Abstract
Spliced variant isoforms of CD44 (CD44v) are a marker of cancer stem cells in solid tumors. They stabilize the xCT subunit of the transporter system xc(–) and thereby promote synthesis of the antioxidant glutathione. Salazosulfapyridine (SASP) is an inhibitor of xCT and suppresses the proliferation of CD44v‐positive cancer cells. Chemotherapy‐naïve patients with advanced non‐squamous non‐small‐cell lung cancer were enrolled in a dose‐escalation study (standard 3 + 3 design) of SASP in combination with cisplatin and pemetrexed. The primary end‐point was the percentage of patients who experience dose‐limiting toxicity. Fifteen patients were enrolled in the study. Dose‐limiting toxicity was observed in one of six patients at a SASP dose of 1.5 g/day (elevation of aspartate and alanine aminotransferase levels, each of grade 3), two of five patients at 3 g/day (hypotension or pneumonitis, each of grade 3), and two of three patients at 4.5 g/day (anorexia of grade 3). The maximum tolerated dose was thus 3 g/day, and the recommended dose was 1.5 g/day. The overall response rate was 26.7% and median progression‐free survival was 11.7 months, much longer than that for cisplatin–pemetrexed alone in previous studies. Exposure to SASP varied markedly among individuals according to ABCG2 and NAT2 genotypes. The serum concentration of free CD44v protein was increased after the first cycle of treatment, possibly reflecting death of cancer stem cells. Salazosulfapyridine was thus given safely in combination with cisplatin–pemetrexed, with the addition of SASP tending to prolong progression‐free survival. This trial is registered in the UMIN Clinical Trials Registry as UMIN000017854.
Keywords: Cancer stem cell, CD44v, non‐small‐cell lung cancer, oxidative stress, salazosulfapyridine
Cancer stem cells constitute a small population of cancer cells that are capable of self‐renewal and cancer initiation.1 Cancer stem cells are also more resistant to cancer treatments, including chemotherapy and radiation therapy, than are other cancer cells.2, 3 Treatment strategies that target CSCs are therefore being pursued in order to improve outcomes in cancer patients. Markers for CSCs are thought to include CD44, CD133, CD90, and aldehyde dehydrogenase, but no agents that specifically target CSCs have yet been established.4
CD44 is an adhesion molecule for the ECM and is implicated in various physiological and pathological processes, including tumor cell growth, invasion, and metastasis.5, 6 It has also been identified as a cell surface marker for CSCs of solid tumors.5 Splice variant isoforms of CD44 are expressed in various tumors7, 8, 9, 10 and have recently been found to bind to xCT, a subunit of a cystine‐glutamate antiporter known as system xc(–). Such binding stabilizes expression of this transporter system at the cell surface and thereby promotes the intracellular synthesis of the antioxidant GSH from imported cystine. Expression of CD44v in tumor cells thus confers resistance to oxidative stress and thereby promotes tumor growth and treatment resistance.11 The stem cell‐like characteristics of CD44v‐positive cancer cells are dependent on xCT‐mediated cystine transport and consequent upregulation of the GSH‐dependent antioxidant system for maintenance of cellular redox homeostasis.
Non‐small‐cell lung cancer is the leading cause of cancer‐related mortality worldwide.12 The standard first‐line treatment for advanced NSCLC has long been platinum‐based combination chemotherapy, with the goal of prolongation of life and relief of symptoms.13, 14 Recently, however, the identification of driver oncogenes, such as those encoding mutant forms of EGFR and fusions of ALK, has led to the development of targeted agents such as EGFR‐TKIs and ALK‐TKIs that have proven superior to chemotherapy for first‐line management of patients with advanced NSCLC positive for these genetic alterations.15 Pembrolizumab, which targets the programmed cell death‐1 immune checkpoint protein on cytotoxic T cells, has also been developed for first‐line treatment of advanced NSCLC.16 However, almost all patients eventually acquire resistance to these various drugs, with the result that the prognosis of individuals with advanced NSCLC remains poor. Innovative drug therapies are therefore needed to improve treatment outcome for advanced NSCLC.
Salazosulfapyridine, a conventional small‐molecule agent used in the treatment of ulcerative colitis and rheumatoid arthritis, is a specific inhibitor of xCT‐mediated cystine transport and has been shown to suppress the proliferation of CD44v‐positive cancer cells.17, 18 A recent clinical study based on PET with (4S)‐4‐(3‐[18F]fluoropropyl)‐L‐glutamate, a probe for xCT‐dependent transport, revealed that the maximized standardized uptake value for the probe was related to the expression levels of CD44 and xCT in human NSCLC.19 These observations suggest that SASP is a potential targeted agent for CD44v‐expressing stem‐like cancer cells in NSCLC.
This is an open‐label, dose‐escalation phase I study for SASP in patients with advanced NSCLC. The primary objective was to determine the RD of SASP in combination with standard doses of CDDP and PEM for first‐line treatment. Secondary objectives included characterization of the safety and pharmacokinetic profiles of SASP as well as preliminary assessment of the antitumor activity of this combination regimen.
Materials and Methods
Study design
This open‐label, dose‐escalation phase I study was undertaken at two centers in Japan. Patients received i.v. PEM (500 mg/m2) followed by i.v. CDDP (75 mg/m2) on day 1 of each treatment cycle as well as oral SASP t.i.d. on days 1–21. Prophylactic anti‐emetic therapy for CDDP was given, with aprepitant, 5‐HT3 receptor antagonists, and dexamethasone being recommended. The study was based on a standard 3 + 3 trial design for detection of DLT in treatment cycle 1. Our findings with a mouse xenograft model revealed that SASP had an additive antitumor effect with CDDP after its i.p. administration at a daily dose of 50 mg/kg (Fig. S1), which corresponds to a dose of 1.6 g/day in humans. Given that the elimination half‐life of SASP is ~10 h in human blood, we anticipated that administration of the drug three times daily would ensure a sufficient blood concentration and be beneficial in terms of treatment adherence. For these reasons, the starting dose of SASP in the present study was set at 1.5 g/day (500 mg t.i.d.).
Patients were assigned to escalating‐dose cohorts in order of their admission into the study. Dose‐limiting toxicity was defined as toxicity according to CTCAE that was at least possibly related to treatment and included neutropenia of grade 4 that was uncomplicated (not associated with fever) for >7 days, febrile neutropenia of grade 4, thrombocytopenia of grade 4, other non‐hematologic toxicity of grade ≥3, pneumonitis of grade ≥2, or any criterion for treatment interruption that resulted in a treatment compliance rate of <70% during a course. Dose‐limiting toxicity observed during the first 21 days of treatment served as the basis for determination of the MTD. If DLT was observed in at least two of three patients during the first treatment cycle, the relevant dose level was determined to be the MTD. If DLT was observed in only one of three patients, up to three new patients were enrolled at that dose level. If DLT was then observed in at least two of six patients, the dose level was determined to be the MTD. When the MTD was determined, the next lowest dose level was determined to be the RD if six patients had been treated at that dose level; however, if only three patients had been treated at that dose level, another three patients were enrolled and this dose level was determined as the RD, unless DLT was observed in more than one of six patients.
Patients who experienced DLT discontinued study medication. After four cycles of combination therapy with CDDP, PEM, and SASP, patients without disease progression were eligible for maintenance therapy with PEM and SASP every 21 days. Salazosulfapyridine was given for up to 365 days.
The trial was carried out in compliance with the study protocol, the Declaration of Helsinki, and Japanese Good Clinical Practice guidelines. All patients provided written informed consent. The trial was carried out at Kyushu University Hospital and National Kyushu Cancer Center (both Fukuoka, Japan) after the protocol was approved by each institutional review board and registered as UMIN000017854.
Study group
Adult (≥20 years of age) patients with pathologically confirmed stage IIIB or IV non‐squamous NSCLC, an ECOG performance status of 0 or 1, and adequate organ function were eligible for the study. Individuals who had previously received cytotoxic chemotherapy for NSCLC were excluded from the trial, although previous treatment with EGFR‐TKIs for EGFR mutation‐positive patients or with ALK‐TKIs for ALK fusion‐positive patients was allowed. Individuals with symptomatic brain metastases were excluded, as were those with spinal metastasis requiring irradiation or surgery. Patients with asymptomatic brain metastasis were eligible. Patients who underwent palliative radiotherapy for metastatic lesions or surgery under general anesthesia within 14 days before enrollment were excluded.
Efficacy assessment
Objective tumor response was assessed according to RECIST (version 1.1)20 every 6 weeks for the first 6 months and every 9 weeks thereafter. Progression‐free survival was assessed for each patient who received at least one dose of SASP.
Pharmacokinetic analysis
For pharmacokinetic analysis, the first individual dose of SASP on day 1 was administered orally together with 200 mL water after the patients had fasted overnight, with a meal being permitted after blood sampling at 4 h after the drug was given. Blood samples were obtained before and at 0.5, 1, 2, 3, 4, 6, 9, 12, and 24 h after SASP administration. The other two doses of SASP were not given on day 1. Plasma was prepared from blood by centrifugation and was stored at −20°C until analysis. The plasma concentration of SASP was determined by a validated ultraperformance liquid chromatography and tandem mass spectrometry method.21 The AUC0–24 for SASP was calculated according to the linear trapezoidal rule.
Genotyping of ABCG2 and NAT2
The single nucleotide polymorphism rs2231142 in ABCG2 (421C→A, Q141K) and NAT2 genotype (NAT2*4, *5B, *6A, *7B), both of which affect the pharmacokinetics of SASP, were evaluated by direct sequencing of genomic DNA isolated from blood samples.
Measurement of free CD44v protein level in serum
Serum samples collected from 14 patients before treatment and on day 21 of treatment cycle 1 were tested with ELISA for human CD44v9 (Cosmo Bio, Tokyo, Japan). The amount of free CD44v in serum was calculated relative to the absorbance of 1 μL culture supernatant of the CD44v9‐positive cell line OSC 19 as 1 unit.
Statistical analysis
The primary end‐point of the study was the percentage of patients who experienced DLT. Secondary end‐points included adverse events, pharmacokinetics of SASP, response rate, and PFS. Changes in the serum level of free CD44v protein were assessed with the paired Student's t‐test as performed with SAS software version 9.3 (SAS Institute, Cary, NC, USA). A P‐value of < 0.05 was considered statistically significant.
Results
Patient characteristics
Fifteen patients at two institutions were enrolled in the study between April 2015 and February 2016. The demographics and clinical characteristics of the study participants are shown in Table 1. The median age was 66 years (range, 42–74 years), 10 patients were men, and 14 individuals had stage IV disease. EGFR mutation status was evaluated in all patients, five of whom were found to harbor activating EGFR mutations (exon 19 deletions or L858R in exon 21) and had been previously treated with EGFR‐TKIs. One patient was positive for the EML4‐ALK fusion gene and had been previously treated with crizotinib and alectinib.
Table 1.
Characteristics of patients with non‐small‐cell lung cancer treated with salazosulfapyridine in combination with cisplatin and pemetrexed (n = 15)
| Characteristics | No. of patients (n = 15) |
|---|---|
| Age, years | |
| Median | 66 |
| Range | 42–74 |
| Sex, n (%) | |
| Male | 10 (67) |
| Female | 5 (33) |
| ECOG performance status, n (%) | |
| 0 | 6 (40) |
| 1 | 9 (60) |
| Clinical stage, n (%) | |
| IIIB | 1 (7) |
| IV | 14 (93) |
| Histology, n (%) | |
| Adenocarcinoma | 15 (100) |
| Smoking status, n (%) | |
| Never smoked | 7 (47) |
| Ex‐smoker | 5 (33) |
| Current smoker | 3 (20) |
| Gene mutation status, n (%) | |
| None | 9 (60) |
| EGFR L858R | 3 (20) |
| EGFR Ex19del | 2 (13) |
| EML4‐ALK | 1 (7) |
| Prior treatment, n (%) | |
| None | 9 (60) |
| Gefitinib | 4 (27) |
| Afatinib | 1 (7) |
| Crizotinib and alectinib | 1 (7) |
Maximum tolerated dose and DLT
Allocation of patients to treatment during the study is summarized in Figure 1, and DLTs apparent at each dose level are listed in Table 2. Dose‐limiting toxicity was not observed in the first three patients treated at dose level 1. At dose level 2, the first patient experienced hives of grade 3 at 9 days after the onset of SASP treatment, and this condition was ameliorated immediately after discontinuation of SASP. The external Efficacy and Safety Review Committee recommended that the patient be excluded from DLT assessment because the hives were regarded as an incidental adverse event. The subsequent three patients at dose level 2 did not experience DLT. At dose level 3, two of three patients experienced DLT (anorexia of grade 3). According to the protocol, two additional patients were enrolled at dose level 2, and two of the total of five patients treated at this dose level experienced DLT (hypotension or pneumonitis, each of grade 3). To confirm the safety of dose level 1, three additional patients were enrolled, with one of the total of six patients treated at this dose level experiencing DLT (elevation of aspartate and alanine aminotransferase levels, each of grade 3). The MTD and RD of SASP, when given in combination with full‐dose CDDP and PEM, were therefore determined to be 3 and 1.5 g/day, respectively. All DLTs were reversible after additional treatment or discontinuation of SASP.
Figure 1.
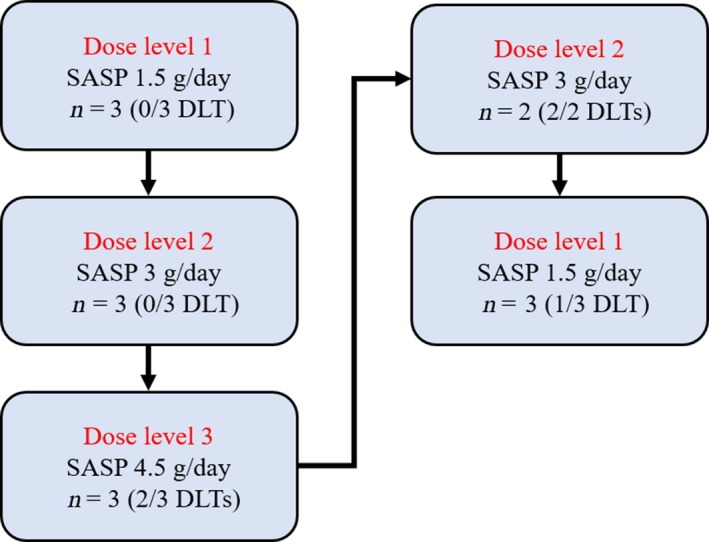
Patient flow in this phase I study of salazosulfapyridine (SASP) with cisplatin and pemetrexed for treatment of advanced non‐small‐cell lung cancer. DLT, dose‐limiting toxicity.
Table 2.
Observed dose‐limiting toxicities (DLTs) at each dose level of salazosulfapyridine (SASP) in patients with advanced non‐small‐cell lung cancer
| Dose level | SASP dose (g/day) | No. of DLTs/patients | DLTs |
|---|---|---|---|
| 1 | 1.5 | 1/6 | (1) ALT and AST elevation |
| 2 | 3.0 | 2/5a | (1) Hypotension; (2) Pneumonitis |
| 3 | 4.5 | 2/3 | (1) Anorexia; (2) Anorexia |
Patients eligible for evaluation of dose‐limiting toxicity. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Safety profile of combination therapy with CDDP, PEM, and SASP
Fifteen patients received combination therapy with CDDP, PEM, and SASP. The median number of treatment cycles was two (range, 1–17 cycles), and the median duration of SASP treatment was 45 days (range, 8–365 days). The most frequent drug‐related adverse events (all CTCAE grades) during protocol treatment were anorexia (n = 14), fatigue (n = 14), nausea (n = 12), anemia (n = 10), vomiting (n = 9), constipation (n = 9), leukopenia (n = 9), and neutropenia (n = 9) (Table 3). Adverse events of grade 3 or 4 observed in ≥20% of patients included neutropenia (n = 8), hyponatremia (n = 7), leukopenia (n = 6), and anorexia (n = 4). There were no treatment‐related deaths.
Table 3.
Frequency of drug‐related adverse events in patients with advanced non‐small‐cell lung cancer during protocol treatment with salazosulfapyridine (SASP) in combination with cisplatin and pemetrexed
| SASP dose (g/day) | All patients (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1.5 (n = 6) | 3.0 (n = 6) | 4.5 (n = 3) | ||||||
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Non‐hematologic | ||||||||
| Anorexia | 5 (83) | 0 (0) | 6 (100) | 2 (33) | 3 (100) | 2 (67) | 14 (93) | 4 (27) |
| Fatigue | 6 (100) | 0 (0) | 5 (83) | 0 (0) | 3 (100) | 0 (0) | 14 (93) | 0 (0) |
| Nausea | 4 (67) | 0 (0) | 5 (83) | 0 (0) | 3 (100) | 1 (33) | 12 (80) | 1 (7) |
| Vomiting | 3 (50) | 0 (0) | 3 (50) | 0 (0) | 3 (100) | 1 (33) | 9 (60) | 1 (7) |
| Constipation | 4 (67) | 0 (0) | 4 (67) | 0 (0) | 1 (33) | 0 (0) | 9 (60) | 0 (0) |
| Hyponatremia | 2 (33) | 2 (33) | 4 (67) | 4 (67) | 2 (67) | 1 (33) | 8 (53) | 7 (47) |
| ALT increased | 5 (83) | 2 (33) | 2 (33) | 0 (0) | 1 (33) | 0 (0) | 8 (53) | 2 (13) |
| AST increased | 4 (67) | 1 (17) | 2 (33) | 1 (17) | 1 (33) | 0 (0) | 7 (47) | 2 (13) |
| GGT increased | 5 (83) | 2 (33) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 7 (47) | 2 (13) |
| Stomach ache | 1 (17) | 0 (0) | 2 (33) | 0 (0) | 2 (67) | 0 (0) | 5 (33) | 0 (0) |
| Hypotension | 0 (0) | 0 (0) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 1 (7) | 1 (7) |
| Pneumonitis | 0 (0) | 0 (0) | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 1 (7) | 1 (7) |
| Febrile neutropenia | 1 (17) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (7) | 1 (7) |
| Hematologic | ||||||||
| Anemia | 6 (100) | 0 (0) | 3 (50) | 1 (17) | 1 (33) | 1 (33) | 10 (67) | 2 (13) |
| Leukopenia | 3 (50) | 2 (33) | 3 (50) | 2 (33) | 3 (100) | 2 (67) | 9 (60) | 6 (40) |
| Neutropenia | 4 (67) | 3 (50) | 3 (50) | 3 (50) | 2 (67) | 2 (67) | 9 (60) | 8 (53) |
| Thrombocytopenia | 2 (33) | 0 (0) | 4 (67) | 0 (0) | 2 (67) | 0 (0) | 8 (53) | 0 (0) |
Data are shown as n (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyl transpeptidase.
Pharmacokinetics of SASP
The C max of SASP after the first single dose on day 1 at levels 1, 2, and 3 ranged from 7.1 to 24.8 μg/mL, from 8.5 to 39.2 μg/mL, and from 6.5 to 13.9 μg/mL, respectively (Table 4). The AUC0–24 after the first single dose on day 1 at levels 1, 2, and 3 ranged from 55.2 to 235.5 μg/h/mL, from 102.8 to 477.4 μg/h/mL, and from 49.1 to 158.4 μg/h/mL, respectively. Both C max and AUC0–24 thus varied markedly among individuals within each dose level. High AUC0–24 values for SASP were apparent in patients with two reduced‐function alleles of ABCG2 (A/A) or NAT2 (*6A/*6A or *6A/*7B).22 In addition, exposure to SASP did not correspond to the occurrence of DLT.
Table 4.
Pharmacokinetic parameters of salazosulfapyridine and genotypes of ABCG2 and NAT2 in patients with advanced non‐small‐cell lung carcinoma
| Dose level | Patient | C max (μg/mL) | AUC0–24 (μg/h/mL) | ABCG2 | NAT2 |
|---|---|---|---|---|---|
| 1 | 1 | 9.5 | 86.8 | C/C | *4/*4 |
| 2 | 7.1 | 55.2 | C/C | *4/*4 | |
| 3 | 10.5 | 119.3 | C/C | *4/*7B | |
| 13a | 10.6 | 96.6 | C/C | *4/*6A | |
| 14 | 7.9 | 87.2 | C/A | *4/*4 | |
| 15 | 24.8 | 235.5 | A/A | *4/*6A | |
| 2 | 4 | 17.9 | 157.3 | C/A | *4/*7B |
| 5 | 8.5 | 102.8 | C/A | *4/*4 | |
| 6 | 39.2 | 477.4 | A/A | *4/*7B | |
| 7 | 17.1 | 225.7 | C/A | *4/*7B | |
| 11a | 13.1 | 132.8 | C/C | *4/*4 | |
| 12a | 21.4 | 247.4 | C/A | *6A/*7B | |
| 3 | 8a | 10.0 | 158.4 | C/C | *6A/*6A |
| 9a | 13.9 | 146.7 | C/C | *4/*4 | |
| 10 | 6.5 | 49.1 | C/C | *4/*4 |
Patients with dose‐limiting toxicities. AUC0–24, area under the concentration–time curve from 0 to 24 h; C max, maximum plasma concentration.
Efficacy
Two of 15 patients were not evaluable for objective response according to RECIST because they had no post‐treatment tumor measurement. Among the 13 assessable patients, four individuals showed a partial response (two at dose level 1, two at dose level 2), yielding an overall response rate of 26.7%. An additional seven patients (46.7%) experienced stable disease, yielding a disease control rate of 73.3%. Median PFS for all 15 patients was 11.7 months (Fig. 2a). At the time of data analysis, 11 of the 15 patients were alive, yielding a 1‐year survival rate of 73%.
Figure 2.
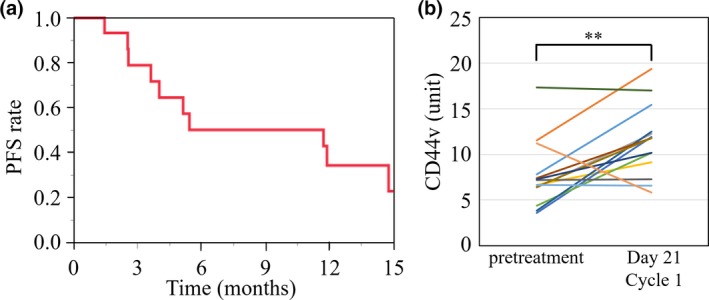
(a) Progression‐free survival (PFS) for all study patients with advanced non‐small‐cell lung cancer treated with salazosulfapyridine in combination with cisplatin and pemetrexed (n = 15). (b) Serum level of free CD44 variant (CD44v) before treatment and at day 21 of treatment cycle 1 for 14 of the study patients. **P < 0.01 (paired Student's t‐test).
Analysis of free CD44v protein in serum
Serum was obtained from 14 patients both before treatment and on day 21 of cycle 1 for measurement of free CD44v protein level. The CD44v level after the first treatment cycle was significantly higher than that before treatment (Fig. 2b).
Discussion
The efficacy of cancer therapies including cytotoxic chemotherapy and radiation therapy is attributable in part to the production of reactive oxygen species and the consequent induction of oxidative stress in cancer cells.2 Variant isoforms of CD44 have recently been found to stabilize the cystine transporter subunit xCT and thereby to promote intracellular formation of the antioxidant GSH and consequent chemoresistance in cancer cells.11 Given that CD44 is a CSC marker, the CD44v–xCT complex is thought to play an important role in chemoresistance in CSCs. This complex is therefore considered a potential novel target for therapy directed against CSCs. Salazosulfapyridine, a drug commonly used to treat ulcerative colitis and rheumatoid arthritis, is a well‐characterized specific inhibitor of xCT.17, 18 Given that SASP has shown efficacy for treatment of CD44v‐positive tumors in animal models,23 it is a potential anticancer drug for targeting of CSCs. This was a phase I trial of SASP in combination with standard chemotherapy for non‐squamous NSCLC. The primary objective of our trial was to determine the RD of SASP.
Our results show that SASP combined with standard‐dose CDDP and PEM has a manageable safety profile when given at dose level 1 (1.5 g/day). Two of three patients experienced anorexia of grade 3 as a DLT at dose level 3 (4.5 g/day), despite adequate prophylactic treatment with anti‐emetic agents. The common adverse events of SASP are gastrointestinal toxicity (nausea, vomiting, gastrointestinal upset, diarrhea, and stomach cramps), fatigue, and headache.24 It is possible that the observed anorexia reflected synergistic toxicity for the combination of SASP and CDDP. One patient treated at dose level 2 (3 g/day) experienced pneumonitis of grade 3 as a DLT. Although respiratory disorders are not common adverse events of SASP treatment, SASP has previously been implicated in the development of pneumonitis.25 Hypotension of grade 3, which has previously been reported as a severe side‐effect of SASP, was observed as a DLT in one patient at dose level 2. The occurrence of DLT for this combination therapy was independent of SASP exposure as reflected by AUC0–24 and Cmax.
Only approximately one‐third of an oral dose of SASP is absorbed by the intestine. The remaining drug is metabolized by intestinal bacteria to 5‐aminosalicylic acid and sulfapyridine, which have no effect on xCT. Sulfapyridine is relatively well absorbed from the intestine and further metabolized by NAT2 in the liver, whereas 5‐aminosalicylic acid is absorbed to a much lesser extent. Breast cancer resistance protein, which is an ATP‐binding cassette transporter encoded by ABCG2, restricts intestinal SASP absorption. Similar to the results of a previous phase I study of SASP monotherapy,21 exposure to SASP after oral doses of 1.5–4.5 g/day in the present study was not dose‐dependent, likely reflecting genetic polymorphism of ABCG2 and NAT2.
A previous phase I/II study of SASP in patients with recurrent or progressive glioma found no tumor shrinkage after treatment at a dose of 1.5–6 g/day.26 A more recent phase I study of SASP at a dose of 8–12 g/day in previously treated patients with advanced gastric cancer also detected no tumor shrinkage.21 In our present phase I study, the response rate (26.7%) according to RECIST did not differ from that previously observed for chemotherapy with CDDP and PEM in patients advanced NSCLC. However, the median PFS of 11.7 months in our study was substantially longer than that (4.0–5.3 months) reported in previous studies of CDDP and PEM for non‐squamous NSCLC.27, 28, 29, 30 Given that tumor relapse after chemotherapy is thought to be driven by CSCs,1 a reduction in the number of CSCs induced by SASP treatment may prolong time to relapse. Indeed, immunohistochemical analysis of tumor tissue obtained before treatment in the present study revealed that the median PFS of patients (n = 5) with a high proportion of CD44v‐positive cells was longer than that of those (n = 8) with a low proportion (>12 vs. 7.9 months), despite the lack of a confirmed partial response in the former patients. Although similar evaluation with a larger sample size is warranted, these data suggest that the proportion of CD44v‐positive cells is a potential predictive biomarker for SASP treatment.
The precise mechanism of CD44v release and the origin of circulating CD44v are not known, although CD44v may be released in at least two distinct types of vesicles, apoptotic blebs and exosomes.31 The adhesion molecule L1 has been shown to be cleaved in exosomes and apoptotic membrane vesicles released from ovarian cancer cells undergoing apoptosis.32 Cleavage of CD44 also occurs in exosomes33 and might be induced by apoptotic stimuli.34 Together, these observations suggest that CD44v might be cleaved in and enter plasma by way of apoptotic blebs and exosomes released from CD44v‐positive stem‐like cancer cells undergoing apoptosis. Consistent with our data, SASP treatment was previously associated with a decrease in the number of CD44v‐positive cells in post‐treatment tumor tissue of patients with advanced gastric cancer.21 It is thus possible that the prolonged PFS observed in the present study was due to a SASP‐induced reduction in the number of CD44v‐positive CSCs that are the origin of disease recurrence.
In conclusion, we found that SASP was safe at the RD of 1.5 g/day in combination with CDDP and PEM. Our results suggest that this triplet regimen may prolong PFS compared with that achieved with CDDP and PEM alone. Given that only approximately one‐third of SASP given orally is absorbed by the intestine, and that the remaining drug induces gastrointestinal toxicity, an i.v. injectable, water‐soluble form of the drug is now under development in order to reduce such toxicity and to facilitate maintenance of an effective blood concentration of SASP. Further development of additional agents that target the CD44v–xCT complex is also warranted to evaluate the efficacy of CSC‐targeted therapy as a novel treatment strategy.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- ALK
anaplastic lymphoma kinase
- AUC0–24
area under the concentration–time curve from 0 to 24 h
- CD44v
spliced variant isoforms of CD44
- CDDP
cisplatin
- Cmax
maximum plasma concentration
- CSC
cancer stem cell
- DLT
dose‐limiting toxicity
- EGFR
epidermal growth factor receptor
- GSH
glutathione
- MTD
maximum tolerated dose
- NAT2
N‐acetyltransferase 2
- NSCLC
non‐small‐cell lung cancer
- PEM
pemetrexed
- PFS
progression‐free survival
- RD
recommended dose
- SASP
salazosulfapyridine
- TKI
tyrosine kinase inhibitor
Supporting information
Fig. S1. Time course of the volume of tumors formed by HCT116 p53−/− cells in nude mice treated with CDDP (2 mg/kg) plus SASP (50, 100, 200, or 400 mg/kg). CDDP was injected intraperitoneally 5, 8, and 11 days after subcutaneous injection of 2 × 106 cells. SASP or saline was injected intraperitoneally once daily from days 5 to 10. Data are means ± SEM for 5 mice per group.
Acknowledgments
This study was supported by AMED (Translational Research Network Program) of the Japan Agency for Medical Research and Development (grant no. 16lm0103008j0005). Pemetrexed was kindly provided by Eli Lilly Japan. We thank Toshihiko Doi (Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa City, Japan) and Jiichiro Sasaki (Department of Respiratory Medicine, Kitasato University School of Medicine, Sagamihara City, Japan) as members of the external Efficacy and Safety Review Committee, as well as Koji Todaka, Ritsuko Taketomi, and other staff of the Center for Clinical and Translational Research, Kyushu University Hospital, for data collection, analysis, and interpretation, as well as helpful advice.
Cancer Sci 108 (2017) 1843–1849
Funding Information
Japan Agency for Medical Research and Development (16lm0103008j0005), Eli Lilly Japan.
References
- 1. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 2011; 17: 313–19. [DOI] [PubMed] [Google Scholar]
- 2. Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS‐mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009; 8: 579–91. [DOI] [PubMed] [Google Scholar]
- 3. Nagano O, Okazaki S, Saya H. Redox regulation in stem‐like cancer cells by CD44 variant isoforms. Oncogene 2013; 32: 5191–8. [DOI] [PubMed] [Google Scholar]
- 4. Zakaria N, Satar NA, Abu Halim NH et al Targeting lung cancer stem cells: research and clinical impacts. Front Oncol 2017; 7: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003; 4: 33–45. [DOI] [PubMed] [Google Scholar]
- 6. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci 2004; 95: 930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okamoto I, Morisaki T, Sasaki J et al Molecular detection of cancer cells by competitive reverse transcription‐polymerase chain reaction analysis of specific CD44 variant RNAs. J Natl Cancer Inst 1998; 90: 307–15. [DOI] [PubMed] [Google Scholar]
- 8. Sasaki JI, Tanabe KK, Takahashi K et al Expression of CD44 splicing isoforms in lung cancers: dominant expression of CD44v8‐10 in non‐small cell lung carcinomas. Int J Oncol 1998; 12: 525–33. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi K, Yamaguchi A, Urano T, Goi T, Nakagawara G, Shiku H. Expression of CD44 variant exons 8‐10 in colorectal cancer and its relationship to metastasis. Jpn J Cancer Res 1995; 86: 292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamaguchi A, Saito M, Gio T et al Expression of CD44 variant exons 8‐10 in gastric cancer. Jpn J Cancer Res 1995; 86: 1166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishimoto T, Nagano O, Yae T et al CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(‐) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387–400. [DOI] [PubMed] [Google Scholar]
- 12. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008; 83: 584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Azzoli CG, Temin S, Aliff T et al 2011 focused update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non‐Small‐Cell Lung Cancer. J Clin Oncol 2011; 29: 3825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reck M, Popat S, Reinmuth N et al Metastatic non‐small‐cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014; 25(Suppl 3): iii27–39. [DOI] [PubMed] [Google Scholar]
- 15. Besse B, Adjei A, Baas P et al 2nd ESMO Consensus Conference on Lung Cancer: non‐small‐cell lung cancer first‐line/second and further lines of treatment in advanced disease. Ann Oncol 2014; 25: 1475–84. [DOI] [PubMed] [Google Scholar]
- 16. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 17. Chen RS, Song YM, Zhou ZY et al Disruption of xCT inhibits cancer cell metastasis via the caveolin‐1/beta‐catenin pathway. Oncogene 2009; 28: 599–609. [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Trachootham D, Liu J et al Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat Cell Biol 2012; 14: 276–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baek S, Choi CM, Ahn SH et al Exploratory clinical trial of (4S)‐4‐(3‐[18F]fluoropropyl)‐L‐glutamate for imaging xC‐ transporter using positron emission tomography in patients with non‐small cell lung or breast cancer. Clin Cancer Res 2012; 18: 5427–37. [DOI] [PubMed] [Google Scholar]
- 20. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 21. Shitara K, Doi T, Nagano O et al Dose‐escalation study for the targeting of CD44v+ cancer stem cells by sulfasalazine in patients with advanced gastric cancer (EPOC1205). Gastric Cancer 2017; 20: 341–9. [DOI] [PubMed] [Google Scholar]
- 22. Yamasaki Y, Ieiri I, Kusuhara H et al Pharmacogenetic characterization of sulfasalazine disposition based on NAT2 and ABCG2 (BCRP) gene polymorphisms in humans. Clin Pharmacol Ther 2008; 84: 95–103. [DOI] [PubMed] [Google Scholar]
- 23. Yoshikawa M, Tsuchihashi K, Ishimoto T et al xCT inhibition depletes CD44v‐expressing tumor cells that are resistant to EGFR‐targeted therapy in head and neck squamous cell carcinoma. Cancer Res 2013; 73: 1855–66. [DOI] [PubMed] [Google Scholar]
- 24. Wiese MD, Alotaibi N, O'Doherty C et al Pharmacogenomics of NAT2 and ABCG2 influence the toxicity and efficacy of sulphasalazine containing DMARD regimens in early rheumatoid arthritis. Pharmacogenomics J 2014; 14: 350–5. [DOI] [PubMed] [Google Scholar]
- 25. Parry SD, Barbatzas C, Peel ET, Barton JR. Sulphasalazine and lung toxicity. Eur Respir J 2002; 19: 756–64. [DOI] [PubMed] [Google Scholar]
- 26. Robe PA, Martin DH, Nguyen‐Khac MT et al Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer 2009; 9: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scagliotti GV, Parikh P, von Pawel J et al Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 28. Barlesi F, Gervais R, Lena H et al Pemetrexed and cisplatin as first‐line chemotherapy for advanced non‐small‐cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07‐01). Ann Oncol 2011; 22: 2466–70. [DOI] [PubMed] [Google Scholar]
- 29. Paz‐Ares L, de Marinis F, Dediu M et al Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non‐squamous non‐small‐cell lung cancer (PARAMOUNT): a double‐blind, phase 3, randomised controlled trial. Lancet Oncol 2012; 13: 247–55. [DOI] [PubMed] [Google Scholar]
- 30. Kawano Y, Ohyanagi F, Yanagitani N et al Pemetrexed and cisplatin for advanced non‐squamous non‐small cell lung cancer in Japanese patients: phase II study. Anticancer Res 2013; 33: 3327–33. [PubMed] [Google Scholar]
- 31. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–79. [DOI] [PubMed] [Google Scholar]
- 32. Gutwein P, Stoeck A, Riedle S et al Cleavage of L1 in exosomes and apoptotic membrane vesicles released from ovarian carcinoma cells. Clin Cancer Res 2005; 11: 2492–501. [DOI] [PubMed] [Google Scholar]
- 33. Stoeck A, Keller S, Riedle S et al A role for exosomes in the constitutive and stimulus‐induced ectodomain cleavage of L1 and CD44. Biochem J 2006; 393: 609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gunthert AR, Strater J, von Reyher U et al Early detachment of colon carcinoma cells during CD95(APO‐1/Fas)‐mediated apoptosis. I. De‐adhesion from hyaluronate by shedding of CD44. J Cell Biol 1996; 134: 1089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Time course of the volume of tumors formed by HCT116 p53−/− cells in nude mice treated with CDDP (2 mg/kg) plus SASP (50, 100, 200, or 400 mg/kg). CDDP was injected intraperitoneally 5, 8, and 11 days after subcutaneous injection of 2 × 106 cells. SASP or saline was injected intraperitoneally once daily from days 5 to 10. Data are means ± SEM for 5 mice per group.


