Abstract
Background
Adolescent intermittent ethanol exposure causes long-lasting alterations in brain epigenetic mechanisms. Melanocortin and neuropeptide Y signaling interact and are affected by ethanol exposure in the brain. Here, the persistent effects of adolescent intermittent ethanol on alpha-melanocyte stimulating hormone, melanocortin 4 receptor, and neuropeptide Y expression and their regulation by histone acetylation mechanisms were investigated in adulthood.
Methods
Male rats were exposed to adolescent intermittent ethanol (2 g/kg, i.p.) or volume-matched adolescent intermittent saline from postnatal days 28 to 41 and allowed to grow to postnatal day 92. Anxiety-like behaviors were measured by the elevated plus-maze test. Brain regions from adult rats were used to examine changes in alpha-melanocyte stimulating hormone, melanocortin 4 receptor, and neuropeptide Y expression and the histone acetylation status of their promoters.
Results
Adolescent intermittent ethanol-exposed adult rats displayed anxiety-like behaviors and showed increased pro-opiomelanocortin mRNA levels in the hypothalamus and increased melanocortin 4 receptor mRNA levels in both the amygdala and hypothalamus compared with adolescent intermittent saline-exposed adult rats. The alpha-Melanocyte stimulating hormone and melanocortin 4 receptor protein levels were increased in the central and medial nucleus of the amygdala, paraventricular nucleus, and arcuate nucleus of the hypothalamus in adolescent intermittent ethanol-exposed compared with adolescent intermittent saline-exposed adult rats. Neuropeptide Y protein levels were decreased in the central and medial nucleus of the amygdala of adolescent intermittent ethanol-exposed compared with adolescent intermittent saline-exposed adult rats. Histone H3K9/14 acetylation was decreased in the neuropeptide Y promoter in the amygdala but increased in the melanocortin 4 receptor gene promoter in the amygdala and the melanocortin 4 receptor and pro-opiomelanocortin promoters in the hypothalamus of adolescent intermittent ethanol-exposed adult rats compared with controls.
Conclusions
Increased melanocortin and decreased neuropeptide Y activity due to changes in histone acetylation in emotional brain circuitry may play a role in adolescent intermittent ethanol-induced anxiety phenotypes in adulthood.
Keywords: alcohol, adolescence, alpha-melanocyte stimulating hormone, melanocortin 4 receptor, neuropeptide Y, histone H3 acetylation
Significance Statement
Adolescence is an important period for the maturation of the brain, and exposure to alcohol during this period can have long-lasting effects on brain biology and function. Here, we show that exposing rats to intermittent, binge-like alcohol exposure during adolescence increases the expression of the stress-related neuropeptide alpha-melanocyte stimulating hormone (α-MSH) and its receptor, the melanocortin 4 receptor (MC4R), and decreases expression of the anti-stress hormone neuropeptide Y (NPY) in emotional circuitry during adulthood. We also observe increased histone H3 acetylation, an epigenetic mark associated with increased transcription, at the promoters of both Pomc (the mRNA precursor to α-MSH) and Mc4r in the amygdala and hypothalamus of adult animals exposed to alcohol in adolescence. On the other hand, decreased histone acetylation at the Npy gene promoter is associated with decreased NPY levels in amygdala of adult rats after adolescent ethanol exposure. The imbalance between the melanocortin and NPY systems in emotional brain circuitry in adulthood after adolescent alcohol exposure may be related to changes in gene-specific histone H3 acetylation and possibly contributes to adult psychopathology.
Introduction
Binge drinking in underage populations is a leading public health concern and societal issue (Brown et al., 2009; Witt, 2010; Patrick et al., 2013). Adolescence is a crucial period for brain maturation, involving the stabilization of synapse formation, grey matter integrity, and axonal projections (Keshavan et al., 2014). Adolescent exposure to alcohol affects the normal trajectory of the developing brain (Keshavan et al., 2014; Spear and Swartzwelder, 2014; Kyzar et al., 2016a). Early onset of alcohol use and adolescent binge consumption lead to an increased risk of alcohol use disorder and comorbid psychiatric diagnoses in adulthood (Grant and Dawson, 1997; DeWit et al., 2000; Witt, 2010).
Recently, stress-related molecules such as those involved in melanocortin signaling have emerged as a novel target of the effects of alcohol on the brain (Olney et al., 2014; Roltsch Hellard et al., 2017). Melanocortins are derived from a prohormone mRNA, pro-opiomelanocortin (Pomc), that is spliced to yield mature protein products including alpha-melanocyte stimulating hormone (α-MSH), along with β-MSH, γ-MSH, and adrenocorticotrophic hormone. α-MSH exerts its effects by binding to and activating melanocortin receptors, such as melanocortin 3 receptor (MC3R) and MC4R. These receptors are G-protein coupled receptors that show wide distribution in key brain areas including the amygdala and hypothalamus (Kishi et al., 2003; Liu et al., 2003; Mountjoy, 2010; Dores et al., 2014). α-MSH induces anxiogenic behavior in rodents, and alcohol withdrawal-induced anxiety-like behavior is attenuated by central administration of a specific MC4R antagonist in adult rats (Kokare et al., 2006). Alcohol withdrawal increases immunoreactivity of α-MSH in specific neurocircuitry including the arcuate nucleus of the hypothalamus (ARC), paraventricular nucleus of the hypothalamus (PVN), and the central nucleus of the amygdala (CeA) (Kokare et al., 2008). These brain circuits are implicated in addictive processes (Koob and Volkow, 2010). The brain melanocortin system, including the lateral hypothalamus and ventral tegmental area, has been shown to be involved in the regulation of ethanol consumption (Olney et al., 2014; Shelkar et al., 2015; Sprow et al., 2016). On the other hand, neuropeptide Y (NPY) is an anti-stress molecule, and several studies suggest that lower NPY levels in the amygdala are associated with anxiety-like behaviors during withdrawal after chronic ethanol exposure and promote alcohol intake in adult rats (Badia-Elder et al., 2001; Thorsell et al., 2007; Gilpin et al., 2008a, 2008b; Zhang et al., 2010; Gilpin, 2012; Sparrow et al., 2012). In fact, α-MSH and NPY interact in opposing ways in the amygdala to regulate alcohol-related behaviors (Kokare et al., 2005).
Epigenetic processes are major regulators of ethanol action in the brain (Starkman et al., 2012; Kyzar and Pandey, 2015; Berkel and Pandey, 2017). Adolescent intermittent ethanol (AIE) exposure of rats causes long-lasting increases in anxiety-like and alcohol drinking behaviors and deficits in global histone H3K9 acetylation in the hippocampus and amygdala, as well as deficits in hippocampal neurogenesis, in adulthood (Vetreno et al., 2014; Pandey et al., 2015; Vetreno and Crews, 2015; Sakharkar et al., 2016). AIE-induced anxiety-like behavior in adulthood coincides with decreased dendritic spine density and expression of brain-derived neurotropic factor (Bdnf) in the central (CeA) and medial nucleus of the amygdala (MeA) (Pandey et al., 2015), which are involved in the negative affective states of alcoholism (Koob and Le Moal, 2005; Koob and Volkow, 2010; Koob et al., 2014; Pandey et al., 2017).
The role of the melanocortin system and its regulation by histone acetylation mechanisms in the persistent effects of AIE in adulthood has not been explored previously. Also, the epigenetic regulation of NPY expression in the amygdala in adulthood after adolescent alcohol exposure is unknown. We therefore investigated the contribution of α-MSH and MC4R in the amygdala and hypothalamus and NPY in the amygdala to the lasting effects of AIE in adulthood. We measured mRNA expression of Pomc and Mc4r in the amygdala and hypothalamus. We measured NPY protein levels in the amygdala and α-MSH and MC4R protein levels in the amygdala and hypothalamus to identify nuclei-specific changes in AIE adult rats compared with AIS adult rats. In addition, we measured the occupancy of acetylated histone H3K9/14 in the promoter region of Mc4r and Npy in the amygdala and the Mc4r and Pomc promoter regions in the hypothalamus to better understand the epigenetic regulation of the melanocortin and NPY systems by AIE in adulthood.
METHODS
Experimental Animals and Behavioral Testing
Pregnant Sprague-Dawley rats were purchased from Harlan Laboratories and housed in a 12-hour-light/-dark cycle with drinking water and food ad libitum. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee. Male rat pups (n = 53) were weaned at postnatal day (PND) 21 and were group-housed (2 or 3 rats) with access to water and food. Rats were randomly assigned for exposure to adolescent intermittent ethanol (AIE) or normal saline (AIS) treatment. Rats received one dose of ethanol (2 g/kg, 20% w/v; AIE) or volume-matched saline (AIS) via i.p. injection per day for 2 consecutive days, followed by 2 days without ethanol or saline treatment for a total of 8 injections during PND 28 to 41, utilizing an exposure paradigm used by our laboratory (Pandey et al., 2015; Kyzar et al., 2016b; Sakharkar et al., 2016) and other laboratories (Pascual et al., 2009; Alaux-Cantin et al., 2013). Both groups of rats were allowed to mature to PND 92 without further treatment and were subjected to the elevated plus maze (EPM) test for anxiety-like behaviors to replicate previous studies (Pandey et al., 2015; Kyzar et al., 2016b) as described by our laboratory and others (File, 1993; Pandey et al., 2006; Sakharkar et al., 2012).
Brain Tissue Collection
On PND 92 immediately after behavioral testing, animals were anesthetized (pentobarbital 50 mg/kg), and brain tissues were dissected and quickly frozen for biochemical studies. Some rats were perfused with normal saline followed by 4% paraformaldehyde solution prepared in phosphate buffer (pH 7.4) as described previously (Pandey et al., 2006, 2015). Brains were isolated and post-fixed overnight in paraformaldehyde and soaked in graded sucrose solutions (10%, 20%, and 30%). All brains were frozen and kept at −80°C until further use for either biochemical studies or immunohistochemical staining of α-MSH, MC4R, and NPY proteins.
mRNA Analysis of Pomc and Mc4r by Quantitative Real-Time PCR
Total RNA was extracted from amygdala and hypothalamic tissues from AIE and AIS adult rats using TRIzol reagent (Life Technologies) followed by the Qiagen miRNAeasy Mini protocol & DNase kit as described previously (Centanni et al., 2014; Kyzar et al., 2016b). Total RNA from each sample was reverse transcribed at 37°C for 2 hours using the high capacity cDNA RT kit (Life Technologies). The reaction was stopped by a 5-minute incubation at 85°C. Pomc and Mc4r mRNA levels were quantified by real-time polymerase chain reaction (PCR) using Maxima SYBR Green (Thermo Scientific). Hypoxanthine phosphoribosyltransferase 1 (Hprt1) was used as a reference gene, and PCR conditions for all primers were 3 minutes at 95°C followed by 30 seconds at 95°C, 30 seconds at 55°C, and 30 seconds at 72°C for 40 cycles. The sequences for each primer set have been provided in Table 1. Quantitative PCR was performed using the Mx3000P qPCR system and analyzed with MxPro software (Agilent Technologies). The c(t) value of the gene of interest was corrected with the c(t) value of the respective Hprt1 internal control gene (Δc(t)), and the respective fold changes were calculated using the 2−ΔΔc(t) method (Livak and Schmittgen, 2001) as reported earlier (Kyzar et al., 2016b). Data are presented as average fold change compared with AIS adult rats.
Table 1.
Primers Used for Measurement of Pomc and Mc4r mRNA and Histone Acetylation Levels of Gene Promoters
| Pomc mRNA Fwd | 5’-TCCTCAGAGAGCTGCCTTTC-3’ |
| Pomc mRNA Rev | 5’-AGCGACTGTAGCAGAATCTCG-3’ |
| Mc4r mRNA Fwd | 5’-TGTCATCATCTGCCTCATTACC-3’ |
| Mc4r mRNA Rev | 5’-GAGGACAGCGATCCTCTTAATG-3’ |
| Hprt1 mRNA Fwd | 5’-TCCTCAGACCGCTTTTCCCGC-3’ |
| Hprt1 mRNA Rev | 5’-TCATCATCACTAATCACGACGCTGG-3’ |
| Mc4r Promoter primer location 1 Fwd | 5’-GTTGGTCAGCTCAAGACGGA-3’ |
| Mc4r Promoter primer location 1 Rev | 5’-TACACATTGGGCCACCTTCC-3’ |
| Mc4r Promoter primer location 2 Fwd | 5’-GTTCCCCACAGCATACCCAT-3’ |
| Mc4r Promoter primer location 2 Rev | 5’-AAAAGCACTCTGTCCTGGCT-3’ |
| Pomc Promoter primer location 1 Fwd | 5’-GGTGCTCTGAAGCAAGACCA-3’ |
| Pomc Promoter primer location 1 Rev | 5’-CCACGTACCAGGAAGGAACC-3’ |
| Pomc Promoter primer location 2 Fwd | 5’-TGGGCTGCCATGATTCTTGA-3’ |
| Pomc Promoter primer location 2 Rev | 5’-TACCTGTGTGCGTCCATCTG-3’ |
| Npy Promoter Fwd | 5’-AGTAGGTCCAGTAGGTCCAGTAGGT-3’ |
| Npy Promoter Rev | 5’-GAAGCAGTCGAGCAAGGTTTT-3’ |
Abbreviations: Rev, reverse; Fwd, forward.
Gold Immunolabeling for α-MSH, MC4R, and NPY
Gold immunolabeling was employed for the measurement of NPY protein levels in the amygdala and α-MSH and MC4R protein levels in the amygdala and hypothalamus, as our laboratory has previously described (Pandey et al., 2008; Moonat et al., 2013; Sakharkar et al., 2014). Briefly, brains were cut into 20-μm-thick coronal sections and immunostained using antibodies against α-MSH (orb13589, Biorbyt Ltd; 1:200 dilution), MC4R (orb214232, Biorbyt Ltd., 1:200 dilution), and NPY (22940, Immunostar; 1:500 dilution). Immunogold particles were quantified using the Computerized Image Analyzer (Loats Associates) connected to a light microscope. The threshold of a non-immunostained area was set to zero. After threshold determination, the software counted the number of immunogold particles/100 μm2 area within 3 object areas from each of 3 bregma-matched brain sections from each rat at high magnification (100x). Values were averaged from 9 total object fields for each rat, and results are represented as number of immunogold particles per 100-μm2 area.
Chromatin Immunoprecipitation (ChIP) Assay
Histone H3K9/14 acetylation at specific loci in the gene promoter regions was measured in the amygdala (Mc4r and Npy) and hypothalamus (Pomc and Mc4r) of AIE and AIS adult rats using ChIP as described previously (Moonat et al., 2013; Pandey et al., 2015; Kyzar et al., 2016b). We evaluated only the epigenetic state of the promoter regions where we observed a change in mRNA levels. Therefore, we did not quantify histone acetylation at the promoter of Pomc in the amygdala. Amygdala and hypothalamic tissue were fixed with methanol-free formaldehyde and subjected to DNA shearing via sonication. The resulting DNA-chromatin complex was immunoprecipitated with an antibody directed to acetylated H3K9/14 (06–599, Millipore), and the precipitated DNA was quantified using qPCR with a SYBR Green master mix (Bio-Rad) and primers designed for distinct regions of the Mc4r, Pomc, and Npy promoter regions (Table 1). After subtraction of input DNA amplification, the 2−ΔΔc(t) method (Livak and Schmittgen, 2001) was used to determine the relative amounts of H3K9/14 acetylation at the promoters as described in our previous studies (Pandey et al., 2015; Kyzar et al., 2016b). Results are expressed as fold change in histone acetylation levels compared with AIS adult rats.
Statistical Analysis
All data between 2 independent groups (AIS and AIE adult rats) were analyzed using a Student’s t test, and each brain region within each group was considered independently as described previously (Pandey et al., 2015; Kyzar et al., 2016b). Significance for all experiments was set at P<.05.
RESULTS
Effects of AIE Exposure on Anxiety and α-MSH and MC4R Expression in the Amygdala and Hypothalamus in Adulthood
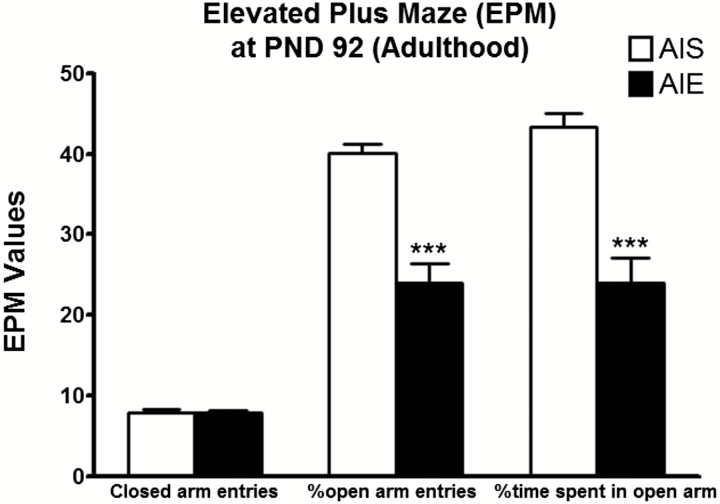
We reported recently that AIE induces anxiety-like behaviors in rats during adulthood (Pandey et al., 2015; Kyzar et al., 2016b; Sakharkar et al., 2016). Here, we replicated our previous findings and found that AIE rats tested at PND 92 displayed decreases in the percentage of time spent in the open arms [t (20) = 5.37, P<.001] and in the percentage of open arm entries [t (20) = 6.37, P<.001] compared with AIS adult rats in the EPM (Figure 1). AIE adult rats displayed no change in closed arm entries compared with AIS adult rats. These results suggest that AIE produces anxiety-like behaviors in adulthood and further confirmed our published findings (Pandey et al., 2015; Kyzar et al., 2016b). There were no significant differences in mean (n=11) body weight (grams) between AIS (PND 28=76.5±4.0; PND 40=137.8±6.2; PND 92= 371.6±5.5) and AIE (PND 28=82.8±4.7; PND 40= 138.9±6.4; PND 92= 365.1± 11.1) rats at various PND.
Figure 1.
Effects of adolescent intermittent ethanol (AIE) on anxiety-like behaviors in adulthood [postnatal day (PND) 92] as measured using the elevated plus maze (EPM) exploration test. Values are represented as mean ± SEM (***P<.001 significantly different from adolescent intermittent saline [AIS] control adult rats, Student’s t test, n = 11).
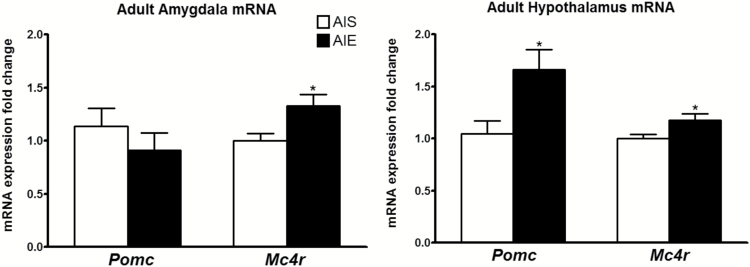
We measured the expression of Pomc and Mc4r mRNA in the amygdala of adult rats exposed to AIE or AIS using qRT-PCR. We found that mRNA levels of Mc4r were significantly [t (15) = -2.74, P<.05] increased in the amygdala of AIE adult rats compared with AIS adult rats (Figure 2). The mRNA expression of Pomc did not change significantly in the amygdala between the 2 groups. Similarly, the mRNA levels of both Pomc [t (9) = -2.56, P<.05] and Mc4r [t (9) = -2.47, P<.05] in the hypothalamus increased significantly in AIE adult rats compared with the AIS adult rats (Figure 2).
Figure 2.
Effects of adolescent alcohol exposure on mRNA expression of pro-opiomelanocortin (Pomc) and melanocortin 4 receptor (Mc4r) in the amygdala and hypothalamus in adulthood. Adolescent intermittent ethanol (AIE)-exposed adult rats display increased Pomc and Mc4r in the hypothalamus and increased Mc4r in the amygdala compared with adolescent intermittent saline (AIS)-exposed adult rats. Values are represented as the mean ± SEM of the fold change derived from the AIS adult rats (*P<.05 significantly different from control AIS rats, Student’s t test, n = 5–9).
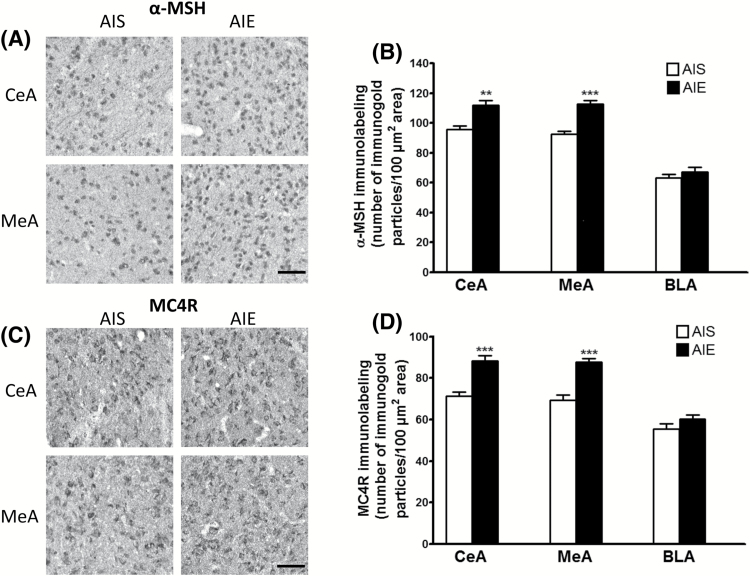
To explore the influence of AIE on the protein products of these transcripts, we measured α-MSH (derived from the precursor mRNA Pomc) and MC4R in AIS- and AIE-exposed adult rats in specific nuclei of the amygdala and hypothalamus. We found a significant increase in both α-MSH and MC4R levels in the CeA [t (10) = -4.18, P<.01 for α-MSH; t (10) = -5.42, P<.001 for MC4R] and MeA [t (10) = -6.69, P<.001 for α-MSH; t (10) = -5.74, P<.001 for MC4R], but not in the basolateral nucleus of the amygdala (BLA) in AIE rats compared with AIS-exposed adult rats (Figure 3).
Figure 3.
Effects of adolescent alcohol exposure on the protein levels of α-melanocyte stimulating hormone (α-MSH) and melanocortin 4 receptor (MC4R) in the amygdaloid structures of rats in adulthood. (A) Representative photomicrographs (scale bar = 50 μm) of α-MSH staining in the central nucleus of the amygdala (CeA) and medial nucleus of the amygdala (MeA) of adolescent intermittent ethanol (AIE)- and saline (AIS)-exposed adult rats. (B) Bar diagram showing protein levels of α-MSH in the CeA, MeA, and basolateral amygdala (BLA) in AIE- and AIS-exposed adult rats as measured by gold immunolabeling. (C) Representative photomicrographs (scale bar = 50 μm) of MC4R gold immunolabeling in the CeA and MeA in AIE- and AIS-exposed adult rats. (D) Bar diagram showing protein levels of MC4R in the CeA, MeA, and BLA of AIS and AIE adult rats as measured by gold immunolabeling. Values are represented as the mean (±SEM) of the number of immunogold particles/100 μm2 area. Values are significantly different from AIS-exposed adult group (**P<.01, *** P<.001, Student’s t test, n = 6).
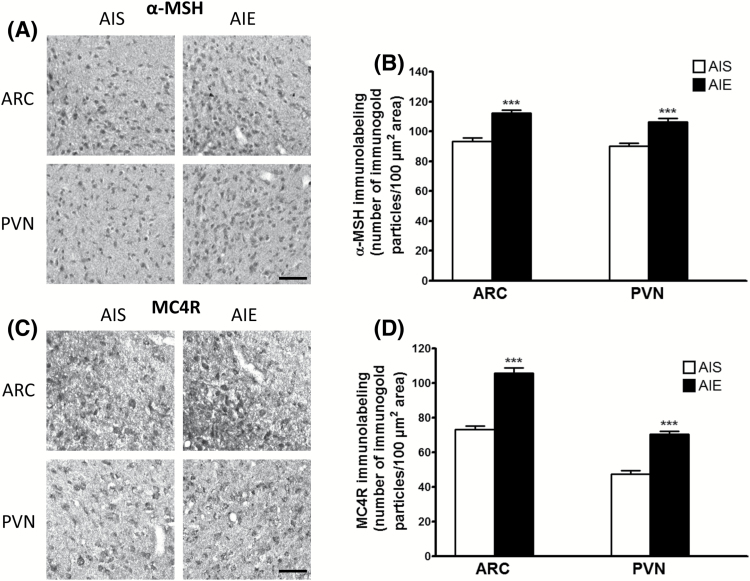
With regard to the hypothalamus, we found significantly increased protein levels of both α-MSH [t (10) = -6.09, P<.001] and MC4R [t (10) = -9.24, P<.001] in the ARC and increased α-MSH [t (10) = -5.48, P<.001] and MC4R [t (10) = -8.18, P<.001] in the paraventricular nucleus (PVN) of the hypothalamus in the AIE animals compared with the AIS-exposed adult rats (Figure 4), correlating with mRNA data. These data suggest that both α-MSH and MC4R protein levels are increased in the amygdala and hypothalamus in adulthood following AIE.
Figure 4.
Effects of adolescent alcohol exposure on the protein levels of α-melanocyte stimulating hormone (α-MSH) and melanocortin 4 receptor (MC4R) in the hypothalamus of rats in adulthood. (A) Representative photomicrographs (scale bar = 50 μm) of α-MSH staining in the arcuate nucleus of the hypothalamus (ARC) and the paraventricular nucleus of the hypothalamus (PVN) of adolescent intermittent ethanol (AIE)- and saline (AIS)-exposed adult rats. (B) Bar diagram showing protein levels of α-MSH in ARC and PVN in AIE- and AIS-exposed adult rats as measured by gold immunolabeling. (C) Representative photomicrographs (scale bar = 50 μm) of MC4R staining in the ARC and PVN in AIE- and AIS-exposed adult rats. (D) Bar diagram showing protein levels of MC4R in ARC and PVN of AIS and AIE adult rats as measured by gold immunolabeling. Values are represented as the mean (±SEM) of the number of immunogold particles/100 μm2. Values are significantly different from AIS-exposed adult group (***P<.001, Student’s t test, n = 6).
Epigenetic Regulation of Pomc and Mc4r Gene Expression following AIE
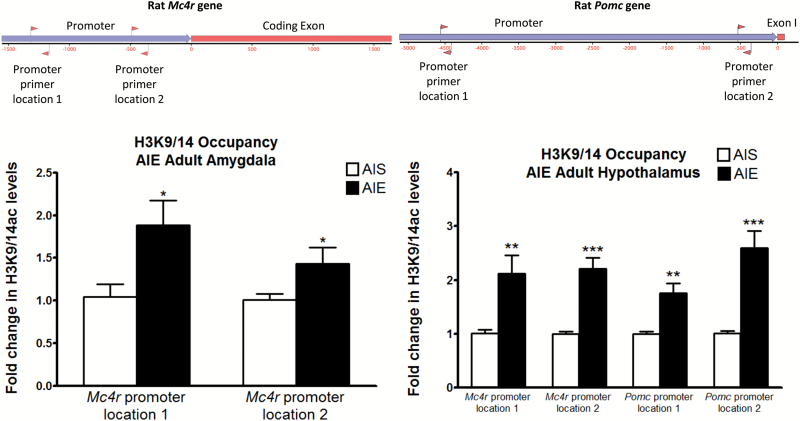
We recently reported that AIE causes a lasting increase in HDAC2 levels in the amygdala, leading to a decrease in acetylated histones globally (H3K9) and locally (H3K9/14) at important synaptic plasticity-related genes including Bdnf (Pandey et al., 2015). Given the involvement of histone acetylation mechanisms in the persistent effects of AIE, we measured occupancy of acetylated histone H3 lysine 9/14 (H3K9/14ac) at the Mc4r and Pomc promoter regions in the amygdala and hypothalamus of AIS and AIE adult rats using the ChIP assay. Here, we found that H3K9/14ac levels were significantly increased in the amygdala of AIE adult rats compared with AIS adult rats at 2 distinct regions [t (7) = -2.77, P<.05 for location 1; t (8) = -2.41, P<.05 for location 2] upstream of the transcription start site of Mc4r (Figure 5). Additionally, H3K9/14ac levels were elevated in the same 2 regions of the Mc4r promoter in the hypothalamus [t (12) = -3.13, P<.01 for location 1; t (12) = -5.67, P<.001 for location 2] and was also increased in 2 regions of the Pomc promoter of AIE adult rats compared with AIS adult rats [t (12) = -3.93, P<.01 for location 1; t (12) = -4.88, P<.001 for location 2]. As acetylation is considered a mark of active transcription (Krishnan et al., 2014), this could explain the increased Mc4r mRNA levels in the amygdala and increased Mc4r and Pomc mRNA levels in the hypothalamus of AIE rats observed here.
Figure 5.
Effects of adolescent alcohol exposure on acetylated histone H3K9/14 occupancy at pro-opiomelanocortin (Pomc) and melanocortin 4 receptor (Mc4r) gene promoters at 2 locations as shown in upper panel. Rats exposed to adolescent intermittent ethanol (AIE) and saline (AIS) were used to measure for H3K9/14 occupancy at the Mc4r promoter region in the amygdala and at both the Mc4r and Pomc promoter regions in the hypothalamus in adulthood by chromatin immunoprecipitation (ChIP) assay. Values are represented as the mean ± SEM of the fold change derived from the AIS adult rats (*P<.05, **P<.01, ***P<.001 significantly different from control AIS rats, Student’s t test, n = 4–6 [amygdala], n = 7 [hypothalamus]).
Effects of AIE on the Expression of NPY and Histone Acetylation of Npy in Adulthood
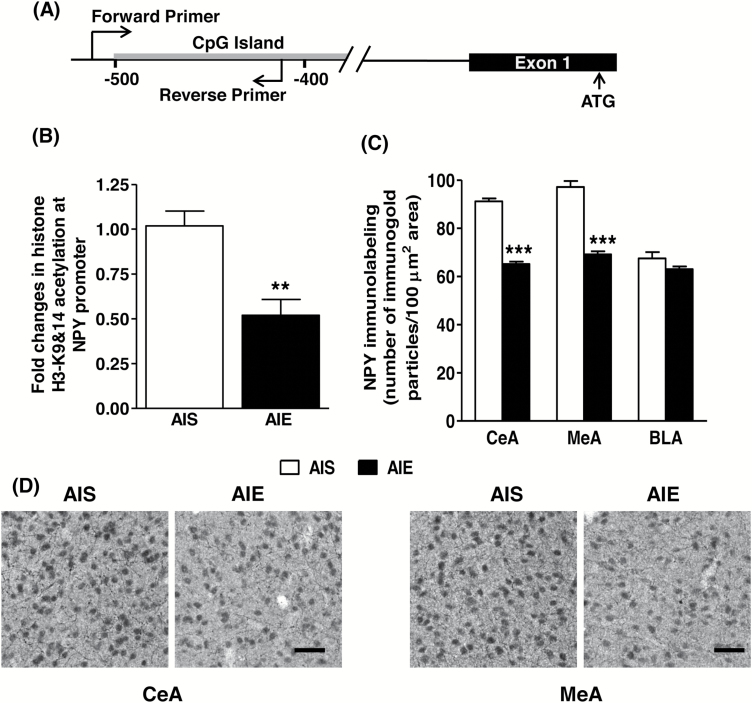
We also measured histone acetylation levels at the Npy gene promoter region that overlaps with a CpG island as shown in Figure 6A. Levels of H3K9/14ac were determined using the ChIP assay. We observed a significant reduction [t (11) = 4.09, P<.01] in H3K9/14ac levels in the Npy gene promoter in the amygdala of AIE adult rats compared with AIS adult rats (Figure 6B). Further, NPY protein levels in the CeA [t (10) = 16.37, P<.001] and MeA [t (10) = 10.21, P<.001], but not in the BLA, were significantly decreased in AIE adult rats compared with AIS adult rats (Figure 6C-D). These results indicate that AIE condenses the chromatin by decreasing local histone acetylation that leads to a reduction in NPY expression in the amygdala at adulthood.
Figure 6.
(A) Schematic representation showing location of forward and reverse primers in the NPY gene promoter used for the determination of histone acetylation overlapping with the DNA sequence that contains stretches of CpG islands. (B) Changes in histone H3K9/14 acetylation levels at the Npy gene promoter in the amygdala of AIS and AIE adult rats as analyzed by the chromatin immunoprecipitation (ChIP) assay. Values are represented as mean (±SEM) of fold change derived from AIS control group (**P<.01 significantly different from control AIS adult group, Student’s t test, n = 6–7). (C) Quantification of gold immunolabeling of NPY protein in the central (CeA), medial (MeA), and basolateral (BLA) nuclei of amygdala of AIS and AIE adult rats. Values are represented as the mean (±SEM) of the number of immunogold particles/100 μm2 area (***P<.001 significantly different from AIS control group, Student’s t test, n = 6). (D) Representative low-magnification photomicrographs (scale bar = 50 μm) of gold immunolabeling showing NPY-positive cells in the CeA and MeA of AIS and AIE adult rats.
Discussion
The present study found that AIE increased mRNA and protein levels of MC4R in both the amygdala and hypothalamus of adult rats compared with AIS-exposed adult rats. Additionally, increased α-MSH protein in the CeA, MeA, ARC, and PVN, along with increased Pomc mRNA in the hypothalamus, was observed in AIE adults compared with AIS adult rats. Epigenetic investigation of Mc4r and Pomc expression showed an increase in acetylated H3K9/14 in the promoter region of Mc4r in the amygdala and both the Mc4r and Pomc promoters in the hypothalamus of AIE-exposed adult rats. AIE produced deficits in histone acetylation of the Npy gene promoter and decreased NPY levels in the CeA and MeA during adulthood. Taken together, these findings suggest that hyperactivity of the melanocortin system due to increased α-MSH and MC4R levels and hypoactive NPY may be involved in anxiety-like behavior seen here and elsewhere after AIE in adulthood (Pandey et al., 2015; Kyzar et al., 2016b; Sakharkar et al., 2016).
In this study, Mc4r mRNA was increased in AIE-exposed adult amygdala and hypothalamic tissue, while Pomc mRNA was increased only in the hypothalamus compared with AIS-exposed control rats. Other groups have shown that chronic ethanol exposure suppressed Pomc mRNA levels in the mediobasal hypothalamus, while ethanol withdrawal gradually increased Pomc levels compared with nonexposed control adult rats (Rasmussen et al., 2002). Alcohol-preferring alko alcohol (AA) adult rats exhibited increased hypothalamic Pomc mRNA expression compared with nonpreferring alko non-alcohol (ANA) adult rats (Gianoulakis et al., 1992). Previous reports have noted an increase in melanocortin tone (i.e., increased α-MSH) in rats during withdrawal after chronic alcohol exposure (Kokare et al., 2006, 2008). Interestingly, the MC4R antagonist HS014 attenuated ethanol withdrawal-associated anxiety-like behaviors in adult rats (Kokare et al., 2006). Conversely, direct injection of α-MSH into the CeA leads to increased anxiety-like behaviors in rats (Kokare et al., 2005). Additionally, alcohol-dependent adult rats exhibit hyperalgesia that is attenuated by both intracerebral and intranasal infusion of HS014, a selective MC4R antagonist, while HS014 has no effect on pain sensitivity in nondependent and alcohol-naive rats (Roltsch Hellard et al., 2017). Adult rats trained to self-administer ethanol in an operant conditioning paradigm exhibited enhanced α-MSH immunoreactivity in the ARC, as well as the nucleus accumbens and bed nucleus of the stria terminalis (Shelkar et al., 2015). Notably, a previous study reported decreased α-MSH immunoreactivity in the CeA, ARC, and PVN of adult rats at PND 63 after AIE (Lerma-Cabrera et al., 2013). Importantly, this group used a higher dose of ethanol (3 g/kg; i.p.) than the one used here, and the animals were allowed to grow only to PND 63 compared with PND 92 in this study. Therefore, the differences between these results could be related to the age and paradigm under which these measurements were performed after AIE.
The melanocortin system is intricately connected to stress and allostatic load (Chaki and Okuyama, 2005; Chaki and Okubo, 2007; Liu et al., 2007; Lim et al., 2012). Importantly, acute restraint stress and forced swimming stress both trigger cfos mRNA induction specifically in a high percentage of Pomc-positive neurons (Liu et al., 2007). Chronic stress causes synaptic changes in dopamine receptor D1-expressing medium spiny neurons in the nucleus accumbens that are mediated by MC4R activation and reversed by MC4R blockade (Lim et al., 2012). Additionally, intracerebral infusion of α-MSH leads to enhanced immobility in the forced swim test that is attenuated by HS014 (Goyal et al., 2006), and chronic restraint increases Mc4r mRNA expression in the hypothalamus (Karami Kheirabad et al., 2015). MC4R blockade by intranasal infusion of HS014 attenuates anxiety-like behaviors and hypothalamic-pituitary-adrenal axis activity in a single prolonged stress model of posttraumatic stress disorder (Serova et al., 2013, 2014). Intracerebroventricular injection of HS014 also blocks social isolation-induced anxiety and depressive-like behaviors, indicating enhanced MC4R tone following stressful life events (Kokare et al., 2010). The increased α-MSH and subsequent activation of MC4R seen in the amygdala and hypothalamus in the present study may be involved in the anxiety-like behaviors in adulthood. Behavioral data showing that AIE produces anxiety-like behaviors in adulthood is similar to our previous studies (Pandey et al., 2015; Kyzar et al., 2016b; Sakharkar et al., 2016), but dissimilar to other studies where AIE appears to attenuate anxiety-like behaviors (Ehlers et al., 2011; Gilpin et al., 2012). The differences in these behavioral results could be related to the use of different rat strains as well as the duration and exposure method of ethanol paradigms.
The interaction between α-MSH/MC4R and other stress-related peptides such as NPY and corticotrophin-releasing factor (CRF), which controls the expression and release of Pomc mRNA and products, has been well documented (Marsh et al., 1999; Bell et al., 2000; Yamano et al., 2004; Kokare et al., 2005; Hsieh et al., 2011). Generally, α-MSH and NPY are functionally antagonistic with regard to food intake and anxiety- and depression-like behaviors, with α-MSH administration eliciting anxiogenic behaviors and NPY causing anxiolysis (Hansen and Morris, 2002; Kokare et al., 2005; Goyal et al., 2006). Like α-MSH, CRF is released in response to stress and is correlated with high-anxiety states (Radulovic et al., 1999; Shekhar et al., 2005). Brain stress systems involving CRF in the amygdala and hypothalamus play crucial roles in alcohol use and addiction (Koob, 2008, 2009; Koob et al., 2014).
The role of NPY, particularly in the amygdala and related structures, in response to alcohol has been extensively studied in adult animals (Heilig and Thorsell, 2002; Gilpin, 2012). Deficits in NPY in the CeA are associated with anxiety-like and alcohol drinking behaviors in rats in multiple alcohol models (Badia-Elder et al., 2001; Thorsell et al., 2007; Gilpin et al., 2008a, 2008b; Zhang et al., 2010). Here, we observed for the first time that AIE significantly decreased NPY levels in CeA and MeA of rats in adulthood. These results suggest that AIE results in increased melanocortin activity and decreased NPY activity in the amygdala in adulthood. These findings are furthered by the observation that the combined treatment of NPY and HS014 (MC4R antagonist) significantly prevented chronic traumatic stress-induced anxiety-like behaviors (Sabban et al., 2015). Another peptide known as agouti-related peptide (AgRP) is produced in NPY-containing neurons in the hypothalamus and acts as an endogenous antagonist at MC4R (Chen et al., 2006). Interestingly, AgRP infusion directly into the CeA of rats leads to an increase in Bdnf mRNA expression when measured 2 hours later (Boghossian et al, 2010), suggesting that antagonism of MC4R may lead to increased BDNF in the amygdala circuitry. BDNF mRNA and protein expression is decreased in the CeA and MeA of AIE adult rats (Pandey et al., 2015), and here we show increased expression of MC4R and its agonist α-MSH and decreased NPY expression in the amygdala. Therefore, it is possible that increased MC4R signaling in the amygdala along with a decrease in BDNF (Pandey et al., 2015) and NPY expression observed in AIE-exposed adult rats together might be operative in the anxiety phenotype seen in AIE adult rats (Figure 7).
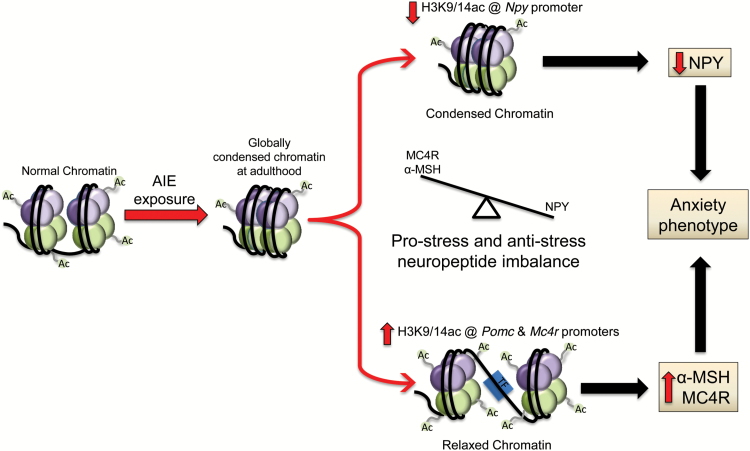
Figure 7.
Adolescent intermittent ethanol (AIE) exposure produces global deficits in histone acetylation due to increased histone deacetylase (HDAC) activity in adulthood, leading to a condensed chromatin structure in the amygdala (Pandey et al., 2015). This model indicates that neuropeptide Y (Npy) gene promoter histone acetylation is decreased, which is associated with decreased NPY expression in the amygdala by AIE in adulthood. On the other hand, AIE produces increased histone acetylation at pro-opiomelanocortin (Pomc) and melanocortin 4 receptor (Mc4r) gene promoters in the hypothalamus and at the Mc4r gene promoter in the amygdala in adulthood. This leads to increased melanocortin activity due to increased alpha-melanocyte stimulating hormone (α-MSH) and MC4R expression. This epigenetically mediated imbalance in NPY and melanocortin activity in the amygdala may be associated with AIE-induced anxiety-like behaviors seen in adulthood. Ac, acetylation; H3K9/14, Histone H3 lysine 9/14; TF, transcription factor.
Epigenetic mechanisms are involved in the lasting effects of AIE, and HDAC inhibitors are able to reverse the increased anxiety-like behavior and decreased activity regulated cytoskeleton-associated protein (Arc) and Bdnf gene-specific histone acetylation (H3K9/14 acetylation) observed in the amygdala of AIE-exposed adult rats (Pandey et al., 2015). Here, we report a paradoxical increase in histone acetylation at the Mc4r promoter in the amygdala of AIE adult rats compared with AIS adult rats, despite the globally increased HDAC2 and decreased H3K9 acetylation seen in the CeA and MeA of AIE adult rats (Pandey et al., 2015). This is possibly due to epigenetic mechanisms working differentially to activate brain pro-stress melanocortin systems, but at the same time inhibiting genes involved in the regulation of synaptic plasticity and anti-stress activity (BDNF and NPY). This is in line with findings that global HDAC inhibition in the brain leads to increased expression of many genes, but also causes decreased expression of a subset of genes (Gräff et al., 2014). Other studies have shown that certain epigenetic enzymes serve dual functions as both transcriptional activators and repressors in the brain (Chahrour et al., 2008; Laurent et al., 2015). We also show that both Mc4r and Pomc show increased histone acetylation at two distinct loci within the promoter in the hypothalamus of AIE adult rats compared with AIS adult rats. Interestingly, fetal alcohol exposure decreases Pomc expression in the hypothalamus of both male and female rats, while also decreasing histone acetylation and increasing repressive H3K9 methylation and DNA methylation in the ARC (Govorko et al., 2012). The decrease in Pomc is additionally transferred intergenerationally to the offspring of male fetal alcohol-exposed rats. This finding potentially represents a divergence between fetal exposure and adolescent exposure to alcohol with regards to Pomc expression.
In summary, we report increased α-MSH and MC4R in the amygdala and hypothalamus of AIE-exposed adult rats that is possibly correlated with anxiety-like behavior and increased alcohol intake (Pandey et al., 2015). The increase in Mc4r expression in the amygdala and both Mc4r and Pomc expression in the hypothalamus of AIE-exposed adult rats may be caused by an increase in H3K9/14 acetylation in the promoters of these genes, which occurs despite a global decrease in histone H3K9 acetylation in the amygdala of AIE-exposed adult rats (Pandey et al., 2015). Interestingly, the anti-anxiety molecule NPY shows decreased expression due to deficits in histone acetylation of the Npy promoter in the amygdala after AIE in adulthood. The epigenetically regulated increase in melanocortin tone and decrease in NPY function may be responsible for the persistent effects of adolescent alcohol exposure on synaptic plasticity and adult psychopathology (Figure 7), and these represent important molecular targets for pharmacological intervention in alcohol addiction.
Statement of Interest
S.C.P. reports that a US patent application entitled “Histone acetyl transferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th, 2006) is currently pending. No biomedical financial interests or potential conflicts of interest were reported by the other authors.
Acknowledgments
The authors thank the following granting agencies for supporting this research.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health UO1AA-019971; U24AA-024605 (NADIA project), RO1AA-010005, P50AA022538 grants, and by the Department of Veterans Affairs (Merit Review Grant, I01BX000143; Senior Research Career Scientist award) to S.C.P., as well as the UGC Raman Post-Doctoral Research Fellowship, Government of India awarded to D.M.K.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M (2013) Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology 67:521–531. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK (2001) Effect of neuropeptide Y (NPY) on oral ethanol intake in wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin Exp Res 25:386–390. [PubMed] [Google Scholar]
- Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF (2000) Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci 20:6707–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkel TDM, Pandey SC (2017) Emerging role of epigenetic mechanisms in alcohol addiction. Alcohol Clin Exp Res 41:666–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghossian S, Park M, York DA (2010) Melanocortin activity in the amygdala controls appetite for dietary fat. Am J Physiol Regul Integr Comp Physiol 298:R385–393. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S (2009) Underage alcohol use: summary of developmental processes and mechanisms: ages 16–20. Alcohol Res Health 32:41–52. [PMC free article] [PubMed] [Google Scholar]
- Centanni SW, Teppen T, Risher ML, Fleming RL, Moss JL, Acheson SK, Mulholland PJ, Pandey SC, Chandler LJ, Swartzwelder HS (2014) Adolescent alcohol exposure alters GABAA receptor subunit expression in adult hippocampus. Alcohol Clin Exp Res 38:2800–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Okubo T (2007) Melanocortin-4 receptor antagonists for the treatment of depression and anxiety disorders. Curr Top Med Chem 7:1145–1151. [DOI] [PubMed] [Google Scholar]
- Chaki S, Okuyama S (2005) Involvement of melanocortin-4 receptor in anxiety and depression. Peptides 26:1952–1964. [DOI] [PubMed] [Google Scholar]
- Chen M, Celik A, Georgeson KE, Harmon CM, Yang Y (2006) Molecular basis of melanocortin-4 receptor for AGRP inverse agonism. Regul Pept 136:40–49. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC (2000) Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 157:745–750. [DOI] [PubMed] [Google Scholar]
- Dores RM, Londraville RL, Prokop J, Davis P, Dewey N, Lesinski N (2014) Molecular evolution of GPCRs: melanocortin/melanocortin receptors. J Mol Endocrinol 52:T29–42. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR, Wills DN, Liu W, Crews FT (2011) Periadolescent ethanol exposure reduces adult forebrain ChAT+IR neurons: correlation with behavioral pathology. Neuroscience 199:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE. (1993) The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res 58:199–202. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, de Waele JP, Kiianmaa K (1992) Differences in the brain and pituitary beta-endorphin system between the alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res 16:453–459. [DOI] [PubMed] [Google Scholar]
- Gilpin NW. (2012) Neuropeptide Y (NPY) in the extended amygdala is recruited during the transition to alcohol dependence. Neuropeptides 46:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Misra K, Koob GF (2008a) Neuropeptide Y in the central nucleus of the amygdala suppresses dependence-induced increases in alcohol drinking. Pharmacol Biochem Behav 90:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE (2008b) Neuropeptide Y suppresses ethanol drinking in ethanol-abstinent, but not non-ethanol-abstinent, Wistar rats. Alcohol 42:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN (2012) Adolescent binge drinking leads to changes in alcohol drinking, anxiety and amygdalar corticotropin releasing factor cells in adulthood in male rats. PloS One 7:e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK (2012) Male germline transmits fetal alcohol adverse effect of hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry 72:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal SN, Kokare DM, Chopde CT, Subhedar NK (2006) Alpha-melanocyte stimulating hormone antagonizes antidepressant-like effect of neuropeptide Y in Porsolt’s test in rats. Pharmacol Biochem Behav 85:369–377. [DOI] [PubMed] [Google Scholar]
- Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, Rei D, Bero AW, Phan TX, Wagner F, Holson E, Xu J, Sun J, Neve RL, Mach RH, Haggarty SJ, Tsai LH (2014) Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell 156:261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA (1997) Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse 9:103–110. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Morris MJ (2002) Evidence for an interaction between neuropeptide Y and the melanocortin-4 receptor on feeding in the rat. Neuropharmacology 42:792–797. [DOI] [PubMed] [Google Scholar]
- Heilig M, Thorsell A (2002) Brain neuropeptide Y (NPY) in stress and alcohol dependence. Rev Neurosci 13:85–94. [DOI] [PubMed] [Google Scholar]
- Hsieh YS, Yang SF, Chen PN, Chu SC, Chen CH, Kuo DY (2011) Knocking down the transcript of protein kinase C-lambda modulates hypothalamic glutathione peroxidase, melanocortin receptor and neuropeptide Y gene expression in amphetamine-treated rats. J Psychopharmacol 25:982–994. [DOI] [PubMed] [Google Scholar]
- Karami Kheirabad M, Namavar Jahromi B, Tamadon A, Ramezani A, Ahmadloo S, Sabet Sarvestan F, Koohi-Hosseinabadi O (2015) Expression of melanocortin-4 receptor mRNA in male rat hypothalamus during chronic stress. Int J Mol Cell Med 4:182–187. [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T (2014) Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry 1:549–558. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK (2003) Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Chopde CT, Subhedar N (2005) Interaction between neuropeptide Y and alpha-melanocyte stimulating hormone in amygdala regulates anxiety in rats. Brain Res 1043:107–114. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Chopde CT, Subhedar NK (2006) Participation of alpha-melanocyte stimulating hormone in ethanol-induced anxiolysis and withdrawal anxiety in rats. Neuropharmacology 51:536–545. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Singru PS, Dandekar MP, Chopde CT, Subhedar NK (2008) Involvement of alpha-melanocyte stimulating hormone (alpha-MSH) in differential ethanol exposure and withdrawal related depression in rat: neuroanatomical-behavioral correlates. Brain Res 1216:53–67. [DOI] [PubMed] [Google Scholar]
- Kokare DM, Dandekar MP, Singru PS, Gupta GL, Subhedar NK (2010) Involvement of alpha-MSH in the social isolation induced anxiety- and depression-like behaviors in rat. Neuropharmacology 58:1009–1018. [DOI] [PubMed] [Google Scholar]
- Koob GF. (2008) A role for brain stress systems in addiction. Neuron 59:11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2009) Brain stress systems in the amygdala and addiction. Brain Res 1293:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the “dark side” of drug addiction. Nat Neurosci 8:1442–1444. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC (2014) The epigenetic landscape of alcoholism. Int Rev Neurobiol 115:75–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Floreani C, Teppen TL, Pandey SC (2016a) Adolescent alcohol exposure: burden of epigenetic reprogramming, synaptic remodeling, and adult psychopathology. Front Neurosci 10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Pandey SC (2015) Molecular mechanisms of synaptic remodeling in alcoholism. Neurosci Lett 601:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Zhang H, Sakharkar AJ, Pandey SC (2016b) Adolescent alcohol exposure alters lysine demethylase 1 (LSD1) expression and histone methylation in the amygdala during adulthood. Addict Biol. doi:10.1111/adb.12404 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent B, Ruitu L, Murn J, Hempel K, Ferrao R, Xiang Y, Liu S, Garcia BA, Wu H, Wu F, Steen H, Shi Y (2015) A specific LSD1/KDM1A isoform regulates neuronal differentiation through H3K9 demethylation. Mol Cell 57:957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma-Cabrera JM, Carvajal F, Alcaraz-Iborra M, de la Fuente L, Navarro M, Thiele TE, Cubero I (2013) Adolescent binge-like ethanol exposure reduces basal α-MSH expression in the hypothalamus and the amygdala of adult rats. Pharmacol Biochem Behav 110:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC (2012) Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK (2003) Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY (2007) The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148:5531–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD (1999) Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nat Genet 21:119–122. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC (2013) Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry 73:763–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG. (2010) Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol 681:29–48. [DOI] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2014) Targeting central melanocortin receptors: a promising novel approach for treating alcohol abuse disorders. Front Neurosci 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K (2006) Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci 26:8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A (2008) Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28:3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H (2015) Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis 82:607–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Kyzar EJ, Zhang H (2017) Epigenetic basis of the dark side of alcohol addiction. Neuropharmacology pii: S0028-3908 (17) 30043–6. doi: 10.1016 (Epub ahead of a print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108:920–931. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD (2013) Extreme binge drinking among 12th-grade students in the United States: prevalence and predictors. JAMA Pediatr 167:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Rühmann A, Liepold T, Spiess J (1999) Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci 19:5016–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR (2002) Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcohol Clin Exp Res 26:535–546. [PubMed] [Google Scholar]
- Roltsch Hellard EA, Impastato RA, Gilpin NW (2017) Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol 22:692–701. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Serova LI, Alaluf LG, Laukova M, Peddu C (2015) Comparative effects of intranasal neuropeptide Y and HS014 in preventing anxiety and depressive-like behavior elicited by single prolonged stress. Behav Brain Res 295:9–16. [DOI] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC (2012) Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res 36:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC (2014) Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol 17:2057–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC (2016) A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct. 221:4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL (2013) Intranasal infusion of melanocortin receptor four (MC4R) antagonist to rats ameliorates development of depression and anxiety related symptoms induced by single prolonged stress. Behav Brain Res 250:139–147. [DOI] [PubMed] [Google Scholar]
- Serova LI, Laukova M, Alaluf LG, Sabban EL (2014) Blockage of melanocortin-4 receptors by intranasal HS014 attenuates single prolonged stress-triggered changes in several brain regions. J Neurochem 131:825–835. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T (2005) Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 8:209–219. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM (2015) Alpha-melanocyte stimulating hormone modulates ethanol self-administration in posterior ventral tegmental area through melanocortin-4 receptors. Addict Biol 20:302–315. [DOI] [PubMed] [Google Scholar]
- Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR, Rinker JA, Jijon AM, Pena J, Navarro M, Kash TL, Thiele TE (2012) Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology 37:1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS (2014) Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neurosci Biobehav Rev 45:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprow G, Rinker J, Lowery-Gointa E, Sparrow A, Navarro M, Thiele TE (2016) Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C57BL/6J mice. Addict Biol. 21:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman BG, Sakharkar AJ, Pandey SC (2012) Epigenetics-beyond the genome in alcoholism. Alcohol Res 34:293–305. [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Repunte-Canonigo V, O’Dell LE, Chen SA, King AR, Lekic D, Koob GF, Sanna PP (2007) Viral vector-induced amygdala NPY overexpression reverses increased alcohol intake caused by repeated deprivations in Wistar rats. Brain 130:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Broadwater M, Liu W, Spear LP, Crews FT (2014) Adolescent, but not adult, binge ethanol exposure leads to persistent global reductions of choline acetyltransferase expressing neurons in brain. PLoS One 9:e113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT (2015) Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci 9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. (2010) Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol 44:119–124. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I (2004) Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci 66:1323–1327. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC (2010) Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol-drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res 34:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]