 4 nonmoral dilemmas. Inevitable scenarios describe situations in which the hypothetical victim dies regardless of the participant’s intervention. Nonmoral dilemmas are scenarios in which the participant chooses between an advantage and a disadvantage for himself without consequences for others. The vignettes were shown on a computer screen, while the dilemmas were presented auditorily via headphones. The participant was asked to take the perspective of a protagonist as all dilemmas are presented in a way that “you” are involved in these scenarios. At the end of each scenario participants were asked to decide if they would choose a utilitarian (sacrifice one or more people to save a higher amount of people) or harm avoidance (no intervention) course of action.
5D-ASC
The 5D-ASC (Dittrich, 1998) was used to assess subjective drug effects in both experimental sessions. The 5D-ASC is a standardized questionnaire comprising 94 items to be answered on visual analogue scales and is an extension of the OAV (Bodmer et al., 1994) containing 66 items. All items from the OAV are incorporated in the 5D-ASC, and the following validated 11 OAV scale scores (Studerus et al., 2010) were computed: experience of unity, spiritual experience, blissful state, insightfulness, disembodiment, impaired control and cognition, anxiety, complex imagery, elementary imagery, audio-visual synesthesia, and changed meaning of percepts. The 5D-ASC scores are expressed as percentage scores of maximum scale values.
PANAS
The PANAS (Watson et al., 1988) was used to assess the self-reported positive and negative affect. Participants are asked to rate the extent to which they experience 20 emotions on a 5-point Likert scale (1=very slightly or not at all; 5= extremely). The questionnaire contains 7 arousal-related items (Russell and Carroll, 1999): active, alert, attentive, excited, distressed, jittery, and upset. To measure drug-induced mood changes, the questionnaire was given before (pre) and 360 minutes (post) after drug intake.
Statistical Analysis
Data were analyzed using STATISTICA 8.0 for Windows (StatSoft). For the MET, repeated-measures ANOVAs were computed to analyze data of each empathy component (cognitive empathy, explicit emotional empathy, implicit emotional empathy) with drug (psilocybin, placebo) and valence (positive stimuli, negative stimuli) as within-subject factors and order (placebo first, psilocybin first) as between-subjects factor. For the MDT, the ratio of utilitarian choice (amount of utilitarian answers/total questions) for each category was computed, and a repeated-measures ANOVA was conducted to analyze the ratio of utilitarian choices with moral dilemma category (personal avoidable dilemma, personal inevitable dilemma, impersonal dilemma) and drug (psilocybin, placebo) as within-subject factors and order (placebo first, psilocybin first) as between-subjects factor. To compare the ratio of correct answers in nonmoral dilemma scenarios between the placebo and psilocybin condition, a paired t test was conducted. To control for psilocybin-induced mood changes on the scores of the MET and MDT, ANCOVAs with changes scores (post minus pre psilocybin condition) of positive affect and negative affect from the PANAS as covariates were performed. Independent-samples t tests were performed between psilocybin-experienced (n=10) and psilocybin-naïve (n=22) participants to compare their MET and MDT scores in the psilocybin condition. A repeated-measures ANOVA with drug (psilocybin, placebo) and 5D-ASC scale scores (experience of unity, spiritual experience, blissful state, insightfulness, disembodiment, impaired control and cognition, anxiety, complex imagery, elementary imagery, audio-visual synesthesiae, changed meaning of percepts) as within-subject factors were computed for the 5D-ASC ratings. A repeated-measures ANOVA with drug (psilocybin, placebo), time (pre, post), and scale scores (positive affect, negative affect) as within-subject factors were computed for the PANAS scores. Tukey posthoc comparisons followed significant main effects or interactions in the ANOVAs. In case of significant drug effects on outcome measures, change scores were computed (psilocybin minus placebo). To test the potential role of increased arousal on significant outcome measures, an arousal change score (post minus pre drug administration) from the mean of the 7 arousal-related PANAS items (Russell and Carroll, 1999) was computed. A moderator analysis was conducted to ascertain whether the relationship between changed meaning of percepts change scores and implicit emotional empathy change scores is influenced by arousal. To represent the interaction between changed meaning of percepts and arousal, the variables were first centered and then multiplied together. PANAS scores of one subject could not be analyzed due to missing data. Finally, the potential effects of altered states of consciousness (5D-ASC scales change scores), mood change (PANAS post minus pre psilocybin condition), and trait empathy (IRI subscales) on significant outcome measures were explored by means of multiple regression analyses using the backward stepwise method. The confirmatory statistical comparisons of all data were carried out on a significance level set at P<.05 (2-tailed).
RESULTS
IRI
Participants’ scores of the 4 IRI subscales are presented in Table 1.
Table 1.
Self-Reported Trait Empathy Scores of the Interpersonal Reactivity Index (IRI) of 32 Subjects
| IRI subscale | Mean | SD | Min | Max |
|---|---|---|---|---|
| Perspective taking (4–20) | 15.19 | 2.18 | 9 | 19 |
| Fantasy (4–20) | 12.56 | 3.23 | 6 | 19 |
| Empathic concern (4–20) | 13.84 | 2.41 | 9 | 20 |
| Personal distress (4–20) | 08.69 | 2.58 | 5 | 15 |
5D-ASC
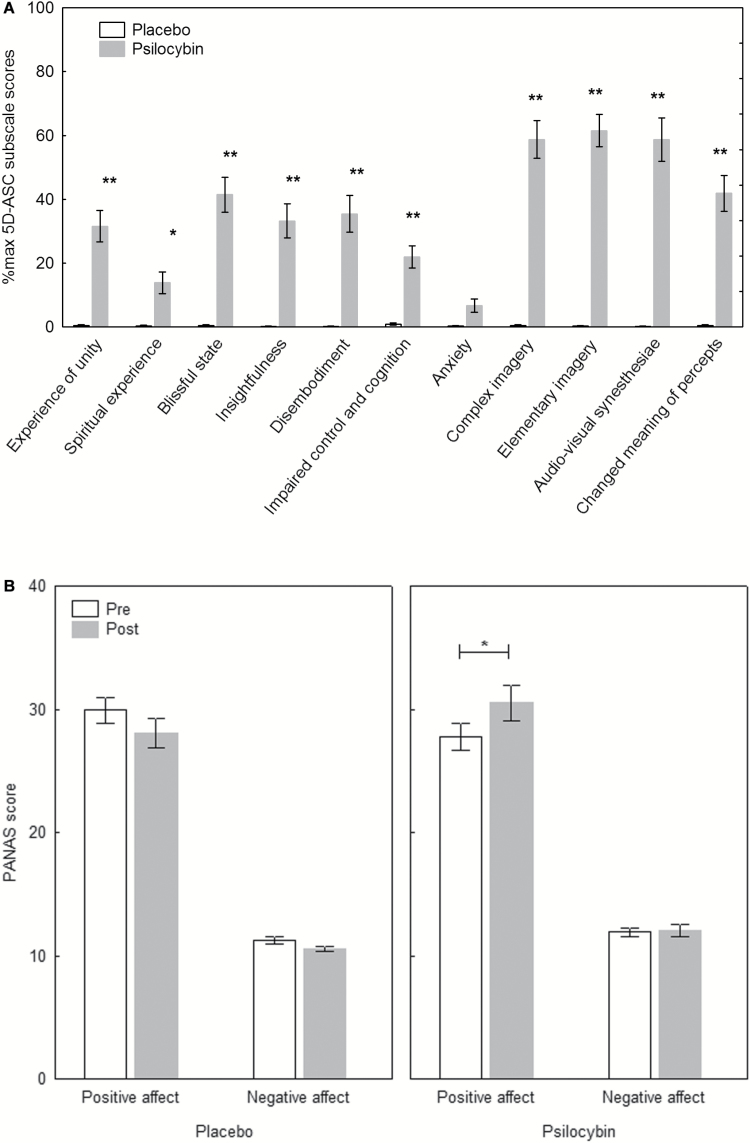
There was a significant drug x scale interaction (F(10,310)=25.06, P<.0001), a significant main effect of scale (F(10,310)=24.57; P<.0001) and a significant main effect of drug (F(1, 31) =100.66, P<.0001). Posthoc tests revealed that except for the anxiety score (P>.8), psilocybin significantly increased all scale scores compared with placebo (all P<.0001, except for spiritual experience P<.05) (Figure 1a).
Figure 1.
Psychological effects of psilocybin. (A) Scores of the Altered States of Consciousness Rating Scale (5D-ASC) scales (n=32). Psilocybin significantly increased all scale scores compared with placebo (all P<.05), except for anxiety (P>.8). (B) Scores of the Positive and Negative Affect Schedule (PANAS) (n=31). Mood states compared from pre to post (360 minutes after drug intake). Psilocybin significantly increased positive mood (P<.05) but not negative mood (P>.8), whereas placebo had no effects on the mood scales (all P>.2). Data are expressed as mean ± SEM. *P<.05, **P<.0001.
PANAS
There was a significant drug x time x scale interaction (F(1,30)=6.58, P<.05), a significant drug x time interaction (F(1,30)=17.34, P<.001), and a significant main effect of scale (F(1,30)=313.64, P<.0001), indicating that ratings on the positive affect scale were higher than on the negative affect scale. Posthoc tests revealed that positive affect ratings were significantly increased under psilocybin (P<.05), but not negative affect ratings (P>.8), whereas placebo did not lead to significant changes in positive affect (P>.2) nor negative affect (P>.8) ratings (Figure 1b). There was a significant difference in the change of arousal level between placebo (M=-0.07; SD=0.32) and psilocybin (M=0.25; SD=0.39) conditions (t(31)=-3.96; P<.001).
MET
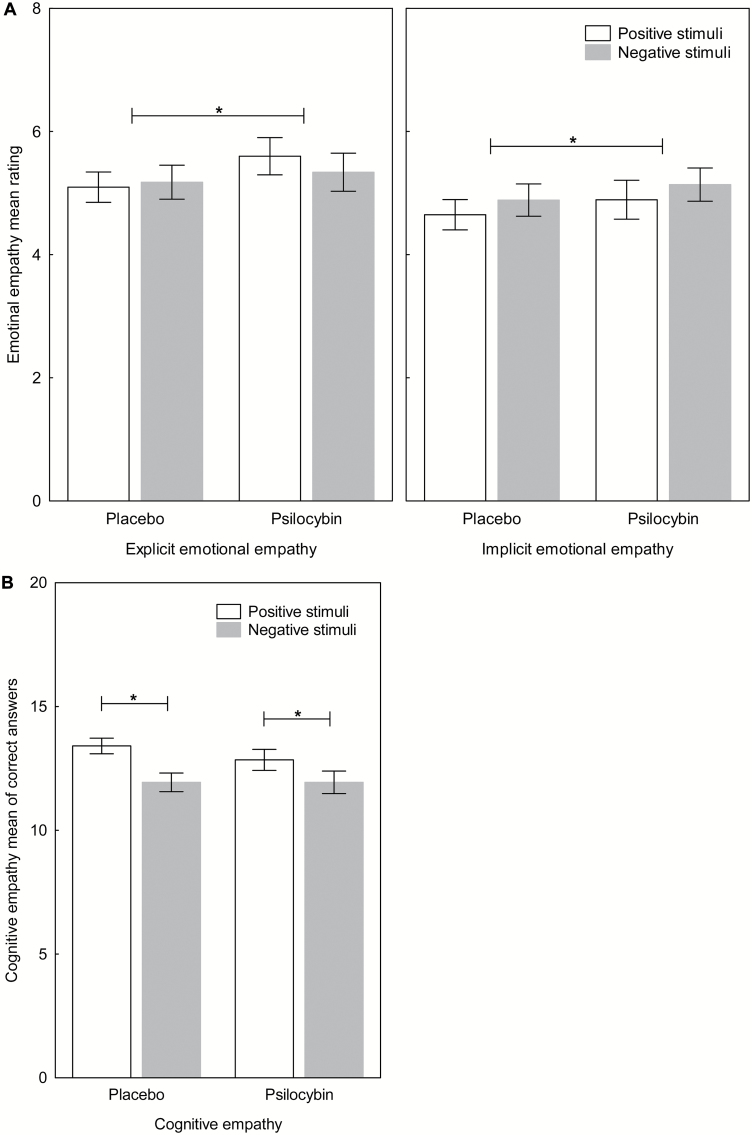
A significant main effect for drug (F(1,30) =7.74, P<.01) revealed that psilocybin increased explicit emotional empathy compared with placebo (Figure 2a). The interaction drug x valence on explicit emotional empathy was not significant (F(1,30)=2.97, P>.09). Neither the interaction drug x valence x order (F(1,30)=1.98, P>.1) nor the interaction drug x order (F(1,30)=3.83, P>.07) was significant. There was no significant main effect for valence (F(1,30)=0.11, P>.7) or order (F(1,30)=2.51, P>.1). For implicit emotional empathy, a significant main effect for drug (F(1,30)= 4.77, P<.05) revealed that psilocybin increased implicit emotional empathy compared with placebo (Figure 2a). The interaction drug x valence was not significant (F(1,30)=0.00, P>.9). Neither the interaction drug x valence x order (F(1,30)=0.01, P>.9) nor the interaction drug x order (F(1,30)= 2.03, P>.1) was significant. There was no significant main effect for valence (F(1,30)=1.05, P>.3), nor order (F(1,30)=3.40, P>.07). There was no significant drug x valence x order interaction (F(1,31)=0.51, P>.4) nor drug x valence interaction (F(1,30)=0.84, P>.3) for cognitive empathy and no significant main effect for drug (F(1,30)=1.45, P>.2). There was a significant drug x order interaction (F(1,30)=5.17, P<.05). Posthoc tests revealed that participants had slightly higher scores in their second test session compared with the first test session independently of drug condition and administration order, as there were neither significant differences in the ratings between drug conditions, nor between administration order (all P>.08). A significant main effect for valence (F(1,30)= 6.58, P<.05) revealed that participants made more mistakes on negative than positive stimuli independent of drug condition (Figure 2b). There was no significant main effect for order (F(1,30)=0.01, P>.9). When adding positive or neg ative mood as a covariate in the analyses, all results remained the same, except for implicit emotional empathy when controlling for positive mood (F(1,30)=2.62, P>.11). Further, independent-samples t tests revealed no significant differences for psilocybin-experienced and psilocybin-naïve participants in the MET scores (all P>.05) in the psilocybin condition.
Figure 2.
Multifaceted empathy test (MET). (A) Psilocybin significantly increased the mean rating of explicit and implicit emotional empathy (each P < .01) compared with placebo regardless of the valence of the stimuli. (B) No significant effect of psilocybin on the mean of correct answers of cognitive empathy (P > .2) compared with placebo were found. In general, participants made significantly more mistakes for negative stimuli than positive stimuli (P < .05). Data are expressed as mean ± SEM in 32 subjects. *P < .05.
Associations between MET Change Scores and 5D-ACS Change Scores, PANAS Change Score and IRI
Multiple linear regression analysis revealed that 5D-ASC scale scores explain a significant amount of the variance in the increase of implicit emotional empathy scores (F(1,31)=11.23, P<.01, R2=.27, R2Adjusted=.24). The analysis showed that only changed meaning of percepts scores significantly predict the increase of implicit emotional empathy scores (Beta=.52, t(31)=3.35, P<.01). No significant results were obtained when the analysis was performed for explicit emotional empathy scores.
As the scale changed meaning of percepts and implicit emotional empathy both contain questions regarding arousal, we tested if this relationship was moderated by arousal as measured with the PANAS. Changed meaning of percepts change scores and arousal were entered in the first step of the regression analysis. The model explained a significant amount of the variance in the increase of implicit emotional empathy scores (F(2,29)=5.31, P<.05, R2=.27, R2Adjusted=.22). It was found that changed meaning of percepts (Beta=.47, t(29)=2.60, P<.05) significantly predicted the increase in implicit emotional empathy but not arousal (Beta=.09, t(29)=0.47, P>.6). In the second step of the regression analysis, the interaction term between changed meaning of percepts and arousal was entered. This model explained a significant amount of the variance in the increase of implicit emotional empathy scores (F(3,28)=4.18, P<.05, R2=.31, R2Adjusted=.24). The analysis shows that only changed meaning of percepts scores significantly predict the increase of implicit emotional empathy scores (Beta=.47, t(28)=2.64, P<.05), but neither arousal (Beta=.07, t(28)=0.38, P>.7) nor the interaction term (Beta=.20, t(28)=1.30, P>.2). Thus, arousal was not a significant moderator of the relationship between changed meaning of percepts and implicit emotional empathy. To investigate if IRI subscale scores or the change scores of the PANAS predict the increase in emotional empathy scores, separate multiple linear regression analyses for explicit and implicit emotional empathy were performed with IRI subscale scores and PANAS scores as predictors using the backward stepwise method. No significant models were obtained (all P> .05).
MDT
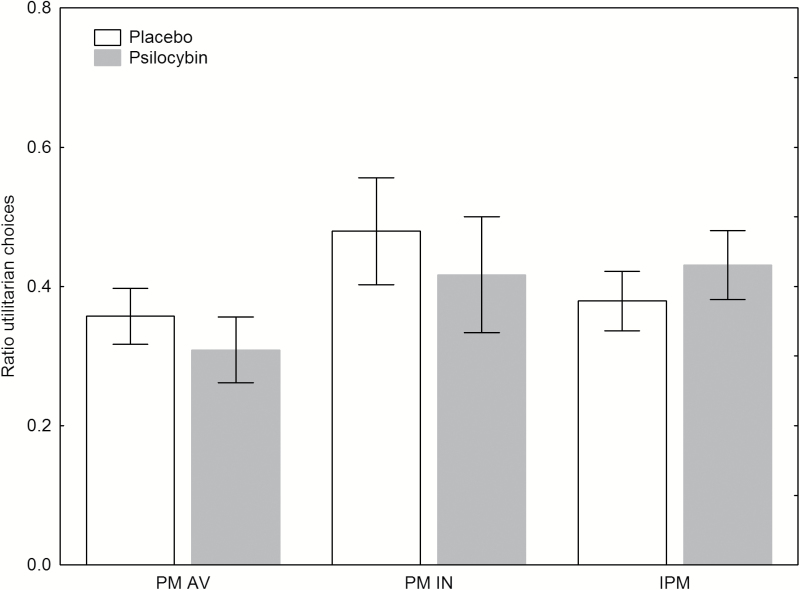
There was no significant drug x category interaction (F(2,44)=0.97, P>.3). Neither the drug x category x order interaction (F(2,44)=0.94, P>.4), nor the drug x order interaction (F(1,22)=0.91, P>.3) was significant. There was no significant main effect for drug (F(1,22)=0.37, P>.5), no significant main effect for order (F1,22)=0.02, P>.8), and no significant main effect for category (F(2,44)=2.77, P>.08) for moral dilemmas (Figure 3). In the nonmoral dilemma scenarios, there was a significant difference between the scores for placebo (M=0.99; SD=0.05) and psilocybin (M=0.91; SD=0.16) condition; t(23)=2.98, P<.01), revealing that participants gave more incorrect answers in the psilocybin condition. When adding positive or negative mood as a covariate in the analyses, all results remained the same. Further, independent-samples t tests revealed no significant differences for psilocybin-experienced and psilocybin-naïve participants in the MDT scores (all P>.1) in the psilocybin condition.
Figure 3.
Mean ration of utilitarian choices in 3 different moral dilemma categories from the moral dilemma task (MDT). No effects of psilocybin on moral dilemmas were found. Categories: personal moral avoidable (PM AV); personal moral inevitable (PM IN); impersonal moral (IPM). Data are expressed as mean ± SEM in 24 subjects.
Discussion
Psilocybin significantly increased explicit and implicit emotional empathy independent of stimuli valence. The increase in implicit emotional empathy was related to alterations in meaning of percepts but not trait empathy. There was no significant change in cognitive empathy between placebo and psilocybin. Although psilocybin led to an increase of emotional empathy, no significant difference in decision-making on hypothetical moral dilemmas was found between placebo and psilocybin.
The present finding suggests that 5-HT2A and 5-HT1A receptor systems may be important in the experience of emotional empathy regardless of the emotional valence of the stimuli. Interestingly, previous work has shown that psilocybin modulates the processing and recognition of negative social and nonsocial stimuli, presumably via 5-HT2A and/or 5-HT1A receptor activation (Kometer et al., 2012; Schmidt et al., 2013; Bernasconi et al., 2014; Kraehenmann et al., 2014; Preller et al., 2016). Specifically, it has been shown that activation of 5-HT2A receptors is implicated in early encoding and recognition of negative facial expressions (Kometer et al., 2012; Schmidt et al., 2013), whereas the 5-HT1A receptor activation seems to influence later processing of both negative and positive facial expression (Kometer et al., 2012; Schmidt et al., 2013; Bernasconi et al., 2014, 2015). Taken together, the present results extend these findings and suggest that the 5-HT2A receptor and possibly also the 5-HT1A receptor are not only implicated in the processing of social and nonsocial emotional stimuli but that they may also be involved in sharing the emotional state of another person (implicit emotional empathy) and the experience of sympathy and prosocial attitudes towards others (explicit emotional empathy). Furthermore, cognitive empathy remained unaffected by psilocybin, indicating that participants completed the task attentively and correctly. In contrast to emotional empathy, which measures one’s current experience of the emotional state of another person, cognitive empathy/Theory of Mind requires the ability to correctly identify the other person’s emotions. It is possible that emotional empathy in contrast to cognitive empathy is dependent on state variables and may therefore be manipulated more easily whereas it probably takes more time to acquire new cognitive empathy skills.
The 5D-ASC scale score changed meaning of percepts assesses a change in the significance of objects or the surroundings and significantly predicted the increase in implicit emotional empathy scores in the psilocybin condition. Some items of this 5D-ASC scale also refer to a change in the relationship between the observer and the observed objects or the environment. This is reflected for example by the item “Objects around me engaged me emotionally much more than usual”. Such an increased emotional engagement seems to be reflected in the boosted implicit emotional empathy ratings in the present study, indicating that the increased sense of significance may not solely refer to surrounding objects but also to the emotional state of other persons. Although psilocybin significantly increased participant’s arousal scores on the PANAS, the relationship between implicit emotional empathy and changed meaning of percepts was not moderated by arousal. It is noteworthy that the acute mood enhancing effects of psilocybin did not significantly predict the increase in emotional empathy. However, as there was no significant drug effect on implicit emotional empathy when controlling for psilocybin-induced positive affect, we cannot rule out that positive mood was associated with increases in implicit emotional empathy. The psilocybin-induced enhancement of emotional empathy was also not significantly predicted by the trait empathy score of the IRI questionnaire, suggesting that the acute empathy enhancing effect of psilocybin may build up independently from the subject’s baseline or trait empathy level. While the IRI scores in our study sample are comparable with scores from a meta-analysis investigating US college students from 1980 to 2009 (Konrath et al., 2011), the finding that psilocybin’s empathy enhancing effect is independent from trait empathy might be especially relevant for the treatment of psychiatric disorders where the affected patient has low trait empathy levels such as in psychopathy (Blair, 2005). In line with this idea studies in the 1960s suggested that psychedelics such as psilocybin and LSD might be useful in the treatment of psychopaths and criminals (Tenenbaum, 1961; Arendsen-Hein, 1963; Leary and Metzner, 1968). Finally it is noteworthy that the implicit and explicit emotional empathy ratings were not associated with the psilocybin-induced visual illusions and hallucinations suggesting that the empathy enhancement is not simply based on visual inaccuracy or disturbances.
A recent fMRI study using the MET showed that emotional empathy in healthy subjects is associated with increased BOLD responses in the brainstem, inferior frontal cortex, posterior superior temporal sulcus, temporal lobe, posterior insular cortex, and posterior cingulate cortex (Dziobek et al., 2011). Whereas prefrontal areas seem to be specifically related to simulating the perspective of others and stepping into their shoes (Mitchell, 2009), regions such as the insula and amygdala appear to be important for enabling the experience of emotional empathy (Blair, 2005; Singer and Lamm, 2009; Dziobek et al., 2011). Given that psilocybin increased neuronal activity as indexed by cerebral glucose metabolism or cerebral blood flow in frontomedial and frontolateral cortices including the anterior cingulate cortex (ACC), the temporomedial cortex, the insula, and the basal ganglia (Vollenweider et al., 1997; Gouzoulis-Mayfrank et al., 1999; Geyer and Vollenweider, 2008) it is conceivable that psilocybin may increase emotional empathy via activation of frontal-temporal and subcortical structures.
The present findings on the effects of psilocybin in the MET endorse the importance of the 5-HT system in empathy. Similar effects were found in previous studies investigating the influence of the 5-HT releasing agent MDMA on the same task (Hysek et al., 2014; Kuypers et al., 2014; Schmid et al., 2014). Specifically, MDMA significantly increased explicit and implicit emotional empathy for all stimuli (Hysek et al., 2014). It is noteworthy that neither oxytocin nor pretreatment with the 5-HT1A receptor antagonist pindolol modulated the empathogenic effects of MDMA (Kuypers et al., 2014). Moreover, a recent study showed that LSD, which acts as an agonist at multiple 5-HT and dopamine receptor sites (Passie et al., 2008), dose-dependently increased explicit and implicit emotional empathy, but in contrast to the present findings with psilocybin, LSD in addition impaired cognitive empathy (Dolder et al., 2016). As the psychological effects of psilocybin are primarily mediated via 5-HT2A receptor activation and partially modulated by 5-HT1A receptor manipulations (Vollenweider et al., 1998; Carter et al., 2007; Kometer et al., 2012; Pokorny et al., 2016), both these 5-HT receptor sites may be crucially implicated in the generation of emotional empathy. However, given that psilocybin acts also on other 5-HT receptor subtypes, further psilocybin studies in combination with selective 5-HT receptor antagonists are warranted to examine the relative contribution of specific 5-HT receptor subtypes on empathy. Although psilocybin also has down-stream effects on the dopamine system (Vollenweider et al., 1998; Vollenweider et al., 1999; Halberstadt and Geyer, 2011), an involvement of the dopamine system in the current results is unlikely since administration of the dopamine reuptake inhibitor methylphenidate did not lead to increased empathy ratings in the MET (Schmid et al., 2014).
Whereas psilocybin increased emotional empathy, it did not affect moral decision-making in any dilemma condition in the MDT. Neuroimaging studies revealed that moral decision-making and empathy are mediated by overlapping networks (Greene et al., 2001; Moll et al., 2002; Greene et al., 2004; Eslinger et al., 2009; Decety et al., 2012), but they also have distinct neuronal correlates (Bzdok et al., 2012). Our finding is well in line with the observation that the serotonin-releasing agent MDMA increased emotional empathy but had no effects on moral judgment in moral dilemma tasks (Schmid et al., 2014). Further, a meta-analysis of fMRI and PET studies investigating the relationship between morality and empathy revealed that affective sharing is unlikely involved during moral decisions (Bzdok et al., 2012). However, our finding is somewhat surprising given that manipulating the serotonergic tone by a single dose of the serotonin reuptake inhibitor citalopram was reported to lead to harm avoidance in the judgment of personal moral dilemmas (Crockett et al., 2010) and to an aversion for painful electric shocks for oneself and others (Crockett et al., 2015). However this apparent discrepancy may be explained by the fact that Crockett et al. (2010) had examined moral judgments (“Is it acceptable to…?”), whereas we investigated moral decision-making with a choice of action (“Would you…?”) condition. A recent study investigating whether evaluative judgments and choices of action differ when people make decisions on dilemmas involving moral issues suggests that judgment and choice of action are mediated, at least in part, by distinct psychological processes (Tassy et al., 2013), which in turn rely on different neural underpinnings (Tassy et al., 2012). Specifically, using identical dilemma tasks it has been suggested that moral judgment is linked to the functional integrity of the right dorsolateral prefrontal cortex, whereas moral action choice may mainly rely on ventromedial prefrontal cortex function (Cima et al., 2010; Tassy et al., 2012). Taken together, these findings and the dissociable effects of psilocybin on empathy and choice of action suggest that the 5-HT2A/1A receptor system may not be involved in moral decision-making. However, it may also be possible that higher doses of psilocybin are needed to alter the functional integrity of the neuronal networks underlying social moral decisions and choices of action.
Furthermore, it is also noteworthy that personal moral scenarios involve emotionally salient violent acts. Such scenarios activate brain regions implicated in emotional processing, including visual pathways and the amygdala (Greene et al., 2001, 2004). Psilocybin has been shown to reduce the neuronal response to social exclusion in the ACC (Preller et al., 2016) and to lead to a decreased amygdala reactivity to threatening stimuli (Kraehenmann et al., 2014). Thus, it is possible that emotionally loaded personal moral scenarios were less emotionally salient in the psilocybin condition and therefore psilocybin did not enhance aversive emotional reactions to harm others as it was found with citalopram (Crockett et al., 2010).
Although participants made significantly more errors in nonmoral dilemmas under psilocybin than under placebo, the error rate in the psilocybin condition (9%) remained very low as well. We are therefore confident that participants could complete the task after psilocybin administration. However, we cannot rule out that this increase could be due to tiredness, as the task was run as the last test of the session. Therefore, it is possible that deficits in attention may have masked potential effects in the MDT.
In conclusion, whereas moral decision-making was unaffected by psilocybin, the results from the MET indicate that psilocybin enhances emotional empathy but not the cognitive component of empathy. This finding highlights the possible role of 5-HT2A/1A receptors in everyday social experience. Therefore, 5-HT2A/1A receptor agonists may have potential beneficial effects in the treatment of mood disorders or psychopathy, which are characterized by deficits in social skills and in particular in the ability to feel with other people.
Statement of Interest
None.
Acknowledgments
We thank the study physician Dr. med. Milan Scheidegger for his work and Dr. Christoph Korn for his technical assistance.
This work was supported by the Heffter Research Institute (grant no. 1-190413) and the Swiss Neuromatrix Foundation (grant no. 2015-0102).
References
- Arendsen-Hein GW. (1963) LSD in the treatment of criminal psychopaths. In: Hallucinogenic drugs and their psychotherapeutic use (Crocket RW, Sandison RA, Walk A, eds). London: H. K. Lewis & Co. Ltd. [Google Scholar]
- Baron-Cohen S. (2012) Zero degrees of empathy. A new theory of human cruelty. London: Penguin. [Google Scholar]
- Bartels DM, Pizarro DA (2011) The mismeasure of morals: antisocial personality traits predict utilitarian responses to moral dilemmas. Cognition 121:154–161. [DOI] [PubMed] [Google Scholar]
- Bernasconi F, Schmidt A, Pokorny T, Kometer M, Seifritz E, Vollenweider FX (2014) Spatiotemporal brain dynamics of emotional face processing modulations induced by the serotonin 1A/2A receptor agonist psilocybin. Cereb Cortex 24:3221–3231. [DOI] [PubMed] [Google Scholar]
- Bernasconi F, Kometer M, Pokorny T, Seifritz E, Vollenweider FX (2015) The electrophysiological effects of the serotonin 1A receptor agonist buspirone in emotional face processing. Eur Neuropsychopharmacol 25:474–482. [DOI] [PubMed] [Google Scholar]
- Blair RJ. (2005) Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn 14:698–718. [DOI] [PubMed] [Google Scholar]
- Bodmer I, Dittrich A, Lamparter D (1994) Aussergewöhnliche Bewusstseinszustände - Ihre gemeinsame Struktur und Messung [Altered states of consciousness - Their common structure and assessment]. In: Welten des Bewusstseins (Hofmann A, Leuner H, eds), pp45–58. Berlin, Germany: VWB. [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB (2012) Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct Funct 217:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Perera M, Verdejo-García A, Young L, Molina-Fernández A, Pérez-García M (2012) Moral decision-making in polysubstance dependent individuals. Drug Alcohol Depend 126:389–392. [DOI] [PubMed] [Google Scholar]
- Carter OL, Hasler F, Pettigrew JD, Wallis GM, Liu GB, Vollenweider FX (2007) Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology (Berl) 195:415–424. [DOI] [PubMed] [Google Scholar]
- Christensen JF, Gomila A (2012) Moral dilemmas in cognitive neuroscience of moral decision-making: a principled review. Neurosci Biobehav Rev 36:1249–1264. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Muccioli M, Làdavas E, Di Pellegrino G (2007) Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Soc Cogn Affect Neurosci 2:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima M, Tonnaer F, Hauser MD (2010) Psychopaths know right from wrong but don’t care. Soc Cogn Affect Neurosci 5:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW (2010) Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci U S A 107:17433–17438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Siegel JZ, Kurth-Nelson Z, Ousdal OT, Story G, Frieband C, Grosse-Rueskamp JM, Dayan P, Dolan RJ (2015) Dissociable effects of serotonin and dopamine on the valuation of harm in moral decision making. Curr Biol 25:1852–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi AM, Macqueen GM, Spreng RN, McKinnon MC (2011) Altered empathic responding in major depressive disorder: relation to symptom severity, illness burden, and psychosocial outcome. Psychiatry Res 188:231–236. [DOI] [PubMed] [Google Scholar]
- Davis MH. (1980) A multidimensional approach to individual differences in empathy. JSAS Cat Selected Doc Psych 85. [Google Scholar]
- Davis MH. (1983) Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol 44:113–126. [Google Scholar]
- Decety J, Cowell JM (2014) The complex relation between morality and empathy. Trends Cogn Sci (Regul Ed) 18:337–339. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD (2012) The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex 22:209–220. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U (2011) Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci 261Suppl 2:145–149. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Unger R (2010) Symptom checklist-90-revised. In: The Corsini Encyclopedia of Psychology (Weiner IB, Craighead WE, eds). Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Dittrich A. (1998) The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry 31:80–84. [DOI] [PubMed] [Google Scholar]
- Dolder PC, Schmid Y, Muller F, Borgwardt S, Liechti ME (2016) LSD acutely impairs fear recognition and enhances emotional empathy and sociality. Neuropsychopharmacology 41:2638–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, Convit A (2008) Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord 38:464–473. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Preissler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S (2011) Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage 57:539–548. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. (2000) Emotion, regulation, and moral development. Annu Rev Psychol 51:665–697. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Robinson-Long M, Realmuto J, Moll J, deOliveira-Souza R, Tovar-Moll F, Wang J, Yang QX (2009) Developmental frontal lobe imaging in moral judgment: Arthur Benton’s enduring influence 60 years later. J Clin Exp Neuropsychol 31:158–169. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS (1997) Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). Washington, DC: American Psychiatric Press, Inc. [Google Scholar]
- Fujino J, Yamasaki N, Miyata J, Kawada R, Sasaki H, Matsukawa N, Takemura A, Ono M, Tei S, Takahashi H, Aso T, Fukuyama H, Murai T (2014) Altered brain response to others' pain in major depressive disorder. J Affect Disord 165:170–175. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX (2008) Serotonin research. Contributions to understanding psychoses. Trend Pharmacol Sci 29:445–453. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Torralva T, Rattazzi A, Marenco V, Roca M, Manes F (2013) Selective impairment of cognitive empathy for moral judgment in adults with high functioning autism. Soc Cogn Affect Neurosci 8:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53:439–452. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, López-Giménez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Büll U, Sass H (1999) Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology 20:565–581. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD (2001) An fMRI investigation of emotional engagement in moral judgment. Science 293:2105–2108. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD (2004) The neural bases of cognitive conflict and control in moral judgment. Neuron 44:389–400. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2011) Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 61:364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Soriano-Mas C, Hernández-Ribas R, López-Solà M, Ortiz H, Alonso P, Deus J, Menchon JM, Real E, Segalàs C, Contreras-Rodríguez O, Blanco-Hinojo L, Cardoner N (2012) Neural correlates of moral sensitivity in obsessive-compulsive disorder. Arch Gen Psychiatry 69:741–749. [DOI] [PubMed] [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, Bär T, Vollenweider FX (1997) Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv 72:175–184. [DOI] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX (2004) Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology (Berl) 172:145–156. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, Preller KH, Quednow BB, Liechti ME (2014) MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci 9:1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiri L, Guterstam J, Franck J, Jayaram-Lindström N (2012) Alcohol dependence associated with increased utilitarian moral judgment: a case control study. PLoS ONE 7:e39882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A (2007) Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 446:908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Kruepke M, Zeier J, Newman JP (2012) Utilitarian moral judgment in psychopathy. Soc Cogn Affect Neurosci 7:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Bachmann R, Studerus E, Seifritz E, Vollenweider FX (2012) Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry 72:898–906. [DOI] [PubMed] [Google Scholar]
- Konrath SH, O’Brien EH, Hsing C (2011) Changes in dispositional empathy in American college students over time: a meta-analysis. Pers Soc Psychol Rev 15:180–198. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Rewerski W, Piechocki T (1972) II. The effects of some hallucinogens on aggressiveness of mice and rats. Pharmacology 7:259–263. [DOI] [PubMed] [Google Scholar]
- Kraehenmann R, Preller KH, Scheidegger M, Pokorny T, Bosch OG, Seifritz E, Vollenweider FX (2014) Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biol Psychiatry 78:572–581. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, de la Torre Rafael, Farre M, Yubero-Lahoz S, Dziobek I, Van den Bos Wouter, Ramaekers JG (2014) No evidence that MDMA-induced enhancement of emotional empathy is related to peripheral oxytocin levels or 5-HT1a receptor activation. PLoS ONE 9:e100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary T, Metzner R (1968) Use of psychedelic drugs in prisoner rehabilitation. Br J Soc Psychiatry 2:27–51. [Google Scholar]
- Mitchell JP. (2009) Inferences about mental states. Philos Trans R Soc Lond B Biol Sci 364:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Oliveira-Souza R de, Bramati IE, Grafman J (2002) Functional networks in emotional moral and nonmoral social judgments. Neuroimage 16:696–703. [DOI] [PubMed] [Google Scholar]
- Passie T, Halpern JH, Stichtenoth DO, Emrich HM, Hintzen A (2008) The pharmacology of lysergic acid diethylamide: a review. CNS Neurosci Ther 14:295–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus C. (2009) Der Saarbrücker Persönlichkeitsfragebogen SPF (IRI) zur Messung von Empathie: Psychometrische Evaluation der deutschen Version des Interpersonal Reactivity Index. [Google Scholar]
- Pokorny T, Preller KH, Kraehenmann R, Vollenweider FX (2016) Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur Neuropsychopharmacol 26:756–766. [DOI] [PubMed] [Google Scholar]
- Preller KH, Pokorny T, Hock A, Kraehenmann R, Staempfli P, Seifritz E, Scheidegger M, Vollenweider FX, Stämpfli P (2016) Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci U S A 113:5119–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Carroll JM (1999) On the bipolarity of positive and negative affect. Psychol Bull 125:3–30. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Hysek CM, Simmler LD, Crockett MJ, Quednow BB, Liechti ME (2014) Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol (Oxford) 28:847–856. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F (2013) The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology (Berl) 225:227–239. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Harari H, Szepsenwol O, Levkovitz Y (2009) Neuropsychological evidence of impaired cognitive empathy in euthymic bipolar disorder. J Neuropsychiatry Clin Neurosci 21:59–67. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33. [PubMed] [Google Scholar]
- Singer T, Lamm C (2009) The social neuroscience of empathy. Ann N Y Acad Sci 1156:81–96. [DOI] [PubMed] [Google Scholar]
- Studerus E, Gamma A, Vollenweider FX (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS ONE 5:e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, Vollenweider FX (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol (Oxford) 25:1434–1452. [DOI] [PubMed] [Google Scholar]
- Tassy S, Oullier O, Duclos Y, Coulon O, Mancini J, Deruelle C, Attarian S, Felician O, Wicker B (2012) Disrupting the right prefrontal cortex alters moral judgement. Soc Cogn Affect Neurosci 7:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassy S, Oullier O, Mancini J, Wicker B (2013) Discrepancies between judgment and choice of action in moral dilemmas. Front Psychol 4:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum B. (1961) Group therapy with LSD-25. (A preliminary report). Dis Nerv Syst 22:459–462. [PubMed] [Google Scholar]
- Uyeno ET. (1978) Effects of psychodysleptics on aggressive behavior of animals. Mod Probl Pharmacopsychiatry 13:103–113. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J (1997) Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16:357–372. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9:3897–3902. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Hell D, Leenders KL (1999) 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man--a PET study with [11C]raclopride. Neuropsychopharmacology 20:424–433. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A (1988) Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]