Abstract
Aggravated behaviors of hepatocellular carcinoma (HCC) will occur after inadequate thermal ablation. However, its underlying mechanisms are not fully understood. Here, we assessed whether the increased matrix stiffness after thermal ablation could promote the progression of residual HCC. Heat‐treated residual HCC cells were cultured on tailorable 3D gel with different matrix stiffness, simulating the changed physical environment after thermal ablation, and then the mechanical alterations of matrix stiffness on cell phenotypes were explored. Increased stiffness was found to significantly promote the proliferation of the heat‐treated residual HCC cells when the cells were cultured on stiffer versus soft supports, which was associated with stiffness‐dependent regulation of ERK phosphorylation. Heat‐exposed HCC cells cultured on stiffer supports showed enhanced motility. More importantly, vitamin K1 reduced stiffness‐dependent residual HCC cell proliferation by inhibiting ERK phosphorylation and suppressed the in vivo tumor growth, which was further enhanced by combining with sorafenib. Increased matrix stiffness promotes the progression of heat‐treated residual HCC cells, proposing a new mechanism of an altered biomechanical environment after thermal ablation accelerates HCC development. Vitamin K1 plus sorafenib can reverse this protumor effect.
Keywords: Hepatocellular carcinoma, matrix stiffness, motility, proliferation, vitamin K1
Radiofrequency ablation (RFA) has been successfully used for unresectable hepatocellular carcinoma (HCC) at early stage. As reported by Facciorusso et al.1, RFA achieves complete ablation in more than 90% of very early and early HCC patients. When RFA is being extensively adopted to the treatment of medium‐sized or large HCC beyond 3 cm, apparent or occult residual HCC after thermal ablation often occurs at the periphery of the tumor where the target treatment temperature is not reached, leading to cancer cell survival at risk for tumor recurrence. Local tumor recurrence after suboptimal RFA is increased with a reported rate ranging from 10% to as high as 52.4%.2, 3, 4 Furthermore, the residual or local recurrent tumor showing a more aggressive progression after RFA is being reported more frequently,5, 6, 7 however, its mechanisms remain unclear.
Multifaceted factors have been reported to be implicated in the acceleration of residual tumor or local recurrence after incomplete RFA. Residual HCC cells after RFA display the sarcomatous transformation,8 or the elevated expression of invasive and stem cell‐like markers.9, 10 Concurrently, activated inflammatory responses in the microenvironment adjacent to the ablated site may also account for the aggressive progression of residual tumor after RFA, including enhanced expression of angiogenesis‐related genes, cytokines, growth factors, and chemokines and the enrichment of inflammatory cells.11, 12, 13, 14 However, RFA not only destroys the tumor, but also alters the biomechanical environment drastically. Similar to the increased tissue stiffness induced by cardiac RFA,15 a significant increase in tissue stiffness at the ablated site after liver RFA has been detected by elastography techniques.16, 17 The potential role of the changed physical attribute of the microenvironment induced by thermal ablation on the malignant behaviors of residual HCC has not been addressed. Given that increase in liver stiffness can favor tumor development,18, 19, 20 it is reasonable to hypothesize that increased matrix stiffness after RFA, which may constitute the mechanical property of the cancer cell niche, could promote the progression of residual tumor after insufficient RFA.
Here, we show that increased matrix stiffness significantly promotes the proliferation of heat‐treated residual HCC cells, which is associated with stiffness‐dependent upregulation of the ERK signaling cascade. High matrix stiffness also enhances the motility of heat‐exposed residual HCC cells. More importantly, vitamin K1 reduces stiffness‐dependent residual HCC cell proliferation and suppressed in vivo tumor growth. This effect is further enhanced by combining with sorafenib treatment. From a new perspective, our work proposes a new mechanism of an altered biomechanical environment after thermal ablation enhances residual HCC development. Vitamin K1 and sorafenib can reverse this protumor effect.
MATERIALS AND METHODS
Cell culture and sublethal in vitro heat treatment
The human HCC cell lines MHCC97H (established by the Liver Cancer Institute, Zhongshan Hospital of Fudan University, Shanghai, China), Hep3B, and HepG2 (ATCC, Manassas, VA, USA), and Huh7 (obtained from the Japanese Cancer Research Resources Bank, Tokyo, Japan) were cultured in the DMEM supplemented with 10% FBS (Gibco, Carlsbad, CA, USA) and 1% penicillin and streptomycin (Invitrogen, Carlsbad, CA, USA) maintained in a humidified atmosphere with 5% CO2 at 37°C. When the cells reached near 70% confluence, they were washed, trypsinized, and resuspended in 1 mL DMEM with 10% FBS in 1.5‐mL micro‐centrifuge tubes (5 × 104 cells). They were then were subjected to heat treatment with predetermined temperatures (37°C, 43°C, 45°C, 46°C, 47°C, 48°C, 50°C, and 55°C) for 10 min in an isothermic water bath incubator (Jing Hong Laboratory Instrument Co., Ltd., Shanghai, China). Thereafter, heat‐treated cells were seeded into 96‐well culture plates in quadruplicate for every temperature and cultured at 37°C for 48 h. Culture media were replaced three times per day to remove dead cells and debris. Absorbance was measured using WST‐1 (Roche Diagnostics, Mannheim, Germany) on a Multiskan Spectrum reader (Thermo Fisher Scientific, Waltham, MA, USA) to determine cell viability. The temperature causing 50% reduction in cell viability relative to the 37°C control (IT50) was calculated using non‐linear regression curve‐fitting by Prism 6.0 (GraphPad Software, San Diego, CA, USA). The IT50 data were used for subsequent experiments to simulate the in vitro sublethal heat treatment condition.
Cell proliferation
The 24‐well plates were precoated with Cell Culture Gel with three different stiffness (soft, medium, and stiff) with strength ranging from 1 kPa to 40 kPa (101 Bio, California, USA) according to the manual's instructions. Heat‐treated residual HCC cells were seeded into the precoated plates and cultured at 37°C for 48 h. Cell proliferation was determined using the WST‐1 cell proliferation assay (Roche Diagnostics). The absorbance was measured at a wavelength of 440 nm with a reference wavelength of 650 nm using a microplate reader.
For individual experiments, heat‐treated residual HCC cells seeded into the precoated medium stiffness plates were exposed to 25 μM U0126 (Cell Signaling Technology, Beverly, MA, USA), 5 μM sorafenib (Santa Cruz Biotechnology, Santa Cruz, CA, USA), 200 μM vitamin K1 (Sigma, St. Louis, MO, USA), 200 μM vitamin K1 plus 25 μM U0126, or 200 μM vitamin K1 plus 5 μM sorafenib for 36 h, then the cell proliferation was determined using the WST‐1 assay as described above.
Quantitative RT‐PCR
Total RNA of samples was extracted using TRIzol reagent (Ambion, Carlsbad, CA, USA), of which 2 μg was synthesized into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Gene expression was measured with Maxima SYBR Green qPCR Master Mix (Thermo Fisher Scientific), and analyzed by the Ct method, normalized to that of human GAPDH and calculated by the 2−ΔΔCt method. The primer sequences are listed in Table S1.
Western blot analysis
Total proteins were extracted with an ice‐cold RIPA lysis buffer containing 1 mM PMSF (Beyotime Institute of Biotechnology, Shanghai, China) and 10% PhosSTOP phosphatase inhibitor Cocktail (Roche). Equivalent amounts of proteins (20 μg) quantified by a bicinchoninic acid protein kit (Millipore, Massachusetts, USA) were separated using 10% SDS‐polyacrylamide gels and then transferred to PVDF membranes (Millipore, USA). Membranes were incubated overnight at 4°C with primary antibodies against proliferating cell nuclear antigen (PCNA) (1:2000; Cell Signaling Technology), ERK1/2 (1:1000; Cell Signaling Technology), Phosopho‐ERK1/2 (Thr202/Tyr204) (1:2000; Cell Signaling Technology), GAPDH (1:1000; Beyotime Institute of Biotechnology) and appropriate HRP‐conjugated secondary antibodies (Jackson Laboratories, West‐Grove, PA, USA) for 1 h at room temperature. Thereafter, the chemiluminescent signals were visualized by Ncm‐ECL Ultra (New Cell & Molecular Biotech Co., Ltd, Suzhou, China).
Extracellular signal‐regulated kinase 1/2 blocking assay
Heat‐treated residual HCC cells plated at medium stiffness were incubated with ERK1/2 inhibitor and/or vitamin K1 for 6 h or 48 h. Then the proteins were extracted for Western blot analysis.
Immunocytochemistry
Heat‐treated residual HCC cells were grown on 6‐well plates precoated with Cell Culture Gel of different stiffness, fixed in 4% paraformaldehyde, and permeabilized with 0.1% Triton X‐100 in PBS for 10 min. Fixed cells were stained with phalloidin‐TRITC (50 μg/mL; Sigma) for 40 min, protected from light. Nuclei were counterstained with DAPI (Beyotime Institute of Biotechnology) for 5 min. Cells images were captured with a fluorescence microscope under appropriate excitation and emission filters (Leica, Wetzlar, Germany).
Immunohistochemistry
Briefly, paraffin‐embedded tissue sections were deparaffinized in xylene, rehydrated through graded ethanol, and underwent antigenic retrieval and endogenous peroxidase blocking. Subsequently, sections were blocked with 2% BSA and incubated with primary antibodies PCNA (1:400; Cell Signaling Technology) and phospho‐ERK1/2 (Thr202/Tyr204, 1:200; Cell Signaling Technology) at 4°C overnight followed by the EnVision two‐step visualization system (GeneTech Company Limited, Shanghai, China). The slides were counterstained with hematoxylin and mounted. The staining was determined by three randomly selected fields under the microscope.
Live‐cell microscopy
Tumor multicellular spheroids were transferred to 6‐well culture plates coated with different stiffness. Following attachment, cells were imaged using a Cell‐IQ cell culturing platform (Chip‐Man Technologies, Tampere, Finland) equipped with a phase‐contrast microscope (Nikon CFI Achromat phase contrast objective with ×200 magnification) and a camera (Nikon, Fukasawa, Japan). Images were captured at 5‐min intervals for 24 h. The images were analyzed using NIH ImageJ software (National Institutes of Health, Bethesda, MD, USA) with the MTrackJ Plugin to depict the cumulative track travelled by the cells with the use of the center of each cell nucleus as the point of tracking.
Animal experiments
Animal experimental protocols were carried out in compliance with the relevant guidelines formulated by the Shanghai Medical Experimental Animal Care Commission and approved by the Animal Ethics Committee of Shanghai Medical College of Fudan University. All mice were purchased from SLAC Laboratory Animal Co., Ltd., Shanghai, China.
Male BALB/c nude mice weighing 18–20 g at 4–6 weeks of age were injected s.c. with 5 × 106 heat‐treated residual HCC cells suspended in Cell Culture Gel of soft or medium stiffness mixed with Matrigel at a ratio of 4:1 (n = 3, each group) into the upper right flank of each mouse. The estimated tumor weight was evaluated with Vernier caliper every 4 days by the calculation formula: length (mm) × (width (mm))2/2. Another 20 mice bearing tumors generated from heat‐treated residual HCC cells with medium stiffness gel were randomly divided into four groups: i.p. injected with DMSO (control; n = 5), vitamin K1 (2 mg/kg, daily; n = 5), sorafenib (1.25 mg/kg, daily; n = 5) or vitamin K1 combined with sorafenib treatment (vitamin K1, 2 mg/kg; sorafenib 1.25 mg/kg; daily; n = 5). The treatments were continued for 2 weeks. Animals were killed 48 h after the last treatment, and tumors were excised, weighed, and placed in liquid nitrogen and formalin for further analysis.
Statistical analysis
Data are presented as means ± standard deviation. Differences between two or multiple groups were processed using Student's t‐test or one‐way anova with spss 17.0 software (SPSS, Chicago, IL, USA) or GraphPad Prism software (GraphPad Software). P‐values below 0.05 were considered statistically significant.
RESULTS
Increased matrix stiffness promoted the proliferation and progenitor‐like traits of heat‐exposed residual HCC cells
Based on IT50 data calculated from heat stress dose–response curves of Hep3B (47.04°C) and HepG2 cells (46.88°C) (Fig. 1a), and MHCC 97H (46.26°C) and Huh7 cells (47.37°C) (data not shown), 47°C for 10 min was selected as the condition of sublethal heat treatment to simulate incomplete thermal ablation in vivo.
Figure 1.
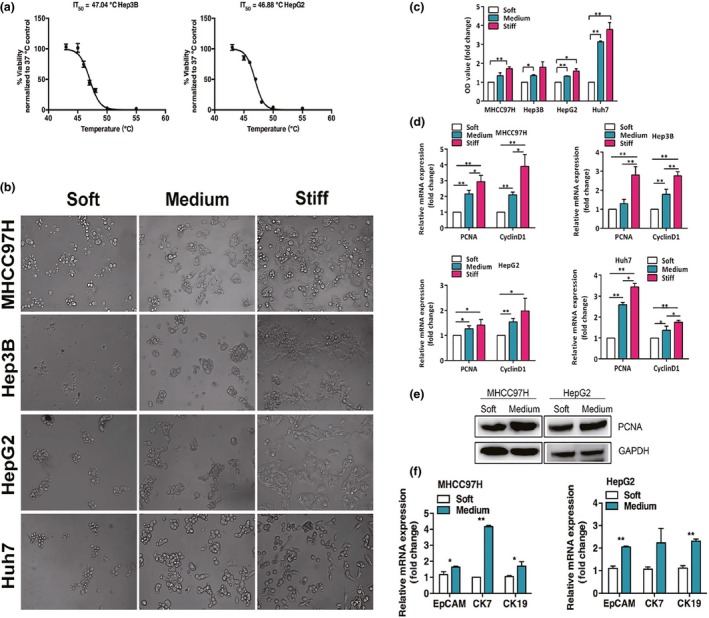
Increased matrix stiffness induces proliferation and progenitor‐like traits in heat‐treated residual hepatocellular carcinoma cells in vitro. (a) Heat stress dose–response curves of Hep3B and HepG2 cells. IT 50, indicating 50% reduction of cell viability, was determined. (b) Morphology changes of heat‐treated residual MHCC97H, Hep3B, HepG2, and Huh7 cells after 48 h of culture on gels of different stiffness. (c) Proliferation of heat‐exposed residual MHCC97H, Hep3B, HepG2, and Huh7 cells cultured on gels of different stiffness were measured using WST‐1 assay. (d) PCNA and CyclinD1 mRNA expressions in heat‐treated residual MHCC97H, Hep3B, HepG2, and Huh7 cells on gels of different stiffness were determined by quantitative RT‐PCR. (e) Proliferating cell nuclear antigen (PCNA) protein expression was analyzed by Western blots. (f) Increased EpCAM,CK7, and CK19 mRNA expressions in heat‐treated residual MHCC97H and HepG2 cells on medium stiffness gels compared with cells cultured on soft gels. *P < 0.05; **P < 0.01.
Morphologies of heat‐exposed residual HCC cells on the different stiffness gels shifted from small and round towards proliferative, outstretched, and spindle‐like in appearance as the culture stiffness increased after 48 h of culture (Fig. 1b). The heat‐exposed residual cells cultured on medium or stiff supports had a higher proliferative activity than those cells on the soft gels (Fig. 1c). This was paralleled by the significant increase of proliferation‐related transcripts (PCNA and CyclinD1) (Fig. 1d). Consistent with this finding, cells proliferated faster as stiffness was elevated, paralleled by a significant increase of PCNA at the protein level (Fig. 1e). The medium stiffness gel was selected for subsequent experiments because its yielded strength ranged from 14 to 20 kPa, which simulates increased liver stiffness after thermal ablation.16, 17 Compared with the heat‐exposed residual cells cultured on the soft gels, transcripts of the putative stem cell markers EpCAM and cholangiocyte markers CK7 and CK19 were dramatically elevated in the cells on the medium stiffness gels (Fig. 1f). These results show that the increased stiffness endows the heat‐treated residual HCC cells a highly proliferative and progenitor‐like cellular phenotype in vitro.
Increased matrix stiffness enhanced the motility of heat‐exposed residual HCC cells
We next sought to assess the motility of the residual HCC cells coordinated by matrix stiffness. Cell migration has been tightly linked to cancer progression and metastasis.21 An in vitro model mimicking the metastatic processes has been developed by the reversal of multicellular spheroids to a monolayer.22 Multicellular spheroids from heat‐treated residual HCC cells were generated under anchorage‐independent growth, then transferred into different stiffness gels. The distance of cell migration from their bases was compared (Fig. 2). When the multicellular spheroids were cultured on the medium stiffness gels, the cells showed vigorous motility and were detached and disseminated extensively into gels. In contrast, the cells cultured on the soft gels showed indolent behavior and compact growth. These results indicate that higher stiffness promotes the motility of heat‐exposed residual HCC cells to show stronger invasive and metastatic propensities.
Figure 2.
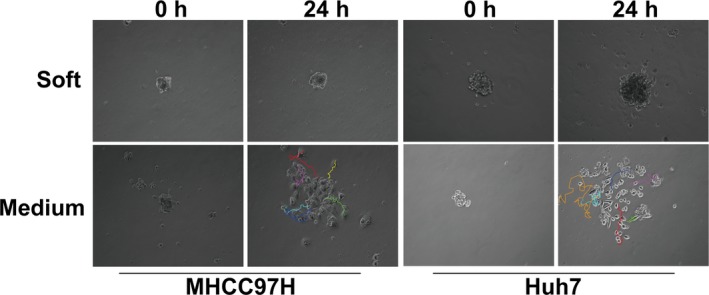
Increased matrix stiffness promotes the motility of heat‐exposed residual hepatocellular carcinoma cells. Representative images (magnification, ×200) of tracks travelled by heat‐treated residual MHCC97H and Huh7 cells on soft and medium stiffness gels were determined by NIH ImageJ software with the MTrackJ Plugin.
Matrix stiffness modulated the cellular phenotypes of heat‐exposed residual HCC cells
To establish bidirectional validation whether matrix stiffness would alter the malignant behaviors of heat‐exposed residual HCC cells, we first cultured residual HCC cells on soft or medium stiffness gels and then transferred them to another stiffness environment (Fig. 3a). We tested the reciprocal combinations including transfer from soft to medium stiffness gel, from medium stiffness to soft gel, from soft to soft gel, and from medium to medium stiffness gel (Fig. 3b). The cell motility of the residual HCC cells was assessed by phalloidin staining of F‐actin. Transfer from soft to medium stiffness gel resulted in higher proliferative growth and spreading migration in the new environment, whereas transfer from medium stiffness to soft gel resulted in a retraction of protrusions and rounded growth. Transfer between soft and soft gel, or between medium and medium stiffness gel resulted in cells sustaining their original morphological features. In parallel with the morphologic changes, the levels of PCNA and CyclinD1 mRNA expression fluctuated when the culture environment was shifted from medium to soft stiffness. These data show that the matrix stiffness microenvironment can modulate the proliferative and motile phenotypes of heat‐exposed residual HCC cells.
Figure 3.
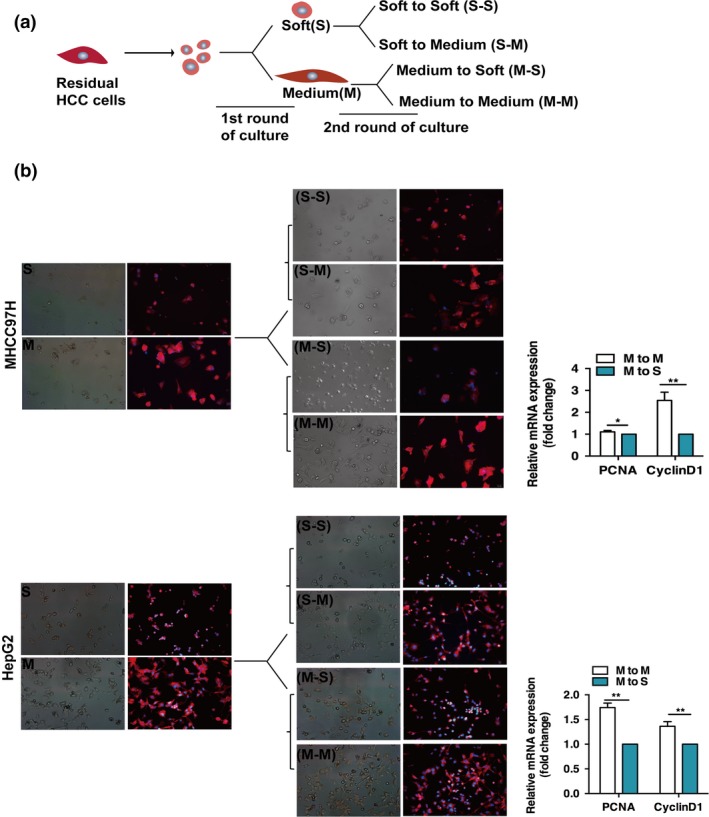
Matrix stiffness regulates the cellular phenotypes of heat‐exposed residual hepatocellular carcinoma cells. (a) Schematic descriptions of the cultures of heat‐treated residual MHCC97H and HepG2 cells on matrices of different stiffness. Cells were cultured switching from soft to soft (S‐S), soft to medium (S‐M), medium to soft (M‐S), and medium to medium (M‐M) stiffness gels. (b) Representative images show the morphological changes of residual MHCC97H and HepG2 cells on soft or medium gels stained with phalloidin‐TRITC and DAPI. In MHCC97H cells, the phase image and corresponding fluorescent image were not taken by the same field. PCNA and CyclinD1 mRNA expressions were determined by quantitative RT‐PCR. *P < 0.05; **P < 0.01.
Activation of ERK was induced by stiffness in heat‐exposed residual HCC cells
We analyzed the stiffness‐associated activation of ERK in the heat‐exposed residual HCC cells. Compared with the residual cells cultured on soft gels, obvious elevation of the phosphorylated ERK was observed in cells on the medium stiffness gels. However, ERK inhibitor (U0126) or vitamin K1 was able to inhibit stiffness‐induced ERK phosphorylation in the residual cells, as well as decrease the expression of the proliferation‐related protein (PCNA). Combined treatment with vitamin K1 plus U0126, substantially decreased ERK activation and PCNA expression (Fig. 4a). Moreover, proliferation and morphology change of heat‐treated residual HCC cells (MHCC97H and Huh7) on the medium stiffness gels were significantly inhibited by U0126, sorafenib, vitamin K1, vitamin K1 plus U0126, and vitamin K1 plus sorafenib (Fig. 4b,c). These data indicate that higher matrix stiffness promotes ERK activation and proliferation in heat‐exposed residual HCC cells, which can be blunted by sorafenib, ERK inhibitor, and/or vitamin K1.
Figure 4.
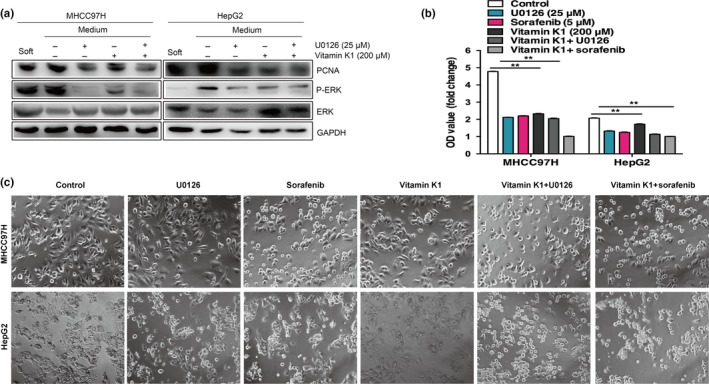
Increased matrix stiffness promotes ERK activation in heat‐exposed residual hepatocellular carcinoma cells. (a) Upregulated proliferating cell nuclear antigen (PCNA) and the phosphorylation level of ERK in heat‐exposed residual MHCC97H and HepG2 cells cultured on medium stiffness gels. The levels of PCNA and ERK phosphorylation were inhibited by ERK inhibitor (U0126) or/and vitamin K1. (b) Proliferative activities of heat‐exposed residual MHCC97H and HepG2 cells on medium stiffness gels were significantly attenuated by U0126, sorafenib, vitamin K1, vitamin K1 plus U0126, or vitamin K1 plus sorafenib. (c) The cell morphology of heat‐treated residual MHCC97H and HepG2 cells on medium stiffness gels was reversed by U0126, sorafenib, vitamin K1 alone, vitamin K1 plus U0126, or vitamin K1 plus sorafenib.
Stiffness promoted in vivo progression of heat‐treated residual HCC cells
To probe the effects of stiffness on the growth dynamics of heat‐exposed residual HCC cells in vivo, residual HCC cells (MHCC97H) embedded in the soft or medium stiffness gel were s.c. inoculated into nude mice. Our results showed that the growth of tumors from residual HCC cells mixed with medium stiffness gels was significantly promoted, compared with that of tumors formed from residual cells with soft gels (Fig. 5a). Real‐time PCR and immunohistochemistry indicated that the levels of PCNA, CyclinD1, EpCAM, CK7, and CK19 mRNA expression and the protein levels of PCNA and ERK phosphorylation were increased in tumors generated from residual HCC cells mixed with medium stiffness gels (Fig. 5b,c). These data suggest that stiffness can promote the in vivo progression of residual HCC cells through ERK activation and enhanced proliferation.
Figure 5.
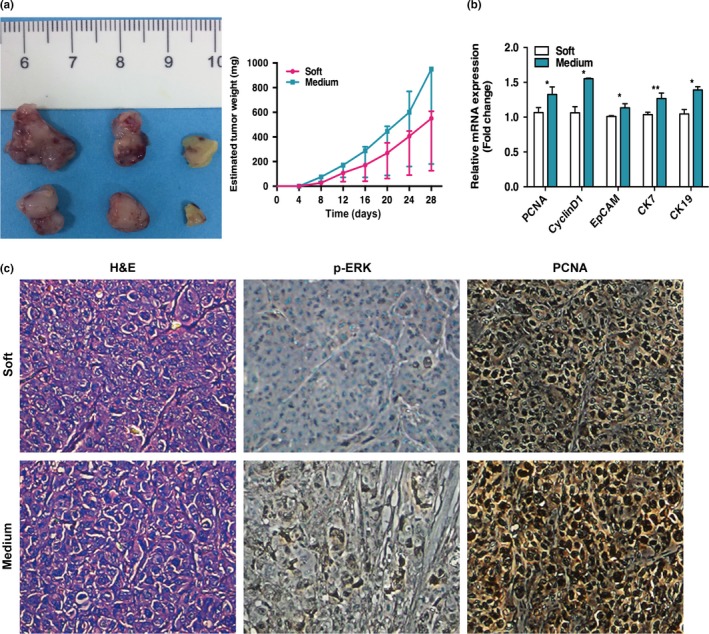
Matrix stiffness promotes the progression of heat‐treated residual hepatocellular carcinoma cells in vivo. (a) Heat‐treated residual MHCC97H cells embedded in the soft or medium stiffness gels were s.c. inoculated into the right flank of nude mice (n = 3 mice per group). Compared to the group of residual MHCC97H cells mixed with soft stiffness gels, faster tumor growth was observed in the group of residual MHCC97H cells with medium stiffness gels. (b) Upregulation of PCNA, CyclinD1, EpCAM,CK7, and CK19 mRNA expression in tumors generated from heat‐treated residual MHCC97H cells with medium stiffness gels was detected by quantitative RT‐PCR. (c) Expression of proliferating cell nuclear antigen (PCNA) and p‐ERK in tumors were compared between the two groups by immunohistochemical analysis. *P < 0.05; **P < 0.01.
Vitamin K1 plus sorafenib suppressed the stiffness‐enhanced progression of heat‐exposed residual HCC in vivo
To validate whether vitamin K1 and/or sorafenib could block the stiffness‐enhanced progression of heat‐exposed residual HCC, residual HCC cells embedded in the medium stiffness gel were transplanted s.c. into nude mice. The mice were treated with either vitamin K1 or sorafenib or the combination of both agents. Our results showed that vitamin K1 or sorafenib alone inhibited tumor growth slightly, whereas the combination of vitamin K1 with sorafenib caused major tumor shrinkage (Fig. 6a). In parallel with the reduction in tumor growth, the levels of PCNA, CyclinD1, EpCAM, CK7, and CK19 mRNA expression and the decreased staining of PCNA and ERK phosphorylation were found in tumors treated with vitamin K1 plus sorafenib (Fig. 6b,c). These findings indicate that vitamin K1 plus sorafenib can abrogate the stiffness‐initiated progression of heat‐exposed residual HCC cells in vivo.
Figure 6.
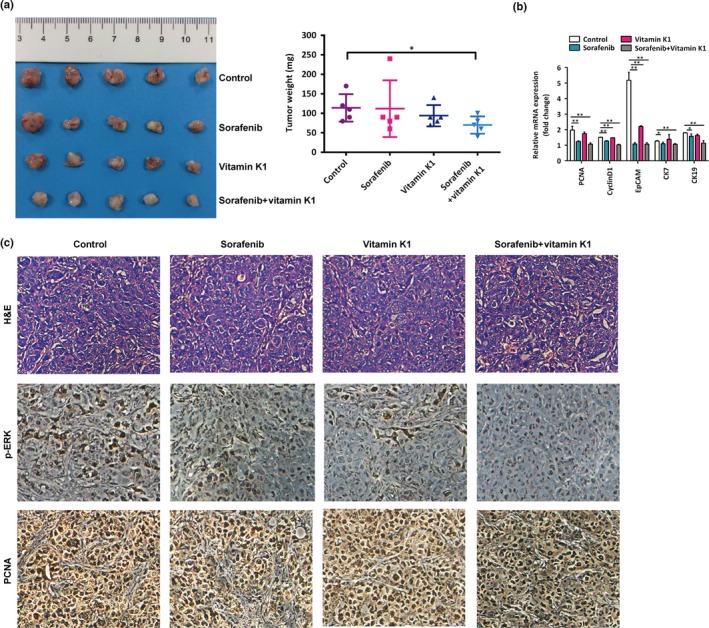
Vitamin K1 plus sorafenib inhibits the stiffness‐induced progression of heat‐exposed residual hepatocellular carcinoma in vivo. (a) Vitamin K1 and sorafenib separately suppressed tumor growth, and the suppression was markedly enhanced by the combination of both agents. (b) PCNA, CyclinD1, EpCAM,CK7, and CK19 mRNA expressions in tumors generated from heat‐treated residual MHCC97H cells with medium stiffness gels were measured using quantitative RT‐PCR. (c) Immunohistochemical analysis was carried out to compare the expression of proliferating cell nuclear antigen (PCNA) and p‐ERK in tumors from the four groups. *P < 0.05; **P < 0.01.
DISCUSSION
Radiofrequency ablation has gained popularity in the management of unresectable small HCC due to its superiority in terms of effectiveness, minimal invasiveness, safety, and repeatability.23 However, when RFA is used for HCCs 3 cm or larger, its treatment efficacy decreases due to the high rate of local tumor recurrence.3 Local tumor recurrence appears to arise from undertreated minimal or occult residual disease, microsatellites after suboptimal RFA, or because of failure to ensure a sufficient safety margin when ablating a larger tumor.24 Moreover, local recurrent tumor after incomplete RFA shows a more invasive progression,5, 6, 7, 25, 26, 27 although its mechanism remains unclear. Here, we reveal that increased matrix stiffness, which will occur after RFA treatment, promotes the proliferation, motility, and progression of heat‐exposed residual HCC cells. More importantly, vitamin K1 and sorafenib can reverse this protumor effect. These findings will help design strategies to treat the local recurrence and prevent its rapid progression after RFA in the treatment of medium‐sized and large HCC.
Most HCCs occur in the setting of cirrhosis. Increased stiffness of liver cirrhosis is caused by excessive ECM deposition. It has been reported that increasing matrix stiffness promotes the proliferation and chemotherapeutic resistance of HCC.18 In our previous work, we showed that higher matrix stiffness increases vascular endothelial growth factor and osteopontin secretion and stem‐like characteristics to promote HCC progression.20, 28, 29 Radiofrequency ablation ablates the tumor and leads to dramatic changes in the mechanical properties of the tumor environment, that is, it further increases the local tissue stiffness.16, 17 In this study, we showed that increased matrix stiffness promotes the progression of heat‐treated residual HCC cells, which will help to explain accelerated disease progression after incomplete RFA. Different from the previous reports that focused on the response of HCC cells themselves to sublethal heat,9, 10, 30, 31, 32, 33, 34 we propose a new mechanism of post‐RFA increased stiffness‐dependent residual tumor progression, namely, heat‐treated residual HCC cells can sense the increased matrix stiffness after RFA to accelerate the tumor progression.
In this study, we found that activation of the ERK pathway contributes to the stiffness‐induced proliferation, motility, and progression of heat‐treated residual HCC cells. Phosphorylation of ERK can mediate mechanotransduction.18 Furthermore, it is also involved in the regulation of cell proliferation and malignant tumor progression including HCC.10, 18, 33, 35 Vitamin K has antitumor activity on oral, pancreatic, and hepatic cancers as a single agent or combined with other therapeutic agents including sorafenib.36, 37, 38 Sorafenib has been approved as the molecular target drug for advanced HCC by suppressing the RAS/RAF/MEK/ERK signal transduction pathway.39, 40
In this study, vitamin K1 plus sorafenib is shown to synergistically and markedly inhibit matrix stiffness‐induced ERK activation and in vivo tumor progression. This result is consistent with previous reports that vitamin K1 with sorafenib exerts an additive inhibitory effect on ERK signaling.39 The combination of sorafenib with RFA significantly decreased post‐RFA HCC recurrence in the treatment of small and medium‐sized HCC.41, 42, 43 Our findings suggest that the potential treatment strategy of RFA combined with vitamin K1 plus sorafenib could further decrease local tumor recurrence after RFA treatment for medium and large HCC (Fig. 7).
Figure 7.
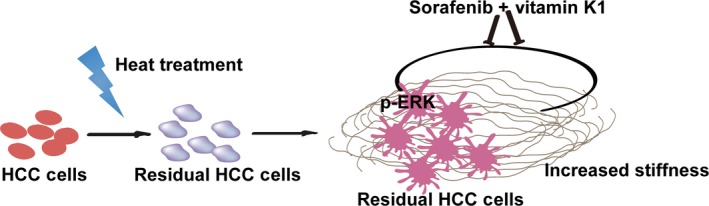
Proposed graphical diagram of increased matrix stiffness promoting tumor progression of heat‐exposed residual hepatocellular carcinoma (HCC) cells. Increased matrix stiffness after heat treatment activates ERK phosphorylation to modulate the progression of heat‐exposed residual HCC cells. Vitamin K1 plus sorafenib can reverse this protumor effect.
In conclusion, our study shows that increased matrix stiffness promotes the progression of heat‐treated residual HCC cells and proposes a new mechanism of the altered biomechanical environment after RFA accelerating residual HCC development. Vitamin K1 and sorafenib disrupt the stiffness‐induced ERK activation to reverse this protumor effect, as potential therapeutic drugs.
DISCLOSURE STATEMENT
The authors have no conflict of interest.
Supporting information
Table S1. Primers for quantitative RT‐PCR.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81472217, 81272723, and 81000909) and the Shanghai Natural Science Fund (Grant No. 09ZR1406400).
Cancer Sci 108 (2017) 1778–1786
Funding information
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81472217, 81272723, and 81000909) and the Shanghai Natural Science Fund (Grant No. 09ZR1406400).
REFERENCES
- 1. Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: an updated review. World J Gastrointest Pharmacol Ther 2016; 7: 477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee J, Lee JM, Yoon JH et al Percutaneous radiofrequency ablation with multiple electrodes for medium‐sized hepatocellular carcinomas. Korean J Radiol 2012; 13(1): 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livraghi T, Goldberg SN, Lazzaroni S et al Hepatocellular carcinoma: radio‐frequency ablation of medium and large lesions. Radiology 2000; 214: 761–8. [DOI] [PubMed] [Google Scholar]
- 4. Seror O, N'Kontchou G, Ibraheem M et al Large (>or=5.0‐cm) HCCs: multipolar RF ablation with three internally cooled bipolar electrodes–initial experience in 26 patients. Radiology 2008; 248: 288–96. [DOI] [PubMed] [Google Scholar]
- 5. Lee HY, Rhim H, Lee MW et al Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol 2013; 23: 190–7. [DOI] [PubMed] [Google Scholar]
- 6. Portolani N, Tiberio GA, Ronconi M et al Aggressive recurrence after radiofrequency ablation of liver neoplasms. Hepatogastroenterology 2003; 50: 2179–84. [PubMed] [Google Scholar]
- 7. Ruzzenente A, Manzoni GD, Molfetta M et al Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol 2004; 10: 1137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koda M, Maeda Y, Matsunaga Y, Mimura K, Murawaki Y, Horie Y. Hepatocellular carcinoma with sarcomatous change arising after radiofrequency ablation for well‐differentiated hepatocellular carcinoma. Hepatol Res 2003; 27: 163–7. [DOI] [PubMed] [Google Scholar]
- 9. Thompson SM, Callstrom MR, Butters KA et al Role for putative hepatocellular carcinoma stem cell subpopulations in biological response to incomplete thermal ablation: in vitro and in vivo pilot study. Cardiovasc Intervent Radiol 2014; 37: 1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshida S, Kornek M, Ikenaga N et al Sublethal heat treatment promotes epithelial‐mesenchymal transition and enhances the malignant potential of hepatocellular carcinoma. Hepatology 2013; 58: 1667–80. [DOI] [PubMed] [Google Scholar]
- 11. Wu L, Fu Z, Zhou S et al HIF‐1alpha and HIF‐2alpha: siblings in promoting angiogenesis of residual hepatocellular carcinoma after high‐intensity focused ultrasound ablation. PLoS One 2014; 9(2): e88913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Rozenblum N, Zeira E, Bulvik B et al Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology 2015; 276: 416–25. [DOI] [PubMed] [Google Scholar]
- 13. Rozenblum N, Zeira E, Scaiewicz V et al Oncogenesis: an “Off‐Target” effect of radiofrequency ablation. Radiology 2015; 276: 426–32. [DOI] [PubMed] [Google Scholar]
- 14. Ouyang Y, Liu K, Hao M et al Radiofrequency ablation‐increased CXCL10 is associated with earlier recurrence of hepatocellular carcinoma by promoting stemness. Tumour Biol 2016; 37: 3697–704. [DOI] [PubMed] [Google Scholar]
- 15. Eyerly SA, Vejdani‐Jahromi M, Dumont DM, Trahey GE, Wolf PD. The evolution of tissue stiffness at radiofrequency ablation sites during lesion formation and in the peri‐ablation period. J Cardiovasc Electrophysiol 2015; 26: 1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwon HJ, Kang MJ, Cho JH et al Acoustic radiation force impulse elastography for hepatocellular carcinoma‐associated radiofrequency ablation. World J Gastroenterol 2011; 17: 1874–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu X, Luo L, Chen J et al Acoustic radiation force impulse elastography for efficacy evaluation after hepatocellular carcinoma radiofrequency ablation: a comparative study with contrast‐enhanced ultrasound. Biomed Res Int 2014; 2014: 901642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schrader J, Gordon‐Walker TT, Aucott RL et al Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology (Baltimore, MD) 2011; 53: 1192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung KS, Kim SU, Ahn SH et al Risk assessment of hepatitis B virus‐related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology (Baltimore, MD) 2011; 53: 885–94. [DOI] [PubMed] [Google Scholar]
- 20. You Y, Zheng Q, Dong Y et al Matrix stiffness‐mediated effects on stemness characteristics occurring in HCC cells. Oncotarget 2016; 7: 32221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci 2013; 70: 1335–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kunjithapatham R, Karthikeyan S, Geschwind JF et al Reversal of anchorage‐independent multicellular spheroid into a monolayer mimics a metastatic model. Sci Rep 2014; 4: 6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg 2009; 249(1): 20–5. [DOI] [PubMed] [Google Scholar]
- 24. Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol 2011; 2011: 104685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shiozawa K, Watanabe M, Takahashi M, Wakui N, Iida K, Sumino Y. Analysis of patients with rapid aggressive tumor progression of hepatocellular carcinoma after percutaneous radiofrequency ablation. Hepatogastroenterology 2009; 56: 1689–95. [PubMed] [Google Scholar]
- 26. Takada Y, Kurata M, Ohkohchi N. Rapid and aggressive recurrence accompanied by portal tumor thrombus after radiofrequency ablation for hepatocellular carcinoma. Int J Clin Oncol 2003; 8: 332–5. [DOI] [PubMed] [Google Scholar]
- 27. Seki T, Tamai T, Ikeda K et al Rapid progression of hepatocellular carcinoma after transcatheter arterial chemoembolization and percutaneous radiofrequency ablation in the primary tumour region. Eur J Gastro Hepatol 2001; 13: 291–4. [DOI] [PubMed] [Google Scholar]
- 28. You Y, Zheng Q, Dong Y et al Higher matrix stiffness upregulates osteopontin expression in hepatocellular carcinoma cells mediated by integrin beta1/GSK3beta/beta‐Catenin signaling pathway. PLoS One 2015; 10(8): e0134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong Y, Xie X, Wang Z et al Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochem Biophys Res Comm 2014; 444: 427–32. [DOI] [PubMed] [Google Scholar]
- 30. Zhang N, Wang L, Chai ZT et al Incomplete radiofrequency ablation enhances invasiveness and metastasis of residual cancer of hepatocellular carcinoma cell HCCLM3 via activating beta‐catenin signaling. PLoS One 2014; 9(12): e115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Brown RE, Martin RC. Incomplete thermal ablation of hepatocellular carcinoma: effects on tumor proliferation. J Surg Res 2013; 181: 250–5. [DOI] [PubMed] [Google Scholar]
- 32. Kong J, Kong J, Pan B et al Insufficient radiofrequency ablation promotes angiogenesis of residual hepatocellular carcinoma via HIF‐1alpha/VEGFA. PLoS One 2012; 7(5): e37266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong S, Kong J, Kong F et al Insufficient radiofrequency ablation promotes epithelial‐mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med 2013; 11: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Obara K, Matsumoto N, Okamoto M et al Insufficient radiofrequency ablation therapy may induce further malignant transformation of hepatocellular carcinoma. Hep Intl 2008; 2(1): 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou SL, Dai Z, Zhou ZJ et al Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology 2012; 56: 2242–54. [DOI] [PubMed] [Google Scholar]
- 36. Okayasu H, Ishihara M, Satoh K, Sakagami H. Cytotoxic activity of vitamins K1, K2 and K3 against human oral tumor cell lines. Anticancer Res 2001; 21: 2387–92. [PubMed] [Google Scholar]
- 37. Showalter SL, Wang Z, Costantino CL et al Naturally occurring K vitamins inhibit pancreatic cancer cell survival through a caspase‐dependent pathway. J Gastroenterol Hepatol 2010; 25: 738–44. [DOI] [PubMed] [Google Scholar]
- 38. Du W, Zhou JR, Wang DL, Gong K, Zhang QJ. Vitamin K1 enhances sorafenib‐induced growth inhibition and apoptosis of human malignant glioma cells by blocking the Raf/MEK/ERK pathway. World J Surg Oncol 2012; 10: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei G, Wang M, Hyslop T, Wang Z, Carr BI. Vitamin K enhancement of sorafenib‐mediated HCC cell growth inhibition in vitro and in vivo. Int J Cancer 2010; 127: 2949–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gao JJ, Shi ZY, Xia JF, Inagaki Y, Tang W. Sorafenib‐based combined molecule targeting in treatment of hepatocellular carcinoma. World J Gastroenterol 2015; 21: 12059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yan SY, Zhang Y, Sun C et al The clinical effect and relevant mechanism of combined sorafenib and radiofrequency ablation in the treatment of early small hepatocellular carcinoma. Oncol Lett 2016; 12: 951–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kan X, Jing Y, Wan QY et al Sorafenib combined with percutaneous radiofrequency ablation for the treatment of medium‐sized hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 2015; 19: 247–55. [PubMed] [Google Scholar]
- 43. Feng X, Xu R, Du X et al Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0‐B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol 2014; 109: 1891–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers for quantitative RT‐PCR.


