Abstract
Background
[11C]Cimbi-36 is a serotonin 2A receptor agonist positron emission tomography radioligand that has recently been examined in humans. The binding of agonist radioligand is expected to be more sensitive to endogenous neurotransmitter concentrations than antagonist radioligands. In the current study, we compared the effect of serotonin releaser fenfluramine on the binding of [11C]Cimbi-36, [11C]MDL 100907 (a serotonin 2A receptor antagonist radioligand), and [11C]AZ10419369 (a serotonin 1B receptor partial agonist radioligand with established serotonin sensitivity) in the monkey brain.
Methods
Eighteen positron emission tomography measurements, 6 for each radioligand, were performed in 3 rhesus monkeys before or after administration of 5.0 mg/kg fenfluramine. Binding potential values were determined with the simplified reference tissue model using cerebellum as the reference region.
Results
Fenfluramine significantly decreased [11C]Cimbi-36 (26–62%) and [11C]AZ10419369 (35–58%) binding potential values in most regions (P < 0.05). Fenfluramine-induced decreases in [11C]MDL 100907 binding potential were 8% to 30% and statistically significant in 3 regions. Decreases in [11C]Cimbi-36 binding potential were larger than for [11C]AZ10419369 in neocortical and limbic regions (~35%) but smaller in striatum and thalamus (~40%). Decreases in [11C]Cimbi-36 binding potential were 0.9 to 2.8 times larger than for [11C]MDL 100907, and the fraction of serotonin 2A receptor in the high-affinity state was estimated as 54% in the neocortex.
Conclusions
The serotonin sensitivity of serotonin 2A receptor agonist radioligand [11C]Cimbi-36 was higher than for antagonist radioligand [11C]MDL 100907. The serotonin sensitivity of [11C]Cimbi-36 was similar to [11C]AZ10419369, which is one of the most sensitive radioligands. [11C]Cimbi-36 is a promising radioligand to examine serotonin release in the primate brain.
Keywords: [11C]Cimbi-36, [11C]AZ10419369, [11C]MDL 100907, fenfluramine, serotonin
Significance Statement
The serotonin (5-HT) neurotransmission system is important for regulating brain functions. Positron emission tomography (PET) imaging could be applied to examine changes in endogenous 5-HT concentration in the living brain if adequate radioligands are available. Agonist radioligands have been suggested to be more sensitive to changes in neurotransmitter concentration than antagonist radioligands to the same target. In the current within-subject comparison study, we demonstrated that the 5-HT2A receptor agonist radioligand [11C]Cimbi-36 was sensitive to fenfluramine-induced 5-HT release in the nonhuman primate brain. The 5-HT sensitivity of [11C]Cimbi-36 was higher than that of [11C]MDL100907, a 5-HT2A receptor antagonist radioligand, and comparable with that of [11C]AZ10419369, a 5-HT1B receptor radioligand and one of the most 5-HT sensitive radioligands. Our results support that [11C]Cimbi-36 is a promising radioligand to detect increases in 5-HT concentration in the primate brain.
Introduction
The serotonin (5-HT) neurotransmission system has an important role in the regulation of basic physiological functions as well as in higher brain functions such as emotion and cognition (Cools et al., 2011; Chou et al., 2012). It is of great interest to develop noninvasive methods to examine the serotonin system in the living primate brain, since a role of this system is implicated in the pathophysiology and treatment of major psychiatric disorders. By consequence, several radioligands have long since been developed for positron emission tomography (PET) imaging of serotonin receptor subtypes and the serotonin transporter (Saulin et al., 2012; Paterson et al., 2013). More recently, it has been of particular interest to develop radioligands that are sensitive to changes in the endogenous neurotransmitter concentration (Laruelle, 2000; Finnema et al., 2015). This PET imaging paradigm is typically interpreted according to the competition model, which postulates that binding of radioligand to neuroreceptor will decrease after increases in neurotransmitter concentration, and vice versa. For that purpose, there is need for radioligands that are sensitive to the endogenous 5-HT concentration in brain.
Some PET radioligands for the 5-HT1A receptor and the 5-HT1B receptor such as [11C]CUMI-101, [11C]AZ10419369, and [11C]P943 have indeed been reported to be sensitive to drug-induced changes in the 5-HT concentration (Finnema et al., 2015). We have previously demonstrated that [11C]AZ10419369, a 5-HT1B receptor partial agonist radioligand, is sensitive to increases in serotonin concentration, as fenfluramine reduced [11C]AZ10419369 binding up to 50% in the monkey brain (Finnema et al., 2010b, 2012). Furthermore, [11C]AZ10419369 allowed detection of the relatively low changes in 5-HT concentration in the human brain induced by escitalopram, a selective serotonin reuptake inhibitor (Nord et al., 2013). As modulation of multiple target proteins is one of the current strategies in the development of novel psychotropic drugs (Bang-Andersen et al., 2011; Varnäs et al., 2016), it is important to develop 5-HT-sensitive PET radioligands targeting 5-HT receptor subtypes other than 5-HT1A and 5-HT1B. It has been suggested that the 5-HT2A receptor could be a promising target for that purpose (Paterson et al., 2010; Tyacke and Nutt, 2015). However, in initial PET studies in the primate brain using 5-HT2A receptor antagonist radioligands such as [11C]MDL100907, no or limited 5-HT sensitivity has been reported (Paterson et al., 2010; Quednow et al., 2012; Talbot et al., 2012; Finnema et al., 2015).
The binding of agonist radioligands has been proposed to be more sensitive to alterations in neurotransmitter concentration than the binding of antagonist radioligands at the same target protein (Paterson et al., 2010; Finnema et al., 2015). This hypothesis has been based on the ternary complex model that suggests that receptors can be in 2 functional states (De Lean et al., 1980; Finnema et al., 2010a). Whereas agonists have high affinity to the functional G-protein coupled receptor and low affinity to the G-protein uncoupled receptor, antagonists have similar affinity to both states of the receptor. Therefore, neurotransmitters (being endogenous agonists) may more markedly compete with radioligand binding to the high-affinity state receptor than to the low-affinity state receptor. This hypothesis has been supported by the higher dopamine sensitivity of dopamine D2/D3 receptor agonist radioligands than antagonists (Narendran et al., 2004, 2010; Seneca et al., 2006; Shotbolt et al., 2012; Gallezot et al., 2014). Considering the low fraction (13%-45%) of 5-HT2A receptors in the high-affinity state in vitro (Sleight et al., 1996; Fitzgerald et al., 1999; Gray et al., 2003; Hazelwood and Sanders-Bush, 2004), there may be prominent differences in 5-HT sensitivity between agonist and antagonist radioligands for this receptor subtype.
[11C]Cimbi-36 is the first 5-HT2A receptor agonist radioligand (Ettrup et al., 2011) that has been characterized both in nonhuman primates (NHPs) (Finnema et al., 2014) and humans (Ettrup et al., 2014). The 5-HT sensitivity of [11C]Cimbi-36 has recently been evaluated in the pig brain (Jørgensen et al., 2016), confirming that [11C]Cimbi-36 binding was sensitive to the 4- to 11-fold increases in 5-HT concentration demonstrated by microdialysis. Therefore, [11C]Cimbi-36 might be a promising radioligand for measurement of increases in 5-HT concentration in the living primate brain.
The aim of this study was to evaluate the 5-HT sensitivity of [11C]Cimbi-36 in the NHP brain. Using a within-subject comparison study design, the sensitivity was directly compared with that of [11C]AZ10419369 binding to the 5-HT1B receptor, a reference radioligand with established serotonin sensitivity, and that of the 5-HT2A receptor antagonist radioligand [11C]MDL 100907 (Ito et al., 1998). Fenfluramine was the drug administered to induce 5-HT release (Rothman and Baumann, 2002). The study design also allowed for an estimation of the fraction 5-HT2A receptors in the high affinity state (Narendran et al., 2004). We hypothesized that the 5-HT sensitivity of [11C]Cimbi-36 would be higher than for [11C]MDL 100907 and different from [11C]AZ10419369 due to differences in the affinity of 5-HT to the target receptors (Paterson et al., 2010).
Methods
Subjects
The NHP study was approved by the Animal Research Ethical Committee of the Northern Stockholm region (Dnr N386/09 and N452/11). Three female rhesus monkeys (Macaca mulatta) with a mean body weight of 7.9 kg (range: 5.2–13.5 kg) were included. The caring and experimental procedures were performed according to the Guidelines for planning, conducting and documenting experimental research (Dnr 4820/06-600) of Karolinska Institutet and the Guide for the Care and Use of Laboratory Animals: Eighth Edition (Council, 2011).
Preparation of Radioligands
[11C]Cimbi-36, [11C]MDL 100907, and [11C]AZ10419369 were prepared according to procedures reported previously (Lundkvist et al., 1996; Pierson et al., 2008; Andersson et al., 2011; Ettrup et al., 2011).
Study Design
A total of 18 PET measurements (6 for each radioligand) were performed on 9 experimental days. On each experimental day, a baseline PET measurement was performed in the morning and repeated after pretreatment with fenfluramine 5 mg/kg. The 2 PET measurements were performed approximately 3 hours apart. The fenfluramine dose was selected to achieve a substantial increase in 5-HT concentration and has been demonstrated to induce a 20-fold increase in a microdialysis study in monkeys (Udo de Haes et al., 2006). Racemic fenfluramine was formulated in saline and was i.v. infused (1 mL/kg) over 10 minutes, starting 30 minutes before injection of radioligand.
PET Experimental Procedures
Anesthesia was initiated by intramuscular injection of ketamine (~10 mg/kg) and, after tracheal intubation maintained by a mixture of sevoflurane (2%-8%), oxygen, and medical air. PET measurements were conducted in High Resolution Research Tomograph. A 6-minute transmission scan (using a single 137Cs source) was followed by acquisition of list-mode data for 123 minutes after i.v. bolus injection of radioligand. During the PET measurements after fenfluramine pretreatment, 7 venous blood samples (at -40, -5, 15, 30, 60, 90, and 120 minutes after injection of radioligand) were collected for determination of the fenfluramine concentration in plasma.
Determination of Plasma Fenfluramine and Norfenfluramine Concentrations
The main metabolite of fenfluramine, norfenfluramine, is also a potent 5-HT releaser (Rothman and Baumann, 2002). Therefore, the plasma concentrations of both fenfluramine and norfenfluramine were determined using liquid chromatography-mass spectrometry as described in supplementary Materials and Methods.
Magnetic Resonance Imaging (MRI)
T1-weighted MRI images were acquired for each monkey on a GE 1.5 Tesla Signa MRI scanner using a 3D spoiled gradient recalled protocol with repetition time 21 milliseconds, flip angle 35°, FOV 12.8, matrix 256 × 256 × 128, and 128 × 1.0 mm2 slices.
Image Data Analysis and Quantification
The MRI images were reoriented to the anterior-posterior commissure plane, and non-brain tissues were removed manually using the Image Processing and VOI Analysis Tool (PBAS) in PMOD (version 3.704; PMOD Technologies). The processed brain MRI images were then corrected for inhomogeneous intensity by applying the N4 algorithm (Tustison et al., 2010) using the Advanced Normalization Tools software package (http://stnava.github.io/ANTs/). PET images were preprocessed according to previously reported methods (Varrone et al., 2009) with reconstructed image frames binned as: 9 × 10 seconds, 2 × 15 seconds, 3 × 20 seconds, 4 × 30 seconds, 4 × 60 seconds, 4 × 180 seconds, and 17 × 360 seconds.
For each of the 9 baseline measurements, a summed PET image was generated for PET-MRI co-registration. Time frames for the summed PET image were based on high tissue counts and optimal tissue contrast to enable PET-MRI co-registration. The applied time frames were 12 to 63 minutes for [11C]Cimbi-36, 21 to 75 minutes for [11C]MDL 100907, and 5 to 18 minutes for [11C]AZ10419369. Each summation image was co-registered to its individual MRI brain image by the Rigid matching algorithm with default settings for primate in the PMOD Fuse It Tool (PFUSEIT). The resulting transformation matrices were applied to the 2 PET data sets obtained for each monkey on the same day.
Fourteen volumes of interest (VOIs) were defined based on the NeuroMaps atlas in the INIA19 rhesus template (Rohlfing et al., 2012), including 3 striatal regions: putamen, caudate nucleus (CN), and ventral striatum (VS); 4 neocortical regions: frontal cortex (FC), parietal cortex (PC), temporal cortex (TC), and occipital cortex (OC); 3 limbic regions: anterior cingulated cortex (ACC), amygdala, and hippocampus; and finally, VOIs for thalamus, midbrain, cerebellum, and whole brain. The VOIs were selected based on regional 5-HT1B and 5-HT2A receptor distribution and are similar as in previous PET studies in NHPs using [11C]Cimbi-36, [11C]MDL 100907, and [11C]AZ10419369 (Finnema et al., 2012, 2014). Each monkey’s brain MRI image was normalized to the INIA19 rhesus template by the Deformable matching algorithm with default settings for primate in the PFUSEIT, and the resulting normalization matrix was used to inversely transform the template VOIs into the individual MRI space.
Calculation of Binding Potential (BPND) and Change after Pretreatment
For each VOI, a decay-corrected time–activity curve was generated from the co-registrated dynamic PET data. BPND values were calculated using the simplified reference tissue model (Lammertsma and Hume, 1996), with cerebellum as the reference region (Meyer et al., 2010; Varnäs et al., 2011; Talbot et al., 2012; Finnema et al., 2014). The identifiability of BPND values was evaluated by the estimate of SE during the fitting process by using the Marquardt-Levenberg algorithm (Marquardt, 1963) and expressed as the percentage of SE (%SE), calculated according to the following equation:
| (1) |
The relative change in BPND values (∆BPND) (%) was calculated using the following equation:
| (2) |
Statistical Analysis
Changes in parameters between the 2 PET measurements performed on the same day were assessed by paired t test. All statistical analyses were performed in GraphPad Prism (version 6.05; GraphPad Software). The threshold of significance set as P<.05 (1-tailed) for fenfluramine induced decreases in BPND and P < .05 (2-tailed) for changes in other parameters.
Results
Radiochemistry
The mean radiochemical purity for each of 3 injected radioligands was 99% (range: 97–100%, n = 6 for each radioligand). Comparing radioligand injection parameters between baseline and pretreatment PET measurements for the same radioligand, a slightly higher injected radioactivity of [11C]AZ10419369 after pretreatment (188 MBq) than baseline (183 MBq) and higher injected mass of [11C]MDL 100907 after pretreatment (0.21 μg) than baseline (0.17 μg) were observed. Otherwise, there were no significant differences (supplementary Table 1).
Plasma Concentrations of Fenfluramine and Norfenfluramine
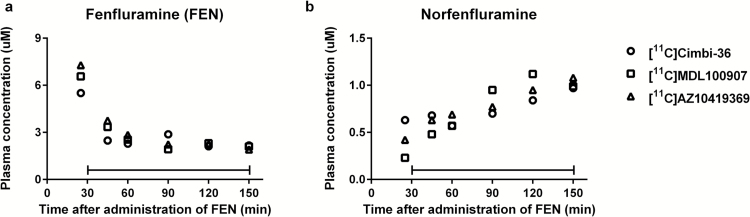
The time courses for the mean plasma concentrations of fenfluramine and norfenfluramine are presented in Figure 1. During the PET measurement, the mean plasma concentration values of fenfluramine and norfenfluramine were similar for the 9 experimental days. The mean (n = 3) values for fenfluramine and norfenfluramine were 2.39 μM and 0.75 μM after [11C]Cimbi-36, 2.44 μM and 0.86 μM after [11C]MDL 100907, and 2.59 μM and 0.83 μM after [11C]AZ10419369, respectively.
Figure 1.
Time-course for mean plasma concentration of (a) fenfluramine (FEN) and (b) norfenfluramine during positron emission tomography (PET) experiments following injection of [11C]Cimbi-36, [11C]MDL 100907, or [11C]AZ10419369 (n = 3 for each radioligand). The capped horizontal lines represent the period of PET measurement.
Fenfluramine-Induced Changes in Radioligand Binding
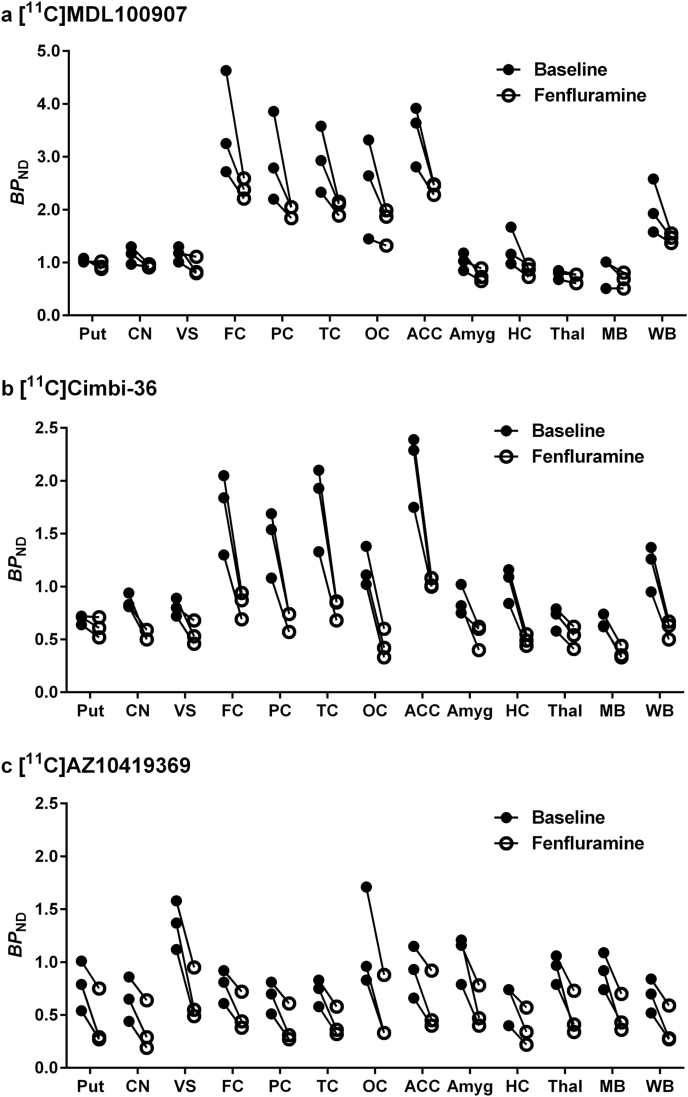
Regional BPND values could be reliably identified (%SE ≤ 10%) for most regions (Table 1), although the identifiability was slightly weaker for the VS (11.3 ± 2.3%, mean ± SD, n = 6), amygdala (10.5 ± 3.5%), and MB (16.5 ± 9.5%) in the [11C]Cimbi-36 experiments. Following administration of fenfluramine, radioactivity concentration in a majority of examined VOIs was reduced when compared with baseline (Figure 2). The reduction in [11C]Cimbi-36 BPND was statistically significant in 12 of the 13 examined brain regions (except putamen). For [11C]AZ10419369, BPND was also significantly reduced in all 13 brain regions. In contrast, the regional reduction in [11C]MDL 100907 BPND was not significant in most regions except for 3 (TC, ACC, and thalamus) (Table 1; Figure 3). For the whole brain, the mean reductions in BPND for [11C]MDL 100907, [11C]Cimbi-36, and [11C]AZ10419369 were 25.8%, 49.4%, and 46.3%, respectively.
Table 1.
Effect of Fenfluramine (FEN) on Regional Binding Potential (BPND) Values (n = 3)
| Region | [11C]MDL 100907 | [11C]Cimbi-36 | [11C]AZ10419369 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | FEN | ∆BPND (%) | Baseline | FEN | ∆BPND (%) | Baseline | FEN | ∆BPND (%) | |
| Put | 1.05 | 0.94 | -10.3 | 0.69 | 0.61 | -11.4 | 0.78 | 0.44 | -46.5* |
| CN | 1.15 | 0.93 | -17.4 | 0.86 | 0.53 | -38.0* | 0.65 | 0.37^ | -46.1* |
| VS | 1.16 | 0.91 | -21.4 | 0.80^ | 0.56^ | -30.4* | 1.36 | 0.66^ | -51.9** |
| FC | 3.53 | 2.39 | -29.9 | 1.73 | 0.83 | -51.3* | 0.78 | 0.51 | -35.2* |
| PC | 2.95 | 1.98 | -30.0 | 1.44 | 0.68 | -51.8* | 0.67 | 0.40 | -42.6* |
| TC | 2.95 | 2.05 | -28.9* | 1.79 | 0.80 | -54.6* | 0.72 | 0.42 | -42.3* |
| OC | 2.47 | 1.73 | -26.1 | 1.17 | 0.45 | -62.2** | 1.17 | 0.51 | -58.2* |
| ACC | 3.46 | 2.41 | -29.3* | 2.14 | 1.03 | -51.1* | 0.91 | 0.59 | -37.0* |
| Amyg | 1.02 | 0.76 | -25.2 | 0.86 | 0.54^ | -36.6* | 1.05 | 0.55 | -47.6* |
| HC | 1.27 | 0.85 | -29.8 | 1.03 | 0.49 | -51.5** | 0.63 | 0.37^ | -41.3* |
| Thal | 0.78 | 0.72 | -8.2* | 0.70 | 0.52 | -25.8** | 0.94 | 0.49 | -48.1* |
| MB | 0.84^ | 0.67 | -16.3 | 0.66^^ | 0.37^^ | -43.3** | 0.92 | 0.50^ | -46.3* |
| WB | 2.03 | 1.46 | -25.8 | 1.19 | 0.60 | -49.4** | 0.69 | 0.38 | -46.3* |
Abbreviations: ACC, anterior cingulated cortex; Amyg, amygdala; ∆BPND (%), ; CN, caudate nucleus; FC, frontal cortex; HC, hippocampus; MB, midbrain; OC, occipital cortex; PC, parietal cortex; Put, putamen; TC, temporal cortex, Thal, Thalamus; VS, Ventral striatum; WB, whole brain.
Data presented as mean (n = 3).
Identifiability (%SE) =
^15% ≥ mean identifiability (%SE) > 10%, ^^20% ≥ mean %SE > 15%.
*P < .05, **P < .01 (1-tailed) by paired t test.
Figure 2.
Magnetic resonance images (MRI) and corresponding coregistrated positron emission tomography (PET) summation images (average of frames from 9 to 123 min) of [11C]MDL 100907, [11C]Cimbi-36, and [11C]AZ10419369 during baseline and post-fenfluramine (FEN) conditions in NHP3. (a) Axial view of images at the level of caudate nucleus. (b) Axial view of images at the level of amygdala/hippocampus; note the difference in the range of the standardized uptake values (SUVs) color bars for the different radioligands. SUVs were calculated from the radioactivity concentration as [kBq/cm3] / (radioactivity injected [MBq] / body weight [kg]). ACC, anterior cingulate cortex; Amyg, amygdala; CN, caudate nucleus; FC, frontal cortex; HC, hippocampus; PC, parietal cortex; OC, occipital cortex; TC, temporal cortex.
Figure 3.
Individual binding potential (BPND) values before and after pretreatment with fenfluramine (FEN) 5.0 mg/kg in 12 predefined volumes of interest: putamen (Put), caudate nucleus (CN), ventral striatum (VS), frontal cortex (FC), parietal cortex (PC), temporal cortex (TC), occipital cortex (OC), anterior cingulate cortex (ACC), amygdala (Amyg), hippocampus (HC), thalamus (Thal), midbrain (MB), and whole brain (WB). (a) [11C]MDL 100907, (b) [11C]Cimbi-36, and (c) [11C]AZ10419369.
Fenfluramine-induced [11C]Cimbi-36 ∆BPND was larger than [11C]MDL 100907 ∆BPND (Table 1). There were regional differences in fenfluramine-induced ∆BPND between [11C]Cimbi-36 and [11C]AZ10419369 as the decreases in [11C]Cimbi-36 BPND were larger than for [11C]AZ10419369 in neocortical and limbic regions (~35%), but smaller in striatum and thalamus (~40%) (Table 1).
Discussion
The aim of the present study in NHPs was to examine the sensitivity of the 5-HT2A receptor agonist radioligand [11C]Cimbi-36 for changes in the endogenous serotonin concentration. A main observation was that the binding of [11C]Cimbi-36 was sensitive to 5-HT release induced by fenfluramine. The results are consistent with a recent PET study in pigs in which fenfluramine reduced the BPND values by 44% (Jørgensen et al., 2016). Moreover, the agonist radioligand [11C]Cimbi-36 was more sensitive to 5-HT release than the antagonist 5-HT2A receptor radioligand [11C]MDL100907. In addition, the 5-HT sensitivity of [11C]Cimbi-36 binding was comparable with that for [11C]AZ10419369 binding to the 5-HT1B receptor. In summary, [11C]Cimbi-36 appears to be one of the most sensitive radioligands so far developed for detection of changes in the endogenous 5-HT concentration in the primate brain.
The larger fenfluramine-induced change in ∆BPND for [11C]Cimbi-36 than for [11C]MDL 100907 is in line with the previously reported higher dopamine sensitivity of dopamine D2/D3 receptor agonist radioligands than antagonist radioligands such as [11C]raclopride (Narendran et al., 2004, 2010; Seneca et al., 2006; Shotbolt et al., 2012; Gallezot et al., 2014). The 92% to 278% larger regional fenfluramine-induced effect on [11C]Cimbi-36 binding than on [11C]MDL 100907 binding in the neocortex is comparable with the 42% to 153% larger regional amphetamine-induced decreases in radioligand binding using different dopamine D2/D3 receptor agonist radioligands in comparison with [11C]raclopride. The results are thus consistent with the view that agonist radioligands are more sensitive to changes in neurotransmitter concentrations than antagonists.
It is worth noting that, in contrast to [11C]MDL 100907 (Pehek et al., 2006), [11C]Cimbi-36 has a relatively poor selectivity for the 5-HT2A receptor subtype. The affinity (Ki) is 0.5 to 0.8 nM for the 5-HT2A receptor, 0.5 nM for the 5-HT2B receptor, and 1.7 nM for the 5-HT2C receptor (Ettrup et al., 2011). We previously reported that in the monkey brain, the relative binding of [11C]Cimbi-36 to [11C]MDL 100907 in several regions, including amygdala, hippocampus, thalamus, and midbrain, is higher than in neocortical regions. These differences are most likely attributable to the binding of [11C]Cimbi-36 to 5-HT2C receptors located either in these regions or in the adjacent choroid plexus (Finnema et al., 2014). Interestingly, similar patterns were also observed in the human brain (Ettrup et al., 2016). Nonetheless, the relative effect of fenfluramine, measured as the ratio of [11C]Cimbi-36 ∆BPND to [11C]MDL 100907 ∆BPND, was in these 4 regions similar to the ratio in neocortical regions where [11C]Cimbi-36 binds selectively to the 5-HT2A receptors (Finnema et al., 2014). These observations for the neocortical regions suggest that there is a limited contribution of 5-HT2C receptor binding to the higher 5-HT sensitivity of [11C]Cimbi-36 when compared with [11C]MDL 100907.
The concept of G-protein coupled receptors being in high- and low-affinity states was originally based on studies in vitro (De Lean et al., 1980; Finnema et al., 2010a). In the present study, the fraction of 5-HT2A receptors in the high-affinity state in vivo was estimated by dividing the [11C]MDL 100907 ∆BPND by the [11C]Cimbi-36 ∆BPND (supplementary Methods). This approach was previously applied to dopamine D2/D3 receptor agonist radioligands and [11C]raclopride (Narendran et al., 2004, 2010; Seneca et al., 2006; Shotbolt et al., 2012). For reliable estimation of the fraction in vivo, we recommend using neocortical regions, where [11C]Cimbi-36 binding is specific to 5-HT2A receptors (Finnema et al., 2014) and where the test-retest variability is favorable for both radioligands (Talbot et al., 2012; Ettrup et al., 2016).The fraction of 5-HT2A receptors in the high-affinity state (54%) was slightly higher than previously reported values from in vitro binding assays (13%-45%) (Sleight et al., 1996; Fitzgerald et al., 1999; Gray et al., 2003; Hazelwood and Sanders-Bush, 2004). The present in vivo estimates should be taken with caution, since they were based on data obtained in only 3 NHPs but may support the view that high- and low-affinity states are valid concepts also in vivo.
[11C]AZ10419369 is one of the most sensitive radioligands for endogenous 5-HT (Paterson et al., 2010; Finnema et al., 2015). In addition to high reduction (~50%) in [11C]AZ10419369 binding to the 5-HT1B receptor after administration of fenfluramine 5 mg/kg, the binding was also demonstrated to be sensitive to smaller changes in 5-HT concentration such as after administration of selective serotonin reuptake inhibitors (Finnema et al., 2010b, 2012; Nord et al., 2013). The present study demonstrates that the 5-HT sensitivity of [11C]Cimbi-36 binding is similar to that of [11C]AZ10419369. However, due to the relatively high expression of 5-HT2A receptors in neocortical and limbic regions (Paterson et al., 2010), [11C]Cimbi-36 may be advantageous to [11C]AZ10419369 for examination of pathophysiology and treatment effects on serotonergic neurotransmission in those regions.
A limitation of the present study is that fenfluramine and norfenfluramine have been reported to bind to 5-HT2 receptors and the potential contribution of direct occupancy, resulting in a decrease in radioligand binding, should therefore be carefully considered (Tyacke and Nutt, 2015). In the present study, the plasma concentrations of fenfluramine and norfenfluramine were measured to estimate the direct occupancy effects (supplementary Methods). The estimated occupancy levels (supplementary Table 2) for the 5-HT2A receptor, the 5-HT2C receptor, and the 5-HT1B receptor (5–13%) were much lower than the observed reductions in radioligand binding (35%-62%). Although the 5-HT2B receptor occupancy was estimated to be relatively high (50%), the low and restricted expression of 5-HT2B receptors in brain (Nichols and Nichols, 2008) suggests that this binding would have negligible effect on [11C]Cimbi-36 binding in the regions examined. In conclusion, a major proportion of the ∆BPND induced by fenfluramine administration can be attributed to 5-HT release and is not likely to represent drug occupancy at the target receptor.
Another potential limitation of the current PET study is the use of anesthesia. Ketamine and isoflurane have previously been shown not to affect 5-HT2A receptor binding of [18F]altanserin in the rodent brain (Elfving et al., 2003). Anesthetic doses of ketamine have however been reported to increase [11C]AZ10419369 binding in NHP brain, but had no effect on fenfluramine-induced decreases in [11C]AZ10419369 binding (Yamanaka et al., 2014). Interestingly, ketamine/xylazine and isoflurane have been reported to elevate the dopamine sensitivity of dopamine D2/D3 receptor agonist radioligands in NHPs and rats, respectively, while isoflurane did not affect [3H]raclopride binding (Ohba et al., 2009; McCormick et al., 2011). However, the increased dopamine sensitivity of dopamine D2/D3 receptor agonist vs antagonist radioligands was confirmed in awake human studies (Narendran et al., 2010; Shotbolt et al., 2012). Future studies to compare the 5-HT sensitivity of [11C]Cimbi-36 and [11C]MDL 100907 in awake humans are warranted to exclude potential anesthesia effects.
The present results may also serve as a starting point for translation to humans, as the NHP brain has higher similarity to the human brain than the pig brain (Capitanio and Emborg, 2008). In one previous study, dexfenfluramine (40 or 60 mg p.o.) was shown to reduce [18F]altanserin antagonist binding to the 5-HT2A receptor in human volunteers (Quednow et al., 2012). Following safety considerations, the proposed maximal dose of dexfenfluramine suitable for human use was 1 mg/kg p.o. (Quednow et al., 2012), which is lower than the 5 mg/kg used in the present study. Therefore, it might be interesting to evaluate the effect of a lower dose of fenfluramine (1 mg/kg) on [11C]Cimbi-36 binding in NHPs in comparison with current results. Nonetheless, since 5 mg/kg reduced [11C]Cimbi-36 by 55%, it may be anticipated that [11C]Cimbi-36 binding may be sensitive to 5-HT release induced also by dexfenfluramine 1 mg/kg in future human studies.
In conclusion, our results support that [11C]Cimbi-36 is a promising radioligand to detect increases in 5-HT concentration in the primate brain. The 5-HT sensitivity of [11C]Cimbi-36 is higher than for [11C]MDL 100907 but similar to [11C]AZ10419369. Future studies are warranted to evaluate the sensitivity of [11C]Cimbi-36 binding to smaller increases in 5-HT concentration and to replicate the current observations in the human brain.
Statement of Interest
Lars Farde is employed part time at the AstraZeneca, PET Science Center at Karolinska Institutet, Personalized Health Care and Biomarkers. The other authors have no conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors thank H Lundbeck A/S (Dr Benny Bang-Andersen) for providing fenfluramine. We also gratefully acknowledge the members of the Karolinska Institutet PET group for their assistance, and in particular Gudrun Nylén for excellent technical assistance.
Part of this work was supported by an International Postdoc grant from the Swedish Research Council (S.J.F.).
References
- Andersson JD, Pierson ME, Finnema SJ, Gulyas B, Heys R, Elmore CS, Farde L, Halldin C. (2011) Development of a PET radioligand for the central 5-HT1B receptor: radiosynthesis and characterization in cynomolgus monkeys of eight radiolabeled compounds. Nucl Med Biol 38:261–272. [DOI] [PubMed] [Google Scholar]
- Bang-Andersen B, Ruhland T, Jorgensen M, Smith G, Frederiksen K, Jensen KG, Zhong H, Nielsen SM, Hogg S, Mork A, Stensbol TB. (2011) Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 54:3206–3221. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Emborg ME. (2008) Contributions of non-human primates to neuroscience research. Lancet 371:1126–1135. [DOI] [PubMed] [Google Scholar]
- Chou YH, Wang SJ, Lirng JF, Lin CL, Yang KC, Chen CK, Yeh CB, Liao MH. (2012) Impaired cognition in bipolar I disorder: the roles of the serotonin transporter and brain-derived neurotrophic factor. J Affect Disord 143:131–137. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. (2011) Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology 36:98–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. (2011) Guide for the care and use of laboratory animals: 8th ed. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 255:7108–7117. [PubMed] [Google Scholar]
- Elfving B, Bjornholm B, Knudsen GM. (2003) Interference of anaesthetics with radioligand binding in neuroreceptor studies. Eur J Nucl Med Mol Imaging 30:912–915. [DOI] [PubMed] [Google Scholar]
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. (2011) Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging 38:681–693. [DOI] [PubMed] [Google Scholar]
- Ettrup A, da Cunha-Bang S, McMahon B, Lehel S, Dyssegaard A, Skibsted AW, Jorgensen LM, Hansen M, Baandrup AO, Bache S, Svarer C, Kristensen JL, Gillings N, Madsen J, Knudsen GM. (2014) Serotonin 2A receptor agonist binding in the human brain with [(1)(1)C]Cimbi-36. J Cereb Blood Flow Metab 34:1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettrup A, Svarer C, McMahon B, da Cunha-Bang S, Lehel S, Moller K, Dyssegaard A, Ganz M, Beliveau V, Jorgensen LM, Gillings N, Knudsen GM. (2016) Serotonin 2A receptor agonist binding in the human brain with [(11)C]Cimbi-36: test-retest reproducibility and head-to-head comparison with the antagonist [(18)F]altanserin. Neuroimage 130:167–174. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Bang-Andersen B, Wikstrom HV, Halldin C. (2010. a) Current state of agonist radioligands for imaging of brain dopamine D2/D3 receptors in vivo with positron emission tomography. Curr Top Med Chem 10:1477–1498. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Gulyas B, Pierson ME, Halldin C, Farde L. (2010. b) Fenfluramine-induced serotonin release decreases [11C]AZ10419369 binding to 5-HT1B-receptors in the primate brain. Synapse 64:573–577. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Varrone A, Hwang TJ, Halldin C, Farde L. (2012) Confirmation of fenfluramine effect on 5-HT(1B) receptor binding of [(11)C]AZ10419369 using an equilibrium approach. J Cereb Blood Flow Metab 32:685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnema SJ, Stepanov V, Ettrup A, Nakao R, Amini N, Svedberg M, Lehmann C, Hansen M, Knudsen GM, Halldin C. (2014) Characterization of [(11)C]Cimbi-36 as an agonist PET radioligand for the 5-HT(2A) and 5-HT(2C) receptors in the nonhuman primate brain. Neuroimage 84:342–353. [DOI] [PubMed] [Google Scholar]
- Finnema SJ, Scheinin M, Shahid M, Lehto J, Borroni E, Bang-Andersen B, Sallinen J, Wong E, Farde L, Halldin C, Grimwood S. (2015) Application of cross-species PET imaging to assess neurotransmitter release in brain. Psychopharmacology (Berl) 232:4129–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Conklin DS, Krause CM, Marshall AP, Patterson JP, Tran DP, Iyer G, Kostich WA, Largent BL, Hartig PR. (1999) High-affinity agonist binding correlates with efficacy (intrinsic activity) at the human serotonin 5-HT2A and 5-HT2C receptors: evidence favoring the ternary complex and two-state models of agonist action. J Neurochem 72:2127–2134. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K, Rabiner EA, Ridler K, Pittman B, Huang Y, Carson RE, Morris ED, Cosgrove KP. (2014) Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology 39:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Bhatnagar A, Gurevich VV, Roth BL. (2003) The interaction of a constitutively active arrestin with the arrestin-insensitive 5-HT(2A) receptor induces agonist-independent internalization. Mol Pharmacol 63:961–972. [DOI] [PubMed] [Google Scholar]
- Hazelwood LA, Sanders-Bush E. (2004) His452Tyr polymorphism in the human 5-HT2A receptor destabilizes the signaling conformation. Mol Pharmacol 66:1293–1300. [PubMed] [Google Scholar]
- Ito H, Nyberg S, Halldin C, Lundkvist C, Farde L. (1998) PET imaging of central 5-HT2A receptors with carbon-11-MDL 100,907. J Nucl Med 39:208–214. [PubMed] [Google Scholar]
- Jørgensen LM, Weikop P, Villadsen J, Visnapuu T, Ettrup A, Hansen HD, Baandrup AO, Andersen FL, Bjarkam CR, Thomsen C, Jespersen B, Knudsen GM. (2016) Cerebral 5-HT release correlates with [11C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J Cereb Blood Flow Metab 37:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158. [DOI] [PubMed] [Google Scholar]
- Laruelle M. (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451. [DOI] [PubMed] [Google Scholar]
- Lundkvist C, Halldin C, Ginovart N, Nyberg S, Swahn CG, Carr AA, Brunner F, Farde L. (1996) [11C]MDL 100907, a radioligland for selective imaging of 5-HT(2A) receptors with positron emission tomography. Life Sci 58:PL 187–192. [DOI] [PubMed] [Google Scholar]
- Marquardt DW. (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11:431–441. [Google Scholar]
- McCormick PN, Ginovart N, Wilson AA. (2011) Isoflurane anaesthesia differentially affects the amphetamine sensitivity of agonist and antagonist D2/D3 positron emission tomography radiotracers: implications for in vivo imaging of dopamine release. Mol Imaging Biol 13:737–746. [DOI] [PubMed] [Google Scholar]
- Meyer PT, Bhagwagar Z, Cowen PJ, Cunningham VJ, Grasby PM, Hinz R. (2010) Simplified quantification of 5-HT2A receptors in the human brain with [11C]MDL 100,907 PET and non-invasive kinetic analyses. Neuroimage 50:984–993. [DOI] [PubMed] [Google Scholar]
- Narendran R, Hwang DR, Slifstein M, Talbot PS, Erritzoe D, Huang Y, Cooper TB, Martinez D, Kegeles LS, Abi-Dargham A, Laruelle M. (2004) In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (-)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 52:188–208. [DOI] [PubMed] [Google Scholar]
- Narendran R, Mason NS, Laymon CM, Lopresti BJ, Velasquez ND, May MA, Kendro S, Martinez D, Mathis CA, Frankle WG. (2010) A comparative evaluation of the dopamine D(2/3) agonist radiotracer [11C](-)-N-propyl-norapomorphine and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. J Pharmacol Exp Ther 333:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD. (2008) Serotonin receptors. Chem Rev 108:1614–1641. [DOI] [PubMed] [Google Scholar]
- Nord M, Finnema SJ, Halldin C, Farde L. (2013) Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol 16:1577–1586. [DOI] [PubMed] [Google Scholar]
- Ohba H, Harada N, Nishiyama S, Kakiuchi T, Tsukada H. (2009) Ketamine/xylazine anesthesia alters [11C]MNPA binding to dopamine D2 receptors and response to methamphetamine challenge in monkey brain. Synapse 63:534–537. [DOI] [PubMed] [Google Scholar]
- Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. (2010) Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab 30:1682–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson LM, Kornum BR, Nutt DJ, Pike VW, Knudsen GM. (2013) 5-HT radioligands for human brain imaging with PET and SPECT. Med Res Rev 33:54–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. (2006) Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology 31:265–277. [DOI] [PubMed] [Google Scholar]
- Pierson ME, Andersson J, Nyberg S, McCarthy DJ, Finnema SJ, Varnas K, Takano A, Karlsson P, Gulyas B, Medd AM, Lee CM, Powell ME, Heys JR, Potts W, Seneca N, Mrzljak L, Farde L, Halldin C. (2008) [11C]AZ10419369: a selective 5-HT1B receptor radioligand suitable for positron emission tomography (PET). Characterization in the primate brain. Neuroimage 41:1075–1085. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Treyer V, Hasler F, Dorig N, Wyss MT, Burger C, Rentsch KM, Westera G, Schubiger PA, Buck A, Vollenweider FX. (2012) Assessment of serotonin release capacity in the human brain using dexfenfluramine challenge and [18F]altanserin positron emission tomography. Neuroimage 59:3922–3932. [DOI] [PubMed] [Google Scholar]
- Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A. (2012) The INIA19 template and NeuroMaps Atlas for primate brain image parcellation and spatial normalization. Front Neuroinform 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. (2002) Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol Ther 95:73–88. [DOI] [PubMed] [Google Scholar]
- Saulin A, Savli M, Lanzenberger R. (2012) Serotonin and molecular neuroimaging in humans using PET. Amino Acids 42:2039–2057. [DOI] [PubMed] [Google Scholar]
- Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C, Innis RB. (2006) Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59:260–269. [DOI] [PubMed] [Google Scholar]
- Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S, Plisson C, Miller SR, Huiban M, Beaver JD, Gunn RN, Laruelle M, Rabiner EA. (2012) Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleight AJ, Stam NJ, Mutel V, Vanderheyden PM. (1996) Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: effects on apparent agonist affinities. Biochem Pharmacol 51:71–76. [DOI] [PubMed] [Google Scholar]
- Talbot PS, Slifstein M, Hwang DR, Huang Y, Scher E, Abi-Dargham A, Laruelle M. (2012) Extended characterisation of the serotonin 2A (5-HT2A) receptor-selective PET radiotracer 11C-MDL100907 in humans: quantitative analysis, test-retest reproducibility, and vulnerability to endogenous 5-HT tone. Neuroimage 59:271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyacke RJ, Nutt DJ. (2015) Optimising PET approaches to measuring 5-HT release in human brain. Synapse 69:505–511. [DOI] [PubMed] [Google Scholar]
- Udo de Haes JI, Harada N, Elsinga PH, Maguire RP, Tsukada H. (2006) Effect of fenfluramine-induced increases in serotonin release on [18F]MPPF binding: a continuous infusion PET study in conscious monkeys. Synapse 59:18–26. [DOI] [PubMed] [Google Scholar]
- Varnäs K, Nyberg S, Halldin C, Varrone A, Takano A, Karlsson P, Andersson J, McCarthy D, Smith M, Pierson ME, Söderström J, Farde L. (2011) Quantitative analysis of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. J Cereb Blood Flow Metab 31:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnäs K, Juréus A, Johnstrom P, Ahlgren C, Schott P, Schou M, Gruber S, Jerning E, Malmborg J, Halldin C, Afzelius L, Farde L. (2016) Integrated strategy for use of positron emission tomography in nonhuman primates to confirm multitarget occupancy of novel psychotropic drugs: an example with AZD3676. J Pharmacol Exp Ther 358:464–471. [DOI] [PubMed] [Google Scholar]
- Varrone A, Sjoholm N, Eriksson L, Gulyas B, Halldin C, Farde L. (2009) Advancement in PET quantification using 3D-OP-OSEM point spread function reconstruction with the HRRT. Eur J Nucl Med Mol Imaging 36:1639–1650. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C, Doi H, Onoe H. (2014) A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry 4:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.